Introduction
Anorectal conditions are among the most common
problems encountered in clinical practice. Anorectal diseases such
as hemorrhoids, anal fissure, anorectal abscesses, anal fistula,
proctalgia fugax and pruritus ani exhibit overlapping symptoms,
which may be distinguished based on consideration of the detailed
history of the patient and anorectal examination (1).
Hemorrhoids represent the most common anorectal
disorder and arise due to engorgement of vascular cushions in the
lower rectum/anus (2,3). Hemorrhoids may be located inside the
anal canal (termed ‘internal hemorrhoids’) or they present at the
anal opening (‘external hemorrhoids’). If external hemorrhoids
become filled with blood clots, this leads to the formation of
thrombosed hemorrhoids. Hemorrhoids are traditionally classified
into grades I-IV, where grade I hemorrhoids are purely internal,
grade II hemorrhoids prolapse on staining but reduce spontaneously,
grade III hemorrhoids are characterized by their prolapsing
requiring manual reduction and grade IV hemorrhoids are prolapsed
and non-reducible (3). According
to current estimates, 75% of the world population will suffer from
bleeding hemorrhoids at a certain point in their lives (4), indicating that hemorrhoids are a
major socioeconomic and medical problem. Hemorrhoids occur commonly
in both genders, with a peak incidence arising between the fourth
and sixth decades of life (5,6).
Even though the precise underlying cause of hemorrhoids has not
been fully elucidated, risk factors for hemorrhoids include
straining during defecation as a consequence of constipation,
obesity, pregnancy, old age, chronic diarrhea, anal intercourse,
cirrhosis with ascites, pelvic floor dysfunction and having a
low-fiber diet (7,8). Although 40-55% of cases exhibit no
symptoms (9), those patients who
are symptomatic frequently exhibit pain, bleeding, prolapse,
soiling, grape-like tissue prolapse, itching or a combination of
these symptoms (3). Various
physiological changes, including abnormal distension of veins,
destruction of collagen fibers and fibroelastic tissues, and damage
of the anal subepithelial muscle occur during hemorrhoidal
progression (10). Inflammatory
reactions in the affected area have been indicated to be associated
with mucosal ulceration, ischemia and thrombosis (11). Several enzymes, including matrix
metalloproteinases, thrombin and plasmin, as well as an array of
signaling factors, are involved in the degradation of the
supporting tissues in the anal cushions, which consist of collagen,
fibronectin and elastin fibers. These events gradually lead to the
promotion of angiogenic and proliferative activity mediated by
transforming growth factor β as part of the healing process
(12,13). Various surgical options, including
stapled hemorrhoidopexy and hemorrhoidectomy, are available as
treatment procedures; however, these methods are highly invasive
and associated with higher costs (2,14).
Furthermore, these surgical options are largely unsuccessful, as
recurrence, pain and bleeding are major concerns after the
procedure (15,16).
An anal fissure is a tear of the anoderm in the anal
canal that is caused by mechanical trauma, sphincter spasm or
ischemia (17). Anal fissures
exhibit overlapping symptoms with hemorrhoids (18) and are always associated with
twinges of pain (19). Chronic
fissures typically require medical treatment or surgical therapy.
Surgical procedures have superior healing rates compared with local
medical therapies, although they may result in persistent
incontinence (20).
An anal fistula is an epithelialized tract or a
connection between the anal canal and the perianal skin. Classical
anal fistulas result from a perineal infection and abscess
formation (21). Fistulas are also
associated with inflammatory bowel disease, radiation, malignancy,
chronic diarrhea or pre-existing incontinence (22). Perineal wounds usually result from
low pelvic tumors, ablation of the tumor, trauma, perineal
infections and electrical or thermal burns (23). Furthermore, various surgical
procedures for fistula-in-ano may also lead to poorly healing
perineal wounds and impaired continence (24). Patient- and surgery-associated
factors, including obesity, being overweight, hypoalbuminemia,
extralevator abdominal resection, intraoperative perforation and
supine position during the second phase of labor, are associated
with delayed perineal wound healing (25-29).
Delayed perineal wounds are associated with morbidity, prolonged
hospital stays, higher costs, home nursing care needs and lower
rates of survival (23,30). Therefore, developing minimally
invasive therapeutic interventions that help to alleviate
inflammation and pain in anal fissures, bleeding hemorrhoids and
perineal wounds would be beneficial for patients.
Herbal products are an important source of medicinal
compounds (31). Traditional
medicinal compounds are known to suppress pain and inflammation
under different pathological conditions (32). Herbal medicines suppress
inflammation by downregulating the recruitment of inflammatory
cells, as well as through suppressing inflammatory cytokine
expression (33). Cytokines such
as regulated upon activation, normal T cell expressed and
presumably secreted (RANTES), IL-1β and VEGF are proinflammatory
cytokines that contribute to inflammation and pain in different
pathological settings (33,34).
Cyclooxygenase-2 (COX-2) is an enzyme that is involved in the
formation of prostaglandins, which are crucially involved in
promoting inflammation (35). The
expression of COX-2 is regulated by growth factors and different
inflammatory cytokines, including IL-1β, IL-6 and tumor necrosis
factor-α (TNF-α), and therefore, its expression is upregulated
during inflammation (35). The use
of natural products for the treatment of these ailments is indeed
cost-effective and minimally invasive (32,36).
A previous study by our group assessed the safety and efficacy of a
polyherbal formulation, AnoSpray® for the treatment of
perineal wounds using a single-center, open-label, randomized
parallel-group trial. The results indicated that the use of
AnoSpray provided a marked improvement in treating perineal wounds
as compared to betadine solution (37). However, at present, the underlying
mechanism through which AnoSpray exerts its effects on anorectal
diseases has remained elusive. The aim of the present study was to
investigate the cytotoxicity and molecular action of AnoSpray in
anorectal diseases. The data obtained on fibroblasts and
macrophages revealed that it was safe to use. Furthermore, it was
demonstrated that AnoSpray suppressed the migration of these cells
and ameliorated the expression of inflammatory factors in both an
in vitro model and in clinical specimens of anorectal
disease. Collectively, the results confirmed the benefit of
AnoSpray treatment in the clinical management of hemorrhoids, anal
fissures and perineal wounds.
Materials and methods
Cell culture
The mouse monocyte/macrophage-like cell line RAW
264.7 and human foreskin fibroblasts (BJ cell line) were obtained
from the American Type Culture Collection. The RAW 264.7 and BJ
cells were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific,
Inc.) and Minimum Essential Medium (Eagle) [MEM(E)] (HiMedia
Laboratories, LLC) media, respectively. The culture media were
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 100 units of penicillin/100 µg/ml
streptomycin (HiMedia Laboratories, LLC), and the cells were grown
in a humidified incubator in an atmosphere with 5% CO2
at 37˚C.
