Introduction
Primary nephrotic syndrome (PNS) is one of the most
common kidney diseases in children. It is induced by various
causes, including weak resistance and weakened immune function,
leading to the increased permeability of the glomerular filtration
membrane and the loss of a large amount of protein in the urine,
consequently leading to a clinical syndrome with a series of
pathophysiological changes (1). At
present, the pathogenesis of PNS remains unknown. Adriamycin (ADR)
nephropathy, which is very similar to early stage minimal change
disease (MCD) and late stage focal segmental glomerulosclerosis, is
a classic animal model of nephrotic syndrome (NS) (2-5).
The P2X7 receptor (P2X7R), which belongs to the P2X
family, is an ATP-gated ion channel that is involved in the
development of numerous diseases, such as HIV infection (6). Compared with other family members,
P2X7R has a specific structure, which has attracted marked
attention (7). P2X7R is involved
in the inflammatory response through numerous signaling pathways,
promoting cellular injury and apoptosis, thus leading to tissue
damage (6). At present,
accumulating evidence from various animal models has shown that
P2X7R participates in the onset and development of kidney disease
through the P2X7R/NLRP3 pathway, which could lead to the downstream
release of IL-1β and IL-18 to play a role in inflammation (8-11).
P2X7R promotes apoptosis in a variety of ways. Previous studies on
several kidney diseases have revealed that P2X7R could lead to
mesangial cell and podocyte apoptosis, which may participate in the
pathogenesis of diseases (12,13).
Furthermore, P2X7R is important for lipid storage and metabolism in
the body (14). Oxidized (ox)-low
density lipoprotein (LDL) has been found to upregulate the
expression of P2X7R in atherosclerosis (15). In addition, ox-LDL is involved in
the onset and development of ADR nephropathy (16), and it has been confirmed that the
expression of P2X7R and ox-LDL is increased in the glomeruli of
children with PNS (Zhu et al, unpublished data). However,
whether P2X7R is involved in the onset and development of ADR
nephropathy has not been reported locally or abroad, to the best of
our knowledge.
In the present study, a mouse model of ADR
nephropathy was established to explore the pathogenesis of PNS, to
investigate the role of P2X7R in ADR-induced nephropathy, and to
determine whether P2X7R inhibition could ameliorate renal injury in
mice with ADR nephropathy.
Materials and methods
Experimental animals
Male BABL/c mice (age, 5 weeks; n=46) with an
average body weight of 17.96 g (Vital River Laboratory Animal
Technology Co. Ltd.) were kept under standard conditions (constant
temperature 21±2˚C, humidity 60±10%, light/dark cycle 12 h) and
received free water and food and standard pellet chow. All animal
procedures were performed according to the guidelines for the care
and use of laboratory animals approved by Shandong Provincial
Hospital. Mice were anesthetized by an intraperitoneal injection of
100 mg/kg sodium pentobarbital. The use of large-dose injections
results in the inhibited breathing of mice. Death was confirmed
when mice did not respond to stimuli, as their hind limbs were
clamped with the hemostatic forceps.
Sample collection
Mice were randomly divided into the following
groups: Normal control group (NC group; n=5), ADR nephropathy group
(ADR group; n=5), ADR + A438079 (100 µmol/kg; Merck KGaA) group
(ADR + A100 group; n=5), ADR + A438079 (200 µmol/kg) group (ADR +
A200 group; n=5), ADR + A438079 (300 µmol/kg) group (ADR + A300
group, n=5) and A438079 (300 µmol/kg) group (A300 group; n=5). The
ADR group was induced by a single, slow intravenous injection of
ADR (10.5 mg/kg body weight, diluted in 0.9% saline) into the tail
vein. After 1 week, mice with massive proteinuria (24-h urinary
protein ratio ≥50 mg/kg) were considered to have ADR nephropathy.
The other groups without ADR received the same dose of saline via
the tail vein. The P2X7R antagonist A438079 (100, 200 and 300
µmol/kg) was injected intraperitoneally 6 h before establishing the
models, after which A438079 (100, 200 and 300 µmol/kg) was
administered every day for 1 week.
After 1 week, all mice were individually placed in
metabolic cages for 24-h urine collection. Following urine
collection, all mice were anesthetized and euthanized by
intraperitoneal injection of 100 mg/kg sodium pentobarbital. Blood
was collected from the heart, and the kidneys were harvested. All
experiments were performed with the approval of the Animal Ethics
Committee of Shandong Provincial Hospital (Jinan, China; approval
no. 2020-107).
Urinary protein and blood sample
analysis
The 24-h urinary protein ratio was detected using
the Coomassie brilliant blue method. Serum albumin (ALB) and total
cholesterol (TC) were evaluated using an Automatic Biochemical
analyzer (AU5400; Olympus Corporation). All specimens were
processed and analyzed according to the manufacturer's
protocol.
