Introduction
Pulmonary arterial hypertension (PAH) is a
progressive disease characterized by increased pulmonary resistance
that leads to right heart failure and in some cases, death
(1,2). Thus far, numerous causes have been
associated with the mechanisms underlying PAH development. For
example, endothelin, nitric oxide and prostaglandin are classic
regulatory factors of PAH (3).
Pulmonary vascular remodeling is the most notable pathological
change in PAH, and pulmonary artery vascular smooth muscle cells
(PA-SMCs) and pulmonary artery endothelial cells (PA-ECs) are key
factors in vascular activity and remodeling (3). However, the pathogenesis of PAH and
associated molecular pathways, such as pulmonary vascular
remodeling, and PA-SMC and PA-EC proliferation, are yet to be fully
elucidated (4-6).
MicroRNAs (miRNAs/miRs) are a class of small
non-coding RNAs (7). miRNAs
participate in a number of key biological processes, such as
differentiation, cell proliferation and apoptosis. miRNAs exert
these biological processes by controlling the 3' untranslated
region of mRNA, which degrades and inhibits the translation of
target genes, as well as regulates the expression levels of target
genes (8).
In PAH, the biological processes of PA-SMCs and
PA-ECs are abnormal, characterized by increased proliferation and
reduced apoptosis (9-11).
miRNAs participate in the regulation of proliferation and apoptosis
in numerous diseases. For example, in retinoblastoma miR-675
promotes glioma cell proliferation and motility by regulating the
RB1 gene (12). miR-34a and
miR-181a have been indicated to participate in apoptosis and
oxidative stress in human osteoarthritic chondrocytes (13). miR-21 promotes breast cancer
proliferation and metastasis (14). We hypothesized that there may be
associations between miRNAs and PAH development. The results of
previous studies have revealed that a number of miRNAs participate
in the regulation of PAH development, such as miR-29b, miR-138, and
miR-222 and miR-204 (15,16). However, the differential expression
of miRNAs in PAH is yet to be fully elucidated.
The aim of the present study was to verify the
relationship between miRNAs and PAH in order to unravel novel
potential therapeutic target for PAH. RNA microarray in patients
with patent ductus arteriosus with or without PAH, reverse
transcription-quantitative PCR in a PAH animal model, flow
cytometry, western blotting, miRNA transfection and MTT assay in
primary cultured PA-SMCs were used for this purpose. The
differential expression levels of miRNAs in patients with PAH were
investigated. Furthermore, the expression level of the miR-30
family was verified in the lung tissue of rats during the
development of PAH. The regulatory functions of miR-30d-5p were
also investigated in the toxicity of PA-SMCs.
Materials and methods
Patient data and blood sample
collection
A total of 6 patients (West China Second University
Hospital of Sichuan University; Chengdu, China) with patent ductus
arteriosus were enrolled in the present study between June 2013 and
January 2014. All the patients exhibited no other lung diseases or
heart diseases. A total of 3 patients diagnosed with severe
pulmonary hypertension by echocardiograms and cardiac catheters
were assigned to the PH group [mean pulmonary artery pressure
(PAP), >70 mmHg]. A further 3 patients without pulmonary
hypertension were included in the control group. The clinical
characteristics of all patients are summarized in Table I. Blood samples from the 6 patients
were collected for RNA extraction and subsequent experiments. All
experiments involving human subjects were approved by the Medical
Ethics Committee of West China Second University Hospital of
Sichuan University (Chengdu, China; approval no. 2015-010). Written
informed consent for using the blood samples of patients was
obtained from the parents of the patients.
 | Table IClinical characteristics of control
patients and patients with PAH. |
Table I
Clinical characteristics of control
patients and patients with PAH.
| A, Control patients
without PAH |
|---|
| Age, months | Sex | Weight, kg | Body surface area,
m2 | RVSP, mmHg | mPAP, mmHg | PVRI, wood units
m2 | PDA diameter,
mm |
|---|
| 34 | Female | 14.0 | 0.590 | 27 | 18 | N/A | 2 |
| 47 | Female | 11.5 | 0.503 | 25 | 15 | N/A | 2 |
| 44 | Female | 9.0 | 0.415 | 22 | 14 | N/A | 2 |
| B, Patients with
PAH |
| Age, months | Sex | Weight, kg | Body surface area,
m2 | RVSP, mmHg | mPAP, mmHg | PVRI, wood units
m2 | PDA diameter,
mm |
| 34 | Female | 13.0 | 0.555 | 105 | 75 | 10.83 | 7 |
| 108 | Male | 26.0 | 1.010 | 116 | 87 | 18.74 | 6 |
| 49 | Male | 12.0 | 0.520 | 112 | 75 | 21.40 | 8 |
miRNA differential expression
spectrum
Total RNA was extracted from blood samples using
PureLink™ RNA extraction kit (cat. no. 12183020; Thermo Fisher
Scientific, Inc.) and purified using mirVana™ PARIS™ RNA and Native
Protein Purification kit (cat. no. AM1556, Ambion; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. RNA
integration was determined using an Agilent Bioanalyzer 2100
(Agilent Technologies, Inc.). A sample was considered qualified
with an RNA integrity number >7.
RNA labeling and array
hybridization
miRNAs in total RNA were labeled using the miRNA
Complete Labeling and Hybridization kit (cat. no. 5190-0456;
Agilent Technologies, Inc.) according to the manufacturer's
protocol. Each slide was hybridized with 100 ng Cy3-labeled RNA
using the aforementioned miRNA Complete Labeling and Hybridization
kit in a hybridization oven (cat. no. G2545A; Agilent Technologies,
Inc.) at 55˚C and low agitation for 20 h, according to the
manufacturer's protocol. Slides were subsequently washed using the
Gene Expression Wash Buffer kit (cat. no. 5188-5327; Agilent
Technologies, Inc.). An Agilent Microarray Scanner (cat. no.
G2565BA; Agilent Technologies, Inc.) was used to scan the slides.
The original data were normalized using the Quantile algorithm,
Gene Spring Software 11.0 (Agilent Technologies, Inc.).
Animal model
All animal experiments animals were approved by the
Medical Ethics Committee of West China Second University Hospital
of Sichuan University (Chengdu, China; approval no. 2015-010).
Sprague-Dawley (SD) rats were purchased from Chengdu Dashuo
Biological Technology Co., Ltd., and raised in
specific-pathogen-free conditions. The room temperature was 25˚C
with 50% humidity. The light and dark cycle was 12 h each. The rats
had free access to food and water. Rats were divided into the
following two groups: i) PAH group; and ii) control group.
In the PAH group, a total of 10 male SD rats
(weight, 300-400 g; age, 9 weeks) underwent left lung resection and
subcutaneous injection of monocrotaline (MCT; 60 mg/kg) one week
after surgery in order to mimic pulmonary hypertension (1). Rats were anesthetized with an
intraperitoneal injection of pentobarbital (30-60 mg/kg) for lung
resection, and the duration of the operation was 10-15 min.
Following the aforementioned procedures, animal health was
monitored daily. In total, 1 rat died 1 day following surgery, and
1 rat died 5 days following surgery. Thus, a total of 8 rats were
used in subsequent procedures. At 5 weeks after the drug injection,
the rats were used for subsequent studies. The results of our
previous study demonstrated that severe PAH formed at 5 weeks
following MCT injection (1).
Furthermore, the control group consisted of 8 healthy male SD rats
(weight, 300-400 g; age, 9 weeks).
PAP was measured through the jugular vein using a
transvenous catheter, and animals were subsequently sacrificed.
Following the aforementioned anesthesia using an intraperitoneal
injection of pentobarbital (30-60 mg/kg), rats were sacrificed by
exsanguination via the jugular veins and carotid arteries. Animal
death was confirmed by an absence of heart rate and lack of
breathing. Lung tissues from both groups were isolated for RNA
extraction. The hearts were dissected, and the weight of the right
ventricle (RV), left ventricle (LV) and ventricular septum (S) were
measured. The right heart hypertrophy index (RVHI) was calculated
using the equation: RV/(LV + S).