Drug preparation
The formulation of AnoSpray/PiloSpray®
(Healing Hands & Herbs Pvt. Ltd.; https://healinghandsandherbs.in/) was mentioned in a
previously published study (37).
Similar to AnoSpray/PiloSpray (see https://pilospray.com/AnoSpray-advanced-piles-spray/)
is an over-the-counter brand name of the formulation; essentially,
AnoSpray/PiloSpray is an Ayurvedic polyherbal formulation in the
form of a spray. AnoSpray/PiloSpray consists of lodhara
(Symplocos racemosa), daruharidra (Berberis
aristata), mocharas (Bombax ceiba), kapur (Cinnamomum
camphora), pudinah (Mentha piperita), til oil
(Sesamum indicum) and kokam oil (Garcinia indica) in
aerosol form. ‘AnoSpray’ or ‘PiloSpray’ is used as the brand name
to refer to the polyherbal spray formulation in the present
study.
Cell viability assay
Cell viability was assessed using an MTT assay,
following the instructions provided in a previously described
protocol (38). In brief, RAW
264.7 and BJ cells (density, 2x104 cells/well) were
seeded into 96-well microplates (with flat bottoms) and treated
with AnoSpray at concentrations of 0-7.5 µl/ml for 24 h. MTT (0.5
mg/ml) solution was added to each well and the plates were
incubated for 4 h at 37˚C. Subsequently, the MTT solution was
carefully aspirated and isopropanol was added to dissolve the
formazan crystals. The optical density of the formazan solution was
subsequently recorded at 570 nm using an automated microplate
reader (EPOCH2; Bio Tek Instruments, Inc.). All experiments were
performed in triplicate.
Wound-closure assay
Cell migration was studied using a conventional
wound-closure/migration assay, as per the standard protocol
described previously (39). In
brief, RAW 264.7 and BJ cells (density, 2x105 cells)
were seeded into 12-well plates and allowed to attain a confluent
monolayer. Upon reaching 100% confluence, the monolayers were
scratched using a sterile 200-µl pipette tip and the old medium was
removed to remove detached cells. Fresh RPMI-1640 complete medium
(1 ml) was added to the cells prior to the treatments. Cells were
subsequently incubated at 37˚C with AnoSpray at concentrations of
0-7.5 µl/ml. Photographs were acquired at 0 and 12/16 h using a
using a phase-contrast microscope (magnification, x100; Nikon
Corporation). The area of wound closure was measured using
Image-Pro Plus 6.0 software (National Institutes of Health).
Western blot analysis
Western blot analysis was performed as per a
standard procedure described previously (40). Specifically, RAW 264.7 and BJ cells
(5x105 cells) were seeded in 60-mm dishes. On the next
day, the cells were treated with different concentrations of
AnoSpray (0-7.5 µl/ml). Cells were harvested by centrifuging the
cells at 1,000 x g at room temperature for 10 min and lysed using
RIPA buffer. The protein concentration was estimated in cell
lysates using Bradford reagent and equal amounts of total protein
(30 µg/lane) were resolved by 10 or 12.5% SDS-PAGE. The separated
proteins were transferred to a polyvinylidene difluoride membrane
(Bio-Rad Laboratories, Inc.) and processed for further analysis
Non-nspecific binding sites were blocked by incubating membranes in
5% skimmed milk at room temperature for 1 h. The membranes were
subsequently incubated with primary antibodies (obtained from Santa
Cruz Biotechnology, Inc.) against COX-2 (cat. no. sc-1746;
1:1,000), RANTES (cat. no. sc-1410; 1:1,000), VEGF (cat. no.
sc-7269, 1:1,000 dilution) and β-actin (Santa Cruz Biotechnology,
Inc., cat. no. sc-1615, 1:2,000) overnight at 4˚C, followed by
incubation with anti-goat HRP (Santa Cruz Biotechnology, Inc.; cat.
no. sc-2020; 1:2,000) or anti-mouse HRP antibodies (Santa Cruz
Biotechnology, Inc.; cat. no. sc-2005; 1:2,000) for 1 h at room
temperature. All blots were visualized using the Clarity Western
ECL reagent (Bio-Rad Laboratories, Inc.). Densitometry analysis was
performed using ImageJ2 software (National Institutes of Health)
and fold-changes were calculated following normalization to
β-actin.
Analysis of clinical specimens
The present study was approved by the Institutional
Ethics Committee of Healing Hands Clinic (Pune, India). Patients
with hemorrhoids were treated thrice a day for at least 15 days
with AnoSpray. Human hemorrhoid specimens derived from the surgical
removal of hemorrhoids (n=10) were collected between March 2020 and
February 2021 with the help of a histopathologist from Healing
Hands Clinic (Pune, India) and written informed consent was
obtained from each of the patients. Paraffin-embedded tissue blocks
were prepared and 5-µm sections were cut and deposited on
poly-L-lysine-coated slides. Immunohistochemical analysis was
performed using the SuperSensitive™ Polymer-HRP IHC
Detection System (BioGenex Laboratories), as per the manufacturer's
protocol. In brief, the sections were deparaffinized in xylene and
rehydrated in an alcohol gradient. Subsequently, the sections were
subjected to antigen retrieval in citrate buffer at 90˚C for 15
min. Sections were covered with peroxide for 10 min to block
endogenous peroxidase activity, followed by power block (provided
as part of the SuperSensitive™ Polymer-HRP IHC Detection
System) to block non-specific binding sites. Sections were then
incubated with primary antibodies against COX-2 (1:100) and RANTES
(1:100) overnight at 4˚C, and subsequently with specific secondary
antibodies for 1 h at room temperature. Liquid DAB chromogen was
added at room temperature for 10 min and images of the tissue
sections were captured using a Nikon Eclipse microscope
(magnification, x200; Nikon Corporation).
