Introduction
Toxoplasmosis is a zoonotic disease caused by the
Toxoplasma gondii (T. gondii) protozoan parasite
(1,2). Toxoplasmosis is widespread and it is
present in every country and seropositivity rates range from less
than 10% to over 90% (3). It is
estimated that onethird of the world's human population is infected
by this parasite (4,5). Human infection results often from the
ingestion or handling of undercooked meat of intermediate hosts
such as sheep, pigs and birds containing tissue cysts. In other
cases, it may result from direct contact with cats or from the
consumption of water or food contaminated by oocysts excreted in
the feces of infected cats (2,6).
Toxoplasmosis is typically an asymptomatic infection resulting in
life-long latent infection in healthy individuals (7). However, this infection is an
opportunistic one that can cause severe complications in
immune-suppressed diseased individuals such as AIDS patients
(8-11).
Several studies that have investigated the human population
infected by T. gondii suggest that this pathogen may play an
etiological role in some mental and behavioral disorders (12-14).
Women infected with T. gondii before pregnancy usually do
not transmit the parasite to their fetuses. Acute toxoplasma
infection during pregnancy can lead to congenital toxoplasmosis for
the fetus and newborn. It can cause abortion, intrauterine fetal
demise and syndromes that include neurologic and neurocognitive
deficits and chorioretinitis (15,16).
Several studies have demonstrated that some
environmental factors, whether occurring as a natural phenomenon or
through human intervention, can strongly influence the occurrence,
transmission and distribution of T. gondii, especially
climate, today's rapid urbanization, global warming, and economic
globalization (7,17,18).
Other important predictors of T. gondii infection are
considered: sociodemographic factors, behavioral and obstetric
factors, geographic location, presence of cats in the household,
agriculture practice (exposure to contaminated soil through farming
or gardening barehanded), history of spontaneous abortion and
maternal age (2,7,19).
Oocysts are resistant to the environment. They can
sporulate in water and become infectious to their hosts, surviving
for a period of up to 6 months in seawater (20,21).
It has been suggested that human infection caused by oocysts is
usually more severe than that caused by the ingestion of tissue
cysts, regardless of the dose (22).
Patients and methods
Participants
The study included 240 pregnant women who were
referred to the Infectious Diseases Office from ‘Dr. Gavril
Curteanu’ Municipal Clinical Hospital, Oradea and to the Infectious
Diseases Outpatient Clinic from the County Emergency Clinical
Hospital, Oradea, Romania during 01/01/2016 to 31/12/2019. The
study was conducted in accordance with the World Medical
Association (WMA) Declaration of Helsinki, Ethical Principles for
Medical Research Involving Human Subjects, and approved by the
Ethics Committee of the University of Oradea, Romania (project
identification code: 17/22.01.2021).
Particular attention was paid to the inclusion,
respectively exclusion criteria in the study. The inclusion
criteria were: i) pregnant women aged between 18 and 40 years; ii)
request to perform serological diagnosis in order to determine the
immune status for this parasite; iii) presence of at least one of
the following criteria at presentation: pregnant women with
clinical signs specific to T. gondii infection with
lymphadenopathy, pregnant women with a history of pregnancy loss
prior to the current pregnancy, pregnant women who kept pets (cats)
at home; iv) pregnant women who could be kept under medical
observation for 1 year in order to monitor in dynamics the
Toxoplasma IgM, IgA, IgG antibody titters; v) stable residence in
Bihor County.
The exclusion criteria were: i) pregnant women under
18 years of age and over 40 years of age; ii) pregnant women who
refused clinical and serological monitoring for 1 year to capture
seroconversion.
Methods
The first stage of the study consisted in describing
the group of pregnant women in terms of age groups as well as in
terms of geographical background (urban/rural). The next stage of
the study aimed to identify several serological changes: pregnant
women with acute toxoplasmosis tested positive for toxoplasma IgM
and IgG antibodies, positive/negative for toxoplasma IgA
antibodies, their evolution being followed in dynamics; pregnant
women with a history of acute infection tested negative for
toxoplasma IgM antibodies and positive for toxoplasma IgG
antibodies. In the case of pregnant women diagnosed with acute
toxoplasmosis, IgG avidity test was also performed in order to
established precisely the moment of infection.
Serological assay
Blood samples to determine the serum level of T.
gondii-specific IgM, IgA and IgG antibodies were drawn in
several stages: the first test at the patient's presentation (onset
of the disease); the following test at 4 weeks; then at 3, 6, 9 and
12 months from the initial sample in order to monitor in dynamics
the T. gondii-specific IgM, IgA and IgG antibody titres.
