Introduction
Coronavirus disease 2019 (Covid-19) is a respiratory
tract infection caused by severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) (1).
Covid-19 has now become a worldwide pandemic, which poses a global
health emergency (2). While the
majority of patients with Covid-19 have mild to moderate symptoms,
~14% of patients may progress to severe pneumonia and exhibit
considerable mortality worldwide (3). To date, 11 vaccines for SARS-CoV-2
have been approved by emergency use authorization worldwide
(4); however, mutations in
SARS-CoV-2 have emerged worldwide, which poses a significant
challenge to the vaccines (5).
Despite several agents, including bamlanivimab, remdesivir,
baricitinib and tocilizumab, being recommended for use in certain
patients with Covid-19, their efficacy has been reported to be
unsatisfactory (4,6); therefore, effective therapeutic
agents for severe Covid-19 are still urgently required.
The immunological characteristics of patients with
severe Covid-19 include markedly elevated serum levels of
proinflammatory cytokines, including interleukin (IL)-6, tumor
necrosis factor-α and IL-1β, characterized as a cytokine storm
(7,8). The cytokine storm has been
hypothesized to serve a critical role in Covid-19 progression,
deterioration and even death (8).
Strategies to dampen inflammatory responses have therefore been
proposed (9). Nafamostat, which is
a serine protease inhibitor, has been reported to effectively
inhibit Middle East respiratory syndrome coronavirus S
protein-initiated membrane fusion (10). Recently, Hoffmann et al
(11) reported that SARS-CoV-2
infection depended on the host cell factors ACE2 and TMPRSS2, which
could be blocked by the clinically used protease inhibitors
camostat mesylate and E-64d. In addition, protease inhibitors have
been suggested as a promising therapeutic option to block the virus
from entering host cells and to prevent future pandemics at the
very beginning (12).
Ulinastatin is a glycoprotein that is extracted and
purified from fresh human urine. Ulinastatin can inhibit the
activity of various proteolytic enzymes and has been widely used
for the treatment of acute pancreatitis (13). Moreover, ulinastatin has been
demonstrated to be an important anti-inflammatory and antioxidative
agent, and has been clinically used as a potential treatment for
circulatory shock, severe sepsis and acute respiratory distress
syndrome (14-16).
As a protease inhibitor, whether ulinastatin has beneficial effects
on Covid-19 is unknown to date. The present study retrospectively
observed the efficacy of high-dose ulinastatin to explore an
effective therapeutic strategy for patients with Covid-19.
Patients and methods
Patients and ulinastatin
administration
The present study was conducted at the Optical
Valley Branch of Maternal and Child Hospital of Hubei Province
(Wuhan, China), which was designated for treating patients with
Covid-19 aged >14 years, between February 19, 2020 and April 5,
2020. Electronic medical records of the patients hospitalized due
to Covid-19 were delivered to Changzheng hospital (Shanghai,
China). The final follow-up date was April 30, 2020. All patients
were diagnosed with Covid-19 according to the guidelines for the
Diagnosis and Treatment of Covid-19 issued by the National Health
Commission of China (version 7.0) (17). A total of 15 patients treated with
standard care, including supplement oxygen, antiviral agents and
antibiotics according to the Chinese guideline for the management
of Covid-19(17) and 12
consecutive patients treated with ulinastatin (Techpool Bio-Pharma
Co., Ltd.) in addition to standard care were enrolled for analysis.
Ulinastatin was diluted with 50 ml normal saline and administered
via intravenous infusion. The infusion time was within 30 min. The
initial dose of ulinastatin was 1,000,000 IU every 8 h and tapered
down to 500,000 IU every 8 h after 4-7 days. The total course of
ulinastatin administration was ~10 days, depending on the overall
status of the patients. The present study was approved by the
Ethics Committee of Shanghai Changzheng Hospital and Optical Valley
Branch of Maternal and Child Hospital of Hubei Province [approval
no. (FYGG(L)-2020-028)].
Disease severity classification
The illness severity of Covid-19 was assessed in
accordance with guidelines issued by the National Health Commission
of China (version 7.0) (17).
Briefly, moderate cases were defined as patients with clinical
symptoms and signs of pneumonia based on CT imaging. Patients with
any of the following conditions were considered severe cases: i)
Respiratory distress, respiratory rate (RR) ≥30 beats/min; ii)
oxygen saturation (SaO2) level <93% while breathing
ambient air; and iii) a ratio of the partial pressure of oxygen
(PaO2) to the fraction of inspired oxygen
(FiO2) (PaO2:FiO2) ≤300 mmHg (1
mmHg=0.133 kPa).
