Introduction
Breast cancer is a malignant tumor and the second
most common cause of cancer-associated mortality worldwide
(1). Breast cancer may spread to
distant organs via metastasis, and 90% of breast cancer mortalities
are attributable to metastasis (2).
At present, the pathogenesis and mechanism of breast cancer remain
unclear; therefore, elucidation of the pathogenesis of breast
cancer is urgently necessary in order to determine specific novel
targets for the diagnosis and treatment of breast cancer.
MicroRNAs (miRs) are single-stranded non-coding RNAs
that may recognize specific target mRNAs at the
post-transcriptional level (3). The
expression levels of the target genes are downregulated via the
promotion of mRNA degradation and/or inhibition of the translation
process (4). Furthermore, there is
evidence to suggest that microRNAs may serve as suppressors or
promoters in the development of various types of cancer. For
example, the overexpression of miR-3196 suppresses breast cancer
cell proliferation and induces apoptosis through targeting
ERBB3(5), whereas miR-24-3p has
been reported to promote breast cancer cell proliferation and
inhibit apoptosis by targeting p27Kip1(6). In a previous study, microRNA-615 was
identified to be downregulated in breast cancer, suggesting that
microRNA-615 is a potential tumor suppressor in breast cancer
(7). Furthermore, miR-615-5p may
also suppress cell proliferation and invasion in lung cancer
(8) and pancreatic ductal
adenocarcinoma (9). However, to the
best of our knowledge, the association between breast cancer and
the ectopic expression of microRNA-615-5p has not yet been
elucidated.
In the present study, heat shock factor (HSF1) was
identified as a target gene of microRNA-615-5p, and the regulatory
mechanism and expression of microRNA-615-5p and HSF1 were examined.
The results of the present study demonstrated that microRNA-615-5p
may function as a tumor suppressor and may provide a theoretical
basis for the early diagnosis and treatment of breast cancer.
Materials and methods
Specimens
A total of 40 pairs of breast cancer tissues and
normal adjacent breast tissues were obtained from Qilu Hospital of
Shandong University (Jinan, China) between June 2015 and January
2016. The patients comprised 40 females, whose mean age was
47.2±7.3 years. All patients were pathologically diagnosed with
breast cancer (Table I) and had not
received chemotherapy or radiotherapy prior to the present study.
Written consent was obtained from each patient. The present study
was approved by the Ethics Committee of Qilu Hospital of Shandong
University.
 | Table IAssociation between miR-615-5p
expression status and clinicopathological features of patients with
breast cancer. |
Table I
Association between miR-615-5p
expression status and clinicopathological features of patients with
breast cancer.
| | | Expression
level | |
|---|
| Clinicopathological
feature | Cases | High miR-615-5p
(n=10) | Low miR-615-5p
(n=30) | P-value |
|---|
| Age | | | | 0.271 |
|
≤60
years | 22 | 4 | 18 | |
|
>60
years | 18 | 6 | 12 | |
| Tumor size | | | | 0.000 |
|
≥2 cm | 29 | 3 | 26 | |
|
>2
cm | 11 | 7 | 4 | |
| Tumor location | | | | 0.361 |
|
Left | 21 | 4 | 17 | |
|
Right | 19 | 6 | 13 | |
| ER status | | | | 0.714 |
|
Negative | 22 | 6 | 16 | |
|
Positive | 18 | 4 | 14 | |
| TNM stage | | | | 0.126 |
|
I/II | 31 | 6 | 25 | |
|
III/IV | 9 | 4 | 5 | |
| Lymph node
metastasis | | | | 0.002 |
|
Negative | 7 | 5 | 2 | |
|
Positive | 33 | 5 | 28 | |
Cell culture
The MCF-7 human breast cancer cell line (Type
Culture Collection of the Chinese Academy of Sciences, Shanghai,
China) was cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.), and 1% penicillin and
streptomycin. The cells were cultured at 37˚C in a 5%
CO2 humidified incubator.
Transfection efficiency of
microRNA-615-5p
The MCF-7 cells were divided into three groups: i)
Control group (untreated); ii) negative control (NC) group
(transfected with microRNA-615-5p mimics NC); and iii) mimics group
(transfected with microRNA-615-5p mimics). The MCF-7 cells were
seeded onto six-well plates at a density of 1x105
cells/well, and 50 nmol/l oligonucleotide (microRNA-615-5p mimics
or NC) were subsequently transfected into cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
microRNA-615-5p mimics and NC oligonucleotides were purchased from
Shanghai GenePharma Co., Ltd. (Shanghai, China). The sequences of
the miR-615-5p mimics were as follows: 5'-GGGGGUCCCCGGUGCUCGGAUC-3'
(sense) and 5'-UCCGAGCACCGGGGACCCCCUU-3' (anti-sense). The
sequences of the NC were as follows: 5'-UUCUCCGAACGUGUCACGUTT-3'
(sense) and 5'-ACGUGACACGUUCGGAGAATT-3' (anti-sense).
Transfection efficiency of HSF1
The MCF-7 cells were divided into three groups: i)
Control group (untreated); ii) pcDNA3.1 group (transfected with
pcDNA3.1); and iii) pcDNA3.1-HSF1 group (transfected with
pcDNA3.1-HSF1). The cells were seeded onto six-well plates at a
density of 2x104 cells/well, and 2 µg/ml pcDNA3.1
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) or pcDNA3.1-HSF1
was subsequently transfected into the cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Vector construction and
co-transfection assay
The HSF1-expression vector was constructed by
inserting HSF1 into a pcDNA3.1 vector. In brief, HSF1 cDNA was
amplified by polymerase chain reaction (PCR) from the cDNA of
MCF-10A cells (Bena Culture Collection, Beijing, China). The HSF1
cDNA was then inserted into pcDNA3.1 (Thermo Fisher Scientific,
Inc.) to construct a pcDNA3.1-HSF1 expression vector. To evaluate
whether or not HSF1 overexpression attenuates the
microRNA-615-5p-induced suppression of HSF1, MCF-7 cells were
divided into three different groups: i) NC group (microRNA-615-5p
mimics NC); ii) mimics group (transfected with microRNA-615-5p
mimics); and iii) mimics + HSF1 group (transfected with
microRNA-615-5p mimics and pcDNA3.1-HSF1). Briefly, cells were
seeded onto six-well plates at a density of 1x105
cells/well, and co-transfected with 50 nmol/l NC or microRNA-615-5p
mimics with or without 2 µg/ml pcDNA3.1-HSF1 using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Analysis of cell proliferation
Following 24 h of transfection, the cells were
washed with PBS, re-seeded onto a 96-well plate at a density of
1x104 cells/well and cultured in fresh RPMI-1640 medium
for 12, 24 or 48 h. Subsequently, a Cell Counting kit-8 (CCK-8)
cell proliferation assay was conducted using the CCK-8 kit (Dojindo
Molecular Technologies Inc., Kumamoto, Japan). The absorbance of
each well was measured at 450 nm using a microplate reader.
