Introduction
Childhood obesity is a major public health problem
worldwide (1). The prevalence of
obesity among children and adolescents in China has increased from
12.3 to 37.3% between 1991 and 2015(2). Excessive accumulation of adipose
tissue, increased inflammatory response, heterotopic deposition of
lipids in other tissues and elevated lipid levels in obese patients
are the main factors responsible for inducing secondary diseases,
such as diabetes, fatty liver, cardiovascular disease and cancer
(3).
Paeonol, also known as peony, is derived from the
dried bark of Paeonia suffruticosa, which belongs to
Paeoniaceae (Ranunculaceae) or the dried root of Xu Chang qing
(Asclepiadaceae) or the whole grass (4). The chemical name of paeonol is
2-hydroxy-4-methoxyacetophenone and it has a wide range of
biological activities (5). For
example, paeonol plays a role in numerous pharmacological actions,
including antiallergic, anti-inflammatory, antitumor,
cardioprotective, neuroprotective, antidiabetic and antimicrobial
effects (6-8).
Paeonol can regulate a variety of physiological and pathological
processes, such as thrombosis, oxidative stress, inflammation and
atherosclerosis (9-11).
Moutan Cortex and Paeoniae Radix Rubra are common traditional
Chinese medicines and reverse high-fat diet-induced metabolic
disorder and restore gut microbiota homeostasis (12). However, as a major component of
Moutan Cortex and Paeoniae Radix Rubra, paeonol in obesity and
lipid metabolism has not been fully elucidated to the best of our
knowledge.
MicroRNAs (miRNAs/miRs) are small non-coding RNA
molecules of 21-23 nucleotides in length. miRNAs play an important
role in the regulation of insulin secretion from pancreatic islet β
cells (13-15).
Meng et al (16)
demonstrated that miR-21 expression was downregulated in the
endothelial progenitor cells of patients with type 2 diabetes,
while Tomé-Carneiro et al (17) demonstrated that miR-21 was involved
in the circulatory immune response of hypertensive drugs in type 2
diabetes. Paeonol can protect vascular endothelial cells from
oxidized low-density lipoprotein-induced injury by downregulating
miR-21 expression (18). The
expression levels of miR-21 are increased during adipocyte
differentiation (19). Therefore,
the present study aimed to explore whether paeonol could inhibit
lipid formation and promote lipid degradation in adipocytes by
decreasing the expression levels of miR-21.
Materials and methods
Cell culture and transfection
3T3-L1 preadipocytes were obtained from the American
Type Culture Collection. The cells were cultured in DMEM (HyClone;
Cytiva) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and incubated at 37˚C with 5% CO2.
3T3-L1 adipocytes were grown for 2 days after
contact inhibition and cultured for 48 h at 37˚C with DMEM
containing 0.5 mM 3-isobutyl-methylxanthine, 1 mM dexamethasone, 10
mg/ml insulin and 10% FBS (induction differentiation solution I).
Induction differentiation solution I was discarded, and cells were
cultured with DMEM containing only 10 mg/ml insulin (induction
differentiation solution II) and differentiated until day 8. For
paeonol treatment, paeonol (purity 99.86%; MedChemExpress) was
dissolved in DMSO (final concentration <0.01%). 3T3-L1
preadipocytes were treated with different concentrations (30, 60
and 120 µM) of paeonol for 24 h along with the induction
differentiation solution for another 48 h, and paeonol was renewed
simultaneously with the induction differentiation solution until
the cells differentiated at day 8.
miR-21 mimic (100 nM) and mimic negative control
(100 nM) were synthesized by Shanghai GenePharma Co., Ltd. The
sequence of miR-21 mimic was 5'-UAGCUUAUC AGACUGAUGUUGA-3'. The
sequence of mimic negative control was
5'-UCUGACAGUUACCAAUGCUUAA-3'. The cells were seeded into 6-well
plates at a density of 3x105 cells/well and cultured for
24 h at 37˚C. The aforementioned oligonucleotides were transfected
into the cells using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) at 37˚C for 6 h. Then, transfected
cells were further incubated for 48 h at 37˚C, according to the
manufacturer's protocol. The transfection efficiency was detected
using reverse transcription-quantitative PCR (RT-qPCR) following 48
h of transfection.
Cell Counting Kit-8 (CCK-8) assay
Trypsin (Beyotime Institute of Biotechnology) was
used to prepare 3T3-L1 cells in a single cell suspension.
Subsequently, the cells were plated into four 96-well plates
(2x103 cells/well) and incubated for 0, 24, 48 or 72 h.
Following each incubation, CCK-8 solution (Dojindo Molecular
Technologies, Inc.) was added to the culture medium in the 96-well
plate and incubated for a further 1 h. The absorbance of the cells
was detected using spectrophotometry (Thermo Fisher Scientific,
Inc.).
Oil Red O staining
Following induction and differentiation, the cells
were analyzed using Oil Red O staining. Briefly, the cells were
washed with PBS solution and subsequently fixed with 4%
formaldehyde for 30 min at room temperature. After 10 min, a new 4%
formaldehyde fixation solution was added, and the fixation was
continued at room temperature for 2 h. The fixation solution was
discarded, and 60% isopropanol solution was added to remove the
residual formaldehyde solution. This was then discarded, and the
cell culture plate was left in an oven at 37˚C to dry. The prepared
Oil Red O working solution was incubated with the cells at room
temperature in the dark for 10 min. Samples were then rinsed 3-4
times with PBS to remove the residual Oil Red O working solution.
The cell culture plate was dried in an oven at 37˚C and a small
amount of ultra-pure water was added for image capture (light
microscope; Nikon Corporation). The ultrapure water was discarded,
and the cell culture plates were dried in an oven at 37˚C.
