Introduction
Pulmonary fibrosis (PF) is characterized by
increased fibroblasts proliferation, interstitial inflammation and
the promotion of extracellular matrix synthesis and deposition
(1). A number of possible
treatments for pulmonary fibrosis have been investigated, but their
effect in clinical trials is suboptimal (2). PF is a major therapeutic challenge for
which novel therapeutic strategies are warranted, and inhibiting
inflammation is increasingly regarded as one of the approaches.
Inflammation is the initial response following lung
injury. Once activated, inflammatory cells, such as neutrophils and
macrophages, accumulate in the lower airways and consequently
release harmful amounts of reactive oxygen species and some
proinflammatory cytokines and growth factors that regulate the
proliferation and secretary activity of alveolar fibroblasts in the
alveolar wall. The activated fibroblasts produce increasing amounts
of matrix proteins, which distort the normal lung architecture and
affect gas exchange. Therefore, inhibition of inflammation and
oxidative stress represents a possible therapeutic strategy
(3-7).
Traditional Chinese medicine has been widely used in
numerous diseases and has shown notable curative effects (8-10).
Feibi decoction (FBD) is a Chinese materia medica product extracted
from 8 Chinese traditional medical herbs and widely used for
treatment of patients with lung diseases, such as pulmonary
fibrosis; however, the precise mechanisms remain to be elucidated.
The present study investigated the effect of FBD-medicated serum
(FBDS) on lipopolysaccharide (LPS)-induced inflammation in
macrophages, and identified that FBDS significantly inhibited the
pro-inflammatory cytokines expression in both RAW264.7 macrophages
and bone marrow-derived macrophages (BMDMs). It was also observed
that FBDS suppressed LPS-induced activation of NF-κB and
Smad2/Smad3. Notably, it was identified that FBDS treatment
decreased the expression of transforming growth factor (TGF)-β1 and
chitinase-3-like protein 1 (CHI3L1). These findings may improve the
function of FBD in the treatment of pulmonary fibrosis and expand
current understanding of the mechanisms underlying Chinese
traditional medicine.
Materials and methods
Animals
A total of 28 clean healthy Sprague-Dawley rats (all
female; 8 weeks old), weighing 200-220 g, were provided by the
Beijing University of Chinese Medicine and housed in a cage at
23±1˚C, 45-55% humidity with a 12-h light/dark cycle. Food and
water were freely available. A total of 20 healthy C57BL/6J mice
(female; 6-8 weeks old; 23.7±1.2 g) were obtained from Beijing
University of Chinese Medicine to generate BMDMs. The mice were
kept at room temperature (controlled at 25˚C) and at 50% humidity.
with a 12-h light/dark cycle. The experiments were carried out
according to the National Institutes of Health Guide for the Care
and Use of Laboratory Animals (11)
and approved by the Animal Ethics Committee of the Scientific
Investigation Board of Beijing university of Chinese Medicine.
Preparation of FBDS
The 28 Sprague-Dawley rats were randomly divided
into FBD-low (n=7), FBD-moderate (n=7), FBD-high (n=7) and control
(n=7) groups. Rats in the FBD groups received intragastric
administration of FBD (low, 5 ml/kg; moderate, 10 ml/kg; high, 20
ml/kg) twice a day for 6 days. The control group received
intragastric perfusion of physiological saline twice a day for 6
days. Then, 1 h following the last administration, rats were
intraperitoneally anesthetized using 3% amobarbital (60 mg/kg) and
blood was sampled from the abdominal aorta and centrifuged (4˚C;
3,500 x g; 10 min). Following anesthesia, the rats exhibited slow
breathing and muscle relaxation, but no respiratory stagnation or
mortality. The serum was aliquoted into 10 ml ampoules and
preserved at -80˚C for future use.
Cell culture
Mouse macrophage cell line RAW264.7 (induced by
leukemia virus) was obtained from the American Type Culture
Collection. The cells were cultured at 37˚C under 5% CO2
in DMEM supplemented with 10% FBS (Invitrogen; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin, and 100 µg/ml streptomycin.
BMDM generation was performed according to a previous study
(12). Briefly, cells were prepared
by flushing the bone marrow from femurs and tibias and then
maintaining in DMEM medium containing 10% FBS and supplemented with
10 ng/ml macrophage colony stimulating factor (M-CSF; Peprotech,
Inc.; cat. no. 315-02). Then, 4-5 days later, adherent cells were
dissociated and cultured in DMEM supplemented with 10% FBS. BMDMs
were identified by flow cytometry. The cells were stained for 20
min at 4˚C with 25 µg/ml of anti-F4/80 and anti-CD11b.
FITC-anti-F4/80, APC-anti-CD11b were purchased from eBioscience
(Thermo Fisher Scientific, Inc.). Data were collected using a
FACSCanto (BD Biosciences) and analyzed using FlowJo version 10
software (FlowJo LLC). F4/80+CD11b+ cells were considered
BMDMs.
Cell viability
Cell viability was assessed using Cell Counting
Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.). Briefly,
5,000 RAW264.7 cells or BMDMs per well were seeded in 96-well
plates and treated with control rats serum or FBDS (10%) at 37˚C
for 24 h, followed by LPS stimulation (100 ng/ml) for 8 h. Then the
suspension was replaced with an equal volume of fresh culture
medium (Invitrogen; Thermo Fisher Scientific, Inc.) containing 10%
CCK-8, followed by 3 h incubation at 37˚C. The absorbance was
determined at 450 nm on a micro-plate reader (Multiskan MK3; Thermo
Fisher Scientific, Inc.).