Determination of IgM and IgG toxoplasma antibodies
were performed in Biostandard Laboratories by the chemiluminescence
immunoassay (CLIA) method. For this we used the Immulite 2000
analyzer (Siemens), together with the necessary reagents, produced
also by Seimens.
Detection of T. gondii-specific IgA
antibodies was carried out by the Bioclinica Laboratories, using
the Chorus analyzer (Diesse Diagnostica Senese S.p.A). The used
method was enzyme immunoassay (EIA).
The IgG avidity test was also carried out by the
Bioclinica Laboratories, using the Biomerieux Vidas analyzer and
reagents. The method used was enzyme linked fluorescent assay
(ELFA). An index <0.200 indicates low avidity. An index between
0.200 and 0.300 indicates intermediate avidity, and an index
>0.300 indicates high avidity, showing that the primary
infection occurred 4 months before.
Following, the study presented the correlation
between the serological profile of the respective pregnant women
and their age, respectively their geographical background.
Next, the pregnant women diagnosed with acute
toxoplasmosis were examined in terms of correlative demographic
parameters, respectively in terms of the medical aspects, the
frequency of lymphadenopathy, the risk of developing
lymphadenopathy, the location of the lymphadenopathy and its degree
of extension, as well as possible correlations with patient age
were assessed.
In the next stage, we performed serological
examinations, determining in all pregnant women the toxoplasma IgM,
IgG, and IgA antibody titres as well as their dynamic evolution
over a period of 12 months, namely: in the first month, at 1-3
months, at 3-6 months, at 6-9 months, at 9-12 months. The IgG
avidity test was carried out in patients with acute toxoplasmosis
to determine as accurately as possible the timing of infection.
Another stage of the research conducted a
comparative monitoring of the course of pregnancy in pregnant women
with acute toxoplasmosis vs. pregnant women without acute
toxoplasmosis in terms of onset, risk of miscarriage in pregnant
women with acute toxoplasmosis and frequency of stillbirths.
Data analysis
MedCalc® version 9.4.2.0
(MedCalc® Software) statistical software was used to
store the information from the patients' medical records in a
database and to perform statistical calculations. The results of
the statistical tests shall be represented by the probability of
the ‘null’ hypothesis (P), its value below 0.05 proves a
statistically significant difference between the studied groups.
Certain results shall be presented graphically using the same
statistical software. Microsoft® Excel® 2010
(Microsoft® Corporation, USA) was used for several
charts. The Kolmogorov-Smirnov test was used to assess each
continuous variable for value distribution compared to the normal
population. Non-parametric tests were conducted (for variables with
asymmetric distribution) depending on the variable. The
non-parametric methods used during the study were: the Mann-Whitney
test where there are only two study groups. Categorical variables
are described by their absolute values and percentages in
parentheses. They were studied using the following tests: the
Chi-square test with Yates' correction for continuity-in the case
of 2x2 contingency tables (categorical variables with 2 possible
values between 2 study groups) with a number of cases over 20;
simple Chi-square test-for other types of frequency tables (3x2,
3x3).
Results
The pregnant women included in the study showed an
increased share of the age groups 21-25 years [86 cases (35.8%)]
and 26-30 years [62 cases (25.8%)], the difference compared to the
other age groups being statistically significant (P<0.0001).
The pregnant women included in the study came mainly
from rural areas [137 cases (57.1%)] compared to urban areas [103
cases (42.9%)], the difference being statistically significant
(P=0.0332).
Of the 240 pregnant women monitored during the four
years of study, 60% (144 cases) did not show serological changes
specific to T. gondii infection. Of the 96 pregnant women
(40%) infected with T. gondii, approximately 1/3 [35 cases
(14.6%)] had acquired acute infection and 2/3 [61 cases (25.4%)]
had a history of prior acute infection (P<0.0001).
The share of pregnant women with acute toxoplasmosis
in the four years studied was 14.6% (35 cases). The distribution of
the reference pregnant women by study years did not show
statistically significant differences (P=0.8563). In addition,
there were no statistically significant differences in the
distribution of pregnant women by age groups (P=0.0997), by years
and by age groups (P=0.4410), by region of origin (P=0.3105) even
if the pregnant women from rural areas were in a higher number, or
by the region of origin and years of study (P=0.4804).
