Introduction
Hypertension is associated with high rates of
incidence worldwide, which is considered to be major risk factor
for stroke, coronary heart disease and chronic kidney disease
(1). A previous study determined
that ~1 billion people had primary hypertension, worldwide
(2). Selection of treatment for
hypertension remains to be a complex medical issue, since patients
may suffer with side effects associated with the medication used to
treat hypertension (3). Although
an increasing number of pathological factors have been associated
with hypertension, the specific mechanism of its pathogenesis
remains unclear. Results of previous studies have demonstrated that
endothelial dysfunction is closely associated with the occurrence
and development of hypertension (4,5).
Endothelial dysfunction is considered to be one of the main causes
of microvascular and macrovascular complications associated with
hypertension (4,5). In addition, endothelial dysfunction
has been reported to be a major factor underlying hypertension in
the heart and kidneys, causing organ dysfunction (6). Endothelial dysfunction is
characterized by changes in permeability and impaired vasodilation,
which have been closely associated with both physiological and
pathological processes, including aging, apoptosis, nitric oxide
(NO) production and autophagy (7).
Results of previous studies have demonstrated that high blood
pressure can affect the function of endothelial cells through a
number of methods (8,9). High blood pressure has been reported
to suppress endothelial-dependent vasodilation by NO, increase the
permeability of endothelial cells, induce endothelial cell
oxidative stress, promote endothelin 1 (ET-1) and angiotensin II
(Ang II) production and promote inflammation (10). In turn, these may lead to
endothelial cell dysfunction to aggravate hypertension and damage
the organ involved (11,12). Endothelial function is a key
prognostic indicator of hypertension, where endothelial dysfunction
limits the auto-repair and regeneration function of endothelial
cells (13). Therefore, it remains
of key importance to explore novel targets of endothelial cells to
protect against endothelial cell injury and to more effectively
diagnose and treat hypertension.
ERK5 is also known as MAPK7 and is a key signaling
molecule in the maintenance of endothelial cell integrity and
homeostasis (14). Atorvastatin, a
type of statin, can be used for the treatment of hypertension
(15). In rat models of
spontaneous hypertension, atorvastatin has been documented to
reduce vascular remodeling by activating protein kinase D/ERK5
(16,17). Pitavastatin is an alternative
statin that is excreted in the feces through the hepatoenteric
circulation (13,14). Only a small portion of pitvastatin
is selectively absorbed by hepatocytes and metabolized by the
cytochrome system, meaning that it can avoid interactions with
other drugs metabolized by this enzyme system (18,19).
However, the effects of pitavastatin in endothelial cell
inflammation and injury induced by hypertension remain to be
elucidated.
Therefore, the aim of the present study was to
investigate the mechanism underlying the effects of pitavastatin on
endothelial cell physiology and its potential association with
ERK5.
Materials and methods
Materials
Ang II was purchased from APExBIO Technology LLC,
whilst pitavastatin was purchased from HL Genomics, Co., Ltd.
Antibodies were purchased as follows: Anti-phosphorylated
(p)-endothelial NO synthase (eNOS; cat. no. ab215717; 1:1,000;
Abcam), anti-eNOS (cat. no. ab76198; 1:1,000; Abcam),
anti-endothelin (ET)1 (cat. no. ab2786; 1:1,000; Abcam), anti-Bcl-2
(cat. no. ab32124; 1:1,000; Abcam), anti-Bax (cat. no. ab32503;
1:1,000; Abcam), anti-cleaved caspase-3 (cat. no. ab2302; 1:1,000;
Abcam), anti-cleaved poly (ADP-ribose) polymerase 1 (PARP1; cat.
no. ab32064; 1:1,000; Abcam), anti-ERK5 (cat. no. ab40809; 1:1,000;
Abcam), anti-GAPDH (cat. no. ab181602; 1:2,000; Abcam),
HRP-conjugated goat anti-mouse IgG H&L (cat. no. ab205719;
1:10,000; Abcam), HRP-conjugated goat anti-rabbit IgG H&L (cat.
no. ab205718; 1:10,000; Abcam) and Alexa Fluor® 488 goat
anti-rabbit IgG H&L (cat. no. ab150077; 1:10,000; Abcam).
The following kits were purchased as follows: NO
Colorimetric assay kit (cat. no. E-BC-K035-S; Elabscience
Biotechnology, Inc.), reactive oxygen species (ROS) colorimetric
assay kit (cat. no. E-BC-K138-F; Elabscience Biotechnology, Inc.),
TNF-α ELISA kit (cat. no. ADI-901-099; Enzo Life Sciences, Inc.),
IL-1β ELISA kit (cat. no. EHC002b.48; NeoBioscience Technology Co.,
Ltd.) and the IL-6 ELISA kit (cat. no. ADI-901-033; Enzo Life
Sciences, Inc.).
Bioinformatics analysis
To search for the potential targets of pitavastatin,
the online database STITCH (https://stitch.embl.de/) was used by entering
‘pitavastatin’ in the ‘Item Name’ box. The minimum required
interaction score was set to >0.4.
Cell lines and transfection
HUVECs (cat. no. BNCC347734; an extensively used
endothelial cell model in vitro) (20) and vascular cell basal medium (cat.
no. BNCC341789) containing 5% FBS, were purchased from BeNa Culture
Collection; Beijing Beina Chunglian Biotechnology Research
Institute. HUVECs were cultured in 5% CO2 with 95%
humidity at 37˚C in a CO2 incubator.
