Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune
disease that is primarily characterized by the destruction of
multiple joints, causing substantial pain, swelling, persistent
synovitis, systemic inflammation and an increase in proinflammatory
cytokines levels (1,2). It affects >1% of the adult
population worldwide and leads to joint damage and the loss of
physical function (1,2). In addition, RA is common in companion
animals, livestock and wild animals, and the joint swelling caused
by RA affects movement and productivity, which has an important
negative impact on the economic value in the farming industry
(3). Although treatment options for
RA have gradually expanded, no effective therapeutic approach
currently exists for the improvement of joint destruction, since
its potential pathogenesis has not been fully elucidated.
Furthermore, although various drugs, such as methotrexate,
leflunomide and aspirin have already been used for RA therapy,
effective drugs with few side effects and low cost are still
lacking (4-6).
Gene therapy has gained considerable interest recently, as it can
effectively prevent these shortcomings to a great extent.
Therefore, identifying the key genes involved in the pathogenic
mechanisms of joint destruction is essential for RA gene therapy
(3).
Numerous studies have demonstrated that
proinflammatory genes, such as interleukin 1 (IL-1) and tumor
necrosis factor-α were markedly increased in the pathogenesis of
RA. Therefore, the inhibition of certain inflammatory cytokines,
especially IL-1, has become the major focus of RA clinical research
since the 1990s (7,8). A previous study revealed that higher
serum IL-1β concentrations were observed in the synovial fluid of
patients with RA (9). An animal
study has also demonstrated that IL-1β injections cause severe
joint destruction, which is a typical symptom of human RA (10). These findings suggested that IL-1β
serves a vital role in the pathogenesis of RA, and that IL-1β
inhibition may be considered a good therapeutic option for RA
treatment.
RNA interference (RNAi), a post-transcriptional gene
silencing method driven by small interfering RNA (siRNA), is a
powerful tool for the prevention and cure of various diseases
(11-15).
Due to its ability to specifically and efficiently silence gene
expression in a sequence-specific manner, RNAi serves major roles
in biological processes (16-19),
and has been considered to be a promising strategy for the therapy
of numerous cancers and cardiovascular diseases, among others
(20,21). However, the clinical application of
siRNA is still in contention due to its inherent instability in
biological fluids along with poor or non-specific cellular uptake
(22-24).
Therefore, the use of IL-1β siRNA alone for RA treatment is
limited. Thus, there is an urgent requirement to elucidate a more
effective tool for target gene transfer in RA gene therapy.
Mesenchymal stem cells (MSCs) are multipotent stem
cells that are considered to be effective cellular delivery
vehicles in numerous types of cell and gene therapies. As a type of
MSCs, bone marrow MSCs (BMSCs) have been extensively investigated
(25-27).
A previous study has demonstrated that protein expression in BMSCs
appeared to be readily altered after the transfection of exogenous
genes (28), suggesting that BMSCs
have high potential as target cells for gene therapy. It has also
been reported that BMSC transplantation exhibited high efficiency
in the treatment of experimental autoimmune encephalomyelitis
(29), indicating that BMSC
transplantation is feasible in the treatment of inflammatory
diseases. Furthermore, it has been demonstrated that BMSCs were
able to regulate the proliferation or survival of T lymphocytes and
exerted an immunosuppressive effect by releasing soluble factors,
such as transforming growth factor β1 (TGF-β1) and hepatocyte
growth factor (30). Based on the
aforementioned advantages, exploring the potential of IL-1β
siRNA-modified BMSCs for RA treatment, and comparing the
therapeutic effect between IL-1β siRNA alone and IL-1β siRNA +
BMSCs requires further elucidation.
The aim of the present study was to establish an RA
rat model by injecting collagen and to further determine the role
of IL-1β in RA pathogenesis. The successfully constructed RA rat
model was used to evaluate the therapeutic effect of IL-1β siRNA
alone or in combination with BMSCs in RA.
Materials and methods
Animals
A total of 40 female Wistar rats (age, 8-10 weeks;
weight, 200-250 g) were purchased from the Comparative Medicine
Center of Yangzhou University [Yangzhou, China; certificate of
quality, SCXK (Su) 2017-0044]. The rats were housed at 23±3˚C with
a relative humidity of 50±10% and subjected to a 12-h light/dark
cycle with free access to food and water. All animal experimental
protocols were approved by the Animal Care and Use Committee of
Yangzhou University (Yangzhou, China).
Experimental design
In the present study, two consecutive experiments
were performed. First, a collagen-induced arthritis (CIA) rat model
was established by injecting animals twice with type II collagen
for 4 weeks (cat. no. C9301; Sigma-Aldrich; Merck KGaA). The first
injection was performed at the beginning of modeling, and the
second injection was administered 1 week after the first injection.
After modeling for 1-3 and 4 weeks, the CIA rat model was
evaluated, and rats injected with the same volume of PBS were used
as controls. After 4 weeks, the successful CIA rats were injected
with PBS and IL-1β siRNA or transplanted with IL-1β siRNA + BMSCs
for an additional 4 weeks. The rats were injected twice over the
4-week period to strengthen the therapeutic effect, once at the
beginning of the 1st week and another time at the beginning of the
2nd week of treatment. The effects were evaluated at 1-3 and 4
weeks post-transplantation.
Induction of CIA model
The CIA rat model was generated as previously
described (31) with certain
modifications. CIA rats were first subcutaneously injected at the
root of tail with 1 ml bovine type II collagen emulsified in
Freund's complete adjuvant (1 mg/ml; cat. no. F5881; Sigma-Aldrich;
Merck KGaA). After 1 week, the rats were immunized a second time
with half the volume of the same substance. Body weight and toe
swelling values were measured once a week after the first
immunization, totally for 4 weeks, to preliminary assess the CIA
rat model. Toe swelling was measured with a vernier caliper at the
same joint. In addition to body weight and toe swelling values,
other parameters such as the immobility time, climbing time, serum
concentrations of IL-1β, histopathology of the knee joint and
expression of certain immune-response related marker genes were
assessed to determine model success.
Forced swimming test
Behavioral changes were measured via forced swimming
tests once a week after the first immunization, totally for 4
weeks. One day before the formal test, rats were placed and trained
in transparent glass cylinders with a height of 93 cm, a bottom
diameter of 54 cm and a depth of 60 cm. The water temperature was
maintained at 22±1˚C. Each cylinder contained 1 rat, and different
cylinders were separated from each other with black organic glass.
Rats swam in the water tank for 15 min to adapt to the swimming
environment prior to the formal experiment, when the rats were
allowed to swim for 6 min. At the end of the experiment, both the
immobility time and climbing time were recorded and analyzed.
Climbing time referred to the time that the rats began having
difficulties to float in the water, especially indicated by the
continuous movement of their front claws on the water surface, or
when limb movements were used to keep the head above the water and
the water surface fluctuated constantly.
ELISA
At 0, 2 and 4 weeks, rats were anesthetized with an
intraperitoneal injection of pentobarbital sodium (65 mg/kg body
weight; Sigma-Aldrich; Merck KGaA) (32-35).
No signs of peritonitis were observed. A total of 1 ml blood sample
was obtained from the orbital venous plexus, then centrifuged at
3,000 x g at 4˚C for 5 min to separate the serum. The serum
concentrations of IL-1β were determined via ELISA (IL-1β Rat ELISA
Kit; cat. no. BMS630; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. The absorbance was measured at 450
nm. Furthermore, BMSCs were lysed on ice for 1 h using 0.5% Triton
X-100 and cell lysate was collected. Both the BMSC cell lysate and
supernatant were also used for detecting IL-1β via ELISA.
Sample collection
Four weeks after modeling, rats were sacrificed by
decapitation after being anesthetized with an intraperitoneal
injection of pentobarbital sodium (65 mg/kg body weight). The
spleen and thymus were subsequently removed and weighed to
calculate the organ index (organ index=tissue wet weight/body
weight). Furthermore, the spleen was collected and stored at -80˚C
to isolate total RNA and detect the mRNA expression levels of
certain immune-response related marker genes. mRNA expression of
programmed death-1 (PD-1), transforming growth factor-β1 (TGF-β1)
and forkhead box protein 3 (Foxp3) in the spleen was determined via
reverse transcription-quantitative PCR (RT-qPCR). Symptoms such as
pain, weight loss, loss of appetite or weakness were set as humane
endpoints for the present study; however, no rats were sacrificed
before the completion of the experiment as a result of displaying
any of these symptoms.
