1. Background
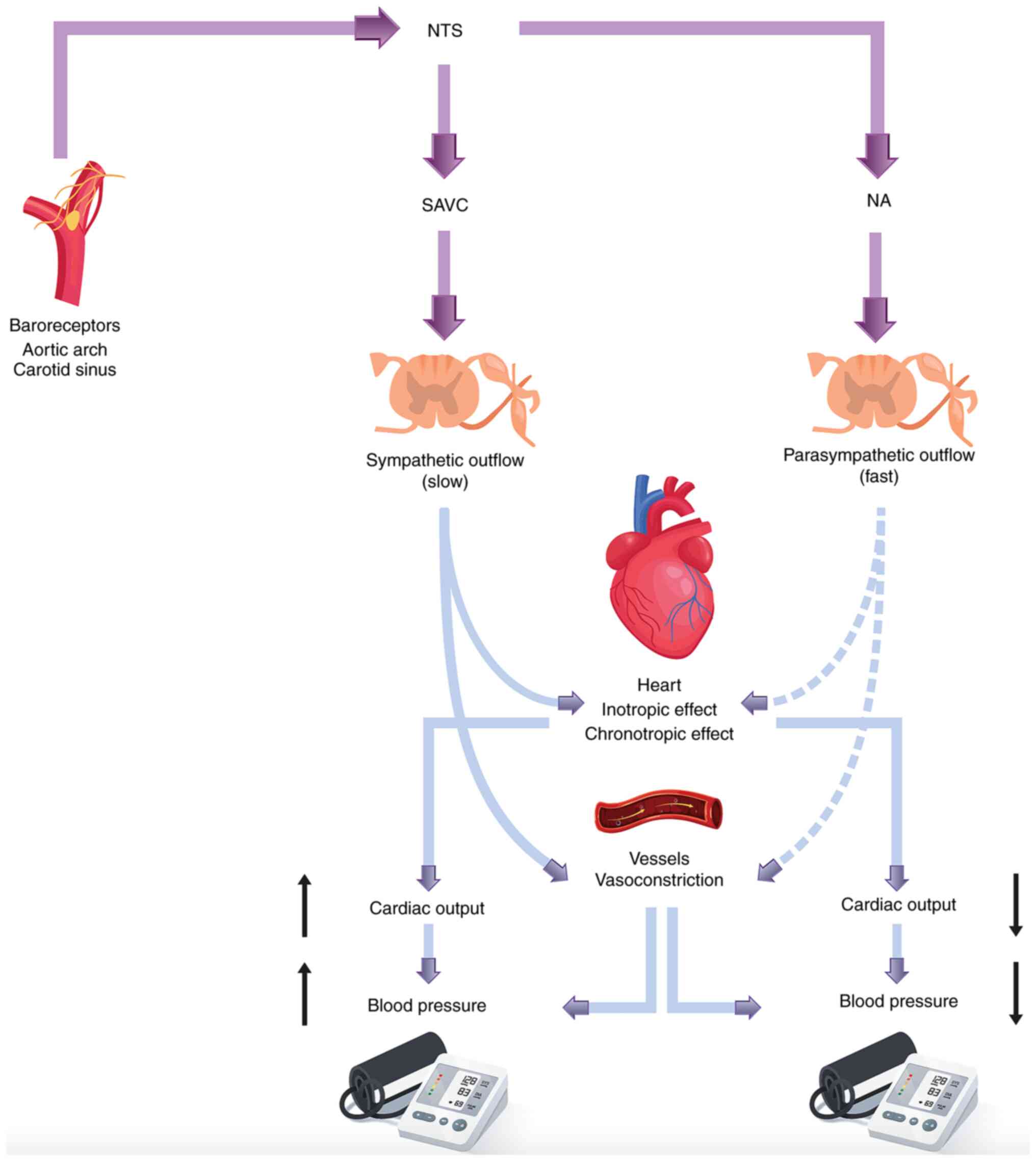
The baroreflex represents a rapid negative feedback
system, which serves an important role in blood pressure
regulation, preventing blood pressure variations via its actions on
peripheral vascular tone and cardiac output (1,2). The
sensor receptors, referred to as baroreceptors, are primarily
located at the nerve endings of the carotid sinus and the aortic
arch vessel wall (3). Dilatation
of these sensors leads to increased vagal activity with a
concomitant decrease in sympathetic nerve activity (SNA), whereas
the opposite effect occurs when blood pressure decreases, achieving
an overall blood pressure adjustment (4). Cardiac function is under the
influence of both the sympathetic and parasympathetic systems,
which modulate its electrophysiological properties, whereas the
conduction system, ventricular myocytes, and the atrium and sinus
nodes are also affected by the action of the autonomous system.
The effects of the baroreflex system on cardiac
function are of paramount importance, particularly in stressful
conditions. When blood pressure increases, the parasympathetic tone
is enhanced and the sympathetic tone is suppressed, protecting the
heart against arrhythmias (5); the
opposite occurs when blood pressure decreases (Fig. 1). Therefore, the baroreflex system
acts as a buffer system by adjusting blood pressure (4). The entire regulatory mechanism is
known as baroreflex sensitivity (BRS).
In case of baroreflex failure, the volume load
tolerance is impaired and may lead to several serious clinical
manifestations, including pulmonary edema (1,6).
Heart failure with reduced left ventricular ejection fraction and
left ventricular diastolic dysfunction are also induced by
baroreflex failure (6). In the
presence of baroreflex dysfunction, risk factors such as diabetes
mellitus (DM), renal insufficiency, aging, atherosclerosis and
hypertension become even more important for the development of
heart failure (7). Notably,
prenatal hypoxia leading to baroreflex failure may result in adult
hypertension (8).
In mathematical terms, BRS can be defined as the
ratio of inter-beat interval change (in msec) over the unit change
of blood pressure (5). Namely,
when inter-beat interval increases by 100 msec and blood pressure
rises by 10 mmHg, the BRS is equal to 100/10, which is referred to
as 10 msec/mmHg. With regard to the aforementioned example, the
increase in inter-beat interval can result from either an increase
in parasympathetic tone, or from a decrease in sympathetic tone. It
has been demonstrated that the joint influence of the sympathetic
and vagal systems on the sinus node contributes to the actual heart
rate. Besides, blood pressure fluctuations are buffered by the
baroreflex system, mainly by adjusting peripheral resistance
(9). In case of changes in blood
pressure, a short time lag is required for baroreflex control
system actions to take effect towards resistance adjustments
(5). Like most negative feedback
systems, the baroreflex exerts a periodic performance with a period
close to 10 sec.
The measurement of BRS is considered a valuable tool
for the evaluation of numerous cardiovascular diseases (10). BRS measurements focus on the
ability of the autonomous system to react to blood pressure changes
at the aortic arch and carotid sinus (11). Cardiovascular tests and analysis of
heart rate variability (HRV) can be used for the diagnosis of
autonomic neuropathy, in which the nerves that control involuntary
bodily functions are damaged (12). In addition, the non-invasive
volume-clamp method allows for quantification of the neural
modulation of the sinus node mediated by arterial baroreceptors
(13).
