Introduction
Renal ischemia-reperfusion injury (RIRI) refers to
renal function damage due to failed functional recovery after
ischemia-reperfusion during kidney operations such as
transplantation and kidney stone surgery. RIRI can sometimes be
irreversible (1). Renal
ischemia-reperfusion causes numerous pathophysiological changes
that results in a poor prognosis. No effective interventions are
available to deal with RIRI. Due to highly vascularized tissues,
unique vasculature and high oxygen consumption, RIRI can often lead
to renal failure and other diseases with high mortality rates
(2,3).
The mechanisms underlying RIRI are complex and have
not been fully elucidated. At present, it is generally hypothesized
that RIRI is associated with Ca2+ overload, production
of oxygen free radicals, activation of cell adhesion molecules,
involvement of chemokines and action of white blood cells (4,5).
Clinically, no targeted drug is currently available to treat RIRI,
although a number of drugs are being used with some curative
effect. These include caspase inhibitors, antioxidants and
P-selectin antagonists (6).
Dexmedetomidine (DEX) is a highly selective
α2-adrenergic agonist; it has anti-sympathetic, sedative and
analgesic effects. Studies have demonstrated that DEX can inhibit
the release of inflammatory factors and suppress the oxidative
stress response to protect organs (7-9).
With regards to RIRI, DEX has been revealed to alleviate
ischemia-reperfusion-induced RIRI in both animal and human
experiments, but its dose-response relationship and underlying
mechanism are still unclear (8).
Moreover, 4-phenylbutyric acid (4-PBA) is a low molecular weight
fatty acid (10). A number of
studies have confirmed that 4-PBA can be used as a molecular
chaperone to reverse the incorrect displacement or incorrect
aggregation of protein molecules to form a normal spatial structure
(11,12). This decreases the overload of
endoplasmic reticulum stress (ERS), suppresses the signal induction
of ERS and alleviates the tissue damage caused by ERS (10). Furthermore, 4-PBA decreases
hepatocyte apoptosis in hepatic ischemia-reperfusion injury and
alleviates cerebral and spinal cord ischemic injury (13). However, the mechanism by which 4-PBA
exerts its therapeutic effect on RIRI is largely unknown.
To the best of our knowledge the present study was
the first to use a hypoxia/reoxygenation (H/R) model of the human
renal tubular epithelial HK-2 cell line and mice and DEX as
positive control to study the therapeutic effect and possible
mechanism of 4-PBA on RIRI at the cellular and animal levels. The
findings could provide evidence to develop improved therapeutic
strategies for the disease.
Materials and methods
Experimental cells and animals
The human renal tubular epithelial HK-2 cell line
(SCSP-511) was purchased from The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences and cultured in 10-cm
adherent culture dishes containing DMEM with 10% FBS (Jiangsu
KeyGEN BioTECH Co., Ltd.) at 37˚C in a 5% CO2
atmosphere. A total of 20 male C57 mice (6-8-weeks; weight, 20-25
g) were obtained from SLEK Lab Animal Center of Shanghai (permit
no. Hunan 2019-0004). The animals were kept at a temperature of
20-26˚C, in 40-70% humidity and a 12/12 h light/dark cycle, with
access to filtered water and food ad libitum. Animal studies
were approved by The Animal Research Ethics Committee of The First
Affiliated Hospital of Nanchang University (approval no. 2019-067).
At the end of experiments, mice were euthanized with an overdose of
CO2 gas with a CO2 replacement rate of 20% of
the cage volume per min (5 l/min), according to the AVMA Guidelines
for Euthanasia (14).
Reagents and instruments
DEX injection (national permit H20130027) was
purchased from Chenxin Pharmaceutical Co., Ltd. 4-PBA (cat. no.
L15M6D1) was sourced from Shanghai YuanYe Biotechnology Co., Ltd.
The Annexin V-FITC/PI Apoptosis kit (cat. no. AP101-100-kit) was
purchased from Multisciences (Lianke) Biotech Co., Ltd. Cell lysis
buffer (cat. no. C1053) was obtained from Applygen Technologies,
Inc. BCA protein quantitative kit (cat. no. CW0014S), neutral resin
(cat. no. CW0136) and Diaminobenzidine Substrate kit (cat. no.
CW00125) were purchased from CoWin Biosciences. Mouse monoclonal
antibody against GAPDH (1:2,000; cat. no. TA-08), goat horseradish
peroxidase-conjugated antibody for mouse IgG (H + L; 1:2,000; cat.
no. ZB-2305) and goat horseradish peroxidase-conjugated antibody
for rabbit IgG (H + L; 1:2,000; cat. no. ZB-2301) were obtained
from OriGene Technologies, Inc. Rabbit monoclonal antibody against
glucose-regulated protein 78 (GRP78; 1:3,000; cat. no. 11587-1-ap)
was purchased from ProteinTech Group, Inc. Rabbit monoclonal
antibodies against eukaryotic translation initiation factor 2α
(eIF2α; 1:1,000; cat. no. AF6087), CypD (1:1,000; cat. no. DF3147),
cytochrome c (1:1,000; cat. no. AF0146), intercellular
adhesion molecule 1 (ICAM-1; 1:1,000; cat. no. AF6088) and vascular
cell adhesion molecule 1 (VCAM-1; 1:1,000; cat. no. DF6082) were
purchased from Affinity Biosciences, Ltd. The hematoxylin staining
kit (cat. no. AR1180-1) was obtained from Boster Biological
Technology. Desktop centrifuge Neofuge 13 was a product of Heal
Force. The ultra-high sensitivity chemiluminescence imaging system
ChemiDocXRS+ was from Bio-Rad Laboratories. The
ultra-high resolution small animal color Doppler ultrasound and
real-time imaging system Vevo®2100 was purchased from
VisualSonics, Inc. The CX41 microscope was obtained from Olympus
Corporation and the NovoCyte™ flow cytometer was obtained from
Agilent Technologies, Inc.