Statistical analysis
Each experiment was performed in triplicate and the
results were expressed as the mean ± SEM. Statistical analysis was
performed using GraphPad Prism 5.0 software (GraphPad Software,
Inc.). An unpaired Student's t-test was utilized to assess
statistical difference between two groups, whereas the
Kruskal-Wallis test was used to measure statistical significance in
the case of multiple doses of drug treatments with the Dunn's
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
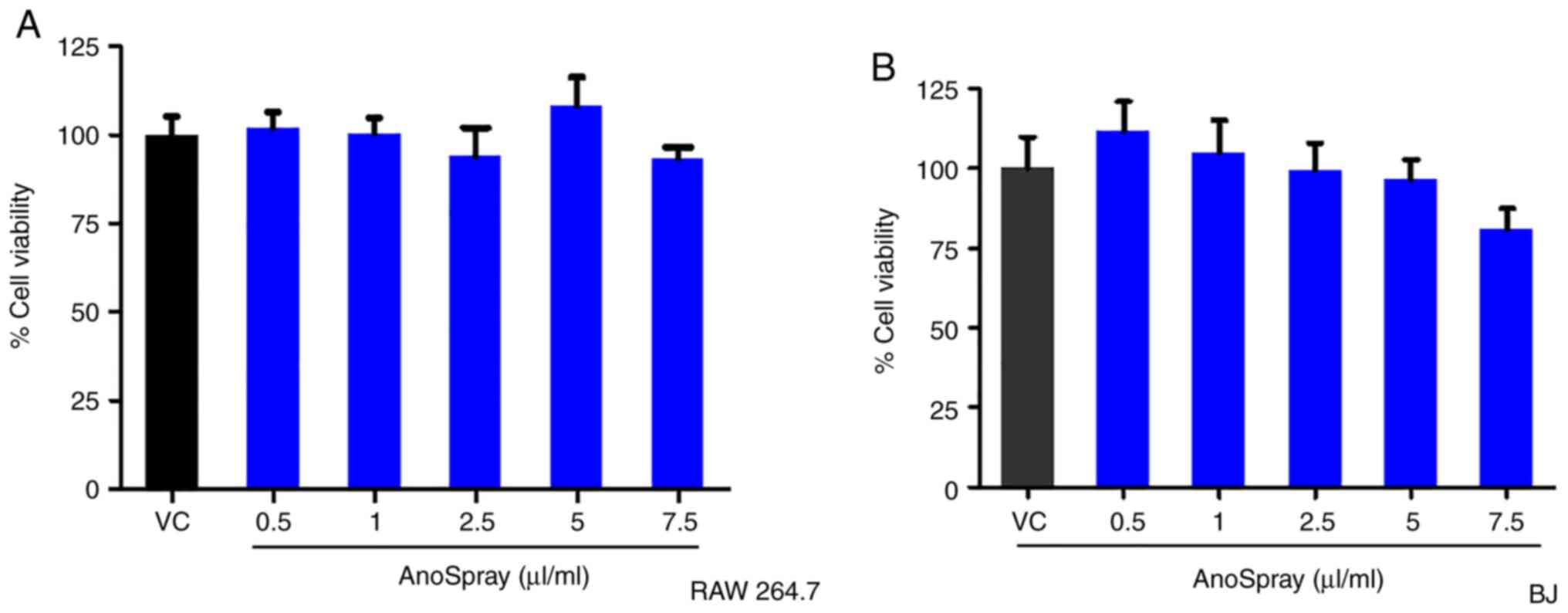
Effect of AnoSpray on the viability of
fibroblasts
A previous study by our group (37) reported on the safety of AnoSpray
treatment in humans for the management of perineal wounds. The
present study aimed to investigate the in vitro cytotoxic
effects of AnoSpray on macrophage and fibroblast cell lines to
assess its safety in therapeutic use. RAW 264.7 (macrophage) and BJ
(fibroblast) cells were used for assessing the effect of AnoSpray
on cell viability. The RAW 264.7 and BJ cell lines in culture were
treated with AnoSpray in a concentration-dependent manner (0-7.5
µl/ml) to study its effect on cell viability using an MTT assay.
The percentage cell viability was measured and the results obtained
were statistically analyzed using the Kruskal-Wallis test. The
results indicated that AnoSpray did not have any significant
effects on the viability of the mouse macrophage cell line RAW264.7
(Fig. 1A). Subsequently, the
effect of AnoSpray on the viability of the human normal fibroblast
cell line BJ was also studied using an MTT assay. Similar to the
results for the RAW 264.7 cell line, AnoSpray did not appear to
significantly affect the viability of the BJ cells (Fig. 1B). Based on these findings, it was
possible to infer that AnoSpray did not affect the viability of
macrophages and fibroblasts in vitro, thereby demonstrating
its safety for therapeutic applications.
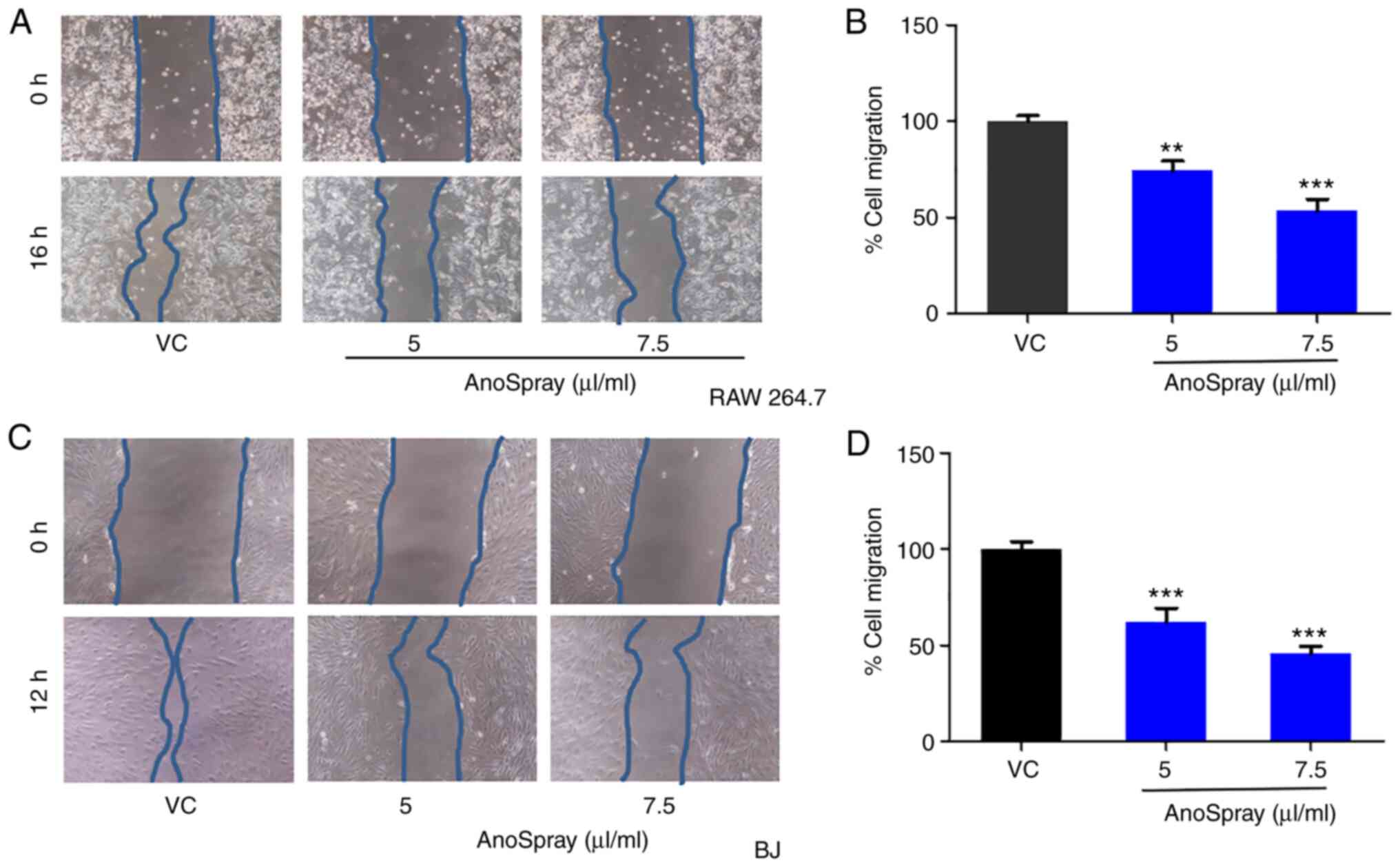
Effect of AnoSpray on the migration of
fibroblasts
Wound healing is a complex and dynamic process that
is involved in the recovery of the structure and functions of
injured tissues (32). Prolonged
inflammation at the site of injury delays the wound-healing
process, inducing pathological pain (41,42).