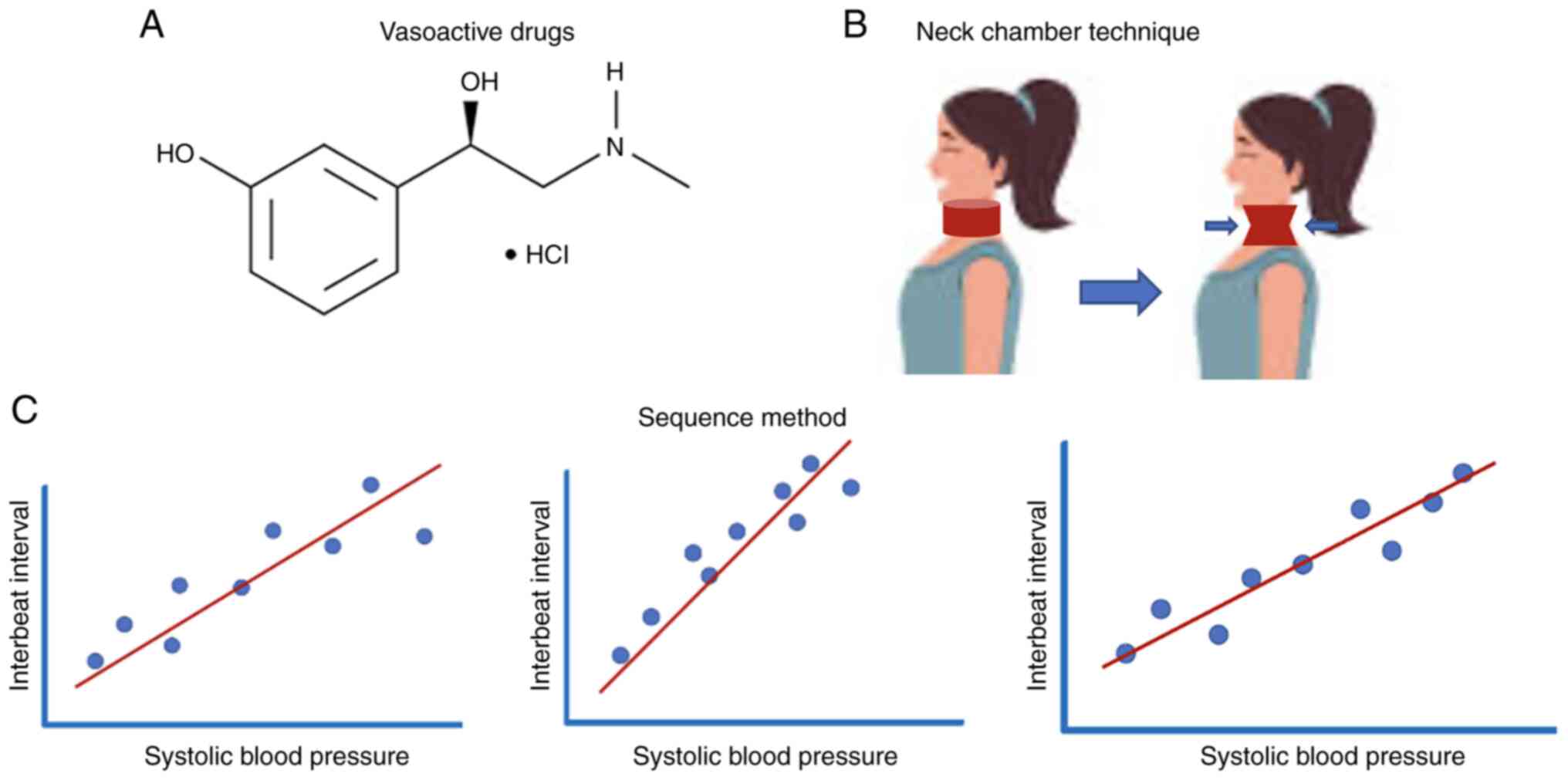
Several methods have been proposed for measurement
of BRS (Fig. 2) (14), for example using vasoactive drugs
(such as phenylephrine) to increase blood pressure, which
suppresses heart rate and subsequently increases the inter-beat
interval (5). The ‘neck chamber
technique’ is another method where a positive/negative pressure is
applied to the neck, leading to deactivation of carotid
baroreceptors (14). During the
Valsalva maneuver, a breathing method used in the diagnosis of
autonomic nervous system dysfunction, increased abdominal and
intrathoracic pressure triggers activation of baroreceptors. The
‘sequence method’ is another approach for the quantification of
BRS, which is based on the application of linear regression
analysis between decreasing/increasing blood pressure and changes
in the R-R interval, namely, the time between two successive
R-waves of the QRS signal of the electrocardiogram (15). Other techniques rely on carotid
ultrasound imaging, such as measurement of the change in the
diameter of the carotid artery after alteration in arterial
pressure (15). Alternatively, the
change in R-R interval (cardiac baroreflex) or muscle SNA
(sympathetic baroreflex) can be measured as a result of barosensory
vessel stretch. Also, several algorithms in time and frequency
domain (when Fast Fourier transform is applied to convert the
signal from time to frequency domain) have been proposed for the
non-invasive measurement of BRS (14), which quantify BRS based on the
association between periodic sequences of the two signals,
specifically inter-beat interval and blood pressure.
BRS is an important homeostatic system the function
of which can be influenced by several risk factors (16). Obesity can markedly decrease BRS,
and can lead to sympathovagal imbalance by decreasing
parasympathetic activity and increasing SNA (17). Notably, high abdominal visceral fat
has been shown to reduce BRS compared with in patients with lower
total and abdominal fat (18).
Similarly, randomized clinical trials have shown that accompanying
features of obesity, such as hyperinsulinemia, insulin resistance
and hypoadiponectinemia, decrease BRS (17). In addition, the adoption of a
hypocaloric diet has been shown to lead to improvement of BRS in
patients with obesity (19).
Furthermore, hypoadiponectinemia in patients with type II DM (T2DM)
has been related to reduced BRS (20). Other factors significantly linked
to reduced BRS include atherosclerosis (21) and aging (22). In addition, dyslipidemia has been
reported to constitute a risk factor that is negatively correlated
with the functionality of the baroreflex system (22).
Specific treatments for improving BRS and subsequent
variations in blood pressure are not currently available (1). A clinical study showed that
angiotensin-converting enzyme inhibitors may provide an improvement
in patients with hypertension (23). Furthermore, a previous animal study
demonstrated that β-blockers can attenuate short-term variability
in blood pressure, and treatment with L-arginine was able to
restore depressed BRS (24).
Another study tested non-pharmacological treatment methods, such as
vagal afferent nerve activation, which was reported to improve BRS
functionality and reverse blood pressure variability (25).
The aim of the present review was to highlight the
relationship between BRS and obesity, including factors closely
associated with obesity, such as the metabolic syndrome,
hypertension, cardiovascular disease and DM. A specific emphasis
has been placed on the role of aging and sex differences, the
impact of exercise and training, as well as changes in BRS in
patients with obesity following bariatric surgery.
2. Methods
Search strategy. A literature search was conducted
using the PubMed database (pubmed.ncbi.nlm.nih.gov) from January 14, 2021 until
August 21, 2021. The searched terms were: ‘baroreflex’ +
‘sensitivity’, ‘baroreflex’ + ‘obesity’, ‘baroreflex: + ‘metabolic
syndrome’, ‘baroreflex’ + ‘hypertension’, ‘baroreflex’ +
‘diabetes’, ‘baroreflex’ + ‘gender’, ‘baroreflex’ + ‘aging’,
‘baroreflex’ + ‘children’ + ‘adolescents’, ‘baroreflex’ + ‘physical
activity’, ‘baroreflex’ + ‘bariatric surgery’, ‘autonomous nervous
system’ + ‘regulation’ and ‘baroreflex sensitivity’ +
‘cardiometabolic risk factors’. The search strategy results are
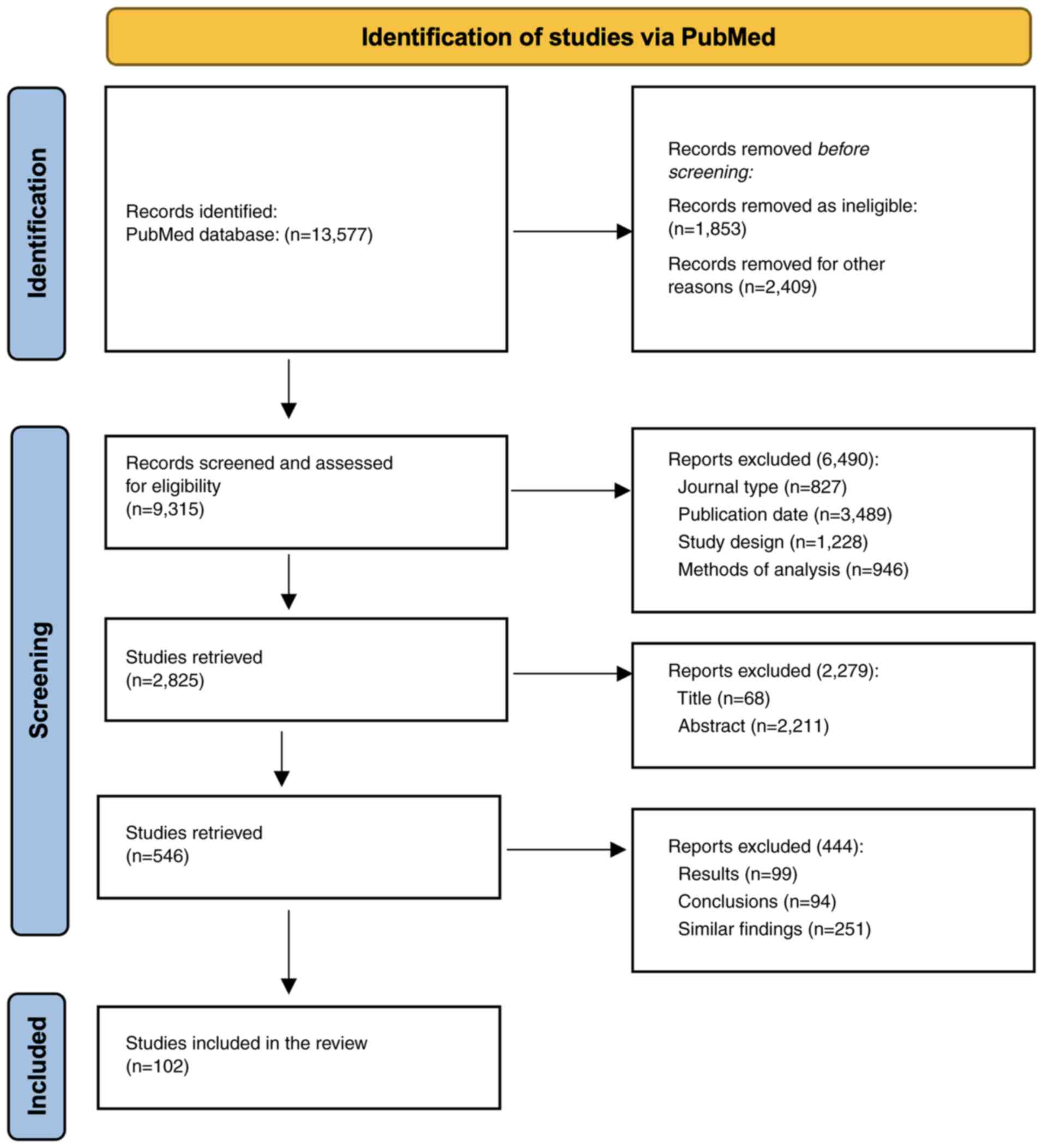
presented in Table I and a
schematic representation of the route of selection is shown in
Fig. 3.