Pregnant women with acute toxoplasmosis showed
lymphadenopathy more frequently [31 cases (88.57%)] than pregnant
women without acute toxoplasmosis in the study group [145 cases
(70.73%)] (Table I), the difference
being statistically significant (P=0.0456). The relative risk of
developing lymphadenopathy in pregnant women with acute
toxoplasmosis was 1.2522 higher than in pregnant women without
acute toxoplasmosis.
 | Table IDistribution by the presence of
lymphadenopathy of pregnant women with acute forms of disease and
of pregnant women without acute forms of disease. |
Table I
Distribution by the presence of
lymphadenopathy of pregnant women with acute forms of disease and
of pregnant women without acute forms of disease.
| | Pregnant women | |
|---|
|
Lymphadenopathy | Without acute
toxoplasmosis n | With acute
toxoplasmosis n | n (%) |
|---|
| No
lymphadenopathy | 60 | 4 | 64 (26.7%) |
| With
lymphadenopathy | 145 | 31 | 176 (73.3%)
(P=0.0456) |
| Total % | 205 (85.4%) | 35 (14.6%) | 240 (100%) |
There was no statistically significant difference
between localized or generalized lymphadenopathy caused by the
presence or absence of acute toxoplasmosis among the reference
pregnant women (P=0.8462). In addition, the presence or absence of
lymphadenopathy was not correlated with the age groups (P=0.0581).
The localized or generalized character of the lymphadenopathy was
not correlated with the age groups either (P=0.8537).
The highest incidence among the pregnant women with
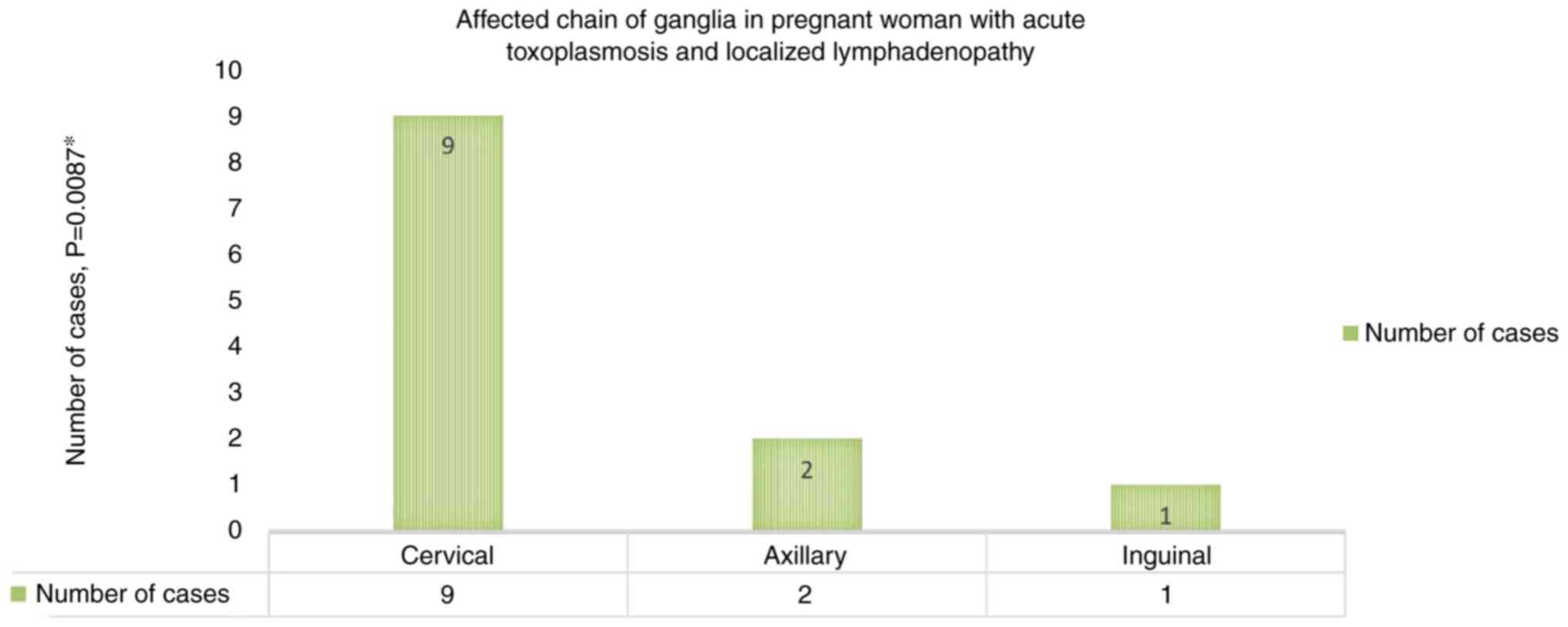
acute toxoplasmosis with a single chain of ganglia affected
(Fig. 1) was represented by those
with the involvement of cervical lymph nodes [9 cases (75%)], the
difference compared to the involvement of other chains of ganglia
being statistically significant (P=0.0087).
