Introduction
The growth and development of the fetus is a complex
phenomenon that can be influenced by several variables. Although
electrolytes are present in the amniotic fluid in trace amounts,
they are considered essential for the health and well-being of the
fetus. Associations between amniotic fluid electrolyte
concentrations and fetal development have been made (1). Common ions found in amniotic fluid
include sodium, potassium, chloride, calcium, magnesium, phosphate
and bicarbonate (2). These ions in
the amniotic fluid serve an important role in a normal pregnancy
and can aid in prevention and early diagnosis of fetal or maternal
pathologies. Through accurate prenatal assessment of the
biochemical composition of the amniotic fluid, overall health
status and fetal maturity can be evaluated (1-3).
Normally, the volume of amniotic fluid increases
steadily until it reaches a maximum of 400-1200 ml at 34-38 weeks.
Afterwards, the volume starts to decline. At 40 weeks, the volume
of amniotic fluid can measure ~800 ml and continues to decrease as
the pregnancy goes on (4-6).
Although the composition of the amniotic fluid does not remain
constant during pregnancy, the bulk volume at all times (~98%) is
water. Other important constituents are urea, creatinine, glucose,
proteins, lipids, bile pigments, fetal epithelial cells and mineral
ions (7).
Common ions have important uses during pregnancy.
Sodium contributes to the regulation of water-electrolyte balance
of the amniotic fluid, high chloride reflects possible renal
pathologies, high potassium and calcium can be signs of
pre-eclampsia, high phosphate indicates reduced antibacterial
activity, magnesium and zinc (Zn) assess the risk of fetal growth
retardation (8-12).
Potassium, phosphate and Zn also affect normal antimicrobial
activity (13-15).
Some heavy metal ions can also be traced in the
amniotic fluid, and small amounts of these elements are normal.
However, large quantities have been shown to have detrimental
effects in humans. For example, lead (Pb) is known for its negative
effects on neural development (16), while cadmium (Cd) is known for its
risk of preterm delivery (17).
Usually, this can be seen in people living in highly
industrialized, highly polluted and poor areas (18-22).
As such, the assessment of these ions in the amniotic fluid is
essential to understanding the risks these people might be facing.
Heavy metals in high concentrations have the ability to disrupt
normal physiological processes. Copper (Cu), arsenic (As), Cd,
nickel (Ni), chromium, mercury, manganese and Pb can lead to fetal
growth retardation, pre-eclampsia, impaired cognitive development
and even cancer (23-28).
The present study had two objectives. The first was
to assess the differences of heavy metal ion concentrations in the
amniotic fluid between preterm (between weeks 15 and 37) and term
(starting week 37) pregnancies. Moreover, the second was to assess
whether pregnant women from two cities with different industrial
levels from Romania would present different heavy metal ion
concentrations in their amniotic fluid.
Materials and methods
Study design
The present retrospective study was conducted in the
‘Bega’ Maternity Clinic in Timisoara, Romania between April 1st
2020 and April 1st 2021. The study design is in accordance with The
Declaration of Helsinki and was approved by the Ethics Committees
of the ‘Bega’ Maternity Clinic (approval no. 260/16IUL2021) and
Petrosani Hospital (approval no. 15990/27.07.2021).
Two cohorts of pregnant patients were examined. The
first included 100 pregnant women admitted in the ‘Bega’ Maternity
Clinic from Timisoara. The second included 60 pregnant women
admitted in the Maternity Clinic of the Petrosani Emergency
Hospital. Written informed consent was provided by all 160
individuals for amniocentesis and use of data for research
purposes. Amniocentesis was performed on all patients, and each
patient cohort was separated in two equal groups.
Participants
The inclusion criteria were as follows: Consenting
adult women with single fetal gestation, patients with medical
indications for amniocentesis (such as maternal age over 35,
unfavorable or uncertain results obtained at screening tests,
previous exposure to infectious agents including Toxoplasma
gondii or cytomegalovirus and known genetic disorders running
in the family). The exclusion criteria were: Pregnancies earlier
than 15 weeks, pregnant patients with severe anemia, hematological,
neoplastic, cardiac or metabolic conditions and patients with
previous perinatal complications or fetal disorders.
Petrosani and Timisoara were selected in order to
determine whether a mountainous, highly industrial city might
harbor higher heavy metal ion concentrations in the amniotic fluid
than a city in the plains, with moderate industry. The patients
were stratified into four groups: Group 1 (n=50), women from
Timisoara with preterm pregnancies (15-37 weeks); group 2 (n=50),
women from Timisoara with term pregnancies (≥37 weeks); group 3
(n=30), women from Petrosani with preterm pregnancies; and group 4
(n=30), women from Petrosani with term pregnancies.
Amniotic liquid sampling
All procedures were performed under careful sterile
and antiseptic conditions. Before the amniocentesis procedure, an
ultrasound evaluation was carried out in order to determine the
location of the placenta, fetus and other characteristics of the
amniotic fluid.
To enter the amniotic cavity, a spinal needle with a
gauge of 20-22 was used under continuous ultrasound guidance. The
entry into the amniotic cavity was done firmly in order to prevent
rupture of the amniotic membrane and avoiding the placenta. Once
the entry into the cavity was confirmed by ultrasound, the amniotic
fluid was slowly aspirated. The first 2 ml were discarded, as they
may be contaminated with maternal cells. A quantity of 20-22 ml was
deemed sufficient, as 18-20 ml were used for genetic, sex and lung
development testing (for pregnancies after week 32). The remaining
2 ml were used for the evaluation of heavy metal ion
concentration.
After removal of the needle, the mothers were kept
under further ultrasound evaluation to confirm proper fetal heart
rate. Intramuscular administration of anti-D immuno-globulin was
also done for Rhesus-negative women in order to prevent fetal
Rhesus disease. After completing the procedure, the mothers were
advised to avoid strenuous and sexual activities for the next 48
h.
Detection of heavy metal ions
The working method used for the detection of heavy
metals in the amniotic fluid involved flame atomic absorption
spectroscopy. Each amniotic fluid sample was nebulized in the gases
of the spectrophotometer's flame. Each of the studied metals have
well-known specific absorption rates and the spectrophotometer was
also equipped with an interference correction system. All
measurements are presented in mg/l.
The method follows national guidelines (SR ISO
8288/2001 standards). All reagents used in this determination were
of high quality and the water used was ultrapure, with no traces of
heavy metals. All spectrophotometer readings were analyzed on a
connected computer running GBC Avanta 1.33 (GBC Scientific
Equipment). The calibration values used for the readings are
presented in Table I.
 | Table ICalibration values for the flame
atomic absorption spectroscopy. |
Table I
Calibration values for the flame
atomic absorption spectroscopy.
| Metal | Zn | Cu | Cd | Ni | Pb | As | Fe |
|---|
| Maximum error | 0.037 | 0.027 | 0.012 | 0.039 | 0.083 | 0.027 | 0.013 |
| R2 | 0.999 | 1.000 | 0.999 | 0.998 | 0.995 | 0.998 | 1.000 |
Data collection
The following clinical and demographical data were
collected for each patient: Age of the patient, gestational age,
concentrations of Pb, Cu, Ni, Cd, As, Zn, iron (Fe) (in mg/l),
femur length (in mm), location of residence (urban or rural) and
smoking status (no smoking, either smoking in the past or quitting
the habit once the patient found out about the pregnancy and active
smoking). The data were recorded in an Excel file (2016 Office
Suite, Microsoft).
Smoker status
Information on tobacco use was also collected.
Active smokers were defined as patients smoking even during
pregnancy. Former smokers were defined as patients that had smoked
and gave up the habit in the past, as well as mothers, which quit
smoking once the pregnancy was suspected and/or confirmed.
Nonsmokers were defined as patients that never smoked.
Statistical analysis
Normal distribution was assessed using the
Shapiro-Wilk test. Descriptive statistics for numerical variables
include means, standard deviations, medians, interquartile range
(IQR, 1st quartile - 3rd quartile) and range. For the comparison of
with non-parametric variables, the Mann-Whitney U test was used.
For categorical variables, frequency (%) and/or count (n) were
included, and Fisher's exact test used to analyze the contingency
tables. The α-level was set at 0.05. P<005 was considered to
indicate a statistically significant difference. All data were
processed using SPSS version 22 for Windows (IBM Corp.).
