Introduction
Myocardial infarction (MI), a cardiovascular
disease, is the primary cause of morbidity and mortality worldwide.
MI is characterized by a reduction in oxygen supply caused by
blocked coronary arteries, resulting in diastolic and systolic
pressure dysfunction. The disease is accompanied by several
mechanical complications, and the primary clinical treatment
strategies aim to restore blood supply to maintain normal oxygen
levels (1-7).
Particularly interesting new cysteine histidine rich (PINCH)
protein, a type of focal adhesion proteins in mammals, is a
LIM-domain-only adapter protein consisting of two isomers, PINCH1
and PINCH2, which share high homology. PINCH serves an important
role in maintaining the structure and function of the heart, and is
involved in several biological processes, including cell migration
and survival. PINCH1 is widely expressed in most tissues and
organs, particularly in the myocardium (8-10).
A study demonstrated that conditional PINCH knockout (KO) in mice
led to severely defective cardiac development. Furthermore, PINCH1
deletion resulted in impaired cell mobility and survival, whereas
the functional complex formed by PINCH1, thymosin β4 (tβ4) and
integrin-linked kinase (ILK), promoted cardiomyocyte migration and
survival following cardiac injury (1-8).
The aforementioned studies indicated that PINCH1 may serve a key
role in cardiac function. However, the potential molecular
mechanism underlying the effects of PINCH1 in the myocardium is not
completely understood.
NF-κB belongs to a family of transcription factors
involved in the regulation of cell differentiation, proliferation
and apoptosis. As an inflammatory factor, NF-κB serves an important
role in the immune system (11,12).
Studies (13-15)
have shown that the NF-κB signaling pathway is activated following
MI and promotes ventricular remodeling in rats (16). The activity of NF-κB can be blocked
by tβ4, a cell penetrating peptide with a variety of biological
functions, thus improving cardiac function (1,17,18).
However, the association between PINCH1 and the NF-κB signaling
pathway in MI in mice is not completely understood. Therefore, the
present study adopted multiple search methods, including western
blotting, reverse transcription-quantitative (RT-q) PCR and flow
cytometry, to find the correlation between PINCH1 and NF-KB
pathway.
Materials and methods
Cell culture
HL1 cardiomyocytes (American Type Culture
Collection) were cultured in high-glucose DMEM (Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Thermo Fisher
Scientific, Inc.) and 1% penicillin and streptomycin at 37˚C with
95% air and 5% CO2.
Plasmid construction and cell
transfection
PINCH1 gene knockdown in cardiomyocytes was
performed by transfecting HL1 cells with PINCH1 shRNA as previously
described (19).
The full-length open reading frame of PINCH1 was
cloned into the pCDH-CMV-MCS-EF1A-GFP-T2A-Puro vector (System
Biosciences, LLC) to generate the corresponding overexpression (OE)
vector, pCDH-PINCH1-OE (System Biosciences, LLC). The shRNA
sequence specifically directed against PINCH1 was designed and
constructed with the following targeting interference sequence
(Sangon Biotech Co., Ltd.): (Forward PINCH1 shRNA 5'-3')
GATCCGGAGACGCAACCAGGTCTTGCTTCAAGAGAGCAAGACCTGGTTGCGTCTCCTTTTTTG;
(Reverse PINCH1 shRNA 5'-3')
AATTCAAAAAAGGAGACGCAACCAGGTCTTGCTCTCTTGAAGCAAGACCTGGTTGCGTCTCCG and
were cloned into PLVX-shRNA1 (Thermo Fisher Scientific, Inc.). The
negative control (NC) shRNA scrambled sequence was (Sangon Biotech
Co., Ltd.): (Forward PINCH1 NC 5'-3'):
GATCCGGAGAAATTCCGGTAGCGCTTGCTTCAAGAGAGCTTGGAAATTCCTGCGTCTCCTTTTTTG;
(Reverse PINCH1 NC 5'-3'):
AATTCAAAAAAGTTCCGGTTAAGCTAGTCTTGCTCTCTTATTGCGCTATTCGGGTTGCGT CTCCG
and were cloned into PLVX-shRNA1 (Thermo Fisher Scientific,
Inc.).
At 60-70% confluence, HL1 cells were transfected
with plasmids using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Briefly, cells were treated with 4 µg PCDH and 4 µg
PLVX-shRNA1 when the HL1 reached 60-70% confluency at 37˚C, 5%
CO2. shRNA NC, shRNA PINCH1, pCDH-NC OE and pCDH-PINCH1
OE vectors were mixed with 500 ml of Opti-MEM and 3.5 ml of
Lipofectamine® RNAiMAX (Invitrogen; Thermo Fisher
Scientific, Inc.), respectively, and then added to a 3.5 cm dish.
At 48 h post-transfection, transfection efficiencies were assessed
via flow cytometric analysis, RT-qPCR and western blotting. The
following successfully transfected cells were generated: shRNA NC,
shRNA PINCH1, pCDH-NC OE and pCDH-PINCH1 OE.
Cell viability assay
To determine HL1 cell viability, a Cell Counting Kit
8 (CCK-8) assay was used according to the manufacturer's
instructions (Beyotime Institute of Biotechnology). Briefly, cells
were seeded (1x105 cells/well) into 96-well plates for
24 h. Subsequently, 10 µl CCK-8 solution was added into each well
at the 72 h. After culture for 4 h, the optical density of each
well was measured at a wavelength of 450 nm using an enzyme mark
analyzer. Cell viability was calculated according to the following
formula: Cell viability (%) = [(mean OD value of PINCH1 KO
group/HL1 group)/(mean OD value of HL1 group)] x100.
Transwell migration assay
The migration abilities of PINCH1 KO HL1 and control
HL1 cells were assessed by performing Transwell assays. Briefly,
cells were plated (1x105 cells/chamber,) into the upper
chamber and were cultured in α-MEM, 10% FBS, 10 U/l penicillin and
10 µg/l streptomycin, whereas α-MEM medium supplemented with 20%
FBS was plated in the lower chamber as a chemoattractant. Following
incubation for 48 h at 37˚C, migratory cells were fixed with 4%
paraformaldehyde at 25˚C for 4 h and then stained with 0.5% crystal
violet at 25˚C for 15 min. The number of migratory cells was
quantified under a microscope (CX43; Olympus Corporation) on five
randomly selected fields.
