Introduction
Osteoarthritis (OA) is a common degenerative joint
disease that is caused by strain, trauma, joint deformity and other
factors (1). At present, it has
been reported that 240 million individuals suffer from OA globally,
mostly affecting those who are middle-aged or elderly (2). The number of patients with OA will
increase with the aging population, which will result in the heavy
economic burden for patients and society (3). Current therapies for OA include
pharmacological treatment and methods to relieve pain, whereas knee
arthroplasty is the final therapeutic option if other methods fail
(4,5). Nevertheless, the pathogenesis of OA
is yet to be fully elucidated and it is important to discover
effective therapeutic methods for patients with OA.
IL-1β, a member of the IL-1 cytokine family, is
involved in articular cartilage degeneration (6). IL-1β is also a vital regulator in the
pathogenesis of OA (7). IL-1β
signaling has been considered a promising target to treat OA
(8). Previous in vivo
models and clinical trials have displayed contradictory results.
Although a number of studies have shown that IL-1 inhibition is an
effective treatment, other studies have also shown that IL-1
inhibition is not an effective analgesic/anti-inflammatory therapy,
especially from previous large-scale clinical studies targeting
IL-1β (9-12).
Chondrocytes are the only cells found in articular cartilage and
serve a key role in maintaining matrix integrity (13). IL-1β-stimulated chondrocytes have
been used to mimic articular cartilage injury in vitro
(14). IL-1β-stimulated CHON-001
cells have been widely used as an in vitro model to study OA
(15-18).
NF-κB, a common transcription factor, serves a key
role in the regulation of cellular responses, including
inflammation, the immune response and apoptosis (19-21).
Under physiological conditions, NF-κB is located in the cytosol and
is combined with its inhibitory protein, IκB. Once IκB is
phosphorylated, the NF-κB hetero-dimer is translocated to the
nucleus where it activates transcription of target genes (21,22).
It has also been reported that NF-κB is crucial for OA occurrence
and progression. Hu et al (23) observed that loganin improves
cartilage degeneration and OA progression in a mouse model by
suppressing the NF-κB pathway in chondrocytes. Moreover, Zhou et
al (24) confirmed that
kinsenoside relieves OA by repolarizing macrophages via
inactivation of the NF-κB/MAPK pathway and protection of
chondrocytes. MAPKs, a group of serine/threonine protein kinases,
are involved in the control of cellular responses, such as
apoptosis and the inflammatory response to cytokines (25). Lee et al (26) reported the protective effects of
aqueous extract of Anthriscus sylvestris on relieving OA
both in vitro and in vivo by inhibiting the MAPK and
NF-κB pathways.
LIM mineralization protein-1 (LMP-1), an
intracellular mediator of bone formation, has been reported to
promote bone formation (27).
LMP-1 has been reported to suppress TNF-α-induced intervertebral
disc degeneration (28). However,
to the best of our knowledge, its role in OA and chondrocytes has
not been previously reported.
The present study aimed to evaluate the role of
LMP-1 in relieving the inflammatory response stimulated by IL-1β
during the pathogenesis of OA. It was hypothesized that after the
OA cell model was established using IL-1β, LMP-1 would exhibit a
protective effect against OA in IL-1β-stimulated chondrocyte
inflammation and that the mechanism underlying the protective
effect of LMP-1 may be associated with the NF-κB and MAPK/JNK
pathways.
Materials and methods
Cell culture
CHON-001 cells were purchased from the American Type
Culture Collection, seeded in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS (HyClone; Cytiva) and 1%
penicillin-streptomycin and maintained at 37˚C with 5%
CO2 in an incubator. CHON-001 cells were exposed to
IL-1β (0.1, 2.0, 5.0 and 10.0 ng/ml; Sigma-Aldrich; Merck KGaA) at
37˚C for 12 h (29).
Construction of pcDNA3.1-LMP-1,
pcDNA3.1, LMP-1 small interfering (si)RNA and negative control (NC)
siRNA
The LMP-1 plasmid (pcDNA3.1-LMP-1), empty plasmid
(pcDNA3.1), LMP-1-siRNA (sense, 5'-GGAAUUUGCACG GACAGGCTT-3' and
anti-sense, 5'-GCCUGUCCGUGCAAA UUCCTT-3') and NC siRNA (forward,
5'-UUCUCCGAACG UGUCACGUTT-3' and reverse. 5'-ACGUGACACGUUCGG
AGAATT-3') were constructed by GenePharm, Inc. The full length of
LMP-1 gene (accession no. AAK30567) was amplified using RT-PCR and
then cloned into vector plasmid using the PCR cloning technique
(30). Briefly, total RNA from
CHON-001 cells was isolated using an TRIzol® reagent
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. The cDNA was obtained using a PrimeScript™ RT kit
(Takara Bio, Inc.). The temperature protocol for the reverse
transcription reaction was as following: 25˚C for 5 min, 42˚C for
60 min and 80˚C for 2 min. Subsequently, amplification of the LMP-1
gene was performed using a PCR Amplification kit (cat. no. R011;
Takara Bio, Inc.) with primers LMP-1-forward,
5'-ATGGATTCCTTCAAAGTAGTGCTG-3' and reverse,
5'-TCACACATGAGAGAAGGCATGG-3'. The thermocycling conditions were as
follows: Initial denaturation at 95˚C for 5 min, followed by 40
cycles of denaturation at 95˚C for 15 sec and annealing/elongation
at 60˚C for 30 sec.
Cell transfection
CHON-001 cells (5x104 cells/well) were
transfected with 0.5 µg pcDNA3.1-LMP-1, 0.5 µg pcDNA3.1, 50 nM
LMP-1-siRNA and 50 nM NC-siRNA at 37˚C for 24 h using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's
instructions. At 24 h post-transfection, reverse
transcription-quantitative (RT-q)PCR was performed to evaluate the
efficiency of cell transfection.
MTT assay
Following transfection for 24 h and/or treatment
with IL-1β for 12 h, CHON-001 cells (2x103 cells per
well) were cultured into 96-well plates at 37˚C. Then, cells were
treated with 10 µl MTT (5 mg/ml) solution and continuously
incubated for a further 4 h. Following treatment, the solution was
removed and 100 µl DMSO was added to each well to dissolve the
formazan product. Finally, the optical density (OD) was measured at
a wavelength of 570 nm using a multifunctional plate reader (BioTek
Instruments, Inc.) following 15 min of vibration mixing, according
to the manufacturer's protocol.
Flow cytometry analysis
Following transfection for 24 h or treatment with
IL-1β for 12 h, CHON-001 cell apoptosis was measured using an
Annexin V-FITC/PI apoptosis detection kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. In brief,
CHON-001 cells (1x106) were collected though
centrifugation (1,000 x g) at 4˚C for 5 min, then the cells were
stained using 5 µl Annexin V-FITC and 5 µl PI for 30 min at room
temperature in the dark. The number of apoptotic cells were
examined using a Cell Lab Quanta™ SC flow cytometer (Beckman
Coulter, Inc.) and analyzed using Kaluza analysis software (version
2.1.1.20653; Beckman Coulter, Inc.).