Data collection
Clinical information of all patients was retrieved
from the hospital electronic medical record system, including
electronic medical records, medication administration records,
laboratory results and radiological examinations. Data were
collected on patient characteristics, including age, sex, symptoms
and comorbidities, including hypertension, diabetes mellitus (DM),
cardio-cerebrovascular diseases (CCVDs), chronic obstructive
pulmonary disease (COPD), malignancy, chronic liver diseases,
chronic kidney diseases, neuropsychiatric diseases and infectious
diseases. Clinical signs (body temperature, RR, concentration of
oxygen inhalation and SaO2) were recorded daily during
hospitalization. For laboratory tests, variables including white
blood cell (WBC) count, lymphocyte count, hypersensitive C-reactive
protein (CRP), total bilirubin (TB), alanine aminotransferase
(ALT), aspartate aminotransferase (AST) and creatinine (Cr) were
obtained on admission and were monitored during hospitalization. In
addition, the incidence of oxygen support requirements (ambient
air, low-flow nasal cannula oxygen therapy, high-flow nasal cannula
oxygen therapy, noninvasive mechanical ventilation and invasive
mechanical ventilation), intensive care unit (ICU) admission,
length of hospital stay, application of extracorporeal membrane
oxygenation (ECMO) and continuous renal replacement therapy were
recorded.
SARS-CoV-2 RNA was detected in nasal and pharyngeal
swab specimens using reverse transcription-PCR. The viral nucleic
acid was closely monitored during hospitalization until discharge
or death. Viral nucleic acid was monitored with nasal and
pharyngeal swab specimens, where the frequency of viral nucleic
acid monitoring was not unified. The criteria for discharge
strictly followed the guidelines issued by the National Health
Commission of China (version 7.0) (17), which required two throat-swab
samples obtained at least 24 h apart negative for SARS-CoV-2
RNA.
Statistical analysis
Data were collected into a Microsoft Excel
spreadsheet (Microsoft Corporation). Continuous variables were
described as the median (interquartile range) and were compared by
unpaired Student's t-test between two groups, or paired Student's
t-test between groups before and after ulinastatin treatment.
Repeated measures ANOVA followed by Bonferroni post hoc tests was
used for comparisons between three groups. Categorical variables
were reported as the subject number with percentage, and
χ2 test or Fisher's exact test was used to compare the
difference in proportions. Graphs were generated using GraphPad
Prism 5.0 (GraphPad Software, Inc.). All statistical procedures
were performed using SPSS version 21.0 (IBM Corp.). Two-tailed
P<0.05 was considered to indicate a statistically significant
difference.
Results
General characteristics of the
patients receiving ulinastatin treatment
A total of 10 patients with severe Covid-19 and two
patients with moderate Covid-19 received ulinastatin treatment
(Table I). The average age of the
patients was 68.0±11.9 years, ranging from 48 to 87 years. Of the
12 patients, 10 were male (83.3%) and two were female (16.7%). The
most common symptoms on admission were fever (8/12, 66.7%), cough
(5/12, 41.7%) and dyspnea (5/12, 41.7%), followed by fatigue (3/12,
25%) and chest distress (3/12, 25%). Other symptoms, including
headache, muscle ache, dizziness and anorexia were rare (1/12,
8.3%). Nine of 12 patients (75.0%) had one or more comorbidities.
Among them, hypertension was the most common comorbidity (8/12,
66.7%), followed by CCVDs (2/12, 16.7%), DM (2/12, 16.7%) and COPD
(1/12, 8.3%).
 | Table IBaseline demographics of patients
receiving ulinastatin treatment. |
Table I
Baseline demographics of patients
receiving ulinastatin treatment.
| Patient no. | Sex | Age, years | Principle
symptoms | Comorbidity | Disease severity | Days of admission
from symptom onset | Days of initiation of
ulinastatin treatment fromhospital admission |
|---|
| 1 | M | 73 | Fever, dyspnea,
headache | Hypertension | Severe | 6 | 1 |
| 2 | F | 72 | Fever, cough | Hypertension, DM,
CCVD | Severe | 2 | 10 |
| 3 | M | 74 | Fever | COPD | Moderate | 8 | 8 |
| 4 | F | 87 | None | Hypertension,
dementia | Moderate | 2 | 0 |
| 5 | M | 55 | Cough, muscle ache,
dyspnea, chest distress | Hypertension | Severe | 9 | 0 |
| 6 | M | 72 | Cough, dyspnea | Hypertension,
CCVD | Severe | 1 | 2 |
| 7 | M | 77 | Fever, cough,
dyspnea | Hypertension,
DM | Severe | 4 | 6 |
| 8 | M | 81 | Fever, chest
distress, fatigue | Hypertension | Severe | 21 | 6 |
| 9 | M | 57 | Fever, cough, chest
distress, dyspnea | None | Severe | 24 | 0 |
| 10 | M | 56 | Dizziness,
fatigue | None | Severe | 18 | 1 |
| 11 | M | 65 | Fever, fatigue,
anorexia | Hypertension | Severe | 19 | 0 |
| 12 | M | 48 | Fever | None | Severe | 6 | 0 |
Table II showed
the results of baseline laboratory tests on admission. Two patients
had an increase in WBC count, whereas one patient showed a decrease
in WBC count in peripheral blood. The percentage of lymphocytes was
decreased in 41.7% of patients (5/12), and 58.3% of patients (7/12)
had elevated CRP levels (mean, 49.70±77.70 mg/l). Nine patients
received umifenovir, lopinavir-ritonavir or oseltamivir antiviral
therapy. Two patients received antifungal treatment (voriconazole)
because of coinfection. Five patients (5/12, 41.7%) received
short-term intravenous methylprednisolone treatment.