Isopropanol solution (100%) was added, and the plates were placed
at room temperature in the dark for 10 min. The absorbance was
measured at 490 nm using a microplate analyzer (MR-96A; Shenzhen
Mindray Bio-Medical Electronics Co., Ltd.).
Glyceride content assay
Using the high-fat sample glycerol enzyme assay kit
(cat. no. E1002; Applygen Technologies, Inc.), all procedures were
performed according to the manufacturer's instructions. The cells
were collected, and the lysate was added to each sample and mixed
well, and incubated at room temperature for 10 min. The upper layer
of liquid from the lysate was transferred to a centrifuge tube was
heated at 70˚C for 10 min and centrifuged for 5 min at 2,795 x g at
room temperature. The supernatant obtained was used for enzymatic
assays. The absorbance was measured at 490 nm using a microplate
analyzer.
RT-qPCR
Total RNA was extracted from cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
Subsequently, PrimeScript™ RT reagent Kit (Takara Bio, Inc.) was
used to reverse transcribe the RNA into cDNA. The reaction
conditions were as follows: 37˚C for 15 min and 85˚C for 5 sec.
qPCR was subsequently performed on an ABI 7500 Real-Time PCR
Detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). qPCR reactions were performed using ChamQ SYBR qPCR Master
Mix (Vazyme Biotech Co., Ltd.). The thermocycling conditions were
as follows: Pre-denaturation at 95˚C for 30 sec; followed by 40
cycles of denaturation at 95˚C for 10 sec, annealing at 55˚C for 30
sec and extension at 72˚C for 1 min. Expression levels were
analyzed using the 2-ΔΔCq method (20). The primer sequences used were as
follows: Fatty acid-binding protein 4 (FABP4) forward, 5'-AAGGTGAAG
AGCATCATAACCCT-3' and reverse, 5'-TCACGCCTTTCA TAACACATTCC-3';
miR-21 forward, 5'-CCGCTCGAG ATCCCAGTAATGGAATGAAG-3' and reverse,
5'-ATA AGAATGCGGCCGCCATCACTTATTATTGCCTATGT-3'; peroxisome
proliferator-activated receptor γ (PPAR-γ) forward,
5'-GGAAGACCACTCGCATTCCTT-3' and reverse,
5'-GTAATCAGCAACCATTGGGTCA-3'; adipocyte protein 2 (Ap2) forward,
5'-CGTCCCGCACGTAGAAGAC-3' and reverse, 5'-CGCCACCGAAGAGGTTGTC-3';
U6 forward, 5'-CTCGCTTCGGCAGCACA-3' and reverse,
5'-AACGCTTCACGAATTTGCGT-3'; GAPDH forward,
5'-AGGTCGGTGTGAACGGATTTG-3' and reverse, 5'-GGG
GTCGTTGATGGCAACA-3'.
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology). Protein
concentration was determined using a BCA protein assay kit and
proteins (40 µg per lane) were separated using 10% gels via
SDS-PAGE (Beyotime Institute of Biotechnology). Following
electrophoresis, the proteins were transferred to polyvinylidene
fluoride membranes (MilliporeSigma) and blocked with 5% skimmed
milk powder for 2 h at room temperature (diluted in PBS-0.1%
Tween-20). The membranes were subsequently incubated overnight at
4˚C with the following primary antibodies: Anti-FABP4 (1:1,000;
cat. no. ab92501; Abcam), anti-CD36 (1:1,000; cat. no. ab221605;
Abcam), anti-glucose transporter 4 (GLUT4; 1:1,000; cat. no.
ab216661; Abcam), anti-PPAR-γ (1:1,000; cat. no. ab272718; Abcam),
anti-Ap2 (1:1,000; cat. no. ab108311; Abcam) and anti-GAPDH
(1:1,000; cat. no. ab8245; Abcam). Following the primary antibody
incubation, the membranes were washed with PBS-0.1% Tween-20 three
times and incubated with goat anti-mouse IgG H&L (HRP)
(1:2,000; cat. no. ab6789; Abcam) or goat anti-rabbit IgG H&L
(HRP) (1:3,000; cat. no. ab6721; Abcam) at room temperature for 2
h. All antibodies were diluted in PBS-0.1% Tween-20. Protein bands
were visualized using Pierce Western Blotting substrate (Thermo
Fisher Scientific, Inc.) and densitometric analysis was performed
using ImageJ software (version 1.48v; National Institutes of
Health).
Statistical analysis
All experiments were independently repeated at least
three times and the data are presented as the mean ± SD.