To construct the lentivirus vector expressing MAPK7
short hairpin (sh)-RNA (shRNA-MAPK7-1 or shRNA-MAPK7-2), shRNA
sequences were annealed and inserted between the EcoRI and
BglII sites of the pHBLV-U6-Puro vector (Hanbio
Biotechnology Co., Ltd.). The vectors were then co-transfected
along with the packaging plasmids including Gal-pol, Rev and VSV-G
(all from Yunzhou Bio, China) in a ratio of 2:1:1:1, into 293T
cells (ATCC) using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.). After incubation at 37˚C for 72 h, the culture
medium was collected and centrifuged at 500 x g for 5 min at room
temperature to obtain the viral supernatant. Finally, HUVECs were
transfected with the lentiviral shRNAs (2rd generation)
at a MOI of ~3 and incubated at 37˚C. Puromycin (Thermo Fisher
Scientific, Inc.) selection (2 µg/ml) was added 2 days
post-transfection and after another 2 days incubation at 37˚C,
transfection efficiency was detected using western blot analysis.
The shRNA sequences were listed as follows: shRNA-MAPK7-1 forward
5'-CCGGACAAGTTACTAAGCCATATTTCTCGAGAAATATGGCTTAGTAACTTG TTTTTTG-3'
and reverse,
5'-AATTCAAAAAACAAGTTACTAAGCCATATTTCTCGAGAAATATGGCTTAGTAA CTTGT-3';
shRNA-MAPK7-2 forward,
5'-CCGGTTGTTAGGAGACAAGTTAAATCTCGAGATTTAACTTGTCTCCTAACAATTTTTG-3'
and reverse,
5'-AATTCAAAAATTGTTAGGAGACAAGTTAAATCTCGAGATTTAACTTGTCTCCTAACAA-3'.
Cell viability assay
Cell Counting Kit-8 (CCK-8; cat. no. C0039; Beyotime
Institute of Biotechnology) assays were performed to measure cell
viability. HUVECs were inoculated into 96-well plates
(2x103 cells/well). After being treated with 1 µM Ang II
with or without 1 µM pitavastatin co-treatment for 24 h at 37˚C,
cells were incubated with 20 µl CCK-8 solution per well at 37˚C for
2 h. The absorbance was measured at 450 nm using a
spectrophotometer.
TUNEL assay
TUNEL staining was performed using the ApopTag
Fluorescein in situ Apoptosis Detection kit (cat. no. S7110;
Sigma-Aldrich; Merck KGaA) following the manufacturer's protocol.
Briefly, after being treated with 1 µM Ang II with or without 1 µM
pitavastatin co-treatment at 37˚C for 24 h, control (untransfected)
or transfected HUVECs (2x104) were washed with PBS and
subsequently fixed using 4% paraformaldehyde for 30 min at room
temperature. Cells were incubated for 90 min at 37˚C with the
terminal deoxynucleotidyltransferase (TdT) incubation buffer. The
negative control slide was incubated without the TdT enzyme. The
reaction was terminated by washing with PBS and the slide was
examined under a fluorescence microscope (Nikon Eclipse 80i; Nikon
Corporation). DAPI (5 µg/ml) was used to stain the nuclei blue at
room temperature for 5 min. Three fields of view per well were
observed (magnification, x200) and the percentage of TUNEL positive
cells was calculated using ImageJ software (version 1.8.0; National
Institutes of Health) as follows: TUNEL-positive cells (%) = the
density of green/blue x100%.
Detection of NO and ROS
HUVECs were inoculated into 96-well plates
(2x103 cells/well). After treatment with 1 µM Ang II
with or without 1 µM pitavastatin co-treatment for 24 h at 37˚C,
detection of NO and ROS levels in the HUVEC culture medium was
performed using the aforementioned commercial kits. Levels of NO
and ROS were measured at 520 nm using a Multiskan Mk3 microplate
reader (Thermo Fisher Scientific, Inc.).
ELISA
Concentrations of TNF-α, IL-1β and IL-6 in the HUVEC
culture medium were detected using the aforementioned ELISA kits
according to the manufacturer's protocol. Expression levels were
quantified using a Multiskan Mk3 microplate reader (Thermo Fisher
Scientific, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from HUVECs was collected using the TRIzol
agent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol, then reversely transcribed into cDNA using
PrimeScript™ RT Master Mix (cat. no. RR036A; Takara Bio, Inc.)
according to the manufacturer's protocol. Subsequently, qPCR was
performed using TB Green™ Fast qPCR Mix (Takara Bio, Inc.) on an
Applied Biosystems 7500 Real-Time PCR System (Thermo Fisher
Scientific, Inc.). The results were analyzed using the
2-ΔΔCq method (21).
The thermocycling conditions used for qPCR were: 95˚C for 2 min,
followed by 40 cycles of 95˚C for 20 sec and 65˚C for 40 sec. The
sequences of the primers were as follows: MAPK7 forward,
5'-ACACGACAACATCATCGCCA-3' and reverse, 5'-TCCAGGACCACGTAGACAGA-3'
and β-actin forward, 5'-ACAGAGCCTCGCCTTTGCC-3' and reverse,
5'-GATATCATCATCCATGGTGAGCTGG-3'. β-actin was used as the internal
reference.
Western blot analysis
Total protein was extracted from the cell lysate
using the RIPA buffer (Beyotime Institute of Biotechnology). The
protein samples were quantified using a BCA assay (Pierce; Thermo
Fisher Scientific, Inc.). Subsequently, extracted proteins (50 µg
per lane) were separated by 10% SDS-PAGE and transferred onto PVDF
membranes (EMD Millipore). Membranes were blocked with 5% skimmed
milk at room temperature for 2 h and subsequently incubated with
the aforementioned primary antibodies overnight at 4˚C. Following
primary antibody incubation, membranes were incubated with the
aforementioned corresponding secondary antibodies at room
temperature for 2 h. Protein bands were visualized using Pierce™
ECL (Thermo Fisher Scientific, Inc.). Protein expression was
quantified using ImageJ software (version 1.8.0; National
Institutes of Health).