Western blot analysis
Total protein was extracted from CHON-001 cells
using RIPA lysis buffer (Beyotime Institute of Biotechnology) and
quantified using a BCA Protein assay kit (Invitrogen; Thermo Fisher
Scientific, Inc.). The protein samples (40 µg per lane) were then
separated via 10% SDS-PAGE and transferred onto PVDF membranes (EMD
Millipore). Following blocking with 5% skimmed milk in PBS-0.1%
Tween-20 at room temperature for 1.5 h, the membranes were cultured
with primary antibodies against GAPDH (1:1,000; cat. no. 5174; Cell
Signaling Technology, Inc.), LMP-1 (1:1,000; cat. no. ab182153;
Abcam), phosphorylated (p)-p65 (1:1,000; cat. no. ab76302; Abcam),
p65 (1:1,000; cat. no. ab32536; Abcam), p-JNK (1:1,000; cat. no.
sc-6254; Santa Cruz Biotechnology, Inc.), JNK (1:1,000; cat. no.
sc-7345; Santa Cruz Biotechnology, Inc.), caspase 3 (1:1,000; cat.
no. 14220; Cell Signaling Technology, Inc.) and cleaved-caspase 3
(1:1,000; cat. no. 9654; Cell Signaling Technology, Inc.) overnight
at 4˚C. After washing in TBS-Tween-20, the membranes were incubated
with horseradish peroxidase-conjugated anti-rabbit or anti-mouse
IgG secondary antibody (both 1:2,000; cat. nos. 7074 and 7076,
respectively; both Cell Signaling Technology, Inc.) at room
temperature for 1.5 h. Finally, the bands were visualized using
Pierce ECL Western Blotting Substrate (Pierce; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
ImageJ v.2.0 software (National Institutes of Health) was used to
quantify the band intensity.
ELISA
The secretion of IL-6 (cat. no. PI330), IL-8 (cat.
no. PI640) and TNF-α (cat. no. PT518) in CHON-001 cell culture
supernatant (collected through centrifugation at 500 x g at 4˚C for
5 min) were quantitatively detected using ELISA kits (Beyotime
Institute of Biotechnology), according to the manufacturer's
protocol. Then, the OD value at 450 nm was measured on a Multiscan
spectrum.
RT-qPCR analysis
LMP-1, p65 and JNK expression levels were measured
via RT-qPCR. Isolation of RNA from CHON-001 cells was performed
using an TRIzol® reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. cDNA was
obtained using a PrimeScript RT kit (Takara Bio, Inc.). The
temperature protocol for the reverse transcription reaction was as
following: 25˚C for 5 min, 42˚C for 60 min and 80˚C for 2 min. qPCR
amplification was then performed with SYBR Premix Ex-Taq (Takara
Bio, Inc.) on an ABI PRISM 7900 sequence detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were as follows: Initial denaturation at 95˚C for 5 min,
followed by 40 cycles of denaturation at 95˚C for 15 sec and
annealing/elongation at 60˚C for 30 sec. GAPDH was used as the
internal control. Primers were obtained from Sangon Biotech Co.,
Ltd. as follows: LMP-1-forward, 5'-TAGCCAGTGTGGGAAGGTCCTGGAAGAG-3'
and reverse, 5'-CTTCTTGCACTTGGCACAGCTGGGTG-3'; p65 forward,
5'-ACAACAACCCCTTCCAAGAAGA-3' and reverse,
5'-CAGCCTGGTCCCGTGAAATA-3'; JNK forward, 5'-CACAGTCCTAAAACGATACC-3'
and reverse, 5'-CCACA CAGCATTTGATAGAG-3' and GAPDH forward,
5'-TCAAC GACCACTTTGTCAAGCTCA-3' and reverse, 5'-GCTGGTG
GTCCAGGGGTCTTACT-3'. Target gene expression was assessed using the
2-ΔΔCq method (31).
Statistical analysis
All experiments were repeated three times.
Statistical analysis was performed using SPSS 20.0 (IBM Corp.).
Data are expressed as the mean ± SD from three independent
experiments. Differences between two groups were assessed using
unpaired Student's t-test; differences between multiple groups were
analyzed by one-way ANOVA followed by post hoc Tukey's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
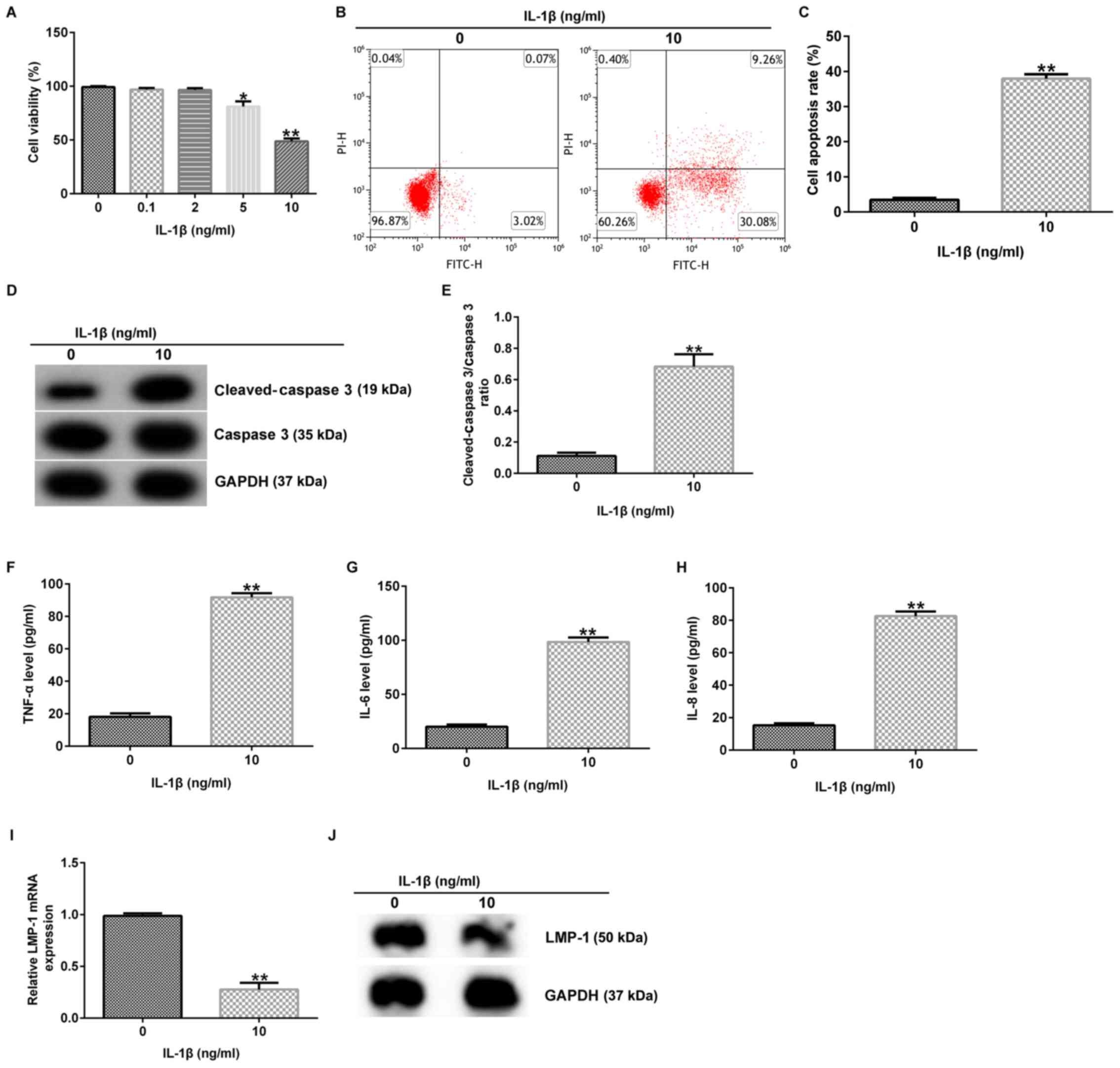
IL-1β induces inflammatory damage and
significantly decreases LMP-1 expression in chondrocytes
Previous reports have shown that IL-1β is involved
in the pathogenesis of OA by causing a cascade of inflammatory and
destructive reactions (7,8). In the present study, various
concentrations of IL-1β (0.1, 2.0, 5.0 and 10.0 ng/ml) were applied
to CHON-001 cells to establish an OA model in vitro.
