Introduction
Ischemic heart disease is one of the leading causes
of morbidity and mortality worldwide (1). Clinically, the most effective
intervention for myocardial ischemia is the restoration of blood
flow perfusion, which reduces myocardial cell apoptosis, narrows
the area of myocardial infarction and reverses cardiac dysfunction
(2). However, reperfusion can also
aggravate myocardial injury and myocardial cell death, in a process
known as myocardial ischemia-reperfusion injury (MIRI) (3). Reperfusion itself can induce the
excessive production of reactive oxygen species, excessive
inflammatory response and cell apoptosis, aggravating myocardial
injury and cell death (4). However,
current available treatment strategies for MIRI are limited
(5). Therefore, it is important to
elucidate novel therapies to reduce MIRI in patients with ischemic
heart disease, which can help to prevent and reverse the occurrence
and development of MIRI, in turn improving their prognoses.
The underlying mechanism of MIRI remains poorly
understood. At the time of MIRI, it is hypothesized that increases
in oxygen free radicals, accumulation of neutrophils, slow blood
flow or no reflow of occluded blood vessels caused by cellular
swelling and myocardial cell calcium overload are all involved in
the occurrence of myocardial injury (6). Changes in cells or tissues during MIRI
can result in the increased production of oxygen free radicals,
which in turn leads to breakage of peptide chains and destruction
of the cellular membrane (7).
Therefore, intracellular ATP production is reduced and cellular
energy metabolism is significantly affected (7). In addition, destruction of cell
membranes makes it easier for inflammatory cells to adhere to blood
vessels, resulting in microcirculatory disorder and cell damage
(8). Therefore, reducing oxidative
stress and the inflammatory response during MIRI is key to
effectively treating this disease.
The galectin (Gal) family represents a group of
endogenous lectins with high affinities for polysaccharides
containing β-galactoside residues (9). To date, 15 members of the lectin
family have been identified, all of which contain highly conserved
sugar recognition domains (9).
Gal-1 is a member of the Gal family that is expressed in various
types of cell, including thymic epithelial cells, endothelial
cells, dendritic cells, macrophages, fibroblasts and bone marrow
stromal cells (9-11).
It is particularly abundant in skeletal muscle, smooth muscle,
myocardium, sensory and motor neurons, and the placenta (10). Gal-1 has been reported to mediate a
variety of biological functions that regulate the level of
inflammation and apoptosis in cells. Huang et al (11) used lipopolysaccharide (LPS) to
induce acute lung injury in mice, and found that Gal-1 attenuated
LPS-induced lung injury and reduced inflammation, oxidative stress
and apoptosis. However, there is a lack of knowledge on the
possible role of Gal-1 in MIRI. Therefore, the present study
constructed a rat MIRI model and a model of MIRI in H9c2 cells.
Gal-1 treatment was then applied to assess the effects of Gal-1 on
MIRI.
Materials and methods
Animals and grouping
A total of 60 2-month-old male Sprague-Dawley rats
(weight, 250-350 g) were purchased from Charles River Laboratories,
Inc. and housed in a temperature-controlled room (21±2˚C) on a 12-h
light/dark cycle (lights on at 06:00) with a relative humidity
range of 30-40%. Animal health and behavior was monitored daily.
The present study was approved by the Animal Ethics Committee of
Chengdu Fifth People's Hospital Animal Center (Chengdu, China).
Rats were housed in a standard environment, receiving rat food and
clean drinking water ad libitum. Rats were randomly divided
into the following groups (n=15 per group): i) Control; ii) Gal-1;
iii) MIRI; and iv) MIRI + Gal-1. Animals in the Gal-1 and MIRI +
Gal-1 groups were injected subcutaneously with Gal-1 (5 µg/g;
Invitrogen; Thermo Fisher Scientific, Inc.) once per day, 1 week
prior to modeling (12). Rats in
the control group underwent the same anesthetic and surgical
procedures but without ligation of the left anterior descending
coronary artery.
MIRI induction
After anesthetizing rats with an intraperitoneal
injection of pentobarbital sodium at a dose of 40 mg/kg, rats were
placed on the operating table in the supine position. A small
animal ventilator was used to maintain breathing. Surgical scissors
were subsequently used to gently cut the left side of the chest to
expose the heart. After locating the left anterior descending
coronary artery, a suture was introduced to perform gentle
ligation. After 30 min, the suture was untied for the
recanalization of the left anterior descending coronary artery for
2 h (6). Echocardiography was
subsequently performed to monitor animal cardiac function, which
included the following parameters: Left ventricular end-systolic
volume (ESV), left ventricular end-diastolic volume (EDV), left
ventricular end-systolic diameter (LVIDs) and left ventricular
end-diastolic diameter (LVIDd). Two rats in each of the MIRI and
MIRI + GAL-1 groups died due to postoperative bleeding. At 2 h
after reperfusion, the remaining 56 rats were euthanized via
cervical dislocation after being anesthetized via intraperitoneal
injection of pentobarbital sodium at a dose of 40 mg/kg, before
heart and blood samples were collected. Cardiac arrest in the rats
indicated that the rats were dead.
Cell culture and treatment
The rat cardiomyocyte H9c2 cell line (American Type
Culture Collection) was cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 1% streptomycin (100 µg/ml) and 1% penicillin
(100 U/ml) at pH 7.4 in a 5% CO2 incubator at 37˚C.
Hypoxia-reoxygenation (HR) was used to construct an in vitro
model of MIRI in H9c2 cells. To mimic myocardial I/R in
vitro, H9C2 cells at 80% confluence were incubated in DMEM
without FBS (previously bubbled with gas mixture containing 95%
N2 and 5% CO2) for 6 h at 37˚C. The cells
were provided with fresh medium and then moved to 95%
O2/5% CO2 for reoxygenation. The control
plates were kept in the incubator with 95% O2/5%
CO2 at 37˚C. Cells were harvested 16 h
post-reoxygenation for analysis.
Ultrasonic cardiogram
At 2 h following recanalization, rats were placed in
the left lateral position and cardiac function was assessed using
Philips ultrasound equipment (Philips iE33; Philips Medical Systems
B.V.). Left ventricular ESV, left ventricular EDV, LVIDs and LVIDd
were all measured. Left ventricular ejection fraction (EF) was
calculated using the following formula: EF=(EDV-ESV)/EDV.
Additionally, fraction shortening (FS) was calculated the following
formula: FS=(LVIDd-LVIDs)/LVIDd.
Hematoxylin and eosin staining
Rat myocardial tissue was fixed with 4%
paraformaldehyde at 4˚C. After 24 h, myocardial tissue was
dehydrated and embedded in paraffin. A microtome was subsequently
used to section the myocardial tissue (thickness, 5 µm). Sections
were dewaxed, hydrated and stained with hematoxylin solution
(Beyotime Institute of Biotechnology) for 1 min at 37˚C. After
rinsing sections for 3 min with running water, excess stain was
removed with hydrochloric acid alcohol (Beyotime Institute of
Biotechnology). Samples were then immersed in eosin solution
(Beyotime Institute of Biotechnology) for 1 min and dehydrated at
37˚C. Neutral gum was used to seal sections. Images were acquired
using a light microscope (Nikon/80i; Nikon Corporation) and a
digital camera (DP71CCD; Olympus Corporation).
