Introduction
Intestinal ischemia/reperfusion (I/R) is a clinical
vascular emergency that is mainly caused by hemorrhagic or septic
shock, cardiac arrest, occlusion of the mesenteric artery and
surgical procedures (1). This
pathophysiological process can cause systemic inflammatory response
syndrome and multiple organ failure, which ultimately results in
high morbidity and mortality rates (2,3).
Intestinal I/R injury is a complex process involving oxidative
stress, inflammation, apoptosis and intestinal barrier function
disruption (4). Increased
microvascular permeability and mucosal barrier damage may occur
following ischemia of the intestine, while reperfusion leads to
serious oxidative stress and inflammatory cell infiltration
(5,6). At present, the strategies for
treating intestinal I/R injury mostly concentrate on the
reperfusion period, since intestinal ischemia is rarely preventable
(7). Several common reagents have
been used to treat intestinal I/R injury, such as glutamine,
palmitoylethanolamide and inducible nitric oxide synthase (iNOS)
inhibitors, but the clinical efficacy of these treatments have not
been determined (8-10).
Novel therapeutic approaches, including natural products for
attenuating inflammation and apoptosis, and protecting the
intestinal mucosal barrier from injury, are under development
(11).
Syringic acid (SA), an abundant phenolic acid
compound, is extracted from olives, spices, grapes, dates and other
plants (12). It has been
demonstrated that SA exerts biological effects in various diseases
due to its anti-inflammatory, antioxidant and antitumor properties
(13). Previous studies have
demonstrated that SA alleviates injury from hypoxia/reoxygenation
or I/R in neuronal, myocardial and kidney cells (14-16).
In addition, SA was revealed to protect from dextran sulfate
sodium-induced experimental colitis in BALB/c mice (17). However, the functional role of SA
in intestinal I/R injury remains unclear. Therefore, the present
study aimed to explore whether SA exhibits a protective role from
intestinal I/R injury in vitro.
Materials and methods
Cell culture and treatment
The human colon carcinoma immortalized cell line
Caco-2 was purchased from the Cell Bank of Shanghai Institutes for
Biological Science. Cells were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 1% penicillin and
streptomycin, and maintained in a humidified atmosphere with 5%
CO2 at 37˚C.
SA was purchased from Sigma-Aldrich (Merck KGaA) and
dissolved in 0.1% DMSO (v/v). Caco-2 cells were cultured for 24 h
at 37˚C and fed with DMEM culture medium supplemented with
different concentrations of SA (0.1, 1.0 and 10.0 µM). After
incubation for 24 h at 37˚C, cells were collected by trypsin
application.
Oxygen-glucose
deprivation/reoxygenation (OGD/R) treatment
To simulate intestinal I/R injury in vitro,
an OGD/R model was established using Caco-2 cells. Caco-2 cells
were cultured in glucose-free DMEM and maintained in an incubator
under conditions of 94% N2, 1% O2 and 5%
CO2 at 37˚C for 8 h. The cells were subsequently placed
in a microaerophilic system in the presence of 95% O2
and 5% CO2 at 37˚C in normal DMEM for 20 h to induce
reoxygenation.
Cell viability assay
Cell Counting Kit-8 (CCK-8) assay was performed to
assess cell viability. Caco-2 cells were seeded into 96-well plates
at a density of 5x103 cells/well and pretreated with
various concentrations of SA (0.1, 1 and 10 µM) for 24 h. After
OGD/R, 10 µl CCK-8 reagent (Dojindo Molecular Technologies, Inc.)
was added to each well, and the cells were incubated for 2 h at
37˚C. The resulting absorbance was detected at 450 nm using a
microplate reader (Bio-Rad Laboratories, Inc.).
Determination of lactate dehydrogenase
(LDH) activity
Cell injury was detected by measuring the LDH
activity using an LDH assay kit (cat. no. A020-2; Nanjing Jiancheng
Bioengineering Institute). Caco-2 cells were seeded into 96-well
plates (5x103 cells/well) and treated with 0.1, 1.0 and
10.0 µM of SA followed by OGD/R injury. The cell culture medium was
then collected, and the LDH activity was measured at 530 nm using a
microplate reader (Benchmark; Bio-Rad Laboratories, Inc.).
ELISA
Caco-2 cells were pretreated with 0.1, 1 and 10 µM
SA for 24 h and underwent OGD/R injury for 8/20 h. The
concentration levels of TNF-α (cat. no. H052-1), IL-6 (cat. no.
H007-1), IL-1β (cat. no. H002) and monocyte chemoattractant
protein-1 (MCP-1; cat. no. H115) in the cell culture medium of
Caco-2 cells were determined using ELISA kits (Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's
instructions. Absorbance at 450 nm was measured using a microplate
reader (BioTek Instruments, Inc.). A total of 6 parallel wells were
set for ELISA.
Western blotting
Total protein was extracted from Caco-2 cells using
RIPA lysis buffer (Beyotime Institute of Biotechnology), after
which the protein concentration was measured using a BCA protein
assay kit (Beijing Dingguo Changsheng Biotechnology Co., Ltd.). A
total of 30 µg/lane protein samples were then separated by 10%
SDS-PAGE and transferred onto PVDF membranes (MilliporeSigma).