Reverse transcription-quantitative
(RT-q) PCR
Following treatment, total RNA from RAW264.7 cells
or BMDMs was extracted with TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. RNA concentration was detected by NanoDrop™ ND-1000
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc.). mRNA (1 µg) was reverse transcribed using PrimeScript RT
Master Mix (Perfect Real Time) kit (Takara Biotechnology Co., Ltd.)
according to the manufacturer's instructions. The cDNA was
denatured for 10 min at 95˚C. A LightCycler (ABI PRISM 7000;
Applied Biosciences; Thermo Fisher Scientific, Inc.) and a SYBR
RT-PCR kit (Takara Biotechnology Co., Ltd.) were used for RT-qPCR
analysis. GAPDH was used as the internal control. Thermocycling
conditions were 1 cycle (95˚C for 5 min) and 40 cycles (95˚C for 15
sec; 57˚C for 30 sec; 72˚C for 30 sec) and the 2-ΔΔCq
method was used to evaluate the relative quantities of each
amplified product in the samples (13). For each qPCR analysis, three
technical replicates were performed. Primer sequences used in qPCR
are shown in Table I.
 | Table IPrimers used in the present study. |
Table I
Primers used in the present study.
| Primer name | Sequence (5'-3') |
|---|
| TNFα
forward |
GCCACCACGCTCTTCTGTCT |
| TNFα
reverse |
TGAGGGTCTGGGCCATAGAAC |
| IL-6
forward |
ACAACCACGGCCTTCCCTAC |
| IL-6
reverse |
CATTTCCACGATTTCCCAGA |
| IL-1β
forward |
ACCTTCCAGGATGAGGACATGA |
| IL-1β
reverse |
AACGTCACACACCAGCAGGTTA |
| IL-8
forward |
GGGTCGTACTGCGTATCCTG |
| IL-8
reverse |
AGACAAGGACGACAGCGAAG |
| TGF-β1
forward |
CACTCCCGTGGCTTCTAGTG |
| TGF-β1
reverse |
GGACTGGCGAGCCTTAGTTT |
| CHI3L1
forward |
CCCCGTTCCTGCGTTCTTAT |
| CHI3L1
reverse |
CAGGTGTTGGGCTATCTGGG |
| GAPDH
forward |
AATGACCCCTTCATTGAC |
| GAPDH
reverse |
TCCACGACGTACTCAGCGC |
ELISA
Mouse tumor necrosis factor (TNF)α (cat. no. MTA00B)
and interleukin (IL)-6 (cat. no. M6000B) ELISA kits (R&D
Systems, Inc.) were used according to the manufacturer's
instructions.
Western blot analysis
Western blot analysis was performed as described
previously (14). The cells were
homogenized, washed with PBS, and lysed in a RIPA buffer (Beyotime
Institute of Biotechnology). The protein concentration of lysates
was measured using Bio-Rad quantification assay (Bio-Rad
Laboratories, Inc.). Proteins (20 µg/lane) were separated using 10%
SDS-PAGE and transferred to a PVDF membrane (EMD Millipore). The
membrane was then blocked with 2.5% non-fat dry milk for 1 h at
room temperature. The antibodies for p65 (1:500; cat. no. 8242),
phosphorylated (p-)p65 (1:500; cat. no. 3033), IκB kinase (IKK)β
(1:500; cat. no. 2678), p-IKKβ (1:500; cat. no. 2694) and β-actin
(1:1,000; cat. no. 3700; endogenous control) were from Cell
Signaling Technology, Inc. Smad2 (1:500; cat. no. ab40855), p-Smad2
(1:500; cat. no. ab53100), Smad3 (1:500; cat. no. ab40854), p-Smad3
(1:500; cat. no. ab52903), TGF-β1 (1:500; cat. no. ab92486) and
CHI3L1 (1:500; cat. no. ab180569) were from Abcam. The primary
antibodies were added and incubated overnight at 4˚C. Following
incubation with the corresponding horseradish peroxidase-conjugated
secondary antibody (goat anti-rabbit IgG-HRP; 1:4,000; cat. no.
sc2004; or goat anti-mouse IgG-HRP; 1:4,000; cat. no. sc2005; Santa
Cruz Biotechnology, Inc.) at 25˚C for 2 h, the target protein was
visualized by enhanced chemiluminescence (cat. no. 32106, Thermo
Fisher Scientific, Inc.). Relative protein expression levels were
determined by scanning densitometry (ChemiDoc XRS + Systems;
Bio-Rad Laboratories, Inc.) and analyzed using Image Lab 5.0
software (Bio-Rad Laboratories, Inc.).