Results
Overview
In the Timisoara cohort, a total of 100 pregnant
women between the age of 20 and 35 were admitted in the maternity
during the study period. The mean maternal ages for groups 1
(preterm) and 2 (term) were 27.76±3.83 and 27.46±3.58 years,
respectively. Mean gestational age was 18.14±1.81 weeks for the
preterm group and 38.66±1.04 weeks for the term group. Mean femur
length for the preterm group was 24.94±6.32 and 74.55±2.65 for the
term group. All demographic data are presented in Table II. The distribution of the area of
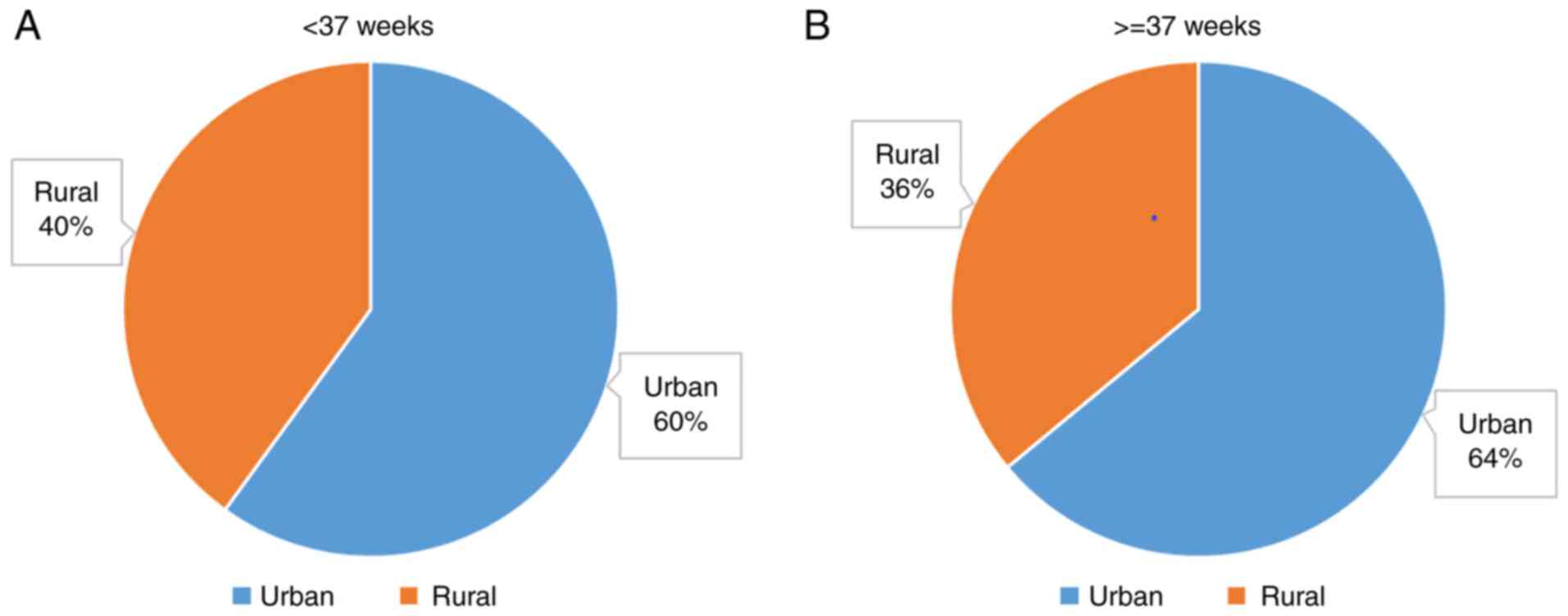
residence distribution is presented in Fig. 1. Statistical analysis of the area of
residence did not reveal a significant difference between the
groups (P=0.83694; Fisher's exact test).
 | Table IIDemographic data for patients from
Timisoara. |
Table II
Demographic data for patients from
Timisoara.
| Parameter | Preterm
pregnancies | Term
pregnancies |
|---|
| Gestational age,
weeks | | |
|
Mean | 18.14 | 38.66 |
|
SD | 1.81 | 1.04 |
|
Median | 18.00 | 39.00 |
|
IQR | 16.88-19.88 | 38.00-39.00 |
|
Range | 6.00 | 4.00 |
| Maternal age,
years | | |
|
Mean | 27.76 | 27.46 |
|
SD | 3.83 | 3.58 |
|
Median | 28.00 | 28.00 |
|
IQR | 24.88-30.13 | 26.00-29.00 |
|
Range | 15.00 | 15.00 |
| FL, mm | | |
|
Mean | 24.94 | 74.55 |
|
SD | 6.32 | 2.65 |
|
Median | 25.30 | 75.05 |
|
IQR | 19.80-29.44 | 72.39-75.63 |
|
Range | 23.50 | 11.70 |
The Petrosani cohort consisted of 60 pregnant women
between the age of 19 and 35 admitted in the maternity. The mean
maternal age was 27.20±4.47 years for group 3 (preterm) and
26.47±4.09 years for group 4 (term). Mean gestational age was
18.33±1.84 weeks for the preterm group and 39.07±0.94 weeks for the
term group. Mean femur length for the preterm group was 25.15±5.98
and 76.13±1.76 for the term group. The demographic data for these
groups are presented in Table
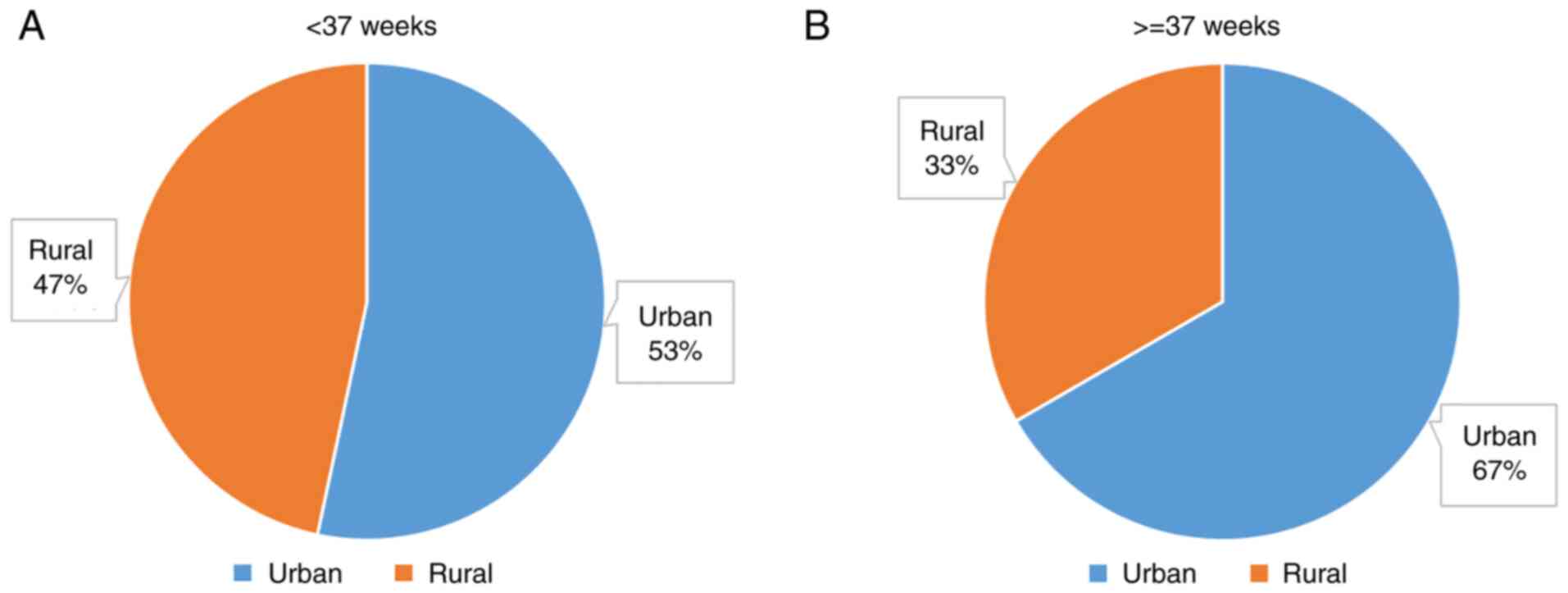
III. The distribution of the area of residence distribution is
presented in Fig. 2. Statistical
analysis of the area of residence using Fisher's exact test showed
no significant difference between the groups (P=0.42956).
 | Table IIIDemographic data for patients from
Petrosani. |
Table III
Demographic data for patients from
Petrosani.
| Parameter | Preterm
pregnancies | Term
pregnancies |
|---|
| Gestational age,
weeks | | |
|
Mean | 18.33 | 39.07 |
|
SD | 1.84 | 0.94 |
|
Median | 18.00 | 39.00 |
|
IQR | 17.00-20.00 | 38.00-39.88 |
|
Range | 6.00 | 3.00 |
| Maternal age,
years | | |
|
Mean | 27.20 | 26.47 |
|
SD | 4.47 | 4.09 |
|
Median | 28.00 | 26.50 |
|
IQR | 23.75-30.13 | 23.88-29.00 |
|
Range | 16.00 | 15.00 |
| FL, mm | | |
|
Mean | 25.15 | 76.13 |
|
SD | 5.98 | 1.76 |
|
Median | 25.35 | 75.50 |
|
IQR | 19.41-30.24 | 75.49-77.10 |
|
Range | 22.30 | 7.90 |
Analysis of the Timisoara cohort
In the Timisoara cohort, there were no statistically
significant differences in the heavy metal ion concentrations
between pre-term and term pregnancies (Table IV).
 | Table IVHeavy metal concentrations in
patients from Timisoara (n=100) with preterm and term
pregnancies. |
Table IV
Heavy metal concentrations in
patients from Timisoara (n=100) with preterm and term
pregnancies.