Reverse transcription-quantitative
(RT-qPCR)
The expression levels of miR-30a-5p, miR-30b-5p,
miR-30c-5p, miR-30d-5p, miR-30e-5p, miR-30a-3p, miR-30b-3p,
miR-30c-1-3p, miR-30c-2-3p, miR-30d-3p and miR-30e-3p in the lung
tissues of the PAH group and control group were verified using
RT-qPCR. Total RNA was extracted from lung tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. RT-qPCR was
performed using the EzOmics™ One-Step qPCR kit (cat. no. BK2100;
Biomics Biotechnologies Co., Ltd.). A total of 1 µl RNA was added
to 25 µl EzOmics™ One-Step qPCR kit components (including 2X master
mix, 50X SYBR Green I, 50 mM MgCl2 and H2O),
1 µl EzQuick™ 50X RT/Taq Mix, 2 µl RT primer and 30 µl
diethylpyrocarbonate-H2O. The primers were included in
the EzOmics™ miRNA qPCR Detection Primer Set (cat. no. BK1010;
Biomics Biotechnologies Co., Ltd.). The thermocycling conditions
were as follows: Initial denaturation at 95˚C for 10 min, followed
by 40 cycles of 95˚C for 15 sec, 55˚C for 30 sec and 72˚C for 30
sec. Data were analyzed using the 2-ΔΔCq method
(17) for relative quantification
to U6. The primer sequences were as follows: miR-30a-5p forward,
5'-AACGAGACGACGACAGAC-3' and reverse, 5'-TGTAAACATCCTCGACTGGAAG-3';
miR-30b-5p forward, 5'-TGTAAACATCCTACACTCAGCT-3' and reverse,
5'-CAGTGCGTGTCGTGGAGT-3'; miR-30c-5p forward,
5'-ACACTCCAGCTGGGTGTAAACATCCTACA CTC-3' and reverse,
5'-CTCAACTGGTGTCGTGGAGTCG GCAATTCAGTTGAGGCTCAGAG-3'; miR-30d-5p
forward, 5'-GCCTATAAACATCCCCGAC-3' and reverse, 5'-GTGCGT
GTCGTGGAGTCG-3'; miR-30e-5p forward, 5'-TGTAAACAT CCTTGACTGGAAGG-3'
and reverse, 5'-CCAGTGCGAATA CCTCGGAC-3'; miR-30a-3p forward,
5'-CCCTGCTCTGGC TGGTCAAACGGA-3' and reverse, 5'-TTGCCAGCCCTGCT
GTAGCTGGTTGAAG-3'; miR-30b-3p forward, 5'-GCTGCG
GTGTAGACATCTAATAC-3' and reverse, 5'-ATCCAGTGCA GGGTCCGACC-3';
miR-30c-1-3p forward, 5'-ACACTCCAG CTGGGCTGGGAGAGGGTTGTTTACTCC-3'
and reverse, 5'-CTCAACTGGTGTCGTGGAG TCGGCAATTCAGTTGA GGGAGTAAA-3';
miR-30c-2-3p forward, 5'-CACGCACTGG GAGAAGGC-3' and reverse,
5'-GTCGTATCCAGTGCAG GGTCCGAGGTATTCGCACTGGATACGAC-3'; miR-30d-3p
forward, 5'-TGGTTTTTTAGTATTATTGTTAGTTGT-3' and reverse,
5'-ATACATACAATCCCAACTATTCAAA-3'; miR-30e-3p forward,
5'-ACGCTTTCAGTCGGATGTTTA CAGC-3' and reverse,
5'-GTGCGTGTCGTGGAGTCG-3'; U6 forward,
5'-GCTTCGGCAGCACATATACTAAAAT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'.
Cell culture
Rat PA-SMCs were isolated and cultured as previously
described by Yin et al (18). Immediately after the rats were
sacrificed, the pulmonary artery was dissected and removed. The
following procedure was performed under aseptic conditions. The
connective tissue, tunica intima and tunica adventitia of the
artery was eliminated. The rest of the tissue was cut into small
pieces ~2 mm2 and transferred to a cell culture flask. A
total of 1 h later, when the tissue attached to the cell culture
flask, it was cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc.) with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.). The cells were cultured in a 37˚C incubator with 5%
CO2.
miRNA transfection
The miR-30d-5p mimics (miR-30d-5p; cat. no.
miR10000807-1-5), miR-negative control (NC) mimics (miR-NC; cat.
no. miR20000807-1-5), miR-30d-5p inhibitor (anti-miR-30d-5p; cat.
no. miR1N0000001-1-5) and miR-NC inhibitor (anti-miR-NC; cat. no.
miR2N0000001-1-5) were purchased from Guangzhou RiboBio Co., Ltd.
Primary cultured PA-SMCs were transfected at 37˚C for 24 h with the
aforementioned miRNAs (100 nM each) using Lipofectamine®
3000 reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The cells were harvested 24 h after
transfection and used in subsequent experiments.
MTT assay
An MTT assay was performed using rat PA-SMCs to
examine cytotoxicity. Cells were cultured in 96-well plates
(5x104) and treated with vehicle or 20 ng/ml
platelet-derived growth factor (PDGF; Sangon Biotech, Co., Ltd.) at
37˚C for 24 h. Cells were further divided into five groups: i)
Control, ii) miR-30d-5p, iii) miR-NC, iv) anti-miR-30d-5p; and v)
anti-miR-NC. MTT reagent (Sigma-Aldrich; Merck KGaA) was added and
cells were incubated for 4 h at 37˚C. Subsequently, a
spectrophotometer was used to assess the formation of colored
formazan with dimethyl sulfoxide at 540 nm. All samples were
analyzed three times.
Flow cytometry
Flow cytometry was performed using rat PA-SMCs
(1x106 cells) to examine cell apoptosis. Annexin V-PE
and PI (BD Biosciences) were used to stain the PA-SMCs. Apoptotic
cells were analyzed using a FACScan flow cytometer (Becton,
Dickinson and Company) with Cell Quest software v1.1 (BD
Biosciences). The apoptosis rate was calculated as the percentage
of early + late apoptotic cells.
Western blot analysis
PA-SMCs were collected 48 h after transfection.
Cells were lysed using phosphatase and proteinase inhibitors.
Proteins were extracted with RIPA lysis buffer (Thermo Fisher
Scientific, Inc.) and protein concentration was determined via the
Bradford method. Proteins (20 µg/lane) were loaded in a 4% 1.0-mm
Bis-Tris gel (Thermo Fisher Scientific, Inc.), and subsequently
transferred onto PVDF membranes (Thermo Fisher Scientific, Inc.).
No blocking was performed before primary antibody incubation. The
membranes were incubated with the primary antibody anti-Notch-3
(1:1,000; cat. no. ab23426; Abcam) at 4˚C for 1 h. Following
primary incubation, membranes were incubated with an anti-rabbit
HRP-conjugated secondary antibody (1:2,000; cat. no. ab7090; Abcam)
for 1 h at room temperature. The integrated optical density of the
samples was measured with a visualization reagent (Luminol;
Sigma-Aldrich; Merck KGaA) using a Gel-Pro analyzer. An
anti-α-tubulin antibody (1:1,000; cat. no. ab7291; Abcam) followed
by incubation with a secondary antibody (1:2,000; cat. no.
ab205719; Abcam) under the same conditions as aforementioned, was
used as the loading control.