Following 12 h stimulation, cell viability and apoptosis were
assessed. The data demonstrated that 5 and 10 ng/ml IL-1β notably
decreased CHON-001 cell viability compared with the control group
(Fig. 1A). Therefore, 10 ng/ml
IL-1β was selected for subsequent analysis. It was also found that
10 ng/ml IL-1β significantly promoted CHON-001 apoptosis (Fig. 1B and C), enhanced cleaved-caspase 3 protein
expression (Fig. 1D) and increased
the ratio of cleaved-caspase 3/caspase 3 (Fig. 1E). Moreover, the secretion of
TNF-α, IL-6 and IL-8 was increased by 10 ng/ml IL-1β (Fig. 1F-H), whereas IL-1β significantly
decreased LMP-1 mRNA (Fig. 1I) and
protein expression (Fig. 1J) in
CHON-001 cells compared with the control group. These findings
revealed that IL-1β induced CHON-001 cellular injury in OA.
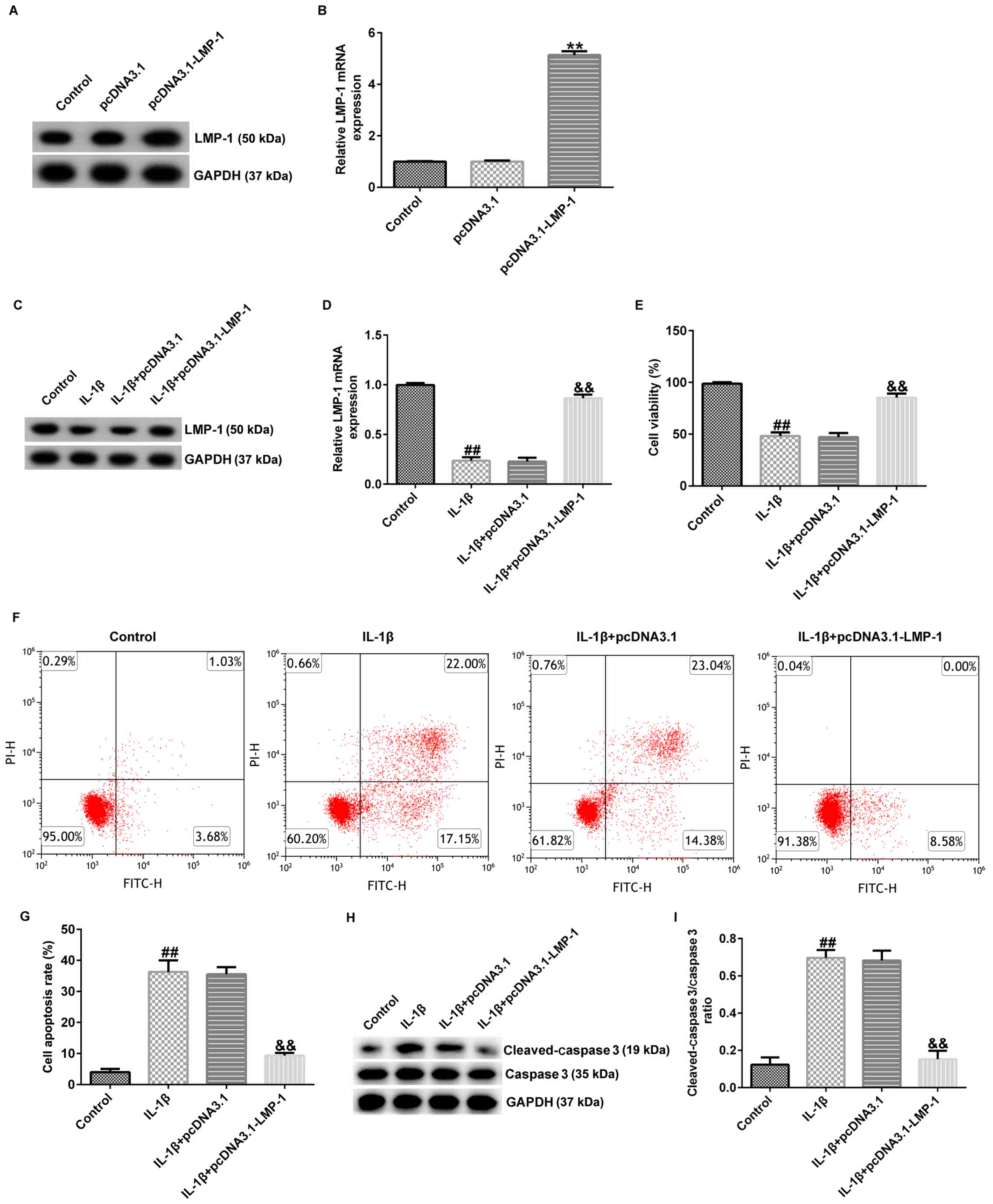
LMP-1 alleviates IL-1β-induced
CHON-001 changes in cell viability and apoptosis
To evaluate whether LMP-1 was associated with
chondrocyte inflammation, CHON-001 cells were transfected with
pcDNA3.1-LMP-1 or pcDNA3.1 for 24 h and stimulated with 10 ng/ml
IL-1β for 12 h. Then, the expression of LMP-1 was measured using
RT-qPCR and western blotting. LMP-1 expression was higher in the
pcDNA3.1-LMP-1 group compared with that in the pcDNA3.1 group
(Fig. 2A and B). LMP-1 enhanced cell viability,
inhibited apoptosis and decreased secretion of inflammatory factors
(TNF-α, IL-6 and IL-8) in CHON-001 cells (Fig. S1). Furthermore, LMP-1 was
downregulated in IL-1β-induced CHON-001 cells compared with the
control group. However, this inhibitory effect was reversed by
pcDNA3.1-LMP-1 (Fig. 2C and
D).
To investigate the role of LMP-1 in IL-1β-induced
CHON-001 cells, the effect of pcDNA3.1-LMP-1 on cell viability and
apoptosis was assessed. IL-1β inhibited cell viability (Fig. 2E), increased the number of
apoptotic cells (Fig. 2F and
G) and enhanced cleaved-caspase 3
protein expression (Fig. 2H) and
the cleaved-caspase 3/caspase 3 ratio (Fig. 2I). However, these effects were
reversed by pcDNA3.1-LMP-1. Thus, the data demonstrated that
upregulation of LMP-1 alleviated IL-1β-induced CHON-001 apoptosis
and inhibition of cell viability.