Histopathology of the knee joint of
CIA rats
A total of 4 weeks after modeling, the whole knee
joints were dissected, fixed in 4% paraformaldehyde solution for 24
h at room temperature, decalcified in 10% ethylene diamine
tetraacetate at room temperature for 30 days (with the solution
renewed once a week) and embedded in paraffin. Standard 4-µm-thick
frontal sections were prepared and stained with hematoxylin for 10
min and eosin for 1.5 min (H&E) at room temperature. The
synovial tissue sections were observed using light microscopy
(CX23; Olympus Corporation; magnification, x20, x100, x200 or x400)
and evaluated in a blinded manner.
Rat BMSC isolation using direct
adherence
A total of 4 healthy 3-4-week-old specific pathogen
free-grade male Wistar rats weighing 100-120 g were sacrificed by
anesthesia with intraperitoneal injection of pentobarbital sodium
(65 mg/kg body weight) followed by decapitation and soaking in 75%
ethanol at room temperature for 10 min. Then, the femur and tibia
were isolated under sterile conditions. Samples were immersed in
DMEM (cat. no. 11965092; Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and 100 µg/ml streptomycin. Joint capsules at
the end of the diaphysis were removed without isolating the
epiphysis, and the diaphysis was then divided, and the connective
tissue was stripped from the diaphysis to avoid a mixed cell
population. A disposable aseptic syringe was used to collect
antibiotic-supplemented DMEM and to repeatedly wash the bone marrow
cavity to collect cells in a sterile petri dish. The obtained BMSC
suspension was centrifuged at 250 x g for 5 min at 26˚C and rinsed
once in DMEM containing 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.). Cells were further resuspended in complete medium (90% DMEM,
10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin) and
transferred to a 25-cm2 plastic culture flask for
incubation at 37˚C in a 5% CO2 incubator. Cells isolated
from one rat were cultured in one flask.
Cell culture and siRNA
transfection
The BMSCs were cultured in DMEM containing 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) at 37˚C in a humidified
incubator with 5% CO2. Cells were seeded at a density of
1x105 cells per well in a six-well plate. When the cells
reached 60-70% confluency, they were treated with
lipopolysaccharide (LPS; cat. no. L8880; Beijing Solarbio Science
& Technology Co., Ltd.; 500 ng/ml in complete DMEM) for 24 h at
37˚C to simulate the RA inflammatory process. IL-1β concentrations
in cells and culture medium were determined using IL-1β Rat ELISA
kit (cat. no. BMS630; Thermo Fisher Scientific, Inc.). IL-1β siRNA
(sense, 5'-GGAAGGCAGUGUCACUCAUTT-3' and antisense,
5'-AUGAGUGACACUGCCUUCCTT-3') and a scrambled siRNA negative control
(sense, 5'-UUCUCCGAACGUGUCACGUTT-3' and antisense,
5'-ACGUGACACGUUCGGAGAATT-3') were purchased from Invitrogen (Thermo
Fisher Scientific, Inc.), and 100 nM siRNA was transfected into
BMSCs for 12 h at 37˚C via Transfast Transfection Reagent (Promega
Corporation), followed by removal of the transfection medium and
addition of the complete medium, and BMSCs were stimulated with LPS
for 24 h to simulate the RA pathogenesis. Next, the cells and
culture medium were harvested, and IL-1β concentration was
determined using IL-1β Rat ELISA kit.
RT-qPCR
Total RNA from spleen tissue or BMSCs was isolated
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
integrity of the extracted total RNA was verified via 1.0% agarose
gel electrophoresis. The purity of the total RNA was determined
according to the A260/A280 nm value using a spectrophotometer.
DNase I was then added to remove genomic DNA contamination at 42˚C
for 3 min. RNA was reverse transcribed into cDNA, and the reaction
components were as follows: Total RNA template (2 µg), M-MLV
reverse transcriptase (Takara Biotechnology Co., Ltd.) (40 U/µl),
oligo(dT) (100 nM), dNTP mix (1 mM), DTT (0.1 M), RNAse inhibitor
(40 U/µl) and deionized water (RNAse-free) to a total volume of 20
µl. The mixture was incubated at 42˚C for 60 min, heated to 70˚C
for 10 min, cooled in iced water and stored at -20˚C. qPCR was
performed by mixing 10 µl Power SYBR Green PCR Master mix (Thermo
Fisher Scientific, Inc.), cDNA (2 µl, diluted 1:20) and forward and
reverse primers (0.2 µM each) to a final PCR volume of 20 µl on an
ABI Prism 7700 sequence detection system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The thermocycling conditions of
qPCR were as follows: Initial denaturation at 95˚C for 5 min;
followed by 40 cycles of denaturation at 95˚C for 30 sec, annealing
at 62˚C for 30 sec and extension at 72˚C for 30 sec; and final
extension at 60˚C for 60 sec. mRNA expression was normalized to
β-actin, and relative gene expression was quantified using the
comparative 2-ΔΔCq method
(36,37). The primers used are presented in
Table I.
 | Table IPCR primers used in the present
study. |
Table I
PCR primers used in the present
study.
| Name | Gene reference
number | Sequence
(5'-3') |
|---|
| PD-1 | NM_001106927 | F:
GCCCGCTTCCAGATCGTAC |
| | | R:
AGGGTCTTCCTTGCGTCCAG |
| TGF-β1 | NM_021578.2 | F:
TGCTGCCGCTTCTGCTCCCACTC |
| | | R:
ATAGATTGCGTTGTTGCGGTCCAC |
| Foxp3 | NM_001108250.1 | F:
GCTTGTTTGCTGTGCGGAGAC |
| | | R:
GTTTCTGAAGTAGGCGAACAT |
| IL-1β | NM_031512 | F:
TTCAAATCTCACAGCAGCAT |
| | | R:
TCCCACGAGTCACAGAGG |
| β-actin | NM_031144.2 | F:
CCTCTGAACCCTAAGGCCAA |
| | | R:
GTCTCCGGAGTCCATCACAA |
Western blotting
BMSC total protein was extracted using RIPA lysis
buffer containing 2 mM EDTA, 100 mM NaCl, 5% SDS, 50 mM NaF, 0.1 mM
Na3VO4, 100 µM 4-benzenesulfonyl fluoride
hydrochloride, 1 mM benzamidine, 50 mM HEPES (pH 7.4) and 10 µg/ml
aprotinin. Protein concentration was then determined with BCA
Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.). A total
of 40 µg protein sample was mixed with loading buffer (187.5 mmol/l
Tris-HCl pH 6.8, 6% SDS, 30% glycerol, 150 mmol/l DTT and 1%
bromophenol blue) and denatured for 5 min by boiling at 100˚C
before being loaded on a 10% SDS-polyacrylamide gel. The proteins
were transferred onto a PVDF membrane after electrophoresis,
blocked with 3% BSA (cat. no. A8020; Beijing Solarbio Science &
Technology Co., Ltd.) for 90 min at room temperature, and then
incubated with primary anti-IL-1β antibodies (1:1,000; ab9722;
Abcam) overnight at 4˚C. After washing three times for 10 min each
with TBS-0.05% Tween-20, the membrane was incubated with goat
anti-rabbit horseradish peroxidase-conjugated antibodies (1:5,000;
cat. no. ab205718; Abcam) for 2 h at room temperature. Finally, via
enhanced chemiluminescence using the SuperSignal West Pico kit
(Pierce; Thermo Fisher Scientific, Inc.), the bands were visualized
and captured with the VersaDoc 4000 MP system (Bio-Rad
Laboratories, Inc.). Quantity One 4.6 software (Bio-Rad
Laboratories, Inc.) was used to calculate the band density values
automatically. β-actin (1:5,000; cat. no. ab8227; Abcam) was used
as a reference protein. IL-1β protein levels were expressed as the
relative-fold change of the LPS + Transfast + BMSCs or the negative
control group. The experiment was performed in triplicate.
In vivo IL-1β siRNA treatment
Successful CIA rats were randomly divided as
follows: i) PBS group, which was used as a negative control; ii)
IL-1β siRNA group and iii) IL-1β siRNA + BMSC group. Both PBS and
100 nM IL-1β siRNA rats were injected via the caudal vein with
Entranster™-in vivo (cat. no. 18668-11-2; Engreen
Biosystem, Ltd.). BMSCs were plated in 24-wells at a density of
2.4x104 cells/well. When cells reached 60-70%
confluency, 100 nM IL-1β siRNA was transfected into BMSCs for 12 h
at 37˚C via Transfast Transfection Reagent (Promega Corporation).