Analysis of cell apoptosis
At 48 h post-transfection, the MCF-7 cells were
washed with PBS. An Annexin V-fluorescein isothiocyanate (FITC)
apoptosis kit (BD Biosciences, San Jose, CA, USA) was used to
quantify apoptotic MCF-7 breast cancer cells by flow cytometry
(FACSCalibur, BD Biosciences) using Cell Quest Pro software
(version 6.0; BD Biosciences), according to the manufacturer's
protocol.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from patient tissue samples
and MCF-7 cells using a microRNAeasy kit (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. To
quantify the microRNA-615-5p and HSF1 mRNA expression in the 40
paired tumor and adjacent tissue samples and MCF-7 cells at 48 h
post-transfection. In total, 2 µl RNA was isolated from the
adjacent and tumor samples and reverse transcribed into cDNA using
the TaqMan MicroRNA RT kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. qPCR
step was subsequently performed using TaqMan Universal Master mix
(Thermo Fisher Scientific, Inc.). In total, 2 µl cDNA, 10 µl TaqMan
Universal Master mix, 1 µl primers and nuclease-free
H2O. The primer sequences used were as follows:
microRNA-615-5p forward, 5'-GCCAGCCACCAAGAAGC-3' and reverse,
5'-GCTCCCGCTGTTTACTCTG-3'; U6 forward,
5'-GCTTCGGCAGCACATATACTAAAAT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'; HSF1 forward,
5'-GCCTTCCTGACCAAGCTGT-3' and reverse, 5'-GTCGAACACGTGGAAGCTGT-3';
Bcl-2 forward, 5'-ACAACATCGCCCTGTGGATGAC-3' and reverse,
5'-ATAGCTGATTCGACGTTTTGCC-3'; cyclin D1 forward,
5'-CCTGTCCTACTACCGCCTCA-3' and reverse, 5'-TCCTCCTCTTCCTCCTCCTC-3';
PCNA forward, 5'-CTCCAACTTCTGGGCTCAAG-3' and reverse,
5'-GTAAACGGACTGCTGGAGGA-3'; Bax forward,
5'-GGAATTCTGACGGCAACTTCAACTGGG-3' and reverse,
5'-GGAATTCTTCCAGATGGTGAGCGAGG-3'; GAPDH forward,
5'-GAAGGTGAAGGTCGGAGTC-3' and reverse 5'-GAAGATGGTGATGGGATTTC-3'.
The thermocycling conditions used were as follows: Initial
denaturation at 95˚C for 10 min; 40 cycles of 95˚C for 10 sec and
62˚C for 15 sec. U6 small nuclear RNA and GAPDH were used as the
endogenous controls. The relative expression level of
microRNA-615-5p was normalized to U6, while the expression levels
of HSF1, B-cell lymphoma 2 (Bcl-2), cyclin D1, proliferating cell
nuclear antigen (PCNA) and Bcl-2-associated X protein (Bax) were
normalized to GAPDH using the 2-ΔΔCq method (10).
Western blot analysis
Total protein was extracted from different groups of
treated MCF-7 cells 48 h post-transfection using M-PER protein
extraction reagent (Pierce; Thermo Fisher Scientific, Inc.)
supplemented with a protease mimics cocktail (Thermo Fisher
Scientific, Inc.). Total protein was quantified using the Bradford
method and 10 µg protein/lane was separated via SDS-PAGE on a 10%
gel (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The separated proteins were transferred
onto polyvinylidene fluoride membranes (Shanghai Ofluorine Chemical
Technology Co., Ltd., Shanghai, China) and blocked in 5% nonfat
milk for 2 h at 37˚C. The membranes were incubated overnight at 4˚C
with the following primary antibodies: Mouse anti-HSF1 (1:1,000;
cat. no. ab201978; Abcam, Cambridge, UK), mouse anti-Bcl-2
(1:1,000; cat. no. ab692; Abcam), rabbit anti-cyclin D1 (1:100;
cat. no. ab16663; Abcam), mouse anti-PCNA (1:1,000; cat. no. ab29;
Abcam), rabbit anti-Bax (1:1,000; cat. no. ab32503; Abcam) and
mouse anti-GAPDH (1:1,000; cat. no. ab8245; Abcam). Subsequent to
washing, the membranes were incubated for 1 h at 37˚C with
horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG (1:1,000;
cat. no. A0208; Beyotime, Shanghai, China) and HRP-labeled goat
anti-mouse secondary antibody (1:1,000; cat. no. A0216; Beyotime,
Shanghai, China). Subsequent to washing, 200 µl Abcam
Chemiluminescent Horseradish Peroxidase Substrate (Abcam) was added
to the membrane surface. The signals were captured and the
intensity of the bands was quantified. ImageJ software (version
1.49; National Institutes of Health, Bethesda, MD, USA) was used to
determine the protein expression levels of HSF1, Bcl-2, PCNA,
cyclin D1 and Bax relative to those of GAPDH. GAPDH served as the
internal control.