Statistical analyses were performed using SPSS 19.0 software (IBM
Corp.). One-way ANOVA followed by Tukey's post hoc test was used to
evaluate statistical differences between groups in all experiments.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression levels of miR-21 are
increased in 3T3-L1 cells following differentiation
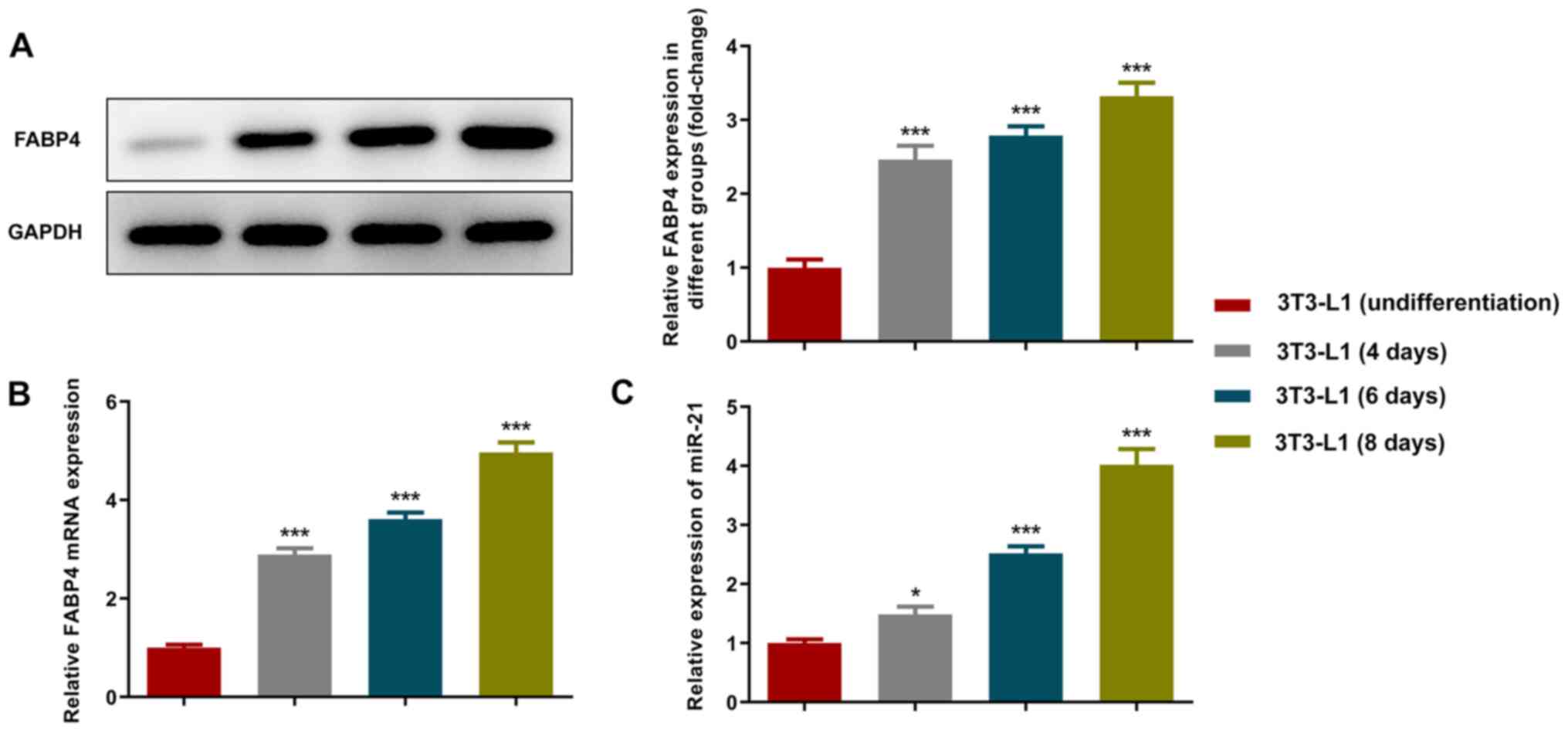
FABP4 is one of the markers of adipocyte
differentiation (21). The
expression levels of FABP4 were detected using RT-qPCR and western
blot analyses. The increase in cell differentiation time resulted
in gradual upregulation in the expression levels of FABP4 (Fig. 1A and B). The results of the RT-qPCR analysis
indicated that the expression levels of miR-21 were also increased
during adipocyte differentiation (Fig.
1C).
Paeonol inhibits the expression levels
of miR-21 in 3T3-L1 differentiated adipocytes
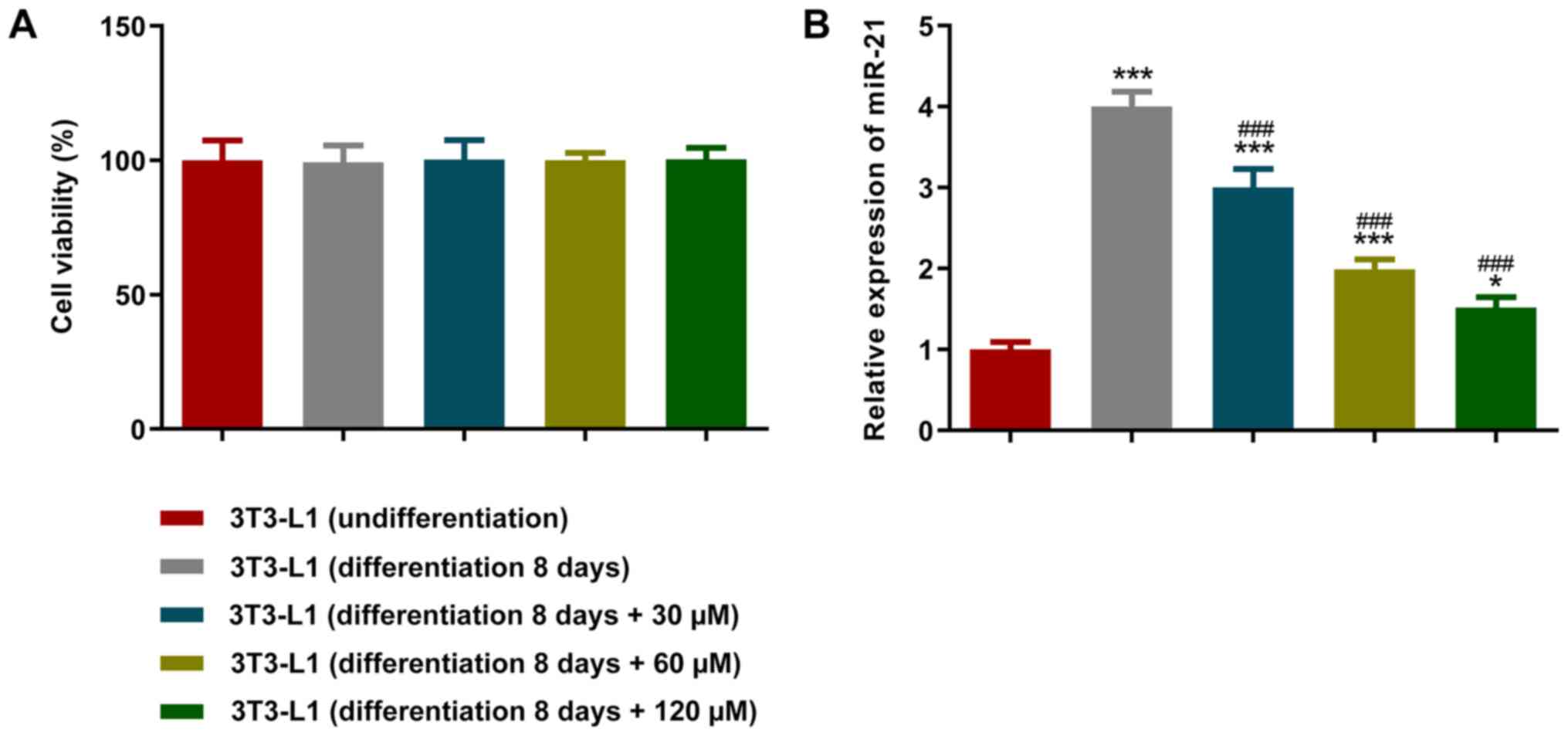
To investigate the effects of paeonol on adipocytes,
different concentrations of this compound were added to the