 | Table IIBaseline clinical characteristics and
other treatments of patients receiving ulinastatin treatment. |
Table II
Baseline clinical characteristics and
other treatments of patients receiving ulinastatin treatment.
| | Routine blood
test | Oxygen
saturation | Drugs
administered |
|---|
| Patient no. | WBC,
x109/l | L, % | CRP, mg/l | Concentration of
oxygen inhalation, % | SaO2,
% | Antiviral | Antibiotic | Antifungal |
Corticosteroids |
|---|
| 1 | 12.6 | 9.3 | 274.09 | 21 | 90 | None | Moxifloxacin | None | None |
| 2 | 3.0 | 21.1 | 116.65 | 41 | 94 |
Lopinavir-Ritonavir | Piperacillin
tazobactam | None |
Methylprednisolone |
| 3 | 4.4 | 20.8 | 7.52 | 21 | 95 | None | Moxifloxacin | None | None |
| 4 | 3.8 | 18.0 | 25.97 | 29 | 96 | None | None | None | None |
| 5 | 4.8 | 6.3 | 45.66 | 41 | 92 | Umifenovir | Moxifloxacin | |
Methylprednisolone |
| 6 | 6.8 | 21.6 | 5.5 | 21 | 92 | Ribavirin,
umifenovir | Levofloxacin,
cefoperazone sulbactam sodium | None |
Methylprednisolone |
| 7 | 5.0 | 29.4 | 20.54 | 21 | 89 | Umifenovir | Moxifloxacin,
cefoperazone sulbactam sodium | Voriconazole | None |
| 8 | 5.2 | 20.5 | 24.36 | 29 | 92 | Umifenovir | Moxifloxacin | None |
Methylprednisolone |
| 9 | 6.1 | 26.2 | 57.71 | 21 | 86 | Oseltamivir | Moxifloxacin | None | None |
| 10 | 11.9 | 7.4 | 9.51 | 21 | 86 | Umifenovir | Moxifloxacin | None | None |
| 11 | 5.6 | 20.7 | 1.92 | 21 | 92 | Umifenovir | None | None | None |
| 12 | 9.0 | 16.7 | 6.95 | 21 | 93 | Umifenovir | Cefoperazone
sulbactam sodium | Voriconazole |
Methylprednisolone |
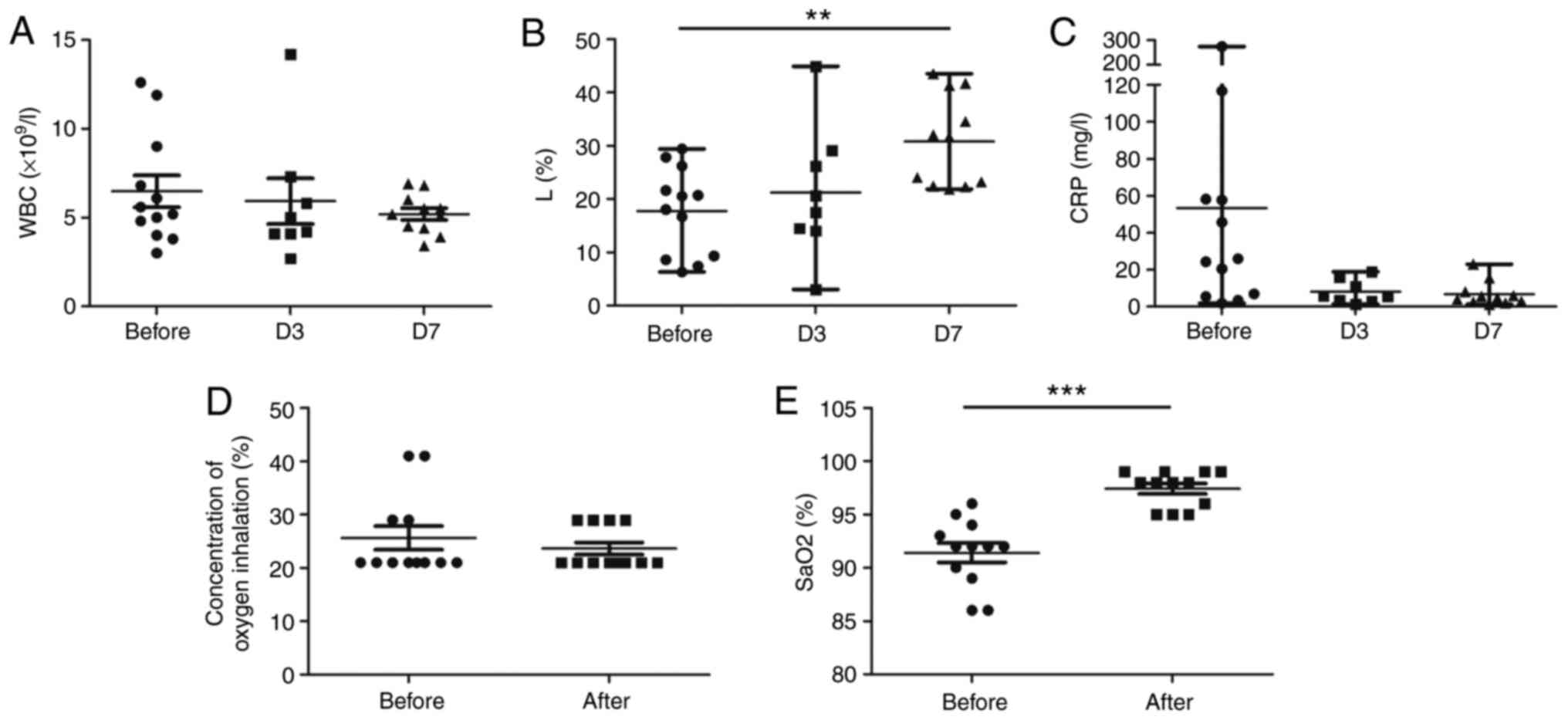
Efficacy of ulinastatin treatment
The WBC levels and the percentage of lymphocytes
