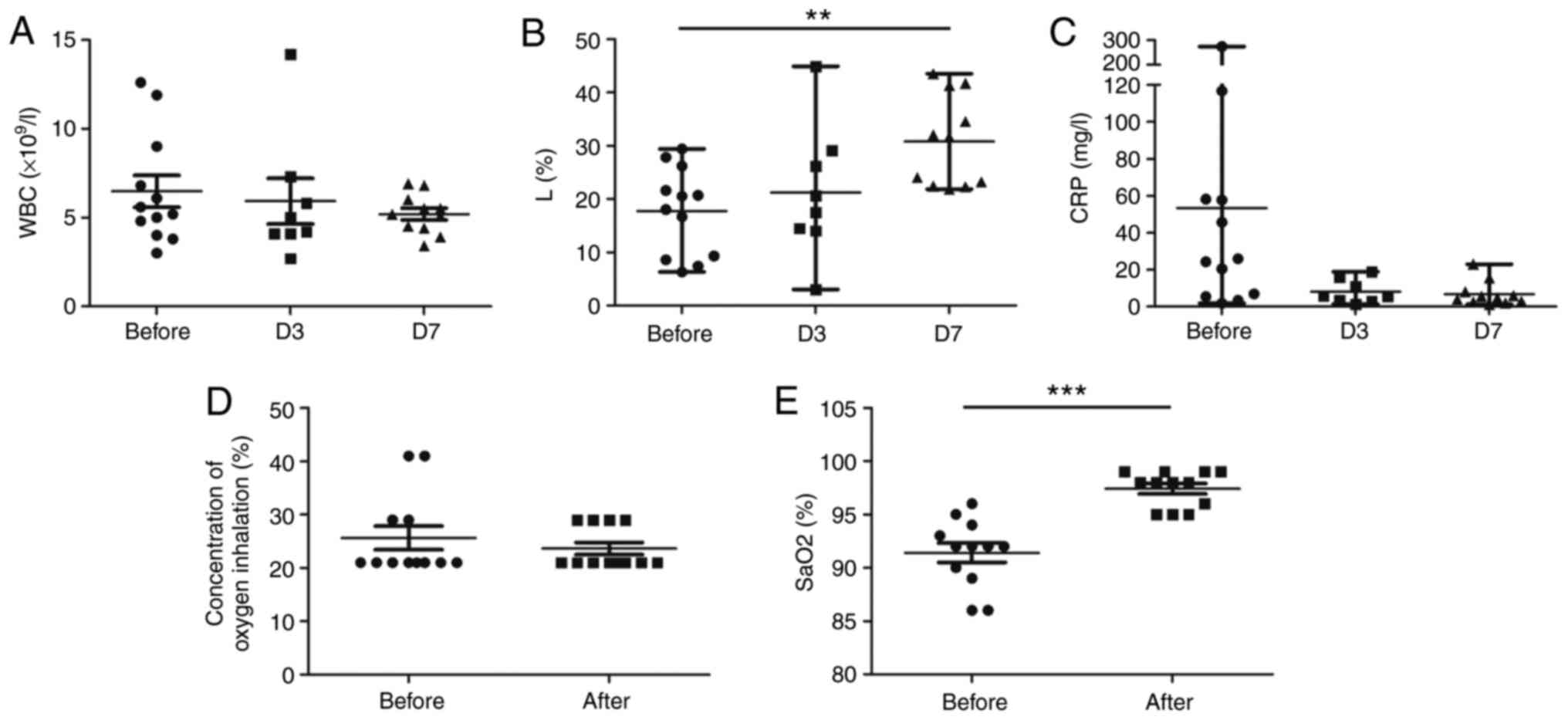
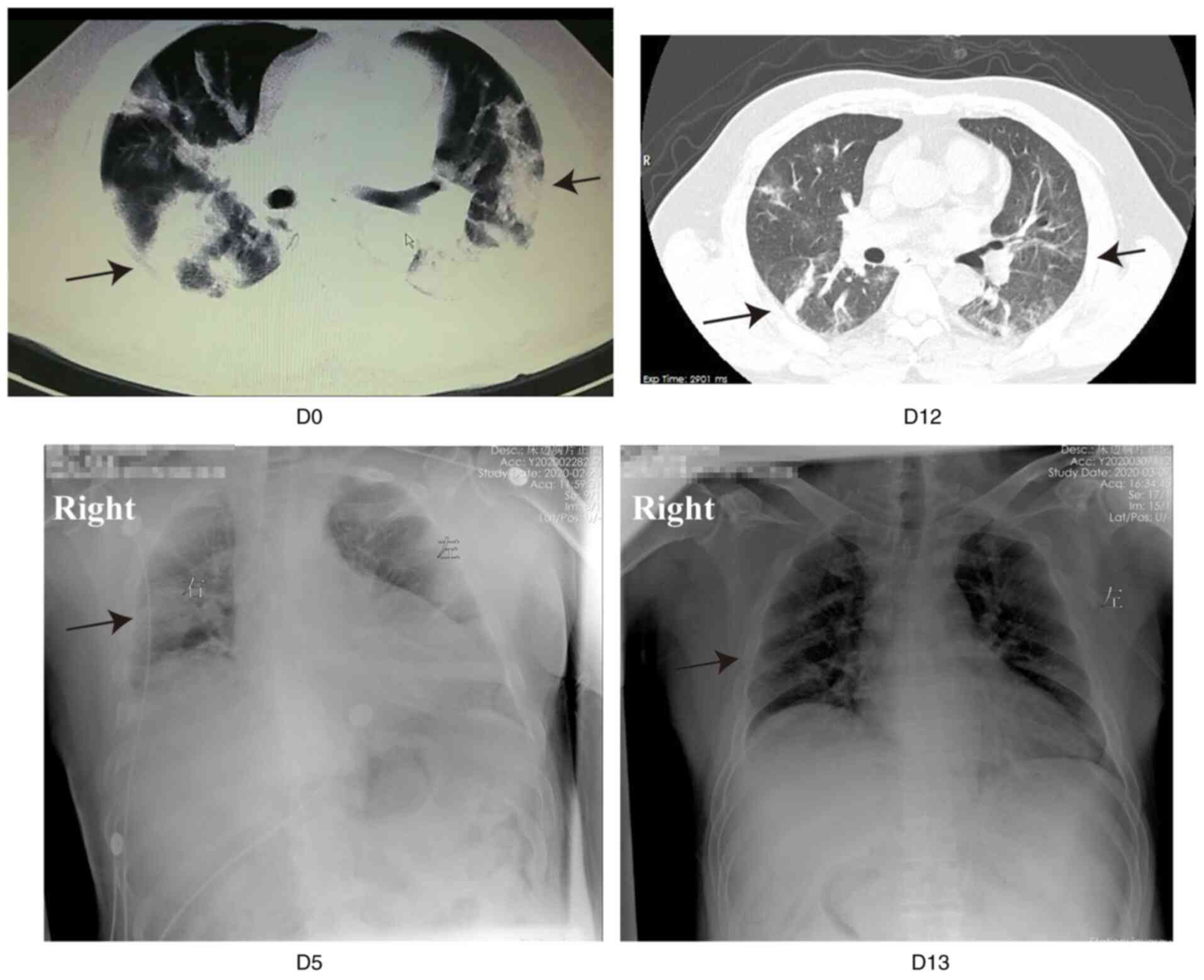
|
1
|
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He
JX, Liu L, Shan H, Lei CL, Hui DSC, et al: Clinical characteristics
of coronavirus disease 2019 in China. N Engl J Med. 382:1708–1720.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cucinotta D and Vanelli M: WHO declares
COVID-19 a pandemic. Acta Biomed. 91:157–160. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H,
Wu Y, Zhang L, Yu Z, Fang M, et al: Clinical course and outcomes of
critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China:
A single-centered, retrospective, observational study. Lancet
Respir Med. 8:475–481. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhou YW, Xie Y, Tang LS, Pu D, Zhu YJ, Liu
JY and Ma XL: Therapeutic targets and interventional strategies in
COVID-19: Mechanisms and clinical studies. Signal Transduct Target
Ther. 6(317)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Calina D, Docea AO, Petrakis D, Egorov AM,
Ishmukhametov AA, Gabibov AG, Shtilman MI, Kostoff R, Carvalho F,
Vinceti M, et al: Towards effective COVID-19 vaccines: Updates,
perspectives and challenges (Review). Int J Mol Med. 46:3–16.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nitulescu GM, Paunescu H, Moschos SA,
Petrakis D, Nitulescu G, Ion GND, Spandidos DA, Nikolouzakis TK,
Drakoulis N and Tsatsakis A: Comprehensive analysis of drugs to
treat SARS-CoV-2 infection: Mechanistic insights into current
COVID-19 therapies (Review). Int J Mol Med. 46:467–488.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang
H, Wang T, Zhang X, Chen H, Yu H, et al: Clinical and immunological
features of severe and moderate coronavirus disease 2019. J Clin
Invest. 130:2620–2629. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cao X: COVID-19: Immunopathology and its
implications for therapy. Nat Rev Immunol. 20:269–270.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mehta P, Mcauley DF, Brown M, Sanchez E,
Tattersall RS and Manson JJ: HLH Across Speciality Collaboration,
UK. COVID-19: Consider cytokine storm syndromes and
immunosuppression. Lancet. 395:1033–1034. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yamamoto M, Matsuyama S, Li X, Takeda M,
Kawaguchi Y, Inoue JI and Matsuda Z: Identification of nafamostat
as a potent inhibitor of Middle East respiratory syndrome
coronavirus S protein-mediated membrane fusion using the
split-protein-based cell-cell fusion assay. Antimicro Agents
Chemother. 60:6532–6539. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hoffmann M, Kleine-Weber H, Schroeder S,
Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH,
Nitsche A, et al: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2
and is blocked by a clinically proven protease inhibitor. Cell.