H/R cell model and drug
treatments
HK-2 cells were suspended in culture medium and
cultured overnight at 37˚C. The medium was refreshed every day
until the cells grew to confluence. For H/R modeling, HK-2 cells
were cultured to 80% confluence and hypoxia was induced by placing
the cell culture in a hypoxic chamber with a 1% O2 + 93%
N2 + 5% CO2 gas mixture in DMEM without serum
at 37˚C for 24 h. After the hypoxic treatment, the cells were
reoxygenated in normal oxygen at 37˚C for 3 h and collected for
further analysis via centrifugation at 500 x g for 10 min at room
temperature. DEX (0.01 nM) and 4-PBA (5 mM) were added to the cells
at 37˚C 1 h prior to modeling. Untreated cells were used as the
control.
Apoptosis analysis
Apoptosis was detected using flow cytometry using
Annexin V-FITC/PI Apoptosis kit. A total of 1-3x106
cells were pelleted and washed twice with 1 ml PBS, resuspended in
300 µl pre-chilled binding buffer, and then 3 µl annexin V-FITC and
5 µl PI-PE were added to cells. After gentle mixing, the cells were
incubated at room temperature in the dark for 10 min, then loaded
with 200 µl precooled binding buffer into the cytometer for
analysis according to the manufacturer's instructions (NovoCyte
Flow Cytometer System; Agilent Technologies, Inc.) using
NovoExpress (v.6.2; Agilent Technologies, Inc.).
Western blotting
Cells were lysed in lysis buffer on ice bath for 30
min and centrifuged at 4˚C at 500 x g for 10 min. Proteins in the
supernatant were quantified using a BCA kit according to the
supplier's protocols. After denaturing, the proteins were separated
on 12% gels using SDS-PAGE for 1-2 h and transferred to PVDF
membranes for western blotting analysis. The membranes were blocked
with 5% nonfat milk powder at room temperature for 2 h and then
incubated with antibodies against eIF2α, CypD, cytochrome c,
ICAM-1, VCAM-1 and GAPDH (used as internal reference) at 4˚C
overnight, followed by incubation and with the secondary goat
horseradish peroxidase-conjugated antibodies for mouse and rabbit
IgG at room temperature for 1-2 h. After visualization using
enhanced chemiluminescence solution (cat. no. 34077; Thermo Fisher
Scientific, Inc.), immunoreactive bands were captured using the gel
imaging system. The gray value of each band was analyzed by
Quantity One software (version 4.6; Bio-Rad Laboratories,
Inc.).
H/R animal model and drug
treatment
After fasting for 16-24 h, mice were anaesthetized
using an intraperitoneal injection of 100 mg/kg ketamine (Jiangsu
Hengrui Medicine Co., Ltd.) and 10 mg/kg xylazine (Hubei Xinmingtai
Chemical Co., Ltd.). An incision along the abdominal midline was
made to expose bilateral renal pedicles. For the sham group, the
right kidney was removed, and the abdominal cavity was closed in 45
min. For the IR model, the right kidney was removed, the left renal
pedicle was clamped with a non-invasive artery clamp for 45 min and
then the clamp was released to re-perfuse for 24 h. For drug
treatments, DEX (10 µg/kg) or 4-PBA (400 mg/kg) was administered by
intraperitoneal injection 30 min before the artery clamping. If the
color of the kidney turned from purple/black to red and the animal
was awake with 3 h of surgery, the re-perfusion was considered
successful. At 24 h after successful modeling, the renal arterial
resistance index (RRI) was determined using high-resolution color
Doppler ultrasound. After the measurement, mice were euthanized as
aforementioned and kidney tissues were collected to measure the
weight and size using an electronic balance and digital Vernier
caliper.
Immunohistochemical assay
Tissues fixed in 4% paraformaldehyde at 4˚C for 12 h
were embedded in paraffin, sectioned at 10 µm thickness, were baked
at 65˚C for 2 h, soaked in xylene for 10 min and rehydrated using a
descending ethanol gradient series. After antigen retrieval with
citrate buffer (cat. no. C1032; Beijing Solarbio Science &
Technology Co., Ltd.) at 100˚C for 20 min and washing with PBS, the
slices were-incubated with diluted antibodies against ICAM-1 and
VCAM-1 overnight at 4˚C and secondary antibody (anti-rabbit IgG
labeled horseradish peroxidase) at room temperature for 10 min. The
diaminobenzidine and hematoxylin chromogen (Dako; Agilent
Technologies, Inc.) were used to stain the slices at 25˚C for 1 h
to visualize immunoreactive bands. The sections were subsequently
mounted with resin (cat. no. CW0136; CoWin Biosciences and examined
under a light microscope and the intensity of staining was
relatively qualified using ImageJ software (v1.4; National
Institutes of Health) to calculate the positive area.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean obtained from at least three independent experiments.