The involvement of the cervical-axillary-inguinal
lymph nodes at the same time [12 cases (63.1%)] was more common
compared to the other combinations (cervical-axillary,
cervical-inguinal, axillary-inguinal) (P=0.0013) in pregnant women
with acute toxoplasmosis and generalized lymphadenopathy.
All 35 pregnant women with acute toxoplasmosis
tested positive for T. gondii-specific IgM antibodies.
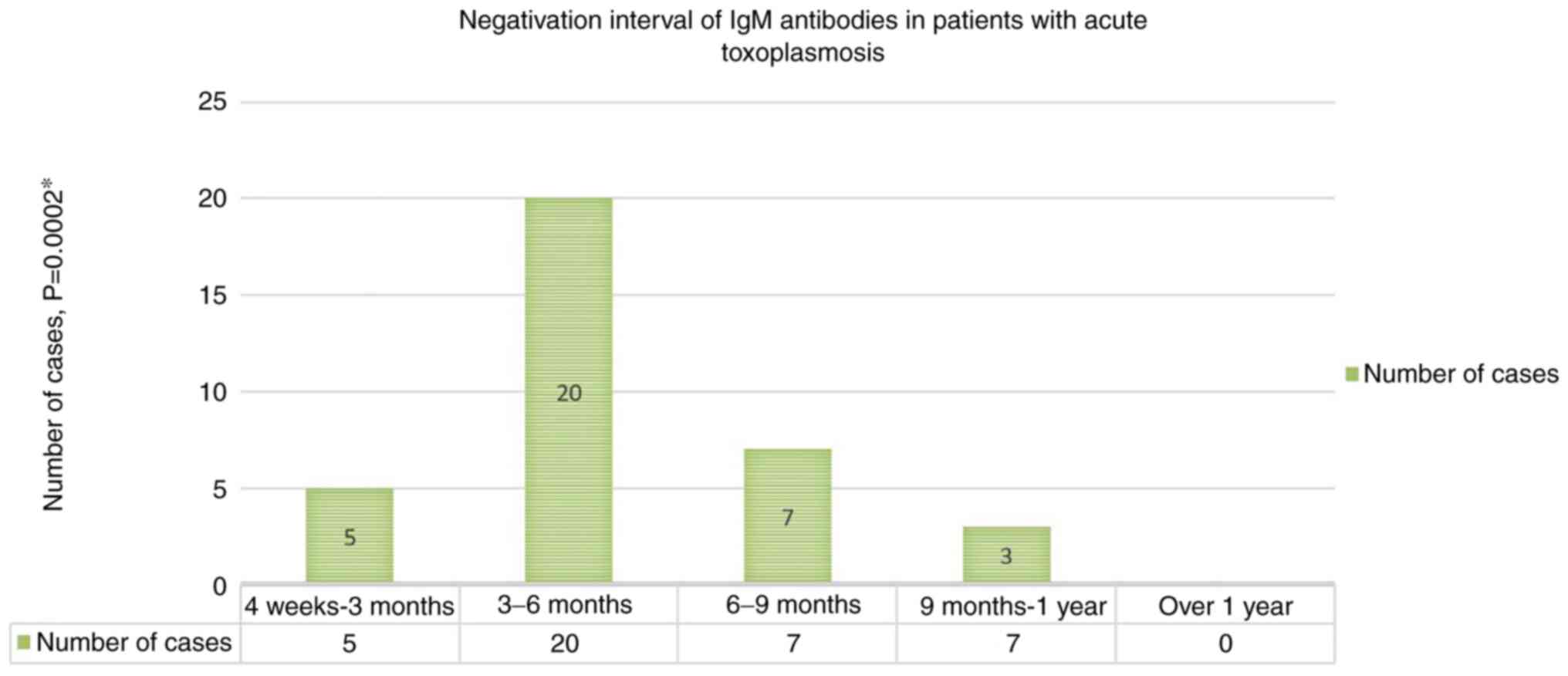
Most pregnant women with acute T. gondii
infection [20 cases (57.1%)] tested negative for T.
gondii-specific IgM antibody serum titres within 3-6 months of
presentation (Fig. 2). The
difference compared to the other shorter (4 weeks-3 months) or
longer intervals (6-9 months, 9-12 months) from the moment pregnant
women sought care was statistically significant (P=0.0002).
No pregnant women in the studied group tested
positive for IgM antibodies for more than 1 year from the time of
diagnosis.
Only 28 cases (80%) of all pregnant women with acute
toxoplasmosis tested positive for T. gondii-specific IgA
antibodies. In the remaining 7 cases (20%), the T.
gondii-specific IgA antibodies had equivocal value. These
antibodies were most frequently negative within 4 weeks to 3 months
[19 cases (75%)] from the moment pregnant women sought care
(P=0.0001).