| Metal | Mean | SD | Median | IQR | Range |
P-valuea |
|---|
| Pb | | | | | | |
|
Preterm | 0.0409 | 0.1582 | 0.0001 | 0.0000-0.0136 | 1.012 | 0.74300 |
|
Term | 0.0570 | 0.1944 | 0.0002 | 0.0000-0.0138 | 1.012 | |
| Cu | | | | | | |
|
Preterm | 0.4736 | 0.5068 | 0.2840 | 0.0829-0.9263 | 1.662 | 0.59780 |
|
Term | 0.5810 | 0.5106 | 0.3690 | 0.0780-1.0339 | 1.738 | |
| Ni | | | | | | |
|
Preterm | 0.4426 | 0.6478 | 0.0131 | 0.0000-0.9954 | 2.057 | 0.09952 |
|
Term | 0.6863 | 0.7292 | 0.7175 | 0.0000-1.2206 | 2.787 | |
| Cd | | | | | | |
|
Preterm | 0.0067 | 0.0354 | 0.0001 | 0.0000-0.0001 | 0.238 | 0.69680 |
|
Term | 0.0103 | 0.0429 | 0.0001 | 0.0000-0.0001 | 0.240 | |
| As | | | | | | |
|
Preterm | 0.9131 | 1.0294 | 0.5530 | 0.0000-1.7294 | 3.769 | 0.17080 |
|
Term | 0.7219 | 0.9954 | 0.0020 | 0.0000-1.4208 | 3.767 | |
| Zn | | | | | | |
|
Preterm | 0.0776 | 0.1518 | 0.0145 | 0.0000-0.0674 | 0.658 | 0.98310 |
|
Term | 0.0534 | 0.1329 | 0.0140 | 0.0000-0.0369 | 0.742 | |
| Fe | | | | | | |
|
Preterm | 0.3072 | 0.3624 | 0.1504 | 0.0414-0.4477 | 1.500 | 0.13470 |
|
Term | 0.3332 | 0.3126 | 0.2333 | 0.1769-0.3369 | 1.382 | |
Analysis of the Petrosani cohort
In the Petrosani cohort, no statistically
significant differences were observed between pre-term and term
pregnancies regarding Pb, Cu, Ni, As and Fe concentrations.
However, the concentrations of Cd and Zn were significantly higher
in the pre-term than in the term pregnancies (Table V).
 | Table VHeavy metal concentrations in
patients from Petrosani (n=60) with preterm and term
pregnancies. |
Table V
Heavy metal concentrations in
patients from Petrosani (n=60) with preterm and term
pregnancies.
| Metal | Mean | SD | Median | IQR | Range |
P-valuea |
|---|
| Pb | | | | | | |
|
Preterm | 0.0728 | 0.2249 | 0.0020 | 0.0000-0.0284 | 1.124 | 0.20840 |
|
Term | 0.1579 | 0.3048 | 0.0190 | 0.0000-0.0865 | 1.202 | |
| Cu | | | | | | |
|
Preterm | 0.0386 | 0.0519 | 0.0265 | 0.0054-0.0468 | 0.265 | 0.78420 |
|
Term | 0.0260 | 0.0200 | 0.0185 | 0.0109-0.0409 | 0.074 | |
| Ni | | | | | | |
|
Preterm | 0.5743 | 0.7706 | 0.0990 | 0.0000-1.2199 | 2.198 | 0.47120 |
|
Term | 0.7173 | 0.8653 | 0.1765 | 0.0000-1.4485 | 2.898 | |
| Cd | | | | | | |
|
Preterm | 0.0135 | 0.0463 | 0.0003 | 0.0000-0.0020 | 0.235 |
0.01512b |
|
Term | 0.0070 | 0.0368 | 0.0001 | 0.0000-0.0001 | 0.202 | |
| As | | | | | | |
|
Preterm | 1.0785 | 0.9899 | 0.7525 | 0.2260-1.7548 | 3.004 | 0.66710 |
|
Term | 1.1075 | 1.2392 | 0.7632 | 0.0086-1.8127 | 4.743 | |
| Zn | | | | | | |
|
Preterm | 0.2202 | 0.2975 | 0.0845 | 0.0344-0.3295 | 1.259 |
0.02606b |
|
Term | 0.0604 | 0.053 | 0.0425 | 0.0199-0.0989 | 0.177 | |
| Fe | | | | | | |
|
Preterm | 0.3377 | 0.4297 | 0.1235 | 0.0704-0.5135 | 1.747 | 0.16690 |
|
Term | 0.4119 | 0.3316 | 0.3760 | 0.1406-0.6029 | 1.500 | |
Analysis between Timisoara and
Petrosani
The Timisoara and Petrosani were also analyzed as a
whole. No significant differences were observed with respect to Ni
and Fe concentrations. However, the median concentrations of Pb,
Cd, As and Zn were significantly higher in the Petrosani cohort,
compared with patients from Timisoara. The median Cu concentration
was significantly higher in the Timisoara cohort, compared with
that in the Petrosani cohort (Table
VI).
 | Table VIComparison of heavy metal
concentrations in the amniotic fluid between the Timisoara (n=100)
and the Petrosani (n=60) groups. |
Table VI
Comparison of heavy metal
concentrations in the amniotic fluid between the Timisoara (n=100)
and the Petrosani (n=60) groups.
| Metal | Mean | SD | Median | IQR | Range |
P-valuea |
|---|
| Pb | | | | | | |
|
Timisoara | 0.0489 | 0.1765 | 0.0001 | 0.0000-0.0130 | 1.012 |
0.04513b |
|
Petrosani | 0.1136 | 0.2671 | 0.0030 | 0.0000-0.0468 | 1.202 | |
| Cu | | | | | | |
|
Timisoara | 0.5281 | 0.5339 | 0.3225 | 0.0805-1.0230 | 1.738 |
<0.00001b |
|
Petrosani | 0.0322 | 0.0392 | 0.0190 | 0.0090-0.0433 | 0.265 | |
| Ni | | | | | | |
|
Timisoara | 0.5645 | 0.6970 | 0.0010 | 0.0000-1.1390 | 2.787 | 0.78150 |
|
Petrosani | 0.6480 | 0.8089 | 0.0504 | 0.0000-1.3100 | 2.898 | |
| Cd | | | | | | |
|
Timisoara | 0.0085 | 0.0392 | 0.0001 | 0.0000-0.0001 | 0.239 |
0.00002b |
|
Petrosani | 0.0101 | 0.0413 | 0.0001 | 0.0000-0.0010 | 0.235 | |
| As | | | | | | |
|
Timisoara | 0.8175 | 1.0120 | 0.2310 | 0.0000-1.5270 | 3.768 |
0.03027b |
|
Petrosani | 1.0751 | 1.1116 | 0.7400 | 0.0685-1.7029 | 4.743 | |
| Zn | | | | | | |
|
Timisoara | 0.0664 | 0.1462 | 0.0140 | 0.0000-0.0560 | 0.742 |
<0.00001b |
|
Petrosani | 0.1394 | 0.2249 | 0.0530 | 0.0238-0.1418 | 1.266 | |
| Fe | | | | | | |
|
Timisoara | 0.3202 | 0.3370 | 0.2219 | 0.0982-0.4240 | 1.500 | 0.44540 |
|
Petrosani | 0.3735 | 0.3793 | 0.2660 | 0.0883-0.5653 | 1.768 | |
Analysis of smoking status
As smoking may affect the concentrations of trace
elements such as heavy metal ions in the blood, urine, hair and
toenail samples, the smoking status of the patients was also
assessed. Fisher's exact test was used to see if the smoking status
was associated with gestational age or with the area of residence.