After blocking with 5% non-fat milk for 1 h at 25 ˚C, the membranes
were incubated at 4˚C overnight with the following primary
antibodies obtained from Abcam: p65 (1:1,000; cat. no. ab16502),
phosphorylated (p)-p65 (1:1,000; cat. no. ab86299), iNOS (1:1,000;
cat. no. ab178945), cyclooxygenase 2 (COX2; 1:1,000; cat. no.
ab179800), Bcl-2 (1:1,000; cat. no. ab32124), Bax (1:1,000; cat.
no. ab32503), cleaved caspase 3 (1:500; cat. no. ab32042), cleaved
peroxisome proliferator-activated receptor (PPAR; 1:1,000; cat. no.
ab178860), Claudin-3 (1:1,000; cat. no. ab214487), Claudin-2
(1:1,000; cat. no. ab53032), zonula occludens 1 (ZO-1; 1:1,000;
cat. no. ab216880) and GAPDH (1:3,000; cat. no. ab125247). The
membranes were washed with 0.05% PBS-Tween-20 thrice and then
incubated with horseradish peroxidase-labeled anti-mouse (cat. no.
7076; Cell Signaling Technology, Inc.; 1:1,000) and anti-rabbit IgG
(cat. no. 7074; 1:1,000; Cell Signaling Technology, Inc.) for 1 h
at 37˚C. The bands were detected using an enhanced
chemiluminescence detection system (Thermo Fisher Scientific,
Inc.). Finally, the protein bands were analyzed using ImageJ
software (Version 1.49; National Institutes of Health) with GAPDH
as a loading control. All the experiments were performed ≥3 times,
and representative data are presented in the corresponding
figures.
Determination of reactive oxygen
species (ROS), superoxide dismutase (SOD) and malondialdehyde (MDA)
levels
Caco-2 cells were seeded in 6-well plates at a
density of 4x105 cells/well, and treated with 0.1, 1 and
10 µM SA for 24 h. Following OGD/R induction, the expression levels
of ROS and MDA, and the level of SOD activity in the culture medium
were evaluated using corresponding commercial kits (cat. nos.
E004-1-1, A003-1-2 and A001-3-2, respectively; Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's
protocols.
TUNEL assay
TUNEL assay was performed to determine apoptosis.
Upon treatment, cells were washed twice with PBS and fixed with 4%
paraformaldehyde at room temperature in the dark for 30 min. Next,
the cells were incubated with proteinase K for 15 min at 37˚C and
placed in 3% H2O2 for 15 min at room
temperature. After washing several times with PBS, the cells were
treated with TUNEL working solution at 37 ˚C for 90 min and
co-labeled with DAPI working solution (1 µg/ml) for 10 min at room
temperature. Next, labeled Caco-2 cells were visualized using a
fluorescence microscope (Leica Microsystems GmbH, x200) and at
least 10 fields per section for each sample were examined.
Transepithelial electrical resistance
(TEER) assay
To investigate the function of the intestinal
barrier in vitro, a TEER assay was carried out to examine
the integrity of the intestinal barrier. Caco-2 cells were treated
with 0.1, 1 and 10 µM SA for 24 h followed by OGD/R induction. The
cells were subsequently harvested and the TEER assay was performed
in 24-well Transwell plates (0.4-µm pore size; Costar; Corning,
Inc.) as previously described (18).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5.0 (GraphPad Software, Inc.). All values are expressed as
the mean ± SD from at least three independent experiments and were
analyzed using one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
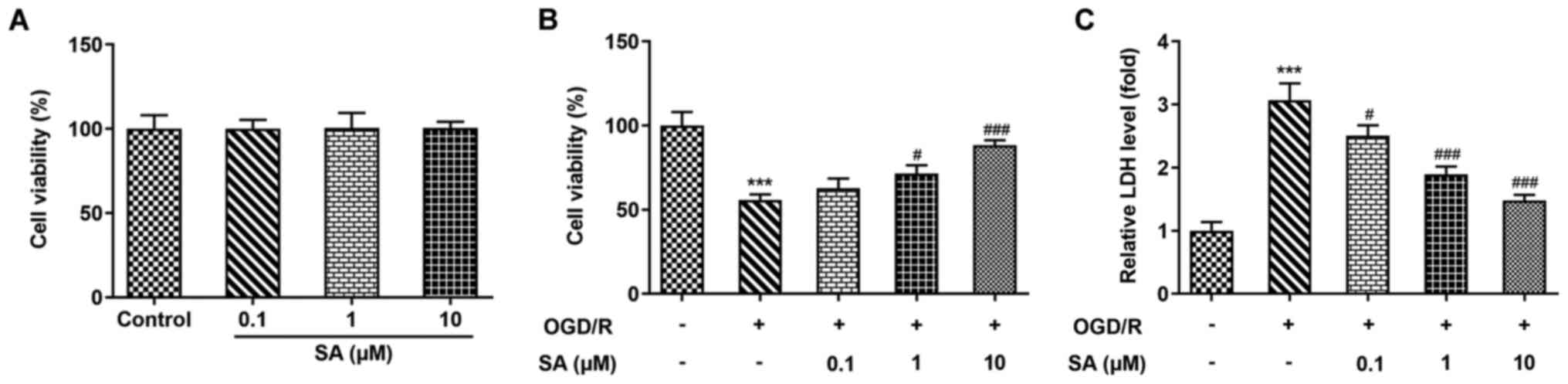
Effects of SA pretreatment on the
viability and injury of OGD/R-stimulated Caco-2 cells
To investigate the role of SA in Caco-2 cell
viability and injury following OGD/R induction, a CCK-8 assay and
LDH activity measurements were carried out. As presented in
Fig. 1A, pretreatment with 0.1-10
µM SA had no significant effect on the viability of Caco-2 cells.