Monitoring the course of pregnancy in women with
acute toxoplasmosis compared to women without acute toxoplasmosis
(Table II) indicated that the
course of pregnancy may be directly correlated with the presence of
acute T. gondii infection (P=0.0072). According to this
study, pregnant women with acute toxoplasmosis had a 3.3 times
higher risk of pregnancy loss than pregnant women without acute
toxoplasmosis.
 | Table IIDistribution of the course of
pregnancy in women with present or absent acute toxoplasmosis. |
Table II
Distribution of the course of
pregnancy in women with present or absent acute toxoplasmosis.
| | Acute
toxoplasmosis | |
|---|
| Course of
pregnancy | Absent n | Present n | n (%) |
|---|
| Termination on
request | 0 | 1 | 1 (0.4%) |
| Birth | 176 | 24 | 200 (83.3%) |
| Birth of a
stillborn fetus | 0 | 1 | 1 (0.4%) |
| Premature
birth | 4 | 0 | 4 (1.7%) |
| Spontaneous
pregnancy loss | 25 | 9 | 34 (14.2%) |
| Total % | 205 (85.4%) | 35 (14.6%)
(P=0.0072) | 240 (100%) |
In 85% of the pregnant women with acute
toxoplasmosis who tested positive for T. gondii-specific
IgM, IgG, and IgA antibodies and who showed increased IgG avidity,
the course of pregnancy was normal. Five percent of the pregnant
women belonging to the respective serological profile underwent
therapeutic abortion, suffered spontaneous abortion, or gave birth
to a stillborn fetus, in the same proportions. All pregnant women
who tested positive for T. gondii-specific IgM, IgG and IgA
antibodies and showed low IgG avidity suffered a spontaneous
pregnancy loss. The course of pregnancy was normal in pregnant
women who tested positive for T. gondii-specific IgM and IgG
antibodies, negative for T. gondii-specific IgA antibodies
and showed increased IgG avidity.
Discussion
In the present study, we considered that pregnant
women, especially those with an obstetric history, marked by
pregnancy loss, spontaneous abortion, stillbirth, preterm birth,
but also pregnant women who develop a clinical picture more or less
suggestive for T. gondii infection should undergo a thorough
examination. We started from the premise that investigations
performed in the first trimester of pregnancy are useful to avoid
the embryopathic effect of a possible infection. Based on Suzuki
et al, we considered the fact that several elaborate
examinations on specific immunology may determine the infection age
and may also establish a certain therapeutic conduct. In their
study related to several serological techniques used for diagnosis
of acute acquired toxoplasmosis, the authors found that
determination of the avidity of T. gondii-specific IgG by
the titration method in patients with detectable IgM antibodies
defines most accurately the stage of infection by T. gondii
(23).
We refer to a selected group in the sense that each
of the pregnant women included in our study presented at least one
risk factor for T. gondii infection, these factors being
decisive for requesting the investigation of the reference pregnant
women in the special ward of the Infectious Diseases Clinic from
Oradea and in the Infectious Diseases Outpatient Clinic from the
County Emergency Clinical Hospital, Oradea, Romania.
The existence of these very restrictive inclusion
criteria makes it difficult to compare our results with the values
established by other studies which either address all pregnant
women in a certain geographical area or in certain groups of
pregnant women selected based on a specific obstetric
pathology.
Our study demonstrated that serum titre for T.
gondii-specific IgM antibodies was most frequently negative
within 3-6 months from the moment of seeking care (57.1%). The
difference compared to the other shorter or longer intervals from
the moment pregnant women sought care was statistically significant
(P=0.0002). Only 80% of the pregnant women with acute toxoplasmosis
tested positive for T. gondii-specific IgA antibodies.
Patients tested negative for these antibodies most frequently
within 4 weeks to 3 months from the time pregnant women sought
care, faster than for T. gondii-specific IgM antibodies; the
difference compared to the other intervals being statistically
significant (P=0.0001). Monitoring the course of pregnancy in women
with acute toxoplasmosis compared to women without acute
toxoplasmosis shows that pregnancy loss may be directly correlated
with the presence of acute T. gondii infection (P=0.0072).
Pregnant women with acute toxoplasmosis had a 3.3 times higher risk
of pregnancy loss than those without acute toxoplasmosis.
According to several data from the literature
consulted as well, the increased prevalence of T. gondii
infection in pregnant women from rural areas may be explained, to a
large extent, by the increased presence of household pets (cats)
which are not under the supervision of a veterinarian, with the
possibility of easier spread of T. gondii oocysts in the
external environment, becoming infective to pregnant women, as well
as by the more frequent consumption of possibly insufficiently
cooked food or insufficiently washed vegetables and fruit on which
oocysts may exist. Accurate information on the quality of water and
soil should increase people's awareness of the importance and the
role of water for human health (24,25).