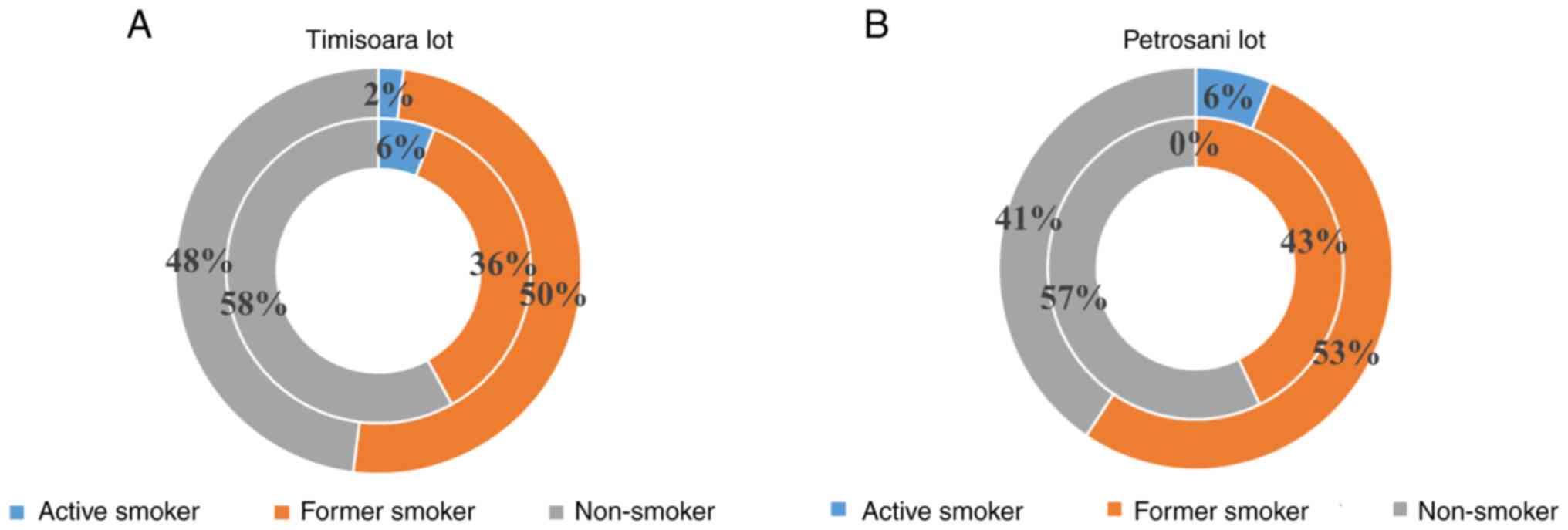
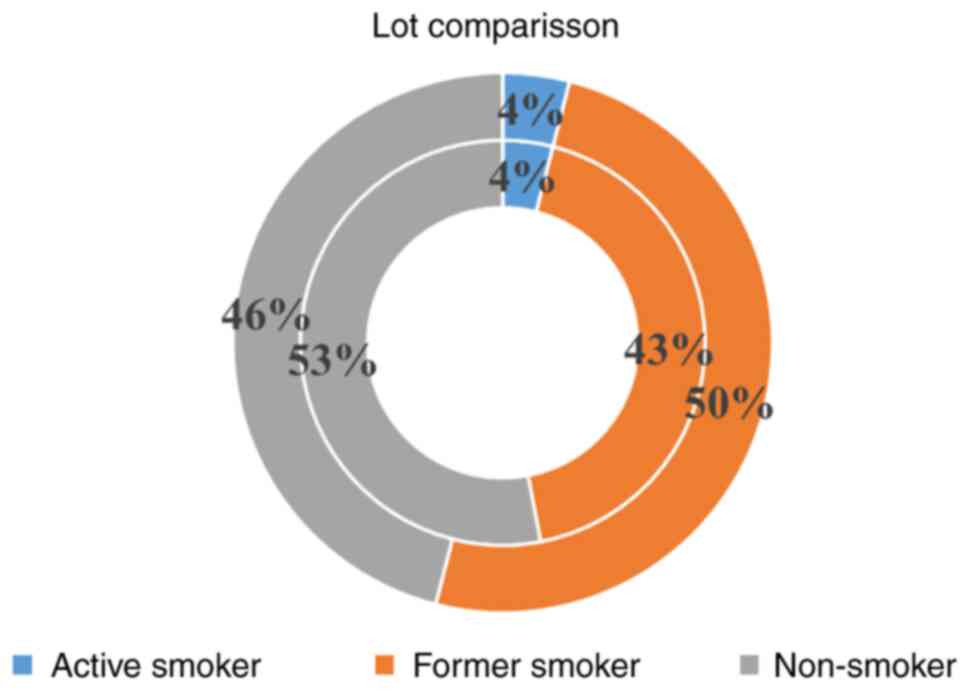
(Table VII; Figs. 3 and 4). There was no association between
smoking status and either of these two parameters.
 | Table VIISmoking status of patients from the
Timisoara and the Petrosani groups. |
Table VII
Smoking status of patients from the
Timisoara and the Petrosani groups.
| Patient group | Active smoker,
n | Former smoker,
n | Non-smoker, n |
P-valuea |
|---|
| Timisoara (all
patients) | 4 | 43 | 53 | 0.7193 |
| Petrosani (all
patients) | 2 | 28 | 30 | |
| Timisoara | | | | |
|
Preterm | 3 | 18 | 29 | 0.26855 |
|
Term | 1 | 25 | 24 | |
| Petrosani | | | | |
|
Preterm | 0 | 12 | 16 | 0.23577 |
|
Term | 2 | 17 | 13 | |
Discussion
Amniotic fluid plays a key role in the development
of the fetus. It protects the fetus from mechanical shock and
insulates it thermally, whilst helping elements from the maternal
plasma reach the fetus, especially in the early pregnancy, before
the formation of the placenta and it participates in keeping the
antibacterial balance. These properties are the result of the
bioactive compounds that take part in its constitution (7).
Since implantation, the extracolemic cavity is
produced, forming the amniotic space. The fetus and the amniotic
fluid are enveloped by the amniotic sac. At weeks 34-38 the
amniotic fluid reaches its maximum value, after which it starts
declining as the pregnancy goes on (4-6).
However, the concentrations of its bioactive compounds changes as
the osmolarity of the fluid decreases. These concentrations are
important as they can provide insight into the status of the fetus
through amniocentesis.
Although they are found in small amounts, mineral
ions can play an important role in the development of the fetus.
Common ions are sodium, chloride, potassium, calcium, magnesium,
bicarbonate and phosphate (2,7,29). In
addition, trace levels of heavy metals, such as Cu, As, Cd, Ni,
chromium, mercury, manganese, Zn and Pb, can also be found in the
amniotic fluid (1,2,7).
Sodium is involved in regulating the
water-electrolyte balance (9,29-31).
Chloride can be used to determine possible renal disorders, such as
the Barter syndrome (8,9,32).
Raised potassium levels may lead to pre-eclampsia and lower
antibacterial activity (11,14).
Elevated calcium levels can result in pre-eclampsia and spina
bifida, while low levels are associated with preterm deliveries
(33-37).
Low levels of magnesium have been associated with pre-eclampsia and
possible fetal growth retardation (12,13,38-40).
High bicarbonate may indicate complicated twin-twin transfusion
syndromes (41), while phosphate
plays a role in antimicrobial activity (42-44).
Heavy metals are metalloids with high density or
atomic number, which are usually found in trace amounts in serum.
Several have well-known physiological functions, such as heme
constitution, hormone production, enzyme regulation or even act as
antioxidants (45). Therefore, it
is normal for these elements to be found in tissues and bodily
fluids. When concentrations are elevated however, certain health
risks may appear. In adults, risks range from acute hepatic injury,
cardiovascular, neuro-psychological disorders to chronic poisoning
or even death (46-48).
Considering the risks adults are exposed to, prudence is advisable
regarding the risks in children.
Zn is essential for embryogenesis and neurogenesis.
Deficiency in this element is associated with fetal growth
retardation and neurodegenerative disorders (28,49-51).
It may also promote antimicrobial activity (15,17).
Due to these concerns, similarly to magnesium and iron,
supplementation for both pregnant and lactating mothers is
recommended. The average intake is in the range of 9.6-11.2 mg/day
(51,52). Another reason to determine the
amniotic fluid levels is that oxidative stress, induced by
menadione, is further exacerbated by high concentrations (80 µM) of
Zn (53). In the Timisoara cohort
of the present study, the differences in Zn concentrations between
term and preterm pregnancies were not statistically significant. In
the Petrosani cohort, the preterm pregnancy group had a median Zn
concentration higher than that of the term pregnancy group. The
differences between the two cohort were also statistically
significant.
Cu is an essential cofactor for several
metalloenzymes. It also has antimicrobial and antiviral properties.
Average intake varies from 0.6 to 1.6 mg/day, although this can be
higher during pregnancy and breastfeeding (54). Deficits may lead to neurological and
immunological abnormalities of the fetus (54). By contrast, high Cu concentrations
may lead to diverse abnormalities. For example, high concentrations
in maternal serum have been linked to pre-eclampsia (55,56),
and elevated levels in the amniotic fluid have been associated with
fetal growth retardation (28).
When considering genetics, Cu is has been implicated in two major
diseases, namely Wilson and Menkes diseases. In both cases,
accumulation of Cu in the fetus can usually be observed prenatally
(57-60).
In the present study, the differences between term and preterm
pregnancies were not statistically significant, both for patients
from Timisoara and from Petrosani. When comparing the two cohorts,
the Timisoara cohort presented higher median concentrations, with
the differences being statistically significant.
Ni is essential for gut bacterial growth, hormone
production, iron absorption and as part of RNA and DNA. Recommended
dietary allowance for women is 400-600 µg/day (61,62).
Large doses of Ni or prolonged exposure can cause harmful effects
such as genotoxicity, hematotoxicity, teratogenicity,
immunotoxicity, carcinogenicity and allergic reactions (62,63).
Smokers and families with lower socio-economic status might be
exposed to higher doses (62-64).
Rodent models have shown that the transfer of Ni through the
placenta occurs mainly from the mother to the fetus, resulting in
its accumulation in the amniotic fluid or fetal organs (65,66).
In the present study, the differences between term and preterm
pregnancies were not considered statistically significant for both
cohorts. There were no statistically significant differences in Ni
concentrations between the two cohorts.
Fe is an important molecule that serves several
functions in the body, with the most important role being in oxygen
transportation, as part of the heme structure (67). Another important role is combating
infections (68). Normal intake for
iron is 16 mg/day for women and 7-11 mg/day for children <14
years (69). Fe deficiency is a
common worldwide problem leading to anemia. Children and pregnant
women are at a high risk for this condition and may need
supplementation. During pregnancy, total intake should reach 27
mg/day, while during lactation, the recommended intake falls to 10
mg/day (70).
Deficiency and anemia in pregnant women has been
associated with low birth weight, preterm delivery and potential
fetal anemia (71,72). During intra-amniotic infection or
inflammation complications, the amniotic levels of Fe are not
significantly increased compared with healthy individuals. This may
be due to hepcidin upregulation, which may result in hypoferremia
in maternal serum, as hepcidin redirects Fe to macrophages
correlated with the infection or inflammation episode (73,74).
Common symptoms of Fe deficiency anemia include paleness, fatigue,
reduced cognitive performance and diminished immune responses.
