Introduction
The current approach to flexor tendon injuries is
complex and is no longer limited to suturing techniques (1). A retrospective study performed in the
United States over a 10 year period estimates that the incidence of
acute flexor tendon injury is ~33.2/100,000 individuals per year
(2). Tendons heal through a
combination of two mechanisms: Intrinsic and extrinsic. Both follow
similar stages to other tissues including inflammation lasting
48-72 h, fibroblast proliferation, collagen production for 3-4
weeks, and remodeling involving cross-linking and reduction of type
III collagen with fiber reorientation (3). Extrinsic healing is characterized by
rapid influx of fibroblasts from the peritendinous tissues, which
favors the formation of adhesions, and intrinsic healing occurs
through fibroblasts originating in the endotendon, without the
formation of adhesions and which by early mobilization stimulates
the synovial pumps, with a higher resistance on the suture line
(4).
The most difficult tendon injury is zone II in which
the adhesions of the flexor tendon radically influence the function
of the hand. In zone II, located from the metacarpo-phalangeal
joint to the base of the middle phalanx, is a narrow osteofibrous
canal through which the two flexor tendons of the fingers pass.
Tendon repair in this area predisposes to the formation of
adhesions, as well as the appearance of diastasis (a weakening of
the sutures with the removal of the tendon ends and not a complete
rupture of them) or rupture. All this can compromise the
postoperative functional result (5).
Strategies to improve hand function currently
include postoperative rehabilitation protocols, appropriate
suturing materials and techniques, changing the gliding surface by
using lubricants and providing growth factors (4). Primary repair is indicated because
secondary interventions involve difficult dissection of the
vascular and nervous elements surrounded by fibrosis (6). When choosing a type of suture it must
meet certain conditions, including: i) Be easy to perform; ii)
reduce tendon manipulation; iii) minimize the amount of foreign
material on the surface of the tendon; and iv) ensure sufficient
strength for early mobilization (6). Regarding the manipulation of the
biological environment, it has been studied on experimental models
at the cellular, molecular and genetic levels, but they do not yet
have large-scale clinical applicability, as described in a previous
study by Evans (7). Reducing the
abrasion resistance by modifying the exogenous surface can be
achieved by applying lubricants, such as hyaluronic acid, lubricin
and phospholipids (8-11).
Lubricin is a unique proteoglycan found in the fluid
that lubricates the surface of intrasynovial tendons and it has
been used in combination with hyaluronic acid to prevent synovial
cell overgrowth. When used alone and not in combination with
hyaluronic acid there were found to be no significant differences
in adhesion prevention (12,13).
The effects of hyaluronic acid on adhesions that
form in the digital channel after reconstruction have been tested
since the 1980s, when St Onge et al (14) applied a paste based on sodium
hyaluronate to the repaired deep flexor tendon of fingers in
monkeys. They compared the functional results with the control
group to which saline was applied and found an improvement in total
active motion in the studied group (14). The disadvantage regarding the
application of hyaluronic acid on tendons is its rapid degradation
by the body (~7 days), thus it requires repeated injection
(15).
Another product, originally used in spinal surgery,
has been shown to be effective in preventing postoperative
adhesions. It is a combination of carboxymethylcellulose and
polyethylene oxide-Dynavisc® (FzioMed, Inc.). It is a
transparent, synthetic, pure gel that does not contain animal
proteins and does not interfere with tissue healing unlike other
compounds of animal origin (16-18).
This gel has proven its effectiveness especially in spinal surgery
and neurolysis (surgical mobilization of the adherent nerve)
(19-23)
and has not been used in acute tendon injuries, opening up a
research perspective in this regard. The aim of the present study
was to test the effect of Dynavisc® on acute injuries of
the intrasynovial flexor tendons in the prevention of postoperative
adhesions and the improvement of functional results.
Materials and methods
Ethical approval
This study was approved by the Ethics Commission of
‘Grigore T. Popa’ University of Medicine and Pharmacy (Iasi,
Romania; approval no. 20311/26.09.2019) in compliance with ethical
and deontological rules for medical and research practice (24).
Experimental conditions
The experimental study was performed on 20 adult
male Wistar laboratory rats (age, 6 months) provided from the
Romanian National Institute of Research, weighing 300-350 g
distributed in two groups. The housing conditions were as follows:
i) Temperature average, 21˚C; ii) humidity, 46-65%; and iii)
light/dark cycle, 12/12 h. Each animal had acces to food and water,
and was kept in their own cage on the first postoperative day.
The control group, represented by 10 rats, in which
after the reconstruction of the flexor tendon, the peritendinous
area was injected with saline solution and the experimental group,
in which the peritendinous area was injected with a single
administration of lubricating gel, Dynavisc®
(carboxymethylcellulose and polyethylene oxide). A total of five
subjects from each group were sacrificed at 4 and 12 weeks when a
biopsy was taken from the operated tendon. The evaluation of the
results was performed by measuring the adhesion score (presence of
peritendinous conjunctival hyperplasias marked with none, one, two
or three +) and by observing histological parameters.