Immunohistochemical (IHC)
staining
After paraffin-embedded myocardial tissue was
dewaxed and hydrated, sections (5 µm) were placed in citrate buffer
and microwaved for 20 min. After the water was naturally cooled,
sections were treated with 3% hydrogen peroxide for 30 min at 37˚C.
Subsequently, 10% goat serum (Beyotime Institute of Biotechnology)
was used to treat sections for 1 h at 37˚C. Samples were then
incubated with the following primary antibodies at 4˚C overnight
(all Abcam, all 1:100): IL-1β (cat. no. ab9722), IL-6 (cat. no.
ab6672), caspase-3 (cat. no. ab4051) and caspase-8 (cat. no.
ab25901). Subsequently, sections were washed with PBS and incubated
with secondary antibodies (Abcam). After DAB and hematoxylin
staining, the slides were dehydrated, cleared with xylene, mounted
with permanent mounting medium, then photographed via light
microscopy (Nikon/80i; Nikon Corporation). The resulting images
were analyzed by Image Pro-plus 6.0 software (Media Cybernetics,
Inc.).
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from left ventricular
anterior wall tissues of rats and H9c2 cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocols. After determining the
concentration of extracted RNA using a spectrophotometer, RNA was
reverse transcribed into cDNA using an RT-qPCR kit (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. The SYBR Green Master Mix (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to amplify cDNA. The total reaction
system volume was 25 µl. The thermocycling conditions were as
follows: Pre-denaturation at 95˚C for 5 min, followed by 35 cycles
of denaturation at 95˚C for 30 sec, annealing at 60˚C for 45 sec,
extension at 72˚C for 3 min and a final extension at 72˚C for 5
min. PCR products were stored at 4˚C. The primers used for cDNA
amplification were constructed by Generay Biotech Co., Ltd. and are
listed in Table I. Using the
2-ΔΔCq method (13), the
expression of endogenous GAPDH was used as the reference gene to
calculate the expression of each mRNA.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Name |
Forward/reverse | Sequence
(5'-3') |
|---|
| IL-1β | Forward |
CCCTTGACTTGGGCTGT |
| | Reverse |
CGAGATGCTGCTGTGAGA |
| IL-8 | Forward |
GAGCAACCCATACCCATCGA |
| | Reverse |
TGGTCCCACCATATCTTCTTAATCT |
| TNF-α | Forward |
CAGCCAGGAGGGAGAAC |
| | Reverse |
GTATGAGAGGGACGGAACC |
| Caspase-3 | Forward |
GGAACGCGAAGAAAAGTG |
| | Reverse |
ATTTTGAATCCACGGAGGT |
| Caspase-8 | Forward |
CACATCCCGCAGAAGAAG |
| | Reverse |
GATCCCGCCGACTGATA |
| Bax | Forward |
GAGGTCTTCTTCCGTGTGG |
| | Reverse |
GATCAGCTCGGGCACTTT |
| Bcl-2 | Forward |
AGGAACTCTTCAGGGATGG |
| | Reverse |
GCGATGTTGTCCACCAG |
| GAPDH | Forward |
ATGGCTACAGCAACAGGGT |
| | Reverse |
TTATGGGGTCTGGGATGG |
Immunofluorescence (IF) staining
H9c2 cells were fixed using 4% paraformaldehyde at
4˚C for 1 h and treated with PBS containing 0.2% Triton X-100 for
15 min at 4˚C. After blocking with 10% goat serum at 4˚C for 30
min, cells were incubated with primary antibodies against caspase-3
and caspase-8 (the same as the above) at 4˚C overnight. After
washing with PBS, cells were incubated with fluorescent secondary
antibodies, including Alexa Fluor 546 goat anti-mouse lgG (H+L;
1:200; cat. no. A11030; Invitrogen; Thermo Fisher Scientific, Inc.)
and donkey anti-rabbit-CY3 (1:200; cat. no. A21206; Molecular
Probes; Thermo Fisher Scientific, Inc.) at 4˚C for 2 h at room
temperature. Samples were then stained with DAPI (Fluoroshield with
DAPI; cat. no. F6057; Gibco; Thermo Fisher Scientific, Inc.) for 5
min at 4˚C, washed with PBS and observed under a fluorescent
microscope (Leica DM3000; Leica Microsystems GmbH; magnification,
x60).
ELISA
Myocardial tissue of the left ventricular anterior
wall of each rat was removed and lysed with RIPA (Beyotime
Institute of Biotechnology). Rat blood samples were collected by
cardiac puncture. Rat serum was then isolated by centrifugation
(2,200 x g for 15 min, 4˚C) and the resultant supernatant was
stored at -80˚C. ELISA kits (all Invitrogen; Thermo Fisher
Scientific, Inc.) were subsequently used to determine the
concentrations of creatine kinase (CK; cat. no. A032-1-1), lactate
dehydrogenase (LDH; cat. no. A020-2-2), IL-6 (cat. no. H007-1-1),
IL-8 (cat. no. H008) and TNF-α (cat. no. H052-1) (all, Nanjing
Jiancheng Bioengineering Institute) in the lysate according to the
manufacturer's protocols. Inflammatory factors in the supernatants
of H9c2 cells were similar to that of myocardial tissue.
Cell counting kit-8 (CCK-8) assay
H9c2 cells were seeded into 96-well plates at a
density of 3.0x104 cells/ml. After confluence reached
50%, various concentrations of Gal-1 (1, 3, 5, 10 and 20 µM) were
used to treat H9c2 cells (after hypoxia/reoxygenation). After 24 h
at 20˚C, 10 µM CCK-8 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) was added into each well of the 96-well plate. After a 2 h
incubation at 20˚C, a microplate reader (cat. no. HBS-1096A;
Nanjing Detie Experimental Equipment Co., Ltd.) was used to measure
the absorbance of each well at 450 nm.
Flow cytometry
Apoptosis rate was determined using flow cytometry.
A cellular sieve (Beyotime Institute of Biotechnology) was utilized
to extract cardiomyocytes from the rat myocardium, after which an
Annexin V-FITC kit (Shanghai GeneChem Co., Ltd.) was used to detect
the apoptotic rate of cardiomyocytes in accordance with the
manufacturer's protocol. H9c2 cells were collected when the cell
density reached 50%. The cells were then resuspended in 500 µl
Binding buffer. Annexin V (5 µl) and PI (10 µl) were added into
Binding buffer, after which cells were incubated in the dark for 5
min at room temperature. Early apoptotic cells were presented in
the lower right quadrant of the plot, while late apoptotic and
necrotic cells were demonstrated in the upper right quadrant of the
plot. A flow cytometer (FACSCalibur; BD Biosciences) was used for
analysis. Data were obtained and analyzed using CellQuest
professional software (Version 3.3; Becton-Dickinson and
Company).