Statistical analysis
All experiments were performed in triplicate. All
data are presented as the mean ± standard deviation and samples
were evaluated using one-way ANOVA followed by Tukey's test using
SPSS 16.0 (SPSS, Inc.). An unpaired Student's t tests were used for
comparisons between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Pitavastatin increases MAPK7
expression in Ang II-induced endothelial cells
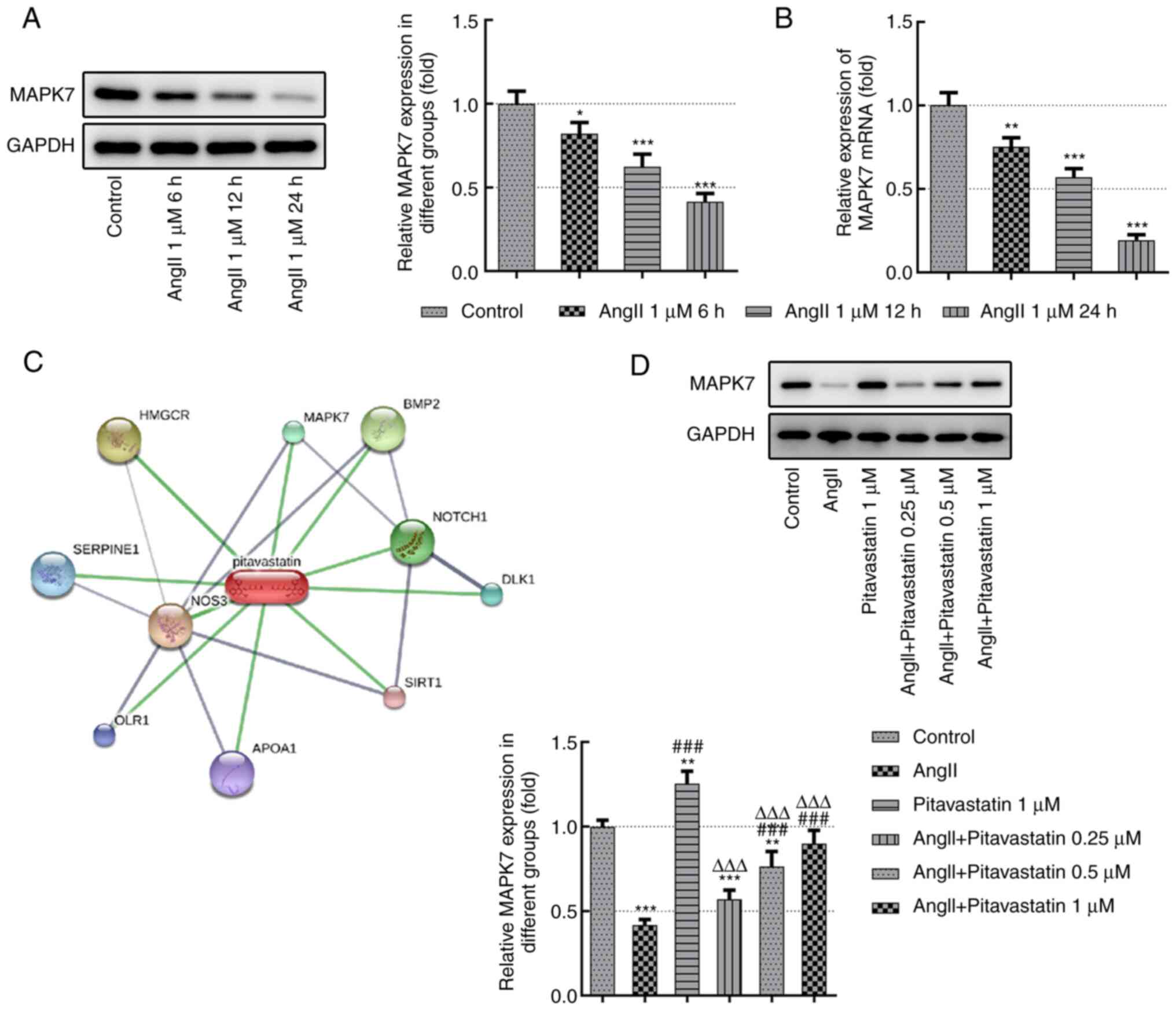
As shown in Fig. 1A
and B, the expression levels of
MAPK7 were detected using RT-qPCR and western blot analyzes. The
results demonstrated that the expression levels of MAPK7 were
significantly decreased following treatment with 1 µM Ang II in a
time-dependent manner. In particular, MAPK7 expression was the
lowest following treatment with 1 µM Ang II for 24 h (Fig. 1A and B). Therefore, this treatment time was
selected for subsequent studies. The STITCH (http://stitch.embl.de/) database was subsequently used
to predict potential pitavastatin targets, which revealed MAPK7 as
one of the hits (Fig. 1C).
Following pitavastatin treatment, the expression levels of MAPK7
were detected using western blot analysis. Results of the present
study demonstrated that the expression levels of MAPK7 in Ang
II-induced HUVECs were increased following treatment with
pitavastatin, especially at higher doses of 0.5 and 1 µM (Fig. 1D). In conclusion, pitavastatin
restored MAPK7 expression in Ang II-induced endothelial cells.