In anorectal diseases, inflammation has a fundamental role in the
aetiology of hemorrhoids (10),
fissures (43) and perineal wounds
(44). Macrophages and fibroblasts
are recruited at the site of wounds and are involved in the
synthesis of extracellular matrix and secretion of proinflammatory
cytokines (41,45). Therefore, the effects of AnoSpray
treatment on the migration of macrophages and fibroblasts were
studied using a conventional wound closure assay. Monolayers of
macrophages and fibroblasts in culture were wounded and
subsequently treated with different concentrations of AnoSpray
(0-7.5 µl/ml). The percentage migration values were then analyzed
and significant differences between the groups were assessed using
Student's t-test. The results revealed that the migration of RAW
264.7 cells was significantly reduced upon AnoSpray treatment in a
concentration-dependent manner (Fig.
2A and B). Similar results
were obtained with the BJ cells. AnoSpray treatment led to a
decrease in the migration rates of these cells (Fig. 2C and D), suggesting that AnoSpray has a
significant role in impeding the migration of fibroblasts and
macrophages.
Effect of AnoSpray on the expression
of inflammatory cytokines
Inflammatory cytokines are highly expressed in
anorectal diseases and are involved in pain, inflammation and
itching (44,46-48). Inflammatory mediators, including
COX-2, RANTES, VEGF, TNF-α and IL1-β, are known to be highly
expressed under different pathological conditions (49,50).
Since fibroblasts and macrophages have been reported to express
proinflammatory mediators (51,52),
macrophages and fibroblasts were used in the present study to
examine changes in the expression levels of these cytokines upon
treatment with AnoSpray (Fig. 3).
Even though the endogenous expression levels of RANTES and VEGF are
low in RAW 264.7 cells, the protein expression levels of these
cytokines in RAW 264.7 cells were significantly suppressed upon
treatment with AnoSpray compared with those in the control cells,
as determined using western blot analysis (Fig. 3A). In addition, the endogenous
expression of COX-2 was reduced in RAW 264.7 cells upon incubation
with AnoSpray (Fig. 3A). The
western blots for RANTES, VEGF and COX-2 were subsequently
quantified and statistically analyzed using one-way ANOVA. The
results revealed that the expression of these cytokines and COX-2
were significantly downregulated in RAW 264.7 cells (Fig. 3B). The expression levels of RANTES
and VEGF were also examined in BJ fibroblasts using western blot
analysis. The results revealed that the expression levels of these