Flow cytometry
Cell apoptosis was evaluated by flow cytometry using
an Annexin V-FITC/PI apoptosis kit (BD Biosciences) according to
the manufacturer's protocol. After cell harvesting, 100 µl binding
buffer was added to prepare a cell suspension (1x106
cells/ml). Subsequently, cells were stained with 5 µl FITC-Annexin
V and 5 µl PI at room temperature for 15 min in the dark. Cells
were then resuspended in 400 µl binding buffer and analyzed by flow
cytometry (FACSCalibur; Bio-Rad Laboratories, Inc.). Cells in early
and late apoptosis was quantified using FlowJo 7.6.1 software
(FlowJo LLC).
Western blotting
Total proteins were extracted from HL1 cells or
mouse myocardial tissues using RIPA lysis buffer (Beyotime
Institute of Biotechnology) supplemented with protease and
phosphatase inhibitors (Beyotime Institute of Biotechnology).
Protein concentrations were determined using the BCA Protein
Detection kit (Beyotime Institute of Biotechnology). Equal amounts
of protein (30 µg) were separated via 10% SDS-PAGE and transferred
onto PVDF membranes. Following blocking with 5% skim milk powder
for 1 h at 25˚C, the membranes were incubated at 4˚C overnight with
primary antibodies targeted against: GAPDH (ab9485,1:5,000; Abcam),
NF-κB (ab32360,1:1,000; Abcam), MYD88 (ab219413,1:1,000; Abcam),
MMP-2 (ab92536,1:1,000; Abcam), MMP-9 (ab76003,1:1,000; Abcam),
TNF-α (ab183218,1:1,000; Abcam),caspase-3 (ab184787,1:1,000; Abcam)
and PINCH1 (ab108609,1:1,000; Abcam). Subsequently, the membranes
were incubated with corresponding secondary antibodies [Goat
Anti-Rabbit IgG H&L (HRP); ab6721, 1:2,000; Abcam] for 1 h at
room temperature. Finally, the ECL detection system was used to
detect the immunoreactive protein signals and analyzed with ImageJ
(v1.8.0.112; National Institutes of Health). GAPDH was used as the
loading control.
TUNEL staining
Cell apoptosis in myocardial tissues was detected by
performing TUNEL staining assays. Briefly, mouse myocardium tissue
was fixed with 4% paraformaldehyde at 25˚C for 4 h. Subsequently,
TUNEL staining solution (Roche Diagnostics) was added at 25˚C for 1
h in a dark humid chamber. DAPI was added dropwise and incubated at
25˚C for 5 min. TUNEL+ cells were counted with mounting
medium (Thermo Fisher Scientific, Inc.) and the cells were observed
under microscope (DM4B; Leica Microsystems GmbH) for three
fields.
MI mouse model
PINCH1 Knockout mice were generated at the
transgenic core facility at University of California at San Diego.
In the present study, all experimental procedures involving
experimental animals were carried out in compliance with the local
animal welfare laws/policies, and the approval from the local
animal ethics committee of Shanghai General Hospital, Affiliated to
Shanghai Jiao Tong University School of Medicine was obtained
(approval number pkj2017-Y38). Adult C57BL/6 mice (weighing 20±2 g,
aged 7±1 weeks) were ordered from Shanghai SLAC Laboratory Animal
Co., Ltd. Mice were maintained in standard cages under a specific
pathogen free (SPF) environment, with 12-h light/dark cycle and
free access to food and water for ~1 week to acclimatize. Mice were
divided into four groups: Wild-type, wild-type-MI, PINCH1 knockout
group and PINCH1 knockout MI group, six mice in each group. In the
wild-type (normal group) only the chests were opened and no heart
surgery performed. Mice were anesthetized with 2% isoflurane
(maintenance, 1.5% isoflurane) through a face mask and placed in a
supine position in the center of the operating table. A midline
neck incision was performed to expose the heart. The left anterior
descending branch of the mouse heart was ligated under a
stereoscopic microscope. After the mice are raised for four weeks,
they are weighed with an electronic scales. An electrocardiograph
was carried out by ultrasound on each mouse.
Histological analysis
Heart tissues were fixed with 4% polyformaldehyde
for 24 h at 25˚C, followed by paraffin embedding. Subsequently, the
paraffin-embedded sections (3 µm) were stained with H&E
following dewaxing in two baths of xylene for 10 min each at 25˚C
and rehydration in a graded alcohol series for 5 min at 25˚C before
rinsing with running water for 5 min, three times at 25˚C.
Hematoxylin staining was for 5 min, followed by 5% acetic acid
differentiation for 1 min and washing in water at 25˚C.
Sections were also stained using Masson's trichrome;
following the operations as aforesaid. The stained sections were
both examined under the Leica DM5000 light microscope (Leica
Microsystems GmbH).