Pitavastatin alleviates damage in Ang
II-induced HUVECs by activating MAPK7
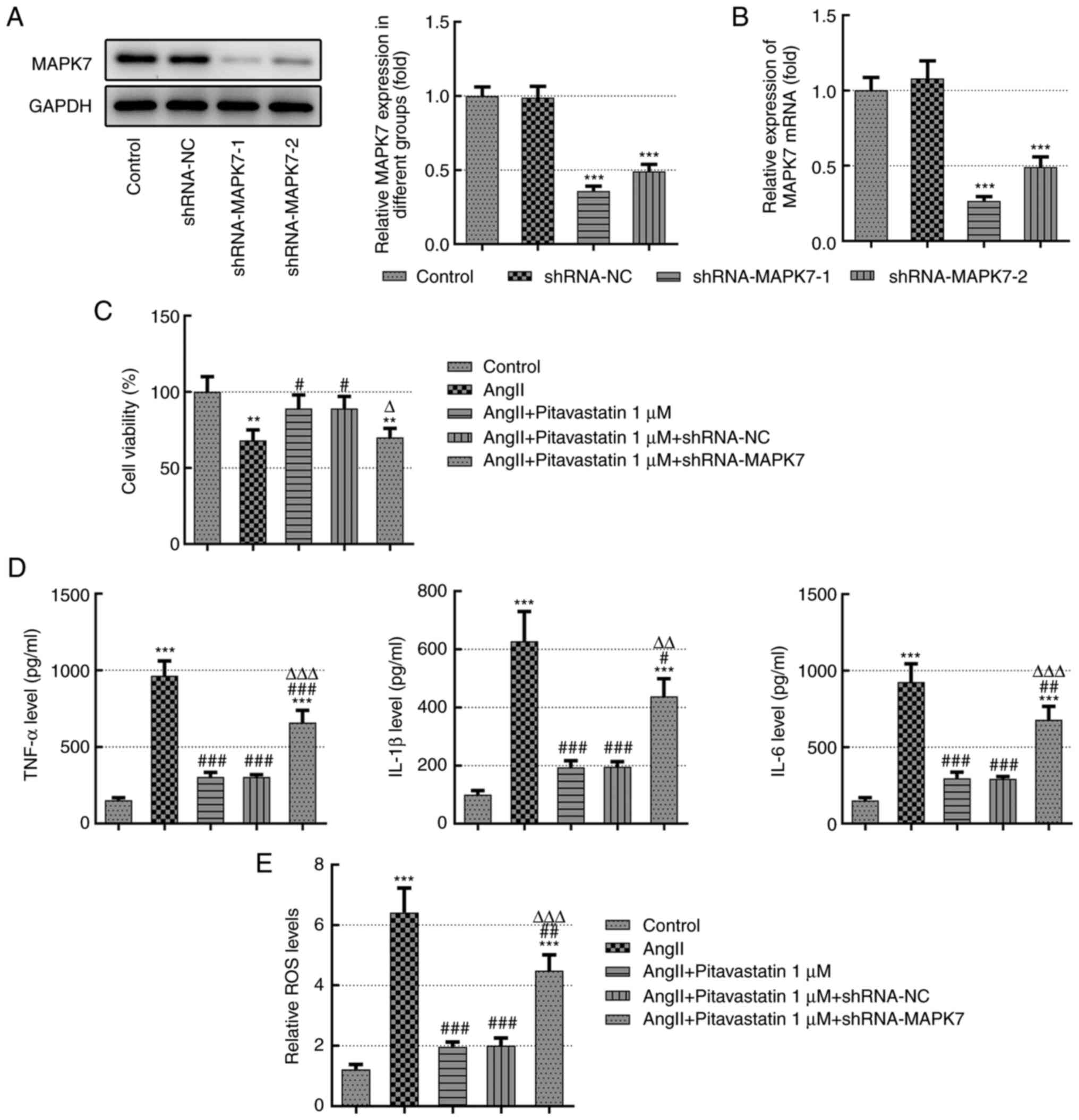
To determine the effects of pitavastatin on Ang
II-induced endothelial cell viability and inflammation, loss of
function experiments were performed. HUVECs were transfected with
shRNA-NC, shRNA-MAPK7-1 or shRNA-MAPK7-2 for MAPK7 silencing. As
demonstrated in Fig. 2A and
B, there was no significant
difference in MAPK7 expression between the sh-negative control (NC)
group compared with that in the control group. However, MAPK7
expression levels were significantly reduced in HUVECs transfected
with sh-MAPK7-1 or sh-MAPK7-2 compared with those in the sh-NC
group (Fig. 2A and B). Since the transfection efficiency was
the highest following transfection with sh-MAPK7-1, this shRNA was
used for subsequent experiments.
In addition, cell viability were significantly
decreased following treatment with Ang II, which was significantly
reversed by pitavastatin treatment (Fig. 2C). By contrast, this
pitavastatin-induced restoration of cell viability was
significantly prevented by MAPK7 knockdown (Fig. 2C). As demonstrated in Fig. 2D, the secretion of IL-1β, IL-6 and
IL-8 were significantly increased following treatment with Ang II
but were significantly reversed by pitavastatin. The
anti-inflammatory effects of pitavastatin on IL-1β, IL-6 and IL-8
were in turn significantly reversed by MAPK7 knockdown (Fig. 2D). In addition, results of the ROS
kit demonstrated that the levels of ROS were significantly
increased following Ang II treatment but were reversed by
concomitant pitavastatin treatment, which was in turn reversed by
transfection with shRNA-MAPK7 (Fig.
2E).