Statistical analysis
All data are presented as the mean ± standard
deviation of three independent experiments. One-way analysis of
variance (ANOVA) was performed to compare three or more groups. If
the ANOVA analysis was significant, the Tukey's post hoc test was
applied for comparison between each two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
FBDS reduces proinflammatory cytokine
expression in LPS-stimulated RAW264.7 macrophages
To investigate the effect of FBD on lung
inflammation, mouse macrophages RAW264.7 cells were incubated with
saline or with FBDS (low, moderate or high dosage) followed by
stimulation with LPS. Subsequently, the production of
proinflammatory cytokines was examined. At first, the cell
viability of RAW264.7 cells following FBDS treatment was examined
and it was identified that RAW264.7 viability was not affected by
FBDS treatment (Fig. S1A). As
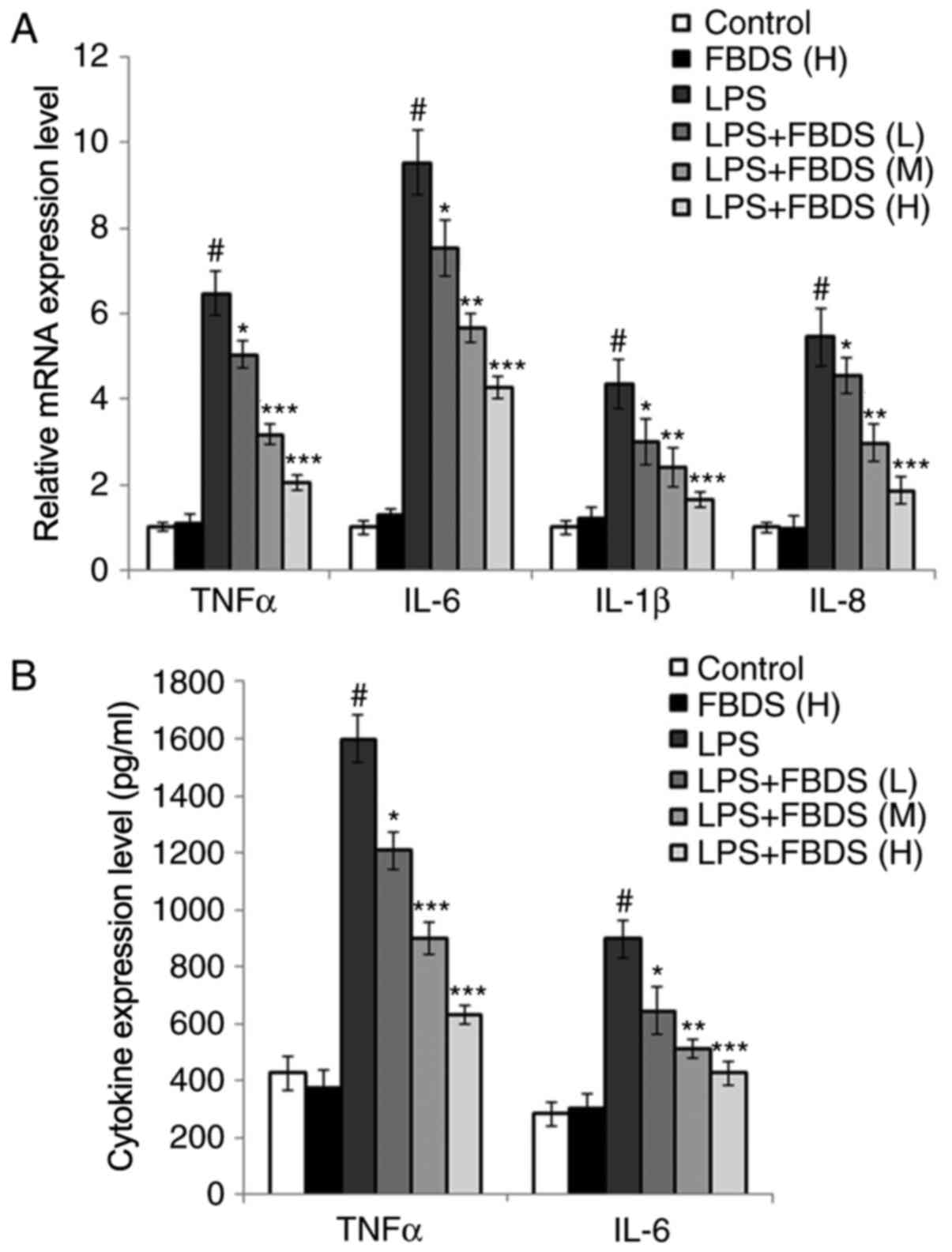
shown in Fig. 1A, treatment with
FBDS significantly decreased the mRNA levels of pro-inflammatory
cytokines induced by LPS stimulation (TNFα, IL-6, IL-1β and IL-8)
compared with the group treated with LPS and control serum in a
dose-dependent manner. In addition, consistent with the mRNA data,
the ELISA data indicated that protein expression levels of TNFα and
IL-6 were also decreased by FBDS incubation compared with the group
treated with LPS and control serum (Fig. 1B).