Electron microscopy
Cardiac ventricles were processed for electron
microscopy analysis as described (19). Mouse myocardium was perfused with
2.5% glutaraldehyde at for 24 h at 25˚C (Tianjin Kemiou Chemical
Reagent Co., Ltd.; 25% glutaraldehyde: 4% PFA=1:9). The harvested
tissues were post-fixed in 2.5% glutaraldehyde for 24 h at 25˚C,
subsequently stained in 1% osmium tetroxide for 72 h and then
cleared in propylene oxide post-dehydration in a series at 25˚C for
7 days, For transmission electron microscopy (TEM; HT7800; Hitachi,
Ltd.), 50 nm ultrathin sections were acquired and double-stained
with 2% uranyl acetate and lead citrate for TEM analysis.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5 software (GraphPad Software, Inc.). All experiments were
repeated at least three times and data are presented as the mean ±
SD. Comparisons between two groups were analyzed using the unpaired
Student's test. Comparison of measurement data between two groups
was analyzed by t-test, while comparison of measurement data among
multiple groups was analyzed by one-way (Tukey) analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
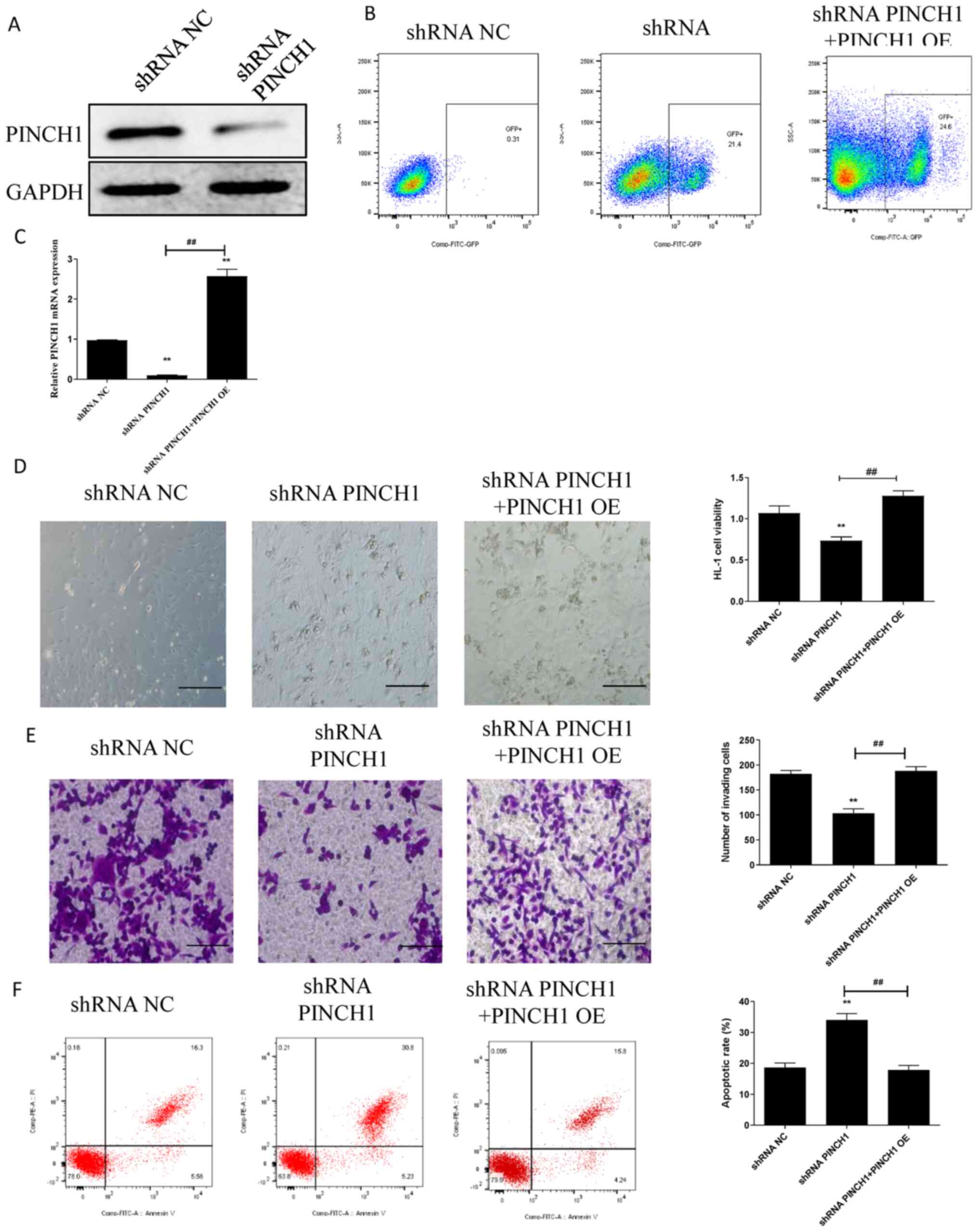
shRNA-mediated PINCH1 knockdown
attenuates cell viability and migration, and increases cell
apoptosis
To explore the significance of PINCH1 in the
function of HL1 cells, cell viability, migration and apoptosis were
assessed in HL1 cells transfected with PINCH1 shRNA. Furthermore,
rescue experiments were performed by inducing PINCH1 overexpression
(PINCH1 OE) in shRNA PINCH1-transfected HL1 cells, as previously
described (20). Successful shRNA
PINCH1 transfection was confirmed by western blotting (Fig. 1A). Following PINCH1 OE, the RT-qPCR
and western blotting results demonstrated that PINCH1 expression
was higher in the PINCH1 OE group compared with that in the NC OE
group (Fig. S1). Moreover, the
transfected cells expressing exogenous GFP were enriched using flow
cytometry (Fig. 1B). PINCH1 OE also
significantly upregulated PINCH1 expression in shRNA
PINCH1-transfected HL1 cells (P<0.05; Fig. 1C). Additionally, compared with
control and shRNA PINCH1 + PINCH1 OE groups, cell viability and
migration were significantly decreased in the shRNA PINCH1 group
(P<0.01) (Fig. 1D and E). Subsequently, the effect of PINCH1 on
HL1 cell apoptosis was significantly evaluated by flow cytometry.
The results showed that the apoptotic rate of HL1 cells transfected
with shRNA PINCH1 was significantly increased compared with control
and shRNA PINCH1 + PINCH1 OE groups (P<0.01; Fig. 1F).