ELISA
Sandwich ELISA was used to detect the levels of
IL-1β (cat. 70-EK201B/3-96, Multi sciences, Wuhan, China) and IL-18
(cat. 70-EK218-96, Multi sciences, Wuhan, China) in kidney tissue
according to the manufacturer's protocol (Multi Sciences Biotech,
Co., Ltd.). Briefly, a section of fresh kidney was collected,
placed in PBS with 0.05% Tween-20 and mixed using ultrasound for
100 sec. Subsequently, the kidney supernatant was harvested.
Standard proteins and samples were subsequently diluted with
standard diluent buffer supplied in the aforementioned kit, after
which 100 µl of standards, controls and diluted samples was added
to the appropriate microtiter 96-wells. The wells were covered and
incubated for 90 min at 37˚C in an incubator. Biotin-conjugated
detection antibody (100 µl) was added into each well after washing
with PBS, incubated for 1 h at 37˚C and washed again with PBS.
Anti-rabbit IgG-HRP (100 µl) was then added to each well, after
which the wells were covered and incubated for 30 min at 37˚C.
Following another PBS wash step, stabilized chromogen (90 µl) was
added to each well and incubated for 15 min at 37˚C in the dark.
Finally, stop solution was added to each well, and the absorbance
of each well at 450 nm was recorded by a microtiter plate reader. A
smooth standard curve was constructed according to standard protein
optical density values. Unknown sample protein concentrations were
derived from this standard curve.
Immunohistochemical assay
The right kidney was fixed at 4˚C for 24 h in 10%
formaldehyde immediately after the mice were sacrificed, after
which it was dehydrated in graded alcohol and then embedded in
paraffin. Paraffin sections (5 µm) were deparaffnized in an
environmentally-safe cleaning agent, such as xylene, rehydrated in
graded alcohol and then washed in PBS (pH 7.4). Antigen retrieval
was performed by boiling the tissue sections in water for 15 min in
0.01 mol/l sodium citrate buffer (pH 6.0) at 100˚C and then cooling
at 25˚C for 2 h. Tissue sections were treated with 3%
H2O2 for 30 min at 37˚C to quench endogenous
peroxide activity. After blocking the sections with 10% goat serum
(Beijing Solarbio Science & Technology Co., Ltd.) for 1 h at
37˚C, the sections were incubated with rabbit anti-mouse P2X7R
antibody (pAb; 1:300; cat. no. ab109054; Abcam), C-X-C motif
chemokine ligand 16 (CXCL16; 1:100; cat. no. bs-1441R; BIOSS),
ox-LDL (1:100; cat. no. bsm-1698M; BIOSS), Bax (1:200; cat. no.
bsm-52316R; BIOSS), caspase-3 (1:200; cat. no. bsm-33277M; BIOSS)
and NLRP3 (1:200; cat. no. ab270449; Abcam) at 4˚C overnight,
followed by incubation with HRP-conjugated secondary goat
anti-rabbit (1:500; cat. no. ab6721; Abcam)/mouse (1:500; cat. no.
ab6789; Abcam) antibody at 37˚C for 30 min. Sections were developed
with DAB reagent, after which the nuclei were counterstained with
Hematoxylin for 1 min at room temperature. The primary antibody was
substituted for PBS as a negative control, while the positive
control was verified by confirming positively-stained tissue
specimens. Images were captured using the white light of an ECLIPSE
Ti scanning microscope (Nikon Corporation) and analyzed by ImageJ
software (version, 6.0; National Institutes of Health) to calculate
%Area.
TUNEL assay
The right kidney was fixed at 4˚C for 24 h in 10%
formaldehyde immediately after the mice were sacrificed, after
which it was dehydrated in graded alcohol and then embedded in
paraffin. Paraffin sections (5 µm) were deparaffnized in an
environmentally-safe cleaning agent, such as xylene, rehydrated in
graded alcohol and then washed in PBS (pH 7.4). Antigen retrieval
was performed by boiling the tissue sections in water for 15 min in
0.01 mol/l sodium citrate buffer (pH 6.0) at 100˚C and then cooling
at 25˚C for 2 h. Tissue sections were treated with 3%
H2O2 for 10 min at 37˚C to quench endogenous
peroxide activity. Then added TdT enzyme reaction solution to the
tissue, and reacted for 1 h at 37˚C in the dark. Then add l
Streptavidin-HRP working solution, and react for 30 min in the dark
at 37˚C. Sections were developed with DAB reagent, after which the
nuclei were counterstained with Hematoxylin for 1 min at room
temperature. Images were captured using the white light of an
ECLIPSE Ti scanning microscope (Nikon Corporation) and analyzed by
ImageJ software (version 6.0; National Institutes of Health) to
calculate the dark brown precipitated cells.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
(IBM Corp.), and the data were expressed as the mean ± SD. The
means between multiple groups were compared using one-way ANOVA.
For the comparison of two groups, an independent sample t-test was
used. P<0.05 was considered to indicate a statistically
significant difference.
