Introduction
Asthma is a chronic lung disease that is often
diagnosed in childhood. It affects ~339 million individuals
worldwide and ~1,000 individuals die each day from asthma (1). Asthma involves chronic tracheal
inflammation, which is mediated T helper 2 cells and exhibits
symptoms including reversible expiratory airflow limitation,
wheezing, shortness of breath, chest tightness and cough.
Hypertrophy and hyperplasia of the tracheal smooth muscle caused by
chronic tracheal inflammation thicken the tracheal wall and goblet
cells, leading to increased mucus production and narrowing of the
trachea (2,3). Although researchers have extensively
investigated this topic, the mechanism has not been fully
elucidated (4). Currently, numerous
commercially available drugs demonstrate good inhibitory efficacy
against asthma, but the incidence and mortality remain high.
Therefore, it is urgently necessary to clarify the mechanism of
asthma and design new therapeutics.
RNA interference (RNAi), also known as
post-transcriptional gene silencing, involves the transfer of
double-stranded RNA into cells to silence or inhibit the expression
of target genes and serves an important role in gene regulation
(5,6). The silencing of target genes with RNAi
has been used for the inhibition of genes associated with cancer
(7).
To date, ~90 types of tyrosine kinase enzymes have
been identified in humans (8,9).
Tyrosine kinase Src, a member of the Src kinase family, belongs to
the non-receptor tyrosine kinase family. Previous studies have
demonstrated that Src kinase regulates cell metabolism, and its
signalling pathway participates in apoptosis, cell proliferation
and development (10,11). Furthermore, Src has been revealed to
participate in the development of lung cancer and HIV infection
(12).
The present study utilized a gene transfer technique
to transfer small interfering RNA (siRNA) into rats with asthma and
thereby interfere with the expression of the Src gene. Through the
analysis of the lung tissues and bronchoalveolar lavage fluid
(BALF) of the rats in different groups, the effect of Src protein
kinase on asthma was elucidated.
Materials and methods
Materials
Concentrated goat serum and Cy3-labeled Goat
Anti-Rabbit IgG (cat. no. BA1034) were purchased from Wuhan Boster
Biological Technology Co., Ltd.; Entranster™-in
vivo (cat. no. 18668-11-1) was purchased from Engreen
Biosystem, Ltd.; Lipofectamine® 2000 was purchased from
Invitrogen (Thermo Fisher Scientific, Inc.); DNA marker was
purchased from TransGen Biotech Co., Ltd.; RNAiso Plus, PrimeScript
RT Reagent kit and SYBR Premix Ex Taq™ II (Tli RNaseH
Plus) were purchased from Takara Bio, Inc.; IL-5 (cat. no.
E-EL-R0558c; Elabscience Biotechnology, Inc.), IL-33 (cat. no.
CSB-E14077r; Cusabio Technology LLC) and IFN-γ (cat. no.
CSB-E04579r; Cusabio Technology LLC) ELISA kits, and CD90 (cat. no.
E-AB-70323) and CD45 (cat. no. E-AB-16319) antibodies were
purchased from Elabscience Biotechnology, Inc.
Animals
A total of 32 healthy male Sprague Dawley (SD) rats,
aged 4 weeks and weighing 200-280 g, were provided by the Animal
Research Centre of Inner Mongolia Medical University. The rats were
acclimated for 1 week prior to the experiments. The SD rats were
provided with continuous standard rodent chow and water and were
housed in a rodent facility at 24±2˚C and 45~65% relative humidity
environment with a 12-h light/dark cycle. All procedures involving
animals and their care were conducted in accordance with the Guide
for the Care and Use of Laboratory Animals (8th Edition), which was
established by the National Academy of Sciences and published by
the National Institutes of Health.
Cultivation and identification of rat
bone marrow mesenchymal stem cells
A healthy male rat (aged 4 weeks and weighing 70 g)
was humanely sacrificed by intraperitoneal anesthetization with 1
g/kg urethane followed by cervical dislocation and then immersed in
75% ethanol for 15 min. Bone marrow was extracted from bilateral
femurs and rinsed with a-Minimum Essential Medium (a-MEM; cat. no.
SH30265; Hyclone; Cytiva) containing 10% foetal calf serum (cat.
no. SH30070; Hyclone; Cytiva) and 1% penicillin-streptomycin (cat.
no. SV30010; Hyclone; Cytiva). The resulting single cell suspension
was centrifuged at 850 x g and 25˚C for 7 min. The supernatant was
removed, and the cells were resuspended with a-MEM containing 10%
foetal calf serum and 1% penicillin-streptomycin. The suspended
cells were cultured and passaged at 37˚C with 5% CO2.
All processes were performed in a sterile environment.
After rinsing with phosphate-buffered saline (PBS),
the cells were fixed with 4% paraformaldehyde for 15 min at room
temperature and rinsed with PBS three times. Then, 0.5% Triton
X-100 was dropped onto the slides, and the slides were maintained
at 20˚C for 20 min. After rinsing and removing the residual
liquids, 5% goat serum (Beijing Solarbio Science & Technology
Co., Ltd.) was dropped onto the slides and the slides were set
aside to block for 30 min at 37˚C. Following this, when the
blocking reagent was absorbed, diluted (1:100) CD90 and CD45
antibodies were dropped onto each slide and the slides were
incubated at 4˚C overnight. The slides were then washed and diluted
(1:100) Cy3-labeled Goat Anti-Rabbit IgG was dropped onto the
slides, which were subsequently incubated at 37˚C for 1 h. After
staining with DAPI at room temperature for 15 min, the slides were
observed under a fluorescence microscope (magnification, x20).
siRNA transfection of rat bone marrow
mesenchymal stem cells
Based on the nucleotide sequence of Src in the
National Centre for Biotechnology Information (NCBI) GenBank
database (13), sequences of siRNA
targeting Src were designed and synthesized by Sangon Biotech Co.,
Ltd., as presented in Table I. A
stock solution (40 µM) was prepared by adding 125 µl diethyl
pyrocarbonate (DEPC) into siRNA. After mixing, the solution was
divided into 6-µl/tube portions and preserved at -20˚C.
Transfection was conducted as follows: Solution A was prepared by
dissolving 0.25 µl siRNA in 50 µl Opti-MEM. Solution B was prepared
by dissolving 0.25 µl Lipofectamine 2000 in 50 µl Opti-MEM, and
leaving to stand for 5 min at 20˚C. The two solutions were mixed in
a 1:1 ratio to form solution C and maintained at room temperature
for 20 min. When the density of rat bone marrow mesenchymal cells
in a 96-well microplate reached >50%, the medium was removed and
replaced with 800 µl/well a-MEM. Solution C was then added.