markers were markedly reduced in the AnoSpray-treated cells
(Fig. 3C). Densitometric analysis
was also performed for the western blot data for RANTES and VEGF,
followed by statistical analysis using one-way ANOVA. This analysis
revealed that the expression levels of these cytokines were
significantly decreased in BJ cells (Fig. 3D). Collectively, these results
indicated that AnoSpray treatment downregulated the expression of
the two proinflammatory cytokines and COX-2 in macrophages and
fibroblasts.
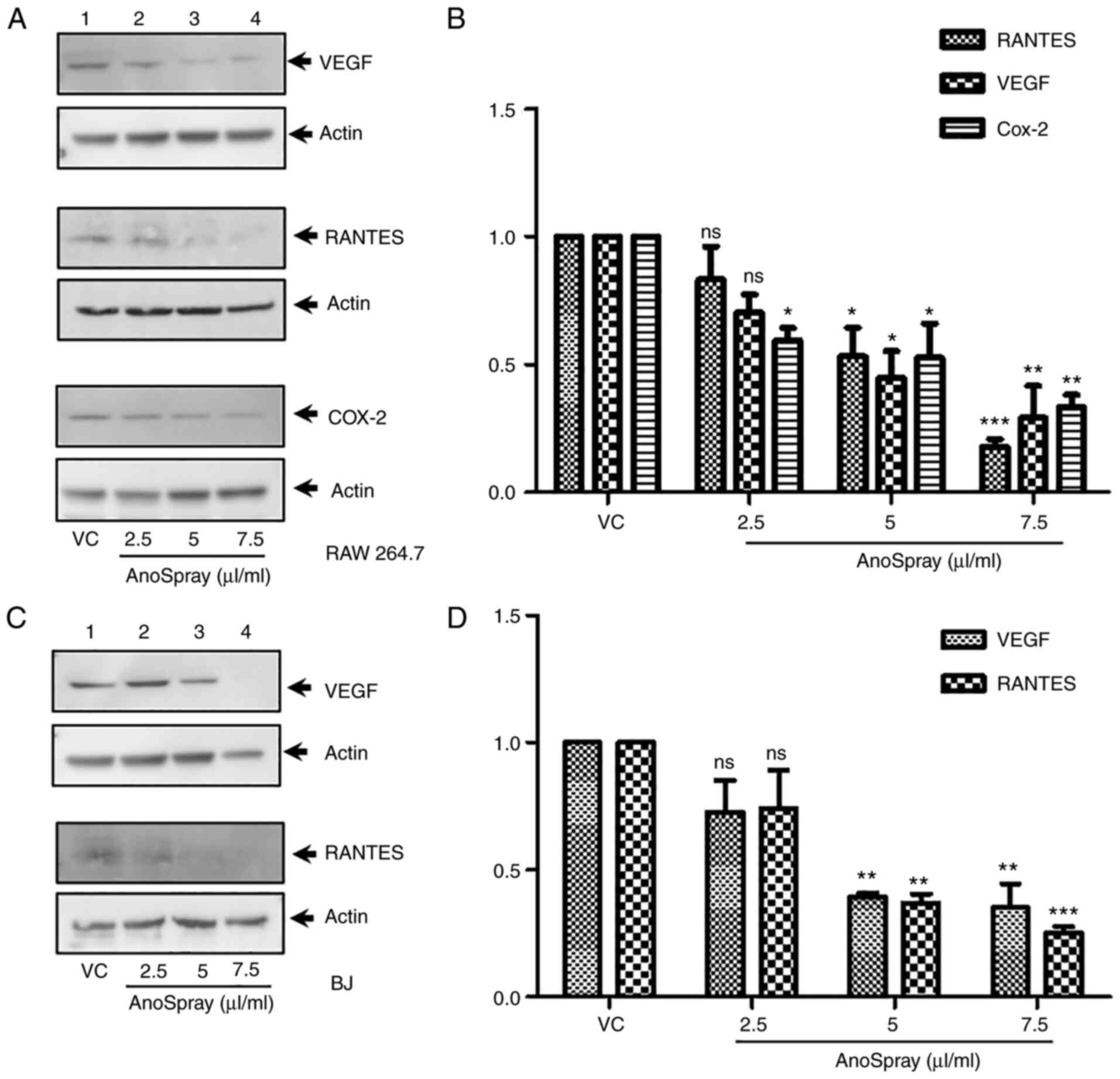 | Figure 3Effect of AnoSpray® on the
expression of proangiogenic and proinflammatory factors. RAW 264.7
and BJ cells were stimulated with VC/AnoSpray® (0-7.5
µg/ml) and immunoblotting was performed to determine the expression
of RANTES, VEGF and COX-2. (A) RAW 264.7 cells were treated with
AnoSpray® and the expression of VEGF, RANTES and COX-2
was determined by western blot. (B) Densitometry analysis was
performed to quantify western blot results for VEGF, RANTES and
COX-2 expression, presented in a bar graph. (C) BJ cells were
treated with AnoSpray® and the expression of VEGF and
RANTES was analyzed by western blot. (D) Densitometry analysis was
performed to quantify western blot data for the expression of VEGF
and RANTES, presented in a bar graph. Values are expressed as the
mean ± standard error of the mean (n=3). Statistical significance
was determined by one-way ANOVA. *P<0.05,
**P<0.01, ***P<0.001. ns, no
significance; VC, vehicle control; RANTES, regulated upon
activation, normal T cell expressed and presumably secreted; COX-2,
cyclooxygenase-2. |
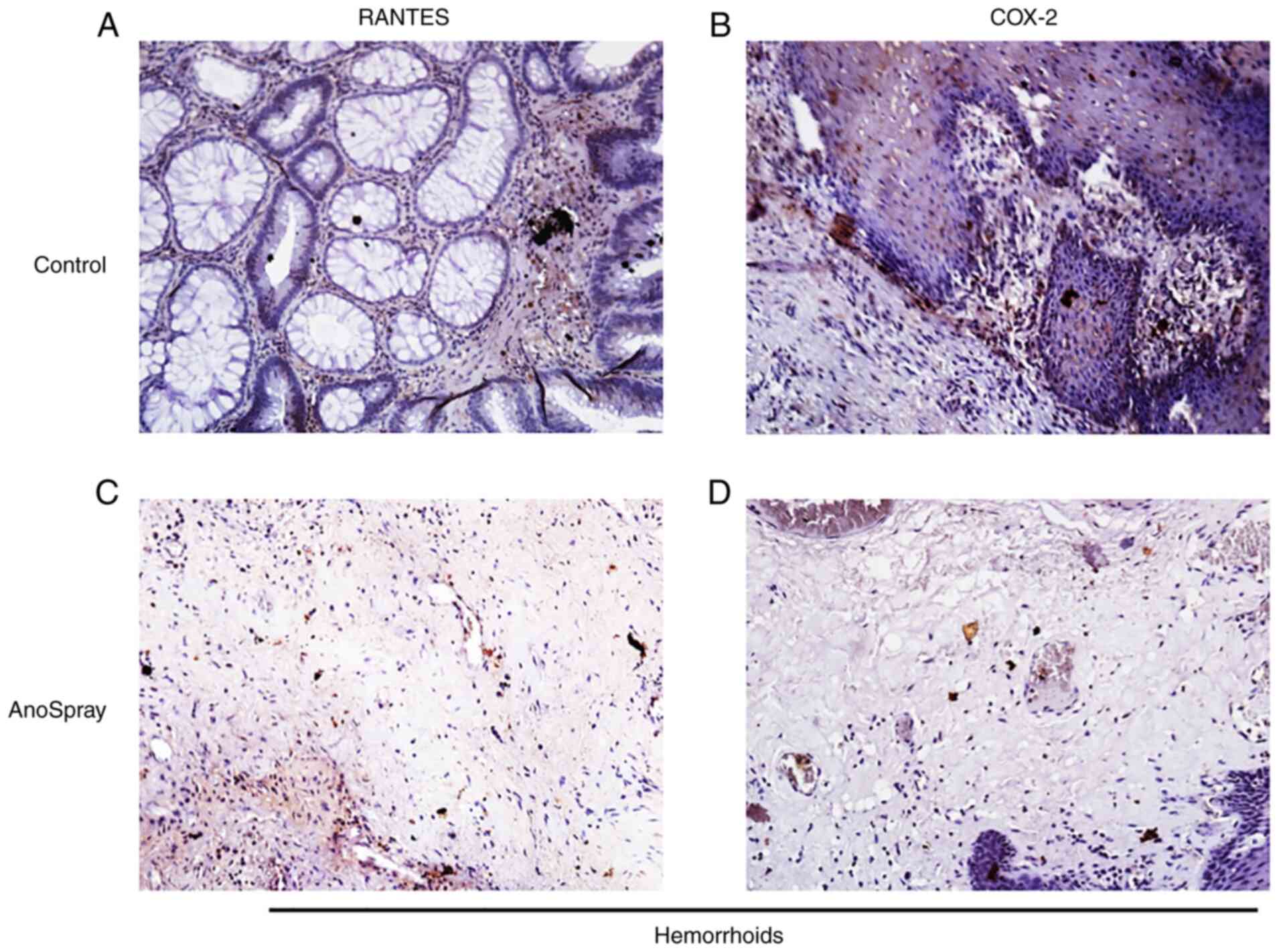
AnoSpray suppresses the expression of
COX-2 and RANTES
To further corroborate the in vitro results
in clinical specimens, the expression levels of the proinflammatory
cytokines RANTES and COX-2 were examined in hemorrhoidal tissues,
where they are known to induce pathological pain. Hemorrhoidal
patients were treated with AnoSpray for at least 15 days. The
expression levels of RANTES as well as COX-2 were examined in both
control (n=5) and AnoSpray-treated (n=5) human hemorrhoid specimens
using immunohistochemical analysis. The results of these
experiments indicated that RANTES and COX-2 were highly expressed
in hemorrhoidal tissues (Fig. 4A
and B). Furthermore, it was noted
that the expression of RANTES and COX-2 was decreased in
AnoSpray-treated (n=5) clinical hemorrhoid specimens (Fig. 4C and D). These results clearly indicated that
AnoSpray treatment suppressed the expression of proinflammatory
cytokines in hemorrhoids.