Then, IL-1β siRNA + BMSCs cells were immediately injected twice via
the caudal vein in rats with BMSCs stock solutions (resuspended in
PBS) of 1x107 cells/kg, on days 3 and 6 after 4 weeks of
CIA modeling. The therapeutic effect was assessed at 1-3 and 4
weeks post-transplantation by means of body weight, toe swelling
value, immobility time, climbing time and serum IL-1β
concentration. The mRNA expression of immune-response related
marker genes in the spleen was determined after therapy for 4
weeks.
Statistical analysis
All data are presented as the mean ± SEM.
Statistical analyses were carried out with SPSS software v20.0 for
Windows (IBM Corp.). Differences between two groups were evaluated
using independent Student's t-test, while differences among three
groups were evaluated using one-way ANOVA followed by Tukey-Kramer
post-hoc test. All the assays conducted were repeated at least
three times. P<0.05 was considered to indicate a statistically
significant difference.
Results
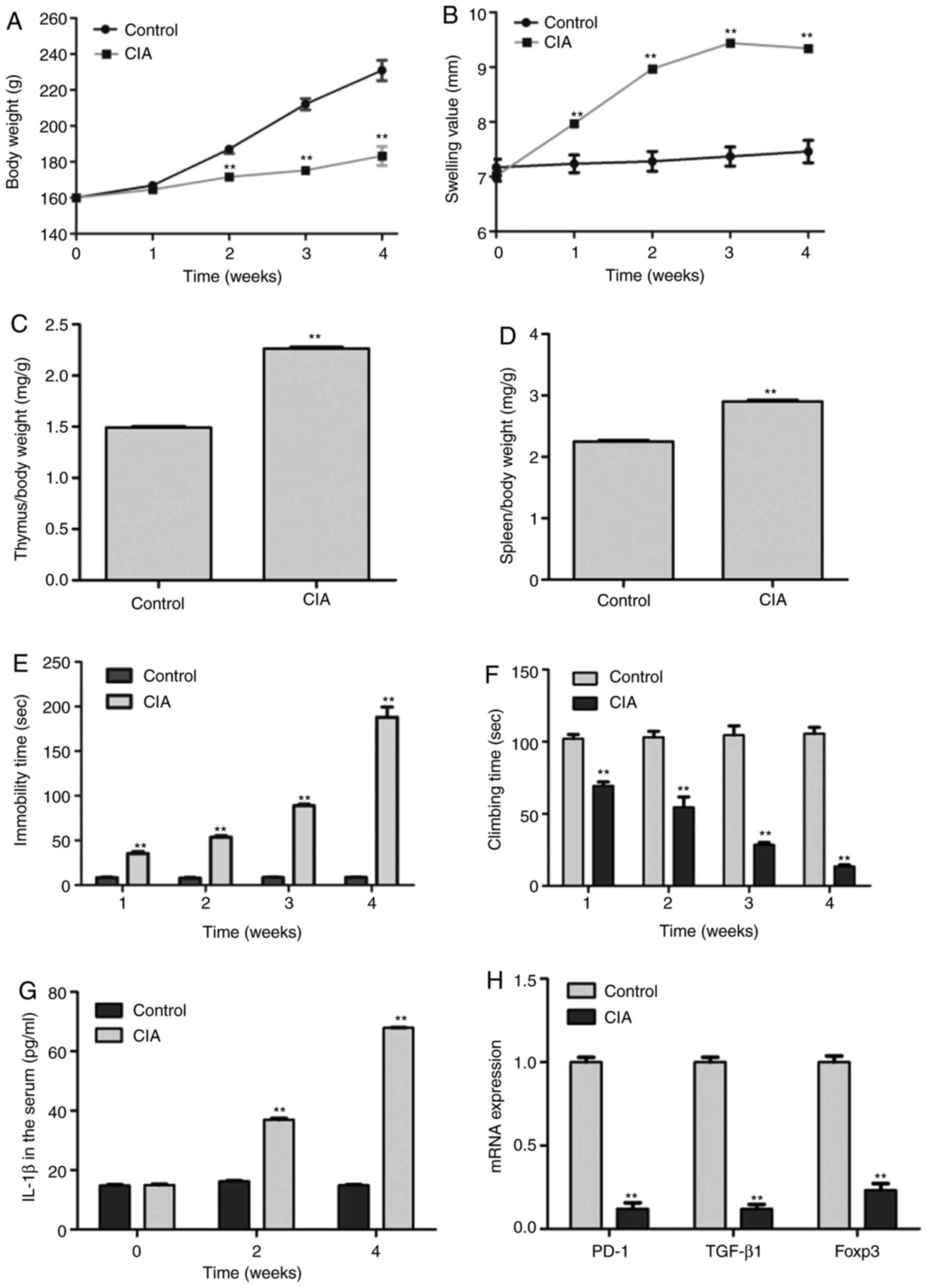
Establishment and evaluation of a CIA
rat model induced by collagen II
Toe swelling value, immobility time and climbing
time are three typical indicators to evaluate whether the CIA rat
model was successfully established (38). After modeling for 4 weeks,
stiffness, deformity and subcutaneous nodules were observed on the
surface of the skin area of CIA rat joints (data not shown). In
addition, although the growth curves of both CIA and control rats
were gradually increased, the body weight of CIA rats was
significantly lower than that of the controls after modeling for 2
weeks (Fig. 1A). Compared with
control rats, CIA rats demonstrated increased foot swelling values,
whose joints could not bear their own body weight, resulting in
crawling difficulties; however, no rats were sacrificed before the
completion of the experiment, as a result of displaying any of the
predetermined following humane endpoints, such as pain, weight
loss, loss of appetite or weakness. Furthermore, during the 1st
week of modeling, the volume of both feet started to swell and
reached a peak at 3 weeks that was then stabilized (Fig. 1B), which indicated a progressively
increasing foot swelling value of CIA rats relative to controls.
Immune organ index analysis demonstrated that both the spleen and
thymus index in CIA rats were significantly elevated compared with
those of control rats (2.91±0.07 vs. 2.25±0.10 mg/g; and
2.27±0.05 vs. 1.49±0.10 mg/g; respectively), indicating that the
pathological changes and immune response in CIA rats were more
severe than those in the control rats (Fig. 1C and D). The forced swimming results revealed
that the immobility time in CIA rats was significantly higher
(Fig. 1E), while the climbing time
was lower than that of the control rats after modeling for 1-3 and
4 weeks, indicating that hind limb movement disorders were becoming
more severe with the extension of the modeling time (Fig. 1F). The results of ELISA demonstrated
that CIA rats exhibited higher serum IL-1β concentrations than that
of the control rats after modeling for 2 and 4 weeks (Fig. 1G). In addition, the RT-qPCR results
revealed that the mRNA expression of the immune-related marker
genes PD-1, TGF-β1 and Foxp3 were significantly decreased in the
spleen compared with those of control rats (Fig. 1H). Overall, the results suggested
that CIA model rats were successfully established, and that the
pathogenesis of CIA was closely associated with elevated serum
IL-1β concentration.
Histopathology of the knee joints of
CIA rats
Compared with the knee joints of control rats, joint
swelling and redness were observed in CIA model rats (Fig. 2A and B) after modeling for 4 weeks. In addition,
the H&E staining results of tissue sections of synovium
demonstrated that the articular synovial cells of the control rats
were normal in morphology and were neatly arranged (Fig. 2C and D), while the CIA model rats presented much
more severe RA symptoms, which were characterized by evident
erosive destruction of the bone and ulceration of the articular
cartilage (Fig. 2E and F), as well as severe mixed infiltration of
inflammatory cells, mainly including neutrophils, lymphocytes and
macrophages, in the synovial membrane, articular cartilage and
joint cavity. In addition, multinucleated giant cells in the
articular cavity, minor bleeding, fibrinous exudation and mild
neovascularization were observed in CIA model rats but not in the
control rats (Fig. 2G and H).