 | Table ILiterature search strategy results
from PubMed. |
Table I
Literature search strategy results
from PubMed.
| Search terms | Citations retrieved
(August 21, 2021) | Studies retrieved
after screening of title and abstract |
|---|
| ‘baroreflex’ +
‘sensitivity’ | 4,571 | 98 |
| ‘baroreflex’ +
‘obesity’ | 257 | 35 |
| ‘baroreflex +
‘metabolic syndrome’ | 87 | 18 |
| ‘baroreflex’ +
‘hypertension’ | 3,149 | 112 |
| ‘baroreflex’ +
‘diabetes’ | 502 | 87 |
| ‘baroreflex’ +
‘gender’ | 494 | 46 |
| ‘baroreflex’ +
‘aging’ | 498 | 37 |
| ‘baroreflex’ +
‘children’ + ‘adolescents’ | 117 | 22 |
| ‘baroreflex’ +
‘physical activity’ | 1,484 | 23 |
| ‘baroreflex’ +
‘bariatric surgery’ | 8 | 6 |
| ‘autonomous nervous
system’ + ‘regulation’ | 2,401 | 64 |
| ‘baroreflex
sensitivity’ + ‘cardiometabolic risk factors’ | 9 | 5 |
Selection criteria
After removing duplicate articles, the authors
evaluated all studies based on the following criteria: i) Journal,
ii) authorship, iii) publication date, iv) study design, v) methods
of analysis, vi) results, and vii) conclusions. The eligibility
criteria were as follows: Articles written in English, which were
relevant to the objective of the review, and the absence of
confounding diseases. One of the authors reviewed the abstracts of
each identified study and excluded them if they did not meet the
eligibility criteria. In order to improve data quality, all studies
that met the inclusion criteria were thoroughly evaluated in terms
of rationale, method design, primary outcome, fatigue assessment,
statistical analysis, results, discussion and conclusions. Those
studies that displayed any bias in the methodology, results or
interpretation of the data, which could be reflected in the overall
analysis of the study, were also excluded (Fig. 3). Finally, 102 articles were
selected for inclusion.
3. Obesity and metabolic syndrome
Obesity represents a chronic relapsing disease
comprising important concurrent metabolic and clinical disorders,
such as DM, dyslipidemia, cardiovascular disease, sleep apnea and
autonomic neuropathy, when the nerves controlling involuntary body
functions are destroyed. It can impact blood pressure, temperature
regulation, digestion and bladder and sexual function (26). Overall, it is associated with
potentiation of the sympathetic nervous system (27). Previously, clinical studies have
identified an association between autonomic nervous system
regulation and obesity (28,29).
Notably, it has been shown that as body weight increases,
baroreflex function is significantly suppressed (28). Furthermore, the guidelines of the
European Society of Hypertension and the European Society of
Cardiology have underlined the role of waist circumference (WC) in
BRS (30,31). A direct association between BRS and
fat mass distribution has been demonstrated in hypertensive
populations, where central obesity led to impaired BRS. Thus, in
patients with central obesity, the enhanced sympathetic
cardiovascular effort may be added to the metabolic risk factors,
such as dyslipidemia, elevated blood pressure and elevated plasma
glucose, which can lead to an increased risk for cardiovascular
complications.
Obesity may contribute to the process of carotid
atherosclerosis via multiple pathogenetic mechanisms, including
carotid media thickening and hyperleptinemia (3). A decrease in BRS has been reported to
be associated with carotid intima media thickness; therefore, it
was hypothesized that obesity-related atherosclerosis may lead to
changes in baroreceptor signaling, which in turn could increase
sympathetic activity and decrease BRS (3). In addition, hormonal factors that are
implicated in the complex biological pathways of long-term
maintenance of body weight and energy balance seem to play a role
in the association between obesity and BRS (32). Leptin is a fundamental hormone that
is secreted primarily by adipose cells and acts as a marker of
total body energy reflecting adipose tissue mass (3). In the obese state, neurons located at
hypothalamic nuclei that express leptin receptors are desensitized
as a result of chronically elevated leptin levels; thus, the
anorexigenic effect of leptin is suppressed (33). Leptin can also exert its action at
the level of the nucleus of the solitary tract, affecting neurons
important to BRS (34). Leptin has
previously been reported to act as a critical signal in activation
of the renal sympathetic nerve by activating the brain
renin-angiotensin system, hypothalamic phosphatidylinositol
3-kinase and melanocortin receptors (3,35).
Leptin receptors also serve an important role in leptin-induced
renal sympathetic nerve activation; the knockout of leptin
receptors in the hypothalamus has been shown to hinder the
activation of sympathetic nerves in patients with obesity and
hypertension (3).
It is known that central obesity and visceral fat
can lead to increased activation of the sympathetic nervous system
compared with peripheral obesity (18,36).
This finding suggests that central obesity is characterized by
autonomic imbalance with hyperactivity of the sympathetic nervous
system. In this context, a previous study aimed to analyze the
relationship between abdominal fat distribution (measured by WC)
and vagal tone (as expressed by spontaneous BRS) (30). The results demonstrated that the
impairment in BRS was greater in patients with central obesity
compared with in those with peripheral adipose distribution. Thus,
the higher risk for cardiovascular complications observed in
patients with central obesity may not only be ascribed to metabolic
factors, but also to the activated sympathetic nervous system. Fat
distribution may also serve an important role in the autonomic
balance and the development of hypertension (30).
The relationship between obesity, BRS and
cardiovascular diseases was further investigated by including the
role of immune system in the exploration (37). It has been hypothesized that there
is a ‘triangle’ between autonomic regulation (including BRS),
cardiovascular diseases (e.g., hypertension, heart failure) and the
immune system. The impact of the autonomic system on cardiovascular
pathology has also been thoroughly studied; sympathetic activation
and parasympathetic suppression has been revealed to worsen heart
failure or hypertension, and subsequently increase morbidity and
mortality (37).
The end-organ damage in hypertension or heart
failure can be worsened or alleviated by pro- or anti-inflammatory
pathways of the immune system, respectively, which are triggered by
neurohumoral transmitters (37).
The strong association between inflammation and obesity has
resulted in studies that aim to unveil the link between
obesity-induced inflammation and other diseases (26,38).
Inflammation is induced by obesity as a result of a chronically
mediated immune response, which is accompanied by the secretion of
cytokines, acute phase proteins (e.g., C-reactive protein) and
chemokines (38). In turn, acute
or chronic inflammation increases the activity of the sympathetic
system, leading to an increased incidence of cardiovascular events
(39). Notably, a previous study
was conducted examining the potential beneficial effects of
administering non-steroidal anti-inflammatory drugs (e.g.,
ibuprofen) to individuals with obesity in order to adjust their BRS
(10); however, an acute dose of
ibuprofen did not exert an effect on BRS.
The importance of studying the role of obesity in
the baroreflex system is reflected in the large number of clinical
trials either ongoing or recently completed (Table II). Since central obesity is one
of the primary risk factors for metabolic syndrome, research has
focused on the association between obesity and aspects of metabolic
syndrome (40-46).
 | Table IIClinical studies investigating the
association between obesity and autonomic disorders, including
baroreflex sensitivity. Information is from ClinicalTrials.gov, US National Library of Medicine
(https://clinicaltrials.gov). |
Table II
Clinical studies investigating the
association between obesity and autonomic disorders, including
baroreflex sensitivity. Information is from ClinicalTrials.gov, US National Library of Medicine
(https://clinicaltrials.gov).