In addition, compared with that of the control group, cell
viability was significantly reduced by OGD/R injury; however, SA
restored the viability of OGD/R-treated cells in a dose-dependent
manner (Fig. 1B). Consistently,
OGD/R injury led to a significant increase in LDH release, while SA
administration significantly reduced LDH release following OGD/R
injury (Fig. 1C). These data
suggest that SA decreased Caco-2 cell viability and cytotoxicity
injury induced by OGD/R.
Effects of SA pretreatment on the
release of inflammatory cytokines in Caco-2 cells subjected to
OGD/R
To explore the protective effect of pretreatment
with SA, the production of inflammatory cytokines was evaluated
in vitro. As presented in Fig.
2A-D, OGD/R significantly enhanced the levels of TNF-α, IL-6,
IL-1β and MCP-1 in Caco-2 cells compared with the normoxia group.
However, the concentrations of the four aforementioned inflammatory
cytokines were significantly reduced following SA treatment in a
dose-dependent manner. Western blot analysis revealed that OGD/R
significantly increased the protein expression of p-p65, iNOS and
COX2, whereas pretreatment with SA reversed this effect in a
dose-dependent manner (Fig. 2E).
The results indicate that SA inhibited inflammatory release in
OGD/R-stimulated Caco-2 cells.
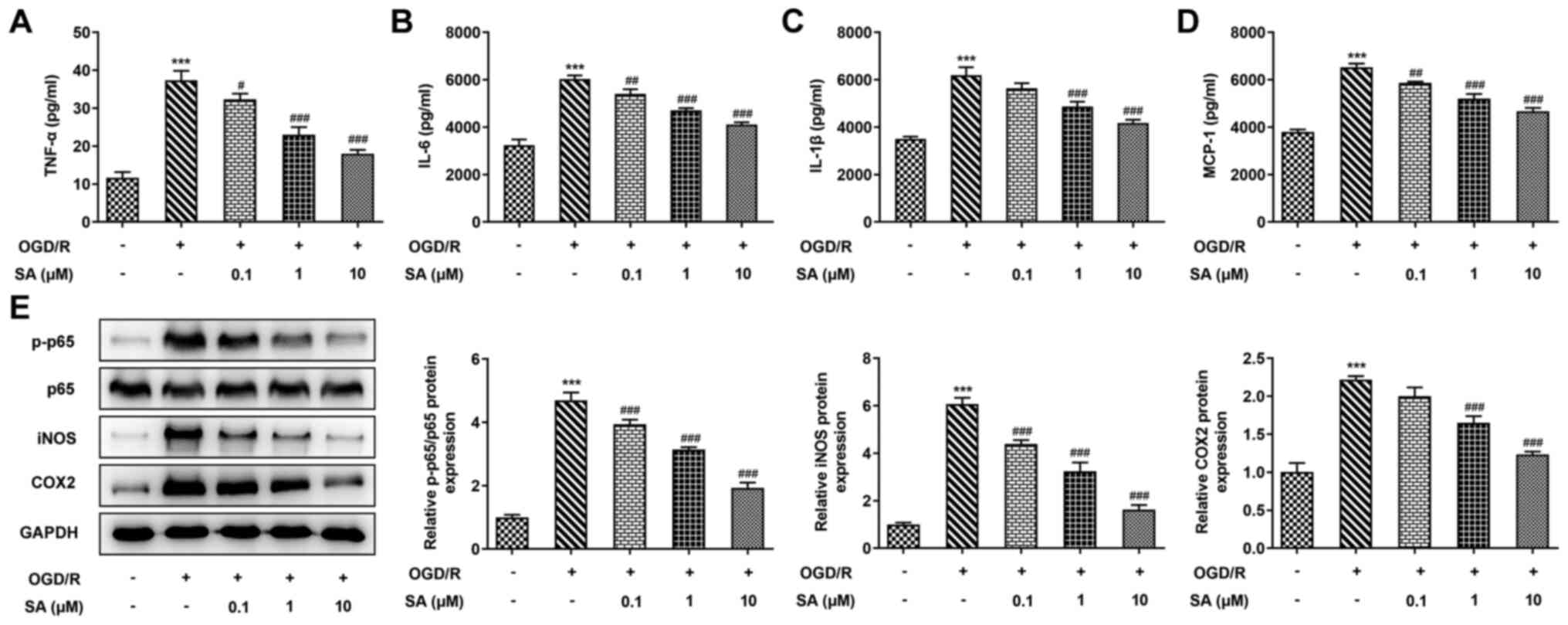 | Figure 2SA pretreatment suppresses the release
of inflammatory cytokines in Caco-2 cells subjected to OGD/R.