returned to normal in all of the patients on day 7 after
ulinastatin treatment (Fig. 1A and
B). CRP decreased markedly and
returned to normal in 83.3% of patients (10/12) on day 7 after
treatment (Fig. 1C). Clinical
symptoms were relieved synchronously in all of the patients. The
peripheral SaO2 improved and 66.7% of the patients
(8/12; P<0.001) did not need further oxygen therapy 7 days after
ulinastatin treatment (Fig. 1D and
E). No patients required ICU
admission, mechanical ventilation, ECMO therapy or renal
replacement therapy. All of the patients were discharged from the
hospital, and the mean hospitalization time was 19.3±6.2 days.
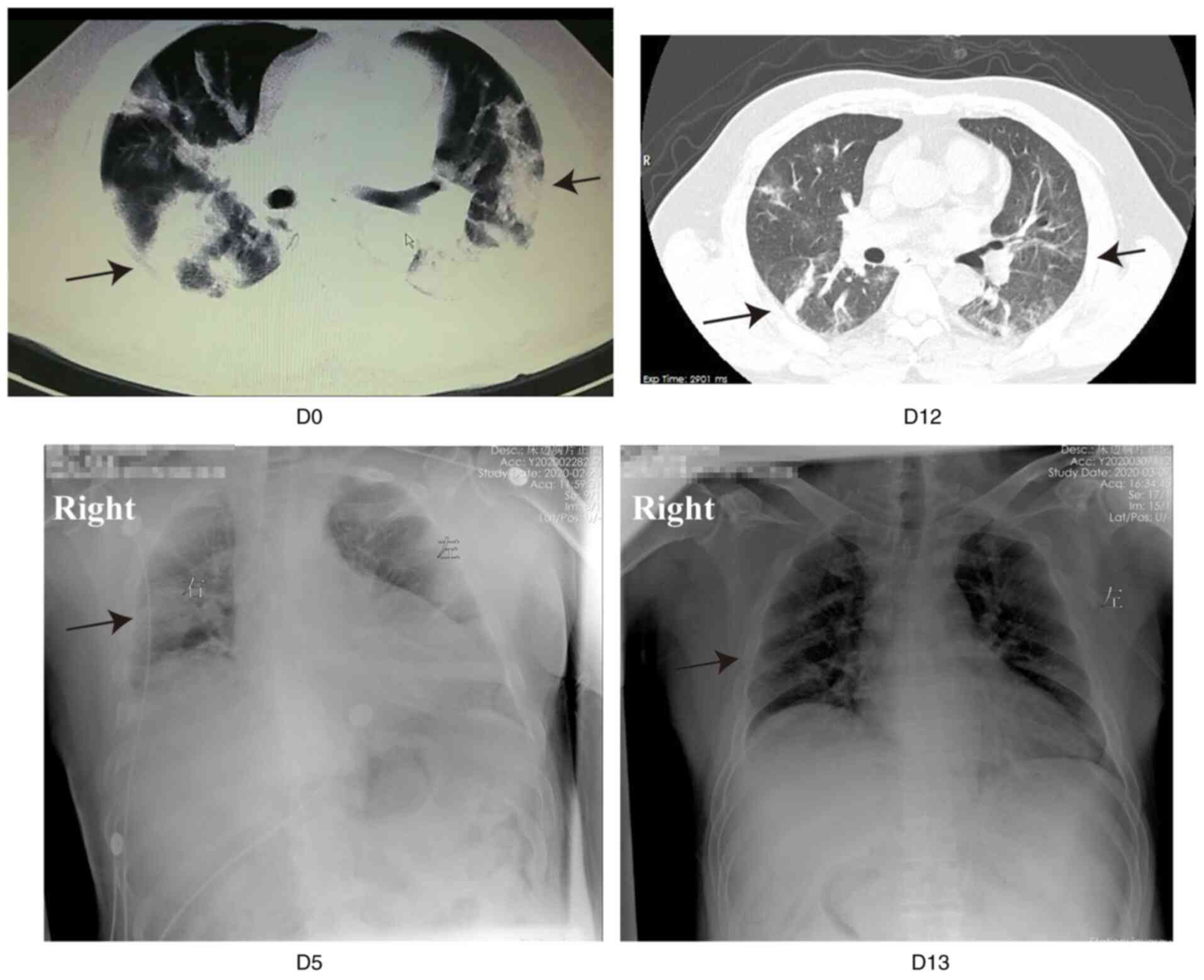
All patients revealed different degrees of
absorption of pulmonary lesions after treatment, according to the
findings on chest imaging. Fig. 2
shows representative images of patient 5. The chest CT scan of
patient 5 showed bilateral, diffuse areas of consolidation, and the
patient was then transferred to the Optical Valley Branch of
Maternal and Child Hospital of Hubei Province. The patient
complained of severe dyspnea on admission, with an RR of 35 beats
per minute. Ulinastatin was administered at a dose of 10,000,000 IU
every 8 h immediately after admission, in addition to standard
therapy. The dyspnea improved considerably the following day and
almost disappeared in the resting state 3 days after ulinastatin
treatment. The peripheral SaO2 reached 100% with
low-flow nasal cannula oxygen therapy thereafter. The bedside chest
X-ray on days 5 and 13 after treatment showed that the lesions were
gradually absorbed (Fig. 2).
Liver and kidney function were also monitored during