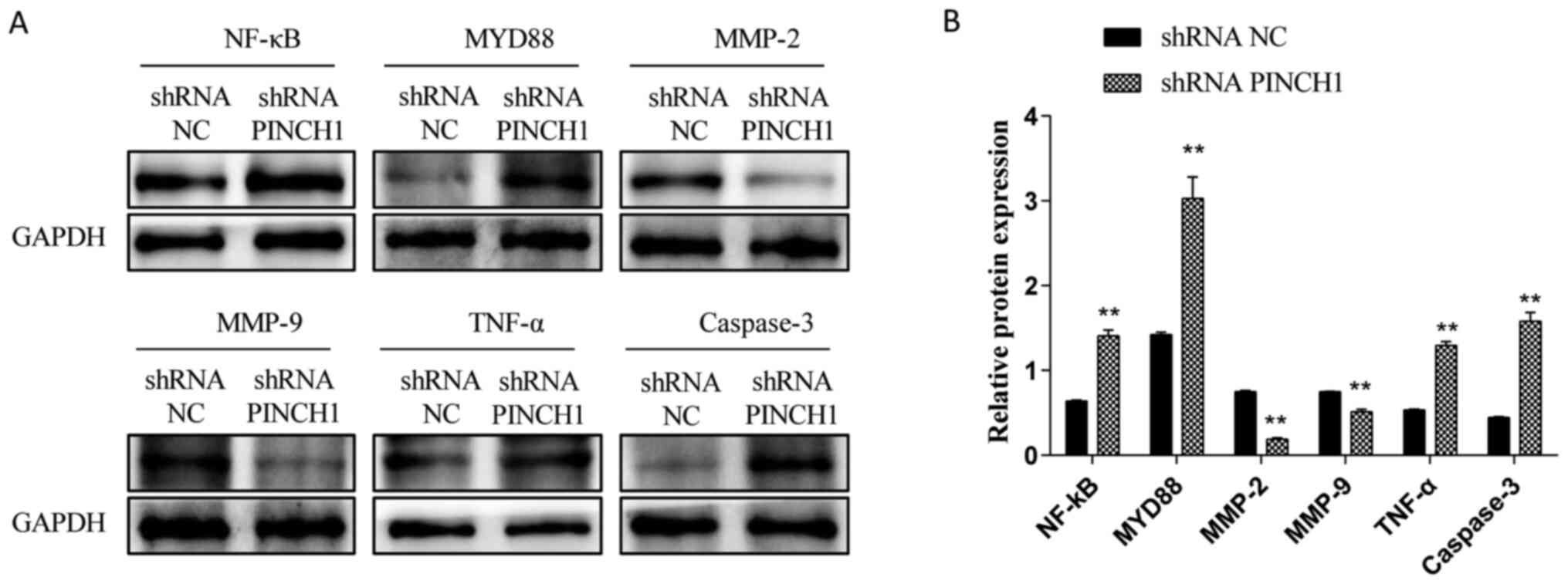
HL1 cell apoptosis is associated with
the NF-κB signaling pathway
To determine whether shRNA PINCH1-mediated HL1
cardiomyocyte apoptosis was associated with the NF-κB signaling
pathway, western blotting was performed to evaluate the expression
levels of NF-κB-related proteins in shRNA NC and shRNA
PINCH1-transfected HL1 cells. Compared with those in the WT group,
the protein expression levels of NF-κB, MYD88, TNF-α and caspase-3
were significantly increased, whereas the expression levels of
MMP-2 and MMP-9 were significantly decreased in shRNA
PINCH1-transfected HL1 cells (P<0.05; Fig. 2A and B). These data suggested that the
biological effect of shRNA PINCH1-transfected HL1 cells might be
mediated by the NF-κB signaling pathway.
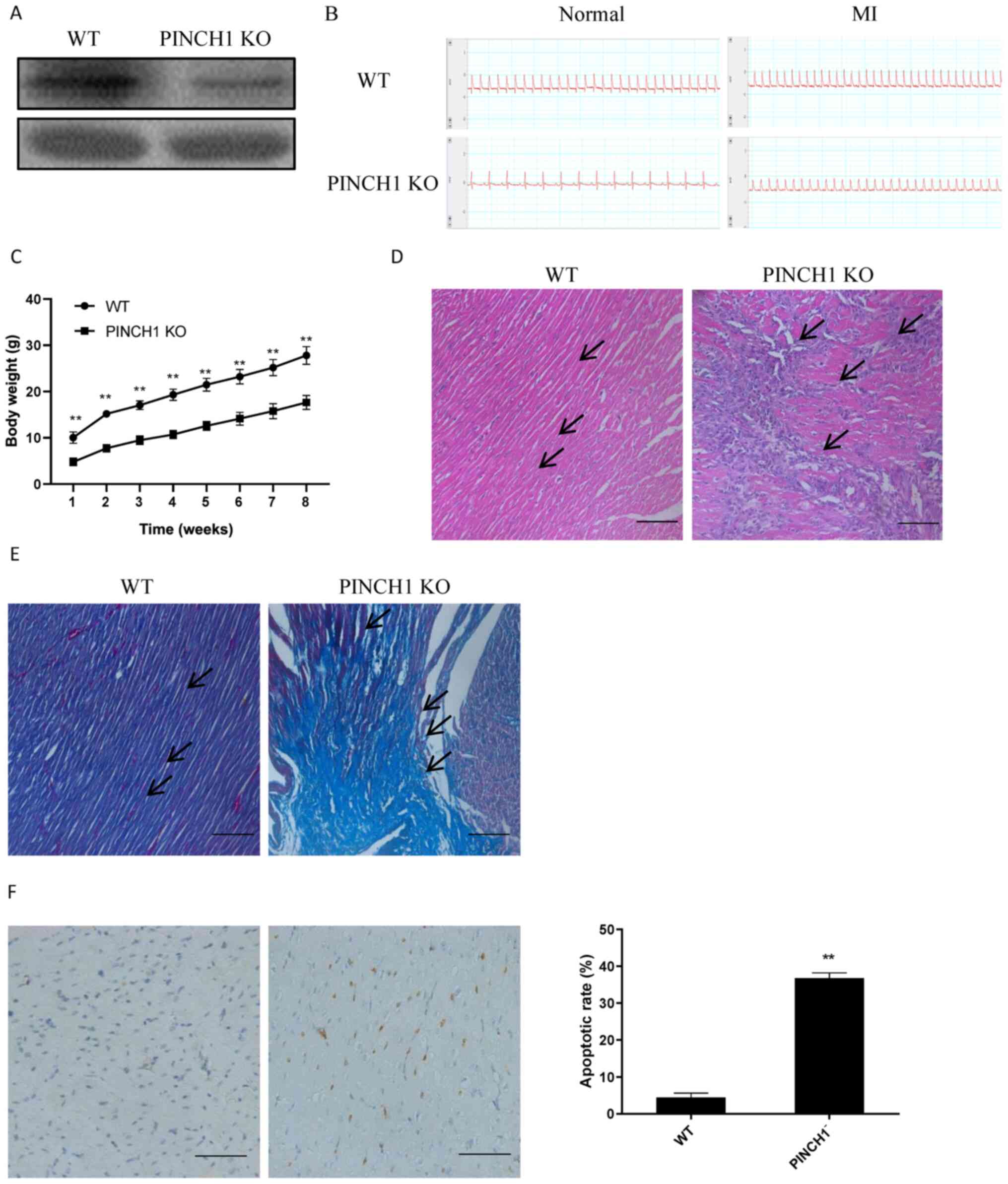
PINCH1 KO may lead to exacerbated
cardiac injury
To further evaluate the potential effects of PINCH1
in the myocardium, a mouse model with PINCH1 KO in cardiac cells
was established. Subsequently, MI was induced in PINCH1 KO and WT
mice at 18 weeks of age. The western blotting results demonstrated
the successful KO of PINCH1 in the myocardial tissue (Fig. 3A). In addition, all mice were
examined for acute MI by electrocardiogram via ultrasound (Fig. 3B). All PINCH1 KO mice gradually
developed severe growth retardation with postnatal development from
one weeks to eight weeks old (Fig.