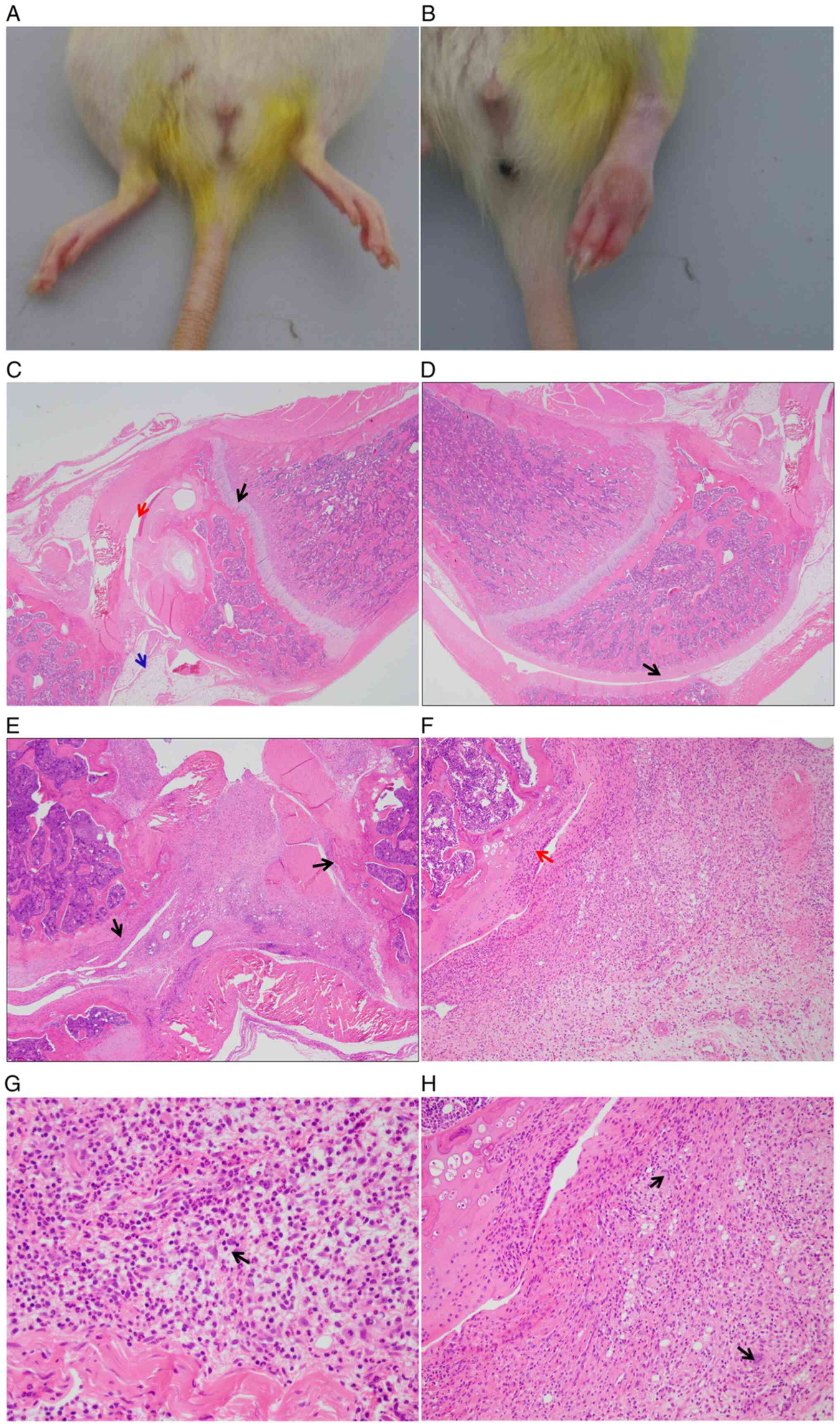 | Figure 2Histopathological analysis of the
knee joints of CIA rats after modeling for 4 weeks. (A) Knee joint
of control rats. (B) Knee joint of CIA rats with obvious swelling
and redness symptoms. (C and D) Hematoxylin and eosin staining of
synovial tissue from the knee joints of control rats, with smooth
articular cartilage surface and neatly arranged synovial tissue
cells. (C) The black arrow indicates the normal growth plate, the
red arrow indicates normal articular cavity and the blue arrow
indicates normal synovium (magnification, x20). (D) The black arrow
indicates normal articular cartilage (magnification, x20). (E)
Erosive bone destruction of CIA model rats. Black arrows indicate
erosion of the articular cartilage (magnification, x20). (F)
Ulceration of the articular cartilage in knee joints. The red arrow
indicates erosion of the articular cartilage (magnification, x100).
(G) Infiltration of inflammatory cells in knee joints, including
neutrophils, lymphocytes and macrophages. The black arrow indicates
mixed cell inflammation, which composed of a large number of
neutrophils and lymphocytes and macrophages (magnification, x400).
(H) Multinuclear giant cells in the articular cavity and deposition
of fibrin clots in knee joints. Black arrows indicate multinuclear
giant cells (magnification, x200). CIA, collagen-induced
arthritis. |
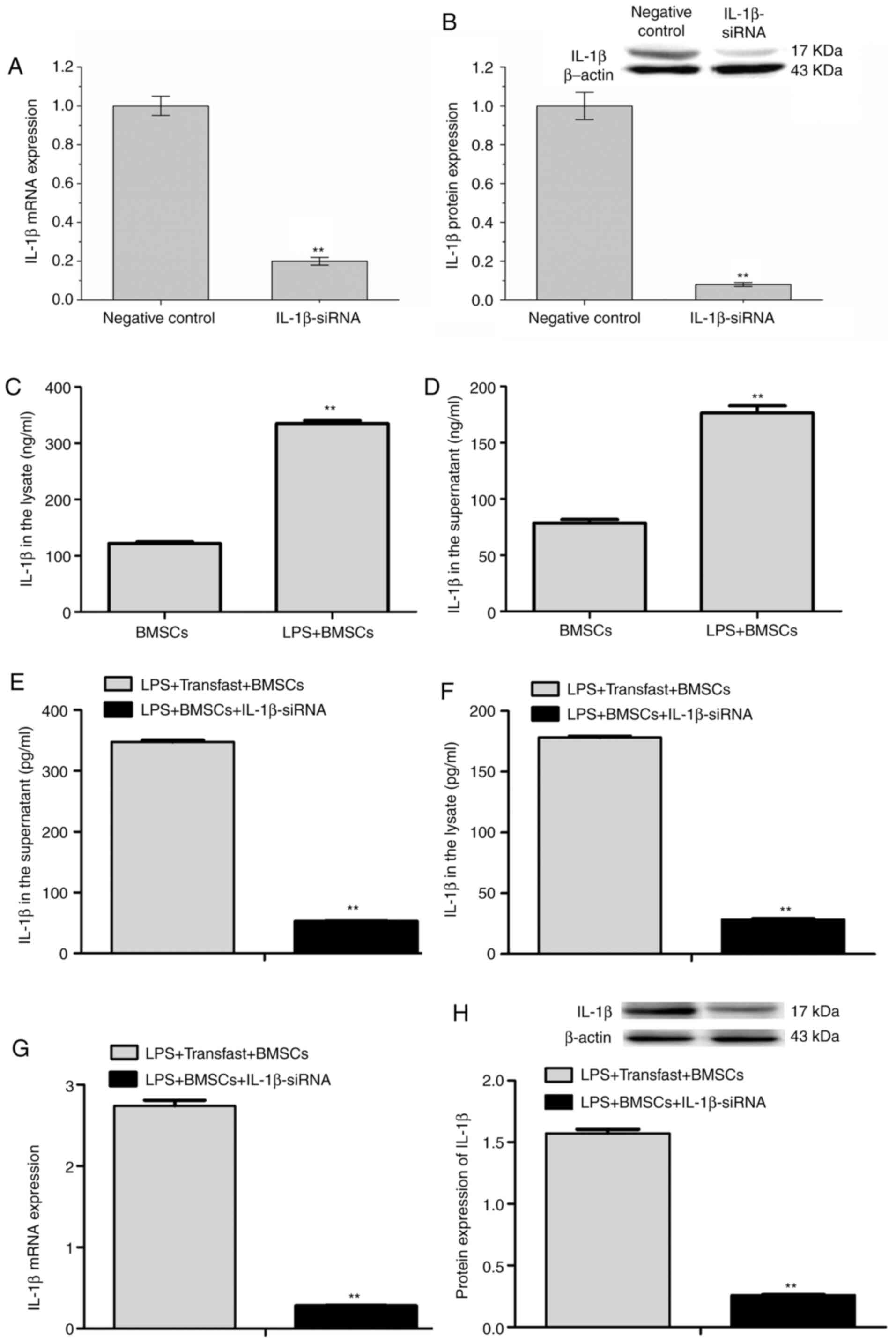
IL-1β expression in LPS-stimulated
BMSCs with or without IL-1β siRNA transfection
In the present study, both RT-qPCR and western
blotting were performed to confirm that IL-1β siRNA transfections
were successful in BMSCs. The results demonstrated that when
compared with scrambled siRNA negative control, IL-1β siRNA
transfection significantly reduced IL-1β mRNA and protein
expressions (Fig. 3A and B). In the current study, BMSCs were
stimulated with LPS (LPS + BMSCs) to simulate the classic
inflammatory symptoms of RA, and IL-1β concentrations in the cell
supernatant and lysate were analyzed by ELISA. The results revealed
that IL-1β concentrations in the cell supernatant (335.29±5.17 vs.