Statistical comparisons between experimental and control groups
were assessed using one-way ANOVA with Tukey's post hoc test. The
data were analyzed by SPSS version 11.5 for Windows (SPSS Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
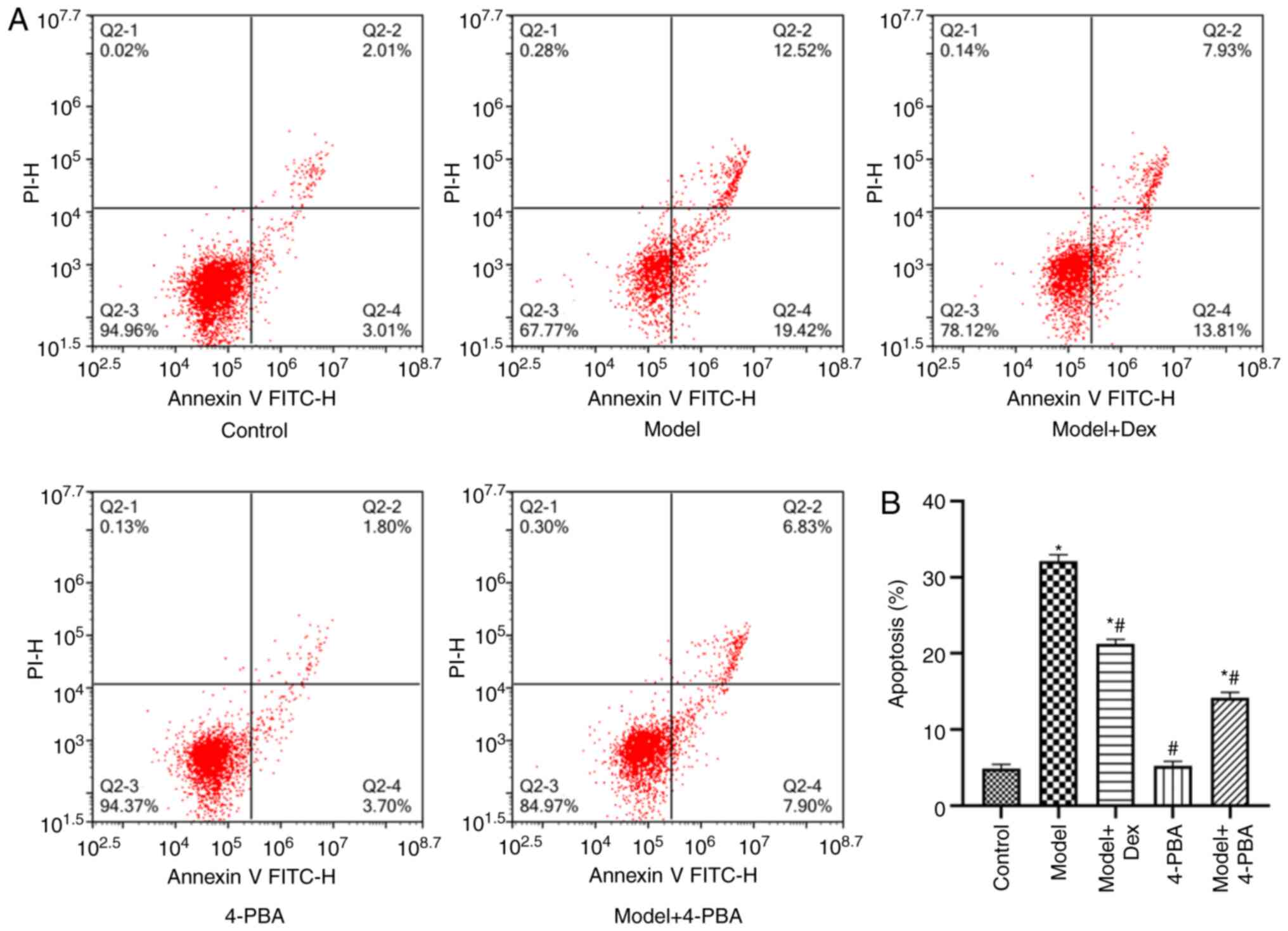
DEX and 4-PBA decrease apoptosis
Flow cytometry studies revealed that compared with
that in normal HK-2 cells, apoptosis was significantly increased in
the model H/R HK-2 cells (P<0.05); furthermore, DEX
significantly lowered apoptosis in the H/R HK-2 cells (P<0.05;
Fig. 1A and B). 4-PBA did not significantly impact the
apoptosis of HK-2 cells, but significantly decreased the apoptosis
of H/R HK-2 cells compared with the model only and control groups
(P<0.05; Fig. 1B). The apoptosis
rate of the model + 4-PBA group was slightly but non-significantly
(P>0.05) lower compared with that of model + DEX group,
indicating that 4-PBA had a significant inhibitory effect on the
apoptosis of HK-2 cells induced by hypoxia reoxygenation and the
effect was equivalent to that of DEX. The data suggested that
combined treatment of DEX and 4-PBA reduced apoptosis-induced by
H/R treatment.