FBDS reduces proinflammatory cytokine
expression in LPS-stimulated BMDMs
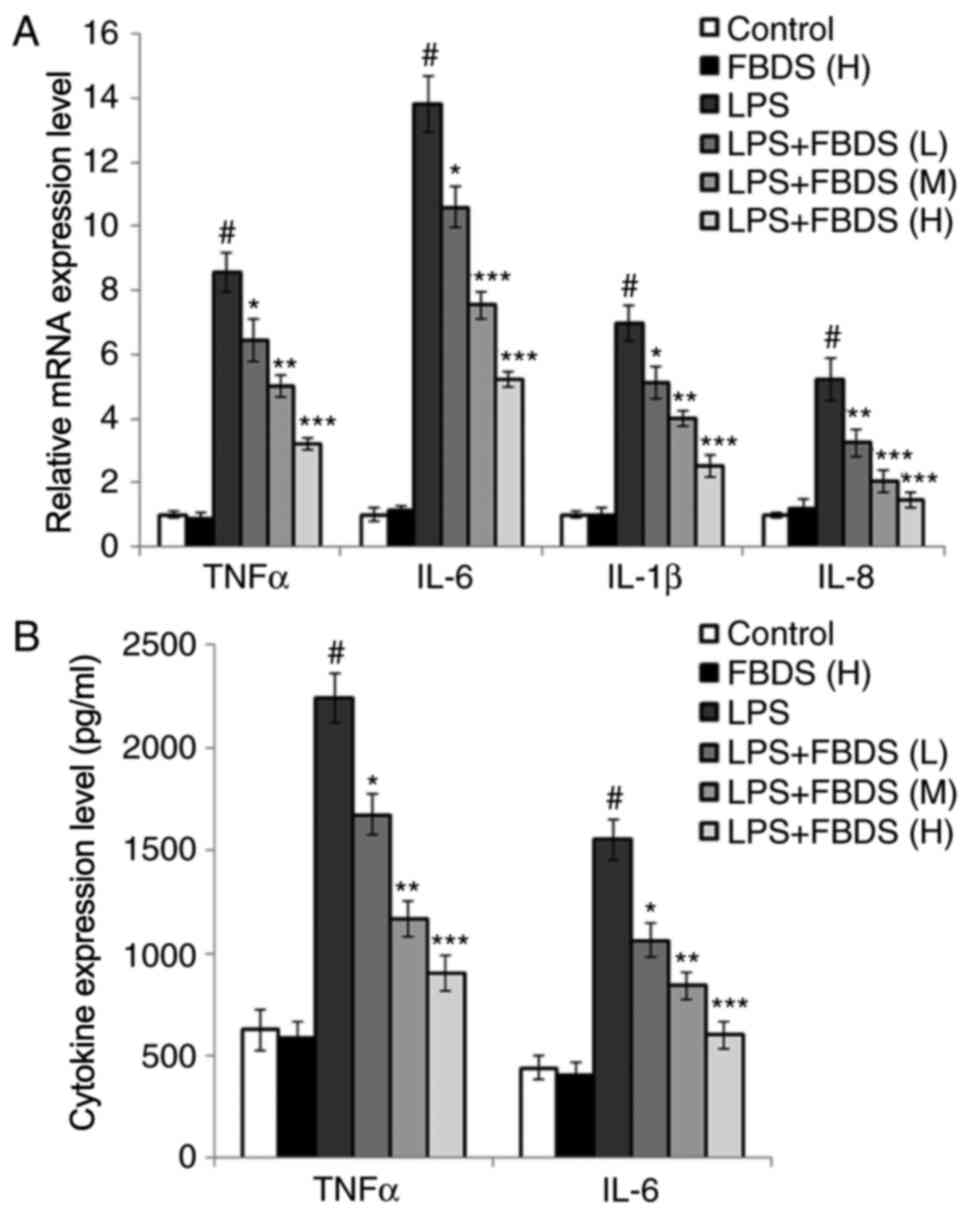
To confirm the results in Fig. 1, BMDMs were used and the experiments
repeated. The cell viability of BMDMs following FBDS treatment and
identification of BMDMs was confirmed (Fig. S1A and B). As shown in Fig. 2 and consistent with the expression
data in Fig. 1, it was identified
that FBDS treatment also decreased the production of
proinflammatory cytokines in both mRNA and protein levels in BMDMs
compared with the group treated with LPS and control serum.