3C). The H&E staining results showed obvious swelling and
hypertrophy of myocardial cells in the myocardial tissue cross
sectional area of PINCH1 KO mice Histological examination showed
myocardial cell arrangement disorders of left ventricle compared
with control hearts (Fig. 3D).
Furthermore, the Masson's trichrome staining results showed that
myocardial fibers in the PINCH1 KO group were disordered and
displayed obvious fibrotic changes compared with those in the WT
group (Fig. 3E). The TUNEL staining
assay results demonstrated that the apoptotic rate of
cardiomyocytes in the PINCH1 KO group was significantly increased
compared with that in the WT group (Fig. 3F). The aforementioned findings
indicated that PINCH1 KO caused myocardial fibrosis and heart
failure in mice.
Heart ultrastructural abnormalities in
PINCH1 KO mice
In representative electron micrographs at day 1,
intercalated disk structures remained largely similar between
control and PINCH1 KO. However, in a few focal regions, gaps of
intercalated disks were widened, with disarrayed sarcomeric
structure and distorted Z lines. At day 10, intercalated disks of
control samples were clearly observed, connecting adjacent myocytes
end to end. In PINCH1 KO, membranes of the intercalated disk were
highly convoluted, and gaps of the intercalated disks were
significantly widened. Compared with WT mice, the muscle layer
structure was disorganized, some myofibrils were broken, the
distance between the membrane and the muscle layer of myocytes was
obviously widened, and the decreased adhesion ability of the
adjacent intercellular myocytes resulted in an increased cell gap
in the PINCH1 KO group (Fig.
4).
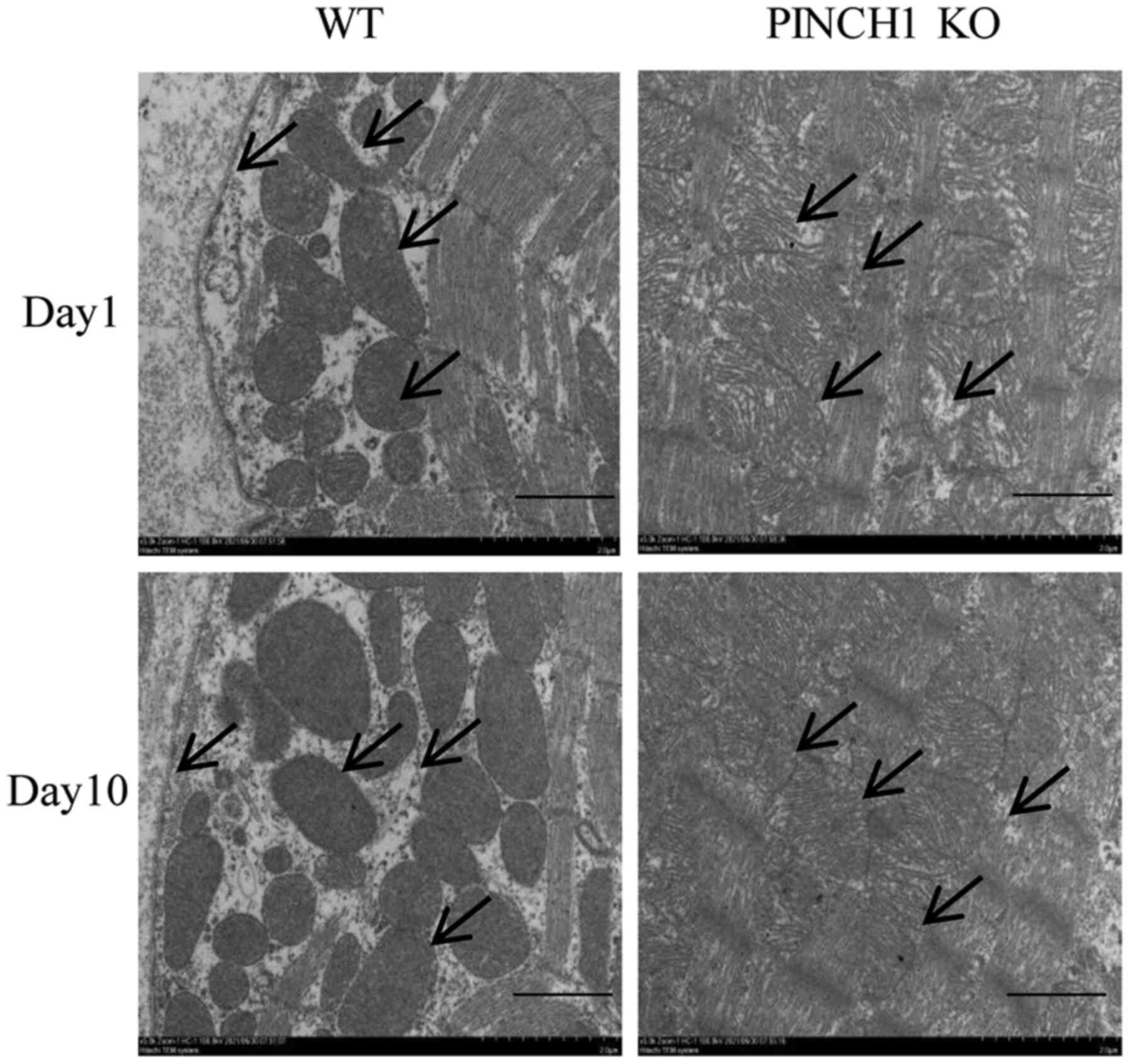 | Figure 4Representative electron micrographs of
PINCH1 KO mice. At day 1 after birth, intercalated disk structures
remained largely similar between control and PINCH1 KO. However, in
a few focal regions, gaps of intercalated disks were widened, with
disarrayed sarcomeric structure and distorted Z lines. At day 10
after birth, intercalated disks of control samples were clearly