| Title | Clinical
design | Sample size | Conditions | Institute | Clinical trials
identifier |
|---|
| Inflammation
Inhibition in Prediabetic Humans | Randomized,
parallel | 21 | Prediabetes,
obesity | University of Iowa,
Iowa City, Iowa, United States | NCT01977417 |
| Comparison of
Gastric Bypass and Sleeve Gastrectomy in Metabolic and
Cardiovascular Indices | Non-randomized,
parallel | 28 | Obesity | National and
Kapodistrian University of Athens, Athens, Greece; Beth Israel
Deaconess Medical Center, Boston, Massachusetts, USA | NCT03851874 |
| Study of the
Cardiometabolic Effects of Obesity Pharmacotherapy | Non-randomized,
parallel | 40 | Obesity, blood
pressure | National and
Kapodistrian University of Athens, Athens, Greece; Athens Medical
Center, Athens, Greece | NCT04575194 |
| Chronotropic
Incompetence During Exercise in Obese Adolescents: Clinical
Implications and Pathophysiology | Non-randomized,
parallel | 120 | Obesity, adolescent
obesity | Hasselt University,
Hasselt, Belgium | NCT03516721 |
| Effects of
Pioglitazone Treatment on Sympathetic Nervous System Function in
Metabolic Syndrome Obesity | Randomized,
parallel | 44 | Metabolic
syndrome | Baker Heart
Research Institute, Melbourne, Victoria, Australia | NCT00408850 |
| Diet and Whole-body
Vibration Training on Cardiovascular and Autonomic Function | Randomized,
parallel | 60 | Obesity,
pre-hypertension, hypertension | Florida State
University, Tallahassee, Florida, United States | NCT01741779 |
| Low-intensity
Resistance Exercise and Diet on Arterial Function and Blood
Pressure | Randomized,
parallel | 41 | Obesity,
pre-hypertension, hypertension | Florida State
University, Tallahassee, Florida, United States | NCT01371370 |
| Whole Body
Vibration Combined With L-citrulline Supplementation on
Cardiovascular Function and Body Composition | Randomized,
parallel | 60 | Obesity,
pre-hypertension, hypertension | FSU College of
Human Sciences, Tallahassee, Florida, United States | NCT02143817 |
| Motivation Makes
the Move | Randomized,
parallel | 120 | Obesity,
cardiovascular diseases, physical conditioning | Helsinki University
Hospital, Helsinki, Finland; Vantaa Health Care and Social
Services, Vantaa, Finland | NCT02686502 |
| The Effects of
Stretching Training on Arterial Function and Autonomic Control | Randomized,
parallel | 30 | Obesity,
pre-hypertension, hypertension | Florida State
University, Tallahassee, Florida, United States | NCT01741766 |
| Sympathetic
Activity in Individuals with the Metabolic Syndrome: Benefits of
Lifestyle Interventions | Randomized,
parallel | 66 | Metabolic
syndrome | Baker Heart
Research Institute, Melbourne, Victoria, Australia | NCT00163943 |
Metabolic syndrome is a term strongly associated
with obesity. It is used to describe a cluster of related metabolic
abnormalities, namely obesity, dyslipidemia, hypertension and
glucose intolerance, which lead to an increased risk of
cardiovascular morbidity and mortality (47). Several definitions of metabolic
syndrome have been provided; however, the term is typically used to
characterize the condition when at least three of the following
situations are present: Increased WC, low high-density lipoprotein
levels, increased triglycerides in the blood, elevated fasting
glucose levels and hypertension (47). A common pathogenetic characteristic
in all these conditions is activation of the sympathetic nervous
system (48). Several
pathophysiological mechanisms are responsible for central
sympathetic overactivity in patients with metabolic syndrome
(32) and these neurogenic
alterations may be attributed to impairment of BRS (49). Notably, a previous study observed
an impairment of baroreceptor control in patients with metabolic
syndrome (49). Other reflexogenic
areas, such as the cardiopulmonary receptors and chemoreceptors,
may also exert a role in baroreflex alterations (49). Notably, this finding was observed
in patients with obesity and sleep apnea, and hypertensive patients
with left ventricular hypertrophy who showed impairment of BRS
(50).
A previous study in 2,835 patients aged 50 to 75
years old evaluated the hypothesis as to whether metabolic syndrome
is associated with the BRS pathway (51). According to this study, patients
with metabolic syndrome had lower neural baroreflex pathway (NBP).
The processes linking carotid artery stiffness and NBP were
inactive in patients with metabolic syndrome, regardless of blood
pressure levels. One study compared arterial baroreflex-deficient
rats and normal rats with BRS differences of ~2.5-fold
(52); this study found that an
intrinsically low BRS caused hypertension and metabolic disorder.
Restoration of defective BRS could be an effective target for
therapeutic intervention in metabolic syndrome
4. Obesity in children and adolescents.
There has recently been an increased interest in
studying BRS in children, examining its relationship with obesity
and its role for the future development of hypertension. Obesity in
childhood is associated with hypertension in adulthood (53). A previous study was performed in 20
children and adolescents with obesity, who were compared with sex-
and age-matched control individuals of a healthy weight (54). BRS was found to be significantly
lower in individuals in the obese range compared with in children
of a healthy weight. In addition, relying on the instantaneous
heart rate measurement of BRS, a statistically significant
reduction in BRS (between obese and healthy weight individuals) was
observed in subjects between 11 and 20 years. Another study
analyzed BRS in normotensive adolescents and children using two
different measurement techniques: Causal and non-causal BRS methods
(55). Causal methods allow the
separation of feedforward and feedback variations; by using causal
analysis it is possible to separate both causal directions of the
system, namely variations within the SBP induced by HR and vice
versa. According to the non-causal BRS method, no significant
differences were found between children and adolescents; however,
the causal BRS method identified significantly lower BRS values in
children in the obese range compared with normal weight children
(55).
The relationship between body weight, BRS and blood
pressure variability was also assessed in a study by Honzíková
et al (56). In this
analysis, an increased body mass index (BMI) in children,
adolescents and young adults was associated with suppression of BRS
and hypertension. The greater the increase in BMI, the deeper the
BRS reduction, the higher the hypertension and the more increased
variability in systolic blood pressure.
A genetic dependency of BRS was identified in
another study (53). Baroreflex
sensitivity is decreased in young normotensive individuals whose
parents have hypertension compared with individuals without a
family history of hypertension. In addition, associations between
several gene polymorphisms and baroreflex heart rate regulation
have been described. Reduced BRS and obesity were referred to as
independent risk factors for hypertension in youths (53). Notably, young normotensive
individuals with hypertensive parents have been shown to exhibit
lower BRS compared with in those without (57,58).
Other studies, focusing on the interplay between gene polymorphisms
and baroreflex regulation, have also found some degree of
association. Notably, polymorphisms in the endothelin-A receptor,
bradykinin B2 receptor, BK channel β1 subunit, aldosterone synthase
and AT1 receptor gene have been reported to be associated with
decreased BRS values (59-63).
In children suffering from DM (type I or II), insulin resistance
also seems to serve an important role in reduced BRS compared with
that in healthy children (53).
Another study in children examined the hypothesis as
to whether BRS is a predictive factor for the short-term outcome of
postural tachycardia syndrome (64). A total of 77 children were enrolled
in the study and were followed clinically for a period of 90 days.
Children with postural tachycardia syndrome exhibited significantly
higher BRS compared with that in healthy individuals. Furthermore,
BRS was found to be positively associated with changes in heart
rate in these children (64).
5. DM and insulin resistance
Several studies have underlined the association
between insulin resistance, hypertension, the autonomous nervous
system and coronary artery disease (3,30,32,53,65).
A high-fat diet over an extended period of time has been reported
to cause insulin resistance, autonomic dysfunction and an increase
in the risk of cardiovascular disease (59). Hyperinsulinemia has also been shown
to be associated with increased norepinephrine levels in patients
with resistant hypertension (53).
In patients with T2DM, BRS evaluation is a tool used
to assess cardiovascular autonomic neuropathy. Cassaglia et
al (65) identified that the
arcuate nucleus in the hypothalamus is a major insulin site of
action responsible for increasing sympathetic activity and BRS. As
aforementioned, BRS is important for the regulation of blood
pressure and reduced BRS can result in an increase in blood
pressure variability, which in turn further reduces BRS, thus
creating a vicious cycle (16).
Additionally, oxidative stress, which is increased in patients with
obesity and insulin resistance, may have a role in lowering cardiac
baroreflex activity (66-68).
Deterioration of BRS has been observed in
experimental models using streptozotocin-induced DM in rats, as
well as in human subjects (69).
The reduced BRS in patients with DM has been linked to changes in
the autonomous system and its regulation of cardiovascular function
(2,70). These changes may take place either
at the central or peripheral levels of the baroreflex circuit and
lead to dysfunction of baroreflex functionality.
Carotid atherosclerosis accompanying DM is another
factor that can contribute to BRS impairment (71). The consequences of BRS
deterioration in patients with DM are very important. A previous
study conducted on hundreds of patients with DM or hypertension
revealed that the mortality risk was almost double in patients with
DM and reduced BRS compared to individuals without diabetes
(72). Another study in 184
patients with DM with no apparent structural heart diseases
identified a relationship between reduced BRS and cardiovascular
incidences of congestive heart failure, myocardial infarction,
stroke and cardiovascular deaths (73). Thus, in patients with DM, early
diagnosis of BRS impairment may be necessary to apply effective
treatment plans and slow the progression of autonomic
dysfunction.
6. Hypertension
Obesity represents an important risk factor for
hypertension, i.e. >60% of patients with hypertension are
overweight in the US (3). It has
been suggested that increased weight leads to an increase in
systolic blood pressure (74).
Under normal conditions, the arterial baroreceptors respond to
acute blood pressure changes through alterations in vascular wall
stretch. Subsequently, in order to buffer these variations in blood
pressure, changes in the SNA are regulated. In patients with
hypertension, baroreceptors are dysfunctional and are not able to
protect against the constantly elevated blood pressure (75).