Following incubation for 6 h at 37˚C in a cell incubator, the
medium was replaced with a-MEM containing 10% foetal calf serum and
1% penicillin-streptomycin and the cells were incubated for a
further 24 h at 37˚C. The cells were collected, and the total RNA
was extracted from the cells and examined by reverse
transcription-quantitative PCR (RT-qPCR).
 | Table ISequences of rat Src siRNAs (11). |
Table I
Sequences of rat Src siRNAs (11).
| Name | Sequence
(5'-3') |
|---|
|
Scrambled-siRNA-F |
AGAGCCGAUUCCUUAACAATT |
|
Scrambled-siRNA-R |
UUGUUAAGGAAGCGGCUCUTT |
| Src-rno-1208-F |
CAGAGCGGCUACUUCUCAATT |
| Src-rno-1208-R |
UUGAGAAGUAGCCGCUCUGTT |
| Src-rno-708-F |
GCGGCUGCAGAUUGUCAAUTT |
| Src-rno-708-R |
AUUGACAAUCUGCAGCCGCTT |
| Src-rno-995-F |
GCCUAAAUGUGAAACACUATT |
| Src-rno-995-R |
UAGUGUUUCACAUUUAGGCTT |
Extraction and quantitative analysis
of mRNA by RT-qPCR
All Eppendorf (EP) tubes and pipette tips used in
this procedure were sterile and RNase-free. Firstly, cells were
rinsed twice with PBS, 200 µl RNAiso Plus was added and the cells
were maintained at 20˚C for 2 min. After mixing the solution, 40 µl
chloroform was added, the sample was kept at 4˚C for 3 min and then
centrifuged at 12,000 x g for 15 min at 4˚C. The supernatant was
transferred into a new EP tube, mixed with 200 µl isopropyl alcohol
and allowed to stand at 20˚C for 10 min. The mixture was then
centrifuged at 12,000 x g for 10 min at 4˚C. Following removal of
the supernatant, the residual layer was washed with 200 µl 75%
ethanol and centrifuged at 7,500 x g and 4˚C for 5 min. The
supernatant was removed and the residual alcohol was volatilized at
20˚C. The RNA residue was dissolved in 30 µl DEPC and preserved at
-80˚C.
The total RNA (1.0 µg) was placed in an RNase-free
tube with 2 µl 5X gDNA eraser buffer and 1 µl gDNA eraser (cat. no.
RR047A; Takara Bio, Inc.), and RNase free distilled water
(dH2O) was added to a total volume of 10 µl. The mixture
was maintained at 42˚C for 2 min and the product was preserved at
4˚C. Then, 1 µl PrimeScript RT Enzyme mix, 1 µl RT Primer mix and 4
µl 5X PrimeScript Buffer from the PrimeScript RT Reagent kit and 4
µl RNase-free dH2O were added to provide a mixture with
a total volume of 20 µl. This mixture was incubated at 37˚C for 15
min followed by 85˚C for 5 sec. The solution of cDNA product was
preserved at 4˚C.
Based on the NCBI GenBank database sequence of the
rat Src gene, primers for Src were designed using the Primer-BLAST
tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE_TYPE=BlastSearch&BLAST_SPEC=OGP__10116__10621&LINK_LOC=blasthome)
and synthesized by Sangon Biotech Co., Ltd. The primer sequences
were as follows: Src forward, 5'-CTTCCTCGTGAGGGAGAGTG-3' and
reverse, 5'-TGGGACACACGGTAGTGAGA-3'. GAPDH served as the internal
control gene with primer sequences as follows: Forward,
5'-TGCTGAGTATGTCGTGGAG-3' and reverse,
5'-GTCTTCTGAGTGGCAGTGAT-3'.
For qPCR, 10 µl SYBR Premix Ex Taq II (Tli RNaseH
Plus) (2X), 4 µl diluted cDNA (200 ng/µl), 0.8 µl forward primer
(10 µM), 0.8 µl reverse primer (10 µM) and 4.4 µl double
dH2O were mixed to provide 20 µl reaction mixture. The
thermocycling protocol for qPCR is presented in Table SI.
Quantitative analysis was based on the
2-ΔΔCq method (14). The
average relative content (%)=relative content of unknown
sample/relative content of control sample = 2-average
ΔCq, where ΔCqunknown sample = Cqunknown
sample - Cqcontrol sample.
Construction of an animal model of
asthma
A total of 32 SD rats were randomly divided into
four groups named the control, model (PBS), empty vector and siRNA
groups. To establish the asthma model, a 100-µl mixture comprising
50 µg ovalbumin (OVA; cat. no. A5503; Sigma-Aldrich; Merck KGaA)
and 2 mg aluminium hydroxide (cat. no. 239186; Sigma-Aldrich; Merck
KGaA) in saline was administered intraperitoneally to rats in the
PBS, empty vector and siRNA groups on days 2, 11 and 22. The rats
in the control group were injected with same volume of saline at
the same time points. In addition, the rats in the PBS, empty
vector and siRNA groups were exposed to 5% OVA inhalation for 20
min each day from day 22 for 5 days. The control group inhaled
atomized saline at the same time points.
Transfection assay
To evaluate the role of Src in the rat model of
asthma, 250 µl Src siRNA (0.5 µg/µl) and 250 µl transfection
compound (Entranster™-in vivo) were diluted with
250 µl PBS, mixed and allowed to stand for 20 min on day 1, prior
to administration to the siRNA group via intravenous injection (100
µl per rat). At the same time point, the rats in the PBS and empty
vector groups were injected with the same volume of PBS and
transfection compound, respectively. On day 2, the 100-µl mixture
comprising 50 µg OVA and 2 mg aluminium hydroxide was injected
intraperitoneally into the rats in the PBS, empty vector and siRNA
groups. Subsequently, the siRNA and transfection compound were
injected intravenously into rats in the siRNA group on days 8, 15
and 22, while rats in the PBS and empty vector group were treated
with the same volume of PBS and transfection compound,
respectively. On day 22, 2 h after injection, the rats in the PBS,
empty vector and siRNA groups were transferred to a closed
container, where they breathed 5% atomized OVA for 20 min. This OVA
inhalation process was performed once a day for 5 days. The control
group inhaled the same quantity of atomized saline instead.
Following the final atomization, the rats in the four groups were
humanely sacrificed by anesthetization with 1 g/kg urethane and
cervical dislocation, and their BALF as well as lung and bronchus
tissues were collected for follow-up examination.
Histochemistry
Lung and bronchus tissues were fixed in 4%
paraformaldehyde at room temperature for 24 h, embedded in
paraffin, sliced into 4 µm-thick sections, stained with
haematoxylin for 5 min and eosin for 3 min at room temperature,
dehydrated and fixed. Slices were examined at high magnification
(x100) using an optical microscope.
Determination of Src mRNA in
tissues
Tissue samples (50 mg) were ground quickly in a
mortar with liquid nitrogen and then transferred to an EP tube.
Following the addition of 1 ml TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.) and mixing, the tube was left to stand at 20˚C
for 5 min. Then, chloroform (200 µl) was added, the mixture was
shaken intensely for 15 sec and set aside for 3 min at 2˚C. After
that, the mixture was centrifuged at 13,800 x g for 15 min at 4˚C.
The RNA-containing supernatant was transferred to a new EP tube,
treated with 500 µl isopropanol for 10 min at room temperature and
then centrifuged at 13,800 x g for 10 min at 4˚C. Following removal
of the supernatant, 1 ml 75% alcohol was added, the mixture was
centrifuged at 5,400 x g for 5 min at 4˚C and the supernatant was
removed. The precipitate was dried at 20˚C, then dissolved with
25-200 µl DEPC H2O for further use. The total RNA was
extracted by this procedure for examination of the mRNA levels by
RT-qPCR as described above.