Dual luciferase reporter assay
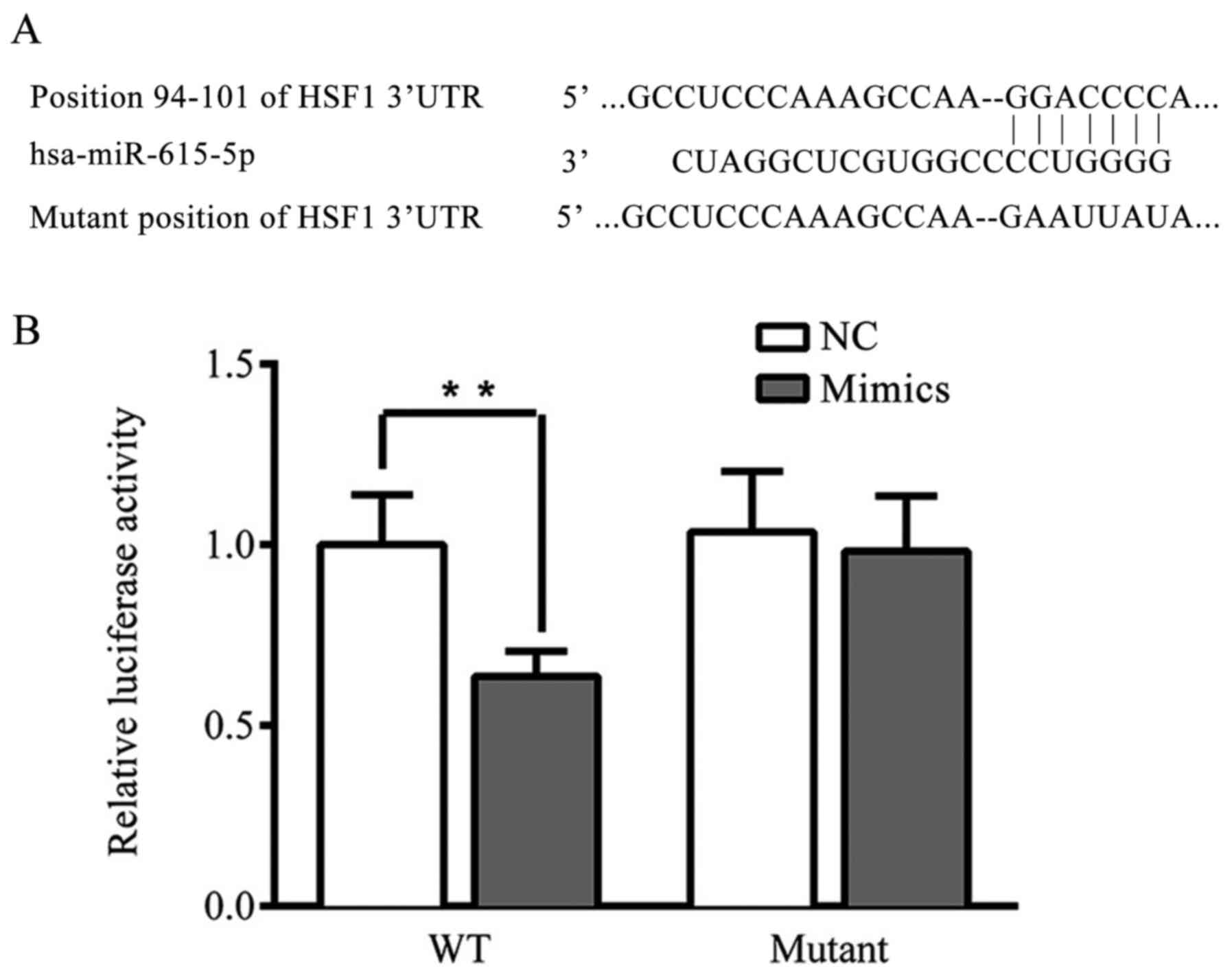
TargetScan bioinformatics analysis (www.targetscan.org) was used to identify HSF1 as a
potential target of miR-615-5p (11). The 293 cells (Type Culture
Collection of the Chinese Academy of Sciences) were seeded onto a
six-well plate at a density of 1x105 cells/well and
transfected with the wild-type (WT) HSF1 3'untranslated region
(UTR; WT HSF1-3'UTR) or mutant HSF1 3'UTR (MUT HSF1 3'UTR) in
combination with either NC or microRNA-615-5p mimics using
Lipofectamine® RNAi Max (Thermo Fisher Scientific,
Inc.). Transfected cells were subsequently incubated at 37˚C for 48
h and the luciferase activity was examined using a Dual-Luciferase
Reporter kit (Beyotime Institute of Biotechnology, Haimen, China).
Firefly luciferase activity was normalized to Renilla
luciferase activity.
Statistical analysis
SPSS version 22.0 (IBM Corp., Armonk, NY, USA) was
used to analyze the results. Data are presented as the mean ±
standard deviation. Statistical comparisons between two groups were
conducted using Student's t-test, while one-way analysis of
variance followed by Newman-Keuls tests was used to analyze
differences among three or more groups. Pearson's correlation
analyses were performed to evaluate the correlation between
miR-615-5p and HSF1. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of microRNA-615-5p and HSF1