Operative protocol
General anesthesia was performed preceded by
intramuscular sedation. Xylazine (2%) was used for sedation (0.1
µg/100 g animal), then ketamine (1 mg/ml-0.1 µg/100 g animal) was
injected every 10-15 min.
All substances were injected intramusculary on the
thigh opposite the operated limb. In addition, for postoperative
pain control, bupivacaine (0.3 µg/animal) was administered
topically at a single dose to each experimental animal.
The animals were placed in a prone position and one
of the hind limbs was disinfected with Betadine and then isolated
with a sterile field. A longitudinal incision was made in the
radius of finger three from the base to the tip of the finger with
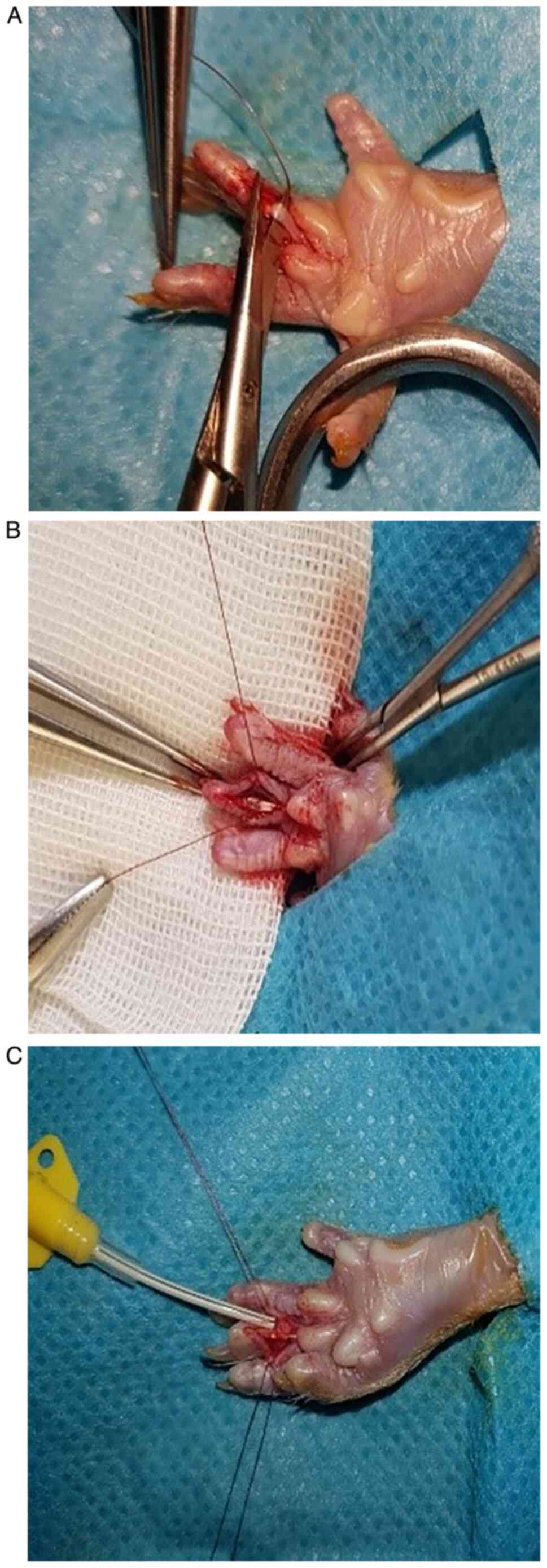
the exposure of the deep flexor tendon. Under magnification the
flexor tendon and pulleys were sectioned, and then sutured with 9-0
thread using the modified Kessler technique (25) augmented with an epitendinous suture
(Fig. 1). In the control group, the
perilesional area and the digital osteo-fibrous canal were injected
with 0.9% saline and in the experimental group, rats were injected
with Dynavisc® (0.05 ml each tendon). The lubricant was
injected through a catheter. Then, the skin was sutured with 7-0
thread and a hydrofilm foil was applied to protect the wound.
Histology
At 4 and 12 weeks, the rats were sacrificed by an
overdose of anesthetic. Halothane (1-5 ml/30-150 g) was used in a
50 ml tube with some tissue to absorb easily and the head was
placed inside the tube. The rats were placed in a closed chamber
for 10 min.
Tissue biopsy consisted of tendon fragments and
adjacent tissue. Immediately after harvesting, the tissue fragments
were fixed in 10% neutral buffered formalin solution for 48 h at
room temperature, then processed by paraffin embedding method
(Leica TP1020; Leica Microsystems GmbH) and sectioned
longitudinally (microtome RM 2235; Leica Microsystems GmbH), to a
thickness of 5 µm. The sections were stained using Masson's stain
at room temperature and examined with a photonic microscope (Leica
DM 1000; Leica Microsystems GmbH), using a digital histological
camera (Leica 5 mpx, full HD; Leica Microsystems GmbH) and LAS
software, version 2016 (Leica Microsystems GmbH) to capture images.