differentiation medium. A CCK-8 assay was used to detect cell
viability and the results revealed that the different
concentrations of paeonol exhibited no effects on cell viability
(Fig. 2A). However, the RT-qPCR
results indicated that the expression levels of miR-21 were
decreased following an increase in paeonol concentration after the
addition of differentiation medium to the cells (Fig. 2B). Therefore, the 120 µM paeonol
group, which exhibited the highest inhibitory effect, was selected
for use in subsequent experiments.
Paeonol inhibits the adipocyte
differentiation of 3T3-L1 cells, while the overexpression of miR-21
reverses this effect
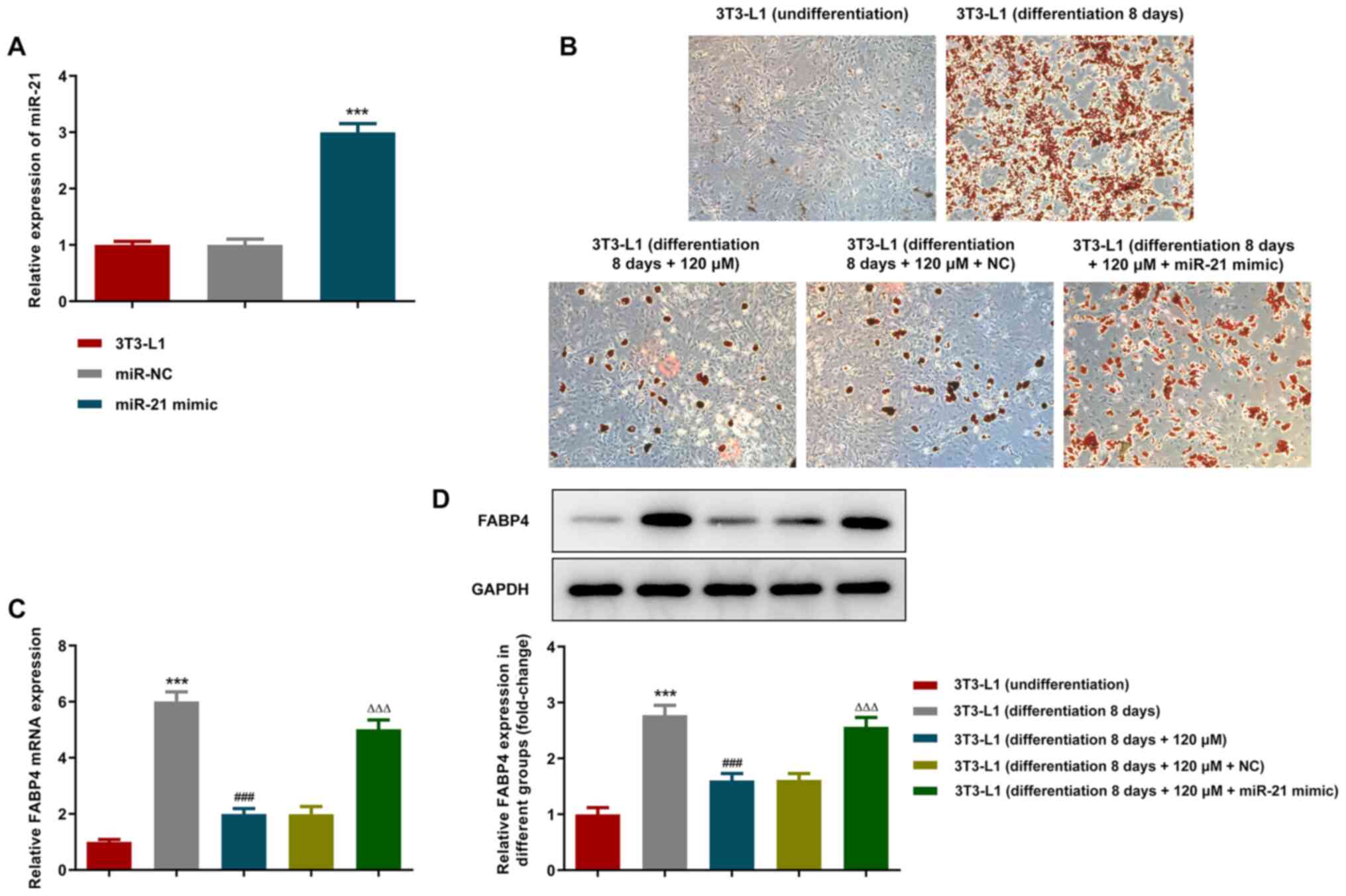
To further explore the specific mechanism of
paeonol-mediated inhibition of 3T3-L1 adipocyte differentiation,
miR-21 mimics were transfected into the cells and the expression
levels of miR-21 were assessed using RT-qPCR. The results indicated
that the expression levels of miR-21 were significantly upregulated
in the miR-21 mimic group (Fig.