Discussion
Hemorrhoids, anal fissures and fistulas are common
benign anorectal diseases (22).
These pathological ailments significantly impact the lifestyles of
patients afflicted with these diseases, and primary or secondary
medical care is usually required, depending on the severity of the
disease. Grade I and, in certain cases, grade II hemorrhoids may be
treated with dietary and lifestyle modifications or medical
treatment options such as sclerotherapy. However, high-grade
hemorrhoids require highly invasive surgical options (10,53),
which are usually associated with postoperative pain, bleeding and
fecal urgency (15,16,54).
Furthermore, owing to the high recurrence rates that ensue after
performing these procedures, these surgical methods only have
partial success (15,16). Anal fissures have several symptoms
that are similar to those of hemorrhoids, including severe pain.
Anal fissures may be divided into acute and chronic categories.
Acute fissures may be effectively treated with conservative
therapies (55), whereas chronic
fissures typically require medical management or surgical therapy
(20). Invasive interventions have
greater healing rates compared with the administration of local
medical therapies; however, they are associated with a risk of
persistent incontinence (20).
Anal fistulas may be treated with operative procedures; however,
recurrence and the formation of perineal wounds limit the success
of operational procedures (24).
In addition, the surgical management of anorectal conditions is
both invasive and associated with higher costs (2,14).
It was observed that delayed wound healing due to inflammation is
responsible for disease-associated morbidities, including pain.
Therefore, developinging novel minimally invasive and economical
therapeutic interventions that exhibit inflammation and
pain-suppressive activities may be beneficial for the betterment of
the lives of patients with anorectal diseases.
Natural products are an important source of
bioactive compounds and have been used since ancient times for the
treatment of various diseases (56). Natural products with medicinal
properties are also able to facilitate the wound-healing process
(32). Several studies on the
wound-healing activities of natural products have been performed.
Herbal products with anti-inflammatory, antioxidant, antibacterial
and pro-collagen synthesis properties have been indicated to elicit
positive effects on wound healing. The medicinal properties of
herbal products may be attributable to the presence of various
bioactive phytochemical constituents, including alkaloids, oils,
flavonoids, tannins, terpenoids, saponins and phenolic compounds
(57). Each bioactive agent may
have a specific function in relation to the different aspects of
the wound-healing process. For instance, saponins are able to
augment the synthesis of pro-collagen from fibroblasts, whereas
tannins and flavonoids have antiseptic and antibacterial
properties, respectively (32,58,59).
Hence, these phytochemicals may regulate one or more aspect(s) of
the wound-healing process and these components may be easily
absorbed by the outer layers of the skin (60). Owing to their anti-inflammatory,
wound healing and analgesic properties, herbal products have
emerged as an important therapeutic option for the treatment of
numerous diseases of different severity. In addition to their
biological activity, they also potentially provide important leads
for the design of novel synthetic compounds (31,46).
A previous study by our group reported that AnoSpray exhibits
wound-healing effects on perineal wounds without causing any side
effects (37). However, the
mechanism underlying this healing activity on perineal wounds was
not identified in that study. The present study demonstrated that
AnoSpray suppresses the migration of fibroblasts and macrophages,
as well as by reducing the expression levels of the proinflammatory
cytokines RANTES and VEGF. COX-2 fulfills a crucial role in
mitigating acute pain by regulating prostaglandin production and
COX-2 inhibitors have been widely used for treating pathological
pain associated with numerous diseases (61,62).
In the present study, it was determined that AnoSpray reduced the
expression of COX-2 in fibroblasts and macrophages. Of note, the
present clinical data also indicated that the expression levels of
COX-2 and RANTES were downregulated in AnoSpray-treated
hemorrhoids. In addition, AnoSpray did not have any apparent
effects on the viability of macrophages and fibroblasts. The major
limitation of the present study was that the expression of RANTES
and COX-2 in anorectal disease tissues was not compared with that
in control anorectal tissues. AnoSpray is a polyherbal formulation
comprising B. aristata, S. racemosa, B. ceiba,
S. indicum, G. indica, C. camphora and M.
piperita extracts. B. aristata and S. racemosa
that was previously reported to exhibit antioxidant,
anti-inflammatory, antiangiogenic and wound-healing properties
(63,64). Another study also reported that
normal and delayed wound healing was enhanced by sesamol derived
from S. indicum in albino rats (65). As a traditional medicine, B.
ceiba has been used in the healing of wounds, exhibiting
anti-inflammatory, antioxidant and antidiabetic activities
(66). An earlier study indicated
that Kokum butter, which is derived from G. indica and has
traditionally been employed for the treatment of wounds and
fissures in hands, restores the elasticity of the skin, acting as a
moisturizer (67). The leaves of
C. camphora have been employed as a therapeutic option for
the treatment of various skin disorders, anti-inflammatory
disorders and antimicrobial diseases, in view of its antioxidant
activities (68). Furthermore, an
extract of C. camphora leaves promoted wound-healing
activity in rats (68). Modarresi
et al (69) demonstrated
that the topical application of essential oil derived from M.
piperita augmented wound healing in an infected mouse model. It
is hypothetically possible that the polyherbal formulation of
AnoSpray acts at different phases of inflammation, wherein pain is
induced or where healing has been initiated, thereby leading to an
improvement in patients' lives. The present study highlighted that
AnoSpray is both safe to use and therapeutically effective in
treating anal fissures, bleeding hemorrhoids and perineal
wounds.
In conclusion, the present study suggested that
AnoSpray does not exhibit any cytotoxic effects on macrophages or
fibroblasts, thereby demonstrating that it is safe to use.
Furthermore, its administration leads to a significant attenuation
of the migration of these cells and also suppresses the expression
of proinflammatory mediators and COX-2, both in vitro and in
clinical specimens. The results of the present study highlighted
the potential implications of AnoSpray as a means of therapy for
clinically controlling bleeding hemorrhoids and in the treatment of
anal fissures and perineal wounds.
Acknowledgements
The authors would like to thank Dr Snehal Porwal,
Founder and Director, Healing Hands and Herbs Pvt. Ltd. (Pune,
India) for valuable support.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The study was conceived and designed by AP, GCK, RB
and GB. Herbal materials were prepared and supplied by AP, GB and
RB. Experiments were performed by RB. The data were analysed and
the manuscript was written and edited by RB, AP, GB and GCK. RB,
AP, GB and GCK confirmed the authenticity of the data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Ethics
Committee of Healing Hands Clinic (Pune, India). Human hemorrhoid
specimens were collected from Healing Hands Clinic (Pune, India)
with informed consent.
Patient consent for publication
Not applicable.
Competing interests
GB and RB are employees of Healing Hands & Herbs
Pvt. Ltd., who provided the pharamceutical product used in this
study.