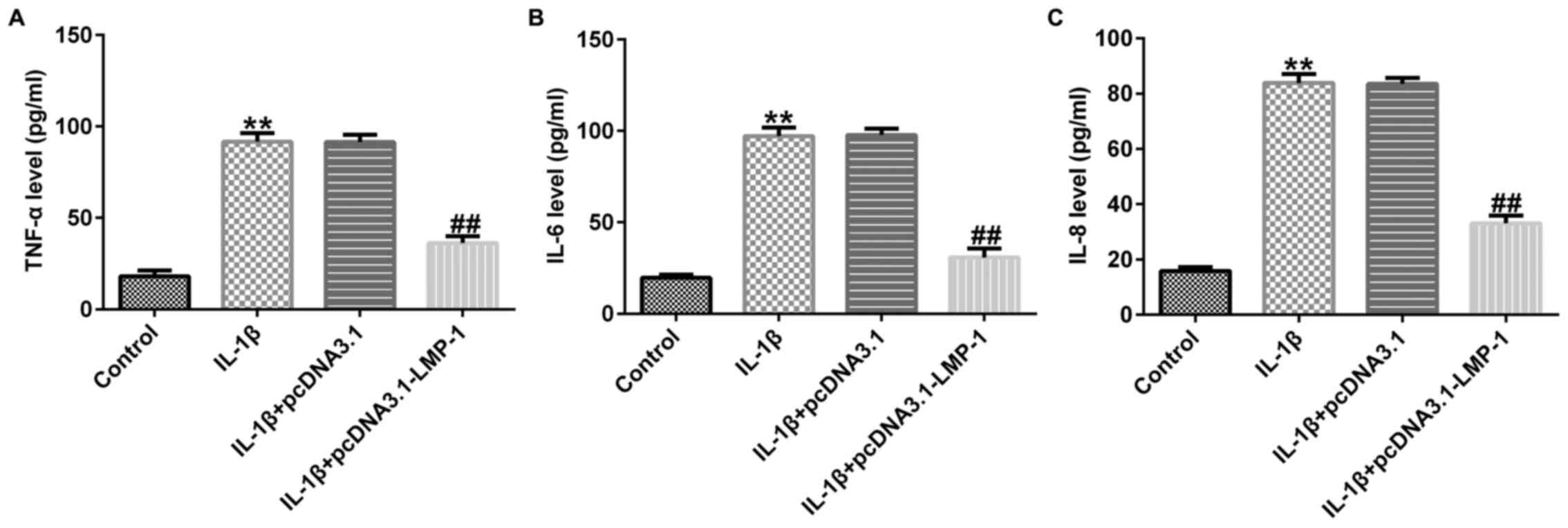
LMP-1 relieves the IL-1β-stimulated
inflammatory response in CHON-001 cells
The effect of LMP-1 on the secretion of inflammatory
factors, including TNF-α, IL-6 and IL-8, was assessed. Levels of
TNF-α, IL-6 and IL-8 were significantly increased in
IL-1β-stimulated CHON-001 cells, whereas pcDNA3.1-LMP-1
significantly decreased inflammatory factor release in
IL-1β-treated CHON-001 cells (Fig.
3A-C). These data suggested that LMP-1 alleviated the
IL-1β-induced inflammatory response in CHON-001 cells.
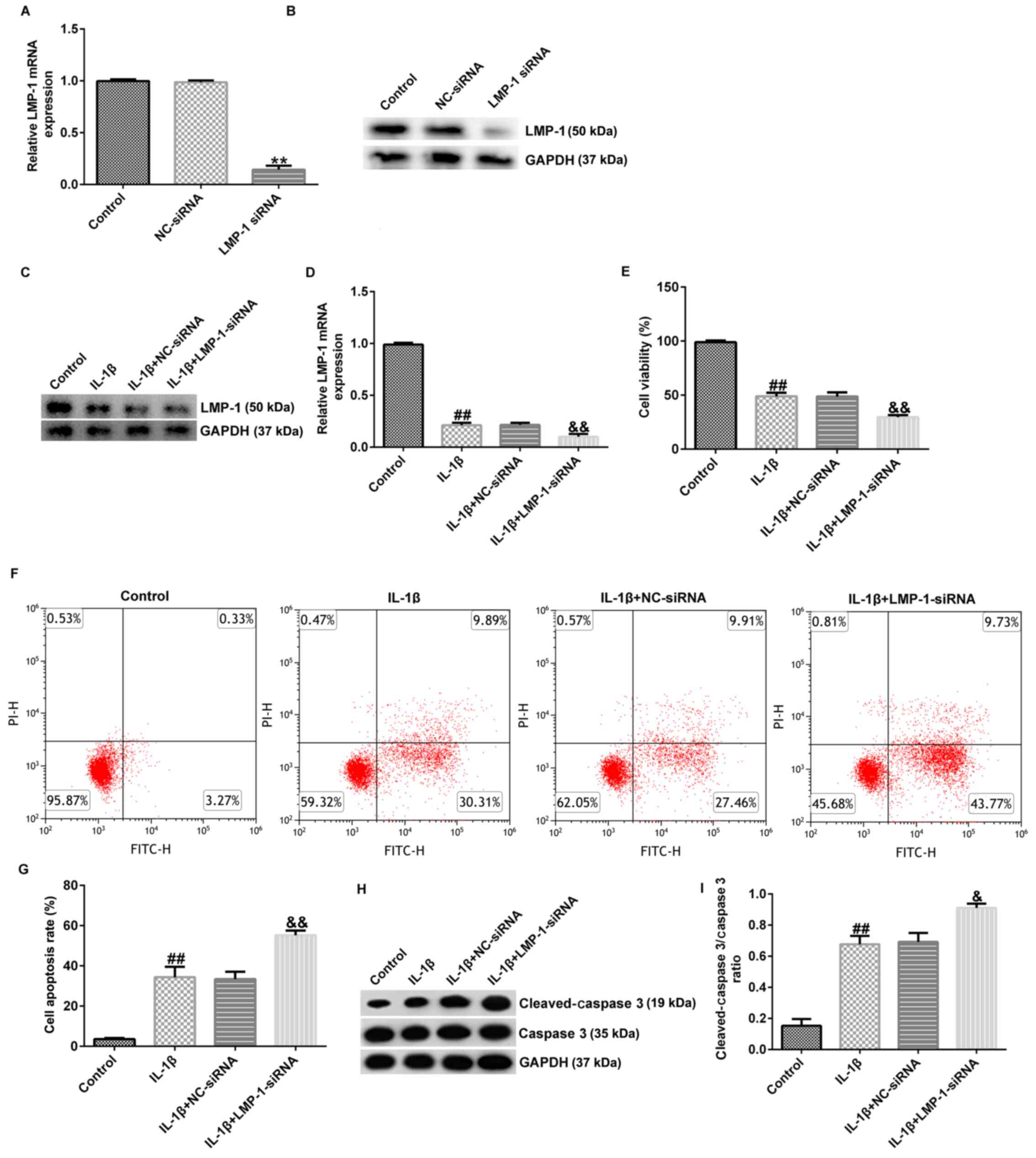
LMP-1-siRNA aggravates IL-1β-induced
CHON-001 apoptosis and decreased cell viability
To determine the protective effects of LMP-1 on
IL-1β-stimulated CHON-001 cells, CHON-001 cells were transfected
with NC-siRNA or LMP-1 siRNA for 24 h and stimulated with 10 ng/ml
IL-1β for 12 h. RT-qPCR and western blotting revealed that LMP-1
siRNA significantly decreased LMP-1 expression (Fig. 4A and B). Compared with the NC-siRNA
transfection group, LMP-1-siRNA significantly decreased cell
viability, enhanced cell apoptosis and increased secretion of
inflammatory factors (TNF-α, IL-6 and IL-8) in CHON-001 cells
(Fig. S2). In addition, LMP-1 was
downregulated in IL-1β-induced CHON-001 cells compared with the
control group. LMP-1 siRNA significantly decreased LMP-1 mRNA and
protein expression (Fig. 4C and
D). The results demonstrated that
IL-1β stimulation significantly inhibited cell viability (Fig. 4E), increased the number of
apoptotic cells (Fig. 4F and
G) and enhanced cleaved-caspase 3
protein expression (Fig. 4H) and
the cleaved-caspase 3/caspase 3 ratio (Fig. 4I). These effects were further
enhanced in the IL-1β+LMP-1 siRNA group compared with the
IL-1β+NC-siRNA group. Collectively, these data suggested that
LMP-1-siRNA aggravated IL-1β-induced CHON-001 apoptosis and
decreased cell viability.