Statistical analysis
SPSS 20.0 (IBM Corp.) and GraphPad Prism 7.0
(GraphPad Software, Inc.) software were used for statistical
analysis. All experiments were repeated in triplicate and
experimental data were expressed as the mean ± standard deviation.
Comparisons between multiple groups were performed using one-way
ANOVA followed by Bonferroni post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Gal-1 treatment attenuates MIRI and
improves cardiac function in rats
To determine the potential effects of Gal-1 on rat
myocardial tissue, H&E staining was performed to detect
morphological changes after inducing MIRI. The results revealed
that the structure of the myocardial tissue from rats in the MIRI
group was disordered, and the number of cardiomyocytes was markedly
decreased (Fig. 1A). By contrast,
morphology of the tissues from rats in the MIRI + Gal-1 group was
visibly improved compared with that in the MIRI group (Fig. 1A). There was no marked difference in
the morphology of myocardial tissues between the Gal-1 and the
control groups. ELISA was subsequently performed to detect the
levels of CK (Fig. 1B) and LDH
(Fig. 1C) in rat serum. The results
demonstrated that Gal-1 reduced the levels of CK and LDH at the
MIRI level. Echocardiography was next performed to determine rat
cardiac function (Fig. 1D-I). The
ESV, EDV, LVIDs and LVIDd of MIRI rats were significantly higher
compared with those in the control group, whereas the EF and FS
were found to be lower when compared with those in the control
group. Gal-1 treatment significantly reversed the effects of MIRI
on each of the aforementioned parameters of rat cardiac function
(Fig. 1D-I).
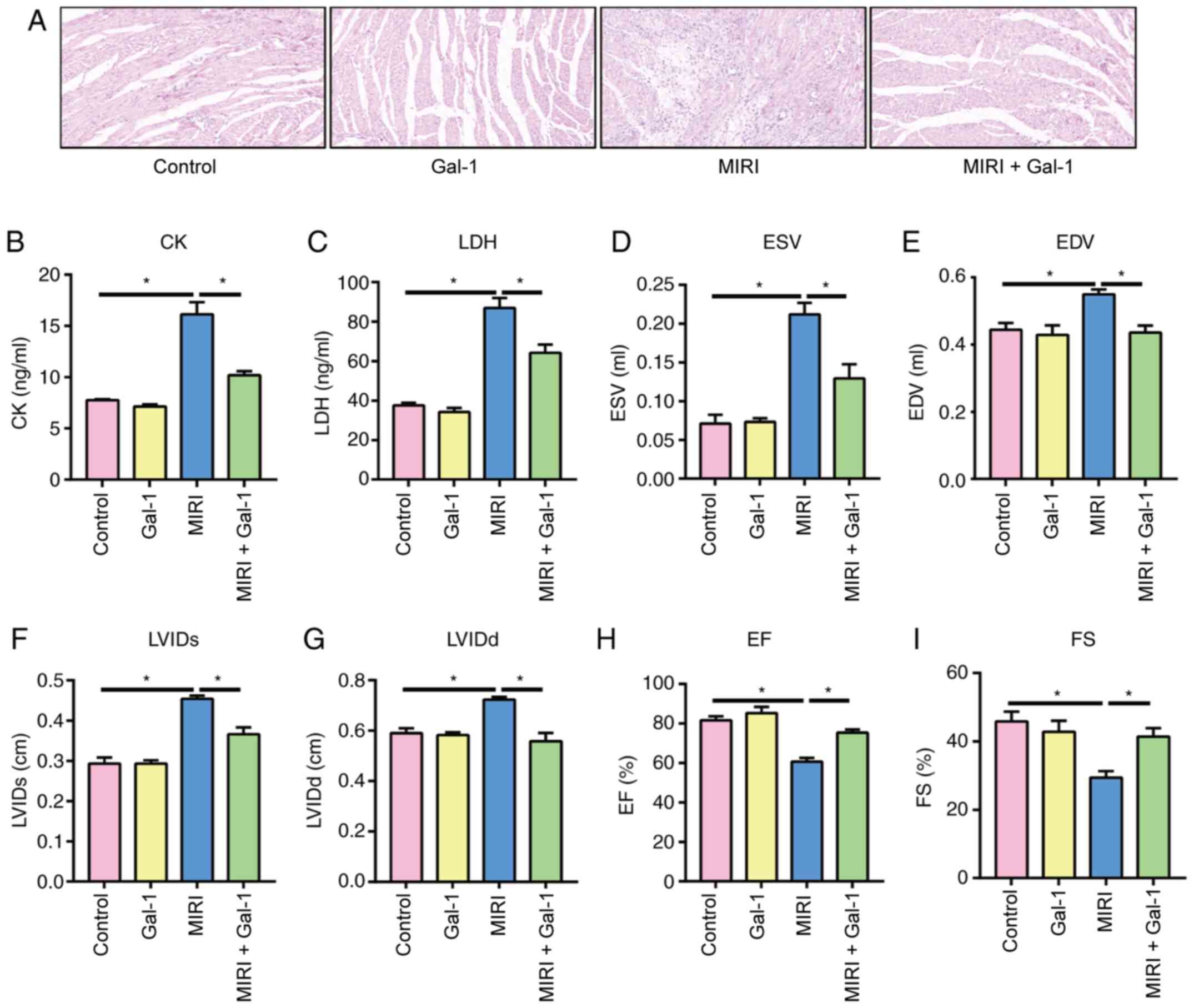 | Figure 1Galectin-1 attenuates myocardial
ischemia-reperfusion injury and improves cardiac function in rats.
(A) Representative H&E staining images of rat myocardial
tissues. Magnification, x200. ELISA results of (B) CK and (C) LDH
in rat serum. Results of rat echocardiography, including (D) ESV,
(E) EDV, (F) LVIDs, (G) LVIDd, (H) EF and (I) FS.