Results
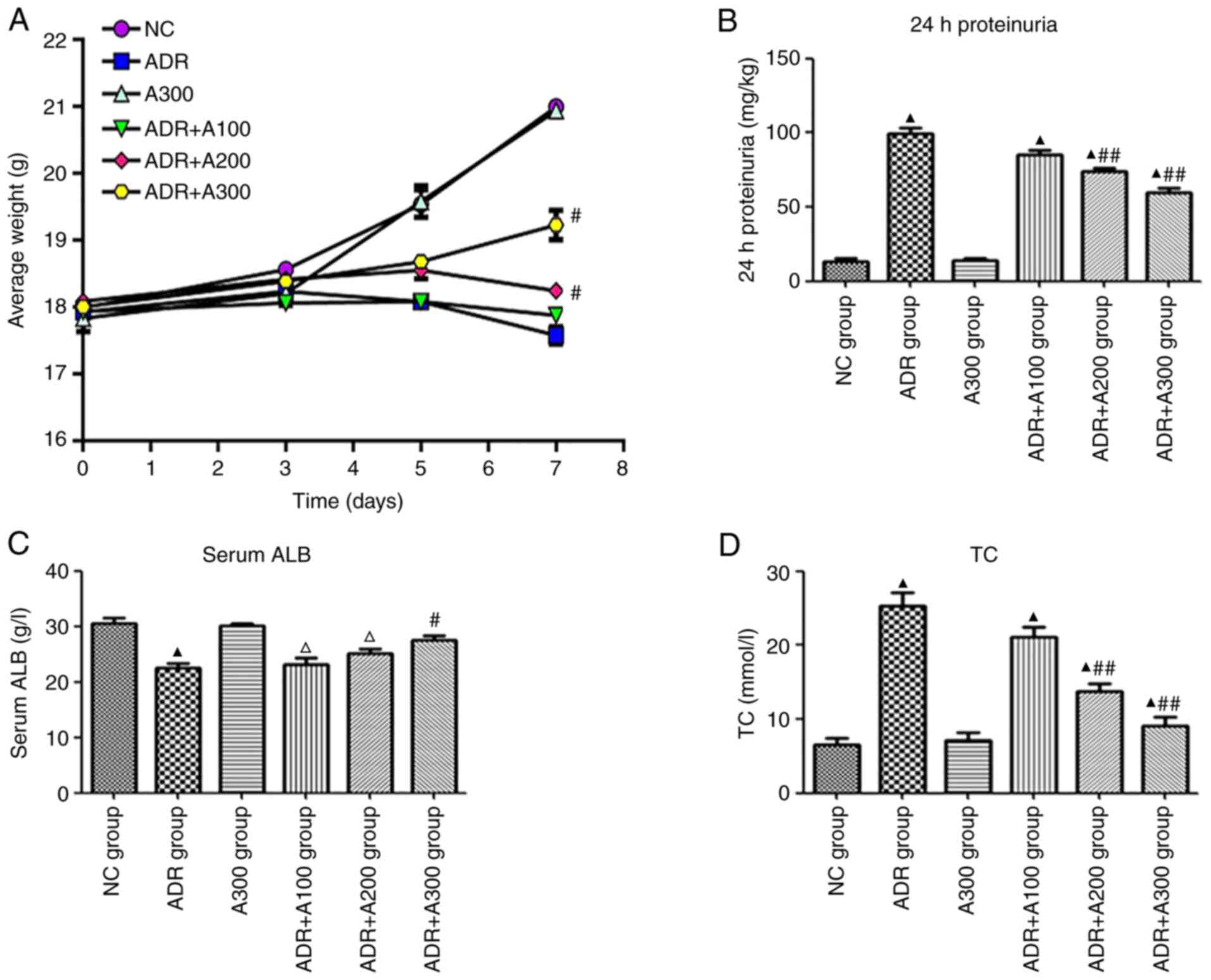
Effect of A438079 on weight, urinary
protein, serum ALB, and TC
As revealed in Fig.
1, compared with the NC group, the weight of mice in the ADR
group gradually decreased. Additionally, compared with the ADR
group, weight loss was significantly reduced in both the ADR + A200
and ADR + A300 groups, while there was no significant difference in
the ADR + A100 group. The ADR group developed significant
proteinuria, hypoalbuminemia and hyperlipidemia, while in the ADR +
A438079 groups, the majority of changes were alleviated to varying
degrees depending on the concentration of A438079. The level of 24
h-proteinuria in the ADR and ADR + A438079 groups was significantly
higher compared with the NC group (Fig. 1B; P<0.01), and the level of 24
h-proteinuria in the ADR + A438079 200 and 300 µmol/kg groups was
significantly lower than that in the ADR group (Fig. 1B; P<0.01). However, no
statistically significant difference was observed in the ADR + A100
group compared with the ADR group (Fig. 1B; P>0.05). Serum ALB levels in
the ADR and ADR + A438079 100 and 200 µmol/kg groups were
significantly lower than those in the NC group (Fig. 1C; P<0.01 or P<0.05).