3A). The differentiation of adipocytes was detected by Oil Red
O staining (Fig. 3B). On the
eighth day of differentiation, lipid accumulation was present in
the cells and the staining was positive. Following addition of
paeonol, the number of positively stained cells was significantly
decreased. Following transfection with the miR-21 mimic, however,
the number of adipocytes stained with Oil Red O was significantly
increased. Then, as demonstrated by RT-qPCR as well as western
blotting results (Fig. 3C and
D), paeonol inhibited the
expression levels of mRNA as well as protein of the adipocyte
differentiation marker FABP4, while miR-21 mimic could partially
reverse the effect of paeonol on FABP4 expression inhibition.
Paeonol promotes lipid degradation and
inhibits triglyceride (TG) synthesis and the expression of
adipogenic transcription factors in adipocytes, whereas
overexpression of miR-21 reverses these effects
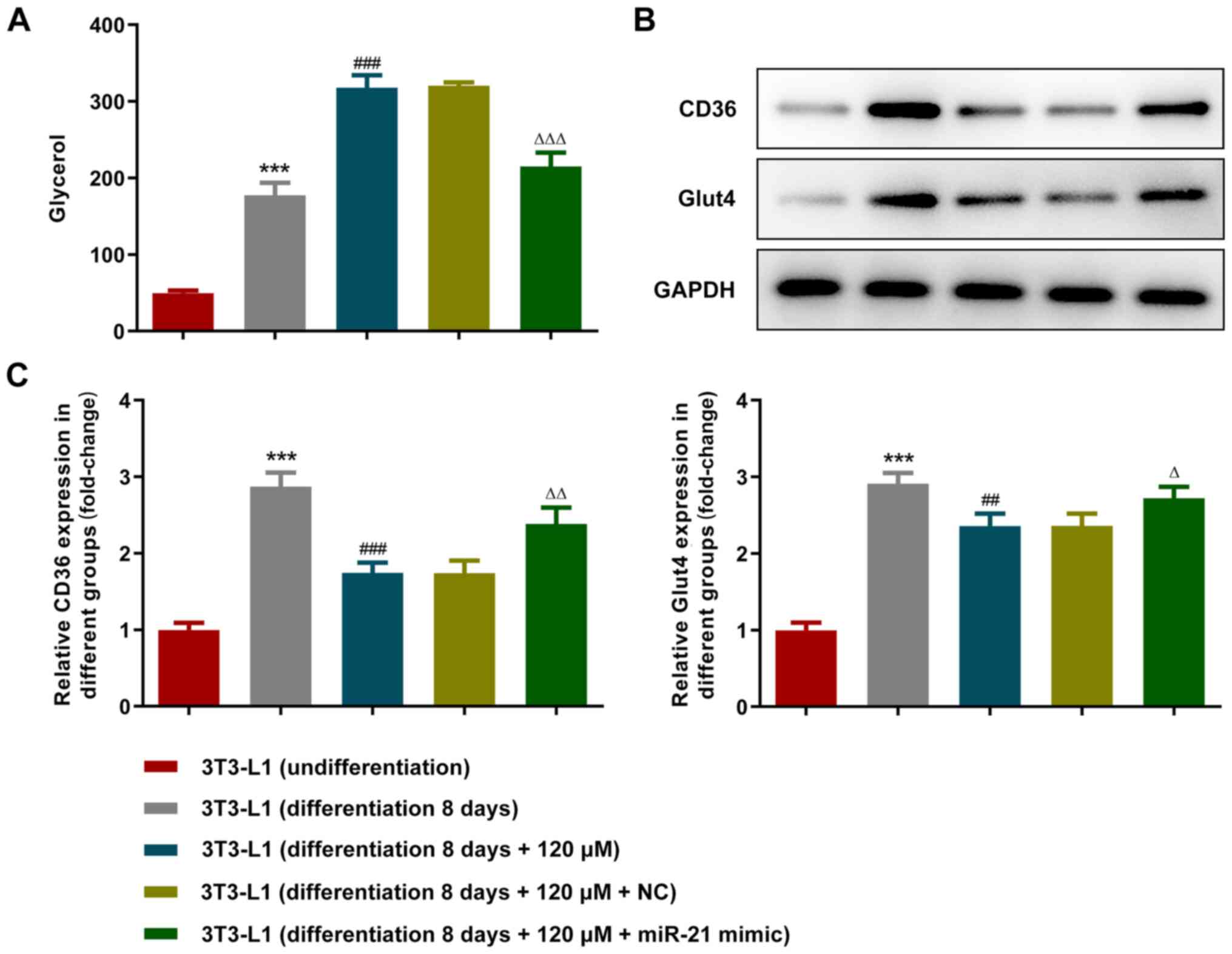
High-fat sample glycerol enzyme assay kit was used
to measure glycerol production in the supernatant (Fig. 4A). Glycerol levels were increased
following the differentiation of 3T3-L1 cells. Paeonol promoted the
glycerol degradation of adipocytes, while the overexpression of
miR-21 reversed its effect. Western blot analysis was used to
detect the expression levels of the TG metabolism-associated
proteins CD36 and GLUT4. Paeonol inhibited TG synthesis, while the
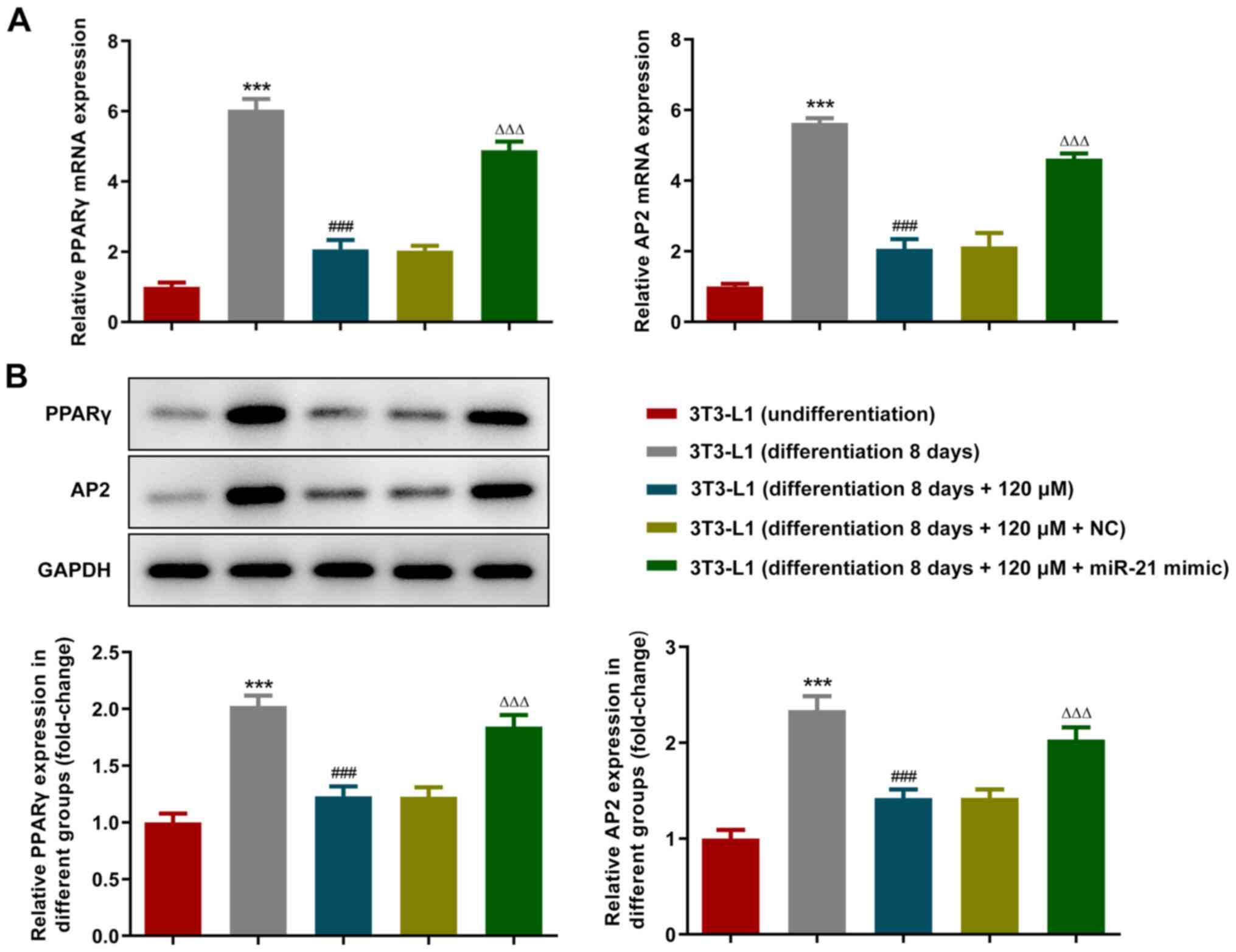
overexpression of miR-21 reversed this effect (Fig. 4B and C). Furthermore, the expression levels of
the adipogenic transcription factors, PPARγ and AP2, were detected