Examination of protein tyrosine kinase
Src
The rat tissue was ground, put into a tube with
lysis buffer (cat. no. P0013; Beyotime Institute of Biotechnology)
and protease inhibitor, and then centrifuged at 13,800 x g and 4˚C
for 10 min. The supernatant was collected and preserved at -20˚C. A
10-µl sample was taken and diluted 10-fold with PBS buffer. The
diluted solution was added to three wells (20 µl/well) and 200 µl
G-250 (Coomassie Brilliant Blue) was added to each well and mixed.
After 2 min, a biophotometer was used to determine the absorbance
value at 595 nm. BSA (1 mg/ml; cat. no. V900933; Sigma-Aldrich;
Merck KGaA) was added the cells of a 96-well plate at volumes of 0,
1, 2, 4, 6, 8 and 10 µl, followed by distilled water to a total
volume of 20 µl. These BSA standards were stained using G-250 using
the aforementioned method. A standard curve was then created for
estimating the protein concentration of the samples. After that,
the samples were examined by western blotting and Scr expression
was analysed by the measurement of its optical density at the
corresponding molecular weight. In brief, for each sample, 30 µg
protein was loaded in each lane, separated by 10% SDS-PAGE, and
then transferred to a PVDF membrane (cat. no. ISEQ00010;
MilliporeSigma). Following blocking with 5% skimmed milk at room
temperature for 30 min, the membranes were incubated with a primary
antibody against SRC (cat. no. ab231081, 1:1,000; Abcam) at 4˚C
overnight or a primary antibody against GAPDH (cat. no. 200306-7E4,
1:1,000; Chengdu Zhengneng Biotechnology Co., Ltd.) at room
temperature for 2 h. The membrane was washed with TBS with Tween-20
(TBST; 10 mM Tris-Cl, pH 7.5, 100 mM NaCl and 1% Tween-20) five
times for 8 min and incubated with a HRP-conjugated goat anti-mouse
antibody (cat. no. A0216; 1:5,000; Beyotime Institute of
Biotechnology) for 1 h at room temperature. After washing with TBST
five times for 8 min, the blots were exposed by using ECL
Chemiluminescent Substrate (cat. no. 180-5001; Tanon Science and
Technology Co., Ltd.) and signals were recorded under the
5200chemiluminescent visualized system (Tanon Science and
Technology Co., Ltd.). Band density was semi-quantified using
ImageJ software V1.8.0 (National Institutes of Health).
Analysis of BALF
Samples of BALF were centrifuged at 1,650 x g for 15
min at 4˚C. The supernatant was separated from the precipitate and
each component was preserved separately. The precipitate was
re-suspended with PBS and cell smears were prepared. After drying
at room temperature, cells were fixed with methanol for 15 min at
room temperature and then stained with Wright-Giemsa staining
solution for 20 min at room temperature. The number of white blood
cells (WBCs), eosinophils (EOSs) and total cells were counted under
a microscope (H550S; Nikon Corporation). The proportion of these
two cells in the total cells was calculated from five fields of
view. The supernatant was employed to determine the concentration
of IL-5, IL-33 and IFN-γ in the BALF using ELISA kits.
Data analysis
SPSS 25.0 (IBM Corp.) was used to perform the
statistical analysis. Results are presented as the mean ± SD from
three independent experiments. Application of Levene's test
confirmed the homogeneity of variance. The statistical significance
of differences among groups was detected using one-way ANOVA
followed by Tukey's post hoc tests. In the siRNA group, the
correlations of Src mRNA expression with Src protein expression,
the number of EOSs and the expression of inflammatory factors were
statistically analysed using Pearson's correlation test. P<0.05
was considered to indicate a statistically significant
difference.
Results
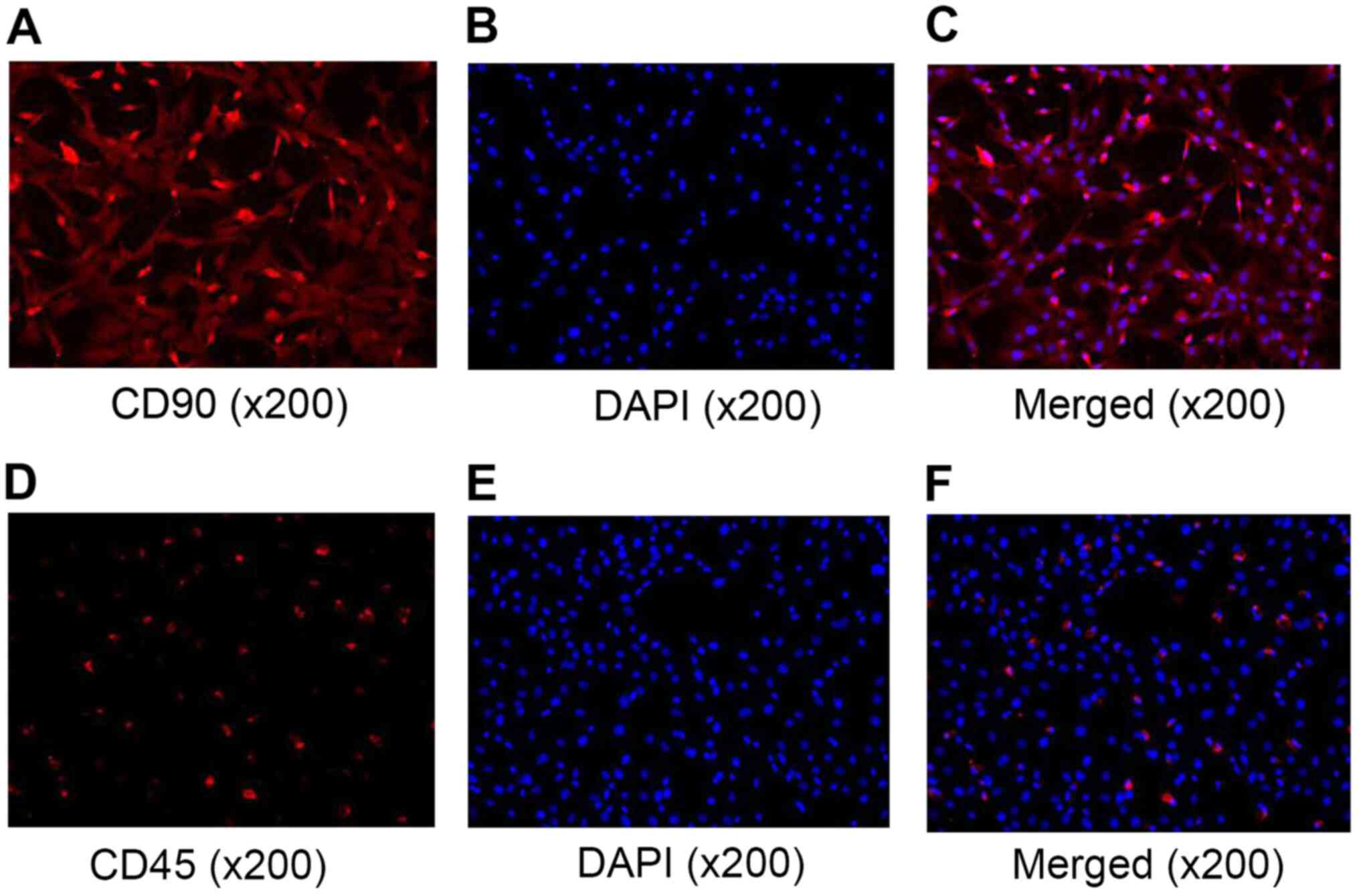
Identification of rat bone marrow
mesenchymal stem cells
Cells isolated from the rat bone marrow and cultured
in vitro were small in volume, with a shuttle-like
morphology and a low cytoplasmic ratio. When examined using
immunofluorescence staining, the cultured cells exhibited positive
expression of CD90 and weak expression of CD45. As shown in
Fig. 1A-F, after counting the
number of positive and total cells, it was found that the CD90
positive rate was >90% and the CD45 positive rate was
<5%.