Caco-2 cells were pretreated with 0.1, 1 and 10 µM of SA for 24 h
and underwent treatment with OGD/R injury for 8/20 h. Levels of (A)
TNF-α, (B) IL-6, (C) IL-1β and (D) MCP-1 were detected using ELISA
kits. (E) Western blotting was used to determine protein levels of
p-p65, p65, iNOS and COX2. ***P<0.001 vs. the
control; #P<0.05, ##P<0.01 and
###P<0.001 vs. the OGD/R group. SA, syringic acid;
OGD/R, oxygen-glucose deprivation/reoxygenation; MCP-1, monocyte
chemoattractant protein-1; p-, phosphorylated; iNOS, inducible
nitric oxide synthase; COX2, cyclooxygenase 2. |
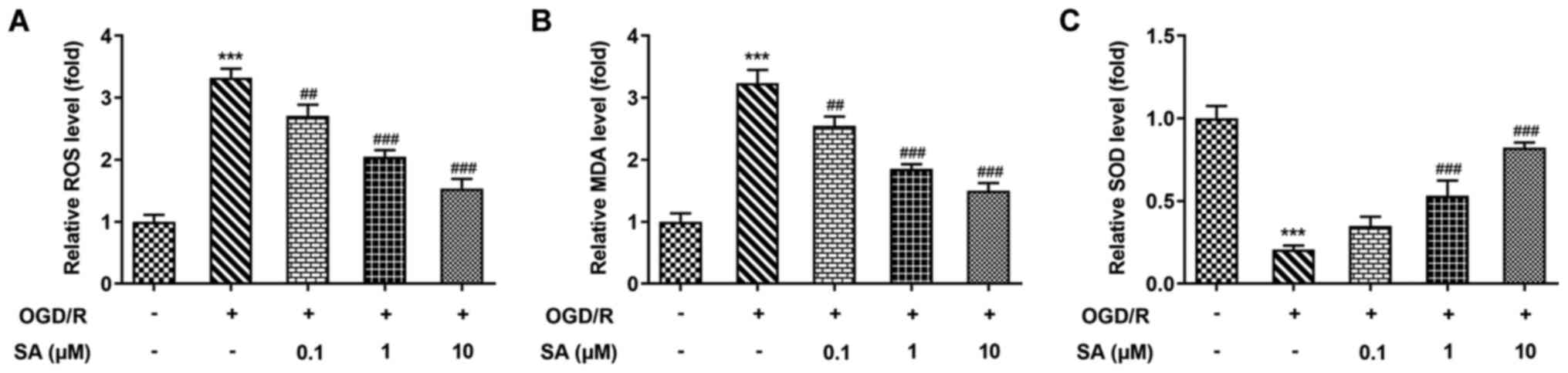
Effects of SA pretreatment on
oxidative stress in OGD/R-treated Caco-2 cells
Oxidative stress plays a notable role in intestinal
cell injury induced by OGD/R. Thus, the present study explored the
protective effect of SA on OGD/R-induced oxidative stress in Caco-2
cells. As presented in Fig. 3,
levels of ROS and MDA were significantly increased, while SOD
activity was significantly decreased after OGD/R induction,
compared with the normoxia group. Conversely, pretreatment with SA
significantly suppressed the production of ROS and MDA, but
elevated SOD activity levels in Caco-2 cells after OGD/R injury.
Therefore, SA may protect intestinal tissue from I/R injury via its
anti-inflammatory and antioxidant activities. These results
suggested that SA suppressed oxidative stress in OGD/R-treated
Caco-2 cells.
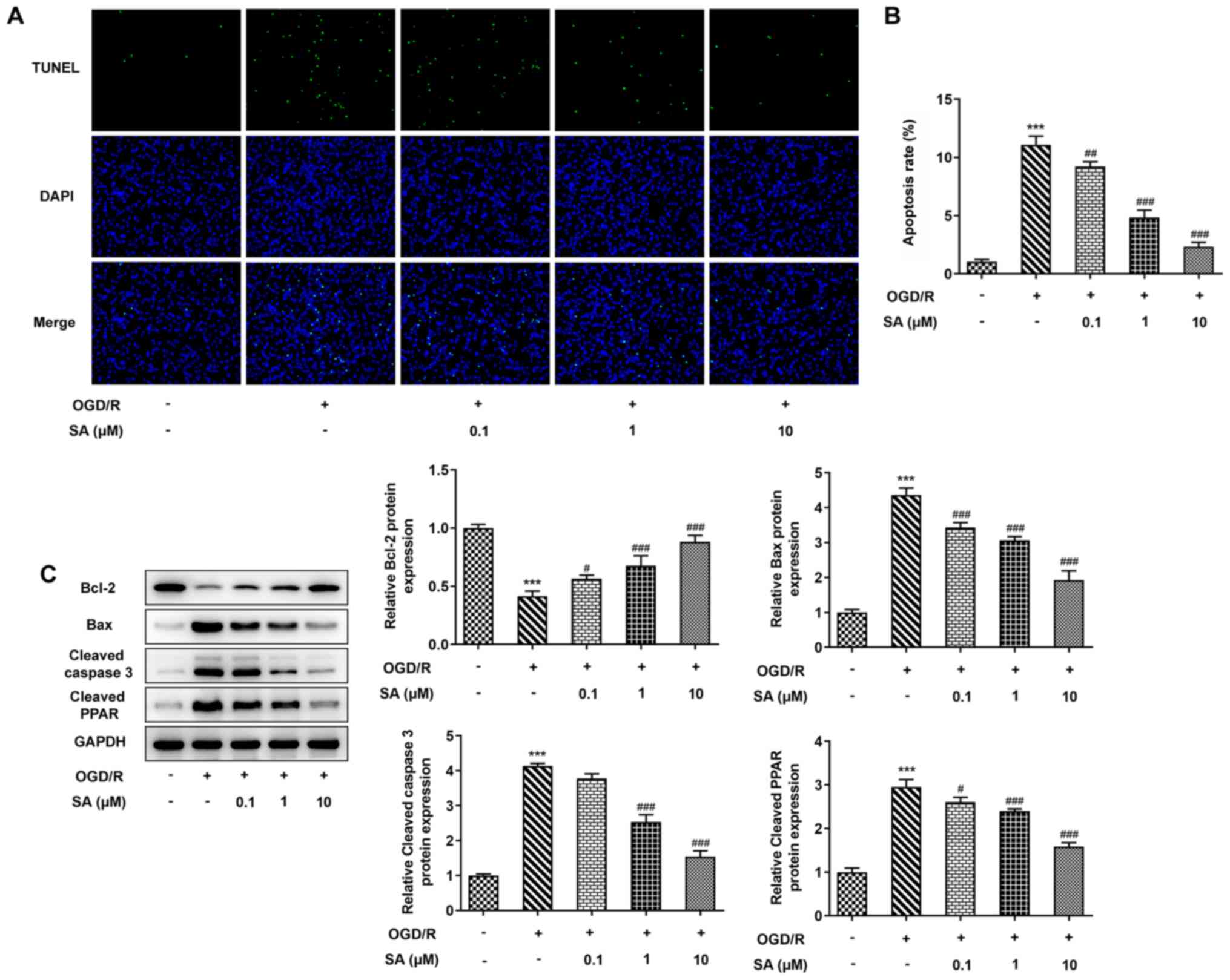
Effects of SA pretreatment on
apoptosis in OGD/R-treated Caco-2 cells
To further analyze the role of SA in intestinal I/R
injury in vitro, apoptosis was evaluated. As presented in
Fig. 4A and B, the results of the TUNEL assay revealed
a significant increase in the apoptosis rate of Caco-2 cells after
OGD/R induction, which was significantly decreased following SA
treatment. Similarly, western blotting revealed that, compared with