using RT-qPCR (Fig. 5A) and
western blot (Fig. 5B) analyses.
The expression levels of PPARγ and AP2 were increased in 3T3-L1
cells following cell differentiation, whereas they were decreased
following treatment with paeonol. Overexpression of miR-21 also
reversed the changes in expression level of PPARγ and AP2.
Discussion
Adipocytes originate from mesenchymal stem cells
(MSCs) (22). MSCs form
preadipocytes, which gradually accumulate fat droplets and
differentiate into mature adipocytes following growth inhibition,
clonal proliferation and gene regulation (23). Previous studies have shown that the
volume of adipocytes cannot be increased infinitely. When the
volume of adipocytes exceeds 1.2-1.6 µg adipocytes/cells,
preadipocytes are activated via paracrine mechanisms. This in turn
induces their proliferation and differentiation into adipocytes
(24). Therefore, the
proliferation and differentiation of preadipocytes leads to the
rapid proliferation of mature adipocytes, which is one of the
mechanisms responsible for causing obesity. In a previous study,
3T3-L1 preadipocytes were cloned and amplified from Swiss3T3 mouse
embryonic fibroblasts. Following induction in vitro, 3T3-L1
preadipocytes differentiated into mature adipocytes, which can be
used as an in vitro model to simulate the adipocyte
differentiation process and the function of viable adipocyte tissue
(25). 3T3-L1 preadipocytes are
internationally recognized cell lines for the study of the
adipogenic process (26).
Therefore, this study used 3T3-L1 cells as the basis to explore the
role of paeonol.