Tendon healing was assessed by using the following indicators: i)
The degree of proliferation of local tenocytes and fibroblasts; ii)
the synthesis of collagen fibers in the perimeter of the tendon
(actual regeneration of the tendon); iii) the presence of
peritendinous conjunctival hyperplasia (fibrous adhesions); and iv)
the presence of peritendinous space with the restoration of the
external sheath of the tendon (epitenon) and the peritendinous
synovial sheath.
Statistical analysis
For statistical analysis SPSS software version 19
(Statistical Packages for the Social Sciences), was used. The
incidence of tendon rupture and the score of adhesions were
calculated using the Fisher's exact test. The quantitative data are
presented as the mean ± SD (the presence of microscopic adhesions).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical observation
In the control group a single rupture at 12 weeks
and two tendons with diastasis at 4 weeks were recorded, while in
the experimental group treated with Dynavisc®, three
rats with diastasis (two in the 12 weeks group) and a single
rupture in the group of 12 weeks were identified (Table I). The Fisher's exact test showed no
significant differences between the two groups (P=0.871).
 | Table IPresence of rupture and diastasis in
the two groups. |
Table I
Presence of rupture and diastasis in
the two groups.
| | Control group, n
(%) | Experimental group, n
(%) |
|---|
| Complications | 4 weeks (n=5) | 12 weeks (n=5) | 4 weeks (n=5) | 12 weeks (n=5) |
|---|
| Diastasis | 2(20) | 0 (0) | 1(10) | 2(20) |
| Rupture | 0 (0) | 1(10) | 0 (0) | 1(10) |
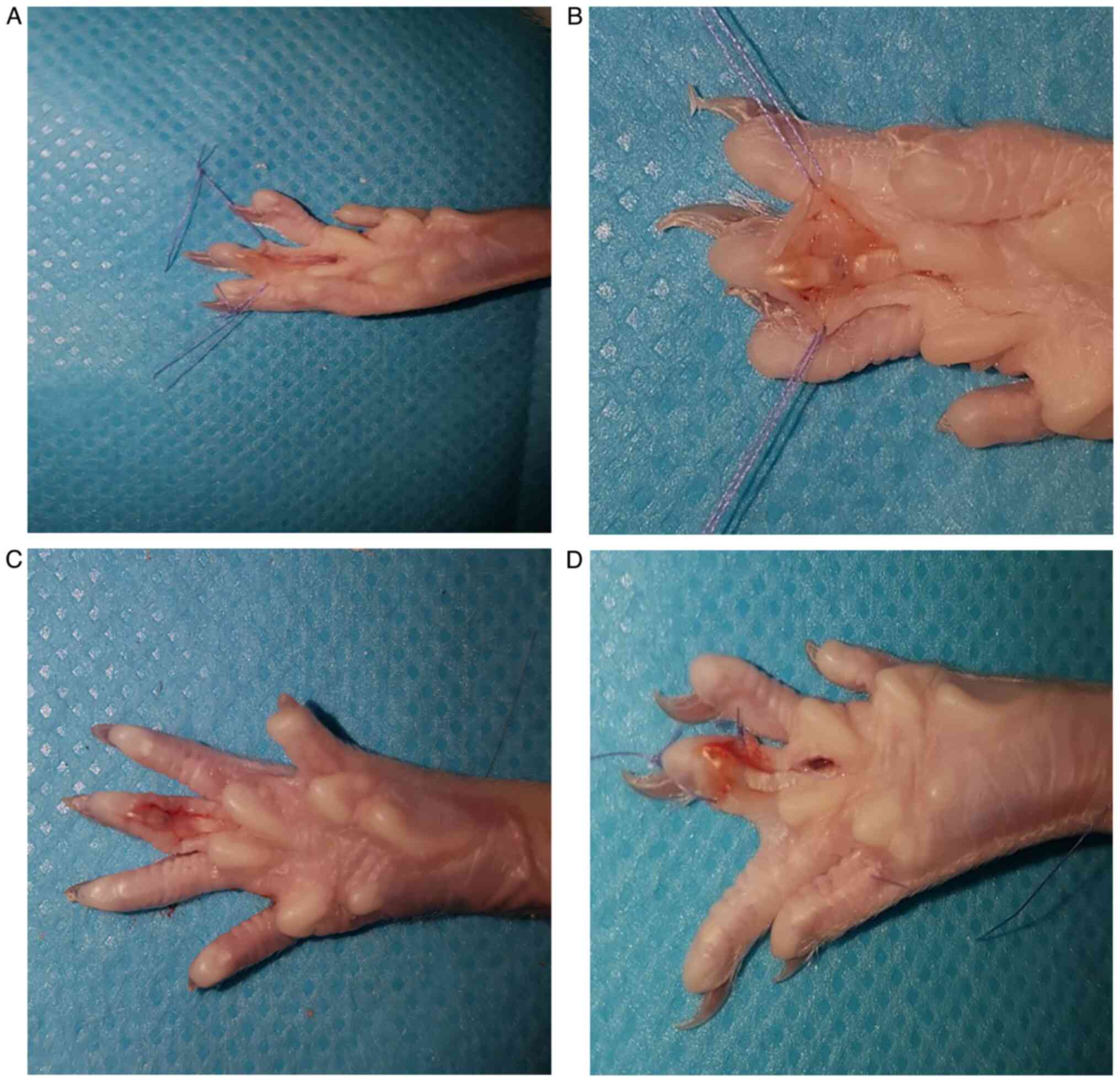
At the time of the biopsy, from an observational
point of view, the presence of important adhesions were found in
the control group, with infiltration of the tendon and adjacent
tissues, which made it difficult to perform the biopsy, and a
rough, opaque tendon with a low degree of mobility. In the
experimental group, the presence of a supple and smooth tendon,
with significantly less adhesions, was observed (Fig. 2).