*P<0.05. CK, creatine kinase; LDH, lactate
dehydrogenase; MIRI, myocardial ischemia-reperfusion injury; ESV,
left ventricular end-systolic volume; EDV, left ventricular
end-diastolic volume; LVIDs, left ventricular end-systolic
diameter; LVIDd, left ventricular end-diastolic diameter; EF, left
ventricular ejection fraction; FS, fraction shortening; Gal-1,
galectin-1. |
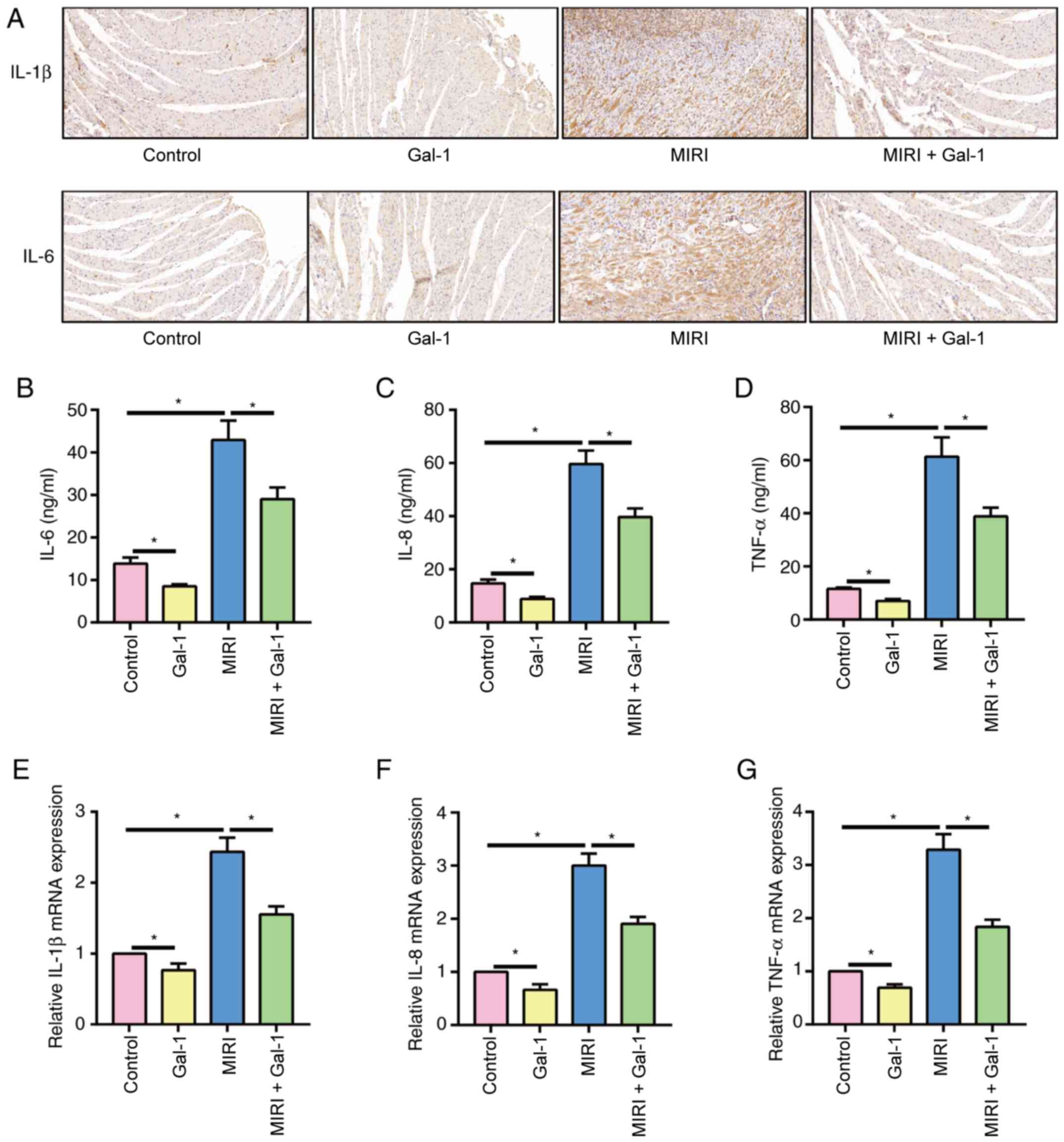
Gal-1 reduces inflammation after
MIRI
The expression of IL-1β and IL-6 was next detected
in rat myocardial tissued via IHC staining (Fig. 2A). The expression of IL-1β and IL-6
in the myocardial tissue of the MIRI group was markedly higher
compared with that in the control group, whilst tissues in the MIRI
+ Gal-1 treatment exhibited decreased IL-1β and IL-6 staining
compared with that in the MIRI alone group. In addition, at basal
level, the expression level of IL-1β and IL-6 in the myocardial
tissue of the Gal-1 group was also lower compared with that in the
control group. The levels of inflammatory factors (IL-6, IL-8 and
TNF-α) in rat serum were next measured by performing ELISA
(Fig. 2B-D). The results revealed
that Gal-1 significantly reduced their expression in rat serum. The
results of RT-qPCR also confirmed the anti-inflammatory effect of
Gal-1 on rat MIRI (Fig. 2E-G). In
summary, IL-1β, IL-6, IL-8 and TNF-α increased after MIRI, but were
reduced after treatment with Gal-1.
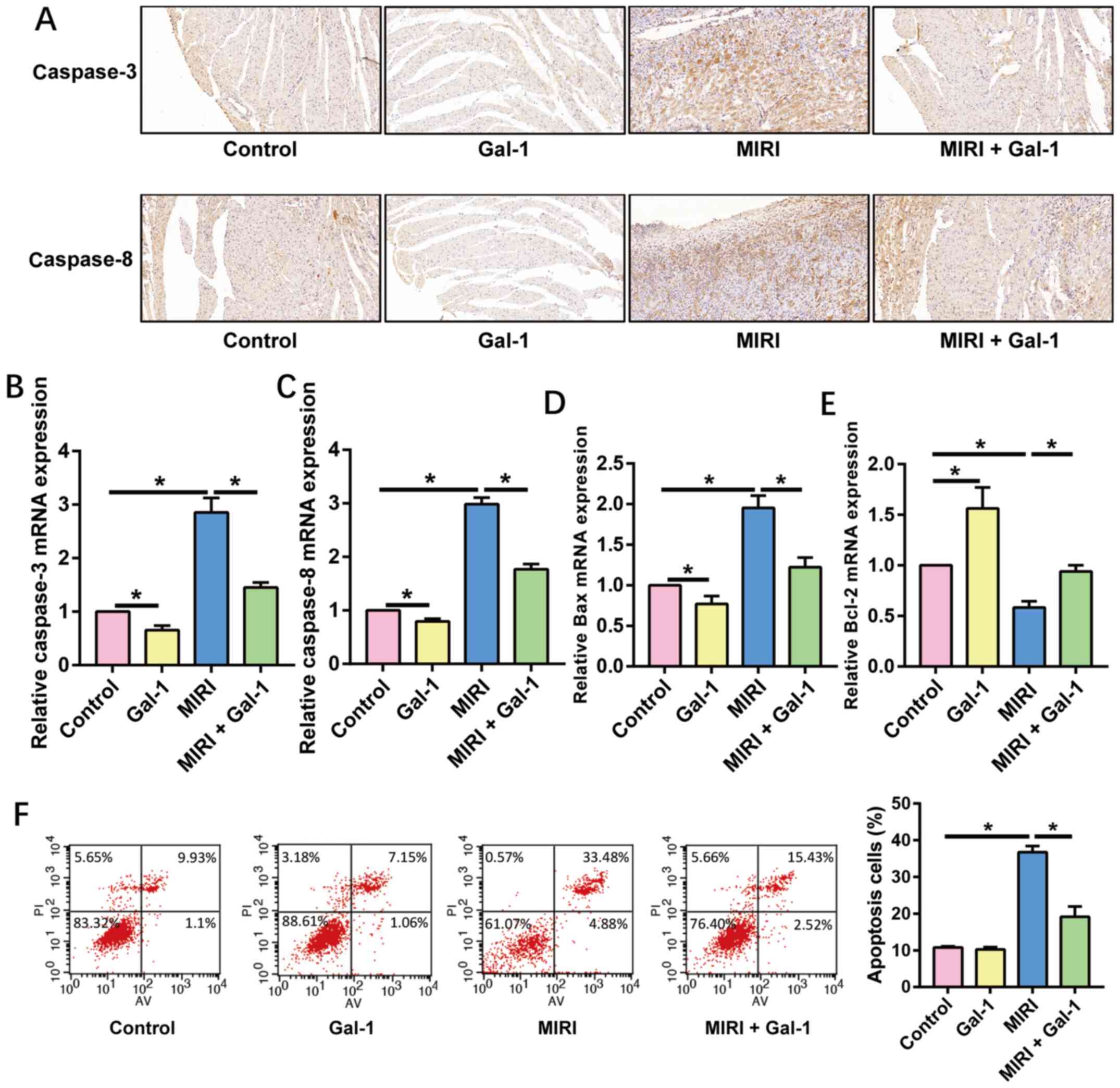
Gal-1 reduces cardiomyocyte apoptosis
after MIRI
The expression of caspase-3 and -8 was assessed in
rat myocardial tissue following IHC staining. The results of IHC
staining revealed that the expression of caspase-3 and -8 was
markedly increased in myocardial tissue after MIRI, whilst Gal-1
treatment decreased the expression of caspase-3 and caspase-8
compared with that at basal and MIRI alone (Fig. 3A). The expression of caspase-3,
caspase-8, Bax and Bcl-2 mRNA in rat myocardial tissue was next
detected using RT-qPCR (Fig. 3B-E).