181:271–280.e8. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Scudellari M: How the coronavirus infects
cells-and why delta is so dangerous. Nature. 595:640–644.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lagoo JY, D'souza MC, Kartha A and Kutappa
AM: Role of ulinastatin, a trypsin inhibitor, in severe acute
pancreatitis in critical care setting: A retrospective analysis. J
Crit Care. 45:27–32. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Karnad DR, Bhadade R, Verma PK, Moulick
ND, Daga MK, Chafekar ND and Iyer S: Intravenous administration of
ulinastatin (human urinary trypsin inhibitor) in severe sepsis: A
multicenter randomized controlled study. Intensive Care Med.
40:830–838. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ji M, Chen T, Wang B, Chen M, Ding Q, Chen
L, Fang Y, Yu X, Chen Y, Wang X, et al: Effects of ulinastatin
combined with mechanical ventilation on oxygen metabolism,
inflammation and stress response and antioxidant capacity of ARDS.
Exp Ther Med. 15:4665–4670. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang X, Zhu Z, Jiao W, Liu W, Liu F and
Zhu X: Ulinastatin treatment for acute respiratory distress
syndrome in China: A meta-analysis of randomized controlled trials.
BMC Pulm Med. 19(196)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
National Health Commission of China.
Guideline for diagnosis and treatment for novel coronavirus
pneumonia (seventh edition). http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml.
Accessed March 4, 2020.
|
|
18
|
Sanders JM, Monogue ML, Jodlowski TZ and
Cutrell JB: Pharmacologic treatments for coronavirus disease 2019
(COVID-19): A review. JAMA. 323:1824–1836. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu
M, Shi Z, Hu Z, Zhong W and Xiao G: Remdesivir and chloroquine
effectively inhibit the recently emerged novel coronavirus
(2019-nCoV) in vitro. Cell Res. 30:269–271. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Holshue ML, DeBolt C, Lindquist S, Lofy
KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural
A, et al: First case of 2019 novel coronavirus in the United
States. N Engl J Med. 382:929–936. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lescure FX, Bouadma L, Nguyen D, Parisey
M, Wicky PH, Behillil S, Gaymard A, Bouscambert-Duchamp M, Donati
F, Le Hingrat Q, et al: Clinical and virological data of the first
cases of COVID-19 in Europe: A case series. Lancet Infect Dis.
20:697–706. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Grein J, Ohmagari N, Shin D, Diaz G,
Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, et
al: Compassionate use of remdesivir for patients with severe
Covid-19. N Engl J Med. 382:2327–2336. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan
G, Ruan L, Song B, Cai Y, Wei M, et al: A trial of
lopinavir-ritonavir in adults hospitalized with severe Covid-19. N
Engl J Med. 382:1787–1799. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z,
Xiang J, Wang Y, Song B, Gu X, et al: Clinical course and risk
factors for mortality of adult inpatients with COVID-19 in Wuhan,
China: A retrospective cohort study. Lancet. 395:1054–1062.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guan WJ, Liang WH, Zhao Y, Liang HR, Chen
ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, et al: Comorbidity and
its impact on 1590 patients with Covid-19 in China: A nationwide
analysis. Eur Respir J. 55(2000547)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Grasselli G, Zangrillo A, Zanella A,
Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G,
Fumagalli R, et al: Baseline characteristics and outcomes of 1591
patients infected with SARS-CoV-2 admitted to ICUs of the lombardy
region, Italy. JAMA. 323:1574–1581. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen Q, Hu C, Liu Y, Liu Y, Wang W, Zheng
H, Rong L, Jia J, Sun S, Yu C and Liu YM: Safety and tolerability
of high-dose ulinastatin after 2-h intravenous infusion in adult
healthy Chinese volunteers: A randomized, double-blind,
placebo-controlled, ascending-dose study. PLoS One.
12(e0177425)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
World Health Organization. Clinical
management of severe acute respiratory infection (SARI) when
COVID-19 disease is suspected. Interim guidance. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected.
Accessed March 13, 2020.
|
|
29
|
Linder A and Russell JA: An exciting
candidate therapy for sepsis: Ulinastatin, a urinary protease
inhibitor. Intensive Care Med. 40:1164–1167. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kanai T, Ishiwata T, Kobayashi T, Sato H,
Takizawa M, Kawamura Y, Tsujimoto H, Nakatani K, Ishibashi N,
Nishiyama M, et al: Ulinastatin, a urinary trypsin inhibitor, for
the initial treatment of patients with Kawasaki disease: A
retrospective study. Circulation. 124:2822–2828. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wagner C, Griesel M, Mikolajewska A,
Mueller A, Nothacker M, Kley K, Metzendorf MI, Fischer AL, Kopp M,
Stegemann M, et al: Systemic corticosteroids for the treatment of
COVID-19. Cochrane Database Syst Rev. 8(CD014963)2021.PubMed/NCBI View Article : Google Scholar
|