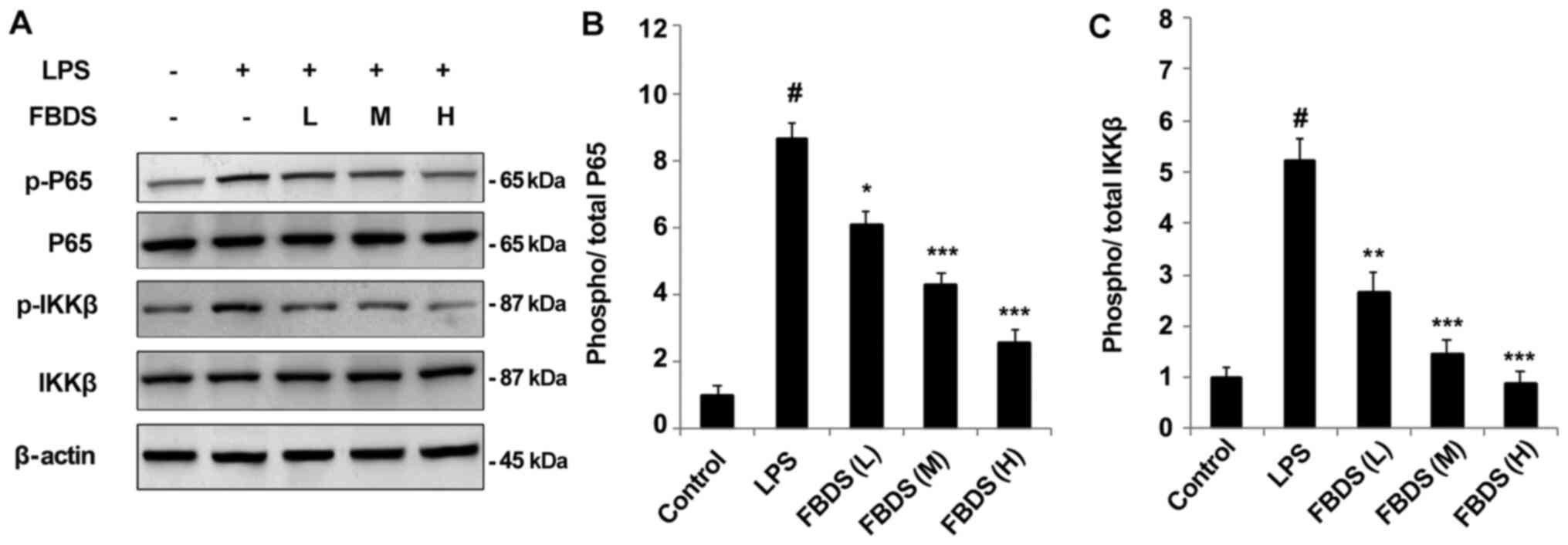
FBDS inhibits the activation of NF-κB
signaling pathway
NF-κB represents a paradigm for signal transduction
and proinflammatory cytokine production (15,16).
Therefore, the present study examined whether FBDS treatment
regulated LPS-induced proinflammatory cytokine production via the
NF-κB signaling pathway. Western blotting demonstrated that
LPS-induced phosphorylation of p65 and IKKβ were all suppressed by
FBDS treatment in BMDMs (Fig. 3A)
or RAW264.7 cells (Fig. S2A).
Western blotting and quantification of protein levels of p-P65 and
p-IKKβ are presented in Fig. 3.
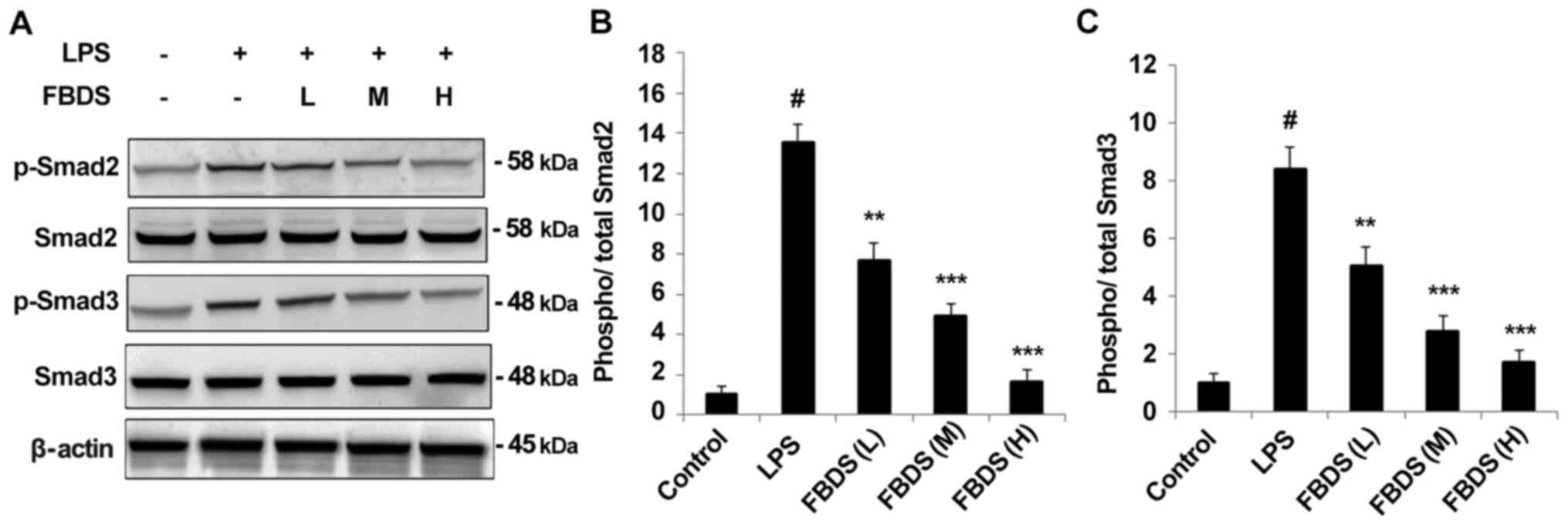
FBDS suppresses the activation of
Smad2/Smad3
A previous study indicated that the Smad signaling
pathways are closely associated with the genesis and development of
pulmonary fibrosis in response to inflammatory stimulation (17). Therefore the effect of FBDS on
Smad2/3 activation was examined. As shown in Fig. 4A, phosphorylation of Smad2/3 induced
by LPS was significantly inhibited in FBDS pre-treated BMDMs in a
dose-dependent manner compared with the group treated with LPS and
control serum, and similar results were also observed in RAW264.7
cells (Fig. S2B). Quantification
of protein levels of p-Smad2 and p-Smad3 are presented as Fig. 4B and C.