Statistical analysis
The miRNA microarray analysis results were analyzed
using the SBC Analysis System (Shanghai BioChip Co., Ltd.),
following the manufacturer's protocol. Additionally, miRNAs with a
fold-change >2 and P<0.05 were considered to indicate a
statistically significant difference. All other data were analyzed
using SPSS software version 23.0 (IBM Corp.). Data following normal
distribution were presented as the mean ± standard deviation. Data
involving two groups following a normal distribution were analyzed
using unpaired Student's t-tests, and data with multiple groups
were analyzed using one-way ANOVA followed by Tukey's post hoc
tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
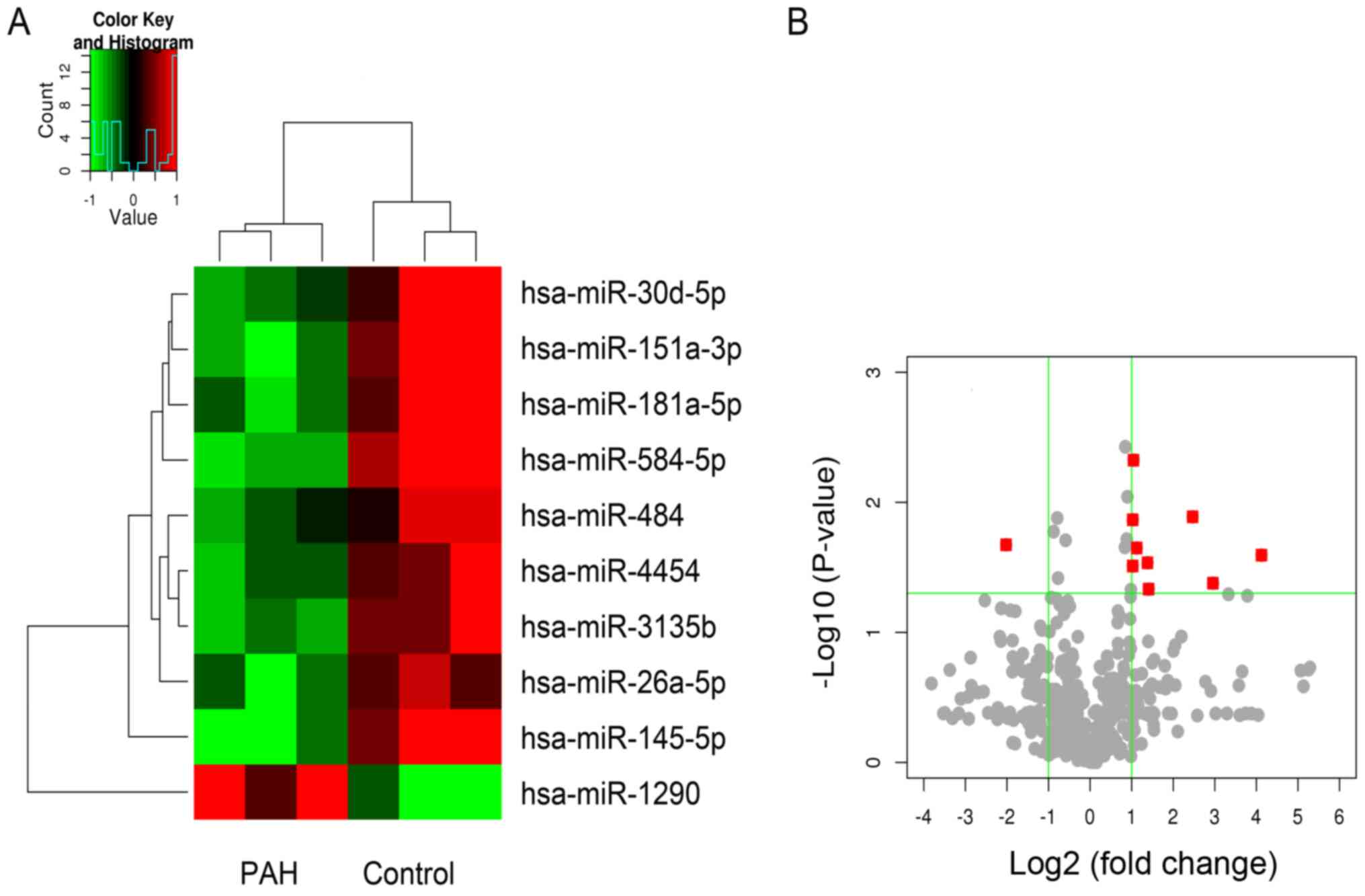
miRNA expression spectrum
A total of 593 differentially expressed miRNAs were
identified and analyzed. The expression levels of a total of nine
miRNAs with a fold-change >2 and P<0.05 were significantly
downregulated, and the expression level of one miRNA was
significantly upregulated in the PAH group, compared with the
control group (Fig. 1B). The
miRNAs with downregulated expression levels included miR-30d-5p,
miR-151q-3p, miR181a-5p, miR-584-5p, miR-484, miR-4454, miR-3135b,
miR-26A-5P and miR-145-5p. The miRNA with an upregulated expression
level was miR-1290 (Fig. 1A).
Circulating miR-30 family expression
levels
In addition to the aforementioned differentially
expressed miRNAs, the expression levels of the circulating miRNA
family were also analyzed (Table
II). The results revealed the upregulation of miR-30c-1-3p and
miR-30c-2-3p, and the downregulation of miR-30c-5p, miR-30d-5p and
miR-30e-3p compared with the control group; however, only the
altered expression level of miR-30d-5p was significant. Moreover,
miR-30a-3p, miR-30a-5p, miR-30b-3p, miR30b-5p, miR-30d-3p and
miR-30e-5p demonstrated a small but not significant fold-change
compared with the control group.
 | Table IIExpression levels of the circulating
miR-30 family. |
Table II
Expression levels of the circulating
miR-30 family.
| Name | Expression in PAH
group vs. control | P-value | Fold-change |
|---|
| miR-30a-3p | Upregulated | 0.823 | 1.01 |
| miR-30a-5p | Upregulated | 0.963 | 1.11 |
| miR-30b-3p | Upregulated | 0.823 | 1.01 |
| miR-30b-5p | Downregulated | 0.672 | 1.05 |
| miR-30c-1-3p | Upregulated | 0.401 | 2.38a |
| miR-30c-2-3p | Upregulated | 0.406 | 1.89 |
| miR-30c-5p | Downregulated | 0.298 | 2.29a |
| miR-30d-3p | Upregulated | 0.823 | 1.01 |
| miR-30d-5p | Downregulated | 0.046b | 2.66a |
| miR-30e-3p | Downregulated | 0.297 | 2.14a |
| miR-30e-5p | Downregulated | 0.428 | 1.33 |
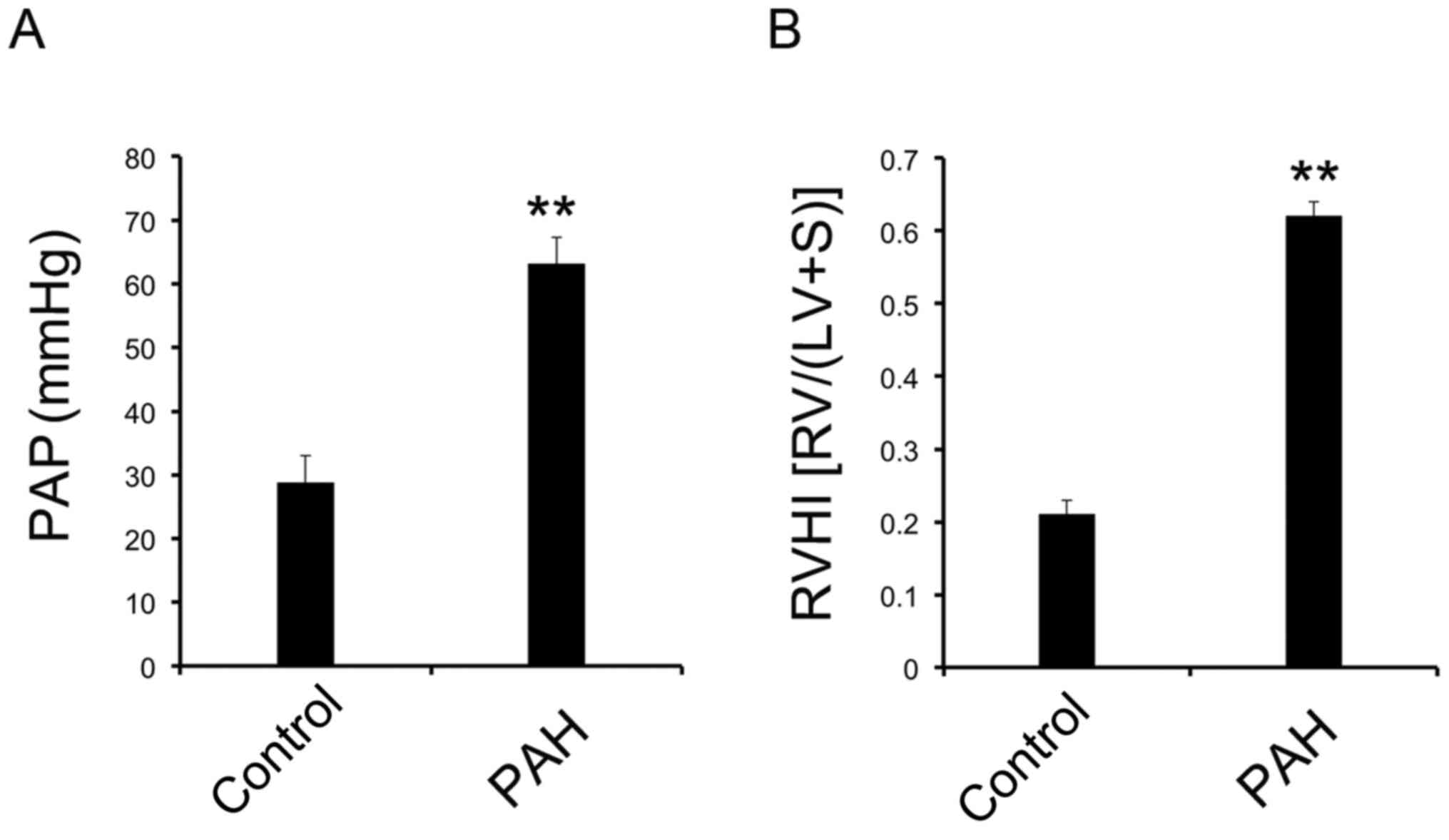
Animal model of PAH with increased PAP
and RVHI
When the animal model was established, PAP was
measured via the jugular vein. RVHI was calculated after the
dissection of the heart. Both PAP (Fig. 2A) and RVHI (Fig. 2B) were significantly increased in
the PAH group compared with the control group. These results
suggested that the PAH model was successfully established.