In patients with obesity and hypertension, the BRS
is depressed, increasing the risk for cardiac arrhythmias (69). No specific therapies are available
to face this dysfunction and moreover the underlying mechanisms
have not yet been fully elucidated. A previous study was performed
in dogs to investigate the impact of progressive weight increase on
the cardiovascular dynamic effects and also to assess baroreflex
activation (76). Body weight
increases resulted in a gradual elevation of arterial pressure. In
order to reverse hypertension, renal denervation and baroreflex
activation were used; however, the attenuation of tachycardia, and
restoration of cardiac and HRV could only be effectively moderated
by baroreflex activation. These findings imply that baroreflex
activation therapy can reduce arterial pressure and lower the risk
factors for arrhythmias (77).
Another point of interest with regard to the origin
of BRS dysfunction in obesity are the kidneys. It is known that
renal sympathetic activation represents an important cause of
hypertension (3). Since obesity
leads to activation of the renal sympathetic system, it may be
hypothesized that obesity also promotes the progression of
hypertension. Even though the exact mechanism is unknown, it may be
associated with altered BRS, activation of the renin-angiotensin
system, dysregulation of adipokines (e.g., leptin) and insulin
resistance (3). A study in rats
demonstrated that obesity induced an inflammatory response in the
kidneys, which led to autonomic dysfunction and contributed to a
decrease in baroreflex regulation (78).
7. Physical activity
Another issue worth mentioning that is related to
obesity and BRS is the role of physical activity. The beneficial
effects of exercise and training are widely known. Notably,
exercise is protective against obesity, hypertension, T2DM,
cardiovascular disease and depression, and promotes positive
self-esteem (79). In addition,
there is evidence that physical activity affects the autonomous
nervous system and improves the condition of patients with
autonomic disorders (72). The
arterial baroreflex influences other neural reflexes in order to
coordinate the autonomic adjustments to training and regulate blood
pressure during exercise (80).
Individuals with hypertension can suffer significant
increases in arterial pressure during physical exercise. Central
command and arterial baroreflex are hypothesized to mediate the
cardiovascular responses to exercise in normotensive healthy
persons (81). Fukuma et al
(82) enrolled patients with heart
disease and studied the relationship between changes in heart rate
and blood pressure increments (or decrements) in response to
exercise. In accordance with Bruce's protocol, a symptom-limited
treadmill exercise test was performed (83). The results revealed that BRS
dysfunction in the presence of blood pressure decreases may result
in insufficient capacity and activation of the sympathetic nervous
system during exercise to adapt to stress.
To elucidate the possible role of sex in the
relationship between training and autonomic regulation, a clinical
study was performed (84). In
this, blood pressure variability, BRS and autonomic modulation were
examined in 14 men and 13 women. No statistically significant
interactions with sex were identified. For both sexes, a profound
impact of training was found on autonomic regulation, variability
in blood pressure and cardiovagal BRS.
8. Sex differences
Differences in BRS have been observed between men
and women. Generally, cardiovagal BRS after a rapid hypertensive
stimulus in healthy women is less intense compared with that in men
(79). However, after hypotensive
stimuli, both sexes appear to exhibit similar behavior. Regarding
the sympathetic BRS, there is no evident difference between men and
women in young individuals; however, as age increases, the
sympathetic BRS of women has been shown to decrease. This finding
may be ascribed to greater arterial stiffness in women compared
with in men of the same age (79).
Another issue worth mentioning is the influence on muscle
sympathetic neural activity of the different sex hormones levels
during the menstrual cycle and pregnancy, or in response to the use
of oral contraceptives (85).
The role of obesity in conjunction with sex has been
investigated. Overall, as aforementioned, obesity leads to
elevation of SNA, which contributes to the development of
hypertension. Both BMI and WC have been reported to be associated
with increased muscle sympathetic neural activity; however, these
findings have only been observed in men and are not present in
women with obesity (86,87). Premenopausal women with obesity can
suppress potentiation of the sympathetic system and hypertension
due to differences in adipose tissue deposition. Possibly, vascular
reactivity may also differ between the sexes (86). Females may be more sensitive to
obesity-induced increases in sympathetic nerve activity and
arterial pressure as they approach menopause, resulting in
decreased estrogen levels and a shift of adipose tissue to the
visceral area.
In addition to sex differences in adipose tissue
distribution, white adipose cells in females are smaller, more
lipogenic, and more insulin-sensitive due to increased
insulin-induced signaling and production of lipid and glucose
synthesis proteins.
A study conducted in order to compare BRS between
men and women in 185 patients with T2DM revealed that women with
depressed BRS exhibited a greater incidence of cardiovascular
events compared with those with normal BRS. By contrast, no such
difference was found in men (88).
Another study demonstrated that women may exhibit lower values of
cardiac and muscle-pump baroreflexes compared with men (89), a finding that may lead to the
conclusion that older women are more vulnerable to orthostatic
intolerance compared with older men.
9. Aging
Aging is generally related to reduced cardiovagal
BRS (22,90). Even though the underlying mechanism
has not been clarified, it is considered that either loss of
arterial distensibility and/or central intervention of the
baroreflex system could be responsible for impaired BRS (91). By contrast, the sympathetic
baroreflex control has not been found to be affected by age. The
underlying mechanisms of decreased cardiovagal baroreflex
sensitivity, may refer to factors such as increased vascular
stiffening, oxidative stress and suppressed cholinergic
responsiveness of the heart. This impairment of cardiovagal BRS
with advanced age may result in reduced ability to buffer blood
pressure changes, hypertension and a greater risk of sudden cardiac
death (68). Regarding the role of
sex, it has been reported that the decrease in cardiovagal BRS with
age is similar between men and women (79). In pathological situations, such as
DM or hypertension, women display a higher reduction than men
(79).
Verma et al (89) assessed the impact of aging on
muscle-pump BRS of the lateral gastrocnemius, tibialis anterior,
medial gastrocnemius and soleus muscles (89). Lower cardiac BRS control was
observed in the older group of patients. In another study, similar
findings were found for all other muscle groups, whereas no
statistically significant changes in mechanical properties were
determined between young and old people, implying that age is only
associated with changes in baroreflex-mediated control (79).
Differences in the connection between muscle SNA and
cardiac output have also been observed. In young men, an inverse
relationship has been determined between the previous two factors;
however, this relationship has not been observed in older men
(85). In women, there are also
differences with aging; β-adrenoreceptor dilation has been shown to
be attenuated with age, which results in adrenergic
vasoconstriction and blood pressure increases. By contrast,
sympathetic vasoconstriction is counterbalanced by
adrenoreceptor-mediated vasodilation (85).
10. Baroreflex system and bariatric
surgery
Obesity is an independent risk factor for
cardiovascular disease, as it affects various inflammatory and
metabolic parameters. It is associated with structural and
functional cardiac alterations, leading to increased cardiac
workload, systolic work stress and left ventricular hypertrophy
(92). Due to the overactivity of
the sympathetic nervous system, BRS can be attenuated (86). Studies have shown that if arterial
compliance is reduced, then BRS is blunted (2,73).
The most common types of bariatric surgery include
Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG),
biliopancreatic diversion with duodenal switch, and gastric
banding, which appear to reduce morbidity and mortality and provide
long-term weight loss (93,94).
RYGB has been proven to increase the postprandial response of the
anorexigenic gut hormones glucagon-like peptide 1 (GLP-1) and
peptide YY (PYY), thus leading to enhanced satiation. Significant
weight loss following RYGB has been shown to be accompanied by left
ventricular mass reduction, thus improving left ventricular
function (94,95). Notably, SG, which is a relatively
newer technique, has also been associated with increased GLP-1 and
PYY, and reduced ghrelin levels, which can be sustained for years
after surgery (94,95). In our previous study, 37 patients
with morbid obesity who underwent RYGB or SG were examined before,
and 3 and 6 months after surgery. BRS and HRV indices improved
significantly and to the same degree after surgery in both groups;
however, RYGB displayed a more beneficial effect on epicardial fat
thickness and left ventricular performance compared with SG
(93).
Resistant hypertension is defined as uncontrolled
hypertension despite optimal doses of at least three
antihypertensive medications including a diuretic, or four or more
antihypertensives, and is more frequent in individuals with obesity
(96). It has been reported that a
weight loss of ≥5 kg can significantly decrease blood pressure and
there have been several studies on the effects of bariatric surgery
on hypertension. The STAMPEDE trial revealed that there was a
significant reduction in the need for antihypertensive medications
following bariatric surgery (97).
Similarly, the GATEAWAY trial demonstrated that blood pressure was
controlled with the need for less antihypertensive medications, and
the improved blood pressure control was maintained 3 years after
the surgery (98).