Our study showed that 80% of all pregnant women with
acute toxoplasmosis tested positive for T. gondii-specific
IgA antibodies. This value is lower than the values reported by
other authors, where 95% of the acquired toxoplasmosis recorded
positive values of T. gondii-specific IgA antibodies
(26). What is specific to our case
study is that all women with acute toxoplasmosis tested negative
for T. gondii-specific IgA antibodies within 9 months after
presentation.
Medical advice regarding termination of the
pregnancy in women suspected of being infected with T.
gondii should consider not only the positivity for T.
gondii-specific IgM, IgG, and IgA antibodies, but also the
results of the IgG avidity test performed in the first trimester of
pregnancy whose increased value indicates an infection prior to
conception. Under these circumstances, there is certainty that the
conception product shall not be infected, thus there shall be no
cases of congenital toxoplasmosis (27,28).
In recent studies on the global prevalence of ATI in pregnant women
infected with T. gondii, it was reported that the global
prevalence of ATI was 1.1% while the studies that used strict
criteria (seroconversion and low IgG avidity) for the definition of
ATI showed that 0.6% of pregnant women had ATI during gestation.
Thus, the prevalence of ATI varied due to many factors such as
income level, human development indices and geographical location
(29,30).
Negative serum serology (both IgG and IgM) is
adequate to rule out T. gondii infection in immunocompetent
patients. Even if serum serology has been widely used for the
diagnosis of toxoplasmosis in both immunocompetent and
immunocompromised patients, some seronegative clinical cases have
been reported especially in immunocompromised patients.
Complimentary investigations can be helpful in these atypical cases
(31-34).
A study carried out by several Chinese authors
showed that anti-T. gondii-specific IgG antibodies were
found in 15.2% of pregnant women; 2.9% of pregnant women were
positive for anti-T. gondii IgM antibodies. Among pregnant
women, 12.6% were positive for IgG antibodies and 0.3% of pregnant
women were positive for IgM antibodies only, while 2.6% pregnant
women were positive for both IgG and IgM antibodies. Univariate
analysis of sociodemographic and risk factors for pregnant women
identified some factors with a value ≤0.25 that may be related to
infection. Four of these were found to be significantly associated
with T. gondii infection: area of residence, cats in home,
contact with cats and dogs, and exposure to soil (35).
There is little information about the epidemiology
of T. gondii infection in pregnant women in Romania.
According to some results, T. gondii seroprevalence in
pregnant women has decreased from 43.79 to 38.81% in the last 10
years in the Western Region of Romania. Our study, unlike other
recent studies carried out in Romania, did not show statistically
significant differences related to the age of the pregnant women
infected with T. gondii,while the prevalence of infections
was higher in urban areas (36,37).
Consistent with the two studies mentioned above, several authors
claim that there is a decreasing trend in T. gondii
infection among pregnant women (38). Epidemiological studies conducted on
T. gondii seroprevalence in pregnant women before the 2000's
revealed rates ranging from 40.4% to 48.7% in two areas of Italy,
whereas some recent studies have found rates of T. gondii
prevalence in pregnant women between 21.5 and 27.5% (39-42).
In conclusion, serum titre for T.
gondii-specific IgM antibodies was most frequently negative
within 3-6 months from the moment of seeking care; 80% of the
pregnant women with acute toxoplasmosis tested positive for T.
gondii-specific IgA antibodies. Pregnant women with acute
toxoplasmosis had a 3.3 times higher risk of pregnancy loss than
those without acute toxoplasmosis.
Acknowledgements
We would like to thank our collaborators from
Bioclinica and Biostandard Laboratories.
Funding
Funding: No funding was received.
Authors' contributions
AC, BMN, CTJP and CB were involved in the conception
of the study and the interpretation of the data. AC, LLV, BIT and
CS contributed to the acquisition of the data and performed
statistical analysis. AC performed serological testing. AC, LLV and
BMN wrote the manuscript. CTJP, CB, BIT and CS revised the
manuscript for important intellectual content. All authors read and
approved the final manuscript for publication.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the first author on reasonable
request.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the University of Oradea, Faculty of Medicine and Pharmacy, Romania
(project identification code: 17/22.01.2021). Informed consent was
obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tenter AM, Heckeroth AR and Weiss LM:
Toxoplasma gondii: From animals to humans. Int J Parasitol.