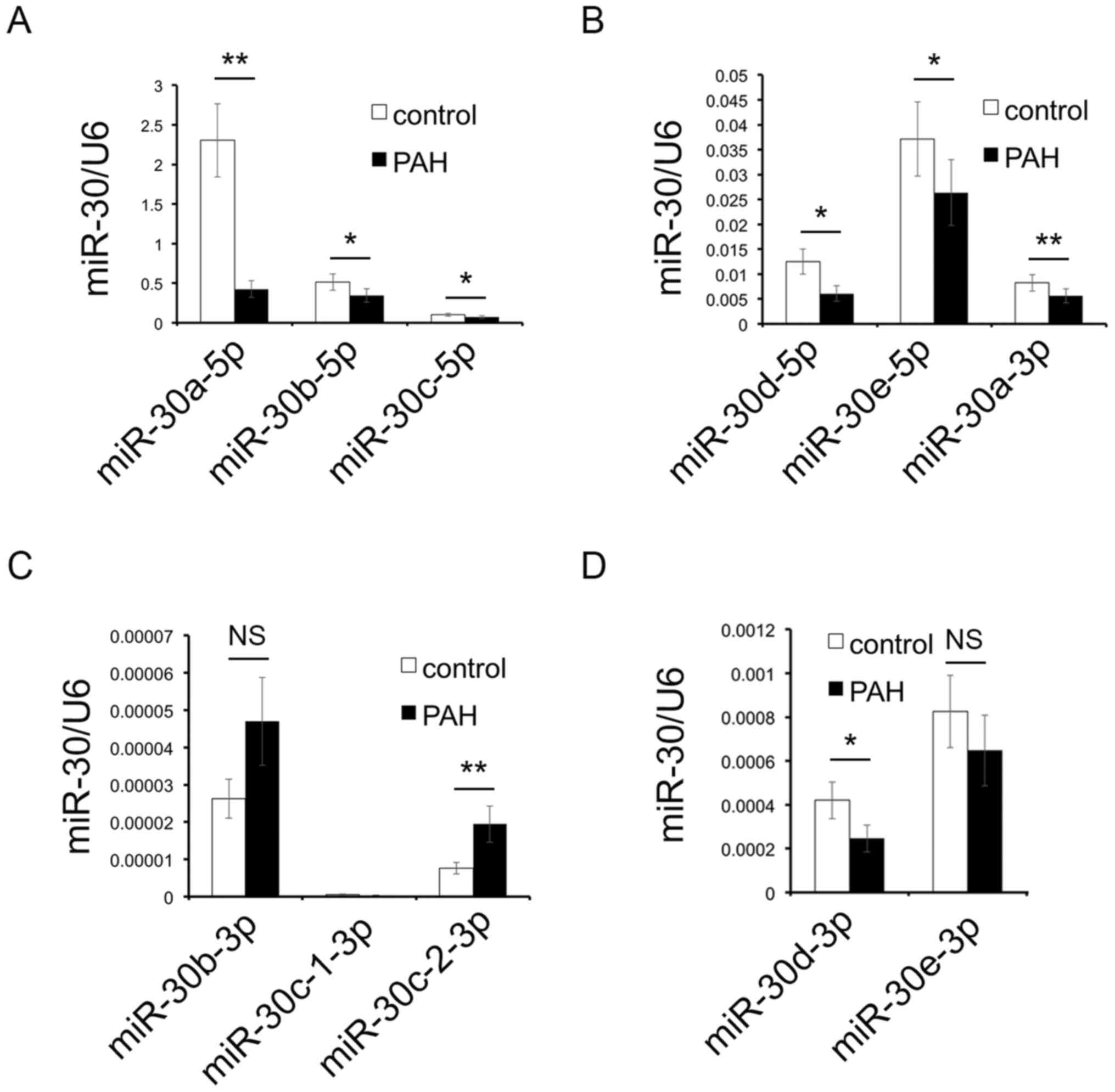
RT-qPCR results of miR-30 family
As demonstrated in Fig.
1, miR-30d-5p was significantly downregulated in the PAH group
compared with the control group; besides, numerous studies have
also revealed the association between the miR-30 family and PAH
(15,16). Moreover, as a previous study has
suggested that the miR-30 family influences vascular smooth muscle
cells (19), the expression of the
miR-30 family was investigated in the lung tissue of a rat PAH
model using RT-qPCR.
The results demonstrated that the expression levels
of miR-30a-5p, miR-30b-5p, miR-30c-5p (Fig. 3A), miR-30d-5p, miR-30e-5p,
miR-30a-3p (Fig. 3B) and
miR-30d-3p (Fig. 3C) were reduced,
and the expression levels of miR-30c-2-3p (Fig. 3C) were increased in the PAH group
compared with the control group. The expression levels of
miR-30c-1-3p (Fig. 3C) and
miR-30e-3p (Fig. 3D) were not
significantly different between the two groups; although a slight
upregulation in miR-30b-3p (Fig.
3C) was observed in the PAH group, compared with the control
group, the difference was not significant.
The results of the present study demonstrated that
the expression levels of miR-30b-5p were downregulated in both the
blood of patients with PAH and the lung tissues of animal models;
therefore, miR-30b-5p was selected for analysis in subsequent
experiments.