in breast cancer and adjacent tissues
Using the online bioinformatics tool TargetScan,
HSF1 was predicted as a potential target gene of microRNA-615-5p.
To verify the biological functions of microRNA-615-5p and HSF1 in
the development of breast cancer, the expression levels of
microRNA-615-5p and HSF1 in the breast cancer tissues and normal
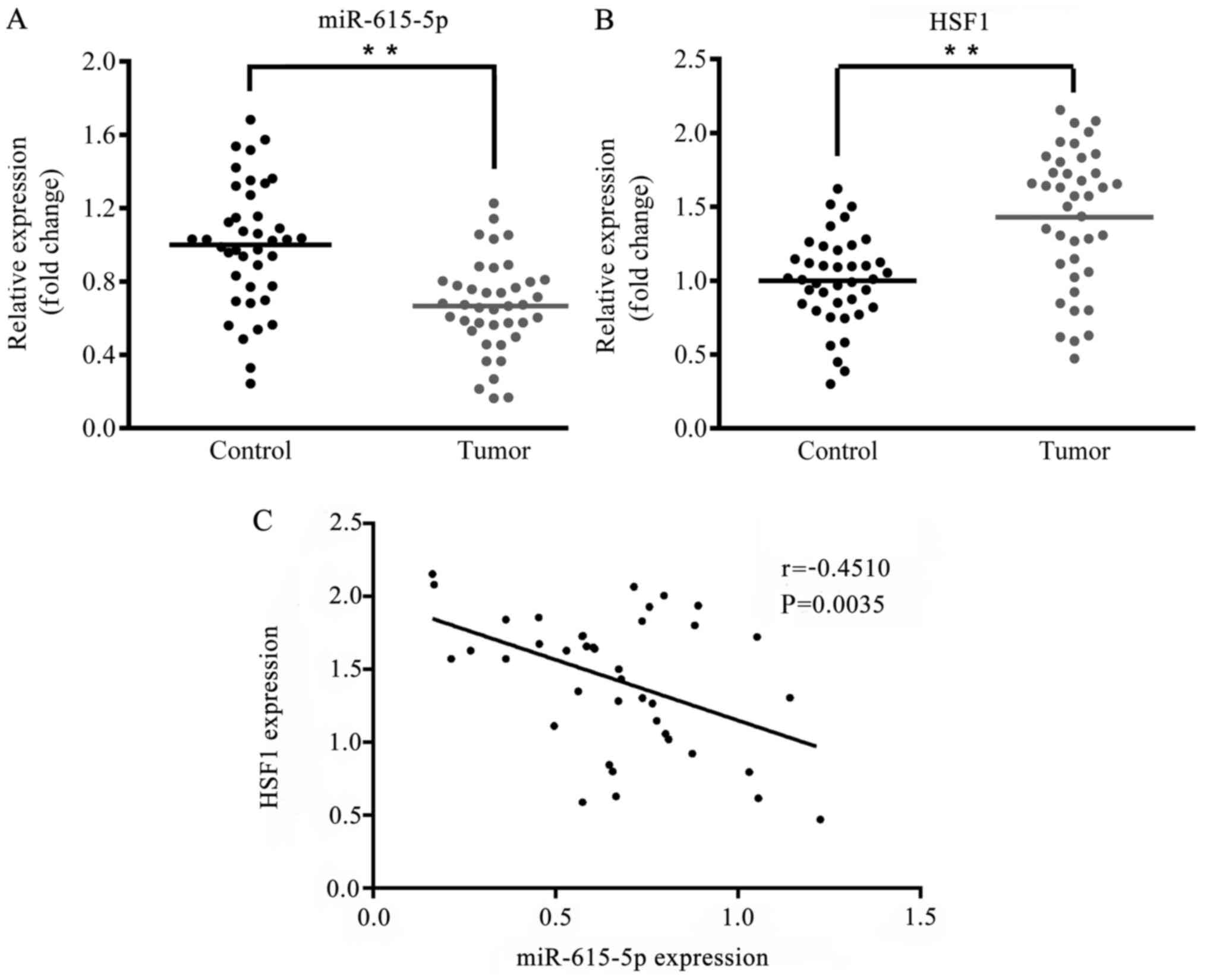
adjacent tissues from 40 patients were compared. As presented in
Fig. 1A and B, in tumor tissues the expression level of
microRNA-615-5p was significantly decreased (P<0.01) whereas the
expression level of HSF1 mRNA was significantly increased
(P<0.01) compared with the respective levels in the normal
adjacent tissues. Furthermore, Pearson's correlation analysis
indicated that the expression levels of microRNA-615-5p and HSF1
were negatively correlated (Fig.
1C; r=-0.4510, P=0.0035).
Transfection efficiency of
microRNA-615-5p and HSF1
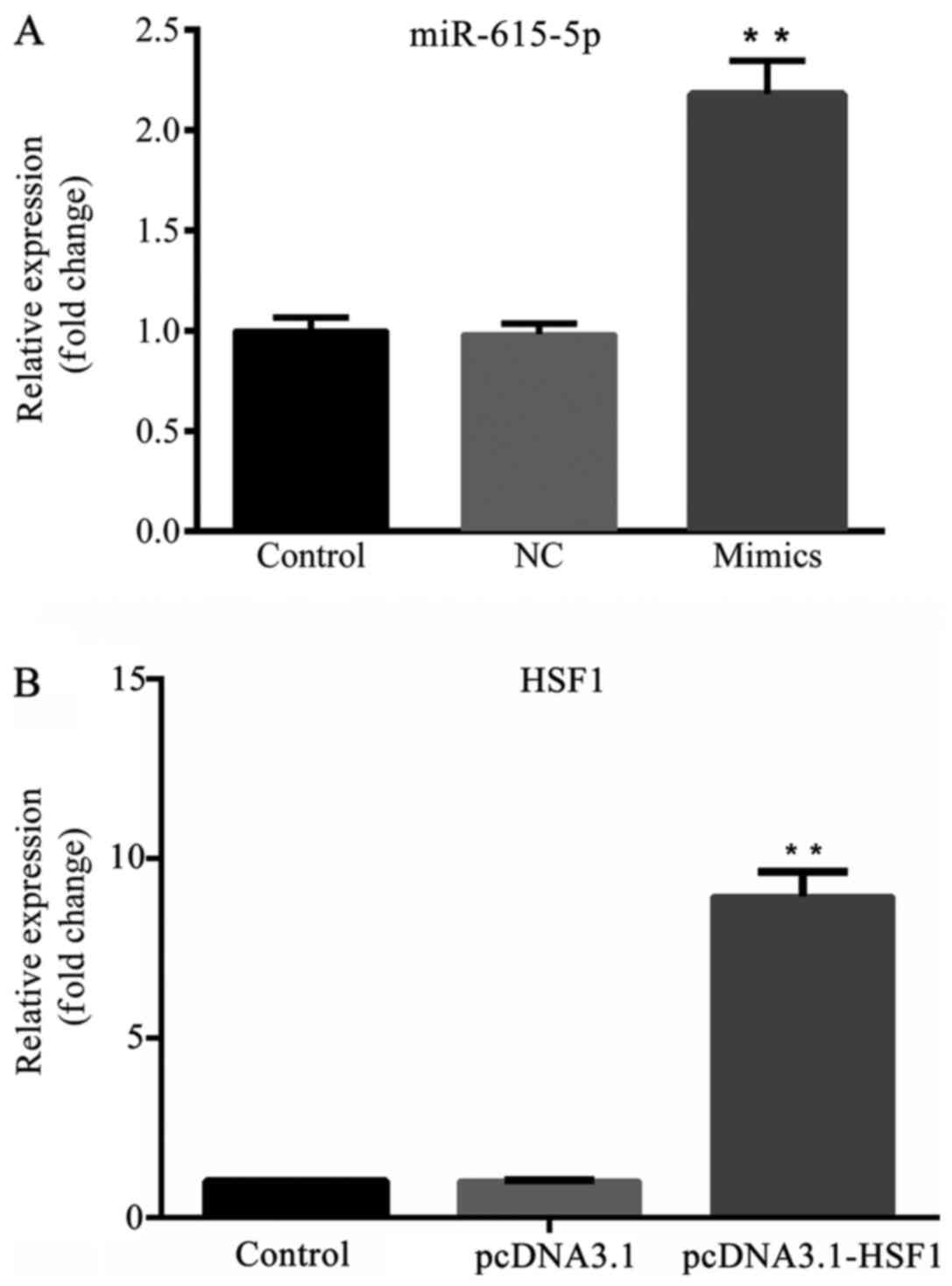
As shown in Fig. 2A,
the expression level of microRNA-615-5p was significantly higher in
the mimics group compared with the NC group (P<0.01), and no
significant difference was detected between the control and NC
groups. Furthermore, the expression level of HSF1 was significantly
increased in the pcDNA3.1-HSF1 group compared with the pcDNA3.1 and
control groups (Fig. 2B;
P<0.01).