the control cells, OGD/R injury significantly inhibited Bcl-2
protein expression, but increased the levels of Bax, cleaved
caspase 3 and cleaved PPAR. However, SA reversed the OGD/R-induced
alteration in the levels of these proteins in a dose-dependent
manner (Fig. 4C). The results
suggested that SA inhibited cell apoptosis in OGD/R-stimulated
Caco-2 cells.
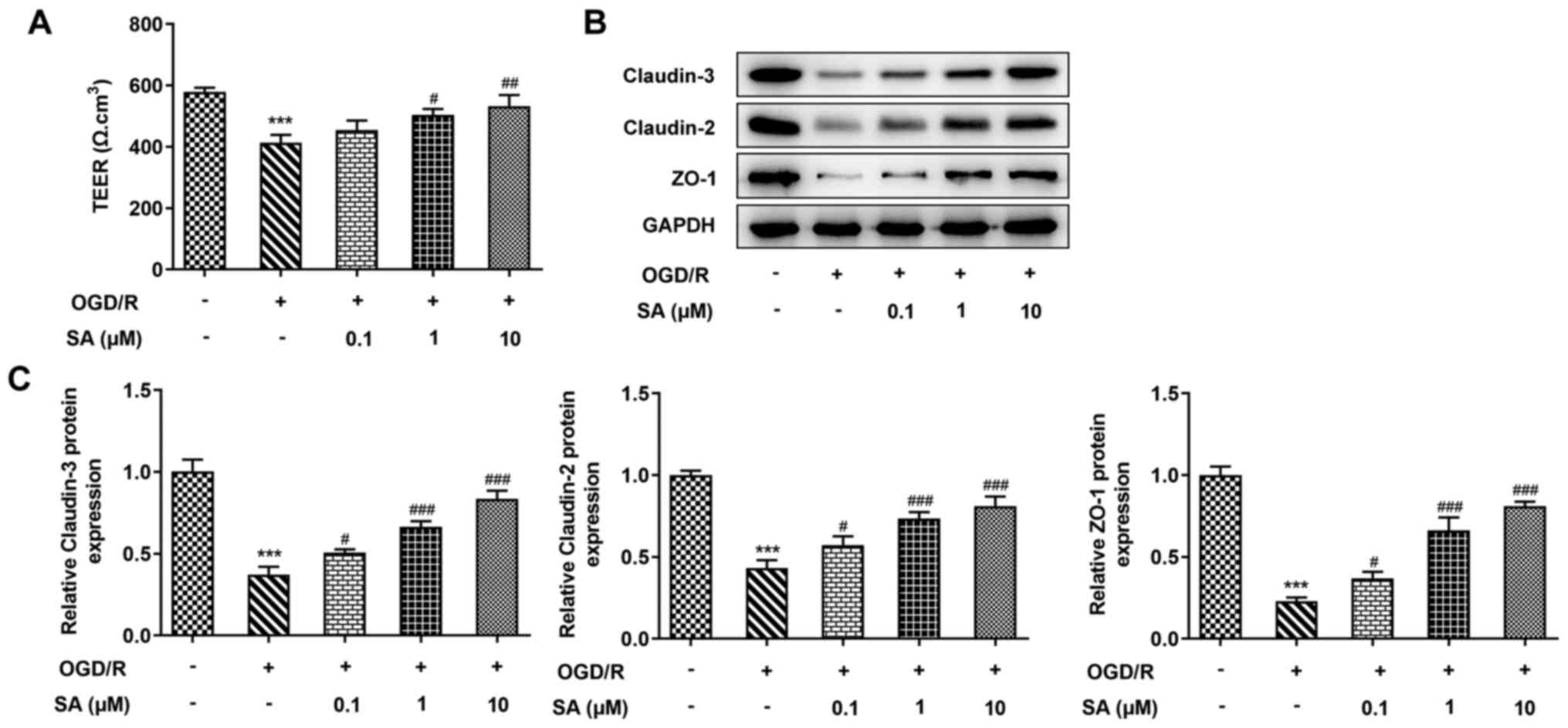
Effects of SA pretreatment on the
intestinal barrier function of Caco-2 cells in response to
OGD/R
The current study assessed whether SA administration
ameliorated OGD/R-induced intestinal barrier disruption. A TEER
assay was performed to examine intestinal epithelial integrity. The
results revealed that the value of TEER was significantly reduced
by OGD/R injury, whereas SA pretreatment significantly increased
TEER in Caco-2 cells (Fig. 5A).
Consistent with these results, OGD/R injury significantly decreased
the protein expression levels of Claudin-3, Claudin-2 and ZO-1, but
these were increased after treatment with SA under OGD/R conditions
in a dose-dependent manner (Fig.
5B and C). The findings
indicated that SA protected Caco-2 cells from OGD/R-induce
intestinal barrier function injury.
Discussion
SA is a type of monomer extracted from traditional
Chinese medicines, including Dendrobium nobile Lindl.
Previous studies have revealed that SA plays a role in disorders
associated with I/R (16,19,20).
However, to the best of our knowledge, the biological role of SA in
intestinal I/R has not been reported thus far. The present study
demonstrated that pretreatment with SA increased cell viability and
abated cytotoxic injury in Caco-2 cells following OGD/R.
Additionally, SA inhibited the OGD/R-induced release of
inflammatory cytokines and oxidative stress, and decreased the rate
of apoptosis in Caco-2 cells. SA also improved epithelial barrier
function and protected intestinal epithelial integrity damaged by
OGD/R.