hospitalization. Two patients showed slightly elevated TB levels
(55.4 and 25.8 µmol/l, respectively; normal range, 3.4-20.5
µmol/l), two patients showed abnormal ALT levels (69.6 and 109.6
U/l, respectively; normal range, 0-55 U/l) and three patients
showed abnormal AST levels (52.2, 51.0 and 37.2 U/l, respectively;
normal range, 5-34 U/l) on admission. These parameters returned to
normal after treatment, with the exception of one patient with a
slightly elevated AST level (70.4 U/l) and one patient with a
slightly elevated ALT level (59.2 U/l; Fig. 3A-C).
Outcomes of patients treated with
ulinastatin compared with standard care
A standard care group was formed by the random
selection of 15 patients from the same hospital. There were no
significant between-group differences regarding baseline
demographics and clinical characteristics (Table III). Only one patient with
moderate Covid-19 on admission progressed to severe Covid-19 during
hospitalization in the ulinastatin treatment group. However, four
patients with moderate Covid-19 on admission progressed to severe
cases, and one patient with moderate Covid-19 and two patients with
severe Covid-19 on admission deteriorated into critically severe
cases during hospitalization in the standard care group (P=0.043).
Furthermore, no mortality was observed in the ulinastatin treatment
group, whereas four of 15 patients (26.7%) died in the standard
care group (P=0.106).
 | Table IIIComparison of clinical features and
outcomes of patients between the ulinastatin treatment group and
standard care group. |
Table III
Comparison of clinical features and
outcomes of patients between the ulinastatin treatment group and
standard care group.