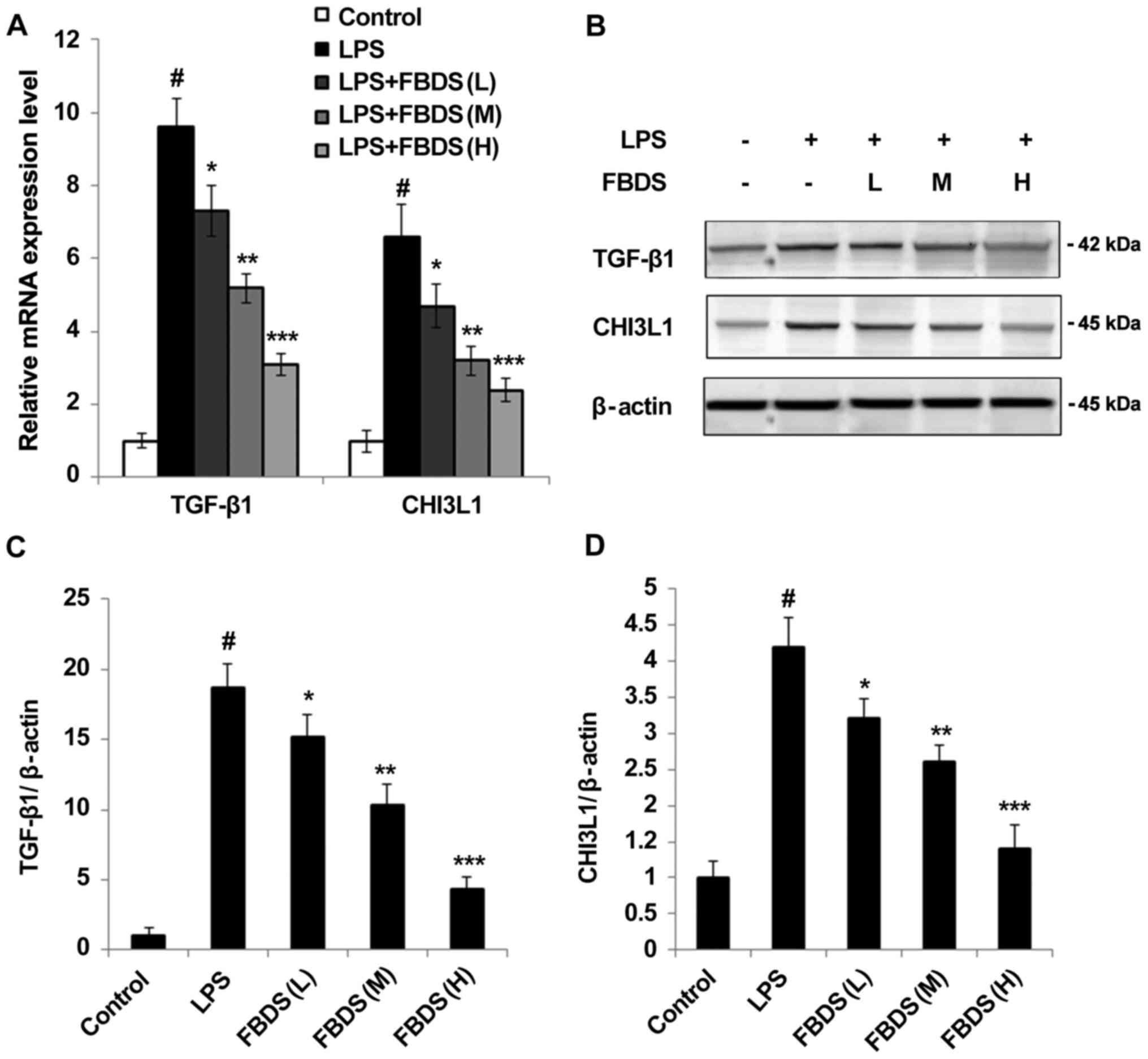
FBDS decreases the expression of
TGF-β1 and CHI3L1
TGF-β is well known as the critical upstream ligand
of Smad signaling pathways. It was observed that FBDS treatment
significantly decreased the mRNA level of TGF-β1 (Fig. 5A), as well as the protein level of
TGF-β1 (Fig. 5B and C). Previous studies suggested CHI3L1 as a
novel biomarker of inflammation, and whether FBDS could also
regulate the expression of CHI3L1 was investigated (1,2).
Notably, it was identified that the mRNA level of CHI3L1 was also
decreased by FBDS treatment compared with the group treated with
LPS and control serum (Fig. 5A).
The protein level of CHI3L1 was also suppressed in FBDS-treated
BMDMs (Fig. 5B and D) compared with the group treated with LPS
and control serum. Similar results were also observed in RAW264.7
cells (Fig. S2C).
Discussion
PF is a chronic, debilitating and often lethal lung
disorder. Although the molecular mechanisms of PF are gradually
becoming clear with numerous researchers' efforts, few effective
drugs have been developed to reverse human PF or even halt the
chronic progression to respiratory failure (8,18,19).