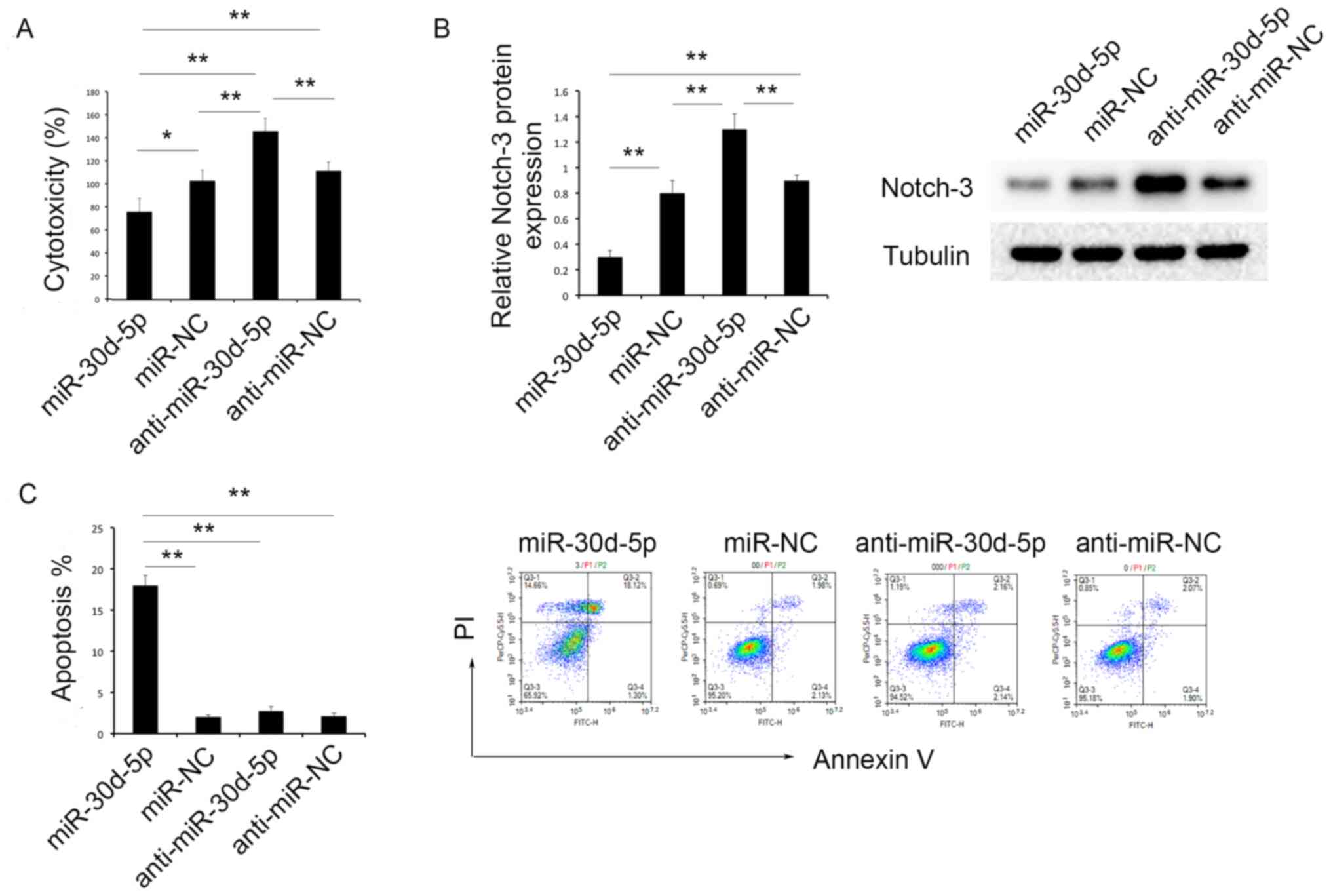
miR-30d-5p inhibits cell toxicity and
promotes the apoptosis of PA-SMCs in vitro
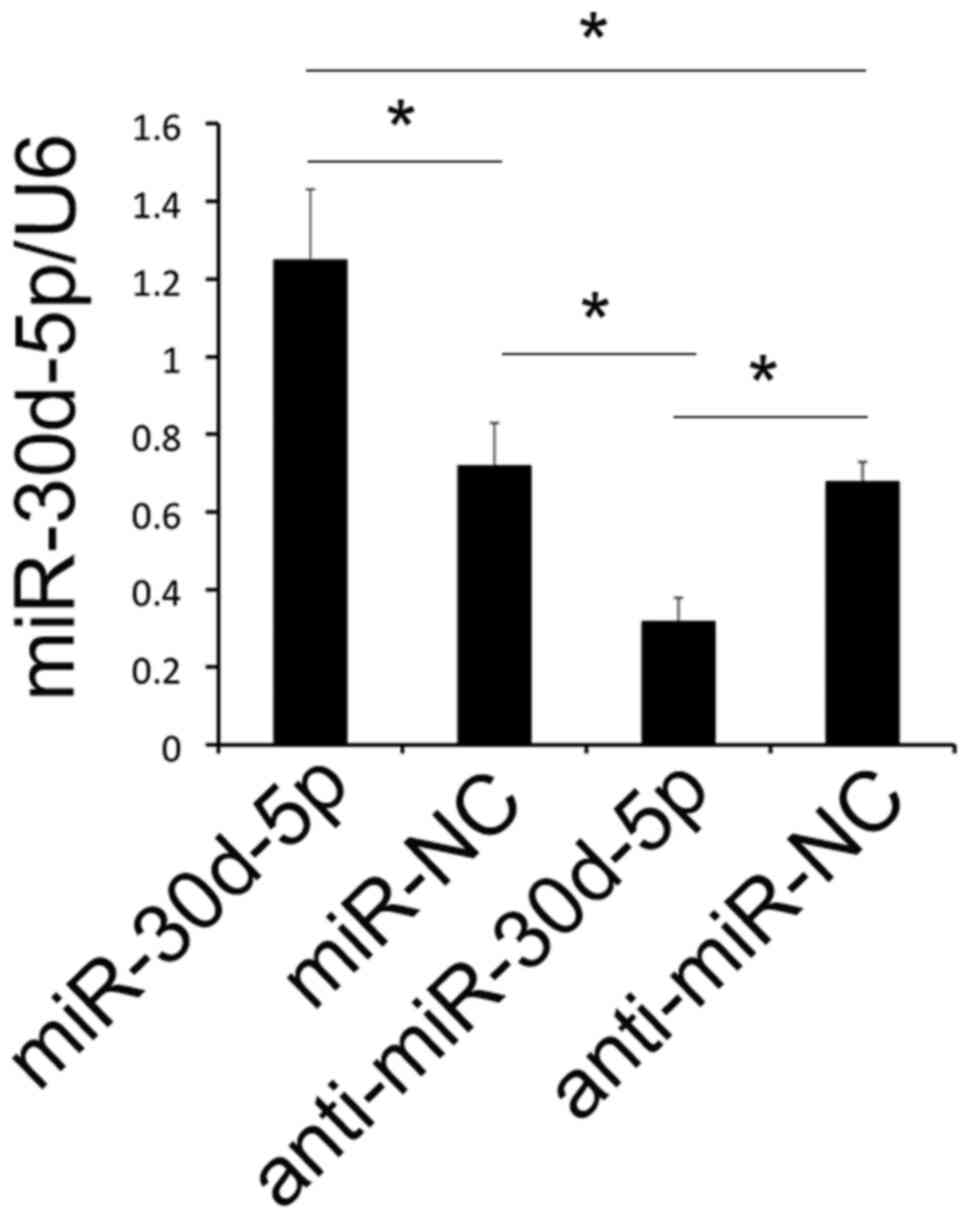
RT-qPCR was used to determine transfection
efficiency in PA-SMCs following cell transfection. As demonstrated
in Fig. 4, the expression levels
of miR-30d-5p were significantly increased in the miR-30d-5p mimics
group compared with the miR-NC group. Moreover, the expression
levels of miR-30d-5p were significant decreased in the
anti-miR-30d-5p group compared with the anti-miR-NC group (Fig. 4).
An MTT assay was used to assess PDGF-induced PA-SMC
toxicity. Following transfection with the miR-30d-5p mimic, the
levels of PDGF-induced toxicity of PA-SMCs were significantly
decreased, compared with the miR-NC group. Moreover, knockdown of
miR-30d-5p resulted in increased levels of toxicity of PA-SMCs,
compared with the anti-miR-NC group (Fig. 5A).