Infants experience these as well, along with an increased risk of
cognitive and psychomotor developmental deficit (75).
Excess Fe can be observed after repeated blood
transfusions or in congenital cases of hemochromatosis. Mutations
of the High Fe2+ protein family and other Fe transport
proteins lead to iron build-up in the organism, resulting in liver
cirrhosis, hepatocellular carcinoma, heart disease and impaired
pancreatic function (76). Although
these symptoms usually appear in later life, infants with Fe
overload may be at risk of developing brain and hematopoiesis
alterations (77).
In the present study, the differences in Fe
concentrations between term and preterm pregnancies were not
considered statistically significant in the Timisoara or Petrosani
cohorts. Nor were there any differences between the two
cohorts.
Cd is an element still being investigated, along
with Pb and As. It is commonly found in cigarettes. Present only in
very small amounts in the body, no physiological role has been
established yet. As a toxic element, previous studies have looked
into its effects on cardiovascular function (78,79),
obesity and ghrelin regulation (80,81),
possible cancer occurrence (82,83),
reproduction and pregnancy (84-87).
Rodent fetal experimentation has suggested cytotoxicity problems,
progesterone disorders, microRNA expression changes, elevated
oxidative stress and DNA damage (84-86).
The toxic effects from Cd, such as high oxidative stress,
cytotoxicity and apoptosis have also been shown in humans (87). Prenatal exposure has been linked
with lower birth weights, preterm deliveries and even possible
spontaneous abortion (88-90).
Elevated amniotic fluid levels have been also linked with
pre-eclampsia (26,91). Children with such prenatal history
might also be prone to cardiometabolic disorders (92). In the present study, the differences
in Cd concentration between term and preterm pregnancies were not
considered statistically significant in patients from Timisoara. In
the Petrosani cohort, the median concentrations for the preterm
pregnancy group were significantly higher, compared with the term
group. Compared with patients from Timisoara, the Petrosani cohort
presented significantly higher median concentrations.
As is an element known since ancient times, where it
was frequently used as a poison. Very small amounts do have some
physiological functions, interacting with the metabolism of
selenium and methionine; the normal dose is in the range of 12-40
µg/day (93,94). Except for the derivative arsenic
trioxide (As2O3), which has antitumor
properties, the levels of As should be maintained in the
recommended levels, as high doses can lead to neuronal insulin
signaling disruption and the development of malignancies, severe
gastrointestinal toxicities, diabetes, cardiac arrhythmias or even
death (95-97).
Related neurological problems include lower IQ levels,
attention-deficit/hyperactivity disorder and autism spectrum
disorders (98,99). Elevated maternal levels have been
associated with maternal hypertension preterm deliveries, low birth
weight and even possible spontaneous abortion (23,24,100,101). In the present study, the
differences between term and preterm pregnancies were not
considered statistically significant, both for patients from
Timisoara and from Petrosani. When comparing the two cohorts, the
Petrosani cohort presented significantly higher median
concentrations of As.
Pb is well-known for its high toxicity and has no
known physiological roles, and as such, avoiding contact with this
metal is advised. Pb poisoning affects the kidneys, the
cardiovascular system, reproduction and especially the neurological
and psychologic systems, as it can pass the blood-brain barrier
(102-106).
In children, the neurological and psychological effects can be
drastic, as their brains and mind undergo much development. This
can lead to problems such as lower IQ levels,
attention-deficit/hyperactivity disorder and antisocial disorders
(107-109).
Other pediatric disorders due to elevated lead concentrations in
the mother's serum could be reduced glomerular filtration, asthma,
immunological and dermatological disorders (110-113).
Perinatal effects of high Pb concentrations are low birth weight,
preterm delivery, pre-eclampsia and pregnancy hypertension
(90,114-117).
Umbilical cord blood analysis has also shown that Pb affects DNA
methylation and has also confirmed the reduced intellectual
abilities of children coming from pregnancies with exposure to Pb
(118,119). In the present study, the
differences in Pb between term and preterm pregnancies were not
statistically significant in either cohort. Compared with the
Timisoara cohort, the patients from Petrosani presented
significantly higher median concentrations of Pb.
For both cohorts, the differences between the
preterm and term pregnancies were minimal. Indeed, no statistically
significant differences were observed in the Timisoara cohorts, and
the patients from Petrosani only showed higher concentrations of Zn
and Cd in the preterm pregnancy group. More importantly, the
comparison between the two cities showed that patients from
Petrosani, a well-known industrial region, had higher
concentrations of Zn, Cd, Pb and As. This is in agreement with
other international studies showing that people living in
industrialized regions are susceptible to accumulation of these
elements, even if they mostly measured the concentrations either in
the mother's serum, urine, toe-nails, hair, fetal placenta and cord
blood (16-22,90,115,120,121).
These elements are important as their concentration may be further
increased by tobacco and are secreted in colostrum and breast milk
(122). These elements are also
some of the more toxic metalloids. Concern regarding their levels
and possible health problems has also been expressed in other
studies (46,47,92,99,111,113,123).
The smoking status of mothers can influence
concentrations in blood, urine, hair or toe-nail samples (124-127).
However, in order to avoid this, the patients in the present study
were separated in three groups. Active smokers were defined as
patients smoking even during pregnancy. Former smokers were defined
as patients that had smoked and gave up the habit in the past, as
well as mothers, which quit smoking once the pregnancy was
suspected and/or confirmed. Nonsmokers were defined as patients
that never smoked. There was no association between smoking status
and gestational age, nor with any of the two cities in particular.
Therefore, smoking status may not be an interfering element with
respect to the concentration of heavy metal ions.
There are some limitations to this study. Larger
sample lots might help produce finer, more accurate results. The
design of the study may result in unequal follow-up, as it involved
two maternity clinics from different cities. All mothers were
recommended supplements such as Elevit 2 (Bayer, Germany) or
Femosun (Sun Wave Pharma, Ascendis Health, South Africa), which may
interfere with some of the recorded elements, especially Fe.
Another limitation, which was not analyzed, may be the frequency of
smoking, represented by the number of cigarettes smoked by an
individual per day. Only the smoker status was assessed under the
labels previously described.
In conclusion, bioactive components found in the
amniotic fluid are important and can be monitored through
amniocentesis. This tool enables healthcare professionals to assess
the condition of the developing fetus. Common ions have been
largely studied in the past. Heavy metal ions require more
attention as minimal differences in concentration might influence
the fetal development. Cd and Pb are elements with high toxicity
and almost no physiological function. Thus, the authors recommend
that these elements be avoided, especially by pregnant women and
children. Metalloids such as Fe, Cu, Zn or Ni are to be discussed
by expecting mothers with their healthcare provider, in order to
check if any supplementation is needed. More in-depth research
should be done in order to outline the effects of these elements
and to determine how they affect antenatal outcomes and childhood
development in the long run.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RIN, GD, AD and AM contributed substantially to the
conception and design of the study, the acquisition, analysis and
interpretation of the data, and were involved in the drafting of
the manuscript. AVP, AGE, DDV, MC and ESB contributed substantially
to the analysis and interpretation of the data and were involved in
the drafting of the manuscript. IC, ICC, FGH and FG contributed
substantially to the interpretation of the data and were involved
in the critical revisions of the manuscript for important
intellectual content. FGH, AM and MC are responsible for confirming
the authenticity of all the raw data and supervision. All authors
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study design followed the international
regulations in accordance with The Declaration of Helsinki. The
study was approved by the Ethics Committees of the ‘Bega’ Maternity
Clinic, (approval no. 260/16IUL2021) and Petrosani Hospital
(approval no. 15990/27.07.2021). Patient informed consent for
publication of the data associated with the manuscript was
obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cassady G and Barnett R: Amniotic fluid
electrolytes and perinatal outcome. Biol Neonat. 13:155–174.
1968.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lind T, Billewicz WZ and Cheyne GA:
Composition of amniotic fluid and maternal blood through pregnancy.
J Obstet Gynaecol Br Commonw. 78:505–512. 1971.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Harman CR: Amniotic fluid abnormalities.
Semin Perinatol. 32:288–294. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Brace RA and Wolf EJ: Normal amniotic
fluid volume changes throughout pregnancy. Am J Obstet Gynecol.
161:382–388. 1989.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Elliott PM and Inman WH: Volume of liquor
amnii in normal and abnormal pregnancy. Lancet. 2:835–840.
1961.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Queenan JT, Thompson W, Whitfield CR and
Shah SI: Amniotic fluid volumes in normal pregnancies. Am J Obstet
Gynecol. 114:34–38. 1972.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Underwood MA, Gilbert WM and Sherman MP:
Amniotic fluid: Not just fetal urine anymore. J Perinatol.
25:341–348. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Curran MA, Nijland MJ, Mann SE and Ross
MG: Human amniotic fluid mathematical model: Determination and
effect of intramembranous sodium flux. Am J Obstet Gynecol.