Histology
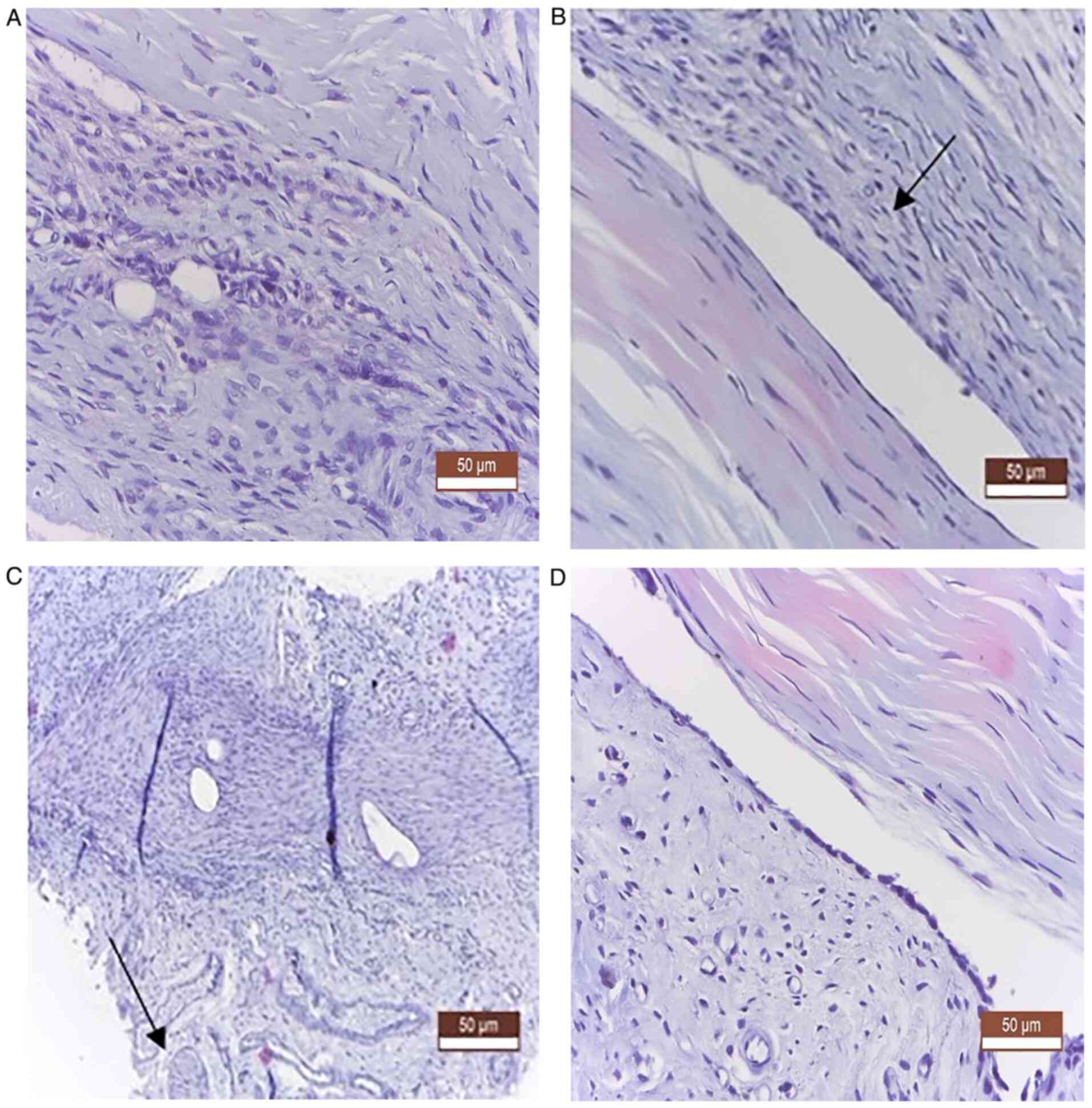
From a histological point of view, in both groups,
at 4 and 12 weeks, no notable differences were observed in terms of
the actual recovery of the tendon. Notable fibrosis in the
peritendinous tissue was noted in the control group compared with
the experimental group. The presence of adhesions was notably lower
in the experimental group, where a higher regeneration process was
observed, characterized by much lower fibrous synechiae, which was
absent in some animals, with the formation of a peritendinous
fibrous tunnel, as a synovial sheath (Fig. 3).