Furthermore, compared with the ADR group, changes in serum ALB were
only reduced in the ADR + A300 group (Fig. 1B; P<0.05). The levels of TC in
the ADR and ADR + A438079 100 and 200 µmol/kg groups were
significantly higher than those in the NC group (Fig. 1D; P<0.01). However, TC levels in
the ADR + A438079 200 and 300 µmol/kg groups were significantly
lower than those in the ADR group (Fig. 1D; P<0.01).
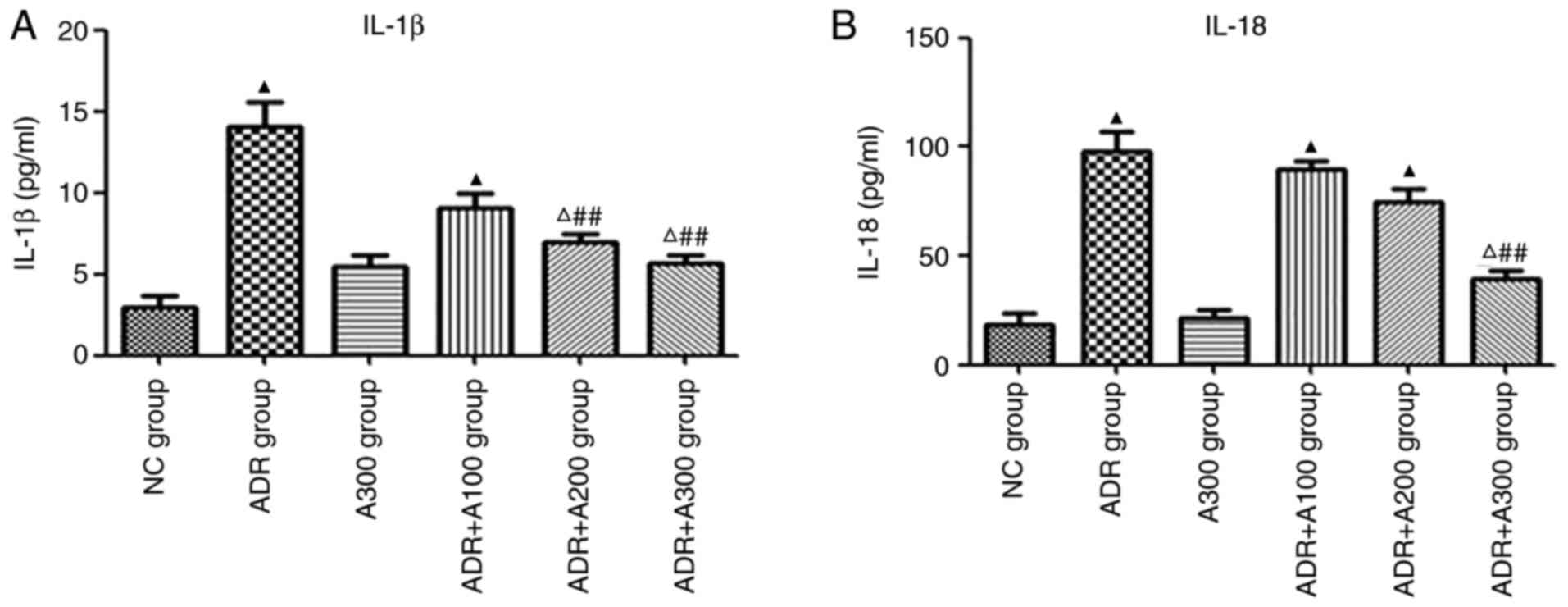
Effect of A438079 on IL-1β and IL-18
expression in renal tissues
IL-1β and IL-18 expression was evaluated in the
kidneys of mice from each treatment group by ELISA. The expression
of IL-1β and IL-18 in the ADR group was significantly higher than
that in the NC group (Fig. 2A and
B; P<0.01). Compared with the
ADR group, IL-1β levels in the ADR + A438079 200 and 300 µmol/kg
groups were alleviated (Fig. 2A;
P<0.01). However, IL-18 levels were only significantly reduced
in the ADR + A300 group (Fig. 2B;
P<0.01).
Effect of A438079 on the expression of
P2X7R, ox-LDL, CXCL16, Bax, caspase-3, and NLRP3 in the
glomeruli
To investigate the expression of P2X7R, ox-LDL,
CXCL16, Bax, caspase-3 and NLRP3 in the glomeruli of different
treatment groups, immunohistochemical analysis was performed. The
expression of P2X7R, ox-LDL, CXCL16, Bax, caspase-3 and NLRP3 in
the glomeruli of the ADR group was significantly increased
(Fig. 3A-L; P<0.01) compared
with that of the NC group. Compared with the ADR group, the
expression of these genes in the ADR + A438079 200 and 300 µmol/kg
groups were significantly reduced (Fig. 3A-L; P<0.05 or P<0.01).