Similar to the results of IHC staining, Gal-1 treatment also
significantly reduced the expression of caspase-3, -8 and Bax and
Bcl-2 mRNA. In addition, the apoptotic rate of rat cardiomyocytes
was assessed via flow cytometry. The results demonstrated that rat
cardiomyocyte apoptosis was significantly increased in the MIRI
group compared with that in the control group, which was
significantly reversed by Gal-1 treatment (Fig. 3F).
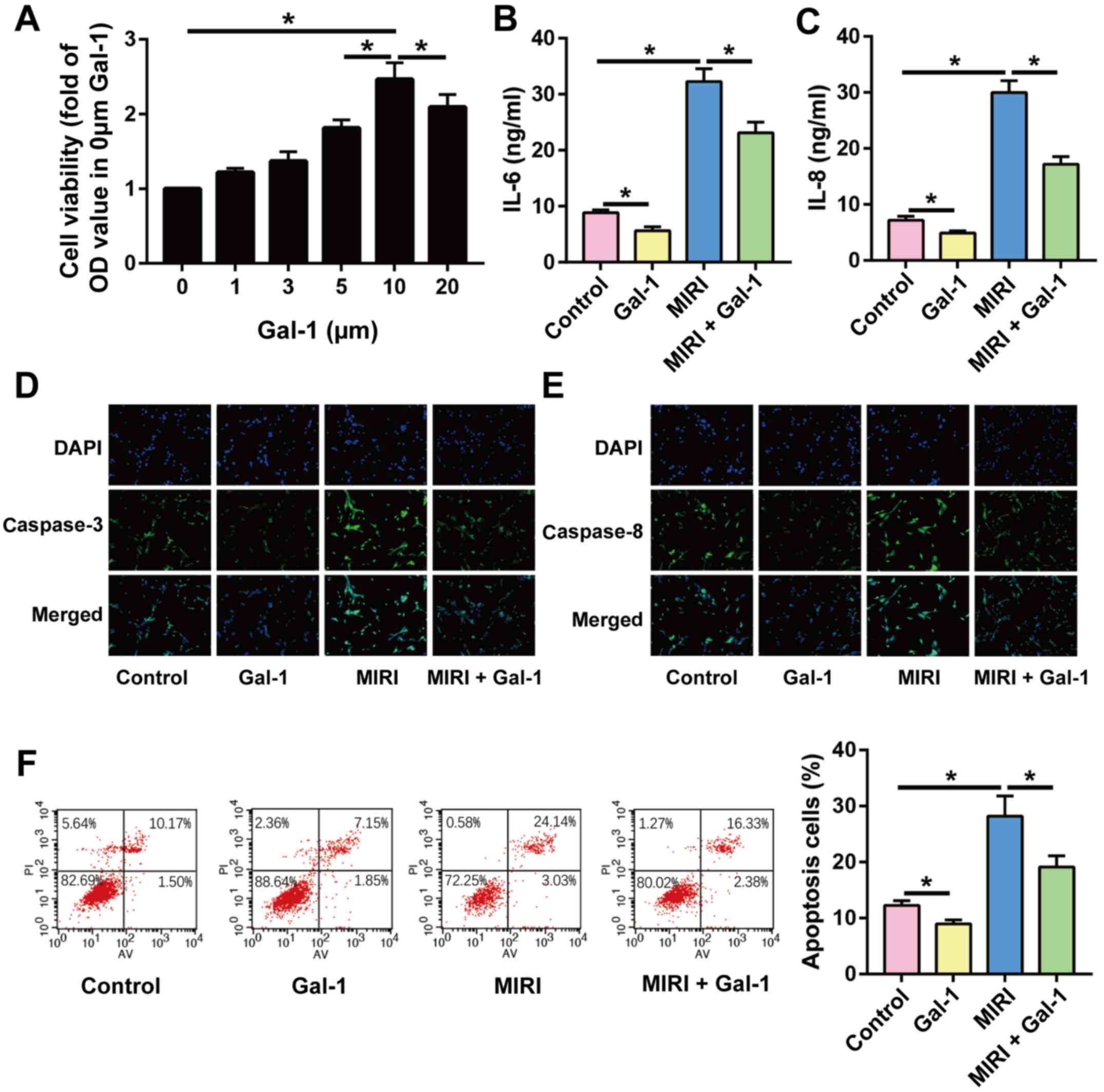
Gal-1 reduces inflammation and
apoptosis in H9c2 cells
To verify the potential effects of Gal-1 on
cardiomyocytes, H9c2 cells were cultured, and the effects of Gal-1
on inflammation and apoptosis were determined. The effect of
different concentrations of Gal-1 (1, 3, 5, 10 and 20 µM) on the
viability of H9c2 cells was assessed by performing a CCK-8 assay.
As the effect of 10 µM Gal-1 on the viability of H9c2 cells under
standard conditions was the most pronounced, this was used for
subsequent experiments (Fig. 4A).
The levels of IL-6 (Fig. 4B) and
IL-8 (Fig. 4C) in H9c2 cell
supernatants were also examined by ELISA. Gal-1 stimulation was
determined to inhibit the inflammatory response of H9c2 cells
induced by HR. Furthermore, IF staining revealed that the
expression of caspase-3 and caspase-8 was decreased in H9c2 cells
following Gal-1 stimulation (Fig.
4D and E). Gal-1 was also found
to reduce the rate of apoptosis in H9c2 cells (Fig. 4F). Therefore, it was concluded that
Gal-1 exerted anti-inflammatory and anti-apoptotic effects on H9c2
cells.