observed, connecting adjacent myocytes end to end. In PINCH1 KO,
membranes of the intercalated disk were highly convoluted, and gaps
of the intercalated disks were significantly widened (scale bar=2
µm). PINCH1, particularly interesting new cysteine histidine rich
1; KO, knockout; WT, wild-type. |
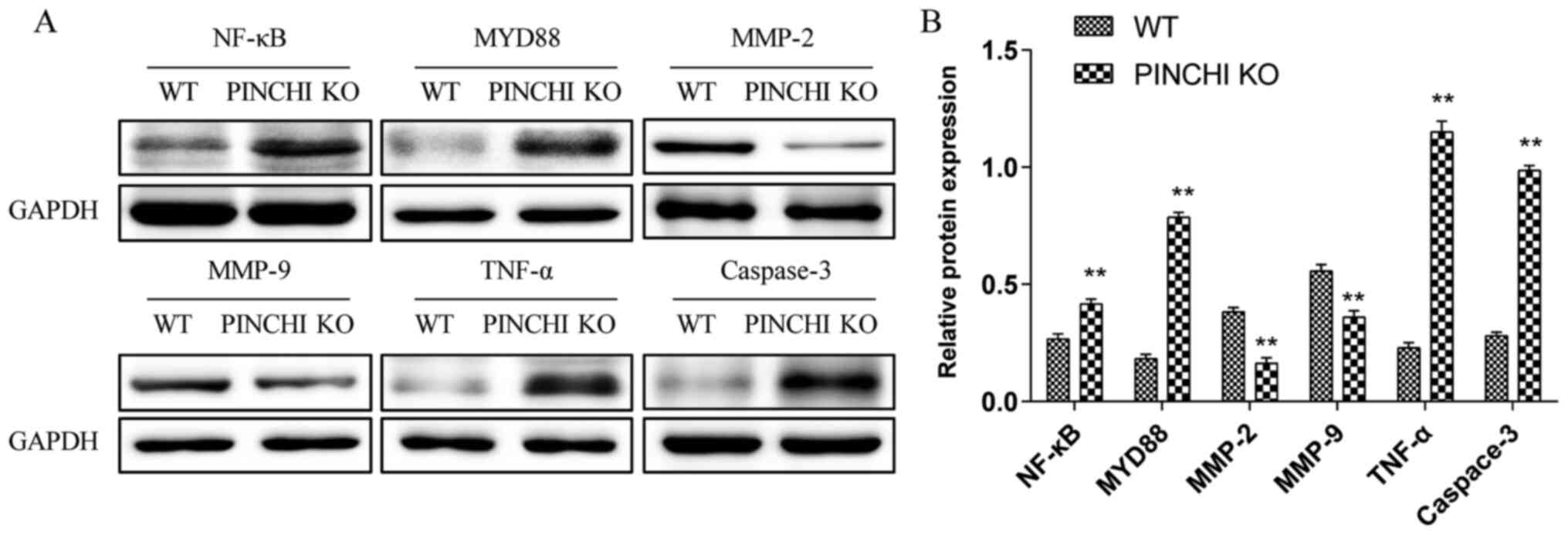
PINCH1 KO in the myocardium may
promote cardiomyopathy in mice via the NF-κB signaling
The results of the in vitro experiments
demonstrated that PINCH1 KO-induced HL1 cardiomyocyte apoptosis was
associated with the NF-κB signaling pathway. Therefore, to
investigate whether the PINCH1 KO-induced cardiomyopathy in mice
was also mediated by NF-κB signaling, the expression levels of the
NF-κB pathway-related proteins were determined in cardiac tissues
by western blotting. Consistent with the in vitro results,
compared with the WT group, the expression levels of NF-κB, MYD88,
TNF-α and caspase-3 were significantly increased (P<0.01;
Fig. 5), whereas the expression
levels of MMP-2 and MMP-9 were notably decreased in the myocardial
tissues of the PINCH1 KO group (P<0.01; Fig. 5).
Discussion
Cardiovascular diseases are a major health problem
worldwide. As the leading cause of death among patients with
cardiovascular disease, MI exhibits high morbidity and mortality
rates worldwide. Furthermore, MI is a very common ischemic heart
disease characterized by impaired cardiomyocyte function, resulting
in progressive heart failure (21-25).
PINCH1 serves a crucial role in maintaining the structure and
function of the heart and is involved in several biological
processes, including cell migration and survival. In zebrafish,
PINCH1 knockdown led to severe heart failure via the
ILK-parvin-PINCH/protein kinase B signaling pathway (26). In addition, PINCH1 KO in mouse
embryos resulted in attenuated ventricular cardiomyocyte
proliferation and excessive cell death (27). The present study demonstrated that
shRNA-mediated PINCH1 knockdown in HL1 cardiomyocytes also
decreased cell viability and migration, but increased apoptosis.