However, there was no statistically significant difference observed
in the ADR + A100 group compared with the ADR group (Fig. 3A-L; P>0.05).
 | Figure 3Effect of A438079 on the expression of
P2X7R, ox-LDL, CXCL16, Bax, caspase-3 and NLRP3 in the glomeruli.
Expression of (A and G) P2X7R, (B and H) ox-LDL, (C and I) CXCL16,
(D and J) Bax, (E and K) caspase-3 and (F and L) NLRP3 in glomeruli
with different groups. ▲P<0.01 vs. NC group;
#P<0.05 and ##P<0.01 vs. ADR group.
ADR, adriamycin; A100/200/300, A438079 100/200/300 µmol/kg; CXCL16,
C-X-C motif chemokine ligand 16; NC, negative control; ox-LDL,
oxidized low density lipoprotein. |
Effect of A438079 on apoptosis in the
glomeruli of mice
The apoptosis of glomeruli in the ADR group was
significantly increased compared with that in the NC group
(Fig. 4; P<0.01). Furthermore,
compared with the ADR group, the apoptosis of glomeruli in the ADR
+ A438079 100, 200 and 300 µmol/kg groups was significantly
decreased (Fig. 4; P<0.01).
Discussion
PNS is a common kidney disease in children, with
glucocorticoids applied as the preferred clinical treatment for NS.
The most common pathological type of PNS is MCD, and the majority
of children with PNS are sensitive to steroid therapy, thus
exhibiting a favorable prognosis. However, certain children with
steroid-dependent or steroid-resistant NS may gradually progress to
focal segmental glomerular sclerosis (FSGS) and eventually to
end-stage renal disease (ESRD) (17). Therefore, it is important to
identify the target of early kidney damage and to find an effective
therapy for the occurrence and development of PNS.
ADR nephropathy in mice, which simulates the
pathological characteristics of human NS with the early
pathological manifestation of MCD and the late pathological
manifestation of FSGS, is a classic animal experimental model of NS
(4,5). In the present study, BALB/c mice were
sensitive to ADR and exhibited similarities to human NS with MCD
and FSGS, such as high urine protein. In addition, it is more
convenient and economical to use mice to conduct research, as they
exhibit a high reproductive ability and are easy to raise (5). BALB/c mice were selected to establish
the model of ADR nephropathy. Mice were injected with ADR (10.5
mg/kg) via the tail vein. After 1 week, the mice displayed massive
proteinuria, hypoalbuminemia and hyperlipidemia. Furthermore,
previous pathological analysis of renal tissues carried out in our
laboratory revealed the distinct proliferation of mesangial cells,
and a marked expansion of the mesangial matrix (16). The results from electron microscopy
suggested that the glomerular basement membrane was pervasively
thickened, and the foot processes were widely fused (16). All changes gradually became
markedly with time. Collectively, these results suggested that the
ADR nephropathy model was successfully established (16). Therefore, BALB/c mice were selected
as the ADR nephropathy animal model in the present study.
P2X7 receptor (P2X7R) is a ligand-gated ion channel
that is a member of the P2X receptor family. Although P2X7R is
similar to other P2X receptors, it has a special structure with a
longer C-terminal (18). Previous
studies have shown that P2X7R was involved in various kidney
diseases, including polycystic kidney disease, diabetic
nephropathy, hypertension nephropathy and renal
ischemia-reperfusion injury (8-11).
In a diabetic nephropathy rat model, Vonend et al (19) revealed that P2X7R expression was
significantly increased in the glomerular cells of diabetic
nephropathy rats, and immunohistochemical staining further
demonstrated that positive cells stained with brown and yellow
precipitation were mainly podocytes. However, P2X7R has not been
reported in regard to ADR nephropathy. In the current study, it was
found that the expression of P2X7R was increased in the glomeruli
of children with PNS (data not yet published), suggesting that
P2X7R may be involved in the pathogenesis of NS. Thus, it was
intended to confirm the result through animal models of NS. A mouse
model was established by ADR and after 1 week, the following
changes were observed in mice: Body weight was significantly
decreased, serum ALB was decreased and TC increased. Furthermore,
the expression of P2X7R in the glomeruli was also increased.
Following treatment with the P2X7R antagonist A438079, the
aforementioned observations in ADR-nephropathy mice were
alleviated. These data suggested that P2X7R may be associated with
the pathogenesis of ADR nephropathy in mice.