Flow cytometry was used to evaluate the levels of
cell apoptosis. Notably, following transfection with the miR-30d-5p
mimic, the levels of apoptosis of PA-SMCs were significantly
increased, compared with the miR-NC group. However, there was no
significant difference in the levels of apoptosis of PA-SMCs
between the anti-miR-30d-5p and anti-miR-NC groups (Fig. 5C).
miR-30d-5p inhibits the expression of
Notch-3
It was previously confirmed that the activation of
the Notch-3 pathway participated in the proliferation of PA-SMCs
(19). Thus, the expression levels
of Notch-3 were determined using western blot analysis. the results
of the present study revealed that the expression levels of Notch-3
were significantly decreased in the miR-30d-5p group, compared with
all other groups; the expression levels of Notch-3 were
significantly increased in the anti-miR-30d-5p group, compared with
all other groups (Fig. 5B).
Discussion
Numerous biological processes involve the
participation of miRNAs (20-22).
However, the role of various miRNAs in the development of PAH is
yet to be fully elucidated. In the present study, miRNAs with
differential expression were screened for in patients with PAH
using a miRNA microarray. In total, 10 miRNAs with significantly
differential expression levels were detected in patients with PAH,
compared with the control group.
The results of previous studies have demonstrated
changes in the expression levels of miR-151a-3p in a number of
diseases (23,24); however, the mechanisms underlying
miR-151a-3p in these diseases have not been further studied, to the
best of our knowledge. Moreover, it has previously been reported
that miR-181a-5p, miR-584-5p, miR-484, miR-145-5p and miR-1290
participated in the modulation of apoptosis and proliferation of
numerous cancer types, such as cervical and gastric cancer
(25-30).
The expression levels of miR-4454 were increased in hypoxic lung
alveolar macrophages (31).
Furthermore, results of a previous study revealed that miR-181a-5p
and miR-4454 participated in cartilage degeneration (32); however, the function of miR-4454
remains to be established. Previous studies have suggested that
miR-3135b is associated with heart disease, such as heart failure
and acute coronary syndrome (33,34);
however, no further study has reported the specific mechanisms
underlying miR-3135b. A study on miR-26a-5p expression indicated
its association with the metastasis of hepatic cellular cancer
(35), but the mechanism remained
unclear.
Results of the present study demonstrated that
circulating miR-30d-5p expression was significantly reduced in
patients with PAH, compared with the control group. Results of
previous studies suggested that miR-30d-5p participates in
hypoxic-ischemic injury (36),
inhibition of prostate cancer cell proliferation (37) and myocardial infarction (38). To the best of our knowledge, this
is the first time an association between miR-30d-5p and PAH has
been reported. Limitations of current microarray analyses include
the generation of false-positive results, and that the levels of
miRNAs in circulation may not be identical to those in tissue.
Thus, the expression of the miR-30 family, including miR-30d-5p was
verified using RT-qPCR analysis in an animal model.
In the animal model, the expression levels of
miR-30d-5p were reduced in the lung tissue of the PAH group
compared with the control group. In addition, alternate miRNAs in
the miR-30 family with significant changes in expression levels
between the two groups were revealed in the present study. These
included reduced expression levels of miR-30a-5p, miR-30b-5p,
miR-30c-5p, miR-30e-5p, miR-30a-3p and miR-30d-3p, and an increase
in the expression levels of miR-30c-2-3p. In patients with PAH,
results of the microarray analysis did not reveal any significant
differential expression of the aforementioned miRNAs. These results
may be due to the following: i) Species differences between humans
and rats; ii) generation of both false negative and positive
results using highly efficient microarray technology; and iii) the
investigation of circulating miRNAs in patients in the present
study, compared with only lung tissues of the animal model. Results
of previous studies demonstrated that the expression levels of
miR-30a-5p, miR-30b-5p and miR-30a-3p were associated with the
proliferation and apoptosis of several types of tumors, such as
hepatocellular and renal cell cancer (39-42).
Moreover, multiple studies have focused on miR-30c-5p and the
associated functions involved in inflammation regulation, tumor
migration and invasion (43,44).
miR-30e-5p participates in carcinogenesis in different types of
tumors through numerous pathways, including the sirtuin 1/JAK/STAT3
signaling and MAPK/nuclear factor of activated T-cells 5 pathway
(45,46). Although miR-30d-3p is associated
with lung cancer and pancreatic stem cell differentiation, the
underlying mechanisms are yet to be elucidated (47,48).
Furthermore, miR-30c-2-3p is associated with cell cycle progression
in breast cancer and the upregulation of hypoxia-inducible
factor-2α activity in renal cell carcinoma (49,50).
Results of the present study demonstrated that the
aforementioned miRNAs exhibited differential expression levels in
patients with PAH and animal models; however, the functions of
these miRNAs in PAH remain unknown. A number of miRNAs are involved
in cell proliferation and apoptosis (51). Results of previous studies have
indicated abnormal proliferation and apoptosis in PA-SMCs and
PA-ECs in both patients with PAH and animal models (1,3,6);
thus, the aforementioned miRNAs may be associated with the
proliferation or apoptosis of cells involved in PAH development.
Results of the present study revealed that miR-30d-5p
overexpression was associated with decreased PA-SMC toxicity and
increased apoptosis compared with control groups. These results may
provide a theoretical basis for the downregulation of miR-30d-5p in
patients with PAH with increased PA-SMC cytotoxicity. In addition,
the effects of miR-30d-5p on cell proliferation and apoptosis have
been established in numerous other diseases, such as myocardial
infarction and lung cancer (38,52).
Several miRNAs have been found to participate in the
regulation of PAH. For example, the inhibition of miR-143 inhibited
the development of PAH (53). In
patients with PAH, miR-124 was downregulated in pulmonary vascular
and circulating progenitor endothelial cells (54). In addition, miR-125a-5p ameliorated
MCT-induced PAH via tumor growth factor-β1(55). These findings suggest that miRNAs
may act as potential therapeutic targets in the treatment of
PAH.
Notch signaling participates in multiple
physiological vascular processes, such as proliferation and
apoptosis (56). Notch-1 is
associated with PA-EC proliferation (57), while Notch-3 is highly expressed in
PA-SMCs and promotes PA-SMC proliferation via vascular endothelial
growth factor (58). As miR-30d-5p
was associated with PA-SMC proliferation and apoptosis, we
hypothesized an association between miR-30d-5p and Notch-3. Results
of the present study demonstrated that overexpression of miR-30d-5p
inhibited Notch-3 expression, while the knockdown of miR-30d-5p
induced higher expression levels of Notch-3.
In the present study, the number of patient samples
was limited; however, results of the microarray analysis provide
novel ideas for further studies. Additional investigations will
involve examining potential changes in the markers of proliferation
and apoptosis to further verify the observed effects of miR-30d-5p
on the regulation of PA-SMC. Furthermore, the sample size of
clinical cases will be increased to verify the observed changes of
miR-30d-5p in patients with PAH. The mechanisms underlying
miR-30d-5p in the regulation of Notch signaling will also be
established. Finally, further investigations will also involve the
use of miR-30d-5p mimics and the miR-30d-5p inhibitor in animal
models of PAH.
In conclusion, miR-30d-5p expression was
downregulated in both patients with PAH and animal models. The
overexpression of miR-30d-5p resulted in increased cytotoxicity and
reduced apoptosis of PA-SMCs. Thus, the mechanisms underlying
miR-30d-5p in PAH may be via the Notch-3 signaling pathway.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from the
Sichuan Province Science and Technology Support Program, China
(grant nos. 2018SZ0180 and 2019YFS0245).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the ArrayExpress database at
EMBL-EBI (www.ebi.ac.uk/arrayexpress; accession no.
E-MTAB-10932).