Effect of microRNA-615-5p on the
proliferation of MCF-7 cells
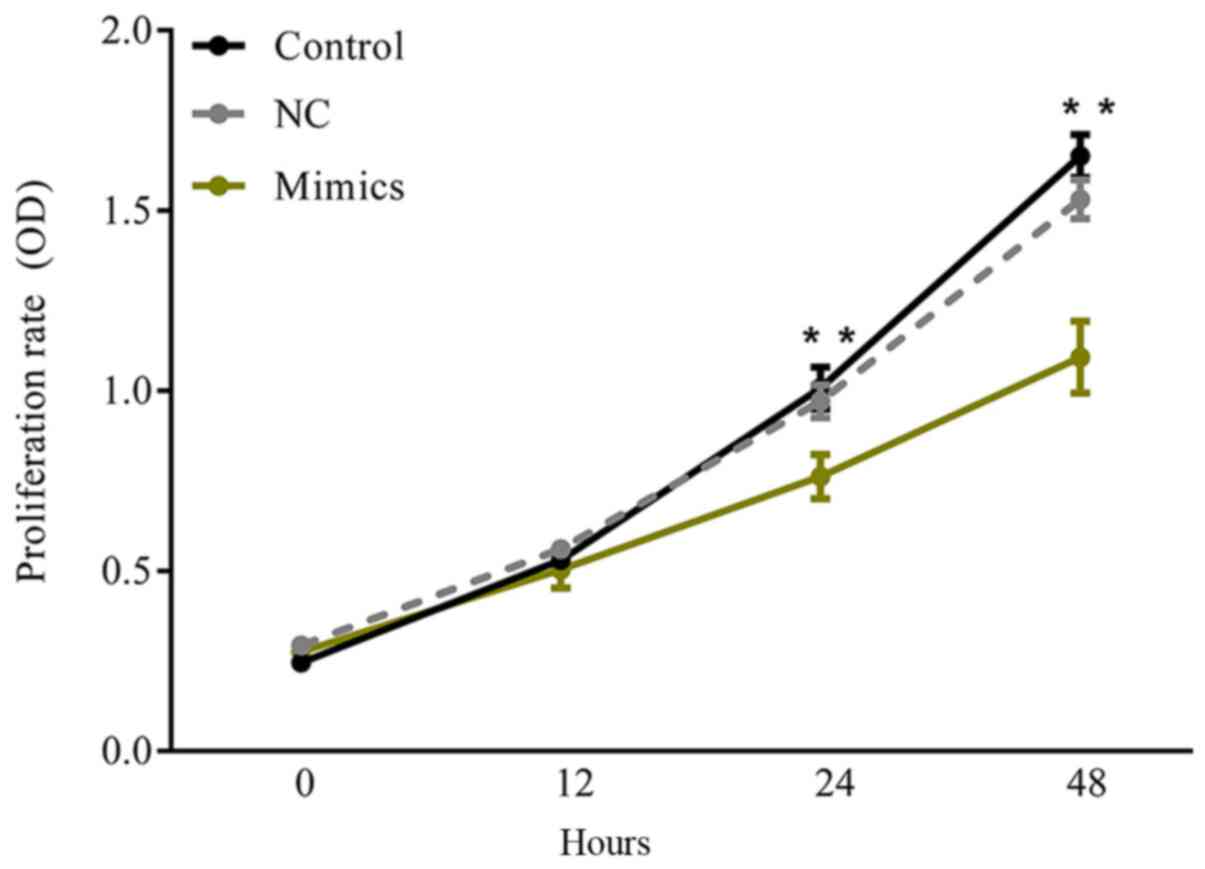
A CCK-8 assay was performed to investigate the
effect of microRNA-615-5p on breast cancer cell growth. The results
demonstrated that there was no significant difference in the
proliferation rate between the control and NC groups during the
48-h test period (Fig. 3;
P>0.05). However, at 24 and 48 h, the cells transfected with
microRNA-615-5p-mimics presented significantly decreased
proliferation compared with the control groups (Fig. 3; P<0.01).
Effect of microRNA-615-5p on the
apoptosis of MCF-7 cells
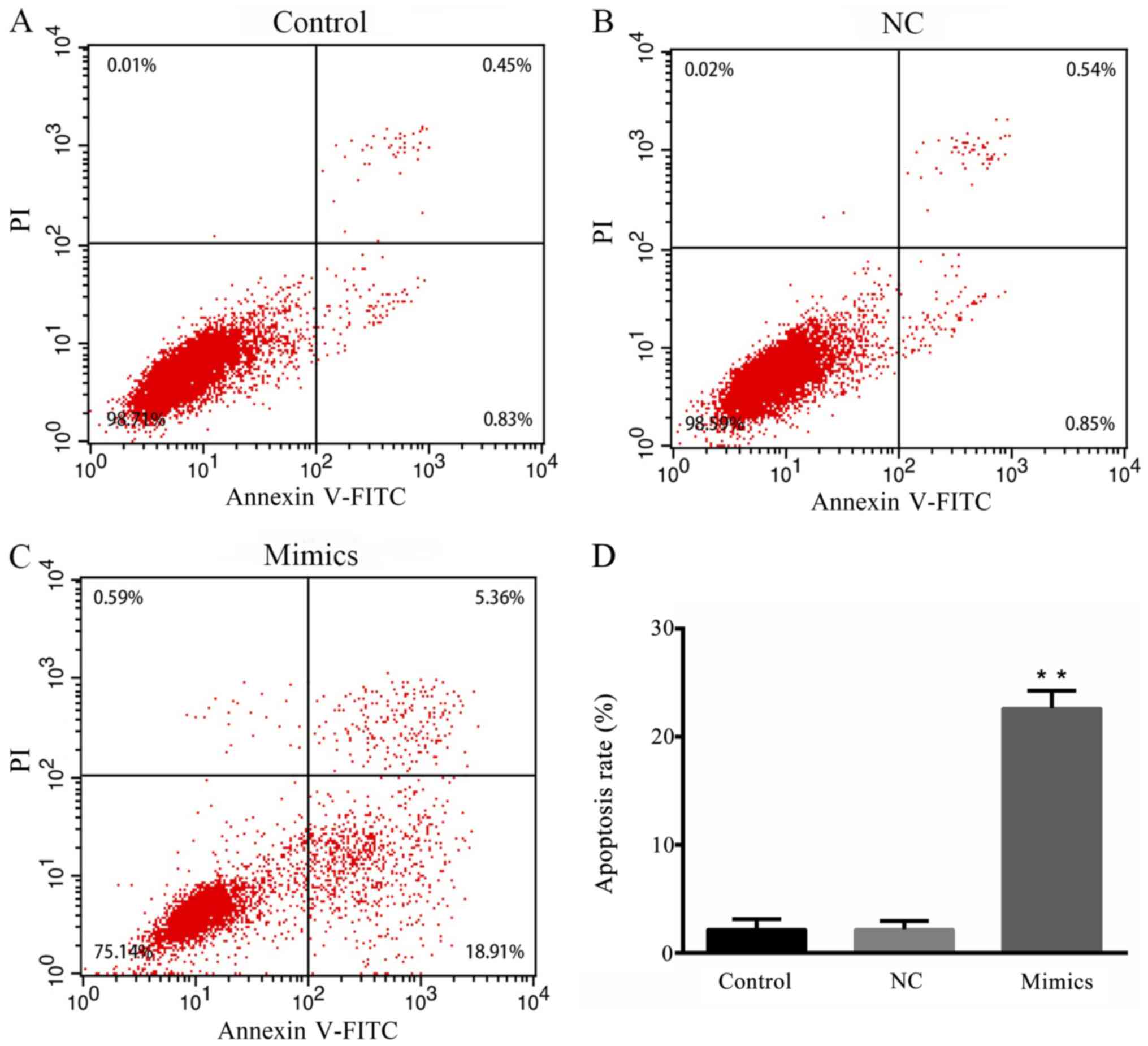
To investigate the effect of microRNA-615-5p on
breast cancer cell apoptosis, an Annexin V-FITC apoptosis kit was
used to examine the apoptosis of the cells in different groups
(Fig. 4A-C). The apoptotic rates of
the control and NC groups were ~1.28 and 1.39%, respectively; the
rate of apoptosis in the microRNA-615-5p mimics group was ~18-fold
higher, and was significantly increased compared that in with the
control groups (P<0.01; Fig.