Pitavastatin increases the production
of NO, eNOS phosphorylation and ET-1 expression in Ang II-induced
HUVECs by activating MAPK7
NO is a key signaling molecule secreted by the
vascular endothelium that serves a key role in vascular smooth
muscle relaxation by activating certain signal transduction
pathways, such as that of hypoxia-inducible factor-1α and VEGF
(22). eNOS is a key enzyme in the
NO production pathway that is an indirect indicator of heart
failure (23). By contrast, ET-1
is mainly secreted by vascular endothelial cells, cardiomyocytes
and endocardial cells and is considered to be a potent
vasoconstrictor (24). ET-1
co-operates with NO to regulate the vasoconstrictive and diastolic
balance of the vascular endothelium (10). As demonstrated in Fig. 3A, treatment with pitavastatin
significantly suppressed the expression levels of eNOS and ET-1 in
Ang II-induced HUVECs, which were significantly reversed following
transfection with the sh-MAPK7 plasmid. Notably, treatment with
pitavastatin in Ang II-induced HUVECs increased the production
levels of NO and p-eNOS, which were significantly reversed
following transfection with sh-MAPK7 (Fig. 3B).
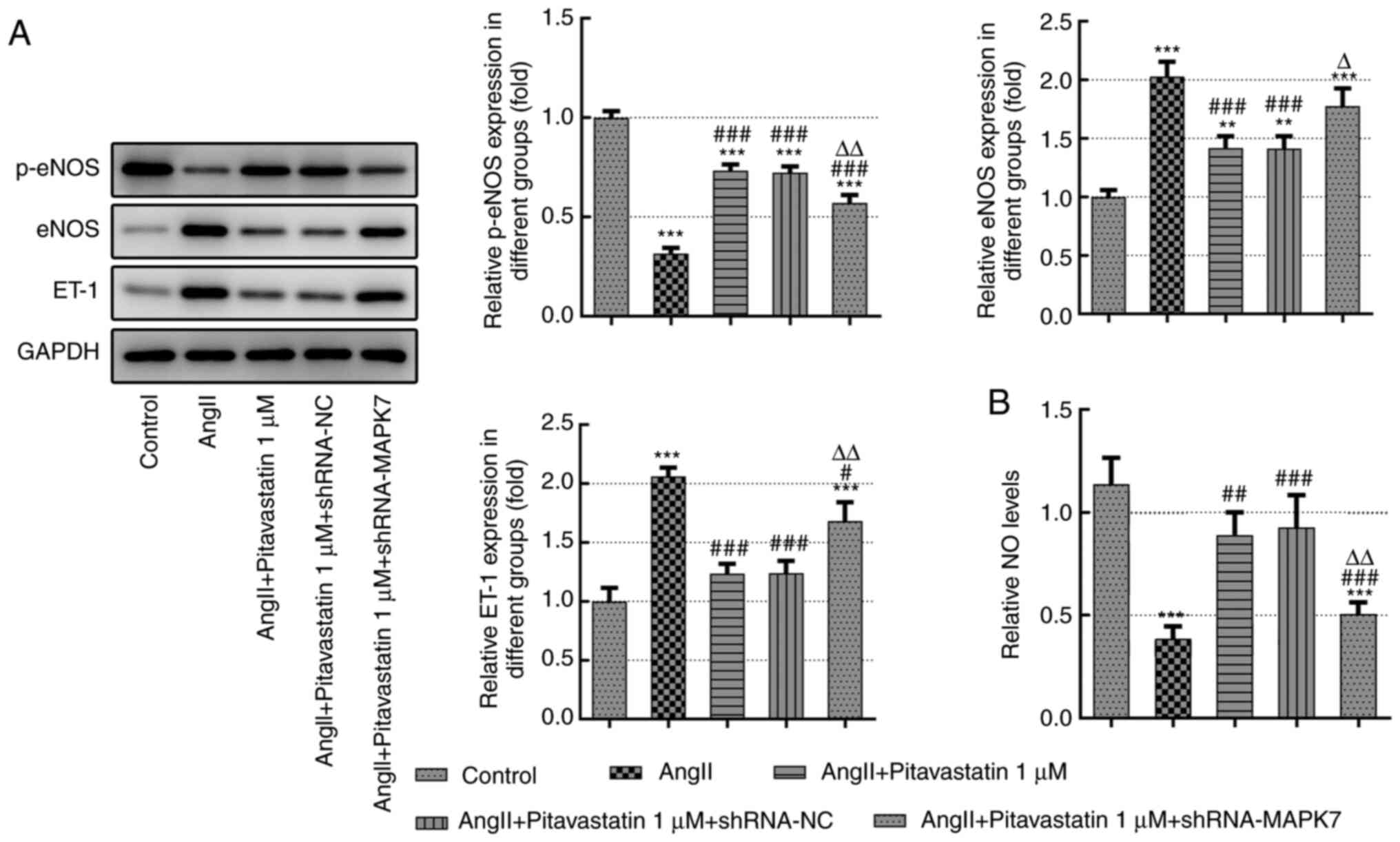 | Figure 3Pitavastatin increases the production
of NO, activation eNOS and ET-1 expression in Ang II-induced HUVECs
by activating MAPK7. (A) Protein expression of eNOS
phosphorylation, eNOS expression and ET-1 expression in Ang
II-induced endothelial cells were measured using western blot
analysis. (B) NO production was measured using a commercial kit.