| Characteristic | Ulinastatin
treatment group (n=12) | Standard care group
(n=15) | Statistics | P-value |
|---|
| Median age, years
(IQR) | 72.0 (56.3,
76.3) | 75.0 (68.0,
83.0) | t=0.970 | 0.341a |
| Male sex, n
(%) | 10 (83.3) | 10 (66.7) | | 0.408b |
| Comorbidities | | | | |
|
Hypertension,
n (%) | 8 (66.7) | 6 (40.0) |
χ2=1.899 | 0.168c |
|
DM, n
(%) | 2 (16.7) | 3 (20.0) | | 1.000b |
|
CCVD, n
(%) | 2 (16.7) | 8 (53.3) | | 0.107b |
|
COPD, n
(%) | 1 (8.3) | 3 (20.0) | | 0.605b |
| Disease severity
status on admission | | | | 0.696b |
|
Mild/moderate,
n (%) | 3 (25.0) | 5 (33.3) | | |
|
Severe, n
(%) | 9 (75.0) | 10 (66.7) | | |
| Baseline laboratory
parameters | | | | |
|
Median WBC
count, x109/l (IQR) | 5.4 (4.2, 8.5) | 6.9 (6.2,
11.3) | t=1.446 | 0.161a |
|
Median
lymphocytes, % (IQR) | 19.3 (8.8,
25.1) | 10.9 (6.9,
22.1) | t=1.168 | 0.254a |
|
Median CRP,
mg/l (IQR) | 25.2(5.9,58.1) | 50.9 (8.6,
89.3) | t=0.189 | 0.851a |
| Transition to more
severe cases, n (%) | 1 (8.3) | 7 (46.7) | | 0.043b |
| Oxygen therapy | | | | 1.000b |
|
Non-invasive
ventilator support, n (%) | 12 (100.0) | 14 (93.3) | | |
|
Ventilator
support, n(%) | 0 (0.0) | 1 (6.7) | | |
| Clinical
outcome | | | | 0.106b |
|
Discharged,
n (%) | 12 (100.0) | 11 (73.3) | | |
|
Death, n
(%) | 0 (0.0) | 4 (26.7) | | |
Safety
No ulinastatin infusion-related adverse events were
observed. Patients with abnormal liver function on admission
improved instead of deteriorating after treatment. Furthermore, the
Cr levels were within reference range (64-104 µmol/l for males and
49-90 µmol/l for females) before and after ulinastatin treatment
(Fig. 3D).
Discussion
The present study retrospectively assessed the
efficacy of ulinastatin in patients with Covid-19. This preliminary
observational study revealed that high-dose ulinastatin treatment
was safe and had a potential beneficial effect on patients with
Covid-19, with rapid improvement of clinical symptoms, blood
parameters and absorption of pulmonary lesions.
Covid-19 is spreading rapidly around the world and
this unprecedented challenge demands that clinicians identify an
effective and safe treatment to protect individuals at high risk
(18). Remdesivir, which has been
reported to exhibit potent in vitro activity against
SARS-CoV-2(19), is considered the
most promising antiviral drug and has been successfully used in
several case series of patients with Covid-19 (20,21).
Recently, the compassionate use of remdesivir without placebo for
53 patients with severe Covid-19 was reported to have 68% efficacy
(22). Notably, 23% of patients
had serious adverse events, and 8% of patients discontinued
remdesivir treatment prematurely. Lopinavir-ritonavir, another
antiviral drug, has been shown to have no benefit for hospitalized
adult patients with severe Covid-19 according to a recent
open-label, individually randomized, controlled trial conducted in
China (23). In addition, in this
previous study lopinavir-ritonavir is also associated with serious
gastrointestinal side effect, where 13.8% patients had to terminate
the use of the drug earlier because of adverse events (23). Notably, older patients, especially
those with comorbidities, are the most vulnerable populations to
severe Covid-19, with a significantly increased risk of
deterioration and fatality (24-26);
therefore, it is of utmost importance to identify effective
therapeutics with high safety, probably from already clinically
approved drugs. Ulinastatin is an intrinsic glycoprotein that has
been widely used in clinical practice (14) and is considered an ideal candidate.
A safety and tolerability study of ulinastatin in adult healthy
volunteers revealed that intravenous infusion of 8,000,000 IU
ulinastatin over a 2 h period was associated with no severe adverse
effects (27). In the present
study, patients received 1,000,000 IU ulinastatin every 8 h through
intravenous injection, and no adverse effects were observed.
It has been reported that ~14% of patients of
Covid-19 will develop into severe cases (28). Inhibition of the deterioration of
the illness is of utmost importance during treatment. Potential
risk factors predicting poor prognosis include older age, high
Sequential Organ Failure Assessment score and d-dimer >1 µg/ml
(24). Therefore, the application
of timely, effective and safe supportive therapies seems critical
for patients with several risk factors (28). In the present study, only one
patient with moderate Covid-19 progressed to a severe case while
receiving ulinastatin treatment, and no mortality was observed
among the patients in the ulinastatin group. By contrast, seven
patients deteriorated into more severe cases during hospitalization
in the standard care group (P=0.043), and four of 15 patients
(26.7%) died in the standard care group.
Cytokine storms have been hypothesized to serve an
essential role in the pathogenesis of Covid-19, and may be
associated with disease severity and fatality (7,8).