Authors' contributions
FH participated in the analysis and interpretation
of the patient data and drafted the manuscript. LQ and HML made
substantial contributions to the conception and design of the
study. CW performed the examinations and contributed to the
acquisition of data. HWL was a major contributor to the
experiments. LQ gave final approval of the version to be published
and agreed to be accountable for all aspects of the work in
ensuring that questions related to the accuracy or integrity of any
part of the work are appropriately investigated and resolved. FH
and LQ confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments involving human subjects and animals
were approved by the Medical Ethics Committee of West China Second
University Hospital of Sichuan University (Chengdu, China; approval
no. 2015-010). Written informed consent for using the blood samples
of patients was obtained from the parents of the patients.
Patient consent for publication
Parents provided written informed consent for the
publication of any data and accompanying images associated with the
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu F, Liu C, Liu H, Xie L and Yu L:
Ataxia-telangiectasia mutated (ATM) protein signaling participates
in development of pulmonary arterial hypertension in rats. Med Sci
Monit. 23:4391–4400. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Goldberg AB, Mazur W and Kalra DK:
Pulmonary hypertension: Diagnosis, imaging techniques, and novel
therapies. Cardiovasc Diagn Ther. 7:405–417. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hansmann G: Pulmonary hypertension in
infants, children, and young adults. J Am Coll Cardiol.
69:2551–2569. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
McClenaghan C, Woo KV and Nichols CG:
Pulmonary hypertension and ATP-sensitive potassium channels.
Hypertension. 74:14–22. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Elia D, Caminati A, Zompatori M, Cassandro
R, Lonati C, Luisi F, Pelosi G, Provencher S and Harari S:
Pulmonary hypertension and chronic lung disease: Where are we
headed? Eur Respir Rev. 28(31636088)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Singh I, Oliveira RKF, Naeije R, Rahaghi
FN, Oldham WM, Systrom DM and Waxman AB: Pulmonary vascular
distensibility and early pulmonary vascular remodeling in pulmonary
hypertension. Chest. 156:724–732. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ye J, Xu M, Tian X, Cai S and Zeng S:
Research advances in the detection of miRNA. J Pharm Anal.
9:217–226. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Wang S, Cao W, Gao S, Nie X, Zheng X, Xing
Y, Chen Y, Bao H and Zhu D: TUG1 Regulates Pulmonary Arterial
Smooth Muscle Cell Proliferation in Pulmonary Arterial
Hypertension. Can J Cardiol. 35:1534–1545. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang Y, Pandey RN, York AJ, Mallela J,
Nichols WC, Hu YC, Molkentin JD, Wikenheiser-Brokamp KA and Hegde
RS: The EYA3 tyrosine phosphatase activity promotes pulmonary
vascular remodeling in pulmonary arterial hypertension. Nat Commun.
10(4143)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ruffenach G, Umar S, Vaillancourt M, Hong
J, Cao N, Sarji S, Moazeni S, Cunningham CM, Ardehali A, Reddy ST,
et al: Histological hallmarks and role of Slug/PIP axis in
pulmonary hypertension secondary to pulmonary fibrosis. EMBO Mol
Med. 11(e10061)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zheng Y, Lu X, Xu L, Chen Z, Li Q and Yuan
J: MicroRNA-675 promotes glioma cell proliferation and motility by
negatively regulating retinoblastoma 1. Hum Pathol. 69:63–71.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cheleschi S, Tenti S, Mondanelli N,
Corallo C, Barbarino M, Giannotti S, Gallo I, Giordano A and
Fioravanti A: MicroRNA-34a and MicroRNA-181a mediate
visfatin-induced apoptosis and oxidative stress via NF-κB pathway
in human osteoarthritic chondrocytes. Cells. 8(874)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang H, Tan Z, Hu H, Liu H, Wu T, Zheng C,
Wang X, Luo Z, Wang J, Liu S, et al: MicroRNA-21 promotes breast
cancer proliferation and metastasis by targeting LZTFL1. BMC
Cancer. 19(738)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu T, Zou XZ, Huang N, Ge XY, Yao MZ, Liu
H, Zhang Z and Hu CP: Down-regulation of miR-204 attenuates
endothelial-mesenchymal transition by enhancing autophagy in
hypoxia-induced pulmonary hypertension. Eur J Pharmacol.
863(172673)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Babicheva A, Ayon RJ, Zhao T, Ek Vitorin
JF, Pohl NM, Yamamura A, Yamamura H, Quinton BA, Ba M, Wu L, et al:
MicroRNA-mediated downregulation of K+ channels in
pulmonary arterial hypertension. Am J Physiol Lung Cell Mol
Physiol. 318:L10–L26. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Δ Δ C(T)) Method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yin Y, Wu X, Yang Z, Zhao J, Wang X, Zhang
Q, Yuan M, Xie L, Liu H and He Q: The potential efficacy of
R8-modified paclitaxel-loaded liposomes on pulmonary arterial
hypertension. Pharm Res. 30:2050–2062. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu YF, Spinelli A, Sun LY, Jiang M,
Singer DV, Ginnan R, Saddouk FZ, Van Riper D and Singer HA:
MicroRNA-30 inhibits neointimal hyperplasia by targeting
Ca(2+)/calmodulin-dependent protein kinase IIδ (CaMKIIδ). Sci Rep.
6(26166)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Morris HE, Neves KB, Montezano AC, MacLean
MR and Touyz RM: Notch3 signalling and vascular remodelling in
pulmonary arterial hypertension. Clin Sci (Lond). 133:2481–2498.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li L, Zhu X, Shou T, Yang L, Cheng X, Wang
J, Deng L and Zheng Y: MicroRNA-28 promotes cell proliferation and
invasion in gastric cancer via the PTEN/PI3K/AKT signalling
pathway. Mol Med Rep. 17:4003–4010. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang Z, Xu J, Zhu R and Liu L:
Down-regulation of miRNA-128 contributes to neuropathic pain
following spinal cord injury via activation of P38. Med Sci Monit.
23:405–411. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mundalil Vasu M, Anitha A, Thanseem I,
Suzuki K, Yamada K, Takahashi T, Wakuda T, Iwata K, Tsujii M,
Sugiyama T and Mori N: Serum microRNA profiles in children with
autism. Mol Autism. 5(40)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang K, Wu X, Wang J, Lopez J, Zhou W,
Yang L, Wang SE, Raz DJ and Kim JY: Circulating miRNA profile in
esophageal adenocarcinoma. Am J Cancer Res. 6:2713–2721.
2016.PubMed/NCBI
|
|
25
|
Yang M, Zhai X, Ge T, Yang C and Lou G:
miR-181a-5p Promotes Proliferation and Invasion and Inhibits
Apoptosis of Cervical Cancer Cells via Regulating Inositol
Polyphosphate-5-Phosphatase A (INPP5A). Oncol Res. 26:703–712.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu Z, Sun F, Hong Y, Liu Y, Fen M, Yin K,
Ge X, Wang F, Chen X and Guan W: MEG2 is regulated by miR-181a-5p
and functions as a tumour suppressor gene to suppress the
proliferation and migration of gastric cancer cells. Mol Cancer.
16(133)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li Q, Li Z, Wei S, Wang W, Chen Z, Zhang
L, Chen L, Li B, Sun G, Xu J, et al: Overexpression of miR-584-5p
inhibits proliferation and induces apoptosis by targeting WW
domain-containing E3 ubiquitin protein ligase 1 in gastric cancer.
J Exp Clin Cancer Res. 36(59)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu J and Li SM: MiR-484 suppressed
proliferation, migration, invasion and induced apoptosis of gastric
cancer via targeting CCL-18. Int J Exp Pathol. 101:203–214.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wu J, He Y, Luo Y, Zhang L, Lin H, Liu X,
Liu B, Liang C, Zhou Y and Zhou J: miR-145-5p inhibits
proliferation and inflammatory responses of RMC through regulating
AKT/GSK pathway by targeting CXCL16. J Cell Physiol. 233:3648–3659.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ma Q, Wang Y, Zhang H and Wang F: miR-1290
contributes to colorectal cancer cell proliferation by targeting
INPP4B. Oncol Res. 26:1167–1174. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Armstrong DA, Nymon AB, Ringelberg CS,
Lesseur C, Hazlett HF, Howard L, Marsit CJ and Ashare A: Pulmonary
microRNA profiling: implications in upper lobe predominant lung
disease. Clin Epigenetics. 9(56)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nakamura A, Rampersaud YR, Sharma A, Lewis
SJ, Wu B, Datta P, Sundararajan K, Endisha H, Rossomacha E, Rockel
JS, et al: Identification of microRNA-181a-5p and microRNA-4454 as
mediators of facet cartilage degeneration. JCI Insight.