**P<0.01 and ***P<0.001 vs. Control;
#P<0.05, ##P<0.01 and
###P<0.001 vs. Ang II; ΔP<0.05 and
ΔΔP<0.01 vs. Ang II + 1 µM pitavastatin. Ang II,
angiotensin II; shRNA, short hairpin RNA; NC, negative control;
ET-1, endothelin-1; NO, nitric oxide; p-, phosphorylated; eNOS,
endothelial NO synthase. |
Pitavastatin inhibits the apoptosis of
Ang II-induced HUVECs by activating MAPK7
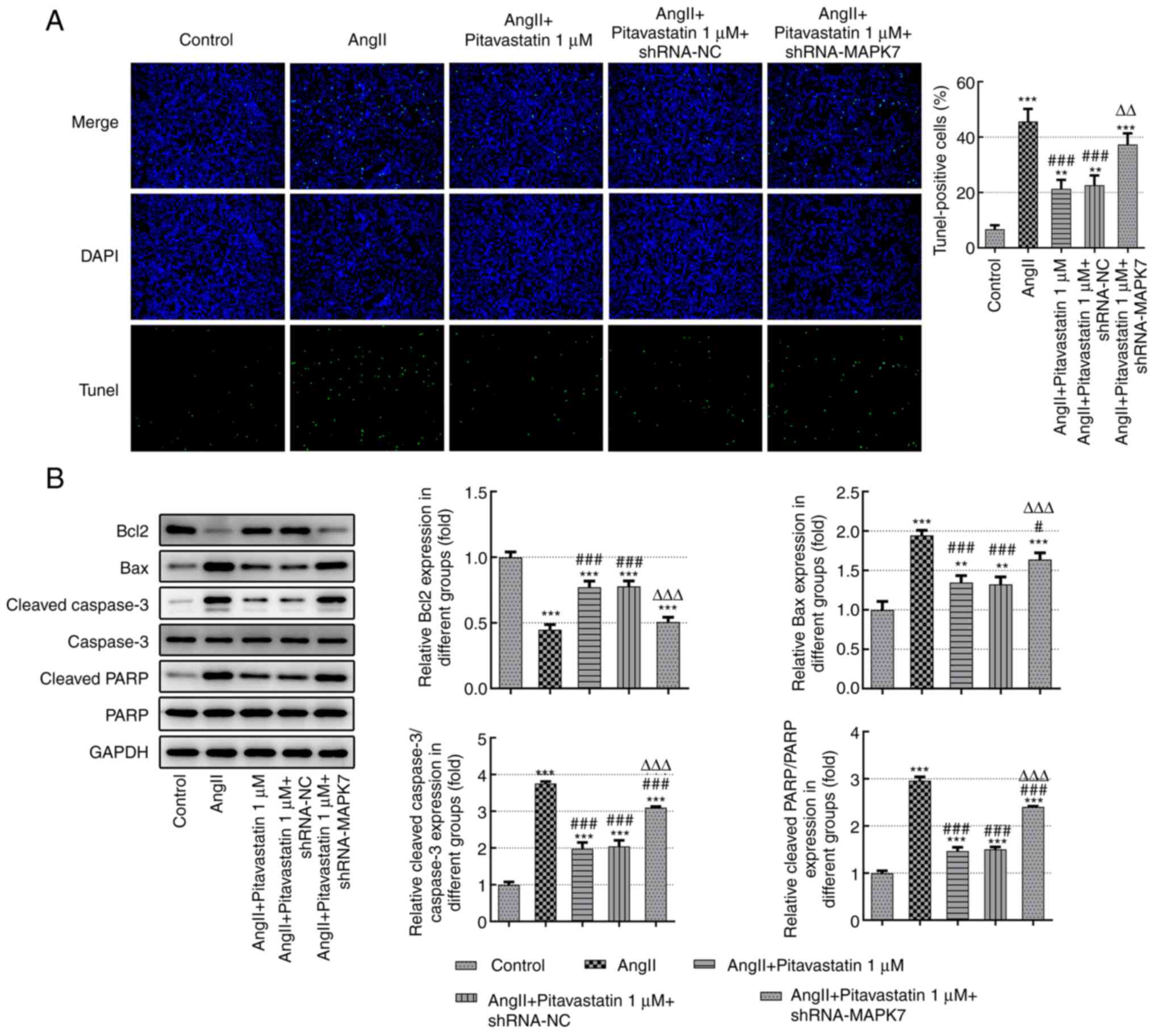
To assess the effects of pitavastatin on the
apoptosis of Ang II-induced HUVECs, TUNEL staining was performed.
The number of TUNEL-positive cells was significantly reduced in the
Ang II + pitavastatin group compared with that in the Ang II-alone
group (green represents apoptotic cells). However, transfection
with shRNA-MAPK7 significantly reversed the inhibitory effects of
pitavastatin on cell apoptosis (Fig.
4A). These results were investigated further by measuring the
expression levels of apoptosis-associated proteins. As demonstrated
in Fig. 4B, the expression levels
of Bax, ratios of cleaved-PARP/PARP and cleaved caspase-3/caspase-3
in the Ang II-induced HUVECs were significantly reduced following
treatment with pitavastatin. However, expression levels of the
anti-apoptotic protein Bcl-2 were significantly increased by
pitavastatin (Fig. 4B). All of the
effects mediated by pitavastatin on the expression of proteins
associated with apoptosis were significantly reversed by
shRNA-MAPK7 transfection (Fig.
4B). These findings suggest that treatment with pitavastatin
inhibited the apoptosis of Ang II-induced HUVECs by activating
MAPK7.
Discussion
Vascular endothelial cell injury is considered to be
one of the first events of atherosclerosis and is closely
associated with the development of cardiovascular diseases,
including coronary heart disease and hypertension (25). Results of a previous study
demonstrated that ischemia injury, oxidative stress and oxidized
low density lipoprotein can all induce endothelial cell injury and
apoptosis (26). In addition, the
expression levels of eNOS and ET-1 were significantly increased
following the induction of vascular endothelial injury, cell
destruction or increased membrane permeability, which were
accompanied with reductions in the levels of NO and may reflect the
degree of cell injury (27,28).