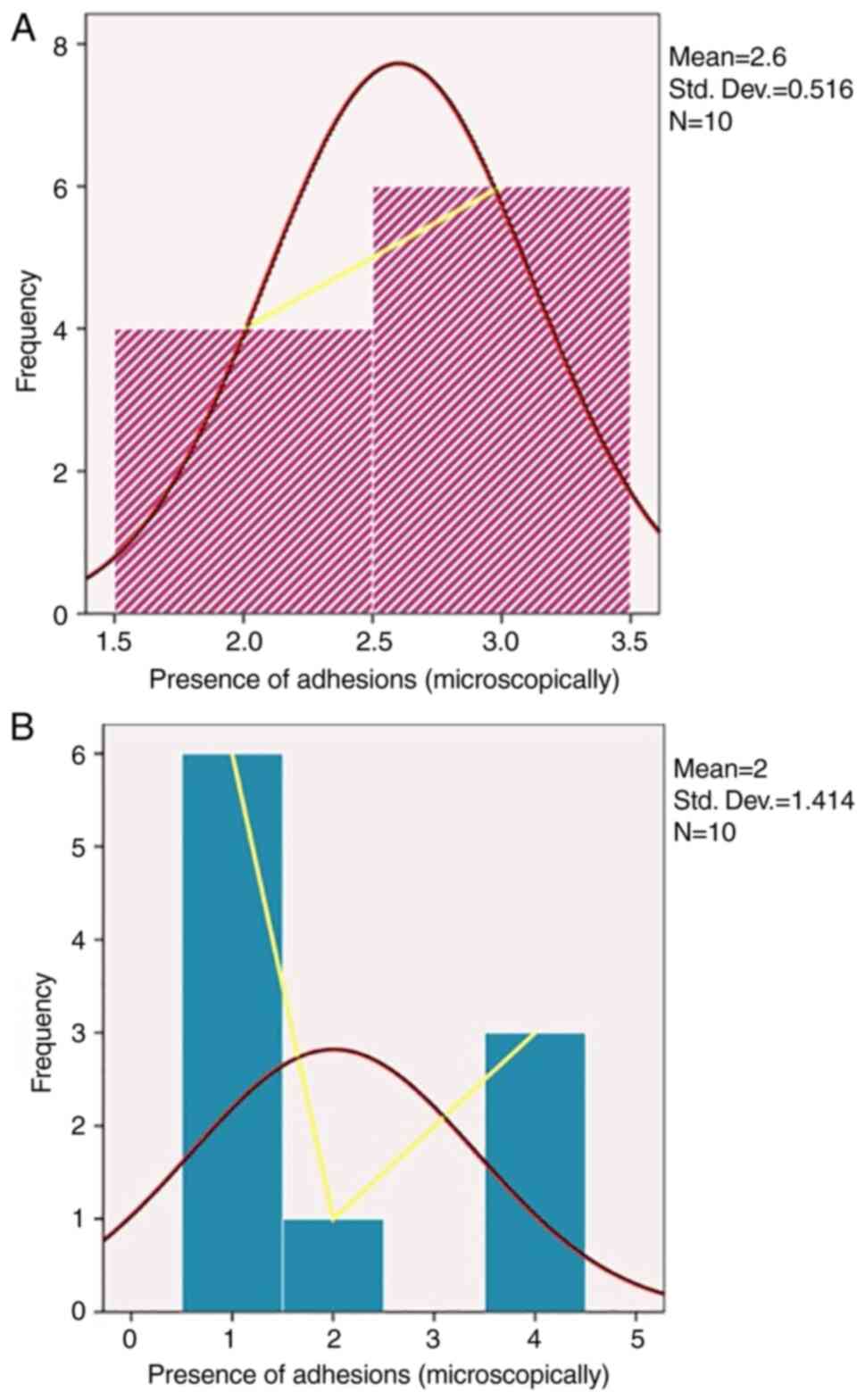
Regarding frequency distribution of adhesions, the
mean was 2.6 in the experimental group with a slight right-handed
distribution compared with the control group where the mean was 2
with a right-tailed distribution (Fig.
4).
Discussion
Over the years, there have been numerous attempts to
improve flexor tendon gliding and avoid complications by improving
suturing techniques and recovery therapy (26). Despite these efforts, Strickland
reported that between 30 and 40% heal through adhesions formation,
which limits finger function (26).