1(e86820)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen F, Yang J, Li Y and Wang H:
Circulating microRNAs as novel biomarkers for heart failure.
Hellenic J Cardiol. 59:209–214. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang A, Kwee LC, Grass E, Neely ML,
Gregory SG, Fox KAA, Armstrong PW, White HD, Ohman EM, Roe MT, et
al: Whole blood sequencing reveals circulating microRNA
associations with high-risk traits in non-ST-segment elevation
acute coronary syndrome. Atherosclerosis. 261:19–25.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liang L, Zeng JH, Wang JY, He RQ, Ma J,
Chen G, Cai XY and Hu XH: Down-regulation of miR-26a-5p in
hepatocellular carcinoma: A qRT-PCR and bioinformatics study.
Pathol Res Pract. 213:1494–1509. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhao F, Qu Y, Zhu J, Zhang L, Huang L, Liu
H, Li S and Mu D: miR-30d-5p plays an important role in autophagy
and apoptosis in developing rat brains after hypoxic-ischemic
injury. J Neuropathol Exp Neurol. 76:709–719. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Song Y, Song C and Yang S:
Tumor-Suppressive Function of miR-30d-5p in Prostate Cancer Cell
Proliferation and Migration by Targeting NT5E. Cancer Biother
Radiopharm. 33:203–211. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jia K, Shi P, Han X, Chen T, Tang H and
Wang J: Diagnostic value of miR-30d-5p and miR-125b-5p in acute
myocardial infarction. Mol Med Rep. 14:184–194. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li WF, Dai H, Ou Q, Zuo GQ and Liu CA:
Overexpression of microRNA-30a-5p inhibits liver cancer cell
proliferation and induces apoptosis by targeting MTDH/PTEN/AKT
pathway. Tumour Biol. 37:5885–5895. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang C, Cai L, Liu J, Wang G, Li H, Wang
X, Xu W, Ren M, Feng L, Liu P, et al: MicroRNA-30a-5p inhibits the
growth of renal cell carcinoma by modulating GRP78 expression. Cell
Physiol Biochem. 43:2405–2419. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liu W, Li H, Wang Y, Zhao X, Guo Y, Jin J
and Chi R: miR-30b-5p functions as a tumor suppressor in cell
proliferation, metastasis and epithelial-to-mesenchymal transition
by targeting G-protein subunit α-13 in renal cell carcinoma. Gene.
626:275–281. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Qi B, Wang Y, Chen ZJ, Li XN, Qi Y, Yang
Y, Cui GH, Guo HZ, Li WH and Zhao S: Down-regulation of
miR-30a-3p/5p promotes esophageal squamous cell carcinoma cell
proliferation by activating the Wnt signaling pathway. World J
Gastroenterol. 23:7965–7977. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ceolotto G, Giannella A, Albiero M,
Kuppusamy M, Radu C, Simioni P, Garlaschelli K, Baragetti A,
Catapano AL, Iori E, et al: miR-30c-5p regulates
macrophage-mediated inflammation and pro-atherosclerosis pathways.
Cardiovasc Res. 113:1627–1638. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Cao JM, Li GZ, Han M, Xu HL and Huang KM:
miR-30c-5p suppresses migration, invasion and epithelial to
mesenchymal transition of gastric cancer via targeting MTA1. Biomed
Pharmacother. 93:554–560. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Xu G, Cai J, Wang L, Jiang L, Huang J, Hu
R and Ding F: MicroRNA-30e-5p suppresses non-small cell lung cancer
tumorigenesis by regulating USP22-mediated Sirt1/JAK/STAT3
signaling. Exp Cell Res. 362:268–278. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Qin X, Li C, Guo T, Chen J, Wang HT, Wang
YT, Xiao YS, Li J, Liu P, Liu ZS, et al: Upregulation of DARS2 by
HBV promotes hepatocarcinogenesis through the miR-30e-5p/MAPK/NFAT5
pathway. J Exp Clin Cancer Res. 36(148)2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wang K, Chen M and Wu W: Analysis of
microRNA (miRNA) expression profiles reveals 11 key biomarkers
associated with non-small cell lung cancer. World J Surg Oncol.
15(175)2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Coskun E, Ercin M and Gezginci-Oktayoglu
S: The role of epigenetic regulation and pluripotency-related
microRNAs in differentiation of pancreatic stem cells to beta
cells. J Cell Biochem. 119:455–467. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Shukla K, Sharma AK, Ward A, Will R,
Hielscher T, Balwierz A, Breunig C, Münstermann E, König R,
Keklikoglou I, et al: MicroRNA-30c-2-3p negatively regulates NF-κB
signaling and cell cycle progression through downregulation of
TRADD and CCNE1 in breast cancer. Mol Oncol. 9:1106–1119.
2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Mathew LK, Lee SS, Skuli N, Rao S, Keith
B, Nathanson KL, Lal P and Simon MC: Restricted expression of
miR-30c-2-3p and miR-30a-3p in clear cell renal cell carcinomas
enhances HIF2α activity. Cancer Discov. 4:53–60. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Jacquin S, Rincheval V, Mignotte B,
Richard S, Humbert M, Mercier O, Londoño-Vallejo A, Fadel E and
Eddahibi S: Inactivation of p53 is sufficient to induce development
of pulmonary hypertension in rats. PLoS One.
10(e0131940)2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Gao L, He RQ, Wu HY, Zhang TT, Liang HW,
Ye ZH, Li ZY, Xie TT, Shi Q, Ma J, et al: Expression Signature and
Role of miR-30d-5p in Non-Small Cell Lung Cancer: A comprehensive
study based on in silico analysis of public databases and in vitro
experiments. Cell Physiol Biochem. 50:1964–1987. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Deng L, Blanco FJ, Stevens H, Lu R,
Caudrillier A, McBride M, McClure JD, Grant J, Thomas M, Frid M, et
al: MicroRNA-143 activation regulates smooth muscle and endothelial
cell crosstalk in pulmonary arterial hypertension. Circ Res.
117:870–883. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Caruso P, Dunmore BJ, Schlosser K, Schoors
S, Dos Santos C, Perez-Iratxeta C, Lavoie JR, Zhang H, Long L,
Flockton AR, et al: Identification of MicroRNA-124 as a major
regulator of enhanced endothelial cell glycolysis in pulmonary
arterial hypertension via PTBP1 (polypyrimidine tract binding
protein) and pyruvate kinase M2. Circulation. 136:2451–2467.
2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Cai Z, Li J, Zhuang Q, Zhang X, Yuan A,
Shen L, Kang K, Qu B, Tang Y, Pu J, et al: miR-125a-5p ameliorates
monocrotaline-induced pulmonary arterial hypertension by targeting
the TGF-β1 and IL-6/STAT3 signaling pathways. Exp Mol Med. 50:1–11.
2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Thistlethwaite PA, Li X and Zhang X: Notch
signaling in pulmonary hypertension. Adv Exp Med Biol. 661:279–298.
2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Skurikhin EG, Krupin VA, Pershina OV, Pan
ES, Ermolaeva LA, Pakhomova AV, Rybalkina OY, Ermakova NN,
Khmelevskaya ES, Vaizova OE, et al: Endothelial progenitor cells
and Notch-1 signaling as markers of alveolar endothelium
regeneration in pulmonary emphysema. Bull Exp Biol Med.
166:201–206. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Guo ML, Kook YH, Shannon CE and Buch S:
Notch3/VEGF-A axis is involved in TAT-mediated proliferation of
pulmonary artery smooth muscle cells: Implications for
HIV-associated PAH. Cell Death Discov. 4(22)2018.PubMed/NCBI View Article : Google Scholar
|