4D).
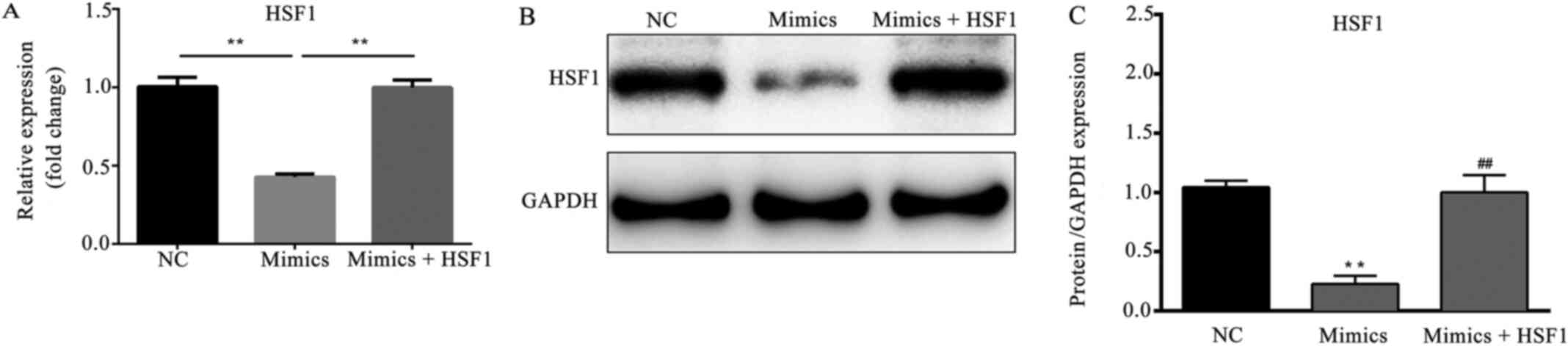
HSF1 overexpression attenuates the
microRNA-615-5p-induced suppression of HSF1
As shown in Fig. 5A,
the mRNA expression level of HSF1 was significantly decreased in
the microRNA-615-5p mimics group compared with the NC group
(P<0.01). However, the reduction in HSF1 expression was
attenuated by the co-transfection of pcDNA3.1-HSF1 (P<0.01). The
western blotting results for HSF1 protein presented a trend of
variation similar to that of the mRNA (P<0.01; Fig. 5B and C).
HSF1 is a direct target of
microRNA-615-5p in breast cancer
The interaction between HSF1 and microRNA-615-5p was
examined using a dual luciferase gene reporter assay. The results
demonstrated that the transfection of microRNA-615-5p significantly
restrained the luciferase activity in the WT HSF1 3'UTR
plasmid-transfected cells (P<0.01), whereas microRNA-615-5p had
no significant effect on the MUT HSF1 3'UTR plasmid-transfected
cells (Fig. 6).