References
|
1
|
Foxx-Orenstein AE, Umar SB and Crowell MD:
Common anorectal disorders. Gastroenterol Hepatol (NY). 10:294–301.
2014.PubMed/NCBI
|
|
2
|
Hollingshead JR and Phillips RK:
Haemorrhoids: Modern diagnosis and treatment. Postgrad Med J.
92:4–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mott T, Latimer K and Edwards C:
Hemorrhoids: Diagnosis and treatment options. Am Fam Physician.
97:172–179. 2018.PubMed/NCBI
|
|
4
|
Guindic LC: Treatment of uncomplicated
hemorrhoids with a Hemor-Rite® cryotherapy device: A
randomized, prospective, comparative study. J Pain Res. 7:57–63.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lorenzo-Rivero S: Hemorrhoids: Diagnosis
and current management. Am Surg. 75:635–642. 2009.PubMed/NCBI
|
|
6
|
Sun Z and Migaly J: Review of hemorrhoid
disease: Presentation and management. Clin Colon Rectal Surg.
29:22–29. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chong PS and Bartolo DC: Hemorrhoids and
fissure in ano. Gastroenterol Clin North Am. 37:627–644, ix.
2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jacobs D: Clinical practice. Hemorrhoids.
N Engl J Med. 371:944–951. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Riss S, Weiser FA, Schwameis K, Riss T,
Mittlböck M, Steiner G and Stift A: The prevalence of hemorrhoids
in adults. Int J Colorectal Dis. 27:215–220. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lohsiriwat V: Hemorrhoids: From basic
pathophysiology to clinical management. World J Gastroenterol.
18:2009–2017. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Morgado PJ, Suárez JA, Gómez LG and
Morgado PJ Jr: Histoclinical basis for a new classification of
hemorrhoidal disease. Dis Colon Rectum. 31:474–480. 1988.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Han W, Wang ZJ, Zhao B, Yang XQ, Wang D,
Wang JP, Tang XY, Zhao F and Hung YT: Pathologic change of elastic
fibers with difference of microvessel density and expression of
angiogenesis-related proteins in internal hemorrhoid tissues.
Zhonghua Wei Chang Wai Ke Za Zhi. 8:56–59. 2005.PubMed/NCBI(In Chinese).
|
|
13
|
Yoon SO, Park SJ, Yun CH and Chung AS:
Roles of matrix metalloproteinases in tumor metastasis and
angiogenesis. J Biochem Mol Biol. 36:128–137. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zagriadskiĭ EA, Bogomazov AM and Golovko
EB: Conservative treatment of hemorrhoids: Results of an
observational multicenter study. Adv Ther. 35:1979–1992.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cerato MM, Cerato NL, Passos P, Treigue A
and Damin DC: Surgical treatment of hemorrhoids: A critical
appraisal of the current options. Arq Bras Cir Dig. 27:66–70.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Santos GD, Coutinho CP, Meyer MM, Sampaio
DV and Cruz GM: Surgical complications in 2,840 cases of
hemorrhoidectomy by Milligan-Morgan, Ferguson and combined
techniques. J Coloproctol (Rio J). 32:271–290. 2012.
|
|
17
|
Higuero T: Update on the management of
anal fissure. J Visc Surg. 152 (Suppl 2):S37–S43. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Villalba H, Villalba S and Abbas MA: Anal
fissure: A common cause of anal pain. Perm J. 11:62–65.
2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Notaras MJ: Anal fissure and stenosis.
Surg Clin North Am. 68:1427–1440. 1988.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tranqui P, Trottier DC, Victor C and
Freeman JB: Nonsurgical treatment of chronic anal fissure:
Nitroglycerin and dilatation versus nifedipine and botulinum toxin.
Can J Surg. 49:41–45. 2006.PubMed/NCBI
|
|
21
|
Nottingham JM and Rentea RM: Anal
Fistulotomy (Seton Placement). In: StatPearls. StatPearls
Publishing, Treasure Island, FL, 2020.
|
|
22
|
Gardner IH, Siddharthan RV and Tsikitis
VL: Benign anorectal disease: Hemorrhoids, fissures, and fistulas.
Ann Gastroenterol. 33:9–18. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sharma RK and Parashar A: The management
of perineal wounds. Indian J Plast Surg. 4:352–363. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Whiteford MH: Perianal abscess/fistula
disease. Clin Colon Rectal Surg. 20:102–109. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bullard KM, Trudel JL, Baxter NN and
Rothenberger DA: Primary perineal wound closure after preoperative
radiotherapy and abdominoperineal resection has a high incidence of
wound failure. Dis Colon Rectum. 48:438–443. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Musters GD, Sloothaak DA, Roodbeen S, van
Geloven AA, Bemelman WA and Tanis PJ: Perineal wound healing after
abdominoperineal resection for rectal cancer: A two-centre
experience in the era of intensified oncological treatment. Int J
Colorectal Dis. 29:1151–1157. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Althumairi AA, Canner JK, Gearhart SL,
Safar B, Sacks J and Efron JE: Predictors of perineal wound
complications and prolonged time to perineal wound healing after
abdominoperineal resection. World J Surg. 40:1755–1762.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Christian CK, Kwaan MR, Betensky RA, Breen
EM, Zinner MJ and Bleday R: Risk factors for perineal wound
complications following abdominoperineal resection. Dis Colon
Rectum. 48:43–48. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Artioukh DY, Smith RA and Gokul K: Risk
factors for impaired healing of the perineal wound after
abdominoperineal resection of rectum for carcinoma. Colorectal Dis.
9:362–367. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chang CC, Lan YT, Jiang JK, Chang SC, Yang
SH, Lin CC, Lin HH, Wang HS, Chen WS, Lin TC and Lin JK: Risk
factors for delayed perineal wound healing and its impact on
prolonged hospital stay after abdominoperineal resection. World J
Surg Oncol. 17(226)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Atanasov AG, Waltenberger B,
Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L,
Schwaiger S, Heiss EH, et al: Discovery and resupply of
pharmacologically active plant-derived natural products: A review.
Biotechnol Adv. 33:1582–1614. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ibrahim N', Wong SK, Mohamed IN, Mohamed
N, Chin KY, Ima-Nirwana S and Shuid AN: Wound healing properties of
selected natural products. Int J Environ Res Public Health.
15(2360)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Santana FP, Pinheiro NM, Mernak MI,
Righetti RF, Martins MA, Lago JH, Lopes FD, Tibério IF and Prado
CM: Evidences of herbal medicine-derived natural products effects
in inflammatory lung diseases. Mediators Inflamm.
2016(2348968)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Llorián-Salvador M and González-Rodríguez
S: Painful understanding of VEGF. Front Pharmacol.
9(1267)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sobolewski C, Cerella C, Dicato M,
Ghibelli L and Diederich M: The role of cyclooxygenase-2 in cell
proliferation and cell death in human malignancies. Int J Cell
Biol. 2010(215158)2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cragg GM and Newman DJ: Natural products:
A continuing source of novel drug leads. Biochim Biophys Acta.