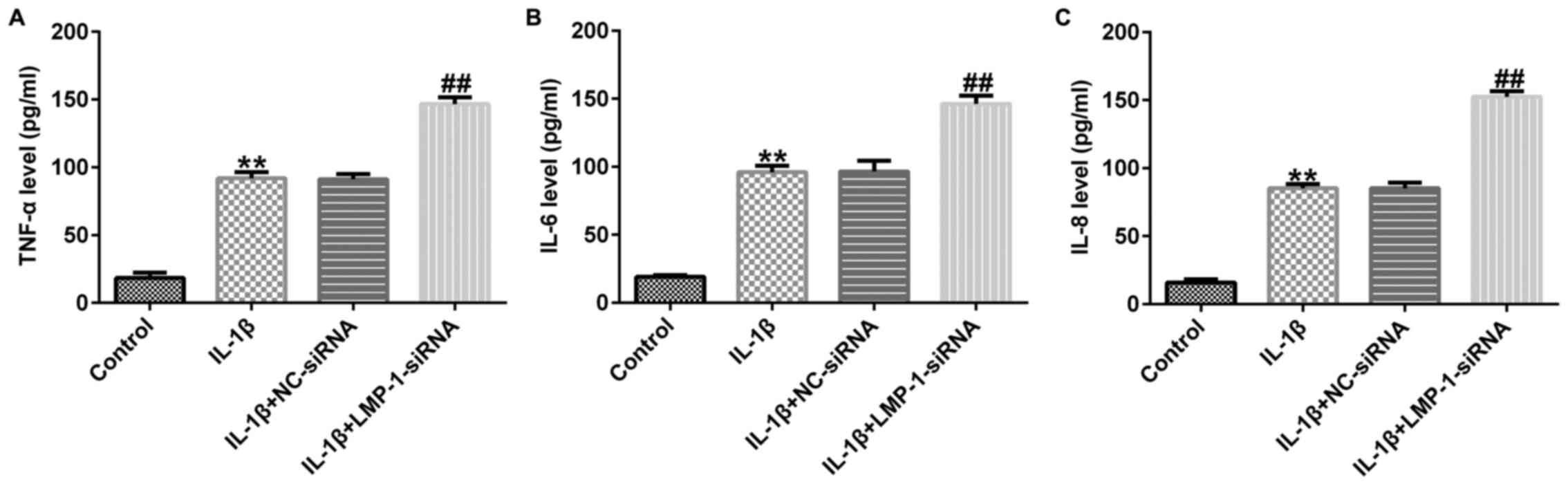
LMP-1-siRNA aggravates the
IL-1β-induced inflammatory response in CHON-001 cells
The effect of LMP-1 on inflammatory factor secretion
in CHON-001 cells was then evaluated. The data revealed that the
levels of TNF-α, IL-6 and IL-8 were significantly enhanced in
IL-1β-treated CHON-001 cells and LMP-1 siRNA further promoted TNF-α
(Fig. 5A), IL-6 (Fig. 5B) and IL-8 (Fig. 5C) release in IL-1β-induced CHON-001
cells. In summary, these data suggested that LMP-1-siRNA aggravated
IL-1β-induced inflammatory response in CHON-001 cells.
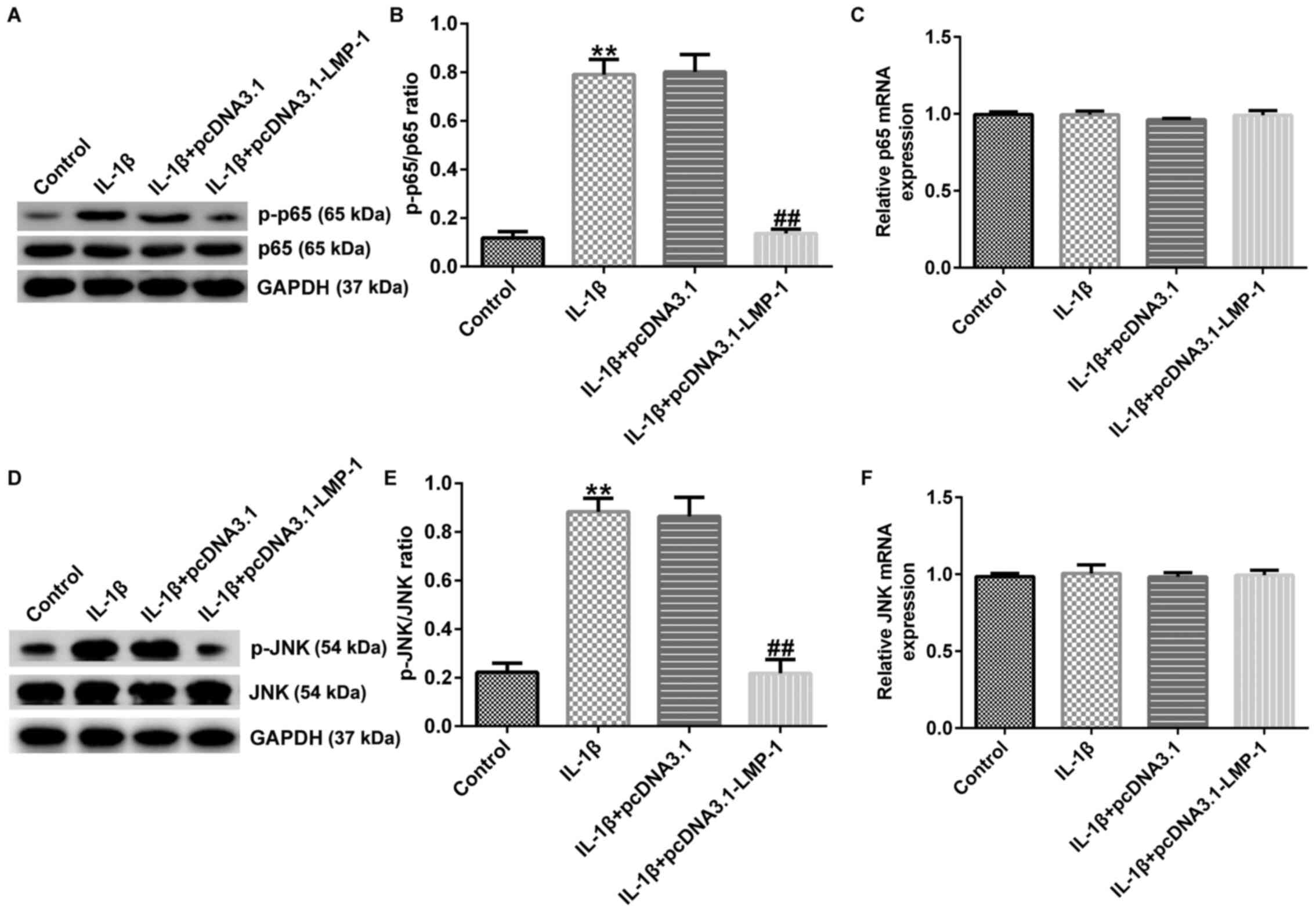
LIM-1 inhibits IL-1β-induced
chondrocyte injury by regulating the NF-κB and MAPK/JNK
pathways
Previous reports have confirmed that NF-κB regulates
the inflammatory response in numerous types of disease (19,21,22).
Thus, the role of LMP-1 in NF-κB and MAPK/JNK pathways was
assessed. CHON-001 cells were transfected with pcDNA3.1-LMP-1 or
pcDNA3.1 for 24 h and stimulated with 10 ng/ml IL-1β for 12 h.