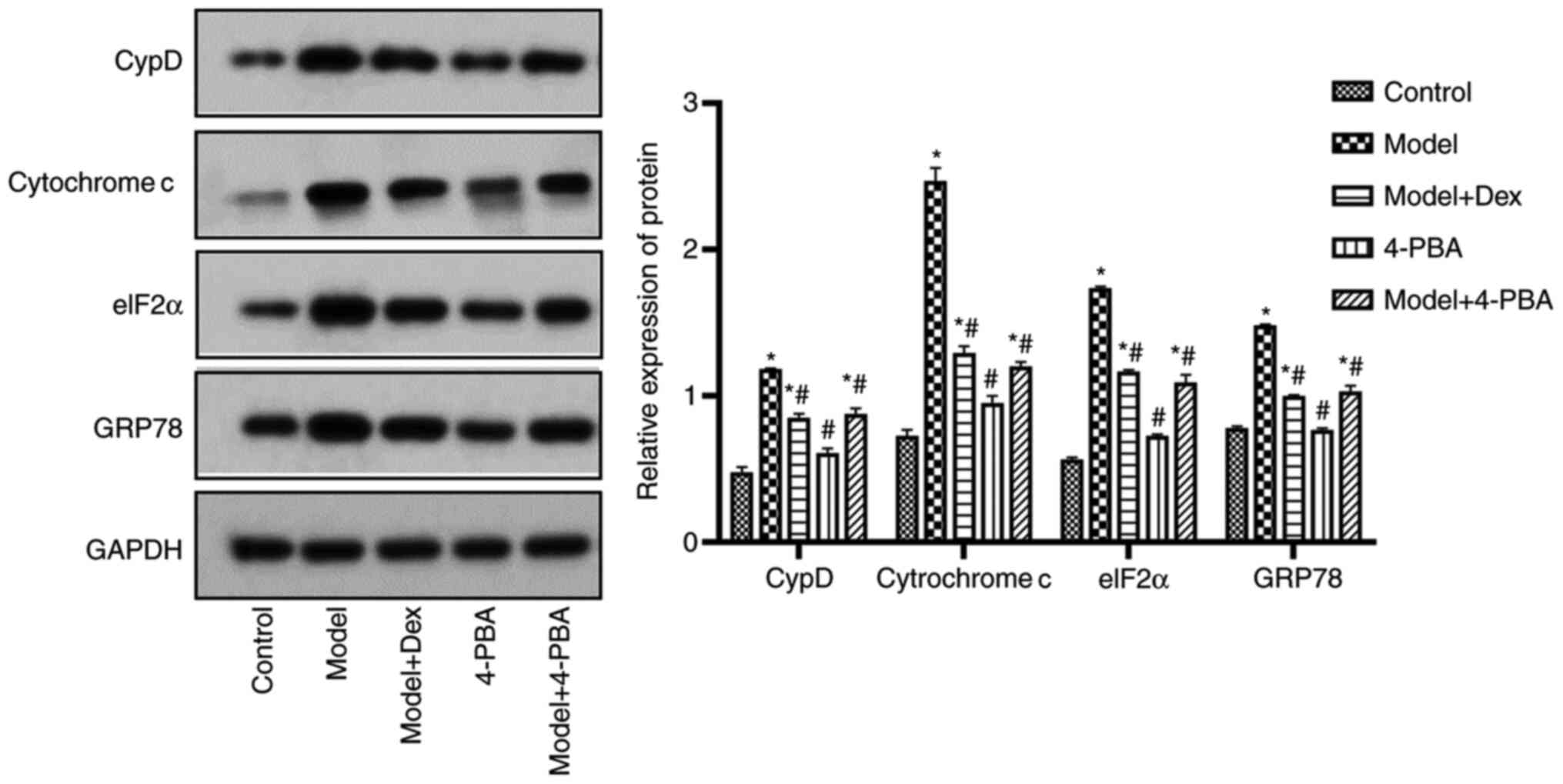
DEX and 4-PBA downregulate the
expression levels of CypD, cytochrome c, eIF2α and GRP78
Western blotting was performed to assess the impact
of DEX and 4-PBA on CypD, cytochrome c, eIF2α and GRP78
expression levels in H/R cells as presented in Fig. 2. The results indicated that 4-PBA
did not significantly change the expression levels of these
proteins compared with the control. However, all of these proteins
were significantly upregulated after H/R modeling (P<0.05;
Fig. 2). DEX and 4-PBA
significantly downregulated the expression levels of these proteins
in H/R HK-2 cells compared with the model group (P<0.05;
Fig. 2). The data indicated that
combined treatment of DEX and 4-PBA reduced the expression of CypD,
cytochrome c, eIF2α and GRP78 after H/R treatment.
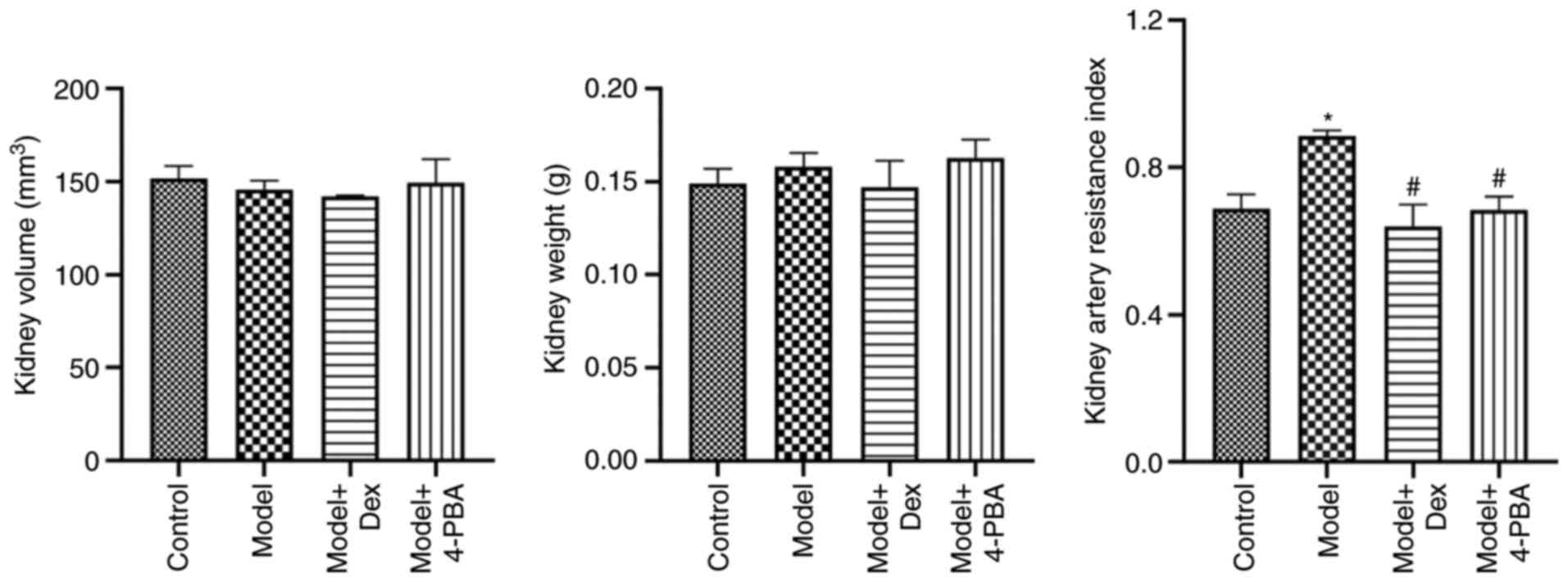
DEX and 4-PBA alleviate
ischemia-reperfusion injury (IRI)
At the time of measurement, the renal volume and
weight were not different among all groups (Fig. 3). The RRI by color Doppler
ultrasound indicated that RRI was significantly increased following
ischemia-reperfusion modeling compared with that in the control
(P<0.05). However, it was decreased significantly after DEX and
4-PBA treatment (P<0.05; Fig.