Discussion
Ischemic heart disease is the main cause of death
worldwide, accounting for >9 million deaths in 2016 according to
the World Health Organization estimates (4). In addition to drug treatment,
reperfusion therapy can improve the symptoms and prognosis of
patients with ischemic heart disease, in turn increasing the cure
rate of acute myocardial infarction (4). However, irreversible damage to the
myocardium may occur prior to treatment, leading to myocardial
necrosis, electrical remodeling and anatomical remodeling of the
ventricle (14). MIRI serves an
important role throughout this process (15). Previous studies have shown that MIRI
may be associated with cardiomyocyte apoptosis, excessive oxygen
free radical production, calcium overload and inflammation
(6,7,14).
Gal-1 is closely associated with cardiac function and metabolism,
which has been previously demonstrated to mediate cardiovascular
inflammation, serving as an important therapeutic target for
cardiovascular related diseases (15). A previous study revealed that Gal-1
alleviated myocardial hypertrophy by regulating calcium channels in
293 cells (16). In addition, Gal-1
inhibited myocardial inflammation and thus served a protective role
in ventricular remodeling in a mouse model of acute myocardial
infarction (17). The present study
investigated the effects of Gal-1 on heart function, inflammation
and apoptosis in the rat myocardium after MIRI. The results
indicated that Gal-1 treatment improved MIRI-induced myocardial
injury, and reduced the extent of inflammation and apoptosis in
myocardial tissue. In addition, the protective effects of Gal-1 on
cardiomyocytes were verified in H9c2 cells. These results may prove
to be helpful for the clinical treatment of MIRI.
The inflammatory response is an important mechanism
of MIRI, where a number of studies have confirmed that
anti-inflammatory therapy is beneficial to MIRI (15,18).
MIRI promotes the production of various inflammatory cytokines,
including IL-1, IL-6 and TNF-α, and promotes the infiltration of
inflammatory cells to the myocardial tissue (15). A continuous inflammatory response
not only affects the ischemic organ itself, but can also cause
damage to other organs or tissues, potentially leading to multiple
organ failure (19). In the present
study, after rats were treated with Gal-1, the expression of
inflammatory factors in the myocardial tissue and serum was
significantly decreased. This suggested that Gal-1 is an important
factor that improves heart function in rats. Related studies have
also previously demonstrated that ischemia-reperfusion not only
causes severe tissue injury, but also excessive inflammatory
reactions by activating both innate and adaptive immune responses
(6,7). Activation of the innate or adaptive
immune response causes a large number of inflammatory cells to be
produced and activated, leading to inflammatory reactions in
several organs.
Studies have previously demonstrated that leukocytes
are involved in the process of ischemia-reperfusion injury
(6,15). The earliest example of leukocyte
involvement in ischemia-reperfusion injury was reported by Romson
et al (20). Their results
revealed that the administration of leukocytes with anti-leukocyte
serum reduced the extent of myocardial infarction in dogs to the
same extent as that mediated by oxygen radical scavengers,
suggesting that leukocytes were involved in ischemia-reperfusion
injury. Furthermore, Frey et al (21) revealed that after 3 h of myocardial
ischemia in dogs, a large number of leukocytes accumulated in the
capillaries, resulting in increased local vascular resistance, no
reflow and tissue edema. In the same study, they also found that
following anti-leukocyte administration, the number of capillaries
without reflow was significantly reduced. In addition, capillary
blockage was significantly reduced and the incidence of ventricular
arrhythmia was also significantly reduced after treatment with
caspase inhibitors in a rabbit cardiomyocyte ischemia reperfusion
model (22). Although cell membrane
damage occurs during ischemia-reperfusion, cell membrane-related
degradation products increase (23). Certain degradation products, such as
reactive oxygen species, have been reported to strongly mediate
inflammatory chemotaxis, which results in a large number of
leukocytes adsorbing to the vascular endothelium, increasing the
number of leukocytes in the microcirculation and circulation
surrounding the organ (24). During
the process of ischemia-reperfusion, the release of a variety of
intercellular adhesion molecules, such as intercellular cell
adhesion molecule-1 and E-selectin, may cause neutrophil
infiltration (25). The
infiltration of neutrophils also embolizes part of the capillary
blood vessel (25). After restoring
blood perfusion, some ischemic tissues remain unable to achieve
blood perfusion, which is the main reason for the occurrence of
no-reflow after tissue ischemia-reperfusion (26). Therefore, the anti-inflammatory
effects of Gal-1 on cardiomyocytes were largely proposed to
ameliorate MIRI in the current study.
Apoptosis, also known as programmed cell death, is a
form of active gene-regulated cell death (27). Cardiomyocyte apoptosis is an
important factor mediating myocardial injury, determining the area
of myocardial infarction and promoting myocardial remodeling
(28,29). The apoptosis of cardiomyocytes may
result in decreased systolic function, thereby leading to a
decrease in cardiac pump function (30). In the present study, Gal-1 reduced
the expression of the caspase family of proteins in cardiomyocytes
and increased the ratio of Bcl-2 and Bax. The results of flow
cytometry also demonstrated that Gal-1 reduced the rate of
apoptosis in rat cardiomyocytes, indicating that cardiomyocyte
apoptosis was suppressed during MIRI by Gal-1. Apoptosis is an
important factor in MIRI (31).
Apoptotic cells have been previously identified in rabbit models of
MIRI, which further confirmed that apoptosis occurs in the marginal
zone of myocardial infarction (32). Other studies using MIRI rabbit
models confirmed that apoptosis did not occur after reperfusion for
4 h following myocardial ischemia for 5 min (33,34).
Additionally, no myocardial apoptosis was detected after 30 min of
ischemia (35). However, after
ischemia for 30 min and reperfusion for 4 h, cardiomyocytes
exhibited marked apoptosis, suggesting that cardiomyocyte apoptosis
is not only dependent on reperfusion injury, but also on the length
of ischemia. A previous study utilizing dogs to establish an MIRI
model revealed no obvious apoptosis after 7 h myocardial ischemia
(36). Apoptosis was observed
following ischemia for 1 h and reperfusion for 6 h. It is
considered that apoptosis is more likely to occur in myocardial
reperfusion (24). In a clinical
study, myocardial tests were conducted in 8 patients who were
diagnosed with acute myocardial infarction (37). Hemorrhagic infarction, spontaneous
subintimal myocardial ischemia and reperfusion injury, and
apoptosis around the infarction area were all found, indicating an
association between MIRI and apoptosis (38). Therefore, apoptosis is an important
mechanism of MIRI. Additionally, the anti-apoptotic effect of Gal-1
was hypothesized to improve myocardial cell survival and MIRI in
the present study.
The activation of inflammatory cells and the release
of inflammatory factors are important for cardiomyocyte apoptosis
during MIRI (39). NF-κB is a key
protein that regulates the immune response and proinflammatory
cytokine expression (39). The
NF-κB signal transduction pathway is closely associated with the
production and release of pro-inflammatory cytokines and the
occurrence of cardiomyocyte apoptosis (40). Previous studies reported that MIRI
induces the activation of NF-κB, the inflammatory cascade of which
promotes the occurrence of cardiomyocyte apoptosis (41,42).