Then, the expression levels of p-p65, p65, p-JNK and JNK were
determined via western blotting and RT-qPCR analysis. IL-1β induced
upregulation of p-p65 and increased the ratio of p-p65/p65
(Fig. 6A and B); these effects were significantly
decreased in the IL-1β+pcDNA3.1-LMP-1 group. The mRNA expression
levels of p65 presented no significant changes between the
different groups (Fig. 6C).
Consistently, IL-1β increased p-JNK protein expression and enhanced
the p-JNK/JNK ratio (Fig. 6D and
E); these effects were inhibited
by pcDNA3.1-LMP-1. However, there were no significant differences
in JNK (Fig. 6F) mRNA expression
levels between groups. Taken together, these data indicated that
LMP-1 inhibited IL-1β-induced human chondrocyte injury by
regulating the JNK/NF-κB signaling pathway.
Discussion
OA is a prevalent inflammatory disease,
characterized by joint dysfunction, cartilage degeneration and
articular cartilage breakdown (1,2). OA
is a global public health problem and a cause of disability
worldwide (32). Previous reports
have revealed that OA is caused by numerous factors, including
strain, trauma and joint deformity (1,33)
and it is estimated that ~3 million new cases of OA occur every
year. With the increasing age of the population, the incidence of
OA has risen year by year (34).
At present, there are no satisfactory therapies for chronic joint
disease. Therefore, the present study aimed to identify an
effective agent for OA treatment.
Previous studies have reported that IL-1β is
associated with OA by regulating chondrocyte apoptosis and the
inflammatory response (14,35). In the present study, CHON-001 cells
were treated with different concentration of IL-1β (0.1, 2.0, 5.0
and 10.0 ng/ml) for 12 h to establish an OA model in vitro.
It was found that the viability of CHON-001 cells was significantly
decreased following treatment with 5 or 10 ng/ml IL-1β, which was
consistent with a previous report (36). Thus, 10 ng/ml IL-1β induced
CHON-001 cells were used as an in vitro OA model for
subsequent experiments. CHON-001 is a fibroblast immortalized cell
line established by hTERT transduction and therefore lacks certain
important characteristics of primary chondrocytes. For example,
primary chondrocytes are quiescent cells that rarely divide under
physiological conditions, but CHON-001 cells are proliferative
cells (37). CHON-001 cells,
collected from an embryo (age, 18 weeks), are distinct from
chondrocytes in adults (38). This
was a limitation of the present study as the majority of patients
with OA are elderly (39).
LMP-1, a regulator of bone formation, is found in
fetal calvarial cells (27,40).
Moreover, previous reports have shown that LMP-1 is involved in
numerous types of disease, including tumor (41), dental caries (42), fracture repair (43) and intervertebral disc degeneration
(28). Nevertheless, the mechanism
by which LMP-1 protects chondrocytes from IL-1β-stimulated damage
in CHON-001 cells remains unknown. Hence, the present study
investigated the underlying mechanism of LMP-1 in IL-1β-induced
CHON-001 cells. The results demonstrated that LMP-1 expression was
significantly decreased in response to IL-1β treatment. The
protective effect of LMP-1 against IL-1β-induced CHON-001 cell
injury was also investigated. The present data indicated that LMP-1
expression was higher in the pcDNA3.1-LMP-1 group compared with
that in the pcDNA3.1 group. LMP-1 was downregulated in
IL-1β-induced CHON-001 cells compared with the control group.
However, this inhibitory effect was reversed by pcDNA3.1-LMP-1.
Furthermore, functional assays revealed that overexpression of
LMP-1 alleviated IL-1β-induced CHON-001 cellular injury, as shown
by increased cell viability, suppressed apoptosis and decreased
cleaved-protein caspase 3 expression levels and cleaved-caspase
3/caspase 3 ratio.
Caspase 3, a member of cysteine-aspartic protease
family, is involved in cellular components and apoptosis (44). Chung et al (45) reported the association between
tacrolimus-induced apoptosis and caspase 3 and 12 in Jurkat cells.
Consistent with earlier results (29), the present study observed that
IL-1β increased cleaved-caspase 3 protein expression and promoted
the cleaved-caspase 3/caspase 3 ratio. Overexpression of LMP-1
decreased cleaved-caspase 3 protein expression and the
cleaved-caspase 3/caspase 3 ratio in IL-1β-induced CHON-001 cells.
Due to caspase 3 cleavage, it was expected that levels of caspase 3
in cells exposed to IL-1β would be decreased but this was not the
case and further research is needed.
Inflammatory factors serve a vital role in OA
development, which results in joint tissue degradation (46). Chunlei et al (46) observed that microRNA-138-5p
silencing protects chondrocytes (ATDC5 and CHON-001 cells) from
IL-1β-induced inflammation by upregulating SOX9. Wang et al
(47) reported that acupuncture
can downregulate the expression of IL-1β and TNF-α in
osteoarthritic chondrocytes. Yao et al (48) also demonstrated that Shenmai
injection protects knee articular cartilage from IL-1β-induced
human chondrocyte damage in OA rabbits. In addition, a previous
study suggested that leonurine inhibits IL-1β-induced inflammation
in a murine OA model (49). LMP-1
also exhibits an anti-inflammatory role in
lipopolysaccharide-induced pre-osteoclasts (50). In the present study, the release of
inflammatory cytokines (including IL-6, IL-8 and TNF-α) in
IL-1β-induced CHON-001 cells was measured. IL-1β notably promoted
the release of IL-6, IL-8 and TNF-α compared with the control
group. Therefore, suppression of chondrocyte apoptosis and the
inflammatory response may be beneficial for OA treatment. In the
present study, overexpression of LMP-1 notably decreased the
secretion of IL-6, IL-8 and TNF-α in IL-1β-stimulated chondrocytes.
These findings demonstrated that LMP-1 alleviated the IL-1β-induced
inflammatory response in chondrocytes cells.
To assess the effect of LMP-1 downregulation in
IL-1β-stimulated OA chondrocytes, LMP-1 expression was knocked down
in CHON-001 cells using LMP-1 siRNA. Functional analysis of LMP-1
siRNA was performed to verify whether it regulated the effects of
IL-1β. The results revealed that knockdown of LMP-1 further
aggravated IL-1β-induced chondrocyte injury, as evidenced by
inhibited CHON-001 cell viability, increased apoptosis and enhanced
IL-6, IL-8 and TNF-α release.
Previously, accumulating evidence confirmed that
activated NF-κB molecules may cause articular joint damage,
resulting in the progression of OA (51,52).
Saito and Tanaka (51) revealed
that inhibiting the Notch and NF-κB pathway prevents the
progression of OA, whilst Lei et al (52) observed that ubiquitin specific
peptidase 19 suppresses TNF-α and IL-1β-induced NF-κB activation by
deubiquitinating mitogen-activated protein kinase kinase kinase 7.
Furthermore, LMP-1 has been shown to promote bone formation and the
association between LMP-1 and the JNK signaling pathway was
confirmed in a previous study (50). The present study demonstrated that
LMP-1 suppressed phosphorylation of p65 and JNK, and thus relieved
the inflammatory response. However, there were no significant
differences in p65 and JNK mRNA expression levels between the
groups. These data suggested that LMP-1 relieved OA development by
altering the NF-κB and MAPK/JNK pathways.
In conclusion, the present study identified that
LMP-1 relieved IL-1β-induced inflammatory injury in CHON-001 cells
via regulating the NF-κB and MAPK/JNK pathways, suggesting that
targeting LMP-1 may alleviate cartilage damage in patients with OA.