3). Compared with that of the DEX-treated group, the RRI of the
4-PBA-treated group was similar and the difference between the
groups was not significant (P>0.05), which indicated that the
treatments produced similar effects.
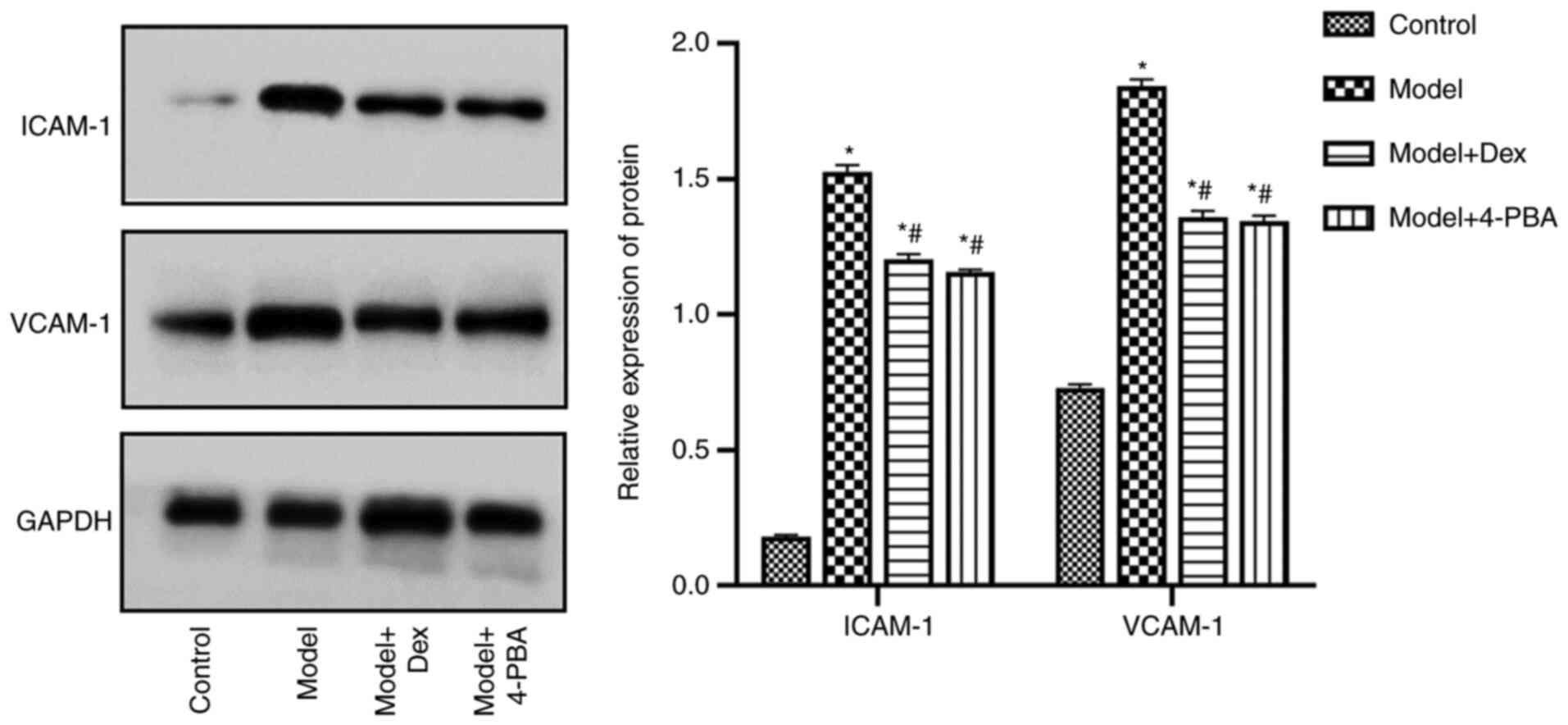
DEX and 4-PBA downregulate the
expression levels of ICAM-1 and vascular adhesion molecule
(VCAM)-1
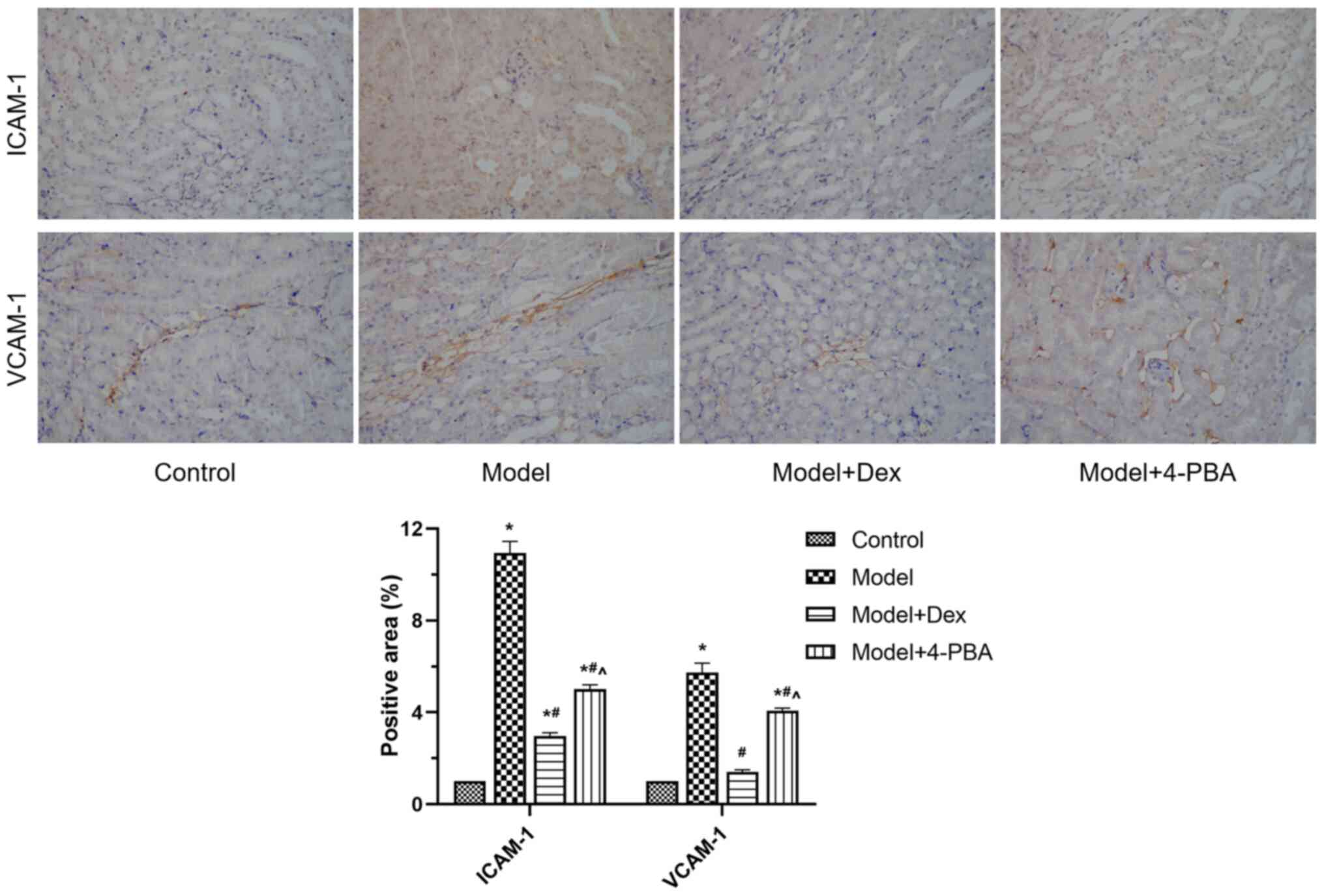
Western blotting and immunohistochemistry were used
to examine the expression of ICAM-1 and VCAM-1 in the kidney
tissues. Western blotting demonstrated that the expression levels
of ICAM-1 and VCAM-1 were significantly increased in the model
compared with those in the control, and that they were
significantly decreased after DEX and 4-PBA treatment (P<0.05;
Fig. 4). The expression levels were
also significantly different between model + 4-PBA and model + DEX
groups in immunohistochemistry assessment of the two proteins
(P<0.05; Fig. 5). These findings
indicated that ICAM-1 and VCAM-1 were downregulated following DEX
and 4-PBA treatment, compared with the model group.
Discussion
At present, the main measures to decrease RIRI and
protect renal function include renal hypothermia, a short surgical
duration and selective blocking of renal artery branches (15). However, these measures are often
insufficiently effective, particularly in cases with complex
conditions, where it is not possible to avoid a long operation time
and the irreversible damage caused by long-term blocking of renal
artery branches (15). The manner
in which to alleviate and decrease RIRI is still a clinical
challenge. The purpose of the present study was to explore the