In addition, TNF-α has also been documented to increase vascular
permeability, enhance neutrophil adhesion and induce cardiomyocyte
apoptosis (43). IL-6 is a
fast-acting inflammatory cytokine that responds to stress within
the heart (44). IL-6 acts directly
on cardiomyocytes to induce myocardial inhibition by altering the
function of the sarcoplasmic reticulum and reducing the
concentration of calcium ions in the cell cytoplasm (44). IL-6 has also been previously
revealed to serve an important regulatory role in myocardial
apoptosis in a rat model of MIRI (35). Therefore, the anti-inflammatory and
anti-apoptotic effects of Gal-1 significantly alleviated MIRI in
rats in the current study.
However, there were some limitations in the present
study. MIRI is the result of multiple pathological mechanisms. The
present study only investigated the effect of Gal-1 on inflammation
and apoptosis during MIRI, but did not investigate the effect of
Gal-1 on other pathological mechanisms of MIRI, such as oxidative
stress and calcium ion overload. In addition, the target of Gal-1
in cardiomyocytes remains unclear. Therefore, the role of Gal-1 in
MIRI requires further study; specifically, the target of Gal-1
needs to be identified through techniques such as gene
sequencing.
In the present study, Gal-1 significantly alleviated
the disorder of cardiomyocytes caused by MIRI and improved cardiac
function in rats. In addition, MIRI was accompanied by an excessive
inflammatory response with increased apoptosis in rat myocardial
tissues, both of which were alleviated by Gal-1 treatment.
Acknowledgements
Not applicable.
Funding
Funding: The current study was funded by the Chengdu Technical
Innovation and Research Project: Mechanism and myocardial
mechanical characteristics of rupture QRS in patients with
hypertrophic cardiomyopathy: CMR multi-mode imaging study (grant
no. 2019-YF05-00111-SN) and the Chengdu High-level Key Clinical
Specialty Construction Project (grant no. 2021-5#).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DO and HL designed the study and performed the
experiments, DO, DN and RL established the animal models, DO and XJ
acquired the data, and HL and XC analyzed the data. All authors
read and approved the final manuscript. DO and HL confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Chengdu Fifth People's Hospital Animal Center
(Chengdu, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Popescu CP, Florescu SA, Hasbun R, Harxhi
A, Evendar R, Kahraman H, Neuberger A, Codreanu D, Zaharia MF,
Tosun S, et al: Prediction of unfavorable outcomes in West Nile
virus neuroinvasive infection-Result of a multinational ID-IRI
study. J Clin Virol. 122(104213)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Henry JJD, Delrosario L, Fang J, Wong SY,
Fang Q, Sievers R, Kotha S, Wang A, Farmer D, Janaswamy P, et al:
Development of injectable amniotic membrane matrix for
postmyocardial infarction tissue repair. Adv Healthc Mater.
9(e1900544)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Murthy SB, Diaz I, Wu X, Merkler AE,
Iadecola C, Safford MM, Sheth KN, Navi BB and Kamel H: Risk of
arterial ischemic events after intracerebral hemorrhage. Stroke.
51:137–142. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
GBD 2017 Disease and Injury Incidence and
Prevalence Collaborators. Global, regional, and national incidence,
prevalence, and years lived with disability for 354 diseases and
injuries for 195 countries and territories, 1990-2017: A systematic
analysis for the global burden of disease study 2017. Lancet.
392:1789–1858. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jackson CA, Kerssens J, Fleetwood K, Smith
DJ, Mercer SW and Wild SH: Incidence of ischaemic heart disease and
stroke among people with psychiatric disorders: Retrospective
cohort study. Br J Psychiatry. 217:442–449. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gul-Kahraman K, Yilmaz-Bozoglan M and
Sahna E: Physiological and pharmacological effects of melatonin on
remote ischemic perconditioning after myocardial
ischemia-reperfusion injury in rats: Role of Cybb, Fas, NfκB,
irisin signaling pathway. J Pineal Res. 67(e12589)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Merz SF, Korste S, Bornemann L, Michel L,
Stock P, Squire A, Soun C, Engel DR, Detzer J, Lörchner H, et al:
Contemporaneous 3D characterization of acute and chronic myocardial
I/R injury and response. Nat Commun. 10(2312)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Stiermaier T, Jensen JO, Rommel KP, de
Waha-Thiele S, Fuernau G, Desch S, Thiele H and Eitel I: Combined
intrahospital remote ischemic perconditioning and postconditioning
improves clinical outcome in ST-elevation myocardial infarction.
Circ Res. 124:1482–1491. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tsai YT, Liang CH, Yu JH, Huang KC, Tung
CH, Wu JE, Wu YY, Chang CH, Hong TM and Chen YL: A DNA aptamer
targeting galectin-1 as a novel immunotherapeutic strategy for lung
cancer. Mol Ther Nucleic Acids. 18:991–998. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kuroi K, Kamijo M, Ueki M, Niwa Y,
Hiramatsu H and Nakabayashi T: Time-resolved FTIR study on the
structural switching of human galectin-1 by light-induced disulfide
bond formation. Phys Chem Chem Phys. 22:1137–1144. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang XT, Liu W, Zhou Y, Sun M, Yang HH,
Zhang CY and Tang SY: Galectin-1 ameliorates
lipopolysaccharide-induced acute lung injury via AMPK-Nrf2 pathway
in mice. Free Radic Biol Med. 146:2222–233. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Carlos CP, Silva AA, Gil CD and Oliani SM:
Pharmacological treatment with galectin-1 protects against renal
ischaemia-reperfusion injury. Sci Rep. 8(9568)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lameijer H, Schutte JM, Schuitemaker NW,
van Roosmalen JJ and Pieper PG: Dutch Maternal Mortality and
Morbidity Committee. Maternal mortality due to cardiovascular
disease in the Netherlands: A 21-year experience. Neth Heart J.
28:27–36. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Seropian IM, González GE, Maller SM,
Berrocal DH, Abbate A and Rabinovich GA: Galectin-1 as an emerging
mediator of cardiovascular inflammation: Mechanisms and therapeutic
opportunities. Mediators Inflamm. 2018(8696543)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fan J, Fan W, Lei J, Zhou Y, Xu H, Kapoor
I, Zhu G and Wang J: Galectin-1 attenuates cardiomyocyte
hypertrophy through splice-variant specific modulation of Ca1.2
calcium channel. Biochim Biophys Acta. 1865:218–229.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Seropian IM, Cerliani JP, Toldo S, Van
Tassell BW, Ilarregui JM, González GE, Matoso M, Salloum FN,
Melchior R, Gelpi RJ, et al: Galectin-1 controls cardiac
inflammation and ventricular remodeling during acute myocardial
infarction. Am J Pathol. 182:29–40. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Han B, Li S, Lv Y, Yang D, Li J, Yang Q,
Wu P, Lv Z and Zhang Z: Dietary melatonin attenuates
chromium-induced lung injury via activating the Sirt1/Pgc-1α/Nrf2
pathway. Food Funct. 10:5555–5565. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang Q, Han B, Xue J, Lv Y, Li S, Liu Y,
Wu P, Wang X and Zhang Z: Hexavalent chromium induces mitochondrial
dynamics disorder in rat liver by inhibiting AMPK/PGC-1α signaling
pathway. Environ Pollut. 265(114855)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Romson JL, Hook BG, Kunkel SL, Abrams GD,
Schork MA and Lucchesi BR: Reduction of the extent of ischemic
myocardial injury by neutrophil depletion in the dog. Circulation.