In the present study, HUVECs were treated with 1 µM Ang II, which
significantly increased proinflammatory cytokine and ROS production
and ET-1 secretion, significantly decreased NO production whilst
significantly increasing the rate of apoptosis. These results
suggest that Ang II is directly involved in the process of vascular
endothelial cell injury and apoptosis.
Statins are lipid-lowering drugs that are frequently
applied in clinical practice (29). They have been previously reported
to exert anti-inflammatory, anti-oxidation, vascular endothelial
protective, plaque stabilizing and anti-platelet properties
(30). Results of the present
study demonstrated that treatment with pitavastatin significantly
increased the production levels of NO and eNOS phosphorylation
whilst significantly decreasing ET-1 expression in Ang II-induced
HUVECs. Furthermore, treatment with pitavastatin alleviated cell
damage and apoptosis of Ang II-induced HUVECs by activating MAPK7,
as demonstrated by MAPK7 knockdown experiments.
MAPK is typically activated by epidermal growth
factors, inflammatory factors and growth factors, which has been
found to regulate the stress response, inflammatory response, cell
proliferation and development (31,32).
Results of a previous study demonstrated that MAPK7 deficiency
alleviated DNA damage in mouse thymus cells and inhibited the
growth of mouse thymus lymphoma (33). Gavine et al (34) revealed that MAPK7 expression was
higher in squamous cell carcinoma and esophageal carcinoma tissues
compared with normal tissues, whilst MAPK7 silencing inhibited the
proliferation, migration and invasion of osteosarcoma cells to
enhance their sensitivity to chemotherapy. In the present study,
treatment with pitavastatin increased MAPK7 expression in Ang
II-induced endothelial cells. In addition, it is known that MAPK7
signaling is activated upon phosphorylation (35), therefore, the effects of
pitavastatin on the phosphorylation of MAPK7 and the effects of an
MAPK7 inhibitor on the effects of pitavastatin should be
investigated in future studies.
To conclude, the present study provided supporting
evidence that pitavastatin can preserve MAPK7 expression to
alleviate Ang II-induced vascular endothelial cell inflammation and
injury. Results of the present study revealed a potentially novel
therapeutic strategy for the treatment of vascular endothelial cell
inflammation and injury. However, the present study solely focused
on the pitavastatin-induced regulation of endothelial cell
proliferation, inflammation and apoptosis. Further investigations
are required to focus on the role of associated signaling
pathways.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
ML and XHL contributed to the conception and design
of the study. ML and XHL performed the experiments and collected
the data. ML and XHL performed the statistical analysis. ML and XHL
completed data interpretation. Both authors contributed to reading
and revising the manuscript and approved the submitted version.
Both authors read and approved the final manuscript. ML and XHL
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Di Palo KE and Barone NJ: Hypertension and
heart failure: Prevention, targets, and treatment. Heart Fail Clin.
16:99–106. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang W, Wang Q, Feng Y, Chen X, Yang L,
Xu M, Wang X, Li W, Niu X and Gao D: MicroRNA-26a protects the
heart against hypertension-induced myocardial fibrosis. J Am Heart
Assoc. 9(e017970)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen Y, Lu W, Yang K, Duan X, Li M, Chen
X, Zhang J, Kuang M, Liu S, Wu X, et al: Tetramethylpyrazine: A
promising drug for the treatment of pulmonary hypertension. Br J
Pharmacol. 177:2743–2764. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhao H, Wang Y, Zhang X, Guo Y and Wang X:
miR-181b-5p inhibits endothelial-mesenchymal transition in
monocrotaline-induced pulmonary arterial hypertension by targeting
endocan and TGFBR1. Toxicol Appl Pharmacol.
386(114827)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dong ZC, Wu MM, Zhang YL, Wang QS, Liang
C, Yan X, Zou LX, Chen C, Han X, Zhang B and Zhang ZR: The vascular
endothelial growth factor trap aflibercept induces vascular
dysfunction and hypertension via attenuation of eNOS/NO signaling
in mice. Acta Pharmacol Sin. 42:1437–1448. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mokotedi L, Millen AME, Mogane C, Gomes M,
Woodiwiss AJ, Norton GR and Michel FS: Associations of inflammatory
markers and vascular cell adhesion molecule-1 with endothelial
dysfunction in collagen-induced arthritis. Eur J Pharmacol.
865(172786)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Canpolat U, Kocyigit D and Yildirim A:
Role of endothelial dysfunction and endocan in atherosclerosis:
Point of origin or end point? Angiology. 71(477)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bhagwani AR, Hultman S, Farkas D, Moncayo
R, Dandamudi K, Zadu AK, Cool CD and Farkas L: Endothelial cells
are a source of nestin expression in pulmonary arterial
hypertension. PLoS One. 14(e0213890)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Luo S, Xia W, Chen C, Robinson EA and Tao
J: Endothelial progenitor cells and hypertension: Current concepts
and future implications. Clin Sci (Lond). 130:2029–2042.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Masi S, Uliana M and Virdis A: Angiotensin
II and vascular damage in hypertension: Role of oxidative stress
and sympathetic activation. Vascul Pharmacol. 115:13–17.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ballard KD, Timsina R and Timmerman KL:
Influence of time of day and intermittent aerobic exercise on
vascular endothelial function and plasma endothelin-1 in healthy
adults. Chronobiol Int. 1–8. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang X, Hu C, Yuan YP, Song P, Kong CY,
Wu HM, Xu SC, Ma ZG and Tang QZ: Endothelial ERG alleviates cardiac
fibrosis via blocking endothelin-1-dependent paracrine mechanism.
Cell Biol Toxicol. 37:873–890. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hu C and Dong ZL: MicroRNA-212 promotes
the recovery function and vascular regeneration of endothelial
progenitor cells in mice with ischemic stroke through inactivation
of the notch signaling pathway via downregulating MMP9 expression.