Thus, upregulation of LMP-1 may be a promising therapeutic target
for OA clinical treatment. However, the present study was a
preliminary in vitro investigation of the role of LMP-1 in
an in vitro model of OA. To confirm the role of LMP-1 in OA,
more research is needed. For example, whether NF-κB and MAPK/JNK
pathways are directly involved in the role of LMP-1 in IL-1β
induced CHON-001 cells still needs to be analyzed, including the
use of NF-κB and MAPK/JNK pathway inhibitors and agonists.
Extracellular matrix degradation in response to IL-1β treatment
also needs further investigation. The role of LMP-1 in other
chondrocyte cell lines or primary chondrocytes should also be
studied. Additional in vivo experiments are required to
verify the precise mechanism of LMP-1 in OA progression in the
future. Also, since OA can be caused by factors other than
chondrocyte injury, investigation of the role of LMP-1 in synovial
tissue or subchondral bone, as well as chondrocytes using a rodent
OA model, should be performed. Moreover, the association between
expression levels of LMP-1 and clinical characteristics of patients
with OA needs to be clarified in the future.
Supplementary Material
Effect LMP-1 on cell viability,
apoptosis and inflammatory factor release in CHON-001 cells.
CHON-001 cells were transfected with pcDNA3.1-LMP-1 or pcDNA3.1 for
24 h. (A) MTT assay was used to assess cell viability. (B) Flow
cytometry was performed to determine the number of apoptotic cells.
(C) Quantification of the number of apoptotic cells. The release of
(D) TNF-α, (E) IL-6 and (F) IL-8 was assessed using ELISA.
**P<0.01 vs. pcDNA3.1. LMP-1, LIM mineralization
protein-1.
Effect of LMP-1 silencing on cell
viability, apoptosis and inflammatory factor release in CHON-001
cells. CHON-001 cells were transfected with NC-siRNA or LMP-1-siRNA
for 24 h. (A) MTT assay was used to assess cell viability. (B) Flow
cytometry was performed to determine the number of apoptotic cells.
(C) Quantification of the number of apoptotic cells. The release of
(D) TNF-α, (E) IL-6 and (F) IL-8 was assessed using ELISA.
**P<0.01 vs. NC-siRNA. LMP-1, LIM mineralization
protein-1; si, small interfering; NC, negative control.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Shenzhen Science
and Technology Research and Development Fund (grant no.
JCYJ20170307153), Project of Education Department of Jiangxi
Province (grant no. GJJ190785) and Science & Technology
Innovation Project of Gannan Medical University (grant no.
TS202003).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DO contributed to the study design, data collection,
statistical analysis, data interpretation and manuscript
preparation. CH contributed to data collection, statistical
analysis and manuscript preparation. SL, CT, HT and YY contributed
to data collection and statistical analysis. DO and CH performed
the experiments. DO and CH confirmed the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Blanco FJ: Osteoarthritis and
atherosclerosis in joint disease. Reumatol Clin Engl Ed.
14:251–253. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hunter DJ and Bierma-Zeinstra S:
Osteoarthritis. Lancet. 393:1745–1759. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhao X, Shah D, Gandhi K, Wei W, Dwibedi
N, Webster L and Sambamoorthi U: Clinical, humanistic, and economic
burden of osteoarthritis among noninstitutionalized adults in the
United States. Osteoarthritis Cartilage. 27:1618–1626.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nees TA and Schiltenwolf M:
Pharmacological treatment of osteoarthritis-related pain. Schmerz.
33:30–48. 2019.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
5
|
Castiello E and Affatato S: Progression of
osteoarthritis and reoperation in unicompartmental knee
arthroplasty: A comparison of national joint registries. Int J
Artif Organs. 43:203–207. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tu Y, Ma T, Wen T, Yang T, Xue L, Cai M,
Wang F, Guan M and Xue H: MicroRNA-377-3p alleviates IL-1β-caused
chondrocyte apoptosis and cartilage degradation in osteoarthritis
in part by downregulating ITGA6. Biochem Biophys Res Commun.
523:46–53. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wojdasiewicz P, Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014(561459)2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jenei-Lanzl Z, Meurer A and Zaucke F:
Interleukin-1β signaling in osteoarthritis - chondrocytes in focus.
Cell Signal. 53:212–223. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fukai A, Kamekura S, Chikazu D, Nakagawa
T, Hirata M, Saito T, Hosaka Y, Ikeda T, Nakamura K, Chung UI, et
al: Lack of a chondroprotective effect of cyclooxygenase 2
inhibition in a surgically induced model of osteoarthritis in mice.
Arthritis Rheum. 64:198–203. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cheng C, Tian J and Zhang F: Can IL-1 be
used as a target for osteoarthritis? Ann Rheum Dis.
79(e88)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kloppenburg M, Peterfy C, Haugen IK, Kroon
F, Chen S, Wang L, Liu W, Levy G, Fleischmann RM, Berenbaum F, et
al: Phase IIa, placebo-controlled, randomised study of lutikizumab,
an anti-interleukin-1α and anti-interleukin-1β dual variable domain
immunoglobulin, in patients with erosive hand osteoarthritis. Ann
Rheum Dis. 78:413–420. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fleischmann RM, Bliddal H, Blanco FJ,
Schnitzer TJ, Peterfy C, Chen S, Wang L, Feng S, Conaghan PG,
Berenbaum F, et al: A phase II trial of lutikizumab, an
anti-interleukin-1α/β dual variable domain immunoglobulin, in knee
osteoarthritis patients with synovitis. Arthritis Rheumatol.
71:1056–1069. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vinod E, Kachroo U, Amirtham SM, Ramasamy
B and Sathishkumar S: Comparative analysis of fresh chondrocytes,
cultured chondrocytes and chondroprogenitors derived from human
articular cartilage. Acta Histochem. 122(151462)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fei J, Liang B, Jiang C, Ni H and Wang L:
Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and
attenuates osteoarthritis progression in a rat model. Biomed
Pharmacother. 109:1586–1592. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang X, Fan J, Ding X, Sun Y, Cui Z and
Liu W: Tanshinone I inhibits IL-1β-induced apoptosis, inflammation
and extracellular matrix degradation in chondrocytes CHON-001 cells
and attenuates murine osteoarthritis. Drug Des Devel Ther.
13:3559–3568. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yang Q, Zhou Y, Cai P, Fu W, Wang J, Wei Q
and Li X: Downregulation of microRNA-23b-3p alleviates
IL-1β-induced injury in chondrogenic CHON-001 cells. Drug Des Devel
Ther. 13:2503–2512. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lian LP and Xi XY: Long non-coding RNA
XIST protects chondrocytes ATDC5 and CHON-001 from IL-1β-induced
injury via regulating miR-653-5p/SIRT1 axis. J Biol Regul Homeost
Agents. 34:379–391. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ou D, Ding W, Tong C and Yi W: Knockdown
of Long Non-coding RNA LINC00473 Protects CHON-001 Cells against
Interleukin-1β-Induced Cell Injury. Biol Pharm Bull. 44:232–237.
2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gasparini C and Feldmann M: NF-κB as a
target for modulating inflammatory responses. Curr Pharm Des.
18:5735–5745. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kumada K, Fuse N, Tamura T, Okamori C and
Kurata S: HSP70/DNAJA3 chaperone/cochaperone regulates NF-κB
activity in immune responses. Biochem Biophys Res Commun.