30:1217–1258. 2000.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Al-Adhroey AH, Mehrass AAO, Al-Shammakh
AA, Ali AD, Akabat MYM and Al-Mekhlafi HM: Prevalence and
predictors of Toxoplasma gondii infection in pregnant women
from Dhamar, Yemen. BMC Infect Dis. 19(1089)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pappas G, Roussos N and Falagas ME:
Toxoplasmosis snapshots: Global status of Toxoplasma gondii
seroprevalence and implications for pregnancy and congenital
toxoplasmosis. Int J Parasitol. 39:1385–1394. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Montoya JG and Liesenfeld O:
Toxoplasmosis. Lancet. 363:1965–1976. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Torgerson PR and Mastroiacovo P: The
global burden of congenital toxoplasmosis: A systematic review.
Bull World Health Organ. 91:501–508. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Montoya JG and Remington JS: Management of
Toxoplasma gondii infection during pregnancy. Clin Infect
Dis. 47:554–566. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Yan C, Liang LJ, Zheng KY and Zhu XQ:
Impact of environmental factors on the emergence, transmission and
distribution of Toxoplasma gondii. Parasit Vectors.
9(137)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tegegne D, Abdurahaman M, Mosissa T and
Yohannes M: Anti-toxoplasma antibodies prevalence and associated
risk factors among HIV patients. Asian Pac J Trop Med. 9:460–464.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Munoz M, Liesenfeld O and Heimesaat MM:
Immunology of Toxoplasma gondii. Immunol Rev. 240:269–285.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Csep A, Vaida L, Bungau S and Todor BI:
Clinical and biological correlations in Toxoplasma gondii
infection in HIV immune suppressed diseased persons. Iran J Public
Health. 44:1012–1013. 2015.PubMed/NCBI
|
|
11
|
Pereira-Chioccola VL, Vidal JE and Su C:
Toxoplasma gondii infection and cerebral toxoplasmosis in
HIV-infected patients. Future Microbiol. 4:1363–1379.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Carruthers VB and Suzuki Y: Effects of
Toxoplasma gondii infection on the brain schizophr. Bull.
33:745–751. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Webster JP, Lamberton PH, Donnelly CA and
Torrey EF: Parasites as causative agents of human affective
disorders? The impact of anti-psychotic, mood-stabilizer and
anti-parasite medication on Toxoplasma gondii's ability to
alter host behaviour. Proc Biol Sci. 273:1023–1030. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fekadu A, Shibre T and Cleare AJ:
Toxoplasmosis as a cause for behaviour disorders-overview of
evidence and mechanisms. Folia Parasitol (Praha). 57:105–113.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jones JL, Lopez A, Wilson M, Schulkin J
and Gibbs R: Congenital toxoplasmosis: A review. Obstet Gynecol
Surv. 56:296–305. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kravetz JD and Federman DG: Toxoplasmosis
in pregnancy. Am J Med. 118:212–216. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mazzillo FF, Shapiro K and Silver MW: A
new pathogen transmission mechanism in the ocean: The case of sea
otter exposure to the land-parasite Toxoplasma gondii. PLoS
One. 8(e82477)2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Afonso E, Germain E, Poulle ML, Ruette S,
Devillard S, Say L, Villena I, Aubert D and Gilot-Fromont E:
Environmental determinants of spatial and temporal variations in
the transmission of Toxoplasma gondii in its definitive
hosts. Int J Parasitol Parasites Wildl. 2:278–285. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Elmore SA, Jones JL, Conrad PA, Patton S,
Lindsay DS and Dubey JP: Toxoplasma gondii: Epidemiology,
feline clinical aspects, and prevention. Trends Parasitol.
26:190–196. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lindsay DS, Collins MV, Mitchell SM, Cole
RA, Flick GJ, Wetch CN, Lindquist A and Dubey JP: Sporulation and
survival of Toxoplasma gondii oocysts in seawater. J
Eukaryot Microbiol. 50 (Suppl):S687–S688. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ribeiro LA, Santos LK, Brito PA Jr, Maciel
BM, Da Silva AV and Albuquerque GR: Detection of Toxoplasma
gondii DNA in Brazilian oysters (Crassostrea rhizophorae).
Genet Mol Res. 14:4658–4665. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jones JL and Dubey JP: Waterborne
toxoplasmosis-recent developments. Exp Parasitol. 124:10–25.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Suzuki LA, Rocha RJ and Rossi CL:
Evaluation of serological markers for the immunodiagnosis of acute
acquired toxoplasmosis. J Med Microbiol. 50:62–70. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Babaie J, Amiri S, Mostafavi E, Hassan N,
Lotfi P, Esmaeili Rastaghi AR and Golkar M: Seroprevalence and risk
factors for Toxoplasma gondii infection among pregnant women
in Northeast Iran. Clin Vaccine Immunol. 20:1771–1773.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sărmăşan C, Drăghici S and Daina L:
Identification, communication and management of risks relating to
drinking water pollution in bihor county. EEMJ. 7:769–774.