67:1016–1023. 1983.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Frey UH, Klaassen M, Ochsenfarth C, Murke
F, Thielmann M, Kottenberg E, Kleinbongard P, Klenke S, Engler A,
Heusch G, et al: Remote ischaemic preconditioning increases serum
extracellular vesicle concentrations with altered micro-RNA
signature in CABG patients. Acta Anaesthesiol Scand. 63:483–492.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li HL, Karwatowska-Prokopczuk E, Mutomba
M, Wu J, Karanewsky D, Valentino K, Engler RL and Gottlieb RA:
Pharmacology of caspase inhibitors in rabbit cardiomyocytes
subjected to metabolic inhibition and recovery. Antioxid Redox
Signal. 3:113–123. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Abassi Z, Armaly Z and Heyman SN:
Glycocalyx degradation in ischemia-reperfusion injury. Am J Pathol.
190:752–767. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gottlieb RA and Engler RL: Apoptosis in
myocardial ischemia-reperfusion. Ann N Y Acad Sci. 874:412–426.
1999.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Amar DN, Epshtein M and Korin N:
Endothelial cell activation in an embolic ischemia-reperfusion
injury microfluidic model. Micromachines (Basel).
10(857)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gonzalez AL, Ciocci PA, Fantinelli JC,
Rojano B, Schinella GR and Mosca SM: Isoespintanol, a monoterpene
isolated from oxandra cf xylopioides, ameliorates the myocardial
ischemia-reperfusion injury by AKT/PKCepsilon/eNOS-dependent
pathways. Naunyn Schmiedebergs Arch Pharmacol. 393:629–638.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li S, Baiyun R, Lv Z, Li J, Han D, Zhao W,
Yu L, Deng N, Liu Z and Zhang Z: Exploring the kidney hazard of
exposure to mercuric chloride in mice:Disorder of mitochondrial
dynamics induces oxidative stress and results in apoptosis.
Chemosphere. 234:822–829. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu B, Yu H, Baiyun R, Lu J, Li S, Bing Q,
Zhang X and Zhang Z: Protective effects of dietary luteolin against
mercuric chloride-induced lung injury in mice: Involvement of
AKT/Nrf2 and NF-κB pathways. Food Chem Toxicol. 113:296–302.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Baiyun R, Li S, Liu B, Lu J, Lv Y, Xu J,
Wu J, Li J, Lv Z and Zhang Z: Luteolin-mediated PI3K/AKT/Nrf2
signaling pathway ameliorates inorganic mercury-induced cardiac
injury. Ecotoxicol Environ Saf. 161:655–661. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhu J, Wang YF, Chai XM, Qian K, Zhang LW,
Peng P, Chen PM, Cao JF, Qin ZH, Sheng R and Xie H: Exogenous NADPH
ameliorates myocardial ischemia-reperfusion injury in rats through
activating AMPK/mTOR pathway. Acta Pharmacol Sin. 41:535–545.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xu XZ, Luo B, Xiao Y and Zheng WQ: Effects
of lncRNA MALAT1-mediated beta-catenin signaling pathway on
myocardial cell apoptosis in rats with myocardial
ischemia/reperfusion injury. Eur Rev Med Pharmacol Sci.
23:9557–9565. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lazou A, Iliodromitis EK, Cieslak D,
Voskarides K, Mousikos S, Bofilis E and Kremastinos DT: Ischemic
but not mechanical preconditioning attenuates ischemia/reperfusion
induced myocardial apoptosis in anaesthetized rabbits: The role of
Bcl-2 family proteins and ERK1/2. Apoptosis. 11:2195–2204.
2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang N, Minatoguchi S, Chen X, Uno Y, Arai
M, Lu C, Takemura G, Fujiwara T and Fujiwara H: Antidiabetic drug
miglitol inhibits myocardial apoptosis involving decreased hydroxyl
radical production and Bax expression in an ischaemia/reperfusion
rabbit heart. Br J Pharmacol. 142:983–990. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhou BZ, Zhang DH, Yu WM and Ning JZ:
Protective effect of cyclosporine A in the treatment of severe
hydronephrosis in a rabbit renal pelvic perfusion model. Turk J Med
Sci. 49:1590–1598. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sheng X, Chen M, Huang B, Liu J, Zhou L,
Bao M and Li S: Cardioprotective effects of low-level carotid
baroreceptor stimulation against myocardial ischemia-reperfusion
injury in canine model. J Interv Card Electrophysiol. 45:131–140.
2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ho MY, Wen MS, Yeh JK, Hsieh IC, Chen CC,
Hsieh MJ, Tsai ML, Yang CH, Wu VC, Hung KC, et al: Excessive irisin
increases oxidative stress and apoptosis in murine heart. Biochem
Biophys Res Commun. 503:2493–2498. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Abhari BA, McCarthy N, Agostinis P and
Fulda S: NF-κB contributes to Smac mimetic-conferred protection
from tunicamycin-induced apoptosis. Apoptosis. 24:269–277.
2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Toldo S, Mauro AG, Cutter Z and Abbate A:
Inflammasome, pyroptosis, and cytokines in myocardial
ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol.
315:H1553–H1568. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nichols TC: NF-kappaB and reperfusion
injury. Drug News Perspect. 17:99–104. 2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Qian Q, Cao X, Wang B, Qu Y, Qian Q, Sun Z
and Feng F: TNF-α-TNFR signal pathway inhibits autophagy and
promotes apoptosis of alveolar macrophages in coal worker's
pneumoconiosis. J Cell Physiol. 234:5953–5963. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen H, Zhang RQ, Wei XG, Ren XM and Gao
XQ: Mechanism of TLR-4/NF-κB pathway in myocardial ischemia
reperfusion injury of mouse. Asian Pac J Trop Med. 9:503–507.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Groot HE, Ali LA, van der Horst IC,
Schurer RA, van der Werf HW, Lipsic E, van Veldhuisen DJ, Karper JC
and van der Harst P: Plasma interleukin 6 levels are associated
with cardiac function after ST-elevation myocardial infarction.
Clin Res Cardiol. 108:612–621. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Moreira DM, da Silva RL, Vieira JL, Fattah
T, Lueneberg ME and Gottschall CA: Role of vascular inflammation in
coronary artery disease: Potential of anti-inflammatory drugs in
the prevention of atherothrombosis. Inflammation and
anti-inflammatory drugs in coronary artery disease. Am J Cardiovasc
Drugs. 15:1–11. 2015.PubMed/NCBI View Article : Google Scholar
|