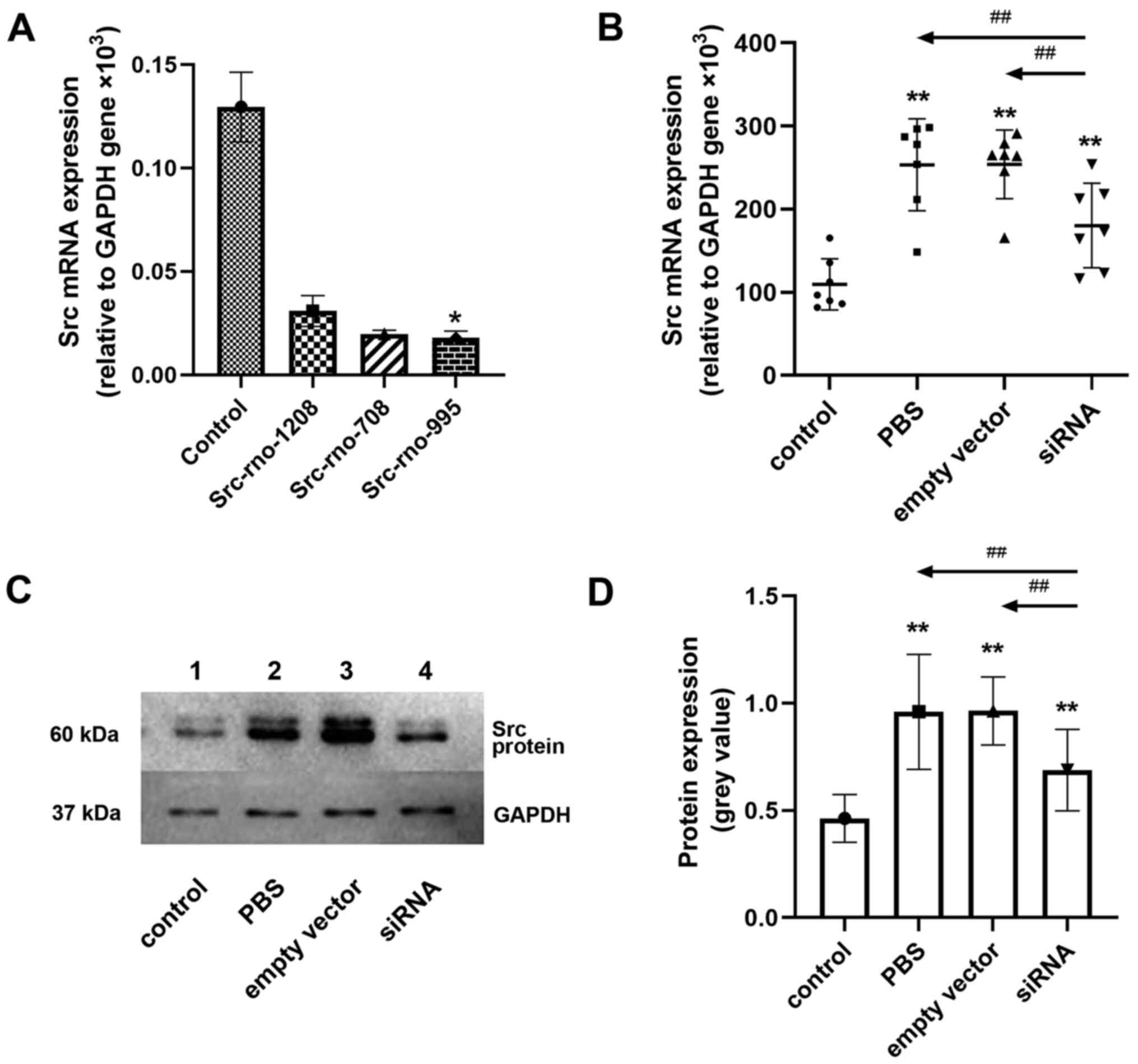
Expression of Src mRNA in vitro
As shown in Fig. 2A,
relative to GAPDH, the expression of Src mRNA in the negative
control group was 0.1295x103, that in the Src-rno-1208
group was 0.0309x103, that in the Src-rno-708 group was
0.0198x103, and that in the Src-rno-995 group was
0.0179x103, which was the lowest (P<0.05). This
indicates that among the siRNAs tested, Src-rno-995 had superior
efficacy in interfering with Src mRNA. Therefore, Src-rno-995 was
selected for use in subsequent experiments.