J Cell Physiol. 234:7090–7103. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gentilini A, Lori G, Caligiuri A, Raggi C,
Maira GD, Pastore M, Piombanti B, Lottini T, Arcangeli A, Madiai S,
et al: Extracellular signal-regulated kinase 5 regulates the
malignant phenotype of cholangiocarcinoma cells. Hepatology.
74:2007–2020. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Curran MP: Amlodipine/Atorvastatin: A
review of its use in the treatment of hypertension and
dyslipidaemia and the prevention of cardiovascular disease. Drugs.
70:191–213. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fang T, Guo B, Xue L and Wang L:
Atorvastatin prevents myocardial fibrosis in spontaneous
hypertension via interleukin-6 (IL-6)/signal transducer and
activator of transcription 3 (STAT3)/endothelin-1 (ET-1) pathway.
Med Sci Monit. 25:318–323. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yuan H, Wang D, Zhang Y and Geng J:
Atorvastatin attenuates vascular remodelling in spontaneously
hypertensive rats via the protein kinase D/extracellular
signal-regulated kinase 5 pathway. Clin Exp Pharmacol Physiol.
47:1429–1438. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nagayama D, Saiki A, Watanabe Y, Yamaguchi
T, Ohira M, Sato N, Kanayama M, Moroi M, Miyashita Y, Shirai K and
Tatsuno I: Prevention of cardiovascular events with pitavastatin is
associated with increased serum lipoprotein lipase mass level:
Subgroup analysis of the TOHO-LIP. J Atheroscler Thromb: Feb 27,
2021. doi: 10.5551/jat.62141.
|
|
19
|
Bhatti H and Tadi P: Pitavastatin. In:
StatPearls, StatPearls Publishing, Treasure Island, FL, 2021.
|
|
20
|
Cao Y, Gong Y, Liu L, Zhou Y, Fang X,
Zhang C, Li Y and Li J: The use of human umbilical vein endothelial
cells (HUVECs) as an in vitro model to assess the toxicity of
nanoparticles to endothelium: A review. J Appl Toxicol.
37:1359–1369. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rajendran S, Shen X, Glawe J, Kolluru GK
and Kevil CG: Nitric oxide and hydrogen sulfide regulation of
ischemic vascular growth and remodeling. Compr Physiol.
9:1213–1247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bonnefont-Rousselot D: Resveratrol and
cardiovascular diseases. Nutrients. 8(250)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jankowich M and Choudhary G: Endothelin-1
levels and cardiovascular events. Trends Cardiovasc Med. 30:1–8.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yamada T, Egashira N, Imuta M, Yano T,
Yamauchi Y, Watanabe H and Oishi R: Role of oxidative stress in
vinorelbine-induced vascular endothelial cell injury. Free Radic
Biol Med. 48:120–127. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang M, Wang X, Yao J and Qiu Z: Long
non-coding RNA NEAT1 inhibits oxidative stress-induced vascular
endothelial cell injury by activating the miR-181d-5p/CDKN3 axis.
Artif Cells Nanomed Biotechnol. 47:3129–3137. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nakamura-Utsunomiya A, Tsumura M, Okada S,
Kawaguchi H and Kobayashi M: Downregulation of endothelial nitric
oxide synthase (eNOS) and endothelin-1 (ET-1) in a co-culture
system with human stimulated X-linked CGD neutrophils. PLoS One.
15(e0230665)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Walshe TE, Ferguson G, Connell P, O'Brien
C and Cahill PA: Pulsatile flow increases the expression of eNOS,
ET-1, and prostacyclin in a novel in vitro coculture model of the
retinal vasculature. Invest Ophthalmol Vis Sci. 46:375–382.
2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Castilla-Guerra L, Fernandez-Moreno MD and
Colmenero-Camacho MA: Statins in Stroke Prevention: Present and
future. Curr Pharm Des. 22:4638–4644. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Koushki K, Shahbaz SK, Mashayekhi K,
Sadeghi M, Zayeri ZD, Taba MY, Banach M, Al-Rasadi K, Johnston TP
and Sahebkar A: Anti-inflammatory action of statins in
cardiovascular disease: The role of inflammasome and toll-like
receptor pathways. Clin Rev Allergy Immunol. 60:175–199.
2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mo S, Qian Y, Zhang W, Qian L, Wang Y,
Cailin G and Ding H: Mitogen-activated protein kinase action in
plant response to high-temperature stress: A mini review.
Protoplasma. 258:477–482. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kim M, Jeong S, Lim CW and Lee SC:
Mitogen-activated protein kinase CaDIMK1 functions as a positive
regulator of drought stress response and abscisic acid signaling in
capsicum annuum. Front Plant Sci. 12(646707)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lochhead PA, Clark J, Wang LZ, Gilmour L,
Squires M, Gilley R, Foxton C, Newell DR, Wedge SR and Cook SJ:
Tumor cells with KRAS or BRAF mutations or ERK5/MAPK7 amplification
are not addicted to ERK5 activity for cell proliferation. Cell
Cycle. 15:506–518. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gavine PR, Wang M, Yu D, Hu E, Huang C,
Xia J, Su X, Fan J, Zhang T, Ye Q, et al: Identification and
validation of dysregulated MAPK7 (ERK5) as a novel oncogenic target
in squamous cell lung and esophageal carcinoma. BMC Cancer.
15(454)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nithianandarajah-Jones GN, Wilm B,
Goldring CE, Müller J and Cross MJ: ERK5: structure, regulation and
function. Cell Signal. 24:2187–2196. 2012.PubMed/NCBI View Article : Google Scholar
|