178:484–490. 1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Massa G, Proesmans W, Devlieger H,
Vandenberghe K, Van Assche A and Eggermont E: Electrolyte
composition of the amniotic fluid in Bartter syndrome. Eur J Obstet
Gynecol Reprod Biol. 24:335–340. 1987.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fotiou M, Michaelidou AM, Masoura S,
Menexes G, Koulourida V, Biliaderis CG, Tarlatzis BC and
Athanasiadis AP: Second trimester amniotic fluid uric acid,
potassium, and cysteine to methionine ratio levels as possible
signs of early preeclampsia: A case report. Taiwan J Obstet
Gynecol. 55:874–876. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Weiss M, Frenkel Y, Dolev E, Barkai G,
Mashiach S and Sela BA: Increased amniotic fluid divalent cation
concentrations in preeclampsia. J Basic Clin Physiol Pharmacol.
6:71–77. 1995.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dawson EB, Evans DR and Nosovitch J:
Third-trimester amniotic fluid metal levels associated with
preeclampsia. Arch Environ Health. 54:412–415. 1999.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Honkonen E, Näntö V, Hyörä H, Vuorinen K
and Erkkola R: Trace elements and antibacterial activity in
amniotic fluid. Neonatology. 50:21–26. 1986.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Scane TM and Hawkins DF: Antibacterial
activity in human amniotic fluid: Relationship to zinc and
phosphate. Br J Obstet Gynaecol. 91:342–348. 1984.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shankar AH and Prasad AS: Zinc and immune
function: The biological basis of altered resistance to infection.
Am J Clin Nutr. 68 (Suppl 2):447S–463S. 1998.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dawson EB, Evans DR, Harris WA and Van
Hook JW: Amniotic fluid B12, calcium, and lead levels associated
with neural tube defects. Am J Perinatol. 16:373–378.
1999.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Asefi Y, Gohari Mahmoudabad A, Habibian
Sezavar A, Mirshahvaladi S, Abyadeh M and Abyareh M: Association
between maternal cadmium exposure and preterm birth: A
meta-analysis. Int J Environ Health Res. 7:1–10. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu L, Dai H, Skuza L and Wei S:
Comprehensive exploration of heavy metal contamination and risk
assessment at two common smelter sites. Chemosphere.
285(131350)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ilechukwu I, Osuji LC, Okoli CP, Onyema MO
and Ndukwe GI: Assessment of heavy metal pollution in soils and
health risk consequences of human exposure within the vicinity of
hot mix asphalt plants in Rivers State, Nigeria. Environ Monit
Assess. 193(461)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Alsubih M, El Morabet R, Khan RA, Khan NA,
Ul Haq Khan M, Ahmed S, Qadir A and Changani F: Occurrence and
health risk assessment of arsenic and heavy metals in groundwater
of three industrial areas in Delhi, India. Environ Sci Pollut Res
Int. 28:63017–63031. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wongsasuluk P, Chotpantarat S, Siriwong W
and Robson M: Human biomarkers associated with low concentrations
of arsenic (As) and lead (Pb) in groundwater in agricultural areas
of Thailand. Sci Rep. 11(13896)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang Y, Qian P, Li D, Chen H and Zhou X:
Assessing risk to human health for heavy metal contamination from
public point utility through ground dust: A case study in Nantong,
China. Environ Sci Pollut Res Int 2021 (Epub ahead of print).
|
|
23
|
Llanos MN and Ronco AM: Fetal growth
restriction is related to placental levels of cadmium, lead and
arsenic but not with an-tioxidant activities. Reprod Toxicol.
27:88–92. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Smeester L, Martin EM, Cable P, Bodnar W,
Boggess K, Vora NL and Fry RC: Toxic metals in amniotic fluid and
altered gene expression in cell-free fetal RNA. Prenat Diagn.
37:1364–1366. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bonithon-Kopp C, Huel G, Moreau T and
Wendling R: Prenatal exposure to lead and cadmium and psychomotor
development of the child at 6 years. Neurobehav Toxicol Teratol.
8:307–310. 1986.PubMed/NCBI
|
|
26
|
Ebrahim K and Ashtarinezhad A: The
association of amniotic fluid cadmium levels with the risk of
preeclampsia, prematurity and low birth weight. Iran J Neonatol.
6:1–6. 2015.
|
|
27
|
Lewis M, Worobey J, Ramsay DS and
McCormack M: Prenatal exposure to heavy metals: Effect on childhood
cognitive skills and health status. Pediatrics. 89 (6 Pt
1):1010–1015. 1992.PubMed/NCBI
|
|
28
|
Pogorelova TN, Linde VA, Gunko VO and
Selyutina SN: The imbalance of metal-containing proteins and free
metal ions in the amniotic fluid during fetal growth. Biomed Khim.
62:69–72. 2016.PubMed/NCBI View Article : Google Scholar : (In Russian).
|
|
29
|
Campbell J, Wathen N, Macintosh M, Cass P,
Chard T and Mainwaring Burton R: Biochemical composition of
amniotic fluid and extraembryonic coelomic fluid in the first
trimester of pregnancy. Br J Obstet Gynaecol. 99:563–565.
1992.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Leela KV and Babu K: Study of biochemical
parameters in amniotic fluid for assessment of foetal maturity in
cases of nor-mal pregnancy. J Evid Based Med Hlthcar. 3:8394–8399.
2016.
|
|
31
|
Gagnon R, Harding R and Brace R: Amniotic
fluid and fetal urinary responses to severe placental insufficiency
in sheep. Am J Obstet Gynecol. 186:1076–1084. 2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gómez de la F CL, Novoa P JM and Caviedes
R N: Bartter syndrome: An infrequent tubulopathy of prenatal onset.
Rev Chil Pediatr. 90:437–442. 2019.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
33
|
Tong XL, Wang L, Gao TB, Qin YG, Qi YQ and
Xu YP: Potential function of amniotic fluid in fetal
development-novel insights by comparing the composition of human
amniotic fluid with umbilical cord and maternal serum at mid and
late gestation. J Chin Med Assoc. 72:368–373. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sabatier M, Garcia-Rodenas CL, Castro CA,
Kastenmayer P, Vigo M, Dubascoux S, Andrey D, Nicolas M, Payot JR,
Bordier V, et al: Longitudinal changes of mineral concentrations in
preterm and term human milk from lactating swiss women. Nutrients.
11(1855)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cruikshank DP, Pitkin RM, Reynolds WA,
Williams GA and Hargis GK: Calcium-regulating hormones and ions in
amniotic fluid. Am J Obstet Gynecol. 136:621–625. 1980.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shah MC, Modi UJ, Bhatt RV and Mistry KP:
Amniotic fluid composition in abnormal pregnancies. Indian J
Pediatr. 50:271–274. 1983.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pettit BR, Baker SP and King GS: The
composition of amniotic fluid in pregnancies complicated by fetal
anencephaly or Spina Bifida. Br J Obstet Gynaecol. 86:637–641.
1979.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mimouni F, Miodovnik M, Tsang RC, Callahan
J and Shaul P: Decreased amniotic fluid magnesium concentration in
diabetic pregnancy. Obstet Gynecol. 69:12–14. 1987.PubMed/NCBI
|
|
39
|
Gortzak-Uzan L, Mezad D, Smolin A, Friger
M, Huleihel M and Hallak M: Increasing amniotic fluid magnesium
concentrations with stable maternal serum levels: A prospective
clinical trial. J Reprod Med. 50:817–820. 2005.PubMed/NCBI
|
|
40
|
Hovdenak N and Haram K: Influence of
mineral and vitamin supplements on pregnancy outcome. Eur J Obstet
Gynecol Reprod Biol. 164:127–132. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Adama van Scheltema PN, In't Anker PS,
Vereecken A, Vandenbussche FP, Kanhai HH and Devlieger R:
Biochemical composition of amniotic fluid in pregnancies
complicated with twin-twin transfusion syndrome. Fetal Diagn Ther.
20:186–189. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Correia-Branco A, Rincon MP, Pereira LM
and Wallingford MC: Inorganic phosphate in the pathogenesis of
pregnancy-related complications. Int J Mol Sci.
21(5283)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Schlievert P, Johnson W and Galask RP:
Bacterial growth inhibition by amniotic fluid. VII. The effect of
zinc supplementation on bacterial inhibitory activity of amniotic
fluids from gestation of 20 weeks. Am J Obstet Gynecol.
127:603–608. 1977.PubMed/NCBI
|
|
44
|
Tomblin J, Davis B and Larsen B: Phosphate
content of human amniotic fluid and its relationship to bacterial
growth inhibition. Am J Reprod Immunol Microbiol. 13:33–35.
1987.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Pier SM: The role of heavy metals in human
health. Tex Rep Biol Med. 33:85–106. 1975.PubMed/NCBI
|
|
46
|
Chowdhury R, Ramond A, O'Keeffe LM,
Shahzad S, Kunutsor SK, Muka T, Gregson J, Willeit P, Warnakula S,
Khan H, et al: Environmental toxic metal contaminants and risk of
cardiovascular disease: Systematic review and meta-analysis. BMJ.
362(k3310)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kim DW, Ock J, Moon KW and Park CH:
Association between Pb, Cd, and Hg exposure and liver injury among
Korean adults. Int J Environ Res Public Health.
18(6783)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Liu YH, Wang CW, Wu DW, Lee WH, Chen YC,
Li CH, Tsai CC, Lin WY, Chen SC, Hung CH, et al: Association of
heavy metals with overall mortality in a Taiwanese population.
Nutrients. 13(2070)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Durá Travé T, da Cunha Ferreira RM,
Monreal I, Ezcurdia Gurpegui M and Villa-Elizaga I: Zinc
concentration of amniotic fluid in the course of pregnancy and its
relationship to fetal weight and length. Gynecol Obstet Invest.