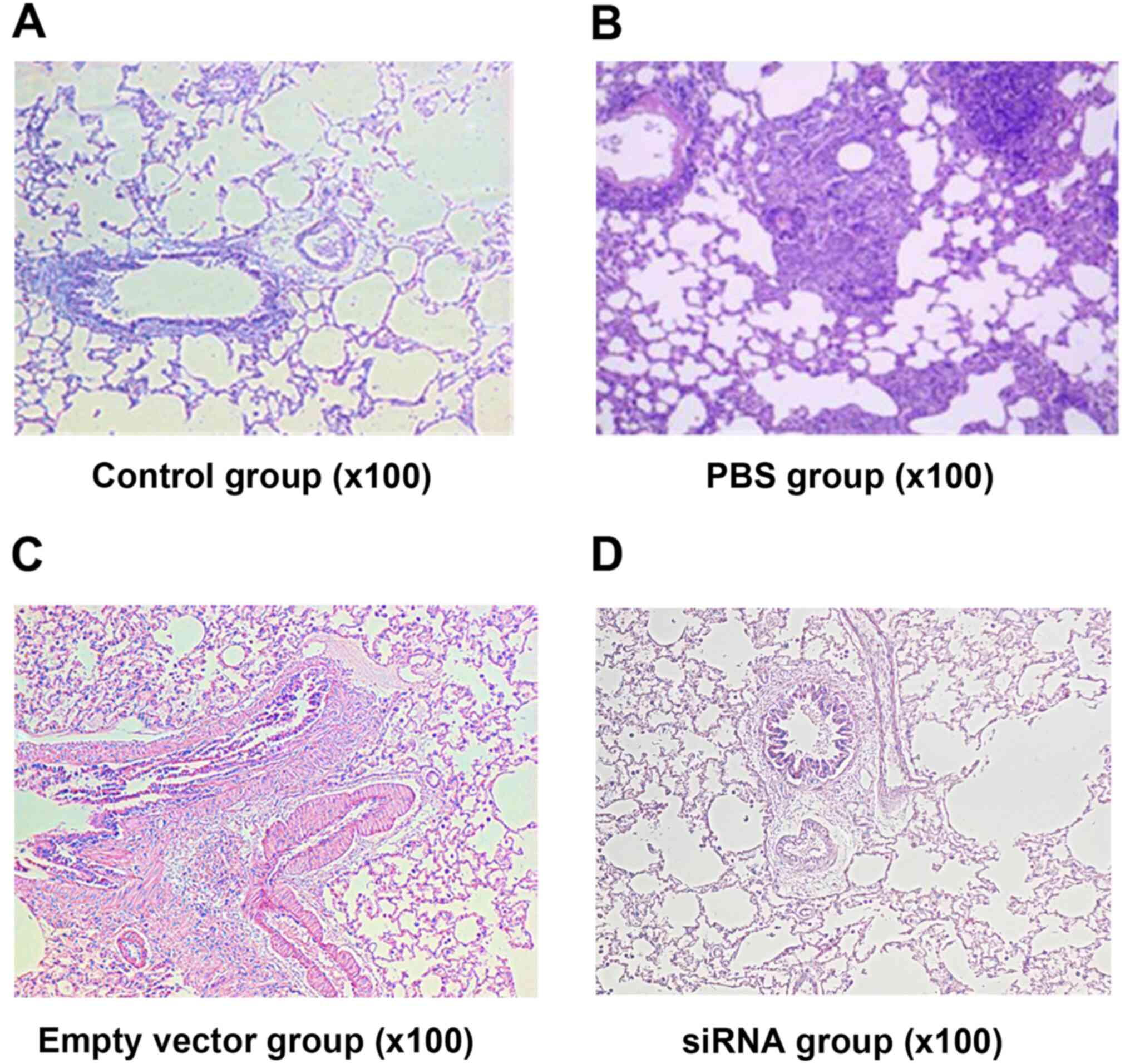
Clinical observation and
histopathology in vivo
Rats in the control group exhibited normal
behaviour, glossy fur and no symptoms of asthma or cough. In the
PBS and empty vector groups, the rats had less glossy fur, poorer
appetites and lower activity levels than the controls. In addition,
following the final OVA atomization challenge, the rats exhibited
anxiety, deep breathing, nose grabbing and purpura. The rats in the
siRNA group displayed glossy fur and obviously improved appetites
and activity levels when compared with the rats in the PBS and
empty vector groups (data not shown).
The H&E staining results were as follows: In the
control group, the bronchial structure and alveolar septa were
normal, the alveolar cavities were clear and no inflammatory cell
infiltration was observed in the lung mucous membrane (Fig. 3A). As shown in Fig. 3B and C, in the PBS and empty vector groups, the
epithelial structure of the trachea was incomplete. Mucous gland
hyperplasia was evident and the secretion of mucus by goblet cells
was increased; mucous plug development was visible in the small
bronchi. The alveolar septa narrowed, the alveolar cavities were
fused and tissue oedema was observed. Inflammatory cell
infiltration was visible at the tracheal wall and surrounding area,
where the inflammatory cells were mainly lymphocytes and EOSs. In
the siRNA group, the bronchial structure was relatively normal and
the epithelial structure was almost complete (Fig. 3D). Destruction of the alveolar
cavities was mild. Furthermore, inflammatory cell infiltration and
mucous secretion were mild in comparison with those in the PBS and
empty vector groups.
Expression of Src mRNA in lung
tissue
As shown in Fig. 2B,
the Src mRNA levels in the control, PBS, empty vector and siRNA
groups were 110±30.7x103, 253±55.4x103,
254±41.3x103 and 180±50.9x103, respectively.
The expression levels of Src mRNA in the PBS and empty vector
groups were significantly higher compared with that in the control
group (P<0.01), and the expression of Src mRNA in the siRNA
group was significantly higher compared with that in the control
group (P<0.01), but significantly lower compared with those in
the PBS and empty vector groups (P<0.01). No difference in Src
mRNA expression was detected between the PBS and empty vector
groups.
Src protein expression in the lung
tissue
Fig. 2C shows
representative western blotting results for the lung tissue and
Fig. 2D presents the greyscale
values of the Src protein compared with those of GAPDH. The
relative greyscale values for the Src protein in the control, PBS,
empty vector and siRNA groups were 0.463±0.111, 0.960±0.268,
0.964±0.159 and 0.688±0.190, respectively. The Src protein levels
in the PBS and empty vector groups were significantly higher
compared with that in the control group (P<0.01). The Src
protein level in the siRNA group was significantly lower compared
with those in the PBS and empty vector groups (P<0.01) and
higher than that in the control group (P<0.01). No difference in
the Src protein level was identified between the PBS and empty
vector groups.
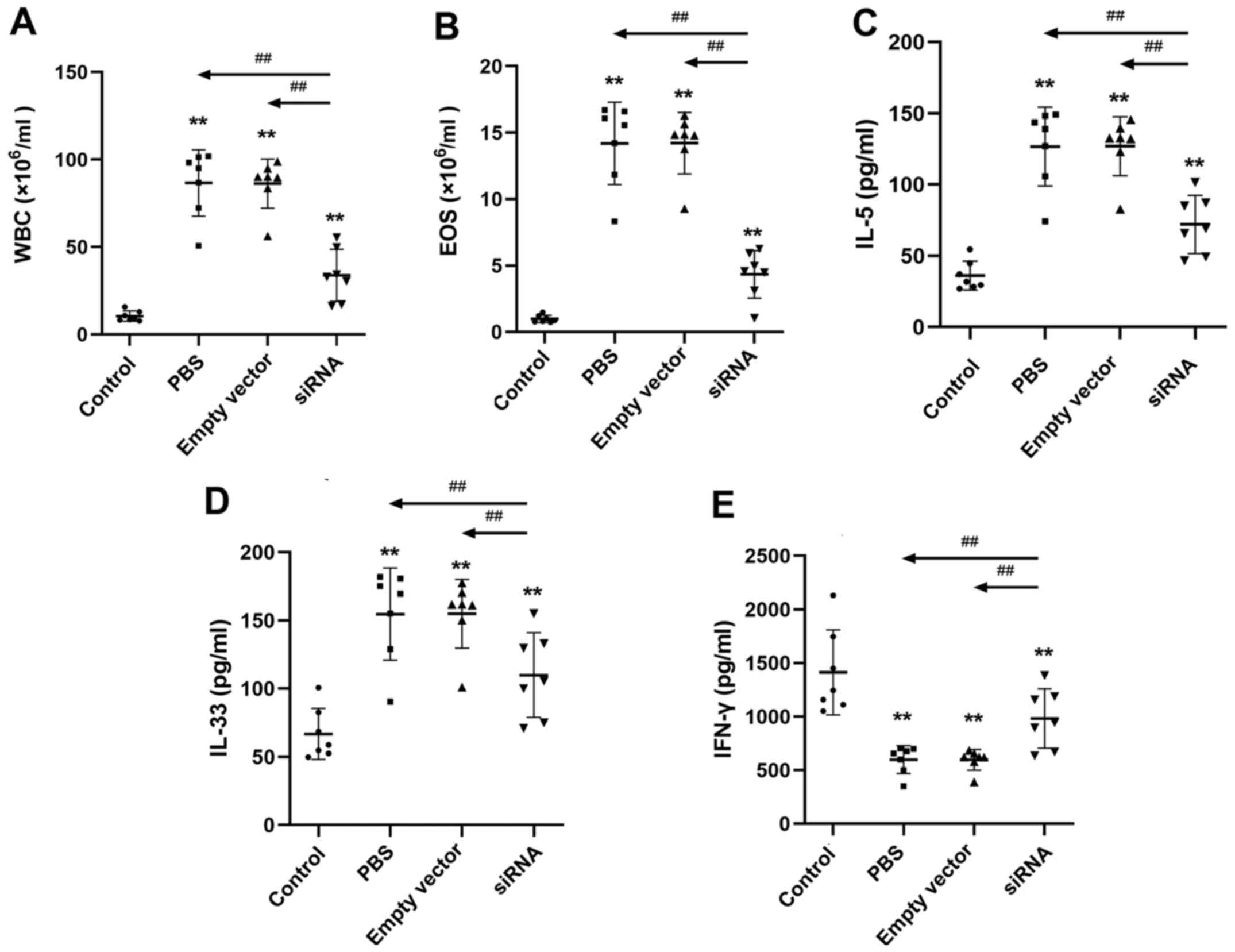
Quantification of WBCs and EOSs in
BALF
As shown in Fig. 4A
and B, in the BALF of the control,
PBS, empty vector and siRNA groups, the numbers of WBCs were
10.5±2.95x106, 86.7±18.9x106,
86.3±14.0x106 and 33.8±14.8x106 cells/ml,
respectively, and the numbers of EOSs were
0.986±0.277x106, 14.2±3.10x106,
14.2±2.31x106 and 4.34±1.78x106 cells/ml,
respectively. The numbers of EOSs and WBCs in the PBS and empty
vector groups were significantly higher compared with those in the
control group (P<0.01); the numbers of these cells in the siRNA
group were also significantly higher than those in the control
group (P<0.01) but significantly lower than those in the PBS and
empty vector groups (P<0.01). No difference was detected between
the PBS and the empty vector groups.