effect of 4-PBA on RIRI and its possible mechanism. The results
indicated that 4-PBA could significantly decrease H/R-induced
apoptosis in HK-2 cells and decrease the renal artery blood flow
resistance in RIRI mice. In addition, 4-PBA could significantly
decrease the expression of ICAM-1 and VCAM-1 in the kidney of RIRI
mice, leading to a significant alleviation of RIRI.
CypD is a component protein of the mitochondrial
permeability transition pore (16).
Mitochondria are the main sites to produce reactive oxygen species
and also the target of oxidative damage. One of the mitochondrial
responses to oxidative stress and thiols is the binding of
adenonucleotide transferase and CypD (16). Studies have revealed that depletion
of CypD in cardiomyocytes results in significantly less
susceptibility to cell death induced by oxidative stress and
calcium overload, and decreased synthesis of apoptosis-related
proteins (16,17). Cytochrome c is a major
component in the respiratory chain and plays a notable role in
redox and energy metabolism. At the same time, cytochrome c
is the key trigger that initiates mitochondrial apoptosis (18). Cytochrome c can activate
downstream caspase 3 through a cascade reaction, leading to
apoptosis (19). EIF2α is a key
regulatory protein in the process of translation initiation, which
is largely dependent on its phosphorylation level (20). PERK is a transmembrane protein
kinase in the endoplasmic reticulum (ER), which can phosphorylate
eIF2α. Under ERS, phosphorylated PERK inactivates eIF2α by
phosphorylating the α-subunit of eIF2, blocking the transformation
from eIF2-GDP to eIF2-GTP (21).