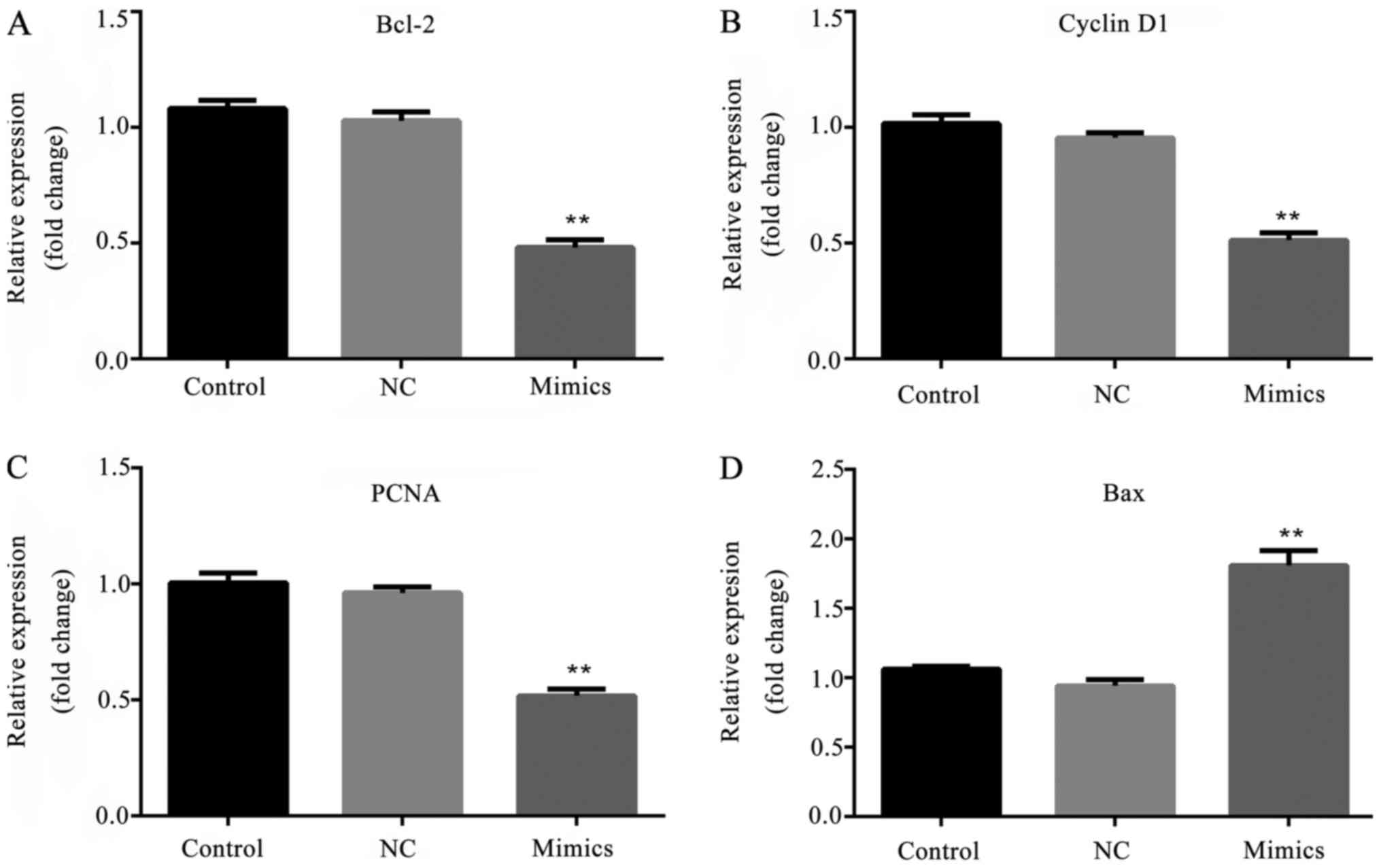
Effect of microRNA-615-5p on the
expression levels of Bcl-2, cyclin D1, PCNA and Bax
In order to investigate the effect of
microRNA-615-5p on proliferation- and apoptosis-associated factors,
the mRNA and protein expression levels of Bcl-2, cyclin D1, PCNA
and Bax were tested. The RT-qPCR data demonstrate that in the
microRNA-615-5p mimics group, the mRNA expression levels of Bcl-2,
cyclin D1 and PCNA were significantly decreased, whereas the
expression level of Bax was significantly increased compared with
the respective levels in the control groups (P<0.01; Fig. 7). Furthermore, the protein
expression levels exhibited the same trend of variation as the mRNA
results (P<0.01; Fig. 8).
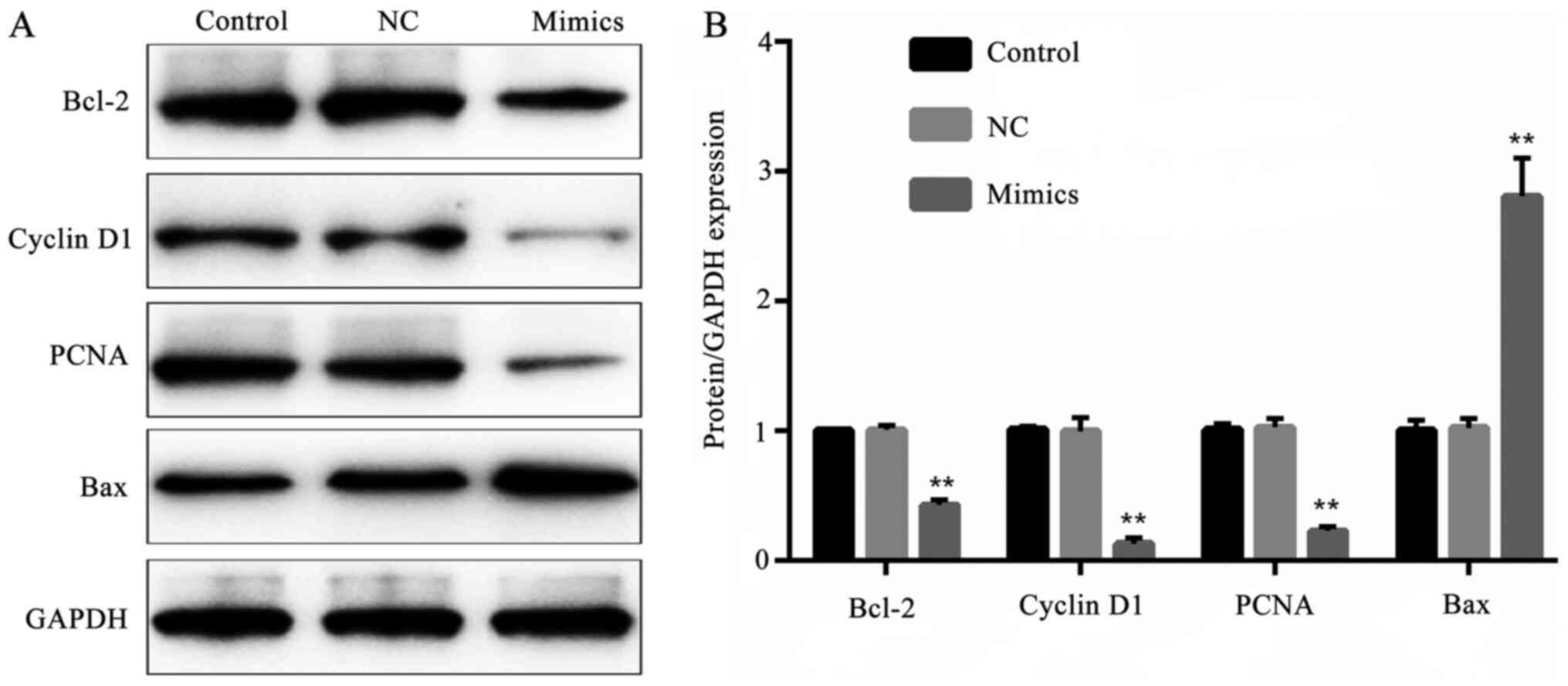 | Figure 8Effect of microRNA-615-5p on protein
expression levels of proliferation- and apoptosis-associated
factors. (A) The protein expression levels of Bcl-2, cyclin D1,
PCNA and Bax detected by western blot assay. (B) Quantified
protein/GAPDH expression levels in different groups.