Expression of IL-5, IL-33 and IFN-γ in
BALF
The concentrations of IL-5, IL-33 and IFN-γ in the
BALF samples from the control, PBS group, empty vector and siRNA
groups are shown in Fig. 4C-E. The
concentrations of IL-5 and IL-33 in the PBS and empty vector groups
were significantly higher compared with those in the control group
(P<0.01); the concentrations of IL-5 and IL-33 in the siRNA
group were also significantly higher than those in the control
group (P<0.01) but significantly lower than those in the PBS and
empty vector groups (P<0.01). The concentrations of IFN-γ in the
PBS and empty vector groups were significantly lower than those in
the control group (P<0.01). The IFN-γ level in the siRNA group
was also significantly lower than that in the control group
(P<0.01) but significantly elevated compared with IFN-γ levels
in the PBS and empty vector groups (P<0.01). No difference was
observed between the PBS and empty vector groups.
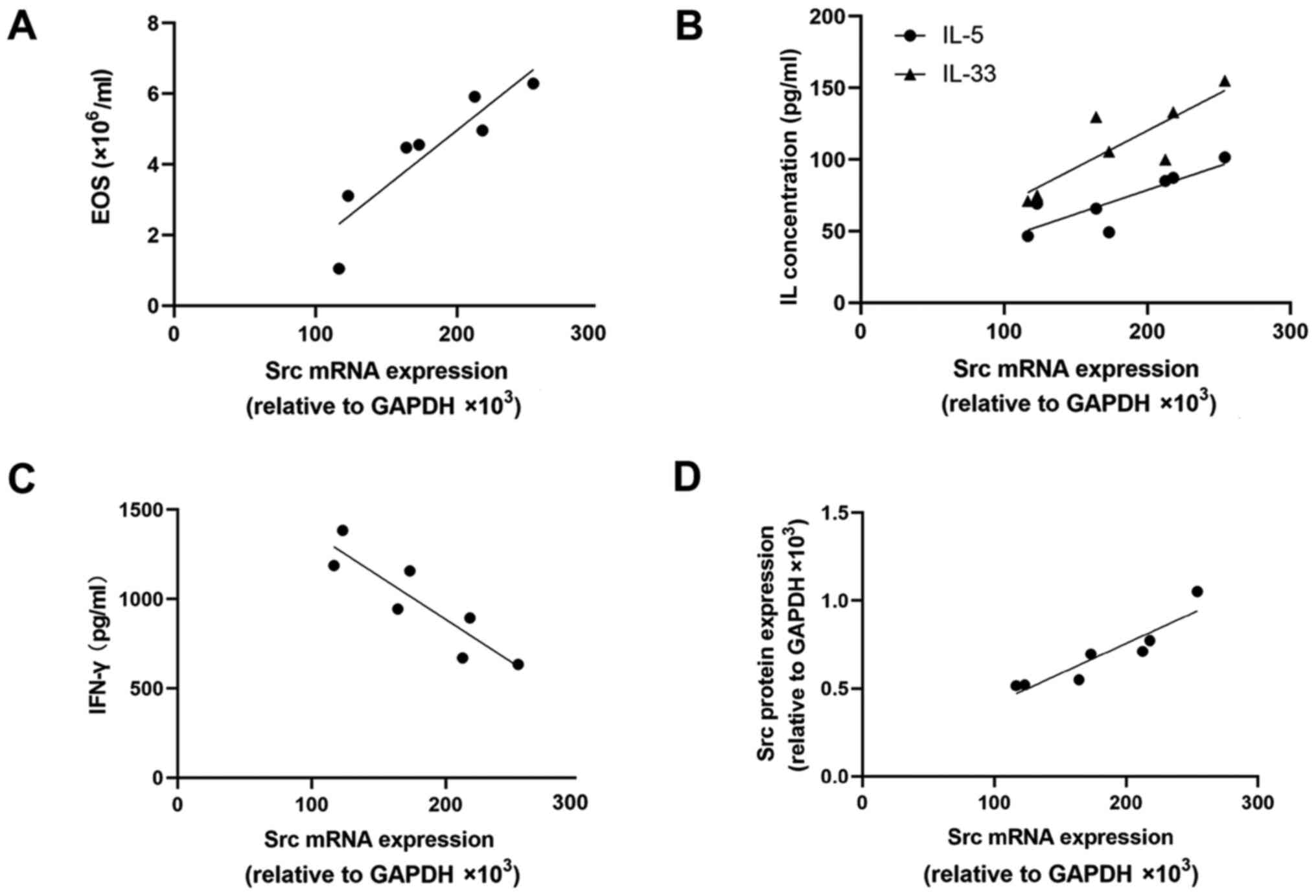
Correlation analysis of Src siRNA
As shown in Fig. 5A
and B, in the siRNA group, the EOS,
IL-5 and IL-33 levels exhibited a positive correlation with Src
mRNA expression (r2=0.824, 0.724 and 0.722,
respectively; P=0.0047, 0.0183 and 0.0155, respectively). IFN-γ
levels were negatively correlated with the Src mRNA level
(r2=0.798, P=0.0067; Fig.
5C). In addition, the Src protein level was positively
correlated with the Src mRNA level (r2=0.8466, P=0.0033;
Fig. 5D).