The anti-inflammatory and antioxidant effects of
paeonol have been noted in specific inflammatory diseases, such as
periodontitis and dermatitis (27). Paeonol significantly inhibits
periodontitis-associated inflammation and oxidative stress levels
by regulating the nuclear factor erythroid-2/NF-κB signaling
pathway (27,28). In a previous study, an emollient
that contained paeonol was found to significantly improve the skin
moisture content, pH and water shunt loss rate of a mouse atopic
dermatitis model, without causing skin irritation (29). These aforementioned studies
demonstrated the anti-inflammatory and antioxidant effects of
paeonol. In the present study, the role of paeonol in lipids
associated with obesity was further investigated. The experimental
results of the current study demonstrated that paeonol promoted
lipid degradation in adipoblast cells, inhibited TG synthesis and
lipid formation in adipoblast cells and promoted lipid
degradation.
The differentiation of adipocytes is a complex
process regulated by transcription factors (30). Members of the PPAR family are major
transcription factors that regulate adipocyte differentiation. A
previous study reported that anthocyanin extract could
significantly decrease the expression levels of the adipocyte
differentiation-related transcription factors, PPARγ and
Ap2(31). The results of the
present study showed that paeonol inhibited the expression of
adipogenic transcription factors in 3T3-L1 adipocytes, and
overexpression of miR-21 reversed its effect. The accumulation of
TGs in adipocytes can reflect the degree of differentiation
(32). Oil Red O can bind to
neutral lipids (33). In the
present study, the data indicated that paeonol inhibited the
adipocyte differentiation of 3T3-L1 cells. Oil Red O staining
analysis indicated that paeonol promoted lipid degradation and
inhibited TG synthesis in adipoblast cells, while the
overexpression of miR-21 reversed this effect.
In conclusion, the current study investigated the
effects of paeonol on the proliferation and differentiation of
3T3-L1 preadipocytes and discussed its effects on lipid formation
and promote lipid degradation in adipocytes. However, due to the
lack of time and funding for this study, research on miR-21 and its
target genes were limited and require further elucidation. Previous
studies have shown that miR-21 regulates the proliferation and
apoptosis of ovarian cancer cells through PTEN/PI3K/Akt, and
bioinformatics analysis has found that there are complementary
binding sites between miR-21 and the 3'-untranslated region of PTEN
(34). Therefore, future studies
will further investigate the effects of miRNAs associated with
paeonol and their target genes on the biological functions of
obesity and lipid metabolism by establishing animal and cell
models.
Acknowledgements
Not applicable.
Funding
Funding: The present study received a grant from the China
Center for Evidence Based Traditional Chinese Medicine (no.
zz13-024-5).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and HG designed the experimental study. JL
analyzed the experiment data and wrote the manuscript. HG aided in
correcting the manuscript. JL carried out the experiments. JL and
HG confirm the authenticity of all the raw data. Both authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Masukume G, O'Neill SM, Baker PN, Kenny
LC, Morton SMB and Khashan AS: The impact of Caesarean section on
the risk of childhood overweight and obesity: new evidence from a
contemporary cohort study. Sci Rep. 8(15113)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang L, Wang H, Zhang B, Popkin BM and Du
S: Elevated fat intake increases body weight and the risk of
overweight and obesity among Chinese adults: 1991-2015 trends.
Nutrients. 12(3272)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Akici N, Onal ZE, Gürbüz T, Sağ C and
Kilinç S: Atherogenic indices in the assessment of cardiovascular
disease risk in children with obesity and subclinical
hypothyroidism. Acta Endocrinol (Bucur). 16:334–338.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Izumi M, Yoshida T, Nakamura T and
Wakamori AM: Paeonol, an Ingredient of Kamishoyosan, Reduces
Intracellular Lipid Accumulation by Inhibiting Glucocorticoid
Receptor Activity in 3T3-L1 Cells. Nutrients. 12(12)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Al-Taher AY, Morsy MA, Rifaai RA, Zenhom
NM and Abdel-Gaber SA: Paeonol attenuates methotrexate-induced
cardiac toxicity in rats by inhibiting oxidative stress and
suppressing TLR4-induced NF-κB inflammatory pathway. Mediators
Inflamm. 2020(8641026)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ramachandhiran D, Vinothkumar V and
Babukumar S: Paeonol exhibits anti-tumor effects by apoptotic and
anti-inflammatory activities in 7,12-dimethylbenz(a)anthracene
induced oral carcinogenesis. Biotech Histochem. 94:10–25.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Adki KM and Kulkarni YA: Neuroprotective
effect of paeonol in streptozotocin-induced diabetes in rats. Life
Sci. 271(119202)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xu F, Xiao H, Liu R, Yang Y, Zhang M, Chen
L, Chen Z, Liu P and Huang H: Paeonol ameliorates glucose and lipid
metabolism in experimental diabetes by activating Akt. Front
Pharmacol. 10(261)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ping M, Xiao W, Mo L, Xiao X, Song S, Tang
W and Yang X: Paeonol attenuates advanced oxidation protein
product-induced oxidative stress injury in THP-1 macrophages.
Pharmacology. 93:286–295. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li H, Dai M and Jia W: Paeonol attenuates
high-fat-diet-induced atherosclerosis in rabbits by
anti-inflammatory activity. Planta Med. 75:7–11. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yu Y, Yan R, Chen X, Sun T and Yan J:
Paeonol suppresses the effect of ox-LDL on mice vascular
endothelial cells by regulating miR-338-3p/TET2 axis in
atherosclerosis. Mol Cell Biochem. 475:127–135. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhong LJ, Xie ZS, Yang H, Li P and Xu XJ:
Moutan Cortex and Paeoniae Radix Rubra reverse
high-fat-diet-induced metabolic disorder and restore gut microbiota
homeostasis. Chin J Nat Med. 15:210–219. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Melkman-Zehavi T, Oren R, Kredo-Russo S,
Shapira T, Mandelbaum AD, Rivkin N, Nir T, Lennox KA, Behlke MA,
Dor Y, et al: miRNAs control insulin content in pancreatic β-cells
via downregulation of transcriptional repressors. EMBO J.