Ulinastatin is an intrinsic broad-spectrum protease inhibitor,
which has been shown to effectively inhibit a variety of cell
proteolytic enzymes and have multifunctional therapeutic mechanisms
(29). Firstly, ulinastatin has
been shown to exert an inhibitory effect on the production of
inflammatory cytokines and adhesion molecules (30). Moreover, ulinastatin may improve
the stability of the lysosomal membrane, and reduce the synthesis
and delivery of lysosomal enzymes, thus scavenging oxygen or
hydroxyl radicals (15). In the
present study, a beneficial effect of ulinastatin was observed on
patients with Covid-19. Unfortunately, the present study did not
investigate the changes in cytokines during treatment due to
limitations of the hospital facility.
There were several limitations in the present study.
Firstly, the number of patients enrolled was limited. Secondly,
this was an observational and retrospective study, and other
treatment factors may interfere with the outcomes. Previous studies
revealed that systemic corticosteroids were frequently administered
in patients with Covid-19 (3,24)
and corticosteroids treatment reduced the all-cause mortality
(31). In the present study, five
of 12 patients (41.7%) in the ulinastatin treatment group and five
of 15 patients (33.3%) in the standard care group received systemic
methylprednisolone treatment. The present preliminary results
revealed the promising efficacy of ulinastatin for patients with
Covid-19. Thus, further randomized controlled trials are
warranted.
In conclusion, the present observational data
revealed that administration of high-dose ulinastatin had a
potential beneficial effect on patients with Covid-19. Considering
the safety of this agent, the sharply rising number of fatalities
due to Covid-19 and the absence of any other approved agent, timely
initiation of ulinastatin treatment is strongly recommended.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HH, PFH, LLS, YBG and WFX analyzed and interpreted
the data and had substantial contributions to conception and design
of the work. QW, ZML, JZY, PMS and ZLY collected all the data from
the patient's electronic medical records and analyzed the data. QW
and PMS assessed the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki (as revised in 2013). The present study
was approved by the Ethics Committee of Shanghai Changzheng
Hospital and Optical Valley Branch of Maternal and Child Hospital
of Hubei Province, and individual consent for this retrospective
analysis was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He
JX, Liu L, Shan H, Lei CL, Hui DSC, et al: Clinical characteristics
of coronavirus disease 2019 in China. N Engl J Med. 382:1708–1720.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cucinotta D and Vanelli M: WHO declares
COVID-19 a pandemic. Acta Biomed. 91:157–160. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H,
Wu Y, Zhang L, Yu Z, Fang M, et al: Clinical course and outcomes of
critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China:
A single-centered, retrospective, observational study. Lancet
Respir Med. 8:475–481. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhou YW, Xie Y, Tang LS, Pu D, Zhu YJ, Liu
JY and Ma XL: Therapeutic targets and interventional strategies in
COVID-19: Mechanisms and clinical studies. Signal Transduct Target
Ther. 6(317)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Calina D, Docea AO, Petrakis D, Egorov AM,
Ishmukhametov AA, Gabibov AG, Shtilman MI, Kostoff R, Carvalho F,
Vinceti M, et al: Towards effective COVID-19 vaccines: Updates,
perspectives and challenges (Review). Int J Mol Med. 46:3–16.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nitulescu GM, Paunescu H, Moschos SA,
Petrakis D, Nitulescu G, Ion GND, Spandidos DA, Nikolouzakis TK,
Drakoulis N and Tsatsakis A: Comprehensive analysis of drugs to
treat SARS-CoV-2 infection: Mechanistic insights into current
COVID-19 therapies (Review). Int J Mol Med. 46:467–488.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang
H, Wang T, Zhang X, Chen H, Yu H, et al: Clinical and immunological
features of severe and moderate coronavirus disease 2019. J Clin
Invest. 130:2620–2629. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cao X: COVID-19: Immunopathology and its
implications for therapy. Nat Rev Immunol. 20:269–270.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mehta P, Mcauley DF, Brown M, Sanchez E,
Tattersall RS and Manson JJ: HLH Across Speciality Collaboration,
UK. COVID-19: Consider cytokine storm syndromes and
immunosuppression. Lancet. 395:1033–1034. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yamamoto M, Matsuyama S, Li X, Takeda M,
Kawaguchi Y, Inoue JI and Matsuda Z: Identification of nafamostat
as a potent inhibitor of Middle East respiratory syndrome
coronavirus S protein-mediated membrane fusion using the
split-protein-based cell-cell fusion assay. Antimicro Agents
Chemother. 60:6532–6539. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hoffmann M, Kleine-Weber H, Schroeder S,
Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH,
Nitsche A, et al: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2
and is blocked by a clinically proven protease inhibitor. Cell.