18:152–155. 1984.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kumar V, Kumar A, Singh K, Avasthi K and
Kim JJ: Neurobiology of zinc and its role in neurogenesis. Eur J
Nutr. 60:55–64. 2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Boskabadi H, Maamouri G, Akhondian J,
Ashrafzadeh F, Boskabadi A, Faramarzi R, Heidar E, Pourbadakhshan
N, Shojaei SRH, Zakerihamidi M, et al: Comparison of birth weights
of neonates of mothers receiving vs. not receiving zinc supplement
at pregnancy. BMC Pregnancy Childbirth. 21(187)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Vickram S, Rohini K, Srinivasan S, Nancy
Veenakumari D, Archana K, Anbarasu K, Jeyanthi P, Thanigaivel S,
Gulothungan G, Rajendiran N and Srikumar PS: Role of zinc (Zn) in
human reproduction: A journey from initial spermatogenesis to
childbirth. Int J Mol Sci. 22(2188)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Jankovic-Karasoulos T, McAninch D, Dixon
C, Leemaqz SY, François M, Leifert WR, McCullough D, Ricciardelli
C, Roberts CT and Bianco-Miotto T: The effect of zinc on human
trophoblast proliferation and oxidative stress. J Nutr Biochem.
90(108574)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Vetchý MPJVKKD: Biological role of copper
as an essential trace element in the human organism. Ceska Slov
Farm. 67:143–153. 2018.PubMed/NCBI
|
|
55
|
Lisa SH, Akhter QS, Nessa A, Munna MA and
Eza LH: Serum copper level and its relation with blood pressure and
urinary protein level in preeclampsia. Mymensingh Med J.
30:473–477. 2021.PubMed/NCBI
|
|
56
|
Sak S, Barut M, Çelik H, Incebiyik A,
Ağaçayak E, Uyanikoglu H, Kirmit A and Sak M: Copper and
ceruloplasmin levels are closely related to the severity of
preeclampsia. J Matern Fetal Neonatal Med. 33:96–102.
2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Schaefer M and Gitlin JD: Genetic
disorders of membrane transport. IV. Wilson's disease and Menkes
disease. Am J Physiol. 276:G311–G314. 1999.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Tümer Z, Tønnesen T, Böhmann J, Marg W and
Horn N: First trimester prenatal diagnosis of Menkes disease by DNA
analysis. J Med Genet. 31:615–617. 1994.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Gu YH, Kodama H, Sato E, Mochizuki D,
Yanagawa Y, Takayanagi M, Sato K, Ogawa A, Ushijima H and Lee CC:
Prenatal diagnosis of Menkes disease by genetic analysis and copper
measurement. Brain Dev. 24:715–718. 2002.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Lorincz MT: Wilson disease and related
copper disorders. Handb Clin Neurol. 147:279–292. 2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Satish K and Trivedi AV: A Review on role
of nickel in the biological system. Int J Curr Microbiol App Sci.
5:719–727. 2016.
|
|
62
|
Savolainen H: Biochemical and clinical
aspects of nickel toxicity. Rev Environ Health. 11:167–173.
1996.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Zambelli B and Ciurli S: Nickel and human
health. Met Ions Life Sci. 13:321–357. 2013.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Moradnia M, Attar HM, Heidari Z, Mohammadi
F and Kelishadi R: Prenatal exposure to chromium (Cr) and nickel
(Ni) in a sample of Iranian pregnant women: Urinary levels and
associated socio-demographic and lifestyle factors. Environ Sci
Pollut Res Int. 28:63412–63421. 2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Wang XW, Gu JY, Li Z, Song YF, Wu WS and
Hou YP: Gestational age and dose influence on placental transfer of
63Ni in rats. Placenta. 31:305–311. 2010.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Hou YP, Gu JY, Shao YF, Song YF, Jing YH,
Wu WS and Pu S: The characteristics of placental transfer and
tissue concentrations of nickel in late gestational rats and
fetuses. Placenta. 32:277–282. 2011.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Kakhlon OR and Cabantchik ZI: The labile
iron pool: Characterization, measurement, and participation in
cellular processes(1). Free Radic Biol Med. 33:1037–1046.
2002.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Ganz T: Hepcidin, a key regulator of iron
metabolism and mediator of anemia of inflammation. Blood.
102:783–788. 2003.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Trumbo P, Yates AA, Schlicker S and Poos
M: Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron,
chromium, copper, iodine, iron, manganese, molybdenum, nickel,
silicon, vanadium, and zinc. J Am Diet Assoc. 101:294–301.
2001.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Taylor CL and Brannon PM: Introduction to
workshop on iron screening and supplementation in iron-replete
pregnant women and young children. Am J Clin Nutr. 106 (Suppl
6):1547S–1554S. 2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Milman N: Iron in pregnancy: How do we
secure an appropriate iron status in the mother and child? Ann Nutr
Metab. 59:50–54. 2011.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Chigladze M: The predictive value of the
mother's risk factors in formation of fetal developmental delay.
Glob Pediatr Health. 8(2333794X21999149)2021.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Fisher AL, Sangkhae V, Presicce P,
Chougnet CA, Jobe AH, Kallapur SG, Tabbah S, Buhimschi CS,
Buhimschi IA, Ganz T and Nemeth E: Fetal and amniotic fluid iron
homeostasis in healthy and complicated murine, macaque, and human
pregnancy. JCI Insight. 5(e135321)2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Gulec S, Anderson GJ and Collins JF:
Mechanistic and regulatory aspects of intestinal iron absorption.
Am J Physiol Gastrointest Liver Physiol. 307:G397–G409.
2014.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Burke RM, Leon JS and Suchdev PS:
Identification, prevention and treatment of iron deficiency during
the first 1000 days. Nutrients. 6:4093–4114. 2014.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Bacon BR, Adams PC, Kowdley KV, Powell LW
and Tavill AS: American Association for the Study of Liver
Diseases. Diagnosis and management of hemochromatosis: 2011
practice guideline by the American association for the study of
liver diseases. Hepatology. 54:328–343. 2011.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Wessling-Resnick M: Excess iron:
Considerations related to development and early growth. Am J Clin
Nutr. 106 (Suppl 6):1600S–1605S. 2017.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Tellez-Plaza M, Guallar E, Howard BV,
Umans JG, Francesconi KA, Goessler W, Silbergeld EK, Devereux RB
and Navas-Acien A: Cadmium exposure and incident cardiovascular
disease. Epidemiology. 24:421–429. 2013.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Tellez-Plaza M, Jones MR, Dominguez-Lucas
A, Guallar E and Navas-Acien A: Cadmium exposure and clinical
cardiovascular disease: A systematic review. Curr Atheroscler Rep.
15(356)2013.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Skolarczyk J, Pekar J,
Skórzyńska-Dziduszko K and Łabądź D: Role of heavy metals in the
development of obesity: A review of research. J Elem. 23:1271–1280.
2018.
|
|
81
|
Dobrescu A, Copaescu C, Zmeu B, Duta C,
Bedreag OH, Stoica L, Tarta C, Rogobete AF and Lazar F: Ghrelin
levels and hunger sensation after laparoscopic sleeve gastrectomy
compared with laparoscopic greater curvature plication in obese
patients. Clin Lab. 66:2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Luevano J and Damodaran C: A review of
molecular events of cadmium-induced carcinogenesis. J Environ
Pathol Toxicol Oncol. 33:183–194. 2014.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Joseph P: Mechanisms of cadmium
carcinogenesis. Toxicol Appl Pharmacol. 238:272–279.
2009.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Liu J, Zeng L, Zhuang S, Zhang C, Li Y,
Zhu J and Zhang W: Cadmium exposure during prenatal development
causes progesterone disruptors in multiple generations via
steroidogenic enzymes in rat ovarian granulosa cells. Ecotoxicol
Environ Saf. 201(110765)2020.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Yi SJ, Xiong YW, Zhu HL, Dai LM, Cao XL,
Liu WB, Shi XT, Zhou GX, Liu AY, Zhao LL, et al: Environmental
cadmium exposure during pregnancy causes diabetes-like phenotypes
in mouse offspring: Association with oxidative stress in the fetal
liver. Sci Total Environ. 777(146006)2021.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Zhu J, Huang Z, Yang F, Zhu M, Cao J, Chen
J, Lin Y, Guo S, Li J and Liu Z: Cadmium disturbs epigenetic
modification and induces DNA damage in mouse preimplantation
embryos. Ecotoxicol Environ Saf. 219(112306)2021.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Liao Y, Zheng H, Wu L, He L, Wang Y, Ou Y,
Yang H, Peng S, Chen F, Wang X and Zhao J: Cadmium cytotoxicity and
possible mechanisms in human trophoblast HTR-8/SVneo cells. Environ
Toxicol. 36:1111–1124. 2021.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Amegah AK, Sewor C and Jaakkola JJK:
Cadmium exposure and risk of adverse pregnancy and birth outcomes:
A systematic review and dose-response meta-analysis of cohort and
cohort-based case-control studies. J Expo Sci Environ Epidemiol.