Seravalle et al (99) measured blood pressure, heart rate
and BRS in hypertensive individuals with severe obesity before and
after surgery. Using direct, intramural recording of SNA in the
skeletal muscle it was revealed that 6 and 12 months after SG a
significant improvement in baroreflex control of the sympathetic
nerve system was observed, as the operation led to profound
sympathoinhibitory effects, in association with reduced plasma
leptin levels. Moreover, SG produced sustained decreases in muscle
SNA, body weight, plasma leptin and systolic blood pressure. Thus,
the authors concluded that the sympathoinhibition may be related to
decreases in plasma leptin associated with a reduction in
adiposity; this previous study was the first, to the best of our
knowledge, to demonstrate the significant and durable
sympathoinhibitory effects of bariatric surgery (99).
da Silva et al (100) conducted a study to assess the
improvement of exercise capacity and peripheral metabaroreflex
(expressed as the area under the curve of vascular resistance)
after bariatric surgery. It was revealed that 3 months after
surgery, exercise capacity was increased, whereas heart rate, blood
pressure and peripheral muscular metabaroreflex were decreased
(100). Furthermore, Limberg
et al (101) concluded
that RYGB surgery improved blood pressure reactivity due to
alterations in the time course of hemodynamic responses. After
surgery, patients showed an attenuation in blood pressure
reactivity, and improved BRS and HRV, thus displaying a decreased
cardiovascular disease risk (101).
The effect of bariatric surgery compared with
non-surgical treatment on blood pressure has been assessed in a
recent review (102). According
to the findings of this previous study, bariatric surgery may be
considered more beneficial than non-surgical treatment in lowering
blood pressure. Moreover, RYGB was revealed to have
sympathoinhibitory effects due to marked reduction in plasma
leptin, indicating the role of leptin in blood pressure and cardiac
output of patients with obesity (99,102).
11. Conclusion
In summary, the baroreflex represents a rapid
negative feedback system the purpose of which is to buffer blood
pressure variabilities. BRS represents an important homeostatic
system; however, its functionality is affected by several risk
factors. Obesity represents a major factor influencing baroreflex
functionality, which can markedly decrease its sensitivity and lead
to sympathovagal imbalance by decreasing parasympathetic activity
and increasing SNA. Specifically, high abdominal visceral fat has
been shown to suppress BRS. Several other factors closely related
to obesity, such as DM, hypertension and cardiovascular disease, as
well as other factors independent of obesity, such as aging, sex
and physical activity status may affect the balance between the
sympathetic and parasympathetic nervous systems. Weight loss
strategies should be globally implemented in order to attenuate the
increasing incidence of autonomic neuropathy.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Onassis
Foundation (grant no. G ZO 011-1/ 2018-2019).
Availability of data and materials
Not applicable.
Author's contributions
SKK conceived and designed the review. SKK, GA, VK,
NT and AK performed the literature review. Data authentication is
not applicable. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kishi T: Baroreflex failure and
beat-to-beat blood pressure variation. Hypertens Res. 41:547–552.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Skrapari I, Tentolouris N and Katsilambros
N: Baroreflex function: Determinants in healthy subjects and
disturbances in diabetes, obesity and metabolic syndrome. Curr
Diabetes Rev. 2:329–338. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen W, Leo S, Weng C, Yang X, Wu Y and
Tang X: Mechanisms mediating renal sympathetic nerve activation in
obesity-related hypertension. Herz. 40 (Suppl 2):S190–S196.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pinna GD, Maestri R and La Rovere MT:
Assessment of baroreflex sensitivity from spontaneous oscillations
of blood pressure and heart rate: Proven clinical value? Physiol
Meas. 36:741–753. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Swenne CA: Baroreflex sensitivity:
Mechanisms and measurement. Neth Heart J. 21:58–60. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Funakoshi K, Hosokawa K, Kishi T, Ide T
and Sunagawa K: Striking volume intolerance is induced by mimicking
arterial baroreflex failure in normal left ventricular function. J
Card Fail. 20:53–59. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mostarda C, Moraes-Silva IC, Moreira ED,
Medeiros A, Piratello AC, Consolim-Colombo FM, Caldini EG, Brum PC,
Krieger EM and Irigoyen MC: Baroreflex sensitivity impairment is
associated with cardiac diastolic dysfunction in rats. J Card Fail.
17:519–525. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Svitok P, Molcan L, Stebelova K, Vesela A,
Sedlackova N, Ujhazy E, Mach M and Zeman M: Prenatal hypoxia in
rats increased blood pressure and sympathetic drive of the adult
offspring. Hypertens Res. 39:501–505. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Drew RC: Baroreflex and neurovascular
responses to skeletal muscle mechanoreflex activation in humans: An
exercise in integrative physiology. Am J Physiol Regul Integr Comp
Physiol. 313:R654–R659. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Al-Khateeb AA, Limberg JK, Barnes JN,
Joyner MJ, Charkoudian N and Curry TB: Acute cyclooxygenase
inhibition and baroreflex sensitivity in lean and obese adults.
Clin Auton Res. 27:17–23. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pankova NB, Alchinova IB, Cherepov AB,
Yakovenko EN and Karganov MY: Cardiovascular system parameters in
participants of Arctic expeditions. Int J Occup Med Environ Health.
33:819–828. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Javorka M, Lazarova Z, Tonhajzerova I,
Turianikova Z, Honzikova N, Fiser B, Javorka K and Baumert M:
Baroreflex analysis in diabetes mellitus: Linear and nonlinear
approaches. Med Biol Eng Comput. 49:279–288. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vinik AI and Ziegler D: Diabetic
cardiovascular autonomic neuropathy. Circulation. 115:387–397.
2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pinheiro A, Vianna LC and Carmo JC:
Noiseless variable-pressure neck chamber device to assess the
carotid baroreflex function. Front Physiol.
11(613311)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Taylor CE, Willie CK, Ainslie PN and Tzeng
YC: Assessment of human baroreflex function using carotid
ultrasonography: What have we learnt? Acta Physiol (Oxf).
211:297–313. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sakamoto M, Matsutani D and Kayama Y:
Clinical implications of baroreflex sensitivity in type 2 diabetes.
Int Heart J. 60:241–246. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Indumathy J, Pal GK, Pal P,
Ananthanarayanan PH, Parija SC, Balachander J and Dutta TK:
Decreased baroreflex sensitivity is linked to sympathovagal
imbalance, body fat mass and altered cardiometabolic profile in
pre-obesity and obesity. Metabolism. 64:1704–1714. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li CH, Sun ZJ, Lu FH, Chou YT, Yang YC,
Chang CJ and Wu JS: Epidemiological evidence of increased waist
circumference, but not body mass index, associated with impaired
baroreflex sensitivity. Obes Res Clin Pract. 14:158–163.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Toschi-Dias E, Trombetta IC, Silva VJD,
Maki-Nunes C, Cepeda FX, Alves MJNN, Carvalho GL, Drager LF,
Lorenzi-Filho G, Negrão CE and Rondon M: Diet associated with
exercise improves baroreflex control of sympathetic nerve activity
in metabolic syndrome and sleep apnea patients. Sleep Breath.
23:143–151. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Takahashi N, Anan F, Nakagawa M, Yufu K,
Shinohara T, Tsubone T, Goto K, Masaki T, Katsuragi I, Tanaka K, et
al: Hypoadiponectinemia in type 2 diabetes mellitus in men is
associated with sympathetic overactivity as evaluated by cardiac
123I-metaiodobenzylguanidine scintigraphy. Metabolism. 56:919–924.
2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pierce GL, Harris SA, Seals DR, Casey DP,
Barlow PB and Stauss HM: Estimated aortic stiffness is
independently associated with cardiac baroreflex sensitivity in
humans: Role of ageing and habitual endurance exercise. J Hum
Hypertens. 30:513–520. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Serhiyenko VA and Serhiyenko AA: Cardiac
autonomic neuropathy: Risk factors, diagnosis and treatment. World
J Diabetes. 9:1–24. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Milovanovic B, Trifunovic D and Djuric D:
Autonomic nervous system adjustment (ANSA) in patients with
hypertension treated with enalapril. Acta Physiol Hung. 98:71–84.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mengal V, Silva PH, Tiradentes RV,
Santuzzi CH, de Almeida SA, Sena GC, Bissoli NS, Abreu GR and
Gouvea SA: Aliskiren and l-arginine treatments restore depressed
baroreflex sensitivity and decrease oxidative stress in
renovascular hypertension rats. Hypertens Res. 39:769–776.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Saku K, Kishi T, Sakamoto K, Hosokawa K,
Sakamoto T, Murayama Y, Kakino T, Ikeda M, Ide T and Sunagawa K:
Afferent vagal nerve stimulation resets baroreflex neural arc and
inhibits sympathetic nerve activity. Physiol Rep.
2(e12136)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Seravalle G and Grassi G: Sympathetic
nervous system, hypertension, obesity and metabolic syndrome. High
Blood Press Cardiovasc Prev. 23:175–179. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tentolouris N, Argyrakopoulou G and
Katsilambros N: Perturbed autonomic nervous system function in
metabolic syndrome. Neuromolecular Med. 10:169–178. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Guarino D, Nannipieri M, Iervasi G, Taddei
S and Bruno RM: The role of the autonomic nervous system in the
pathophysiology of obesity. Front Physiol. 8(665)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Russo B, Menduni M, Borboni P, Picconi F
and Frontoni S: Autonomic nervous system in obesity and
insulin-resistance-the complex interplay between leptin and central
nervous system. Int J Mol Sci. 22(5187)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Man T, Tegegne BS, van Roon AM, Rosmalen
JGM, Nolte IM, Snieder H and Riese H: Spontaneous baroreflex
sensitivity and its association with age, sex, obesity indices and
hypertension: A population study. Am J Hypertens 2021 (Epub ahead
of print).
|
|
31
|
European Society of Hypertension-European
Society of Cardiology Guidelines Committee. 2003 European Society
of Hypertension-European Society of Cardiology guidelines for the
management of arterial hypertension. J Hypertens. 21:1011–1053.