The animal model of ADR nephropathy is a classic
model as the observed histological changes resemble those of human
MCD and focal glomerulosclerosis (20). ADR induces thinning of the
glomerular endothelium and podocyte effacement associated with loss
of size- and charge-specific barrier to filtration of plasma
proteins. These changes are seen as early as 1-2 weeks after ADR
injection, and are severe by 4 weeks (21). Evidence has shown that P2X7R plays
a critical role in regulating lipid storage and metabolism in
vivo (14). Additionally,
ox-LDL may be the potential mediator for upregulating the
expression of P2X7R in atherosclerosis (15). Numerous studies have suggested that
lipid deposition is present in the kidney tissue of patients with
end-stage and chronic kidney diseases as well as in animal models
of NS (22-25).
It is broadly known that hyperlipidemia is one of the clinical
manifestations of PNS in children. Studies have revealed that
circulating LDL in hyperlipidemia, particularly ox-LDL, may be
deposited in kidney forming foam cells, upregulating the expression
of inflammatory factors, increasing the secretion of chemokines and
the infiltration of inflammatory cells, as well as the
proliferation of renal intrinsic cells, eventually leading to
glomerulosclerosis (26,27). Previous research has indicated that
during the progression of ADR nephropathy, CXCL16 could promote the
uptake of ox-LDL by podocytes, cause the accumulation of lipid in
podocytes, and eventually lead to the injury of podocytes (16). In consistency, the expression of
ox-LDL and CXCL16 were increased in the glomeruli of mice with ADR
nephropathy in the current study, while treatment with P2X7R
antagonist A438079, significantly decreased their expression.
Furthermore, Hu et al (28)
previously indicated that P2X7R may participate in lipid metabolic
disorders by regulating the CXCL16 pathway. Therefore, it was
hypothesized that P2X7R, ox-LDL and CXCL16 may be associated with
ADR nephropathy in mice. P2X7R inhibition may therefore reduce the
expression of ox-LDL by downregulating the CXCL16 pathway to
alleviate kidney injury of mice with ADR nephropathy.
P2X7R is involved in the in vivo inflammatory
response via a variety of signaling pathways to promote cellular
injury and apoptosis, and thus induce tissue damage (6). The P2X7R/NLPR3 pathway, which
promotes the release of downstream cytokines (IL-1β and IL-18) to
induce the immune inflammatory response, is the key pathway in
which P2X7R plays its pathophysiological role (29,30).
However, whether P2X7R/NLRP3 is also involved in the occurrence of
ADR nephropathy is unknown. Therefore, in the present study, the
expression of NLRP3 was detected in murine renal tissues by
immunohistochemistry, and the levels of IL-1β and IL-18 were
determined by ELISA. The results revealed that NLRP3 levels were
increased in the glomeruli of ADR nephropathy mice, and the levels
of IL-1β and IL-18 were also increased in renal tissues. While
using the P2X7R antagonist, A438079, the expression of NLRP3, IL-1β
and IL-18 was decreased, suggesting that the P2X7R/NLRP3 pathway
may be involved in the occurrence and development of ADR
nephropathy in mice.
Turner et al (31) indicated that the mRNA expression of
P2X7R was increased in the renal tissue of rats with proliferative
glomerulonephritis, as well as the mRNA expression of the
pro-apoptotic marker, Bax, and apoptosis in the glomeruli. In
addition, caspase-3, a member of the caspase protein family, is
located downstream of the caspase cascade reaction and is a key
executor of the apoptotic response, playing an active role in
apoptosis (32). In an animal
experimental study of renal toxicity induced by cisplatin in male
C57BL/6 mice, it was revealed that the expression of caspase-3 in
renal tissue was upregulated and that apoptosis was increased in
the model group, while the expression of caspase-3 was
significantly reduced after the preliminary administration of P2X7R
antagonist A438079(33). The
expression of podocyte apoptosis in diabetic nephropathy was
increased, as was the expression of the pro-apoptotic factor
caspase-3(34). In the present
study, immunohistochemistry was used to detect apoptotic protein
expression in the renal tissues of ADR-induced nephropathy mice.
Additionally, the TUNEL method to determine the number of apoptotic
cells. The expression of Bax and caspase-3 in the glomeruli of ADR
nephropathy mice increased with increased apoptosis, while
administration of A438079 significantly decreased the expression of
Bax and caspase-3 in the glomeruli. Furthermore, the number of
apoptotic cells was significantly decreased, suggesting that P2X7R
may promote apoptosis via Bax and caspase-3 to participate in the
occurrence and development of ADR nephropathy in mice.
In conclusion, P2X7R, ox-LDL and CXCL16 may be
associated with ADR nephropathy in mice. Inhibition of P2X7R may
reduce the expression of ox-LDL by downregulating the CXCL16
pathway to alleviate kidney injury in mice with ADR nephropathy.
Activated P2X7R may promote the release of inflammatory cytokines
IL-1β and IL-18 through the downstream P2X7R/NLRP3 pathway and
upregulate the expression of Bax and caspase-3 to promote
apoptosis, which participates in the process of ADR nephropathy.