30:835–845. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ma E, Fu Y and Garvey WT: Relationship of
circulating miRNAs with insulin sensitivity and associated
metabolic risk factors in humans. Metab Syndr Relat Disord.
16:82–89. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lang H, Xiang Y, Lin N, Ai Z, You Z, Xiao
J, Liu D and Yang Y: Identification of a panel of miRNAs as
positive regulators of insulin release in pancreatic β-cells. Cell
Physiol Biochem. 48:185–193. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Meng S, Cao JT, Zhang B, Zhou Q, Shen CX
and Wang CQ: Downregulation of microRNA-126 in endothelial
progenitor cells from diabetes patients, impairs their functional
properties, via target gene Spred-1. J Mol Cell Cardiol. 53:64–72.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tomé-Carneiro J, Larrosa M, Yáñez-Gascón
MJ, Dávalos A, Gil-Zamorano J, Gonzálvez M, García-Almagro FJ, Ruiz
Ros JA, Tomás-Barberán FA, Espín JC, et al: One-year
supplementation with a grape extract containing resveratrol
modulates inflammatory-related microRNAs and cytokines expression
in peripheral blood mononuclear cells of type 2 diabetes and
hypertensive patients with coronary artery disease. Pharmacol Res.
72:69–82. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu YR, Chen JJ and Dai M: Paeonol
protects rat vascular endothelial cells from ox-LDL-induced injury
in vitro via downregulating microRNA-21 expression and TNF-α
release. Acta Pharmacol Sin. 35:483–488. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kang M, Yan LM, Zhang WY, Li YM, Tang AZ
and Ou HS: Role of microRNA-21 in regulating 3T3-L1 adipocyte
differentiation and adiponectin expression. Mol Biol Rep.
40:5027–5034. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mitterberger MC, Lechner S, Mattesich M,
Kaiser A, Probst D, Wenger N, Pierer G and Zwerschke W: DLK1(PREF1)
is a negative regulator of adipogenesis in
CD105+/CD90+/CD34+/CD31-/FABP4-
adipose-derived stromal cells from subcutaneous abdominal fat pats
of adult women. Stem Cell Res. 9:35–48. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee SC, Lee YJ, Shin MK and Sung JS:
Regulation of CXCR6 expression on adipocytes and osteoblasts
differentiated from human adipose tissue-derived mesenchymal stem
cells. Stem Cells Int. 2020(8870133)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jiang R, Yang T, Zhang Y, Wang Z and Zhang
T: LKB1 promotes the transformation of bone marrow mesenchymal stem
cells into adipocytes under oxidative stress via AMPK-mTOR
signaling pathway. J Interferon Cytokine Res. 40:370–376.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang J, Cai B, Ma M, Luo W, Zhang Z,
Zhang X and Nie Q: ALDH1A1 inhibits chicken preadipocytes'
proliferation and differentiation via the PPARγ pathway in vitro
and in vivo. Int J Mol Sci. 21(3150)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pacifici F, Farias CLA, Rea S, Capuani B,
Feraco A, Coppola A, Mammi C, Pastore D, Abete P, Rovella V, et al:
Tyrosol may prevent obesity by inhibiting adipogenesis in 3T3-L1
preadipocytes. Oxid Med Cell Longev. 2020(4794780)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Son Y, Cox JM, Stevenson JL, Cooper JA and
Paton CM: Angiopoietin-1 protects 3T3-L1 preadipocytes from
saturated fatty acid-induced cell death. Nutr Res. 76:20–28.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kurt S, Gürkan CG, Keleş Tezal GC, Çiftçi
A, Gürgör PN, Güler Ş and Çetinkaya BÖ: Histopathological and
biochemical evaluation of the effect of Paeoniflorin on the
periodontium during and after periodontitis formation in rats. Arch
Oral Biol. 102:135–140. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ni J, Yang D, Song L and Li C: Protective
effects of paeoniflorin on alveolar bone resorption and soft-tissue
breakdown in experimental periodontitis. J Periodontal Res.
51:257–264. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Qiu J, Chen M, Liu J, Huang X, Chen J,
Zhou L, Ma J, Sextius P, Pena AM, Cai Z, et al: The
skin-depigmenting potential of Paeonia lactiflora root extract and
paeoniflorin: In vitro evaluation using reconstructed pigmented
human epidermis. Int J Cosmet Sci. 38:444–451. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Baek K and Baek JH: The transcription
factors myeloid elf-1-like factor (MEF) and distal-less homeobox 5
(Dlx5) inversely regulate the differentiation of osteoblasts and
adipocytes in bone marrow. Adipocyte. 2:50–54. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang P, Ji R, Ji J and Chen F: Changes of
metabolites of acrylamide and glycidamide in acrylamide-exposed
rats pretreated with blueberry anthocyanins extract. Food Chem.
274:611–619. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kim JH, Lee S, Kim HY and Cho EJ: Acer
okamotoanum inhibits adipocyte differentiation by the regulation of
adipogenesis and lipolysis in 3T3-L1 cells. Int J Mol Med.
45:589–596. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Curcio CA, Johnson M, Rudolf M and Huang
JD: The oil spill in ageing Bruch membrane. Br J Ophthalmol.
95:1638–1645. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu HY, Zhang YY, Zhu BL, Feng FZ, Yan H,
Zhang HY and Zhou B: miR-21 regulates the proliferation and
apoptosis of ovarian cancer cells through PTEN/PI3K/AKT. Eur Rev
Med Pharmacol Sci. 23:4149–4155. 2019.PubMed/NCBI View Article : Google Scholar
|