It is widely accepted that I/R contributes to the
inflammatory response that occurs during the occurrence and
development of intestinal I/R (21). Inflammation can aggravate
intestinal I/R injury (22,23).
The present study established an in vitro intestinal I/R
model using OGD/R injury. The data revealed that OGD/R
significantly promoted the production of inflammatory cytokines,
including TNF-α, IL-6, IL-1β and MCP-1 in Caco-2 cells, as well as
increasing the expression levels of p-p65, iNOS and COX2. SA
pretreatment inhibited the OGD/R-induced increase in these
inflammatory cytokines in a dose-dependent manner. In addition,
evidence indicates that inflammation is associated with oxidative
stress in intestinal I/R injury (24,25).
The antioxidant enzyme system is required to mitigate injuries
induced by I/R (26). The present
study revealed that OGD/R injury resulted in higher levels of
oxidative stress by increasing the production of ROS and MDA, and
by reducing SOD activity. Therefore, SA may protect intestinal
tissue from I/R injury via its anti-inflammatory and antioxidant
activities.
Reperfusion after ischemia in intestinal tissues
leads to the activation of caspases and an imbalance of
pro-/anti-apoptotic proteins (27). Apoptosis, as an active gene
directed cell death process, is easily induced by external stimuli
and is relevant to intestinal reperfusion injury (28). Bcl 2 is combined with Bax to form a
heterodimer that prevents Bax homodimerization and the activation
of caspase 3 (29,30). Caspase 3 and PPAR serve an
important role in the apoptosis of intestinal epithelial cells
(31,32). In the present study OGD/R
specifically downregulated the expression of Bcl 2 and upregulated
expression of Bax, cleaved caspase 3 and cleaved PPAR in Caco-2
cells. Nevertheless, pretreatment of SA reversed the effects of
OGD/R on the expression levels of Bcl 2, Bax, cleaved caspase 3 and
cleaved PPAR.