Wong et al (27) described
the mechanism of adhesion formation on murine model and found that
although intrasynovial tendons heal like a classic wound through
inflamation, proliferation, synthesis and apoptosis, the greatest
cellular activity is in the surrounding tissue where substantial
scarring appears between tendon and the surrounding sheath
(28).
If in the past only the surgical technique was given
special importance, the current concepts also include the
manipulation of the biological and biochemical environment to
improve the response in intrinsic repair and diminish extrinsic
healing as well as efforts to reduce frictional resistance of the
tendon (28). Several adjuvants
have been used to find the answer for rapid recovery after tendon
surgery in experimental studies, but at present no human trials
have been conducted (29). These
adjuvants can be grouped in two categories: Drugs and barriers. The
most popular drugs that have been invesigated include non-steroidal
anti-inflammatory medications, 5-Fluorouracil, transforming growth
factor β inhibitors and lubricin. Among the barriers, the
hyaluronic acid membrane, hydroxyapatite and bovine pericardia have
been investigated the most (30-32).
Flexor tendons are intrasynovial tendons with a
surface covered with a lubricating fluid that ensures the gliding
in the narrow digital channel of the fingers, decreasing the
frictional resistance between it and its sheath (33). Hagberg et al (34) studied the composition of fluid in
the synovial sheath and found that it has a composition similar to
the synovial fluid of the joints, being rich in hyaluronic acid
with a role in lubrication and nutrition.
Hyaluronic acid has been shown to not influence
tendon healing and not increase the risk of diastasis or rupture.
This was proved by Özgenel and Etöz in a clinical study on 22
patients and they concluded that repetitive injections of
hyaluronic acid can improve clinical outcomes when compared with a
single dose administration (35).
The combination of
carboxymethylcellulose-polyethylene oxide (Dynavisc®)
has been shown to be safe and effective in spinal and abdominal
surgery and does not interfere with tissue healing. This has been
confirmed by both experimental and clinical studies that have
demonstrated the role of the barrier (36,37).
The product is not metabolized by the body as in the case of
hyaluronic acid so no reinjection is required.
In the present study, the activation and
proliferation of tenocytes and resident fibroblasts at both ends of
the tendon and the synthesis of new connective tissue between its
ends were noted in both groups, so Dynavisc® did not
inhibit tendon healing. In the control group, conjunctival
hyperplasia with a repairing role was more intense and at the same
time aberrant, occurring on the surface of the epitenon, invading
the adjacent anatomical structures, surrounding the peripheral
nerves. The synovial sheath disappeared by welding its two sheets
and the disappearance of the mesothelium (internal visceral and
parietal epithelium). There was also a large
epithelioid-inflammatory reaction around the sutures in the
thickness of the tendon. In the experimental group treated with
Dynavisc®, a superior regeneration process was observed,
characterized by much smaller fibrous synechiae, which was absent
in some animals, with the constitution of a peritendinous fibrous
tunnel with the role of synovial sheath. There were also areas of
fibrillar neogenesis from the epitenon, but smaller, in terms of
fibrous spicules or thin fibrous clamps.
It has been suggested that some products used to
prevent adhesions may interfere with scar reshaping and
complications. In the current study, the statistical analysis
showed no significant differences between the two groups, which
indicated that Dynavisc® did not influence the
occurrence of complications during surgery, in terms of tendon
healing.
From a histological point of view, in the present
study case, in both groups, both at 4 and 12 weeks, no notable
differences were noticed in terms of the actual recovery of the
tendon. However, significant fibrosis in the peritendinous tissue
was observed in the control group, compared with the experimental
group. The major differences were found at 12 weeks where, in the
control group, aberrant peritendinous conjunctival hyperplasias
were identified, with a fibrous block appearance, with welding the
body of the tendon at the adjacent tissues, also tightening
regional nerve fibers (peripheral nerves). The existence of a
peritendinous space with the role of synovial sheath was not
noticed. In the experimental group at 12 weeks, these adhesions
were notably reduced compared with the control group, so the
lubricant did not delay the remodeling of the tendon scar.
The results of the current study suggested that a
lack of lubricant administration leads to a high presence of
macroscopic and microscopic adhesions. To the best of our
knowledge, there are no other studies evaluating the effect of
Dynavisc® on the prevention of flexor tendon adhesions
after primary repair, which is one of the strengths of the present
study. In the future, this product could be tested in clinical
practice, opening novel perspectives for improving tendon gliding
after flexor tendon repair. The study has several limitations and
in the future it could be improved by quantitative assays,
particularly to evaluate the degree of proliferation of tenocytes
and fibroblasts, and collagen synthesis. Also, this study did not
include any biomechanical tests to evaluate the friction forces
necessary to mobilize the tendon inside the digital canal, a
parameter that can predict the limb function.