Additionally, inhibition of P2X7R may reduce the release of IL-1β
and IL-18 by downregulating the P2X7R/NLRP3 pathway, downregulating
the expression of Bax and caspase-3, and reducing apoptosis,
thereby alleviating kidney injury in mice with ADR nephropathy.
Therefore, P2X7R antagonists may potentially be used in the
treatment of NS, but more specific studies are required.
Since the initial changes of ADR nephropathy are
similar to minimal change type nephropathy, the changes in the
model were only observed for 1 week in animal experiments,
therefore only the index data for diagnosing NS was recorded in the
laboratory at that time. In the future, changes and effects of
P2X7R in ADR nephropathy should be further investigated and all
relevant biochemical indicators should be evaluated.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Teachers' research
of Jining Medical University (grant no. JYFC2018FKJ042).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ designed the research. YZ and XN performed the
experiments. YZ and XN analyzed the data. XN drafted the manuscript
and analyzed data. ML, WX and KL collected and interpreted data,
and revised the final manuscript. YZ wrote the manuscript. YZ and
XN confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were performed with the approval of
the Animal Ethics Committee of Shandong Provincial Hospital (Jinan,
China; approval no. 2020-107)
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hao S, Wu Y, Kang Y, Niu X, Zhu G and
Huang W: A single-center analysis of primary nephrotic syndrome
with acute pancreatitis in children. Medicine (Baltimore).
99(e21056)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen A, Sheu LF, Ho YS, Lin YF, Chou WY,
Chou TC and Lee WH: Experimental focal segmental glomerulosclerosis
in mice. Nephron. 78:440–452. 1998.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Manabe N, Kinoshita A, Yamaguchi M, Furuya
Y, Nagano N, Yamada-Uchio K, Akashi N, Miyamoto-Kuramitsu K and
Miyamoto H: Changes in quantitative profile of extracellular matrix
components in the kidneys of rats with adriamycin-induced
nephropathy. J Vet Med Sci. 63:125–133. 2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lydia A, Asanuma K, Nonaka K, Takagi M,
Jeong KH, Kodama F, Asao R, Asanuma E, Prodjosudjadi W and Tomino
Y: Effects of 22-oxa-calcitriol on podocyte injury in
adriamycin-induced nephrosis. Am J Nephrol. 35:58–68.
2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang YM, Wang Y, Harris DCH, Alexander SI
and Lee VWS: Adriamycin nephropathy in BALB/c mice. Curr Protoc
Immunol. 108:15.28.1–15.28.6. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Adinolfi E, Giuliani AL, De Marchi E,
Pegoraro A, Orioli E and Di Virgilio F: The P2X7 receptor: A main
player in inflammation. Biochem Pharmacol. 151:234–244.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jiang LH, Baldwin JM, Roger S and Baldwin
SA: Insights into the molecular mechanisms underlying mammalian
P2X7 receptor functions and contributions in diseases, revealed by
structural modeling and single nucleotide polymorphisms. Front
Pharmacol. 4(55)2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Arkhipov SN and Pavlov TS: ATP release
into ADPKD cysts via pannexin-1/P2X7 channels decreases ENaC
activity. Biochem Biophys Res Commun. 513:166–171. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang C, Hou XX, Rui HL, Li LJ, Zhao J,
Yang M, Sun LJ, Dong HR, Cheng H and Chen YP: Artificially
cultivated ophiocordyceps sinensis alleviates diabetic nephropathy
and its podocyte injury via inhibiting P2X7R expression and NLRP3
inflammasome activation. J Diabetes Res.
2018(1390418)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Franco M, Bautista-Pérez R, Cano-Martínez
A, Pacheco U, Santamaría J, Del Valle Mondragón L, Pérez-Méndez O
and Navar LG: Physiopathological implications of P2X1 and P2X7
receptors in regulation of glomerular hemodynamics in angiotensin
II-induced hypertension. Am J Physiol Renal Physiol. 313:F9–F19.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Koo TY, Lee JG, Yan JJ, Jang JY, Ju KD,
Han M, Oh KH, Ahn C and Yang J: The P2X7 receptor antagonist,
oxidized adenosine triphosphate, ameliorates renal
ischemia-reperfusion injury by expansion of regulatory T cells.
Kidney Int. 92:415–431. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Harada H, Chan CM, Loesch A, Unwin R and
Burnstock G: Induction of proliferation and apoptotic cell death
via P2Y and P2X receptors, respectively, in rat glomerular
mesangial cells. Kidney Int. 57:949–958. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sha W, Shen L, Zhou L, Xu DY and Lu GY:
Down-regulation of miR-186 contributes to podocytes apoptosis in
membranous nephropathy. Biomed Pharmacother. 75:179–184.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Beaucage KL, Xiao A, Pollmann SI, Grol MW,
Beach RJ, Holdsworth DW, Sims SM, Darling MR and Dixon SJ: Loss of
P2X7 nucleotide receptor function leads to abnormal fat
distribution in mice. Purinergic Signal. 10:291–304.