2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Thorp AA and Schlaich MP: Relevance of
sympathetic nervous system activation in obesity and metabolic
syndrome. J Diabetes Res. 2015(341583)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Myers MG, Cowley MA and Münzberg H:
Mechanisms of leptin action and leptin resistance. Annu Rev
Physiol. 70:537–556. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ciriello J: Leptin in nucleus of the
solitary tract alters the cardiovascular responses to aortic
baroreceptor activation. Peptides. 44:1–7. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fantin F, Giani A, Zoico E, Rossi AP,
Mazzali G and Zamboni M: Weight loss and hypertension in obese
subjects. Nutrients. 11(1667)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lakkis JI and Weir MR: Obesity and kidney
disease. Prog Cardiovasc Dis. 61:157–167. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Abboud FM and Singh MV: Autonomic
regulation of the immune system in cardiovascular diseases. Adv
Physiol Educ. 41:578–593. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lumeng CN and Saltiel AR: Inflammatory
links between obesity and metabolic disease. J Clin Invest.
121:2111–2117. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pal GK, Adithan C, Ananthanarayanan PH,
Pal P, Nanda N, Thiyagarajan D, Syamsunderkiran AN, Lalitha V and
Dutta TK: Association of sympathovagal imbalance with
cardiovascular risks in young prehypertensives. Am J Cardiol.
112:1757–1762. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Engin A: The definition and prevalence of
obesity and metabolic syndrome. Adv Exp Med Biol. 960:1–17.
2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
McCracken E, Monaghan M and Sreenivasan S:
Pathophysiology of the metabolic syndrome. Clin Dermatol. 36:14–20.
2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Koren D and Taveras EM: Association of
sleep disturbances with obesity, insulin resistance and the
metabolic syndrome. Metabolism. 84:67–75. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Dieli-Conwright CM, Courneya KS,
Demark-Wahnefried W, Sami N, Lee K, Buchanan TA, Spicer DV,
Tripathy D, Bernstein L and Mortimer JE: Effects of aerobic and
resistance exercise on metabolic syndrome, sarcopenic obesity, and
circulating biomarkers in overweight or obese survivors of breast
cancer: A randomized controlled trial. J Clin Oncol. 36:875–883.
2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Demidowich AP, Levine JA, Apps R, Cheung
FK, Chen J and Fantoni G: CHI Consortium. Patel TP and Yanovski JA:
Colchicine's effects on metabolic and inflammatory molecules in
adults with obesity and metabolic syndrome: Results from a pilot
randomized controlled trial. Int J Obes (Lond). 44:1793–1799.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Jais A and Brüning JC: Hypothalamic
inflammation in obesity and metabolic disease. J Clin Invest.
127:24–32. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Rubio-Ruiz ME, Guarner-Lans V,
Pérez-Torres I and Soto ME: Mechanisms underlying metabolic
syndrome-related sarcopenia and possible therapeutic measures. Int
J Mol Sci. 20(647)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Alberti KG, Eckel RH, Grundy SM, Zimmet
PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM and
Smith SC Jr: International Diabetes Federation Task Force on
Epidemiology and Prevention; Hational Heart, Lung, and Blood
Institute; American Heart Association; World Heart Federation;
International Atherosclerosis Society; International Association
For The Study of Obesity. Harmonizing the metabolic syndrome: A
joint interim statement of the international diabetes federation
task force on epidemiology and prevention; national heart, lung,
and blood institute; American Heart Association; World Heart
Federation; International Atherosclerosis Society; and
International Association for the Study of Obesity. Circulation.
120:1640–1645. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Quarti Trevano F, Dell'Oro R, Biffi A,
Seravalle G, Corrao G, Mancia G and Grassi G: Sympathetic overdrive
in the metabolic syndrome: Meta-analysis of published studies. J
Hypertens. 38:565–572. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Grassi G, Dell'Oro R, Quarti-Trevano F,
Scopelliti F, Seravalle G, Paleari F, Gamba PL and Mancia G:
Neuroadrenergic and reflex abnormalities in patients with metabolic
syndrome. Diabetologia. 48:1359–1365. 2005.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Grassi G, Facchini A, Trevano FQ, Dell'Oro
R, Arenare F, Tana F, Bolla GB, Monzani A, Robuschi M and Mancia G:
Obstructive sleep apnea-dependent and -independent adrenergic
activation in obesity. Hypertension. 46:321–325. 2005.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zanoli L, Empana JP, Estrugo N, Escriou G,
Ketthab H, Pruny JF, Castellino P, Laude D, Thomas F, Pannier B, et
al: The neural baroreflex pathway in subjects with metabolic
syndrome: A sub-study of the paris prospective study III. Medicine
(Baltimore). 95(e2472)2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zhang LL, Zhang Y, Cheng YQ, Zhang JM, Liu
HQ, Wang WZ, Mehta JL, Xiong ZG, Su DF and Liu AJ: Metabolic
syndrome emerges after artificial selection for low baroreflex
sensitivity. CNS Neurosci Ther. 24:828–836. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Honzíková N and Závodná E: Baroreflex
sensitivity in children and adolescents: Physiology, hypertension,
obesity, diabetes mellitus. Physiol Res. 65:879–889.
2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Lazarova Z, Tonhajzerova I, Trunkvalterova
Z, Brozmanova A, Honzíková N, Javorka K, Baumert M and Javorka M:
Baroreflex sensitivity is reduced in obese normotensive children
and adolescents. Can J Physiol Pharmacol. 87:565–571.
2009.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Javorka M, Tonhajzerova I, Turianikova Z,
Czippelova B and Chladekova L: Causal baroreflex sensitivity
analysis in obese children and adolescents. Can J Diabetes.
37(S285)2013.
|
|
56
|
Honzíková N, Nováková Z, Závodná E,
Paderová J, Lokaj P, Fiser B, Balcárková P and Hrstková H:
Baroreflex sensitivity in children, adolescents, and young adults
with essential and white-coat hypertension. Klin Padiatr.
218:237–242. 2006.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Parmer RJ, Cervenka JH and Stone RA:
Baroreflex sensitivity and heredity in essential hypertension.
Circulation. 85:497–503. 1992.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lopes HF, Silva HB, Consolim-Colombo FM,
Barreto Filho JA, Riccio GM, Giorgi DM and Krieger EM: Autonomic
abnormalities demonstrable in young normotensive subjects who are
children of hypertensive parents. Braz J Med Biol Res. 33:51–54.
2000.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ormezzano O, Poirier O, Mallion JM, Nicaud
V, Amar J, Chamontin B, Mounier-Véhier C, François P, Cambien F and
Baguet JP: A polymorphism in the endothelin-A receptor gene is
linked to baroreflex sensitivity. J Hypertens. 23:2019–2026.
2005.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Xing-Sheng Y, Yong-Zhi L, Jie-Xin L,
Yu-Qing G, Zhang-Huang C, Chong-Fa Z, Zhi-Zhong T and Shu-Zheng L:
Genetic influence on baroreflex sensitivity in normotensive young
men. Am J Hypertens. 23:655–659. 2010.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Gollasch M, Tank J, Luft FC, Jordan J,
Maass P, Krasko C, Sharma AM, Busjahn A and Bähring S: The BK
channel beta1 subunit gene is associated with human baroreflex and
blood pressure regulation. J Hypertens. 20:927–933. 2002.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ylitalo A, Airaksinen KE, Hautanen A,
Kupari M, Carson M, Virolainen J, Savolainen M, Kauma H, Kesäniemi
YA, White PC and Huikuri HV: Baroreflex sensitivity and variants of
the renin angiotensin system genes. J Am Coll Cardiol. 35:194–200.
2000.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Jíra M, Závodná E, Honzíková N, Nováková
Z, Vašků A, Izakovicová Hollá L and Fiser B: Association of A1166C
polymorphism in AT(1) receptor gene with baroreflex sensitivity.
Physiol Res. 59:517–528. 2010.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Li H, Liao Y, Wang Y, Liu P, Sun C, Chen
Y, Tang C, Jin H and Du J: Baroreflex sensitivity predicts
short-term outcome of postural tachycardia syndrome in children.
PLoS One. 11(e0167525)2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Cassaglia PA, Hermes SM, Aicher SA and
Brooks VL: Insulin acts in the arcuate nucleus to increase lumbar
sympathetic nerve activity and baroreflex function in rats. J
Physiol. 589:1643–1662. 2011.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Pan Y, Rong Y, Huang J, Zhu K, Chen J, Yu
C and Chen M: Lower cardiovagal tone and baroreflex sensitivity
associated with hepatic insulin resistance and promote
cardiovascular disorders in Tibetan minipigs induced by a high fat
and high cholesterol diet. J Diabetes Complications. 33:278–288.