To conclude, biological approaches to prevent
adhesion formation represent the future of acute flexor tendon
surgery to obtain improved functional results. This study supported
the important role of biological lubricant in the regeneration, not
only of the tendon, but of the peritendinous structures, by
limiting aberrant fibrous proliferation in the regeneration process
and helping to build a peritendinous space, which will later form a
true synovial sheath. In conclusion Dynavisc® can be
used safely in acute flexor tendon injuries.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AMCL, CS and DCM performed the experiments in the
present study. AMCL, SAP, CT, TS and BC performed data analysis.
AMCL, SAP, DCM, BC, IHJ, AT and TS wrote the manuscript. CS, DCM,
IHJ and AT made substantial contributions to conception and design,
acquisition, analysis and interpretation of data. AMCL, CS and DCM
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Commission of
‘Grigore T. Popa’ University of Medicine and Pharmacy (Iasi,
Romania; approval no. 20311/26.09.2019) in compliance with ethical
and deontological rules for medical and research practice.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen CH, Chen SH, Shalumon KT and Chen JP:
Prevention of peritendinous adhesions with electrospun polyethylene
glycol/polycaprolactone nanofibrous membranes. Colloids Surf B
Biointerfaces. 133:221–230. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
de Jong JP, Nguyen JT, Sonnema AJ, Nguyen
EC, Amadio PC and Moran SL: The incidence of acute traumatic tendon
injuries in the hand and wrist: A 10-year population-based study.
Clin Orthop Surg. 6:196–202. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wu YF and Tang JB: Tendon healing, edema,
and resistance to flexor tendon gliding: Clinical implications.
Hand Clin. 29:167–178. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sammer DM and Chung KC: Advances in the
healing of flexor tendon injuries. Wound Repair Regen. 22 (Suppl
1):S25–S29. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Loiselle AE, Kelly M and Hammert WC:
Biological augmentation of flexor tendon repair: A challenging
cellular landscape. J Hand Surg Am. 41:144–149. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Giesen T, Calcagni M and Elliot D: Primary
flexor tendon repair with early active motion: Experience in
Europe. Hand Clin. 33:465–472. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Evans RB: Managing the injured tendon:
Current concepts. J Hand Ther. 25:173–190. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Moro-oka T, Miura H, Mawatari T, Kawano T,
Nakanishi Y, Higaki H and Iwamoto Y: Mixture of hyaluronic acid and
phospholipid prevents adhesion formation on the injured flexor
tendon in rabbits. J Orthop Res. 18:835–840. 2000.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sun Y, Chen MY, Zhao C, An KN and Amadio
PC: The effect of hyaluronidase, phospholipase, lipid solvent and
trypsin on the lubrication of canine flexor digitorum profundus
tendon. J Orthop Res. 26:1225–1229. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Taguchi M, Zhao C, Sun YL, Jay GD, An KN
and Amadio PC: The effect of surface treatment using hyaluronic
acid and lubricin in the gliding resistance of human extrasynovial
tendons in vitro. J Hand Surg Am. 34:1276–1281. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Oryan A, Moshiri A and Meimandiparizi AH:
Effects of sodium-hyaluronate and glucosamine-chondroitin sulfate
on remodeling stage of tenotomized superficial digital flexor
tendon in rabbits: A clinical, histopathological, ultrastructural,
and biomechanical study. Connect Tissue Res. 52:329–339.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rhee DK, Marcelino J, Baker M, Gong Y,
Smits P, Lefebre V, Jay GD, Stewart M, Wang H, Warman ML and
Carpten JD: The secreted glycoprotein lubricin protects cartilage
surfaces and inhibits synovial cell overgrowth. J Clin Invest.
115:622–631. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Amadio PC: Gliding resistance and
modifications of gliding surface of tendon: Clinical perspectives.
Hand Clin. 29:159–166. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
St Onge R, Weiss C, Denlinger JL and
Balazs EA: A preliminary assessment of Na-hyaluronate injection
into ‘no man's land’ for primary flexor tendon repair. Clin Orthop
Relat Res. 146:269–275. 1980.PubMed/NCBI
|
|
15
|
Amiel D, Ishizue K, Billings E Jr, Wiig M,
Vande Berg J, Akeson WH and Gelberman MD: Hyaluronan in flexor
tendon repair. J Hand Surg Am. 14:837–843. 1989.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hayashi M, Zhao C, Thoreson AR, Chikenji
T, Jay GD, An KN and Amadio PC: The effect of lubricin on the
gliding resistance of mouse intrasynovial tendon. PLoS One.