Additionally, targeted PINCH1 KO in the myocardium of mice
aggravated cardiomyopathy and heart failure.
MI has been associated with inflammatory responses
in the myocardium. Although, the myocardial tissue inflammatory
response promotes myocardial repair and wound healing, excessive
inflammation may also lead to heart failure. Following MI,
surviving myocardial and inflammatory cells promote NF-κB
activation to participate in the inflammatory repair response and
initiate the inflammatory cascade. However, the release of
excessive inflammatory cytokines, MMPs and other
inflammatory-associated factors also expands the MI area (24,25).
TNF-α is a proinflammatory cytokine involved in the regulation of
numerous autoimmune diseases. The downstream target of
TNF-α-mediated inflammation is the transcription factor NF-κB.
Therefore, NF-κB activation in the heart tissue is considered as a
hallmark of MI (10). It has been
reported that toll-like receptors promote heart failure in response
to injury or stress by activating the NF-κB signaling pathway via
the adapter protein MYD88. MYD88 serves an important role in the
signaling and activation of NF-κB downstream signals (18). Furthermore, MMPs are proteolytic
inflammation-related enzymes involved in the degradation and
remodeling of extracellular matrix. It has been reported that MMPs
promote the ventricular remodeling process (28-31).
The results from the in vivo and in vitro experiments
conducted in the present study revealed that the expression levels
of NF-κB, MYD88, TNF-α, and caspase-3 were significantly increased,
whereas MMP-2 and MMP-9 expression levels were decreased in the
shRNA PINCH1 and PINCH1 KO groups.
In summary, the present study demonstrated that
targeted PINCH1 shRNA attenuated cell viability and migration, and
increased cell apoptosis in vitro, thus leading to
myocardial disease and heart failure in mice via the NF-κB
signaling pathway. These findings provided a theoretical basis for
the clinical treatment of MI. However, since two homologous PINCH
isomers have been identified, the mechanism of action underlying
PINCH in MI requires further investigation.
Supplementary Material
Transfection efficiency of PINCH1 OE
in HL-1 cells. (A) Western blotting and (B) reverse
transcription-quantitative PCR were performed to assess the
transfection efficiency of PINCH1 OE. PINCH1, particularly
interesting new cysteine histidine rich 1; OE, overexpression; NC,
negative control.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Key Discipline
Groups of Shanghai Pudong New Area (grant no. PWZxq2017-01), the
Natural Science Foundation of Shanghai of China (grant no.
17ZR1425800) and Shanghai Municipal Health Commission of China
(grant no. 202040159).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ conceived and designed the experiments. XW and JS
contributed to designing the study and drafted the manuscript. XW
and YZ confirm the authenticity of all the raw data. XW, JS, ZL and
KL performed the data analysis. YZ made substantial contributions
to proofreading the manuscript and gave final approval of the
version to be published. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal experiments involved in the present study
were approved by the Ethics Committee of Zhoupu Hospital (approval
no. 2017-C-040-E01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sopko N, Qin Y, Finan A, Dadabayev A,
Chigurupati S, Qin J, Penn MS and Gupta S: Significance of thymosin
β4 and implication of PINCH-1-ILK-α-parvin (PIP) complex in human
dilated cardiomyopathy. PLoS One. 6(e20184)2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zou L, Ma X, Lin S, Wu B, Chen Y and Peng
C: Bone marrow mesenchymal stem cell-derived exosomes protect
against myocardial infarction by promoting autophagy. Exp Ther Med.
18:2574–2582. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang HR, Bai H, Yang E, Zhong ZH, Chen
WY, Xiao Y, Gu YH and Lu SF: Effect of moxibustion preconditioning
on autophagy-related proteins in rats with myocardial ischemia
reperfusion injury. Ann Transl Med. 7(559)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang R, Wang M, Zhou J, Ye T, Xie X, Ni D,
Ye J, Han Q, Di C, Guo L, et al: Shuxuening injection protects
against myocardial ischemia-reperfusion injury through reducing
oxidative stress, inflammation and thrombosis. Ann Transl Med.
7(562)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jiang T, Zhang L, Ding M and Li M:
Protective effect of vasicine against myocardial infarction in rats
via modulation of oxidative stress, inflammation, and the PI3K/Akt
pathway. Drug Des Devel Ther. 13:3773–3784. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang C, Liang R, Gan X, Yang X, Chen L
and Jian J: MicroRNA-384-5p/Beclin-1 as potential indicators for
epigallocatechin gallate against cardiomyocytes ischemia
reperfusion injury by inhibiting autophagy via PI3K/Akt pathway.
Drug Des Devel Ther. 13:3607–3623. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Belete S, Punjabi K, Afoke J and Anderson
J: Surgical management of post-infarction ventricular septal
defect, mitral regurgitation and ventricular aneurysm. J Surg Case
Rep. 2019(rjz256)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liang X, Sun Y, Ye M, Scimia MC, Cheng H,
Martin J, Wang G, Rearden A, Wu C, Peterson KL, et al: Targeted
ablation of PINCH1 and PINCH2 from murine myocardium results in
dilated cardiomyopathy and early postnatal lethality. Circulation.
120:568–576. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Braun A, Bordoy R, Stanchi F, Moser M,
Kostka GG, Ehler E, Brandau O and Fässler R: PINCH2 is a new five
LIM domain protein, homologous to PINCH and localized to focal
adhesions. Exp Cell Res. 284:239–250. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liang X, Zhou Q, Li X, Sun Y, Lu M, Dalton
N, Ross J Jr and Chen J: PINCH1 plays an essential role in early
murine embryonic development but is dispensable in ventricular
cardiomyocytes. Mol Cell Biol. 25:3056–3062. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pan SP, Pirker T, Kunert O, Kretschmer N,
Hummelbrunner S, Latkolik SL, Rappai J, Dirsch VM, Bochkov V and
Bauer R: C13 megastigmane derivatives from epipremnum pinnatum:
β-Damascenone inhibits the expression of pro-inflammatory cytokines
and leukocyte adhesion molecules as well as NF-κB signaling. Front
Pharmacol. 10(1351)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang J, Lei JR, Yuan LL, Wen R and Yang
J: Response gene to complement-32 promotes cell survival via the
NF-κB pathway in non-small-cell lung cancer. Exp Ther Med.