2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Lucini D, Cusumano G, Bellia A, Kozakova
M, Difede G, Lauro R and Pagani M: Linosa Study Group. Is reduced
baroreflex gain a component of the metabolic syndrome? Insights
from the LINOSA study. J Hypertens. 24:361–370. 2006.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Hendricks AS, Lawson MJ, Figueroa JP,
Chappell MC, Diz DI and Shaltout HA: Central ANG-(1-7) infusion
improves blood pressure regulation in antenatal
betamethasone-exposed sheep and reveals sex-dependent effects on
oxidative stress. Am J Physiol Heart Circ Physiol. 316:H1458–H1467.
2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Rowaiye OO, Jankowska EA and Ponikowska B:
Baroreceptor sensitivity and diabetes mellitus. Cardiol J.
20:453–463. 2013.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Kück JL, Bönhof GJ, Strom A, Zaharia OP,
Müssig K, Szendroedi J, Roden M and Ziegler D: Impairment in
baroreflex sensitivity in recent-onset type 2 diabetes without
progression over 5 years. Diabetes. 69:1011–1019. 2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Yakhou L, Constant I, Merle JC, Laude D,
Becquemin JP and Duvaldestin P: Noninvasive investigation of
autonomic activity after carotid stenting or carotid
endarterectomy. J Vasc Surg. 44:472–479. 2006.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Gerritsen J, Dekker JM, TenVoorde BJ,
Kostense PJ, Heine RJ, Bouter LM, Heethaar RM and Stehouwer CD:
Impaired autonomic function is associated with increased mortality,
especially in subjects with diabetes, hypertension, or a history of
cardiovascular disease: The Hoorn Study. Diabetes Care.
24:1793–1798. 2001.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Okada N, Takahashi N, Yufu K, Murozono Y,
Wakisaka O, Shinohara T, Anan F, Nakagawa M, Hara M, Saikawa T and
Yoshimatsu H: Baroreflex sensitivity predicts cardiovascular events
in patients with type 2 diabetes mellitus without structural heart
disease. Circ J. 74:1379–1383. 2010.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Ozemek C, Tiwari S, Sabbahi A, Carbone S
and Lavie CJ: Impact of therapeutic lifestyle changes in resistant
hypertension. Prog Cardiovasc Dis. 63:4–9. 2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Guyenet PG, Stornetta RL, Souza GMPR,
Abbott SBG and Brooks VL: Neuronal networks in hypertension: Recent
advances. Hypertension. 76:300–311. 2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Iliescu R, Tudorancea I, Irwin ED and
Lohmeier TE: Chronic baroreflex activation restores spontaneous
baroreflex control and variability of heart rate in obesity-induced
hypertension. Am J Physiol Heart Circ Physiol. 305:H1080–H1088.
2013.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Alnima T, Kroon AA and de Leeuw PW:
Baroreflex activation therapy for patients with drug-resistant
hypertension. Expert Rev Cardiovasc Ther. 12:955–962.
2014.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Khan SA, Sattar MZA, Abdullah NA, Rathore
HA, Ahmad A, Abdulla MH and Johns EJ: Improvement in baroreflex
control of renal sympathetic nerve activity in obese Sprague Dawley
rats following immunosuppression. Acta Physiol (Oxf). 221:250–265.
2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Fu Q and Ogoh S: Sex differences in
baroreflex function in health and disease. J Physiol Sci.
69:851–859. 2019.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Raven PB, Young BE and Fadel PJ: Arterial
baroreflex resetting during exercise in humans: Underlying
signaling mechanisms. Exerc Sport Sci Rev. 47:129–141.
2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Dombrowski M, Mannozzi J and O'Leary DS:
Neural control of cardiovascular function during exercise in
hypertension. Front Physiol. 9(1829)2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Fukuma N, Kato K, Munakata K, Hayashi H,
Kato Y, Aisu N, Takahashi H, Mabuchi K and Mizuno K: Baroreflex
mechanisms and response to exercise in patients with heart disease.
Clin Physiol Funct Imaging. 32:305–309. 2012.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Hamlin MJ, Draper N, Blackwell G, Shearman
JP and Kimber NE: Determination of maximal oxygen uptake using the
bruce or a novel athlete-led protocol in a mixed population. J Hum
Kinet. 31:97–104. 2012.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Kingsley JD, Tai YL, Marshall EM, Glasgow
A, Oliveira R, Parks JC and Mayo X: Autonomic modulation and
baroreflex sensitivity after acute resistance exercise: Responses
between sexes. J Sports Med Phys Fitness. 59:1036–1044.
2019.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Baker SE, Limberg JK, Ranadive SM and
Joyner MJ: Neurovascular control of blood pressure is influenced by
aging, sex, and sex hormones. Am J Physiol Regul Integr Comp
Physiol. 311:R1271–R1275. 2016.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Brooks VL, Shi Z, Holwerda SW and Fadel
PJ: Obesity-induced increases in sympathetic nerve activity: Sex
matters. Auton Neurosci. 187:18–26. 2015.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Straznicky NE, Grima MT, Sari CI, Eikelis
N, Nestel PJ, Dixon JB, Lambert GW, Schlaich MP, Phillips SE and
Lambert EA: Neck circumference is associated with muscle
sympathetic nerve activity in overweight and obese men but not
women. Front Physiol. 8(203)2017.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Yufu K, Takahashi N, Okada N, Wakisaka O,
Shinohara T, Nakagawa M, Hara M, Yoshimatsu H and Saikawa T: Gender
difference in baroreflex sensitivity to predict cardiac and
cerebrovascular events in type 2 diabetic patients. Circ J.
75:1418–1423. 2011.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Verma AK, Xu D, Garg A, Blaber AP and
Tavakolian K: Effect of aging on muscle-pump baroreflex of
individual leg muscles during standing. front Physiol.
10(845)2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Monahan KD: Effect of aging on baroreflex
function in humans. Am J Physiol Regul Integr Comp Physiol.
293:R3–R12. 2007.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Bonyhay I, Jokkel G and Kollai M: Relation
between baroreflex sensitivity and carotid artery elasticity in
healthy humans. Am J Physiol. 271:H1139–H1144. 1996.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Nakamura M and Sadoshima J: Cardiomyopathy
in obesity, insulin resistance and diabetes. J Physiol.
598:2977–2993. 2020.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Kokkinos A, Alexiadou K, Liaskos C,
Argyrakopoulou G, Balla I, Tentolouris N, Moyssakis I, Katsilambros
N, Vafiadis I, Alexandrou A and Diamantis T: Improvement in
cardiovascular indices after Roux-en-Y gastric bypass or sleeve
gastrectomy for morbid obesity. Obes Surg. 23:31–38.
2013.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Koshino Y, Villarraga HR, Somers VK,
Miranda WR, Garza CA, Hsiao JF, Yu Y, Saleh HK and Lopez-Jimenez F:
Changes in myocardial mechanics in patients with obesity following
major weight loss after bariatric surgery. Obesity (Silver Spring).
21:1111–1118. 2013.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Lakhani M and Fein S: Effects of obesity
and subsequent weight reduction on left ventricular function.
Cardiol Rev. 19:1–4. 2011.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Schiavon CA, Pio-Abreu A and Drager LF:
Bariatric surgery for resistant hypertension: Working in progress!
Curr Hypertens. Rep. 22(55)2020.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Schauer PR, Bhatt DL, Kirwan JP, Wolski K,
Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE,
Nissen SE, et al: Bariatric surgery versus intensive medical
therapy for diabetes - 5-year outcomes. N Engl J Med. 376:641–651.
2017.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Schiavon CA, Bersch-Ferreira AC, Santucci
EV, Oliveira JD, Torreglosa CR, Bueno PT, Frayha JC, Santos RN,
Damiani LP, Noujaim PM, et al: Effects of bariatric surgery in
obese patients with hypertension: The GATEWAY randomized trial
(Gastric Bypass to Treat Obese Patients with Steady Hypertension).
Circulation. 137:1132–1142. 2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Seravalle G, Colombo M, Perego P, Giardini
V, Volpe M, Dell'Oro R, Mancia G and Grassi G: Long-term
sympathoinhibitory effects of surgically induced weight loss in
severe obese patients. Hypertension. 64:431–437. 2014.PubMed/NCBI View Article : Google Scholar
|
|
100
|
da Silva RP, Martinez D, Faria CC, de
Carli LA, de Souza WI, Meinhardt NG, Souto KE, Trindade MR and
Ribeiro JP: Improvement of exercise capacity and peripheral
metaboreflex after bariatric surgery. Obes Surg. 23:1835–1841.
2013.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Limberg JK, Guo W, Joyner MJ, Charkoudian
N and Curry TB: Early blood pressure response to isometric exercise
is attenuated in obese individuals who have undergone bariatric
surgery. J Appl Physiol (1985). 124:960–969. 2018.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Wang L, Lin M, Yu J, Fan Z, Zhang S, Lin
Y, Chen X and Peng F: The impact of bariatric surgery versus
non-surgical treatment on blood pressure: Systematic review and
meta-analysis. Obes Surg. 31:4970–4984. 2021.PubMed/NCBI View Article : Google Scholar
|