Therefore, novel strategies are urgently required. Traditional
Chinese medicine, which is the main component of the medical
practice used for >5,000 years in China, has been demonstrated
to be effective in the treatment of a diverse range human diseases
(20-22).
FBD has been widely used for treatment of patients with lung
diseases such as cough and PF. For the first time, to the best of
the authors' knowledge, it was identified in the present study that
FBDS significantly inhibit proinflammatory cytokine production in
macrophages, which suggested that FBD may also serve an
anti-inflammatory role.
NF-κB, is reported as a central dimeric
transcription factor, regulating the expression of genes
responsible for innate and adaptive immunity, cell proliferation
and apoptosis (15,16). Previous studies have also reported
that NF-κB signaling participates in the regulation of PF (23,24).
In present study, it was observed that FBDS treatment significantly
alleviated the inflammatory cytokines production induced by LPS in
a dose-dependent manner in RAW264.7 cells and BMDMs. In addition,
it was identified that FBDS treatment individually did not affect
the cell viability in RAW264.7 cells and BMDMs. Consistently, the
inflammatory cytokines expression levels were not changed following
LPS stimulation (data not shown), which indicated that FBDS may
inhibit inflammation by affecting downstream signaling pathways
stimulated by LPS, and NF-kB signaling pathway is the most
important downstream pathway in response to the LPS stimulation. It
was identified that FBDS suppressed the phosphorylation of P65 and
IKKβ, indicating that FBD may inhibit the activation of NF-κB.
Phosphorylation of Smad2 and Smad3 induces fibrosis and was
hypothesized to be the main cause of PF (25). The effect of FBDS on LPS-induced
phosphorylation of Smad2 and Smad3 was also detected, and it was
identified that the levels of phosphorylation were significantly
decreased by FBDS treatment; these data suggested that FBDS
alleviated LPS-induced inflammation primarily through the NF-κB and
Smad2/3 signaling pathways.
Notably, it was also identified that the mRNA and
protein expression of TGF-β1, the critical upstream ligand of Smad
signaling pathways, were significantly inhibited in FBDS-treated
macrophages. In addition, the expression of CHI3L1, a novel
biomarker of inflammation, was also inhibited at both mRNA and
protein levels. However, the precise mechanisms by which FBD
regulates the mRNA and protein expression of TGF-β1 and CHI3L1
requires further study. Although RAW264.7 cells and BMDMs were used
to illustrate the effect of FBDS in inflammation, both of these
cells are widely used in LPS-related inflammatory studies, so the
results of the present study may also need to be repeated in
another different type of macrophage cell line to prove the
robustness of the hypothesis.
Supplementary Material
Effect of FBDS on RAW264.7 and BMDMs
viability. and identification of BMDMs. RAW264.7 cells and BMDMs
were incubated with blank serum or FBDS (10%) for 24 h, followed by
LPS stimulation (100 ng/ml) for 8 h. (A) Cell viability of RAW264.7
and BMDMs. (B) BMDMs identified by flow cytometry using F4/80 and
CD11b antibodies. Data are representative of three independent
experiments (mean ± standard deviation). FBDS, Feibi
decoction-medicated serum; BMDMs, bone marrow derived macrophages;
LPS, lipopolysaccharide.
NF-κB, Smad2/Smad3 activation and
TGF-β1, CHI3L1 expression levels are suppressed by FBDS treatment
in RAW264.7 cells. RAW264.7 cells were incubated with blank serum
or FBDS (10%) for 24 h, followed by LPS stimulation (100 ng/ml) for
8 h. (A) Phosphorylation of p65 and IKKβ and (B) Smad2 and Smad3
were examined by western blot assay. (C) The protein levels of
TGF-β1 and CHI3L1. Data are representative of three independent
experiments. TGF, transforming growth factor; CHI3L1,
chitinase-3-like protein 1; FBDS, Feibi decoction-medicated serum;
LPS, lipopolysaccharide; p-, phosphorylated.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Natural Science
Foundation of China (grant no. 81573970).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW, GL and YJ contributed to the conception and
design of the study, data acquisition, analysis and revised the
manuscript. ZL and XZ contributed to data collection and
statistical analysis. HY and SL contributed to data collection,
statistical analysis and manuscript preparation. XZ and YJ
confirmed the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The protocols were performed in accordance with the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals (26). The
experiments were carried out according to the NIH Guide for the
Care and Use of Laboratory Animals (26) and approved by the Animal Ethics
Committee of the Scientific Investigation Board of Beijing
university of Chinese medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li Y, Liang J, Yang T, Monterrosa Mena J,
Huan C, Xie T, Kurkciyan A, Liu N, Jiang D and Noble PW: Hyaluronan
synthase 2 regulates fibroblast senescence in pulmonary fibrosis.
Matrix Biol. 55:35–48. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lasky JA and Ortiz LA: Antifibrotic
therapy for the treatment of pulmonary fibrosis. Am J Med Sci.