This affects the recruitment and initiation of initiator
methionyl-transfer RNA and the 40S ribosomal subunit, resulting in
the suspension of protein synthesis to decrease protein load in the
ER and to restore cell homeostasis (22,23).
GRP78 is a notable molecular chaperone located in the ER, which
plays a role in protein folding, transport and ERS response
(24). As an ERS marker, GRP78 can
bind to ERS-activated pro-apoptotic receptors to inhibit their
signal transduction, thus protecting cells (25).
The present study revealed that 4-PBA could
significantly decrease the expression of CypD, cytochrome c,
eIF2α and GRP78. In addition, H/R-induced apoptosis in HK-2 cells
was decreased. We hypothesized that downregulation of these genes
is likely due to 4-PBA suppressing the release of H/R-induced
inflammatory factors. However, further studies are needed to
elucidate the possible molecular mechanisms.
ICAM-1 and VCAM-1 are members of the immunoglobulin
superfamily. Under the stimulation of inflammatory cytokines and
bacterial endotoxins, they are expressed on the surface of
activated endothelial cells to mediate the adhesion and exudation
of leukocytes; they also have close relationship with the
inflammatory mechanism of IRI (26,27).
The animal experiments of the present study demonstrated that 4-PBA
could significantly decrease the expression of ICAM-1 and VCAM-1.
In the IRI lesion, inflammatory cytokines are released locally,
resulting in increased expression of ICAM-1 and VCAM-1, and
increased cell adhesion. As a consequence, a large number of
leukocytes adhere to vascular endothelial cells, resulting in
obstruction of blood vessels and decreased blood flow (26,27).
Meanwhile, leukocytes outside blood vessels may produce free
radicals, proteolytic enzymes and other toxic substances, causing
vascular injury and bleeding (28,29).
Taken together, the evidence suggests that 4-PBA could downregulate
the expression of CypD, cytochrome c, eIF2α and GRP78 to
decrease cellular oxygen free radicals, leading to a decrease in
apoptosis and ERS. As a consequence, IRI and intercellular adhesion
are decreased to healthy levels; moreover, the expression levels of
ICAM-1 and VCAM-1 are reversed by downregulation of the expression
of CypD, cytochrome c, eIF2α and GRP78 proteins to healthy
levels.
There are limitations to the present study. For
example, the renal function of mice was not assessed using
hematoxylin-eosin or Periodic acid-Schiff staining to detect
pathological changes in the kidney, and the ERS was addressed using
only two indicators, eIF2α and GRP78. In further studies, more
indicators and parameters, including pathological examination,
should be applied for an improved understanding of changes in renal
function following treatment.
In conclusion, to the best of our knowledge, the
present study has demonstrated that 4-PBA can decrease RIRI in HK-2
cells and mice for the first time. The therapeutic effects were
mediated via downregulation of CypD, cytochrome c, eIF2α,
GRP78, ICAM-1 and VCAM-1, and were validated with human cell and
animal studies.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by Jiangxi Provincial
Department of Education (grant no. 180061).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW and HG designed the study. XW, YZ and KW
performed the experiments and the data analysis. XW and KW confirm
the authenticity of all the raw data. XW, YZ and HG drafted the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments and animal care were
conducted in accordance with the criteria of the Laboratory Animals
Welfare Act and the Guide for the Care and Use of Laboratory
Animals (30) provided by the
Animal Research Ethics Committee of the First Affiliated Hospital
of Nanchang University. Animal studies were approved by The Animal
Research Ethics Committee of The First Affiliated Hospital of
Nanchang University (approval no. 2019-067).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kusch A, Hoff U, Bubalo G, Zhu Y, Fechner
M, Schmidt-Ullrich R, Marko L, Müller DN, Schmidt-Ott KM, Gürgen D,
et al: Novel signalling mechanisms and targets in renal ischaemia
and reperfusion injury. Acta Physiol (Oxf). 208:25–40.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
de Vries DK, Khairoun M, Lindeman JH,
Bajema IM, de Heer E, Roest M, van Zonneveld AJ, van Kooten C,
Rabelink TJ, Schaapherder AF and Reinders ME: Renal
ischemia-reperfusion induces release of angiopoietin-2 from human
grafts of living and deceased donors. Transplantation. 96:282–289.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Perico N, Cattaneo D, Sayegh MH and
Remuzzi G: Delayed graft function in kidney transplantation.
Lancet. 364:1814–1827. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kalyanaraman B: Teaching the basics of
redox biology to medical and graduate students: Oxidants,
antioxidants and disease mechanisms. Redox Biol. 1:244–257.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang Y, He J, Pei X and Zhao W: Systematic
review and meta-analysis of mesenchymal stem/stromal cells therapy
for impaired renal function in small animal models. Nephrology
(Carlton). 18:201–208. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Song JY, Meng LQ and Li XM: Therapeutic
application and prospect of Astragalus membranaceus and Angelica
sinensis in treating renal microvascular lesions. Zhongguo Zhong Xi
Yi Jie He Za Zhi. 28:859–861. 2008.PubMed/NCBI(In Chinese).
|
|
7
|
Afonso J and Reis F: Dexmedetomidine:
Current role in anesthesia and intensive care. Rev Bras Anestesiol.
62:118–133. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shukry M and Miller JA: Update on
dexmedetomidine: Use in nonintubated patients requiring sedation
for surgical procedures. Ther Clin Risk Manag. 6:111–121.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Patil RH, Naveen Kumar M, Kiran Kumar KM,
Nagesh R, Kavya K, Babu RL, Ramesh GT and Chidananda Sharma S:
Dexamethasone inhibits inflammatory response via down regulation of
AP-1 transcription factor in human lung epithelial cells. Gene.