121.76±3.10 ng/ml) and lysate (176.65±6.33 vs. 78.52±3.24 ng/ml)
were both significantly elevated in LPS + BMSCs compared with those
of BMSCs (Fig. 3C and D).
In addition, IL-1β concentrations in both the cell
supernatant (347.84±6.17 vs. 52.66±1.72 pg/ml) and lysate
(178.29±2.18 vs. 28.47±1.27 pg/ml) were reduced in the LPS + BMSCs
+ IL-1β siRNA group compared with those in the LPS + BMSCs +
Transfast group (Fig. 3E and
F), suggesting that IL-1β siRNA
transfection was able to successfully reverse the increased IL-1β
concentration in both the supernatant and lysate of LPS-stimulated
BMSCs.
Furthermore, the RT-qPCR and western blotting
results revealed that IL-1β mRNA (0.28±0.02 vs. 2.74±0.15) and
protein expression were significantly reduced in the LPS + BMSCs +
IL-1β siRNA group relative to the LPS + BMSCs + Transfast group
(Fig. 3G and H), further demonstrating that IL-1β
expression was able to be successfully silenced after IL-1β siRNA
transfection in LPS-stimulated BMSCs.
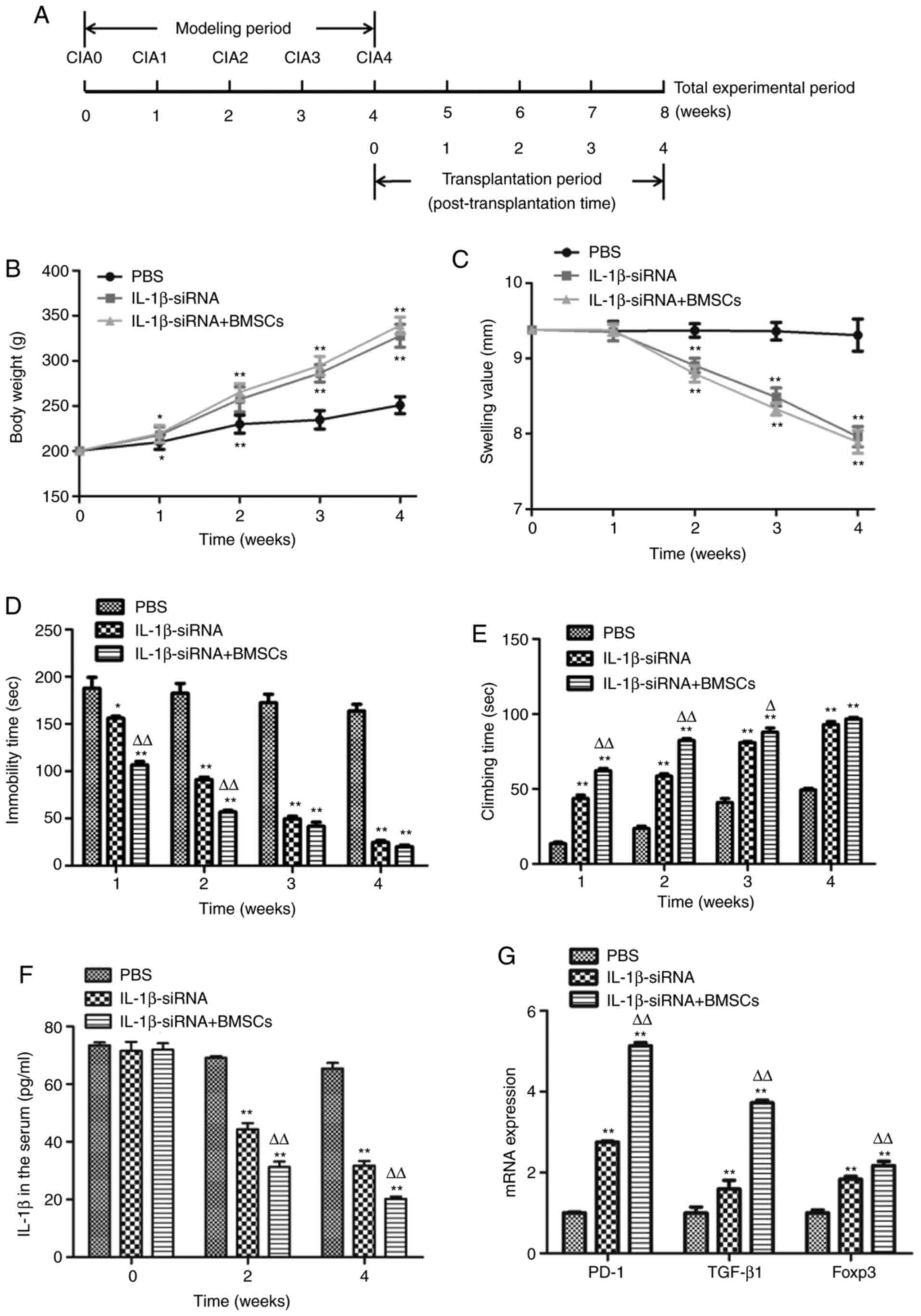
Therapeutic effect of IL-1β siRNA
combined with BMSCs on CIA model rats
The timeline of modeling and transplantation therapy
is presented in Fig. 4A. The
results demonstrated that compared with PBS-injected rats, the body
weight of both IL-1β siRNA-injected rats and IL-1β siRNA +
BMSCs-transplanted rats was significantly increased after therapy
for 1-3 and 4 weeks (Fig. 4B).
Furthermore, the toe swelling of rats that received IL-1β siRNA and
IL-1β siRNA + BMSCs was significantly lower than that of PBS rats
after therapy for 2, 3 and 4 weeks (Fig. 4C). The forced swimming results
demonstrated that compared with PBS rats, the immobility time in
rats treated with IL-1β siRNA and IL-1β siRNA + BMSCs was
significantly reduced (187.90±5.4 vs. 156.02±4.58 vs. 106.45±7.97
sec), while the climbing time was significantly increased
(13.62±2.17 vs. 43.68±4.74 vs. 62.22±3.36 sec) after therapy for 1
week. Furthermore, with the prolongation of the treatment, the
immobility time was reduced gradually in the 3rd and 4th weeks
compared with the 1st and 2nd weeks of treatment in both IL-1β
siRNA and IL-1β siRNA + BMSCs rats, while the climbing time was
increased gradually. Furthermore, compared with IL-1β siRNA rats,
IL-1β siRNA + BMSCs rats demonstrated lower immobility time and
higher climbing time in the 4th week of treatment (Fig. 4D and E), suggesting that both IL-1β siRNA and
IL-1β siRNA + BMSCs resulted in a gradually improved recovery over
time, and the combination treatment achieved a more prominent
improvement compared with that of IL-1β siRNA treatment alone.
The ELISA results revealed that the serum IL-1β
concentrations in both IL-1β siRNA and IL-1β siRNA + BMSCs rats
were significantly lower than those of PBS rats after treatment for
2 (44.34±2.19 vs. 31.27±1.87 vs. 69.15±0.55 pg/ml, respectively)
and 4 (31.76±1.59 vs. 20.21±0.83 vs. 65.44±1.94 pg/ml,
respectively) weeks. Furthermore, IL-1β siRNA + BMSCs rats
presented notably lower IL-1β concentration than IL-1β siRNA rats
(Fig. 4F). The RT-qPCR results
demonstrated that the mRNA expression of PD-1, TGF-β1 and Foxp3 in
spleen was significantly increased in both IL-1β siRNA and IL-1β
siRNA + BMSCs rats compared with that in PBS rats, and IL-1β siRNA
+ BMSCs rats exhibited a higher increase than IL-1β siRNA rats
(Fig. 4G). Overall, the above
behavioral tests and inflammation assessment indicated that both
IL-1β siRNA and IL-1β siRNA + BMSCs were able to significantly
ameliorate the CIA symptoms, while IL-1β siRNA + BMSCs
transplantation exhibited a higher therapeutic efficacy compared
with that of IL-1β siRNA injection alone in CIA rats.