It has been reported that intestinal I/R injury
causes dysfunction of the intestinal mucosal barrier, which is
regarded as a complication that may lead to life-threatening
bacterial translocation from the gut, multi-system organ failure
and mortality (10,33). Thus, protecting the intestinal
barrier from injury or enhancing the ability of the organism to
repair the intestinal barrier is an important step for blocking
pathophysiological processes from intestinal I/R (34). Tight junction proteins, such as
Claudin-2, Claudin-3 and ZO-1, are associated with intestinal
barrier function and intestinal permeability (35). In the present study,
transepithelial electrical resistance and western blotting were
detected to measure intestinal barrier function in vitro.
The results revealed that OGD/R injury reduced the value of
transepithelial electrical resistance and decreased the expression
levels of Claudin-3, Claudin-2 and ZO-1 in Caco-2 cells. Treatment
with SA for 24 h increased the transepithelial electrical
resistance and enhanced the levels of these tight junction
proteins, indicating the regulatory role of SA in epithelial
barrier function. However, in the present study, the effects of SA
on intestinal I/R were explored in vitro. The role of SA in
animal experiments and clinical trials need to be performed in
further study.
In summary, the present study demonstrated that SA
increased cell viability and abrogated OGD/R cell injury in Caco-2
cells. Furthermore, the current study demonstrated that SA exerted
a protective role in OGD/R-treated Caco-2 cells by inhibiting
inflammation, oxidative stress, apoptosis and mucosal barrier
injury. These results may provide a novel therapeutic drug for the
treatment of disorders derived from intestinal I/R injury.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SX and JX designed the study, performed the
experiments, drafted and revised the manuscript. SX analyzed the
data and examined the literature. SX and JX confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lehtimäki TT, Kärkkäinen JM, Saari P,
Manninen H, Paajanen H and Vanninen R: Detecting acute mesenteric
ischemia in CT of the acute abdomen is dependent on clinical
suspicion: Review of 95 consecutive patients. Eur J Radiol.
84:2444–2453. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vollmar B and Menger MD: Intestinal
ischemia/reperfusion: Microcirculatory pathology and functional
consequences. Langenbecks Arch Surg. 396:13–29. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Berlanga J, Prats P, Remirez D, Gonzalez
R, Lopez-Saura P, Aguiar J, Ojeda M, Boyle JJ, Fitzgerald AJ and
Playford RJ: Prophylactic use of epidermal growth factor reduces
ischemia/reperfusion intestinal damage. Am J Pathol. 161:373–379.
2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mallick IH, Yang W, Winslet MC and
Seifalian AM: Ischemia-reperfusion injury of the intestine and
protective strategies against injury. Dig Dis Sci. 49:1359–1377.
2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Koike K, Moore EE, Moore FA, Read RA, Carl
VS and Banerjee A: Gut ischemia/reperfusion produces lung injury
independent of endotoxin. Crit Care Med. 22:1438–1444.
1994.PubMed/NCBI View Article : Google Scholar
|
|
6
|
de Groot H and Rauen U:
Ischemia-reperfusion injury: processes in pathogenetic networks: a
review. Transplant Proc. 39:481–484. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang Z, Sun R, Wang G, Chen Z, Li Y, Zhao
Y, Liu D, Zhao H, Zhang F, Yao J, et al: SIRT3-mediated
deacetylation of PRDX3 alleviates mitochondrial oxidative damage
and apoptosis induced by intestinal ischemia/reperfusion injury.
Redox Biol. 28(101343)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Di Paola R, Impellizzeri D, Torre A,
Mazzon E, Cappellani A, Faggio C, Esposito E, Trischitta F and
Cuzzocrea S: Effects of palmitoylethanolamide on intestinal injury
and inflammation caused by ischemia-reperfusion in mice. J Leukoc
Biol. 91:911–920. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang AL, Niu Q, Shi N, Wang J, Jia XF,
Lian HF, Liu Z and Liu CX: Glutamine ameliorates intestinal
ischemia-reperfusion Injury in rats by activating the Nrf2/Are
signaling pathway. Int J Clin Exp Pathol. 8:7896–7904.
2015.PubMed/NCBI
|
|
10
|
Kannan KB, Colorado I, Reino D, Palange D,
Lu Q, Qin X, Abungu B, Watkins A, Caputo FJ, Xu DZ, et al:
Hypoxia-inducible factor plays a gut-injurious role in intestinal
ischemia reperfusion injury. Am J Physiol Gastrointest Liver
Physiol. 300:G853–G861. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ates B, Yilmaz I, Geckil H, Iraz M,
Birincioglu M and Fiskin K: Protective role of melatonin given
either before ischemia or prior to reperfusion on intestinal
ischemia-reperfusion damage. J Pineal Res. 37:149–152.
2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Campos FM, Couto JA, Figueiredo AR, Tóth
IV, Rangel AO and Hogg TA: Cell membrane damage induced by phenolic
acids on wine lactic acid bacteria. Int J Food Microbiol.
135:144–151. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Srinivasulu C, Ramgopal M, Ramanjaneyulu
G, Anuradha CM and Suresh Kumar C: Syringic acid (SA) - A review of
its occurrence, biosynthesis, pharmacological and industrial
importance. Biomed Pharmacother. 108:547–557. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cao Y, Zhang L, Sun S, Yi Z, Jiang X and
Jia D: Neuroprotective effects of syringic acid against
OGD/R-induced injury in cultured hippocampal neuronal cells. Int J
Mol Med. 38:567–573. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ding SK, Wang LX, Guo LS, Luo P, Du JJ,
Zhao ZL and Wang GG: Syringic acid inhibits apoptosis pathways via
downregulation of p38MAPK and JNK signaling pathways in H9c2
cardiomyocytes following hypoxia/reoxygenation injury. Mol Med Rep.