181:271–280.e8. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Scudellari M: How the coronavirus infects
cells-and why delta is so dangerous. Nature. 595:640–644.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lagoo JY, D'souza MC, Kartha A and Kutappa
AM: Role of ulinastatin, a trypsin inhibitor, in severe acute
pancreatitis in critical care setting: A retrospective analysis. J
Crit Care. 45:27–32. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Karnad DR, Bhadade R, Verma PK, Moulick
ND, Daga MK, Chafekar ND and Iyer S: Intravenous administration of
ulinastatin (human urinary trypsin inhibitor) in severe sepsis: A
multicenter randomized controlled study. Intensive Care Med.
40:830–838. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ji M, Chen T, Wang B, Chen M, Ding Q, Chen
L, Fang Y, Yu X, Chen Y, Wang X, et al: Effects of ulinastatin
combined with mechanical ventilation on oxygen metabolism,
inflammation and stress response and antioxidant capacity of ARDS.
Exp Ther Med. 15:4665–4670. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang X, Zhu Z, Jiao W, Liu W, Liu F and
Zhu X: Ulinastatin treatment for acute respiratory distress
syndrome in China: A meta-analysis of randomized controlled trials.
BMC Pulm Med. 19(196)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
National Health Commission of China.
Guideline for diagnosis and treatment for novel coronavirus
pneumonia (seventh edition). http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml.
Accessed March 4, 2020.
|
|
18
|
Sanders JM, Monogue ML, Jodlowski TZ and
Cutrell JB: Pharmacologic treatments for coronavirus disease 2019
(COVID-19): A review. JAMA. 323:1824–1836. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu
M, Shi Z, Hu Z, Zhong W and Xiao G: Remdesivir and chloroquine
effectively inhibit the recently emerged novel coronavirus
(2019-nCoV) in vitro. Cell Res. 30:269–271. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Holshue ML, DeBolt C, Lindquist S, Lofy
KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural
A, et al: First case of 2019 novel coronavirus in the United
States. N Engl J Med. 382:929–936. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lescure FX, Bouadma L, Nguyen D, Parisey
M, Wicky PH, Behillil S, Gaymard A, Bouscambert-Duchamp M, Donati
F, Le Hingrat Q, et al: Clinical and virological data of the first
cases of COVID-19 in Europe: A case series. Lancet Infect Dis.
20:697–706. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Grein J, Ohmagari N, Shin D, Diaz G,
Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, et
al: Compassionate use of remdesivir for patients with severe
Covid-19. N Engl J Med. 382:2327–2336. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan
G, Ruan L, Song B, Cai Y, Wei M, et al: A trial of
lopinavir-ritonavir in adults hospitalized with severe Covid-19. N
Engl J Med. 382:1787–1799. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z,
Xiang J, Wang Y, Song B, Gu X, et al: Clinical course and risk
factors for mortality of adult inpatients with COVID-19 in Wuhan,
China: A retrospective cohort study. Lancet. 395:1054–1062.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guan WJ, Liang WH, Zhao Y, Liang HR, Chen
ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, et al: Comorbidity and
its impact on 1590 patients with Covid-19 in China: A nationwide
analysis. Eur Respir J. 55(2000547)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Grasselli G, Zangrillo A, Zanella A,
Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G,
Fumagalli R, et al: Baseline characteristics and outcomes of 1591
patients infected with SARS-CoV-2 admitted to ICUs of the lombardy
region, Italy. JAMA. 323:1574–1581. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen Q, Hu C, Liu Y, Liu Y, Wang W, Zheng
H, Rong L, Jia J, Sun S, Yu C and Liu YM: Safety and tolerability
of high-dose ulinastatin after 2-h intravenous infusion in adult
healthy Chinese volunteers: A randomized, double-blind,
placebo-controlled, ascending-dose study. PLoS One.
12(e0177425)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
World Health Organization. Clinical
management of severe acute respiratory infection (SARI) when
COVID-19 disease is suspected. Interim guidance. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected.
Accessed March 13, 2020.
|
|
29
|
Linder A and Russell JA: An exciting
candidate therapy for sepsis: Ulinastatin, a urinary protease
inhibitor. Intensive Care Med. 40:1164–1167. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kanai T, Ishiwata T, Kobayashi T, Sato H,
Takizawa M, Kawamura Y, Tsujimoto H, Nakatani K, Ishibashi N,
Nishiyama M, et al: Ulinastatin, a urinary trypsin inhibitor, for
the initial treatment of patients with Kawasaki disease: A
retrospective study. Circulation. 124:2822–2828. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wagner C, Griesel M, Mikolajewska A,
Mueller A, Nothacker M, Kley K, Metzendorf MI, Fischer AL, Kopp M,
Stegemann M, et al: Systemic corticosteroids for the treatment of
COVID-19. Cochrane Database Syst Rev. 8(CD014963)2021.PubMed/NCBI View Article : Google Scholar
|