Discussion
RA modeling is essential for understanding the
pathogenesis of RA and for finding effective treatments. Current
methods for establishing RA models include: Adjuvant arthritis
(AA), CIA, ovalbumin-induced arthritis and avridine-induced
arthritis (AIA), among which the AA and CIA modeling methods are
widely used (39,40). In our previous preliminary study,
both CIA and AA modeling methods were used, and it was revealed
that when compared with the AA method, the CIA rat model was more
likely to present the typical pathological symptoms of human RA in
several aspects, including synovial inflammation and slow and late
onset of chronic persistent inflammation, suggesting that CIA is an
ideal model for RA (data not shown). This is in concordance with
previous research, which indicated that CIA is widely used as an RA
model in antirheumatic drug screening, since it exhibits various
similarities to human RA (41).
The present study improved the CIA modeling method
by first injecting combined emulsions of type II collagen and
complete Freund's adjuvant, while a previous study demonstrated
that the successful rate of CIA modeling was 40% (38). A total of 7 days after the first
immunization, the mixed antigen was injected again, which
guaranteed the high success rate (>80%; data not shown) and
stability of the CIA model. Furthermore, 200-250 g male adult rats
were selected for CIA modeling, mainly based on a previous study,
which demonstrated that age serves a key role in the construction
of the CIA model and rats <21 days or >9 months of age were
not easy to be modeled because of the effect of age and sex
(42). After modeling, CIA rats
were assessed by testing important indicators associated with RA.
The results revealed that the swelling value, immune organ index,
immobility time and serum IL-1β concentration were significantly
increased, while the body weight, climbing time and PD-1, TGF-β1
and Foxp3 mRNA expression in the spleen were reduced, suggesting
that a stable and reliable CIA model was established, which
contributed to the follow-up gene and cell therapy experiment. In
addition, histopathology analyses also demonstrated severe joint
destruction of CIA rats, which also indicated successful RA
modeling.
Abnormal inflammatory cytokines are considered to be
involved in RA pathogenesis, among which the most important one
that is associated with articular cartilage injury is IL-1β. IL-1β
mainly exists in the synovial fluid and serum of patients with RA
and serves important regulatory roles in synovial inflammation. It
is highly expressed in the synovial membrane during inflammation in
RA disease and can be used as an indicator to judge RA severity
(43,44). A previous study has demonstrated
that IL-1β was able to induce endothelial cells to promote the
aggregation of lymphocytes, neutrophils and macrophages, and
increase the secretion of prostaglandins by neutrophils, thereby
increasing the expression of collagenase, chondrocytes and
fibroblasts in synovial cells, as well as the secretion of JAK2 and
JAK3 kinases, which led to the occurrence of local inflammatory
response in joints (45,46). Therefore, the examination of IL-1β
concentration is important for the evaluation of RA modeling
success. The present study demonstrated that the serum IL-1β
concentration of CIA rats was elevated, suggesting that IL-1β
served crucial roles in RA development, and may have induced the
production of proteinases and osteoclast activation, thus leading
to joint destruction. RA is also caused by the imbalance of T
cells, which in turn leads to increased proinflammatory and reduced
anti-inflammatory cytokines (47,48). A
previous study demonstrated that PD-1 null mice developed
late-onset inflammatory arthritis and mild glomerulonephritis,
indicating that PD-1 was important for in vivo peripheral
self-tolerance and was involved in the negative regulation of the
immune response (49). Furthermore,
another study in human and murine arthritis demonstrated that the
PD-1 gene was closely associated with RA pathogenesis, while a PD-1
agonist ameliorated RA activity by reducing inflammatory cytokine
production (50). TGF-β1 and Foxp3
are also key molecules of the immune system apparatus (51). In the present study, the mRNA
expression of PD-1, TGF-β1 and Foxp3 in the spleen was
significantly reduced in CIA rats, which confirmed the imbalance of
cytokines in the CIA rat model and the successful establishment of
the RA model. These results suggested that the blockade of these
three genes may have increased T and B cell activation and
proliferation, leading to IL-1β production.
RNAi is a relatively novel technology for gene
silencing, and RNAi-based therapy is a promising strategy that
reduces joint inflammation in experimental arthritis (52,53).
BMSCs, as matrix stem cells, undergo chondrogenic differentiation
and are capable of promoting cartilage repair and tissue
regeneration (54). BMSCs can not
only differentiate into multiple tissue cells, but also enhance the
repair of tissue damage caused by inflammation by secreting various
matrix molecules, which suggests that they may aid in regenerating
RA cartilage (54-56).
Mature articular cartilage has a limited capacity for
self-regeneration after injury due to the lack of blood and nerve
supply, resulting in poor articular cartilage recovery after damage
(57). RA is mainly caused by
cartilage destruction and synovial damage (58). In the present study, in order to
simulate the inflammatory process of RA, BMSCs were treated with
LPS. The results demonstrated that IL-1β expression was
significantly induced, suggesting the important role of IL-1β in
the pathogenesis of RA. IL-1β siRNA with high silencing efficiency
was subsequently transfected into LPS-stimulated BMSCs. The results
revealed that the mRNA and protein expression of IL-1β was
significantly inhibited in LPS-stimulated BMSCs, indicating that
the exogenous IL-1β siRNA could be successfully transfected into
BMSCs and produced a high and stable silencing effect. Previous
animal studies have determined that allogeneic BMSC transplantation
was able to successfully repair cartilage defects in the local
microenvironment (26,27). Another study demonstrated that BMSC
co-therapy with TGF-β1 can repair cartilage defects more
effectively than treatment with TGF-β1 alone (59), suggesting an enhanced effect of
BMSCs in the gene therapy of arthritic diseases.
In the current study, in vivo injections of
IL-1β siRNA alone and IL-1β siRNA + BMSCs were used to treat RA
rats. Based on the unique superiority of BMSCs compared with
differentiated cells, it was hypothesized that IL-1β siRNA
transfection and BMSC transplantation could produce a better
therapeutic effect. Compared with the effects observed in
PBS-injected rats, both IL-1β siRNA-injected rats and IL-1β
siRNA-BMSCs-transplanted rats exhibited a significant therapeutic
effect at the morphological, behavioral, histopathological and
immunological level, and IL-1β siRNA-BMSCs transplantation
exhibited a better therapeutic effect on the remission of symptoms
compared with that of IL-1β siRNA injection alone, indicating that
IL-1β siRNA-BMSCs achieved a more prominent improvement of RA.
However, in the present study, the therapeutic effects were
evaluated by comparisons with PBS-injected CIA rats only. The lack
of a control (vehicle treatments only for both modeling and therapy
stages) as the baseline is a limitation to be considered.
The present study demonstrated that the blockade of
IL-1β was able to ameliorate RA-associated clinical symptoms by
increasing body weight and reducing swelling time and immobility
time and increasing climbing time, which is consistent with
previous results (60). As an
effective immunosuppressive factor, TGF-β1 exhibits an
anti-inflammatory effect and serves an important role in the
pathogenesis of several inflammatory diseases. A previous study
demonstrated that inhibiting the expression of TGF-β1 led to
autoimmune disorders in RA, while its overexpression resulted in RA
improvement (47).
The results of the present study revealed that the
mRNA expression of TGF-β1 was significantly lower in the spleen of
RA rats than that of control rats, suggesting that decreased
expression of TGF-β1 may not be adequate to inhibit the development
of autoimmune inflammation, which may also be one of the reasons
for RA incidence. In addition, the mRNA expression of Foxp3 was
also decreased significantly in the spleen of RA rats, which may
have promoted CD4+CD25+ regulatory T cell
populations and contributed to the decreased secretion of
TGF-β1(61). In the therapeutic
experiment, both TGF-β1 and Foxp3 mRNA expression levels were
significantly increased in the spleen of IL-1β siRNA-injected rats
and IL-1β siRNA-BMSCs-transplanted rats, indicating that RA may be
potentially improved by the promotion of
CD4+CD25+ regulatory T cell population and
function (62,63).