16:2290–2294. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sancak EB, Akbas A, Silan C, Cakir DU,
Turkon H and Ozkanli SS: Protective effect of syringic acid on
kidney ischemia-reperfusion injury. Ren Fail. 38:629–635.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fang W, Zhu S, Niu Z and Yin Y: The
protective effect of syringic acid on dextran sulfate
sodium-induced experimental colitis in BALB/c mice. Drug Dev Res.
80:731–740. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu L, Yao J, Li Z, Zu G, Feng D, Li Y,
Qasim W, Zhang S, Li T, Zeng H, et al: miR-381-3p knockdown
improves intestinal epithelial proliferation and barrier function
after intestinal ischemia/reperfusion injury by targeting nurr1.
Cell Death Dis. 9(411)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tokmak M, Yuksel Y, Sehitoglu MH, Guven M,
Akman T, Aras AB, Cosar M and Abbed KM: The neuroprotective effect
of syringic acid on spinal cord ischemia/reperfusion injury in
rats. Inflammation. 38:1969–1978. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tokmak M, Sehitoglu MH, Yuksel Y, Guven M,
Akman T, Aras AB, Yaka U, Gomleksiz C, Albayrak SB and Cosar M: The
axon protective effects of syringic acid on ischemia/reperfusion
injury in a rat sciatic nerve model. Turk Neurosurg. 27:124–132.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang Z, Li Z, Feng D, Zu G, Li Y, Zhao Y,
Wang G, Ning S, Zhu J, Zhang F, et al: Autophagy induction
ameliorates inflammatory responses in intestinal
ischemia-reperfusion through inhibiting NLRP3 inflammasome
activation. Shock. 52:387–395. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vieira AT, Pinho V, Lepsch LB, Scavone C,
Ribeiro IM, Tomassini T, Ribeiro-dos-Santos R, Soares MB, Teixeira
MM and Souza DG: Mechanisms of the anti-inflammatory effects of the
natural secosteroids physalins in a model of intestinal ischaemia
and reperfusion injury. Br J Pharmacol. 146:244–251.
2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Souza DG, Vieira AT, Pinho V, Sousa LP,
Andrade AA, Bonjardim CA, McMillan M, Kahn M and Teixeira MM:
NF-kappaB plays a major role during the systemic and local acute
inflammatory response following intestinal reperfusion injury. Br J
Pharmacol. 145:246–254. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Stefanutti G, Pierro A, Vinardi S, Spitz L
and Eaton S: Moderate hypothermia protects against systemic
oxidative stress in a rat model of intestinal ischemia and
reperfusion injury. Shock. 24:159–164. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Turan I, Ozacmak HS, Ozacmak VH, Barut F
and Araslı M: Agmatine attenuates intestinal ischemia and
reperfusion injury by reducing oxidative stress and inflammatory
reaction in rats. Life Sci. 189:23–28. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sasaki M and Joh T: Oxidative stress and
ischemia-reperfusion injury in gastrointestinal tract and
antioxidant, protective agents. J Clin Biochem Nutr. 40:1–12.
2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang HC, Zhang HF, Guo WY, Su H, Zhang KR,
Li QX, Yan W, Ma XL, Lopez BL, Christopher TA, et al: Hypoxic
postconditioning enhances the survival and inhibits apoptosis of
cardiomyocytes following reoxygenation: Role of peroxynitrite
formation. Apoptosis. 11:1453–1460. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fliss H and Gattinger D: Apoptosis in
ischemic and reperfused rat myocardium. Circ Res. 79:949–956.
1996.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Burlacu A: Regulation of apoptosis by
Bcl-2 family proteins. J Cell Mol Med. 7:249–257. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104.
1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schwab M, Reynders V, Ulrich S, Zahn N,
Stein J and Schröder O: PPARgamma is a key target of
butyrate-induced caspase-3 activation in the colorectal cancer cell
line Caco-2. Apoptosis. 11:1801–1811. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pellerito O, Notaro A, Sabella S, De
Blasio A, Vento R, Calvaruso G and Giuliano M: WIN induces
apoptotic cell death in human colon cancer cells through a block of
autophagic flux dependent on PPARγ down-regulation. Apoptosis.
19:1029–1042. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tian R, Wang RL, Xie H, Jin W and Yu KL:
Overexpressed miRNA-155 dysregulates intestinal epithelial apical
junctional complex in severe acute pancreatitis. World J
Gastroenterol. 19:8282–8291. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wattanasirichaigoon S, Menconi MJ, Delude
RL and Fink MP: Lisofylline ameliorates intestinal mucosal barrier
dysfunction caused by ischemia and ischemia/reperfusion. Shock.
11:269–275. 1999.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Slifer ZM and Blikslager AT: The integral
role of tight junction proteins in the repair of injured intestinal
epithelium. Int J Mol Sci. 21(E972)2020.PubMed/NCBI View Article : Google Scholar
|