Discussion
Bronchial asthma causes airway obstruction and
chronic inflammation and is one of the most common chronic diseases
in developed countries (15). In
2016, the Global Burden of Disease collaboration estimated that
420,000 individuals worldwide died from asthma, which is >1,000
per day (1). It has been reported
that 20% of patients exhibit worsening symptoms and 10% lack an
effective method of control (16).
When asthma reaches an advanced level, the quality of life of the
patient declines. At present, commonly used methods to control
asthma are progressive treatments, with strategies differing
according to the severity of the disease. Glucocorticoids,
leukotriene modifiers, β2-agonists and anti-IgE therapy serve as
frequently used treatment methods for asthma; however, effective
targeted drugs are lacking (17,18).
Therefore, new technologies and therapies for asthma are popular
topics of research worldwide.
Cell proliferation in airway smooth muscle (ASM) is
a key contributor to chronic asthma. According to previous
research, Src proteins can promote the generation and metastasis of
ASM cells (19). Therefore, these
molecules are potential targets in the treatment of asthma. siRNA
is a double-stranded RNA that typically comprises 19-29 nucleotides
in length (20). When siRNA enters
target cells, it induces the degradation of mRNA and thereby
inhibits gene expression (21,22).
Therefore, the use of siRNA technology is a powerful strategy for
studying the function of genes in vitro. Xie et al
(23) designed a novel pulmonary
delivery system for siRNA, comprising transferrin-polyethylenimine
(Tf-PEI) for the selective delivery of siRNA to activated T cells
(ATCs) in the lung. Their results demonstrated that Tf-PEI
polyplexes efficiently and selectively deliver siRNA to ATCs. In
another study, in which dexamethasone-conjugated polyethylenimine
(DEXA-PEI) was combined with anti-vitamin D binding protein (VDBP)
treatment, DEXA-PEI served as an siRNA carrier molecule for the
delivery of VDBP siRNA. Treatment with DEXA-PEI/VDBP siRNA
effectively reduced airway inflammation, goblet cell hyperplasia
and the expression of inflammatory factors such as IL-4, IL-13 and
eotaxin-1(24). The use of RNAi
technology has become a standard method for silencing target genes
in vitro. Due to its small size, ability to easy pass
through the cell membrane and high resistance to nuclease
degradation, siRNA has become the most popular tool for gene
silencing (20). In the present
study, RT-qPCR was employed to select the most effective siRNA for
targeting the Src gene in rats.
The SD rats in the present study developed asthma
following the administration of OVA. H&E staining revealed that
the rats that were exposed to OVA and treated with PBS or empty
vector had lung tissue damage, inflammatory cell effusion,
increased mucus secretion and narrowed alveolar intervals. These
findings indicated that the asthmatic models were successfully
established. siRNA targeting Src was injected intravenously into
the rats in the siRNA group, and OVA and aluminium hydroxide were
further administered to the rats in the PBS, empty vector and siRNA
groups. The results of H&E staining in the siRNA group showed
that inflammatory cell effusion and mucous secretion were markedly
decreased compared with those in the PBS and empty vector groups.
The levels of EOSs and WBCs in the BALF of all OVA-exposed rats
were higher than those in the control group, but the levels in the
siRNA group were lower than those in the PBS and empty vector
groups, and no difference was detected between the PBS and empty
vector groups. These results indicate that interfering with Src
expression ameliorated the pathological conditions of asthma to a
certain extent.
RT-qPCR and western blot analyses were performed,
which indicated that the expression of Src mRNA and protein in the
lung tissues of the asthmatic rats in the PBS and empty vector
groups were significantly higher than those in the control group,
indicating that Src was activated in the rat model of asthma. The
Src mRNA and protein expression levels in the siRNA group were
lower than those in the PBS and empty vector group, although higher
than those in the control group. Furthermore, Src protein
expression significantly positively correlated with Src mRNA
expression in the siRNA group, indicating that the siRNA
transfection reduced the expression of Src mRNA and suppressed the
expression of Src protein in asthmatic rats, although the effect
was limited.
EOSs play a key role in several chronic airway
diseases, including asthma, as they promote the immune response of
the airway to foreign substances, maintain a partial immune
response and release granule proteins that cause tissue damage
(25-27).
IL-5 is an important pro-inflammatory cytokine and inhibitor of
eosinophil apoptosis. Previous research has indicated that IL-5
inhibitors can effectively reduce the concentration of EOSs in the
airways and blood (28). IL-33 is
an activator in type 2 inflammation. IL-33 and type 2 cytokines are
induced by rhinovirus in asthmatic airways and their expression
levels are associated with the severity of asthma; previous studies
have shown that IL-33 is an important mechanistic link in the
association between rhinovirus infection and aggravation of asthma
(29,30). Immune responses induced by IFN-γ are
also dominant in severe asthma in adults (31,32).
IFN-γ has a regulatory effect on immune and non-immune cells and
the ability to regulate mast cells in asthma. IFN-γ has been shown
to induce airway epithelial cells to release prostaglandins and
stimulate β adrenergic receptors on airway smooth muscle, thereby
regulating airway function (33).
In the present study, following the use of siRNA transfection
technology to interfere with Src expression in rats, the
concentrations of IL-5, IL-33, and IFN-γ in the BALF were detected
using ELISAs. The expression levels of IL-5 and IL-33 in the PBS
and empty vector groups were higher than those in the control
group. However, the administration of Src siRNA reduced the
expression levels of IL-5 and IL-33 compared with those in the PBS
and empty vector groups, indicating that the interference with Src
expression inhibited the release of tracheal inflammatory factors.
The IFN-γ level in the siRNA group was higher than those in the PBS
and empty vector groups but lower than that in the control group.
These findings suggest that the expression of Src mRNA inhibits
IFN-γ, leading to destruction of the tracheal structure, and this
was attenuated by the knockdown of Src.
Through the correlation analysis of Src mRNA
expression with EOSs, IL-5, IL-33 and IFN-γ, the levels of EOSs,
IL-5 and IL-33 were shown to be positively correlated with Src mRNA
expression, while IFN-γ was negatively correlated with Src mRNA
expression. However, as the sample size was small, the accuracy of
the correlation analysis may be limited. Therefore, the results
require validation by a study with a larger sample size.
In conclusion, the present study utilized gene
transfer techniques to interfere with the expression of Src in rats
and indicated that siRNA transfection decreased the levels of IL-5
and IL-33 and increased the levels of IFN-γ in lung tissue, reduced
the levels of WBCs and EOSs in the BALF, and effectively
ameliorated tracheal tissue pathology in asthmatic rats. These
results suggest that Src protein tyrosine kinase inhibitors have
potential as a novel method for the treatment of asthma in the
future.
Supplementary Material
Experimental conditions of
quantitative PCR.
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by a grant from the Natural
Science Foundation of Inner Mongolia of China (grant no.
2013MS1169).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX and RW conceived the study, contributed to the
interpretation of the results and edited the manuscript. All
authors participated in the design of the study. MW and JY
contributed to study design, the development of methods, specific
experiments and drafting the article. TL, PX and BB performed
experiments and collected data. TL performed the statistical
analysis and interpretation of the results. All authors have read
and approved the final manuscript. XX and RW confirm the
authenticity of all the raw data. XX and RW are guarantors of this
study, had full access to all the data in the study and take
responsibility for the integrity of the data and the accuracy of
the data analysis.