In summary, the current study demonstrated the
important role of IL-1β in the pathogenesis of RA and IL-1β
siRNA-BMSCs transplantation significantly decreased the severity of
RA by reducing IL-1β concentration and improving biological
behaviors. The results suggested that IL-1β was implicated in the
pathogenesis of RA, and that IL-1β siRNA was effective in RA
therapy, while its combination with BMSCs exerted a synergistic
therapeutic effect. The findings of the present study may provide a
theoretical basis for the improvement of RA using IL-1β siRNA +
BMSC transplantation. The present findings provide the first
evidence, to the best of our knowledge, that IL-1β siRNA-BMSCs can
repair cartilage defects of RA more effectively and may represent a
novel strategy for RA treatment.
Acknowledgements
Not applicable.
Funding
Funding: The present work was supported by the National Natural
Science Foundation of China (grant nos. 32072809, 31172284 and
31501923), the Natural Science Foundation of Jiangsu Province
(grant nos. BK20211119 and BK20150443), China Postdoctoral Science
Foundation Funded Project (grant no. 2015M581872) and Postdoctoral
Science Foundation Funded Project of Jiangsu Province (grant no.
1501073A), the Priority Academic Program Development of Jiangsu
Higher Education Institutions (PAPD), Yangzhou University Top-level
Talents Support Program (2018) (grant no. 137080146) and Science
and Technology Innovation Cultivation Fund of Yangzhou University
(grant no. 2019CXJ140).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HX and SP designed the experiments, supervised the
laboratory work and critically revised the manuscript. XD mainly
performed the experiments and analyzed the data. YW assisted with
data analysis, discussion of results and writing of the manuscript.
TZ, YL and AZ provided samples and carried out the detection of the
serum parameters. XD and YW confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval (approval no. SYXK-SU-2007-0005) was
obtained from the Institute of Animal Care and Use Committee of
Yangzhou University (Yangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Atabaki M, Hashemi M, Daneshvar H and
Alijani E: Association between interleukin-1 receptor associated
kinase 1 rs3027898 A/C gene polymorphism and rheumatoid arthritis.
Biomed Rep. 6:335–338. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Scott DL, Wolfe F and Huizinga TW:
Rheumatoid arthritis. Lancet. 376:1094–1108. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lee WJ, Kim JY, Wu TP and Park LS: The
establishment of a porcine rheumatoid arthritis model: Collagen
induced arthritis minipig model. J Pharmacol Sci. 132:41–47.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shea B, Swinden MV, Tanjong Ghogomu E,
Ortiz Z, Katchamart W, Rader T, Bombardier C, Wells GA and Tugwell
P: Folic acid and folinic acid for reducing side effects in
patients receiving methotrexate for rheumatoid arthritis. Cochrane
Database Syst Rev: May 31, 2013 (Epub ahead of print). doi:
10.1002/14651858.CD000951.pub2.
|
|
5
|
Wang W, Zhou H and Liu L: Side effects of
methotrexate therapy for rheumatoid arthritis: A systematic review.
Eur J Med Chem. 158:502–516. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Howard SC, McCormick J, Pui CH, Buddington
RK and Harvey RD: Preventing and managing toxicities of high-dose
methotrexate. Oncologist. 21:1471–1482. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Corrigall VM and Panayi GS: Autoantigens
and immune pathways in rheumatoid arthritis. Crit Rev Immunol.
22:281–293. 2002.PubMed/NCBI
|
|
8
|
Iwakura Y: Roles of IL-1 in the
development of rheumatoid arthritis: Consideration from mouse
models. Cytokine Growth Factor Rev. 13:341–355. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shoda H, Nagafuchi Y, Tsuchida Y, Sakurai
K, Sumitomo S, Fujio K and Yamamoto K: Increased serum
concentrations of IL-1 beta, IL-21 and Th17 cells in overweight
patients with rheumatoid arthritis. Arthritis Res Ther.
19(111)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ghivizzani SC, Kang R, Georgescu HI,
Lechman ER, Jaffurs D, Engle JM, Watkins SC, Tindal MH, Suchanek
MK, McKenzie LR, et al: Constitutive intra-articular expression of
human IL-1 beta following gene transfer to rabbit synovium produces
all major pathologies of human rheumatoid arthritis. J Immunol.
159:3604–3612. 1997.PubMed/NCBI
|
|
11
|
McManus MT and Sharp PA: Gene silencing in
mammals by small interfering RNAs. Nat Rev Genet. 3:737–747.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Shiver JW, Fu TM, Chen L, Casimiro DR,
Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, et
al: Replication-incompetent adenoviral vaccine vector elicits
effective anti-immunodeficiency-virus immunity. Nature.
415:331–335. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Dykxhoorn DM, Novina CD and Sharp PA:
Killing the messenger: Short RNAs that silence gene expression. Nat
Rev Mol Cell Biol. 4:457–467. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Lieberman J, Song E, Lee SK and Shankar P:
Interfering with disease: Opportunities and roadblocks to
harnessing RNA interference. Trends Mol Med. 9:397–403.
2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Song E, Lee SK, Wang J, Ince N, Ouyang N,
Min J, Chen J, Shankar P and Lieberman J: RNA interference
targeting Fas protects mice from fulminant hepatitis. Nat Med.
9:347–351. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Kalluri R and Kanasaki K: RNA
interference: Generic block on angiogenesis. Nature. 452:543–545.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Okumura A, Pitha PM and Harty RN: ISG15
inhibits Ebola VP40 VLP budding in an L-domain-dependent manner by
blocking Nedd4 ligase activity. Proc Natl Acad Sci USA.
105:3974–3979. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen Y, Cheng G and Mahato RI: RNAi for
treating hepatitis B viral infection. Pharm Res. 25:72–86.
2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kim SH, Jeong JH, Lee SH, Kim SW and Park
TG: Local and systemic delivery of VEGF siRNA using polyelectrolyte
complex micelles for effective treatment of cancer. J Control
Release. 129:107–116. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lucas T, Bonauer A and Dimmeler S: RNA
therapeutics in cardiovascular disease. Circ Res. 123:205–220.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shen H, Sun T and Ferrari M: Nanovector
delivery of siRNA for cancer therapy. Cancer Gene Ther. 19:367–373.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lu PY, Xie F and Woodle MC: In vivo
application of RNA interference: From functional genomics to
therapeutics. Adv Genet. 54:117–142. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Oishi M, Nagasaki Y, Itaka K, Nishiyama N
and Kataoka K: Lactosylated poly(ethylene glycol)-siRNA conjugate
through acid-labile beta-thiopropionate linkage to construct
pH-sensitive polyion complex micelles achieving enhanced gene
silencing in hepatoma cells. J Am Chem Soc. 127:1624–1625.
2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kim SH, Mok H, Jeong JH, Kim SW and Park
TG: Comparative evaluation of target-specific GFP gene silencing
efficiencies for antisense ODN, synthetic siRNA, and siRNA plasmid
complexed with PEI-PEG-FOL conjugate. Bioconjug Chem. 17:241–244.
2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Luque-Campos N, Contreras-López RA, Jose
Paredes-Martínez M, Torres MJ, Bahraoui S, Wei M, Espinoza F,
Djouad F, Elizondo-Vega RJ and Luz-Crawford P: Mesenchymal stem
cells improve rheumatoid arthritis progression by controlling
memory T cell response. Front Immunol. 10(798)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Richardson SM, Kalamegam G, Pushparaj PN,
Matta C, Memic A, Khademhosseini A, Mobasheri R, Poletti FL,
Hoyland JA and Mobasheri A: Mesenchymal stem cells in regenerative
medicine: Focus on articular cartilage and intervertebral disc
regeneration. Methods. 99:69–80. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhu Y, Wu X, Liang Y, Gu H, Song K, Zou X
and Zhou G: Repair of cartilage defects in osteoarthritis rats with
induced pluripotent stem cell derived chondrocytes. BMC Biotechnol.
16(78)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kurozumi K, Nakamura K, Tamiya T, Kawano
Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, et al:
BDNF gene-modified mesenchymal stem cells promote functional
recovery and reduce infarct size in the rat middle cerebral artery
occlusion model. Mol Ther. 9:189–197. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jorgensen C, Djouad F, Apparailly F and
Noël D: Engineering mesenchymal stem cells for immunotherapy. Gene
Ther. 10:928–931. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Di Nicola M, Carlo-Stella C, Magni M,
Milanesi M, Longoni PD, Matteucci P, Grisanti S and Gianni AM:
Human bone marrow stromal cells suppress T-lymphocyte proliferation
induced by cellular or nonspecific mitogenic stimuli. Blood.