31:299–317. 2021.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Gonzalez-Nahm S, Nihlani K, S House J, L
Maguire R, G Skinner H and Hoyo C: Associations between maternal
cadmium exposure with risk of preterm birth and low after birth
weight effect of mediterranean diet adherence on affected prenatal
outcomes. Toxics. 8(90)2020.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Kaur M, Sharma P, Kaur R and Khetarpal P:
Increased incidence of spontaneous abortions on exposure to cadmium
and lead: A systematic review and meta-analysis. Gynecol
Endocrinol. 25:1–6. 2021.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Chan TF, Su JH, Chung YF, Hsu YH, Yeh YT,
Jong SB and Yuan SS: Amniotic fluid and maternal serum leptin
levels in pregnant women who subsequently develop preeclampsia. Eur
J Obstet Gynecol Reprod Biol. 108:50–53. 2003.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Akhtar E, Roy AK, Haq MA, von Ehrenstein
OS, Ahmed S, Vahter M, Ekstrom EC, Kippler M, Wagatsuma Y and Raqib
R: A longitudinal study of rural Bangladeshi children with
long-term arsenic and cadmium exposures and biomarkers of
cardiometabolic diseases. Environ Pollut.
271(116333)2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Hunter P: A toxic brew we cannot live
without, Micronutrients give insights into the interplay between
geochemistry and evolutionary biology. EMBO Rep. 9:15–18.
2008.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Uthus EO: Evidence for arsenic
essentiality. Environ Geochem Health. 14:55–58. 1992.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Platanias LC: Biological responses to
arsenic compounds. J Biol Chem. 284:18583–18587. 2009.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Wisessaowapak C, Watcharasit P and
Satayavivad J: Arsenic disrupts neuronal insulin signaling through
in-creasing free PI3K-p85 and decreasing PI3K activity. Toxicol
Lett. 349:40–50. 2021.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Navas-Acien A and Guallar E: Measuring
Arsenic Exposure, Metabolism, and Biological effects: The role of
urine proteomics. Toxicol Sci. 106:1–4. 2008.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Wang SX, Wang ZH, Cheng XT, Li J, Sang ZP,
Zhang XD, Han LL, Qiao XY, Wu ZM and Wang ZQ: Arsenic and fluoride
exposure in drinking water: Children's IQ and growth in Shanyin
county, Shanxi province, China. Environ Health Perspect.
115:643–647. 2007.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Skogheim TS, Weyde KVF, Engel SM, Aase H,
Surén P, Øie MG, Biele G, Reichborn-Kjennerud T, Caspersen IH,
Hornig M, et al: Metal and essential element concentrations during
pregnancy and associations with autism spectrum disorder and
attention-deficit/hyperactivity disorder in children. Environ Int.
152(106468)2021.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Vahter M: Effects of arsenic on maternal
and fetal health. Annu Rev Nutr. 29:381–399. 2009.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Farzan SF, Chen Y, Wu F, Jiang J, Liu M,
Baker E, Korrick SA and Karagas MR: Blood pressure changes in
relation to arsenic exposure in a US pregnancy cohort. Environ
Health Perspect. 123:999–1006. 2015.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Ekong EB, Jaar BG and Weaver VM:
Lead-related nephrotoxicity: A review of the epidemiologic
evidence. Kidney Int. 70:2074–2084. 2006.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Navas-Acien A, Guallar E, Silbergeld EK
and Rothenberg SJ: Lead exposure and cardiovascular disease-a
systematic review. Environ Health Perspect. 115:472–482.
2007.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Park SK, O'Neill MS, Vokonas PS, Sparrow
D, Wright RO, Coull B, Nie H, Hu H and Schwartz J: Air pollution
and heart rate variability: Effect modification by chronic lead
exposure. Epidemiology. 19:111–120. 2008.PubMed/NCBI View Article : Google Scholar
|
|
105
|
White LD, Cory-Slechta DA, Gilbert ME,
Tiffany-Castiglioni E, Zawia NH, Virgolini M, Rossi-George A,
Lasley SM, Qian YC and Basha MR: New and evolving concepts in the
neurotoxicology of lead. Toxicol Appl Pharmacol. 225:1–27.
2007.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Bouchard MF, Bellinger DC, Weuve J,
Matthews-Bellinger J, Gilman SE, Wright RO, Schwartz J and
Weisskopf MG: Blood lead levels and major depressive disorder,
panic disorder, and generalized anxiety disorder in US young
adults. Arch Gen Psychiatry. 66:1313–1319. 2009.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Meyer PA, McGeehin MA and Falk H: A global
approach to childhood lead poisoning prevention. Int J Hyg Environ
Health. 206:363–369. 2003.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Lanphear BP, Hornung R, Khoury J, Yolton
K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein
R, Greene T, et al: Low-level environmental lead exposure and
children's intellectual function: An international pooled analysis.
Environ Health Perspect. 113:894–899. 2005.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Bellinger DC: Very low lead exposures and
children's neurodevelopment. Curr Opin Pediatr. 20:172–177.
2008.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Saylor C, Tamayo-Ortiz M, Pantic I,
Amarasiriwardena C, McRae N, Estrada-Gutierrez G, Parra-Hernandez
S, Tolentino MC, Baccarelli AA, Fadrowski JJ, et al: Prenatal blood
lead levels and reduced preadolescent glomerular filtration rate:
Modification by body mass index. Environ Int.
154(106414)2021.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Hsieh CY, Jung CR, Lin CY and Hwang BF:
Combined exposure to heavy metals in PM2.5 and pediatric
asthma. J Allergy Clin Immunol. 147:2171–2180 e13. 2021.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Wang M, Xia W, Zeng Q, Zhang W, Qian X,
Bao S, Zhou A, Li Y and Xu S: Associations between prenatal and
postnatal lead exposure and preschool children humoral and cellular
immune responses. Ecotoxicol Environ Saf.
207(111536)2021.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Lee S, Park SK, Park H, Lee W, Kwon JH,
Hong YC, Ha M, Kim Y, Lee B and Ha E: Prenatal heavy metal
exposures and atopic dermatitis with gender difference in
6-month-old infants using multipollutant analysis. Environ Res.
195(110865)2021.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Bellinger DC: Teratogen update: Lead and
pregnancy. Birth Defects Res A Clin Mol Teratol. 73:409–420.
2005.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Goto Y, Mandai M, Nakayama T, Yamazaki S,
Nakayama SF, Isobe T, Sato T and Nitta H: Association of prenatal
maternal blood lead levels with birth outcomes in the Japan
Environment and Children's Study (JECS): A nationwide birth cohort
study. Int J Epidemiol. 50:156–164. 2021.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Wu SZ, Xu HY, Chen Y, Chen Y, Zhu QL, Tan
MH and Zhang MM: Association of blood lead levels with
preeclampsia: A cohort study in China. Environ Res.
195(110822)2021.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Irwinda R, Wibowo N and Putri AS: The
concentration of micronutrients and heavy metals in maternal serum,
placenta, and cord blood: A cross-sectional study in preterm birth.
J Pregnancy. 2019(5062365)2019.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Park J, Kim J, Kim E, Kim WJ and Won S:
Prenatal lead exposure and cord blood DNA methylation in the Korean
Exposome Study. Environ Res. 195(110767)2021.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Tatsuta N, Nakai K, Kasanuma Y,
Iwai-Shimada M, Sakamoto M, Murata K and Satoh H: Prenatal and
postnatal lead exposures and intellectual development among
12-year-old Japanese children. Environ Res.
189(109844)2020.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Tomska N, Kosik-Bogacka DI,
Łanocha-Arendarczyk N, Szylińska A, Kotfis K, Sipak-Szmigiel O and
Rotter I: Relationship between concentrations of elements and
geographic location in Poland. Ann Agric Environ Med. 28:283–290.
2021.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Khanam R, Kumar I, Oladapo-Shittu O, Twose
C, Islam AA, Biswal SS, Raqib R and Baqui AH: Prenatal
environmental metal exposure and preterm birth: A scoping review.
Int J Environ Res Public Health. 18(573)2021.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Szukalska M, Merritt TA, Lorenc W,
Sroczyńska K, Miechowicz I, Komorowicz I, Mazela J, Barałkiewicz D
and Florek E: Toxic metals in human milk in relation to tobacco
smoke exposure. Environ Res. 197(111090)2021.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Karakis I, Landau D, Gat R, Shemesh N,
Tirosh O, Yitshak-Sade M, Sarov B and Novack L: Maternal metal
concentration during gestation and pediatric morbidity in children:
An exploratory analysis. Environ Health Prev Med.
26(40)2021.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Riaz M, Lewis S, Naughton F and Ussher M:
Predictors of smoking cessation during pregnancy: A systematic
review and meta-analysis. Addiction. 113:610–622. 2018.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Gomez-Roig MD, Marchei E, Sabra S, Busardò
FP, Mastrobattista L, Pichini S, Gratacós E and Garcia-Algar O:
Maternal hair testing to disclose self-misreporting in drinking and
smoking behavior during pregnancy. Alcohol. 67:1–6. 2018.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Benowitz NL, Hukkanen J and Jacob P III:
Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp
Pharmacol. 29–60. 2009.PubMed/NCBI View Article : Google Scholar
|
|
127
|
White AJ, O'Brien KM, Jackson BP and
Karagas MR: Urine and toenail cadmium levels in pregnant women: A
reliability study. Environ Int. 118:86–91. 2018.PubMed/NCBI View Article : Google Scholar
|