Ethics approval and consent to
participate
All procedures involving animals and their care were
conducted in accordance with the Guide for the Care and Use of
Laboratory Animals, established by the National Academy of Sciences
and published by the National Institutes of Health. The study was
approved by the Ethics Committee of The Third Affiliated Hospital
of Baotou Medical College (Baotou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Global Asthma Network. Global asthma
report 2018. http://www.globalasthmanetwork.org. Accessed October
14, 2020.
|
|
2
|
British Thoracic Society; Scottish
Intercollegiate Guidelines Network. British guideline on the
management of asthma. Thorax. 58 (Suppl 1):i1–i94. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Turner S, Paton J, Higgins B and Douglas
G: British Guidelines on the Management of Asthma. British
guidelines on the management of asthma: what's new for 2011?
Thorax. 66:1104–1105. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kang JY, Kim JW, Kim JS, Kim SJ, Lee SH,
Kwon SS, Kim YK, Moon HS, Song JS, Park SH and Lee SY: Inhibitory
effects of anti-immunoglobulin E antibodies on airway remodeling in
a murine model of chronic asthma. J Asthma. 47:374–380.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Deng YM, Xie QM, Tang HF, Sun JG, Deng JF,
Chen JQ and Yang SY: Effects of ciclamilast, a new PDE 4 PDE4
inhibitor, on airway hyperresponsiveness, PDE4D expression and
airway inflammation in a murine model of asthma. Eur J Pharmacol.
547:125–135. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sun JG, Deng YM, Wu X, Tang HF, Deng JF,
Chen JQ, Yang SY and Xie QM: Inhibition of phosphodiesterase
activity, airway inflammation and hyperresponsiveness by PDE4
inhibitor and glucocorticoid in a murine model of allergic asthma.
Life Sci. 79:2077–2085. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Charbe NB, Amnerkar ND, Ramesh B,
Tambuwala MM, Bakshi HA, Aljabali AAA, Khadse SC, Satheeshkumar R,
Satija S, Metha M, et al: Small interfering RNA for cancer
treatment: Overcoming hurdles in delivery. Acta Pharm Sin B.
10:2075–2109. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Corren J: Cytokine inhibition in severe
asthma: Current knowledge and future directions. Curr Opin Pulm
Med. 17:29–33. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Barnes PJ: Cytokine-directed therapies for
the treatment of chronic airway diseases. Cytokine Growth Factor
Rev. 14:511–522. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Holgate ST: Cytokine and anti-cytokine
therapy for the treatment of asthma and allergic disease. Cytokine.
28:152–157. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Walsh GM: Targeting eosinophils in asthma:
Current and future state of cytokine- and chemokine-directed
monoclonal therapy. Expert Rev Clin Immunol. 6:701–704.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Strasner AB, Natarajan M, Doman T, Key D,
August A and Henderson AJ: The Src kinase Lck facilitates assembly
of HIV-1 at the plasma membrane. J Immunol. 181:3706–3713.
2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
NCBI: Rattus norvegicus SRC
proto-oncogene, non-receptor tyrosine kinase (Src), mRNA. NCBI
Reference Sequence NM_031977.1. https://www.ncbi.nlm.nih.gov/nuccore/14010834/.
Accessed June 5, 2018.
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lee JU, Kim JD and Park CS:
Gene-environment interactions in asthma: Genetic and epigenetic
effects. Yonsei Med J. 56:877–886. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gallelli L, Busceti MT, Vatrella A,
Maselli R and Pelaia G: Update on anticytokine treatment for
asthma. Biomed Res Int. 2013(104315)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Guo S and Kemphues KJ: par-1, a gene
required for establishing polarity in C. elegans embryos, encodes a
putative Ser/Thr kinase that is asymmetrically distributed. Cell.
81:611–620. 1995.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in caenorhabditis elegans. Nature. 391:806–811.
1998.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Krymskaya VP, Goncharova EA, Ammit AJ, Lim
PN, Goncharov DA, Eszterhas A and Panettieri RA Jr: Src is
necessary and sufficient for human airway smooth muscle cell
proliferation and migration. FASEB J. 19:428–430. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
O'Keefe EP: siRNAs and shRNAs: Tools for
protein knockdown by gene silencing. Mater Methods. 3(197)2013.
|
|
21
|
McManus MT and Sharp PA: Gene silencing in
mammals by small interfering RNAs. Nat Rev Genet. 3:737–747.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
McManus MT, Haines BB, Dillon CP,
Whitehurst CE, van Parijs L, Chen J and Sharp PA: Small interfering
RNA-mediated gene silencing in T lymphocytes. J Immunol.
169:5754–5760. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xie Y, Kim NH, Nadithe V, Schalk D, Thakur
A, Kılıç A, Lum LG, Bassett DJP and Merkel OM: Targeted delivery of
siRNA to activated T cells via transferrin-polyethylenimine
(Tf-PEI) as a potential therapy of asthma. J Control Release.
229:120–129. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Choi M, Gu J, Lee M and Rhim T: A new
combination therapy for asthma using dual-function
dexamethasone-conjugated polyethylenimine and vitamin D binding
protein siRNA. Gene Ther. 24:727–734. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Leo C and Chen JD: The SRC family of
nuclear receptor coactivators. Gene. 245:1–11. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liao L, Kuang SQ, Yuan Y, Gonzalez SM,
O'Malley BW and Xu J: Molecular structure and biological function
of the cancer-amplified nuclear receptor coactivator SRC-3/AIB1. J
Steroid Biochem Mol Biol. 83:3–14. 2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ
and O'Malley BW: Partial hormone resistance in mice with disruption
of the steroid receptor coactivator-1 (SRC-1) gene. Science.
279:1922–1925. 1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Karelina T, Voronova V, Demin O, Colice G
and Agoram BM: A mathematical modeling approach to understanding
the effect of anti-interleukin therapy on eosinophils. CPT
Pharmacometrics Syst Pharmacol. 5:608–616. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jackson DJ, Makrinioti H, Rana BM, Shamji
BW, Trujillo-Torralbo MB, Footitt J, Del-Rosario J, Telcian AG,
Nikonova A, Zhu J, et al: IL-33-dependent type 2 inflammation
during rhinovirus-induced asthma exacerbations in vivo. Am J Respir
Crit Care Med. 190:1373–1382. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Barlow JL, Peel S, Fox J, Panova V,
Hardman CS, Camelo A, Bucks C, Wu X, Kane CM, Neill DR, et al:
IL-33 is more potent than IL-25 in provoking IL-13-producing
nuocytes (type 2 innate lymphoid cells) and airway contraction. J
Allergy Clin Immunol. 132:933–941. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Raundhal M, Morse C, Khare A, Oriss TB,
Milosevic J, Trudeau J, Huff R, Pilewski J, Holguin F, Kolls J, et
al: High IFN-γ and low SLPI mark severe asthma in mice and humans.
J Clin Invest. 125:3037–3050. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Leavy O: Asthma and allergy: An IFNγ bias
in severe asthma. Nat Rev Immunol. 15:466–467. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kawakami T and Galli SJ: Regulation of
mast-cell and basophil function and survival by IgE. Nat Rev
Immunol. 2:773–786. 2002.PubMed/NCBI View
Article : Google Scholar
|