2008.
|
|
26
|
Bessierès MH, Roques C, Berrebi A, Barre
V, Cazaux M and Séguéla JP: IgA antibody response during acquired
and congenital toxoplasmosis. J Clin Pathol. 45:605–608.
1992.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lappalainen M and Hedman K: Serodiagnosis
of toxoplasmosis. The impact of measurement of IgG avidity. Ann Ist
Super Sanita. 40:81–88. 2004.PubMed/NCBI
|
|
28
|
Horváth KN, Szénási Z, Danka J and Kucsera
I: Value of the IgG avidity in the diagnosis of recent
toxoplasmosis: A comparative study of four commercially available
anti-Toxoplasma gondii IgG avidity assays. Acta Parasitol.
50:255–260. 2005.
|
|
29
|
Rostami A, Riahi SM, Contopoulos-Ioannidis
DG, Gamble HR, Fakhri Y, Shiadeh MN, Behniafar H, Taghipour A,
Maldonado YA, Mokdad AH, et al: Acute toxoplasma infection in
pregnant women worldwide: A systematic review and meta-analysis.
PLoS Negl Trop Dis. 13(e0007807)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nogareda F, Le Strat Y, Villena I, De Valk
H and Goulet V: Incidence and prevalence of Toxoplasma
gondii infection in women in France, 1980-2020: Model-based
estimation. Epidemiol Infect. 142:1661–1670. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yates WB, Chiong F, Zagora S, Post JJ,
Wakefield D and McCluskey P: Ocular toxoplasmosis in a tertiary
referral centre in Sydney Australia-clinical features, treatment,
and prognosis. Asia Pac J Ophthalmol (Phila). 8:280–284.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ozgonul C and Besirli CG: Recent
developments in the diagnosis and treatment of ocular
toxoplasmosis. Ophthalmic Res. 57:1–12. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Garweg JG, De Groot-Mijnes JD and Montoya
JG: Diagnostic approach to ocular toxoplasmosis. Ocul Immunol
Inflamm. 19:255–261. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sigle M, El Atrouni W and Ajlan RS:
Seronegative ocular toxoplasma panuveitis in an immunocompetent
patient. Am J Ophthalmol Case Rep. 19(100745)2000.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cong W, Dong XY, Meng QF, Zhou N, Wang XY,
Huang SY, Zhu XQ and Qian AD: Toxoplasma gondii infection in
pregnant women: A seroprevalence and case-control study in Eastern
China. Biomed Res Int. 2015(170278)2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Motoi S, Navolan DB, Malita D, Ciohat I,
Nemescu D, Manciuc C, Gorun F, Vilibic-Cavlek T, Boda D, Craina M
and Dobrescu A: A decreasing trend in Toxoplasma gondii
seroprevalence among pregnant women in Romania-results of a
large-scale study. Exp Ther Med. 20:3536–3540. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Olariu TR, Petrescu C, Darabus G, Lighezan
R and Mazilu O: Seroprevalence of Toxoplasma gondii in
Western Romania. Infect Dis (Lond). 47:580–583. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fanigliulo D, Marchi S, Montomoli E and
Trombetta CM: Toxoplasma gondii in women of childbearing age
and during pregnancy: Seroprevalence study in central and Southern
Italy from 2013 to 2017. Parasite. 27(2)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Buffolano W, Gilbert RE, Holland FJ,
Fratta D, Palumbo F and Ades AE: Risk factors for recent toxoplasma
infection in pregnant women in Naples. Epidemiol Infect.
116:347–351. 1996.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Valcavi PP, Natali A, Soliani L, Montali
S, Dettori G and Cheezi C: Prevalence of anti-Toxoplasma
gondii antibodies in the population of the area of Parma
(Italy). Eur J Epidemiol. 11:333–337. 1995.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Capretti MG, De Angelis M, Tridapalli E,
Orlandi A, Marangoni A, Moroni A, Guerra B, Arcuri S, Marsico C and
Faldella G: Toxoplasmosis in pregnancy in an area with low
seroprevalence: Is prenatal screening still worthwhile? Pediatr
Infect Dis J. 33:5–10. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Dalmartello M, Parazzini F, Pedron M,
Pertile R, Collini L, La Vecchia C and Piffer S: Coverage and
outcomes of antenatal tests for infections: A population based
survey in the Province of Trento, Italy. J Matern Fetal Neonatal
Med. 32:2049–2055. 2019.PubMed/NCBI View Article : Google Scholar
|