513:947–951. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jing H and Lee S: NF-κB in cellular
senescence and cancer treatment. Mol Cells. 37:189–195.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen Z, Yao L, Liu Y, Pan Z, Peng S, Wan
G, Cheng J, Wang J and Cao W: Astragaloside IV regulates
NF-κB-mediated cellular senescence and apoptosis of hepatic
stellate cells to suppress PDGF-BB-induced activation. Exp Ther
Med. 18:3741–3750. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hu J, Zhou J, Wu J, Chen Q, Du W, Fu F, Yu
H, Yao S, Jin H, Tong P, et al: Loganin ameliorates cartilage
degeneration and osteoarthritis development in an osteoarthritis
mouse model through inhibition of NF-κB activity and pyroptosis in
chondrocytes. J Ethnopharmacol. 247(112261)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhou F, Mei J, Han X, Li H, Yang S, Wang
M, Chu L, Qiao H and Tang T: Kinsenoside attenuates osteoarthritis
by repolarizing macrophages through inactivating NF-κB/MAPK
signaling and protecting chondrocytes. Acta Pharm Sin B. 9:973–985.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen C, Nelson LJ, Ávila MA and Cubero FJ:
Mitogen-activated protein kinases (MAPKs) and cholangiocarcinoma:
The Missing Link. Cells. 8(1172)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lee SA, Moon SM, Han SH, Hwang EJ, Park
BR, Kim JS, Kim DK and Kim CS: Chondroprotective effects of aqueous
extract of Anthriscus sylvestris leaves on osteoarthritis in
vitro and in vivo through MAPKs and NF-κB signaling inhibition.
Biomed Pharmacother. 103:1202–1211. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pan H, Li X, Wang J, Zhang K, Yang H, Li
Z, Zheng Z and Liu H: LIM Mineralization Protein-1 Enhances Bone
Morphogenetic Protein-2-Mediated Osteogenesis Through Activation of
ERK1/2 MAPK Pathway and Upregulation of Runx2 Transactivity. J Bone
Miner Res. 30:1523–1535. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu H, Pan H, Yang H, Wang J, Zhang K, Li
X, Wang H, Ding W, Li B and Zheng Z: LIM mineralization protein-1
suppresses TNF-α induced intervertebral disc degeneration by
maintaining nucleus pulposus extracellular matrix production and
inhibiting matrix metalloproteinases expression. J Orthop Res.
33:294–303. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang X, Zhang Q, Gao Z, Yu C and Zhang L:
Baicalin alleviates IL-1β-induced inflammatory injury via
down-regulating miR-126 in chondrocytes. Biomed Pharmacother.
99:184–190. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ning BT and Tang YM: Establishment of the
cell line, HeLa-CD14, transfected with the human CD14 gene. Oncol
Lett. 3:871–874. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Δ Δ C(T)) Method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Conaghan PG, Cook AD, Hamilton JA and Tak
PP: Therapeutic options for targeting inflammatory osteoarthritis
pain. Nat Rev Rheumatol. 15:355–363. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Poulet B, Westerhof TA, Hamilton RW,
Shefelbine SJ and Pitsillides AA: Spontaneous osteoarthritis in
Str/ort mice is unlikely due to greater vulnerability to mechanical
trauma. Osteoarthritis Cartilage. 21:756–763. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Martel-Pelletier J, Barr AJ, Cicuttini FM,
Conaghan PG, Cooper C, Goldring MB, Goldring SR, Jones G, Teichtahl
AJ and Pelletier JP: Osteoarthritis. Nat Rev Dis Primers.
2(16072)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xue H, Tu Y, Ma T, Wen T, Yang T, Xue L,
Cai M, Wang F and Guan M: miR-93-5p attenuates IL-1β-induced
chondrocyte apoptosis and cartilage degradation in osteoarthritis
partially by targeting TCF4. Bone. 123:129–136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xiao P, Zhu X, Sun J, Zhang Y, Qiu W, Li J
and Wu X: MicroRNA-613 alleviates IL-1β-induced injury in
chondrogenic CHON-001 cells by targeting fibronectin 1. Am J Transl
Res. 12:5308–5319. 2020.PubMed/NCBI
|
|
37
|
Charlier E, Relic B, Deroyer C, Malaise O,
Neuville S, Collée J, Malaise MG and De Seny D: Insights on
Molecular Mechanisms of Chondrocytes Death in Osteoarthritis. Int J
Mol Sci. 17(2146)2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Archer CW and Francis-West P: The
chondrocyte. Int J Biochem Cell Biol. 35:401–404. 2003.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xia B, Di Chen Zhang J, Hu S, Jin H and
Tong P: Osteoarthritis pathogenesis: A review of molecular
mechanisms. Calcif Tissue Int. 95:495–505. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Viggeswarapu M, Boden SD, Liu Y, Hair GA,
Louis-Ugbo J, Murakami H, Kim HS, Mayr MT, Hutton WC and Titus L:
Adenoviral delivery of LIM mineralization protein-1 induces
new-bone formation in vitro and in vivo. J Bone Joint Surg Am.
83:364–376. 2001.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liu H, Huang L, Zhang Z, Zhang Z, Yu Z,
Chen X, Chen Z, Zen Y, Yang D, Han Z, et al: LIM mineralization
protein-1 inhibits the malignant phenotypes of human osteosarcoma
cells. Int J Mol Sci. 15:7037–7048. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wang XY, Zhang Q and Chen Z: A possible
role of LIM mineralization protein 1 in tertiary dentinogenesis of
dental caries treatment. Med Hypotheses. 69:584–586.
2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Strohbach CA, Rundle CH, Wergedal JE, Chen
ST, Linkhart TA, Lau KH and Strong DD: LMP-1 retroviral gene
therapy influences osteoblast differentiation and fracture repair:
A preliminary study. Calcif Tissue Int. 83:202–211. 2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104.
1999.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chung YW, Chung MW, Choi SK, Choi SJ, Choi
SJN and Chung SY: Tacrolimus-induced apoptosis is mediated by
endoplasmic reticulum-derived calcium-dependent Caspases-3,-12 in
Jurkat cells. Transplant Proc. 50:1172–1177. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chunlei H, Chang Z, Sheng L, Yanchun Z,
Lulin L and Daozhang C: Down-regulation of miR-138-5p protects
chondrocytes ATDC5 and CHON-001 from IL-1β-induced inflammation via
up-regulating SOX9. Curr Pharm Des. 25:4613–4621. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wang DH, Bao F, Wu ZH, Sun H and Zhang YX:
Influence of acupuncture on IL-1beta and TNF-alpha expression in
the cartilage of rats with knee osteoarthritis. Zhongguo Gu Shang.
24:775–778. 2011.PubMed/NCBI(In Chinese).
|
|
48
|
Yao N, Chen N, Xu X, Sun D, Liu W, Li G,
Bi X, Li S, Chen Z, Chen G, et al: Protective effect of Shenmai
injection on knee articular cartilage of osteoarthritic rabbits and
IL-1β-stimulated human chondrocytes. Exp Ther Med. 13:3013–3020.
2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yin W and Lei Y: Leonurine inhibits IL-1β
induced inflammation in murine chondrocytes and ameliorates murine
osteoarthritis. Int Immunopharmacol. 65:50–59. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Liu H, Bargouti M, Zughaier S, Zheng Z,
Liu Y, Sangadala S, Boden SD and Titus L: Osteoinductive LIM
mineralization protein-1 suppresses activation of NF-kappaB and
selectively regulates MAPK pathways in pre-osteoclasts. Bone.
46:1328–1335. 2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Saito T and Tanaka S: Molecular mechanisms
underlying osteoarthritis development: Notch and NF-κB. Arthritis
Res Ther. 19(94)2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lei CQ, Wu X, Zhong X, Jiang L, Zhong B
and Shu HB: USP19 Inhibits TNF-α- and IL-1β-Triggered NF-κB
Activation by Deubiquitinating TAK1. J Immunol. 203:259–268.
2019.PubMed/NCBI View Article : Google Scholar
|