2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Peng K, Liu L, Wei D, Lv Y, Wang G, Xiong
W, Wang X, Altaf A, Wang L, He D, et al: P2X7R is involved in the
progression of atherosclerosis by promoting NLRP3 inflammasome
activation. Int J Mol Med. 35:1179–1188. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang C, Li Q, Zhen J, Xu Y and Sun S:
Simvastatin ameliorates renal lipidosis through the suppression of
renal CXCL16 expression in mice with adriamycin-induced
nephropathy. Int J Clin Exp Pathol. 8:15696–15707. 2015.PubMed/NCBI
|
|
17
|
Gauckler P, Shin JI, Alberici F, Audard V,
Bruchfeld A, Busch M, Cheung CK, Crnogorac M, Delbarba E, Eller K,
et al: Rituximab in adult minimal change disease and focal
segmental glomerulosclerosis-what is known and what is still
unknown? Autoimmun Rev. 19(102671)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gentile D, Natale M, Lazzerini PE,
Capecchi PL and Laghi-Pasini F: The role of P2X7 receptors in
tissue fibrosis: A brief review. Purinergic Signal. 11:435–440.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vonend O, Turner CM, Chan CM, Loesch A,
Dell'Anna GC, Srai KS, Burnstock G and Unwin RJ: Glomerular
expression of the ATP-sensitive P2X receptor in diabetic and
hypertensive rat models. Kidney Int. 66:157–166. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mansouri E, Assarehzadegan MA,
Nejad-Dehbashi F and Kooti W: Effects of Pravastatin in
adriamycin-induced nephropathy in rats. Iran J Pharm Res.
17:1413–1419. 2018.PubMed/NCBI
|
|
21
|
Lee VW and Harris DC: Adriamycin
nephropathy: A model of focal segmental glomerulosclerosis.
Nephrology (Carlton). 16:30–38. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee HS: Oxidized LDL, glomerular mesangial
cells and collagen. Diabetes Res Clin Pract. 45:117–122.
1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Korhonen M, Ylänne J, Laitinen L and
Virtanen I: The alpha 1-alpha 6 subunits of integrins are
characteristically expressed in distinct segments of developing and
adult human nephron. J Cell Biol. 111:1245–1254. 1990.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lee HS, Jeong JY, Kim BC, Kim YS, Zhang YZ
and Chung HK: Dietary antioxidant inhibits lipoprotein oxidation
and renal injury in experimental focal segmental
glomerulosclerosis. Kidney Int. 51:1151–1159. 1997.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang G, Li Q, Wang L, Chen Y, Zhang W and
Yang H: The effects of inflammation on lipid accumulation in the
kidneys of children with primary nephrotic syndrome. Inflammation.
34:645–652. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Moorhead JF, Chan MK, El-Nahas M and
Varghese Z: Lipid nephrotoxicity in chronic progressive glomerular
and tubulo-interstitial disease. Lancet. 2:1309–1311.
1982.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Vaziri ND, Navab M and Fogelman AM: HDL
metabolism and activity in chronic kidney disease. Nat Rev Nephrol.
6:287–296. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hu ZB, Chen Y, Gong YX, Gao M, Zhang Y,
Wang GH, Tang RN, Liu H, Liu BC and Ma KL: Activation of the
CXCL16/CXCR6 pathway by inflammation contributes to atherosclerosis
in patients with end-stage renal disease. Int J Med Sci.
13:858–867. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Di Virgilio F: The therapeutic potential
of modifying inflammasomes and NOD-like receptors. Pharmacol Rev.
65:872–905. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ogura Y, Sutterwala FS and Flavell RA: The
inflammasome: First line of the immune response to cell stress.
Cell. 126:659–662. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Turner CM, Tam FW, Lai PC, Tarzi RM,
Burnstock G, Pusey CD, Cook HT and Unwin RJ: Increased expression
of the pro-apoptotic ATP-sensitive P2X7 receptor in experimental
and human glomerulonephritis. Nephrol Dial Transplant. 22:386–395.
2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Eamshaw WC, Martins LM and Kaufinann SH:
Mammalian caspases: Structure, activation, substrates, and
functions during apoptosis. Annu Rev Biochem. 68:383–424.
1999.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang Y, Yuan F, Cao X, Zhai Z, Huang G,
Du X, Wang Y, Zhang J, Huang Y, Zhao J and Hou W: P2X7 receptor
blockade protects against cisplatin-induced nephrotoxicity in mice
by decreasing the activities of inflammasome components, oxidative
stress and caspase-3. Toxicol Appl Pharmacol. 281:1–10.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu L, Yang L, Chang B, Zhang J, Guo Y and
Yang X: The protective effects of rapamycin on cell autophagy in
the renal tissues of rats with diabetic nephropathy via
mTOR-S6K1-LC3II signaling pathway. Ren Fail. 40:492–497.
2018.PubMed/NCBI View Article : Google Scholar
|