8(e83836)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhao C, Hashimoto T, Kirk RL, Thoreson AR,
Jay GD, Moran SL, An KN and Amadio PC: Resurfacing with chemically
modified hyaluronic acid and lubricin for flexor tendon
reconstruction. J Orthop Res. 31:969–975. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhao C, Sun YL, Kirk RL, Thoreson AR, Jay
GD, Moran SL, An KN and Amadio PC: Effects of a lubricin-containing
compound on the results of flexor tendon repair in a canine model
in vivo. J Bone Joint Surg Am. 92:1453–1461. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kitano T, Zerwekh JE, Edwards ML, Usui Y
and Allen MD: Viscous carboxymethylcellulose in the prevention of
epidural scar formation. Spine (Phila Pa 1976). 16:820–823.
1991.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fransen P: Safety of
carboxymethylcellulose/polyethylene oxide for the prevention of
adhesions in lumbar disc herniation-consecutive case series review.
Ann Surg Innov Res. 2(2)2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rodgers KE, Robertson JT, Espinoza T,
Oppelt W, Cortese S, diZerega GS and Berg RA: Reduction of epidural
fibrosis in lumbar surgery with Oxiplex adhesion barriers of
carboxymethylcellulose and polyethylene oxide. Spine J. 3:277–284.
2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gill C, Klufmoeller F and Noack W:
Experience with Oxiplex/SP gel for the prevention of post-surgical
adhesions in decompressive spine surgery. Paper presented at North
American Spine Society 18th Annual Meeting. October 21-25, San
Diego, CA, 2003.
|
|
23
|
Tos P, Crosio A, Pellegatta I, Valdatta L,
Pascal D, Geuna S and Cherubino M: Efficacy of anti-adhesion gel of
carboxymethylcellulose with polyethylene oxide on peripheral nerve:
Experimental results on a mouse model. Musce Nerve. 53:304–309.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Balls M, Goldberg A, Fentem J, Broadhead
CL, Burch RL, Festing MF, Frazier JM, Hendriksen CF, Jennings M,
van der Kamp MD, et al: The three Rs: The way forward: The report
and recommendations of ECVAM Workshop 11. Altern Lab Anim.
23:838–866. 1995.PubMed/NCBI
|
|
25
|
Savage R: The search for the ideal tendon
repair in zone 2: Strand number, anchor points and suture
thickness. J Hand Surg Eur Vol. 39:20–29. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Strickland JW: The scientific basis for
advances in flexor tendon surgery. J Hand Ther. 18:94–111.
2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wong JKF, Lui YH, Kapacee Z, Kadler KE,
Fergusos MWJ and McGrouther DA: The cellular biology of flexor
tendon adhesion formation: An old problem in a new paradigm. Am J
Pathol. 175:1938–1951. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ozgenel GY: The effects of a combination
of hyaluronic and amniotic membrane on the formation of
peritendinous adhesions after flexor tendon surgery in chickens. J
Bone Joint Surg Br. 86:301–307. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Khanna A, Friel M, Gougoulias N, Longo UG
and Maffulli N: Prevention of adhesions in surgery of the flexor
tendons of the hand: What is the evidence? Br Med Bull. 90:85–109.
2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Loiacono C, Palermi S, Massa B, Belviso I,
Romano V, Gregorio AD, Sirico F and Sacco AM: Tendinopathy:
Pathophysiology, therapeutic options, and role of nutraceutics. A
narrative literature review. Medicina (Kaunas).
55(447)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Megerle K, Woon C, Kraus A, Raghavan S,
Pham H and Chang J: Flexor tendon sheath engineering using
decellularized porcine pericardium. Plast Reconstr Surg.
138:630e–641e. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Linderman SW, Gelberman RH, Thomopoulos S
and Shen H: Cell and biologic-based treatment of flexor tendon
injuries. Oper Tech Orthop. 26:206–215. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ji X, Reisdorf RL, Thoreson AR, Berglund
LR, Moran SL, Jay JD, An KN, Amadio PC and Zhao C: Surface
modification with chemically modified synovial fluid for flexor
tendon reconstruction in a canine model in vivo. J Bone Joint Surg
Am. 97:972–978. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hagberg L, Heinegård D and Ohlsson K: The
contents of macromolecule solutes in flexor tendon sheath fluid and
their relation to synovial fluid. A quantitative analysis. J Hand
Surg Br. 17:167–171. 1992.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Özgenel GY and Etöz A: Effects of
repetitive injections of hyaluronic acid on peritendinous adhesions
after flexor tendon repair: A preliminary randomized,
placebo-controlled clinical trial. Ulus Travma Acil Cerrahi Derg.
18:11–17. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
diZerega GS, Cortese S, Rodgers KE, Block
KM, Falcone SJ, Juarez TG and Berg R: A modern biomaterial for
adhesion prevention. J Biomed Mater Res B Appl Biomater.
81:239–350. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lei W, Ehmsen RJ, Chiaccherini RP, Krelle
JL and diZerega GS: Reduction of leg pain by Oxiplex gel after
lumbar discectomy in patients with predominant leg pain and
elevated levels of lower back pain: A prospective, randomized,
blinded, multicenter clinical study. J Spine Disord Tech.
28:301–307. 2015.PubMed/NCBI View Article : Google Scholar
|