19:107–114. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ge ZW, Wang BC, Hu JL, Sun JJ, Wang S,
Chen XJ, Meng SP, Liu L and Cheng ZY: IRAK3 gene silencing prevents
cardiac rupture and ventricular remodeling through negative
regulation of the NF-κB signaling pathway in a mouse model of acute
myocardial infarction. J Cell Physiol. 234:11722–11733.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Han A, Lu Y, Zheng Q, Zhang J, Zhao Y,
Zhao M and Cui X: Qiliqiangxin attenuates cardiac remodeling via
inhibition of TGF-β1/Smad3 and NF-κB signaling pathways in a rat
model of myocardial infarction. Cell Physiol Biochem. 45:1797–1806.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
He Q, Zhou W, Xiong C, Tan G and Chen M:
Lycopene attenuates inflammation and apoptosis in post-myocardial
infarction remodeling by inhibiting the nuclear factor-κB signaling
pathway. Mol Med Rep. 11:374–378. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jin JL, Deng ZT, Lyu RG, Liu XH and Wei
JR: Expression changes of Notch and nuclear factor-κB signaling
pathways in the rat heart with myocardial infarction. Zhonghua Xin
Xue Guan Bing Za Zhi. 45:507–512. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
17
|
Gupta S, Kumar S, Sopko N, Qin Y, Wei C
and Kim IK: Thymosin β4 and cardiac protection: Implication in
inflammation and fibrosis. Ann N Y Acad Sci. 1269:84–91.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Qiu P, Wheater MK, Qiu Y and Sosne G:
Thymosin beta4 inhibits TNF-alpha-induced NF-kappaB activation,
IL-8 expression, and the sensitizing effects by its partners
PINCH-1 and ILK. FASEB J. 25:1815–1826. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen J, Kubalak SW, Minamisawa S, Price
RL, Becker KD, Hickey R, Ross J Jr and Chien KR: Selective
requirement of myosin light chain 2v in embryonic heart function. J
Biol Chem. 273:1252–1256. 1998.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sandfort V, Eke I and Cordes N: The role
of the focal adhesion protein PINCH1 for the radiosensitivity of
adhesion and suspension cell cultures. PLoS One.
5(e13056)2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang W, Tian Y, Gao Q, Li X, Li Y, Zhang
J, Yao C, Wang Y, Wang H, Zhao Y, et al: Inhibition of apoptosis
reduces diploidization of haploid mouse embryonic stem cells during
differentiation. Stem Cell Reports. 15:185–197. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yokokawa T, Yoshihisa A, Kanno Y, Abe S,
Misaka T, Yamada S, Kaneshiro T, Sato T, Oikawa M, Kobayashi A, et
al: Circulating acetoacetate is associated with poor prognosis in
heart failure patients. Int J Cardiol Heart Vasc.
25(100432)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Luo ZR, Li H, Xiao ZX, Shao SJ, Zhao TT,
Zhao Y, Mou FF, Yu B and Guo HD: Taohong siwu decoction exerts a
beneficial effect on cardiac function by possibly improving the
microenvironment and decreasing mitochondrial fission after
myocardial infarction. Cardiol Res Pract.
2019(5198278)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Neyrinck K, Breuls N, Holvoet B,
Oosterlinck W, Wolfs E, Vanbilloen H, Gheysens O, Duelen R, Gsell
W, Lambrichts I, et al: The human somatostatin receptor type 2 as
an imaging and suicide reporter gene for pluripotent stem
cell-derived therapy of myocardial infarction. Theranostics.
8:2799–2813. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Puddighinu G, D'Amario D, Foglio E, Manchi
M, Siracusano A, Pontemezzo E, Cordella M, Facchiano F, Pellegrini
L, Mangoni A, et al: Molecular mechanisms of cardioprotective
effects mediated by transplanted cardiac ckit+ cells
through the activation of an inflammatory hypoxia-dependent
reparative response. Oncotarget. 9:937–957. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang B, Zhang L, Cao H, Yang J, Wu M, Ma
Y, Fan H, Zhan Z and Liu Z: Myoblast transplantation improves
cardiac function after myocardial infarction through attenuating
inflammatory responses. Oncotarget. 8:68780–68794. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Meder B, Huttner IG, Sedaghat-Hamedani F,
Just S, Dahme T, Frese KS, Vogel B, Köhler D, Kloos W, Rudloff J,
et al: PINCH proteins regulate cardiac contractility by modulating
integrin-linked kinase-protein kinase B signaling. Mol Cell Biol.
31:3424–3435. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Singh MV, Swaminathan PD, Luczak ED,
Kutschke W, Weiss RM and Anderson ME: MyD88 mediated inflammatory
signaling leads to CaMKII oxidation, cardiac hypertrophy and death
after myocardial infarction. J Mol Cell Cardiol. 52:1135–1144.
2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gu C, Wang F, Zhao Z, Wang H, Cong X and
Chen X: Lysophosphatidic acid is associated with atherosclerotic
plaque instability by regulating NF-κB dependent matrix
metalloproteinase-9 expression via LPA2 in macrophages.
Front Physiol. 8(266)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang Q, Sui X, Sui DJ and Yang P:
Flavonoid extract from propolis inhibits cardiac fibrosis triggered
by myocardial infarction through upregulation of SIRT1. Evid Based
Complement Alternat Med. 2018(4957573)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ibarra-Lara L, Sánchez-Aguilar M,
Soria-Castro E, Vargas-Barrón J, Roldán FJ, Pavón N, Torres-Narváez
JC, Cervantes-Pérez LG, Pastelín-Hernández G and Sánchez-Mendoza A:
Clofibrate treatment decreases inflammation and reverses myocardial
infarction-induced remodelation in a rodent experimental model.
Molecules. 24(270)2019.PubMed/NCBI View Article : Google Scholar
|