322:213–221. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhao L, Wang X, Chang Q, Xu J, Huang Y,
Guo Q, Zhang S, Wang W, Chen X and Wang J: Neferine, a
bisbenzylisoquinline alkaloid attenuates bleomycin-induced
pulmonary fibrosis. Eur J Pharmacol. 627:304–312. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang HD, Yamaya M, Okinaga S, Jia YX,
Kamanaka M, Takahashi H, Guo LY, Ohrui T and Sasaki H: Bilirubin
ameliorates bleomycin-induced pulmonary fibrosis in rats. Am J
Respir Crit Care Med. 165:406–411. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen CY, Peng WH, Wu LC, Wu CC and Shihlan
H: Luteolin ameliorates experimental lung fibrosis both in vivo and
in vitro: Implications for therapy of lung fibrosis. J Agric Food
Chemy. 58:11653–11661. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tsai KD, Yang SM, Lee JC, Wong HY, Shih
CM, Lin TH, Tseng MJ and Chen W: Panax notoginseng attenuates
Bleomycin-Induced pulmonary fibrosis in mice. Evid Based Complement
Alternat Med. 2011(404761)2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sener G, Topaloglu N, Sehirli AO, Ercan F
and Gedik N: Resveratrol alleviates bleomycin-induced lung injury
in rats. Pulm Pharmacol Ther. 20:642–649. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li LC and Kan LD: Traditional Chinese
medicine for pulmonary fibrosis therapy: Progress and future
prospects. J Ethnopharmacol. 198:45–63. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xiang J, Cheng S, Feng T, Wu Y, Xie W,
Zhang M, Xu X and Zhang C: Neotuberostemonine attenuates
bleomycin-induced pulmonary fibrosis by suppressing the recruitment
and activation of macrophages. Int Immunopharmacol. 36:158–164.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xiong X, Yang X, Liu Y, Zhang Y, Wang P
and Wang J: Chinese herbal formulas for treating hypertension in
traditional Chinese medicine: Perspective of modern science.
Hypertens Res. 36:570–579. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals, 8th
edition. National Academies Press, Washington, DC, 2011.
|
|
12
|
Ferri F, Parcelier A, Petit V, Gallouet
AS, Lewandowski D, Dalloz M, van den Heuvel A, Kolovos P, Soler E,
Squadrito ML, et al: TRIM33 switches off Ifnb1 gene transcription
during the late phase of macrophage activation. Nat Commun.
6(8900)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lee GT, Kwon SJ, Lee JH, Jeon SS, Jang KT,
Choi HY, Lee HM, Kim WJ, Kim SJ and Kim IY: Induction of
interleukin-6 expression by bone morphogenetic protein-6 in
macrophages requires both SMAD and p38 signaling pathways. J Biol
Chem. 285:39401–39408. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Karin M and Lin A: NF-kappaB at the
crossroads of life and death. Nat Immunol. 3:221–227.
2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li Q and Verma IM: NF-kappaB regulation in
the immune system. Nat Rev Immunol. 2:725–734. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Wang P, Nie X, Wang Y, Li Y, Ge C, Zhang
L, Wang L, Bai R, Chen Z, Zhao Y and Chen C: Multiwall carbon
nanotubes mediate macrophage activation and promote pulmonary
fibrosis through TGF-β/Smad signaling pathway. Small. 9:3799–3811.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Meyer KC: Pulmonary fibrosis, part I:
Epidemiology, pathogenesis, and diagnosis. Expert Rev Respir Med.
11:343–359. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Smith ML: Update on pulmonary fibrosis:
Not all fibrosis is created equally. Arch Pathol Lab Med.
140:221–229. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu X, Li D, Gao H, Gao Y, Zhang L, Du Y,
Wu J and Gao P: Protective effect of the traditional Chinese
medicine xuesaitong on intestinal ischemia-reperfusion injury in
rats. Int J Clin Exp Med. 8:1768–1779. 2015.PubMed/NCBI
|
|
21
|
Ding Z and Lian F: Traditional Chinese
medical herbs staged therapy in infertile women with endometriosis:
A clinical study. Int J Clin Exp Med. 8:14085–14089.
2015.PubMed/NCBI
|
|
22
|
Yin D, Liu Z, Peng D, Yang Y, Gao X, Xu F
and Han L: Serum containing Tao-Hong-Si-Wu decoction induces human
endothelial cell VEGF production via PI3K/Akt-eNOS signaling. Evid
Based Complement Alternat Med. 2013(195158)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Qin Y, Li S, Zhao G, Fu X, Xie X, Huang Y,
Cheng X, Wei J, Liu H and Lai Z: Long-term intravenous
administration of carboxylated single-walled carbon nanotubes
induces persistent accumulation in the lungs and pulmonary fibrosis
via the nuclear factor-kappa B pathway. Int J Nanomedicine.
12:263–277. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang D, Yuan W, Lv C, Li N, Liu T, Wang L,
Sun Y, Qiu X and Fu Q: Dihydroartemisinin supresses inflammation
and fibrosis in bleomycine-induced pulmonary fibrosis in rats. Int
J Clin Exp Pathol. 8:1270–1281. 2015.PubMed/NCBI
|
|
25
|
Zhou Y, He Z, Gao Y, Zheng R, Zhang X,
Zhao L and Tan M: Induced pluripotent stem cells inhibit
bleomycin-induced pulmonary fibrosis in mice through suppressing
TGF-β1/Smad-mediated epithelial to mesenchymal transition. Front
Pharmacol. 7(430)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
National Research Council (US) Institute
for Laboratory Animal Research: Guide for the Care and Use of
Laboratory Animals. National Academies Press (US), Washington, DC,
1996.
|