645:85–94. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Luo T, Chen B and Wang X: 4-PBA prevents
pressure overload-induced myocardial hypertrophy and interstitial
fibrosis by attenuating endoplasmic reticulum stress. Chem Biol
Interact. 242:99–106. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang Z, Zheng S, Gu Y, Zhou L, Lin B and
Liu W: 4-PBA enhances autophagy by inhibiting endoplasmic reticulum
stress in recombinant human beta nerve growth factor-induced PC12
cells after mechanical injury via PI3K/AKT/mTOR signaling pathway.
World Neurosurg. 138:e659–e664. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zeng M, Sang W, Chen S, Chen R, Zhang H,
Xue F, Li Z, Liu Y, Gong Y, Zhang H and Kong X: 4-PBA inhibits
LPS-induced inflammation through regulating ER stress and autophagy
in acute lung injury models. Toxicol Lett. 271:26–37.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bhardwaj R, Tandon C, Dhawan DK and Kaur
T: Effect of endoplasmic reticulum stress inhibition on
hyperoxaluria-induced oxidative stress: Influence on cellular ROS
sources. World J Urol. 35:1955–1965. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Association AVM: AVMA guidelines for the
euthanasia of animals. 2013.
|
|
15
|
Ng CK, Gill IS, Patil MB, Hung AJ, Berger
AK, de Castro Abreu AL, Nakamoto M, Eisenberg MS, Ukimura O,
Thangathurai D, et al: Anatomic renal artery branch microdissection
to facilitate zero-ischemia partial nephrectomy. Eur Urol.
61:67–74. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ye F, Li X, Liu Y, Jiang A, Li X, Yang L,
Chang W, Yuan J and Chen J: CypD deficiency confers neuroprotection
against mitochondrial abnormality caused by lead in SH-SY5Y cell.
Toxicol Lett. 323:25–34. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ramachandran A, Lebofsky M, Baines CP,
Lemasters JJ and Jaeschke H: Cyclophilin D deficiency protects
against acetaminophen-induced oxidant stress and liver injury. Free
Radic Res. 45:156–164. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu K, Shu D, Song N, Gai Z, Yuan Y, Li J,
Li M, Guo S, Peng J and Hong H: The role of cytochrome c on
apoptosis induced by Anagrapha falcifera multiple nuclear
polyhedrosis virus in insect Spodoptera litura cells. PLoS One.
7(e40877)2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
McManus MJ and Franklin JL: Dissociation
of JNK activation from elevated levels of reactive oxygen species,
cytochrome c release, and cell death in NGF-Deprived sympathetic
neurons. Mol Neurobiol. 55:382–389. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Palam LR, Baird TD and Wek RC:
Phosphorylation of eIF2 facilitates ribosomal bypass of an
inhibitory upstream ORF to enhance CHOP translation. J Biol Chem.
286:10939–10949. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dias-Teixeira KL, Calegari-Silva TC,
Medina JM, Vivarini ÁC, Cavalcanti Á, Teteo N, Santana AKM, Real F,
Gomes CM, Pereira RMS, et al: Emerging role for the PERK/eIF2α/ATF4
in human cutaneous leishmaniasis. Sci Rep. 7(17074)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Page AB, Owen CR, Kumar R, Miller JM,
Rafols JA, White BC, DeGracia DJ and Krause GS: Persistent
eIF2alpha(P) is colocalized with cytoplasmic cytochrome c in
vulnerable hippocampal neurons after 4 hours of reperfusion
following 10-minute complete brain ischemia. Acta Neuropathol.
106:8–16. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chesnokova E, Bal N and Kolosov P: Kinases
of eIF2a switch translation of mRNA subset during neuronal
plasticity. Int J Mol Sci. 18(2213)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Teng J, Liu M, Su Y, Li K, Sui N, Wang S,
Li L, Sun Y and Wang Y: Down-regulation of GRP78 alleviates
lipopolysaccharide-induced acute kidney injury. Int Urol Nephrol.
50:2099–2107. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Aksoy MO, Kim V, Cornwell WD, Rogers TJ,
Kosmider B, Bahmed K, Barrero C, Merali S, Shetty N and Kelsen SG:
Secretion of the endoplasmic reticulum stress protein, GRP78, into
the BALF is increased in cigarette smokers. Respir Res.
18(78)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Small DL, Morley P and Buchan AM: Biology
of ischemic cerebral cell death. Prog Cardiovasc Dis. 42:185–207.
1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chaves KC, Peron JP, Chammas R, Turaça LT,
Pesquero JB, Braga MS, Foguer K, Schor N and Bellini MH: Endostatin
gene therapy stimulates upregulation of ICAM-1 and VCAM-1 in a
metastatic renal cell carcinoma model. Cancer Gene Ther.
19:558–565. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xing H, Sun S, Mei Y and Herman D: The
protective effect of rosuvastatin on ischemic brain injury and its
mechanism. J Huazhong Univ Sci Technolog Med Sci. 26:667–669.
2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shen A, Yang J, Gu Y, Zhou D, Sun L, Qin
Y, Chen J, Wang P, Xiao F, Zhang L and Cheng C:
Lipopolysaccharide-evoked activation of p38 and JNK leads to an
increase in ICAM-1 expression in Schwann cells of sciatic nerves.
FEBS J. 275:4343–4353. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cardon AD, Bailey MR and Bennett BT: The
animal welfare Act: From enactment to enforcement. J Am Assoc Lab
Anim Sci. 51:301–305. 2012.PubMed/NCBI
|