1830:3670–3695. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Porwal A, Gandhi P and Kulkarni D: An open
label, randomized, comparative, parallel group, single center study
to evaluate safety and efficacy of Ano spray in a comparison with
betadine solution application in acute perineum wounds. Ayushdhara.
4:1409–1412. 2017.
|
|
38
|
Kumar D, Haldar S, Gorain M, Kumar S,
Mulani FA, Yadav AS, Miele L, Thulasiram HV and Kundu GC:
Epoxyazadiradione suppresses breast tumor growth through
mitochondrial depolarization and caspase-dependent apoptosis by
targeting PI3K/Akt pathway. BMC Cancer. 18(52)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chakraborty G, Jain S and Kundu GC:
Osteopontin promotes vascular endothelial growth factor-dependent
breast tumor growth and angiogenesis via autocrine and paracrine
mechanisms. Cancer Res. 68:152–161. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kumar D, Kumar S, Gorain M, Tomar D, Patil
HS, Radharani NN, Kumar TV, Patil TV, Thulasiram HV and Kundu GC:
Notch1-MAPK signaling axis regulates CD133+ cancer stem
cell-mediated melanoma growth and angiogenesis. J Invest Dermatol.
136:2462–2474. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Landén NX, Li D and Ståhle M: Transition
from inflammation to proliferation: A critical step during wound
healing. Cell Mol Life Sci. 73:3861–3885. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Guo S and Dipietro LA: Factors affecting
wound healing. J Dent Res. 89:219–229. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen S and Yu Q: A new theory on the cause
of anal fissure-impaction theory. J Coloproctol (Rio J).
40:321–325. 2020.
|
|
44
|
Segre D, Dal Corso HM, Landra M and
Giuffrida MC: Management of the unhealed perineal wound. In:
Inflammatory bowel disease and familial adenomatous polyposis.
Springer, Milano, pp463-472, 2006.
|
|
45
|
Bertone AL: Principles of wound healing.
Vet Clin North Am Equine Pract. 5:449–463. 1989.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Derakhshan AR: Natural treatments for
fissure in ano used by traditional persian scholars, Razi (Rhazes)
and Ibn Sina (Avicenna). J Evid Based Complementary Altern Med.
22:324–333. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Dey YN, Wanjari MM, Kumar D, Lomash V and
Jadhav AD: Curative effect of Amorphophallus paeoniifolius tuber on
experimental hemorrhoids in rats. J Ethnopharmacol. 192:183–191.
2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Azeemuddin M, Viswanatha GL, Rafiq M,
Thippeswamy AH, Baig MR, Kavya KJ, Patki PS and Shyam R: An
improved experimental model of hemorrhoids in rats: Evaluation of
antihemorrhoidal activity of an herbal formulation. ISRN Pharmacol.
2014(530931)2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wojdasiewicz P, Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014(561459)2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sprague AH and Khalil RA: Inflammatory
cytokines in vascular dysfunction and vascular disease. Biochem
Pharmacol. 78:539–552. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Fitzgerald SM, Lee SA, Hall HK, Chi DS and
Krishnaswamy G: Human lung fibroblasts express interleukin-6 in
response to signaling after mast cell contact. Am J Respir Cell Mol
Biol. 30:585–593. 2004.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Arango Duque G and Descoteaux A:
Macrophage cytokines: Involvement in immunity and infectious
diseases. Front Immunol. 5(491)2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lohsiriwat V: Treatment of hemorrhoids: A
coloproctologist's view. World J Gastroenterol. 21:9245–9252.
2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Mounsey AL, Halladay J and Sadiq TS:
Hemorrhoids. Am Fam Physician. 84:204–210. 2011.PubMed/NCBI
|
|
55
|
Bhardwaj R and Parker MC: Modern
perspectives in the treatment of chronic anal fissures. Ann R Coll
Surg Engl. 89:472–478. 2007.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Dias DA, Urban S and Roessner U: A
historical overview of natural products in drug discovery.
Metabolites. 2:303–336. 2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Thakur R, Jain N, Pathak R and Sandhu SS:
Practices in wound healing studies of plants. Evid Based Complement
Alternat Med. 2011(438056)2011.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Chandel RS and Rastogi RP: Triterpenoid
saponins and sapogenins: 1973-1978. Phytochemistry. 19:1889–1908.
1980.
|
|
59
|
Harbone JB: Phytochemical methods: A guide
to modern techniques of plants analysis. Fakenham Press Limited:
New York, NY, USA, 1973.
|
|
60
|
Tsala DE, Amadou D and Habtemariam S:
Natural wound healing and bioactive natural products.
Phytopharmacology. 4:532–560. 2013.
|
|
61
|
Ricciotti E and FitzGerald GA:
Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol.
31:986–1000. 2011.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Merecz-Sadowska A, Sitarek P, Śliwiński T
and Zajdel R: Anti-inflammatory activity of extracts and pure
compounds derived from plants via modulation of signaling pathways,
especially PI3K/AKT in macrophages. Int J Mol Sci.
21(9605)2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Potdar D, Hirwani RR and Dhulap S:
Phyto-chemical and pharmacological applications of Berberis
aristata. Fitoterapia. 83:817–830. 2012.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Acharya N, Acharya S, Shah U, Shah R and
Hingorani L: A comprehensive analysis on Symplocos racemosa
Roxb.: Traditional uses, botany, phytochemistry and pharmacological
activities. J Ethnopharmacol. 181:236–251. 2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Shenoy RR, Sudheendra AT, Nayak PG, Paul
P, Kutty NG and Rao CM: Normal and delayed wound healing is
improved by sesamol, an active constituent of Sesamum
indicum (L.) in albino rats. J Ethnopharmacol. 133:608–612.
2011.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Rameshwar V, Kishor D, Tushar G, Siddharth
G and Sudarshan G: A pharmacognostic and pharmacological overview
on Bombax ceiba. Sch Acad J Pharm. 3:100–107. 2014.
|
|
67
|
Ananthakrishnan R and Rameshkumar KB:
Phytochemicals and bioactivities of Garcinia indica
(thouars) choisy-a review. Divers Garcinia Species West Ghats:
Phytochem Perspect. 142:151–161. 2016.
|
|
68
|
Sen PK and Garg S: Wound repair and
regenerating effect of ethyl acetate soluble fraction of ethanolic
extract of Cinnamomum camphora leaves in wistar albino rats.
J Drug Deliv Ther. 9:1173–1176. 2019.
|
|
69
|
Modarresi M, Farahpour MR and Baradaran B:
Topical application of Mentha piperita essential oil
accelerates wound healing in infected mice model.
Inflammopharmacology. 27:531–537. 2019.PubMed/NCBI View Article : Google Scholar
|