99:3838–3843. 2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang Y, Lu S, Zhang G, Wu S, Yan Y, Dong Q
and Liu B: Anti-inflammatory effects of HDL in mice with rheumatoid
arthritis induced by collagen. Front Immunol.
9(1013)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lau WA, King RG and Boura AL:
Methoxyphenamine inhibits basal and histamine-induced nasal
congestion in anaesthetized rats. Br J Pharmacol. 101:394–398.
1990.PubMed/NCBI View Article : Google Scholar
|
|
33
|
França AS, Rossoni LV, Amaral SM and
Vassallo DV: Reactivity of the isolated perfused rat tail vascular
bed. Braz J Med Biol Res. 30:891–895. 1997.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Murakami M, Niwa H, Kushikata T, Watanabe
H, Hirota K, Ono K and Ohba T: Inhalation anesthesia is preferable
for recording rat cardiac function using an electrocardiogram. Biol
Pharm Bull. 37:834–839. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Guarino MP, Santos AI, Mota-Carmo M and
Costa PF: Effects of anaesthesia on insulin sensitivity and
metabolic parameters in Wistar rats. In Vivo. 27:127–132.
2013.PubMed/NCBI
|
|
36
|
Castañeda-Delgado JE, Bastián-Hernandez Y,
Macias-Segura N, Santiago-Algarra D, Castillo-Ortiz JD,
Alemán-Navarro AL, Martínez-Tejada P, Enciso-Moreno L, Garcia-De
Lira Y, Olguín-Calderón D, et al: Type I interferon gene response
is increased in early and established rheumatoid arthritis and
correlates with autoantibody production. Front Immunol.
8(285)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Trentham DE, Townes AS and Kang AH:
Autoimmunity to type II collagen an experimental model of
arthritis. J Exp Med. 146:857–868. 1977.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mia MY, Zhang L, Hossain A, Zheng CL,
Tokunaga O and Kohashi O: Dimethyl dioctadecyl ammonium bromide
(DDA)-induced arthritis in rats: A model of experimental arthritis.
J Autoimmun. 14:303–310. 2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Silván AM, Abad MJ, Bermejo P and Villar
AM: Adjuvant-carrageenan-induced inflammation in mice. Gen
Pharmacol. 29:665–669. 1997.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Holmdahl R, Andersson M, Goldschmidt TJ,
Gustafsson K, Jansson L and Mo JA: Type II collagen autoimmunity in
animals and provocations leading to arthritis. Immunol Rev.
118:193–232. 1990.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wilson-Gerwing TD, Pratt IV, Cooper DM,
Silver TI and Rosenberg AM: Age-related differences in
collagen-induced arthritis: Clinical and imaging correlations. Comp
Med. 63:498–502. 2013.PubMed/NCBI
|
|
43
|
Dayer JM: The pivotal role of
interleukin-1 in the clinical manifestations of rheumatoid
arthritis. Rheumatology (Oxford). 42 (Suppl 2):ii3–ii10.
2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sun WK, Bai Y, Yi MM, Wu LJ, Chen JL, Wu
DM, Wu HW, Wan L, Meng Y and Zhang QL: Expression of T follicular
helper lymphocytes with different subsets and analysis of serum
IL-6, IL-17, TGF-β and MMP-3 contents in patients with rheumatoid
arthritis. Eur Rev Med Pharmacol Sci. 23:61–69. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ishigame H, Nakajima A, Saijo S, Komiyama
Y, Nambu A, Matsuki T, Nakae S, Horai R, Kakuta S and Iwakura Y:
The role of TNFalpha and IL-17 in the development of excess IL-1
signaling-induced inflammatory diseases in IL-1 receptor
antagonist-deficient mice. Ernst Schering Res Found Workshop.
129–153. 2006.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang Q, Zhou X, Yang L, Zhao Y, Chew Z,
Xiao J, Liu C, Zheng X, Zheng Y, Shi Q, et al: The natural compound
notopterol binds and targets JAK2/3 to ameliorate inflammation and
arthritis. Cell Rep. 32(108158)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Goldring MB: Anticytokine therapy for
osteoarthritis. Expert Opin Biol Ther. 1:817–829. 2001.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Mateen S, Zafar A, Moin S, Khan AQ and
Zubair S: Understanding the role of cytokines in the pathogenesis
of rheumatoid arthritis. Clin Chim Acta. 455:161–171.
2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Nishimura H, Nose M, Hiai H, Minato N and
Honjo T: Development of lupus-like autoimmune diseases by
disruption of the PD-1 gene encoding an ITIM motif-carrying
immunoreceptor. Immunity. 11:141–151. 1999.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Raptopoulou AP, Bertsias G, Makrygiannakis
D, Verginis P, Kritikos I, Tzardi M, Klareskog L, Catrina AI,
Sidiropoulos P and Boumpas DT: The programmed death 1/programmed
death ligand 1 inhibitory pathway is up-regulated in rheumatoid
synovium and regulates peripheral T cell responses in human and
murine arthritis. Arthritis Rheum. 62:1870–1880. 2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Nakagawa S, Arai Y, Mori H, Matsushita Y,
Kubo T and Nakanishi T: Small interfering RNA targeting CD81
ameliorated arthritis in rats. Biochem Biophys Res Commun.
388:467–472. 2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Inoue A, Takahashi KA, Mazda O, Arai Y,
Saito M, Kishida T, Shin-Ya M, Morihara T, Tonomura H, Sakao K, et
al: Comparison of anti-rheumatic effects of local RNAi-based
therapy in collagen induced arthritis rats using various cytokine
genes as molecular targets. Mod Rheumatol. 19:125–133.
2009.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Tamir A, Petrocelli T, Stetler K, Chu W,
Howard J, Croix BS, Slingerland J and Ben-David Y: Stem cell factor
inhibits erythroid differentiation by modulating the activity of
G1-cyclin-dependent kinase complexes: A role for p27 in erythroid
differentiation coupled G1 arrest. Cell Growth Differ. 11:269–277.
2000.PubMed/NCBI
|
|
55
|
Jiang Y, Jahagirdar BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, et al: Pluripotency of mesenchymal stem cells
derived from adult marrow. Nature. 418:41–49. 2002.PubMed/NCBI View Article : Google Scholar
|
|
56
|
El Qashty RM, Mohamed NN, Radwan LR and
Ibrahim FM: Effect of bone marrow mesenchymal stem cells on healing
of temporomandibular joints in rats with induced rheumatoid
arthritis. Eur J Oral Sci. 126:272–281. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
DiFederico E, Shelton JC and Bader DL:
Complex mechanical conditioning of cell-seeded agarose constructs
can influence chondrocyte biosynthetic activity. Biotechnol Bioeng.
114:1614–1625. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Smolen JS, Aletaha D and McInnes IB:
Rheumatoid arthritis. Lancet. 388:2023–2038. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wu G, Cui Y, Ma L, Pan X, Wang X and Zhang
B: Repairing cartilage defects with bone marrow mesenchymal stem
cells induced by CDMP and TGF-β1. Cell Tissue Bank. 15:51–57.
2014.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Feldmann M, Brennan FM and Maini RN: Role
of cytokines in rheumatoid arthritis. Annu Rev Immunol. 14:397–440.
1996.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Pericolini E, Gabrielli E, Alunno A,
Bartoloni Bocci E, Perito S, Chow SK, Cenci E, Casadevall A, Gerli
R and Vecchiarelli A: Functional improvement of regulatory T cells
from rheumatoid arthritis subjects induced by capsular
polysaccharide glucuronoxylomannogalactan. PLoS One.
9(e111163)2014.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ali S, Leonard SA, Kukoly CA, Metzger WJ,
Wooles WR, McGinty JF, Tanaka M, Sandrasagra A and Nyce JW:
Absorption, distribution, metabolism, and excretion of a respirable
antisense oligonucleotide for asthma. Am J Respir Crit Care Med.
163:989–993. 2001.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Fiset PO, Soussi-Gounni A,
Christodoulopoulos P, Tulic M, Sobol SE, Frenkiel S, Lavigne F,
Lamkhioued B and Hamid Q: Modulation of allergic response in nasal
mucosa by antisense oligodeoxynucleotides for IL-4. J Allergy Clin
Immunol. 111:580–586. 2003.PubMed/NCBI View Article : Google Scholar
|