**P<0.01, mimics vs. NC group. Control, untransfected
cells; NC, microRNA-615-5p negative control-transfected cells;
mimics, microRNA-615-5p mimics-transfected cells; Bcl-2, B-cell
lymphoma 2; PCNA, proliferating cell nuclear antigen; Bax,
bcl-2-like protein 4; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
Discussion
In the present study, the effects of microRNA-615-5p
on the progression of breast cancer at the cellular level were
investigated via the transfection of microRNA-615-5p mimics into
MCF-7 cells. The results demonstrated that the overexpression of
microRNA-615-5p suppressed the growth and promoted the apoptosis of
MCF-7 cells. In addition, bioinformatic analysis predicted that
HSF1 was a target gene of microRNA-615-5p, which was confirmed by a
dual luciferase reporter assay. The present study is consistent
with previous studies, which demonstrated that microRNA-615-5p
functions as a tumor suppressor (12), whereas HSF1 serves as a tumor
promoter (13).
In previous studies, it has been identified that
microRNA-615-5p functions as a tumor suppressor in pancreatic
ductal adenocarcinoma (9,14). Furthermore, microRNA-615 may inhibit
prostate cancer cell proliferation and invasion by directly
targeting cyclin D2(15).
MicroRNA-615 has also been indicated to be a tumor suppressor that
may inhibit breast cancer carcinogenesis by suppressing RAC-b
serine/threonine-protein kinase expression (7). In the present study, the results of
the cell proliferation and apoptosis assays demonstrated that
microRNA-615-5p serves as a tumor suppressor in breast cancer,
which is consistent with the aforementioned previous studies.
MicroRNAs typically regulate cell proliferation and
apoptosis by targeting specific genes. An association of HSF1 with
oncogenesis has previously been identified (16). A number of previous studies have
demonstrated that HSF1 is overexpressed in various types of cancer,
including hepatocellular carcinoma (17), colorectal cancer (18), breast cancer (19) and others (20-24).
In the present study, proliferation- and apoptosis-associated
factors, namely Bcl-2, cyclin D1, PCNA and Bax, were evaluated
using RT-qPCR and western blot analysis. The results of the western
blot analysis indicate that HSF1 may function as an oncogene in
breast cancer.
PCNA is a cell cycle marker that serves a role in
the regulation of the cell proliferation process, and was first
identified by Miyachi et al (25). Several studies have demonstrated
that PCNA upregulation may promote breast cancer cell proliferation
(26-28).
Furthermore, a study observed that loss of HSF1 reduced the
expression of PCNA in vivo (29). Cyclin D1 overexpression has been
reported to correlate with early cancer onset and tumor progression
(30). Furthermore, cyclin D1 was
found to be overexpressed in breast cancer tissues, suggesting that
it may serve as a potential biomarker (31). A number of studies have indicated
that the suppression of cyclin D1 may inhibit breast cancer cell
proliferation (32-34).
Furthermore, the forced expression of HSF1 has been shown to
increase the expression of cyclin D1(35). In the present study, it was
identified that the expression levels of HSF1, PCNA and cyclin D1
were decreased following transfection with microRNA-615-5p-mimics.
Therefore, it is hypothesized that microRNA-615-5p may inhibit
breast cancer cell proliferation by targeting HSF1, which may
decrease the expression levels of PCNA and cyclin D1.
Bcl-2 is recognized as an important factor with
anti-apoptotic effects and was originally identified in human
follicular B cell lymphoma (36).
Bcl-2 expression has been revealed to be downregulated in
triple-negative breast cancer (37,38).
Wang et al (39)
demonstrated that HSF1 knockdown was able to restrain the
expression of Bcl-2 in breast cancer. Bax is a proapoptotic protein
associated with cell apoptosis (40,41).
The increased expression of Bax has been confirmed to promote
breast cancer cell apoptosis (42,43).
In addition, Lou et al (44)
demonstrated that HSF1 knockdown increased the expression of Bax.
The results of the present study demonstrated that the expression
of Bcl-2 was decreased whereas that of Bax was increased when the
cells were transfected with microRNA-615-5p mimics. Therefore, it
is hypothesized that microRNA-615-5p may promote breast cancer cell
apoptosis by targeting HSF1, which may decrease the expression of
the anti-apoptotic protein Bcl-2 and increase the expression of the
proapoptotic protein Bax.
However, a previous study reported that insulin-like
growth factor 2 (IGF2) is directly downstream of microRNA-615-5p
(45). MicroRNA-615-5p may inhibit
hepatocellular carcinoma cell invasion by directly suppressing
IGF2(46). In addition, IGF2 has
been found to be upregulated in breast cancer (47), indicating that microRNA-615-5p may
also regulate breast cancer cell proliferation and apoptosis via
interaction with IGF2. Therefore, whether or not the
microRNA-615-5p/IGF2 axis participates in the regulation of breast
cancer cell proliferation and apoptosis requires further
investigation. Other studies have indicated that HSF1 is downstream
of human epidermal growth factor receptor 2 (HER2) and could be
activated by HER2 (48,49). Therefore, the interaction of
microRNA-615-5p and HER2 in the regulation of breast cancer cell
proliferation and apoptosis remains unclear and also requires
further investigation.
In conclusion, the present results demonstrated that
microRNA-615-5p promoted the apoptosis of breast cancer cells and
inhibited their growth by downregulating the expression levels of
HSF1, Bcl-2, PCNA and cyclin D1, and increasing the expression of
Bax. Therefore, targeting microRNA-615-5p is potentially a
promising method for the treatment of breast cancer. However, two
limitations remain: i) The underlying targeted relationship between
microRNA-615-5p and HER2 requires further elucidation, and ii)
further investigation of HER-positive breast cancer cell lines is
necessary.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KL designed the experiments, analyzed the patient
data and purchased the reagents. RM performed the examination, and
was a major contributor in writing the manuscript. Both authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Qilu Hospital of Shandong University (Jinan, China).
Written consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hesari A, Azizian M, Darabi H, Nesaei A,
Hosseini SA, Salarinia R, Motaghi AA and Ghasemi F: Expression of
circulating miR-17, miR-25, and miR-133 in breast cancer patients.
J Cell Biochem, Nov 28, 2018 (Epub ahead of print). doi:
10.1002/jcb.27984.
|
|
3
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dalmay T: Mechanism of miRNA-mediated
repression of mRNA translation. Essays Biochem. 54:29–38.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ji CZ, Han SH and Xing YF: Overexpression
of miR-3196 suppresses cell proliferation and induces cell
apoptosis through targeting ERBB3 in breast cancer. Eur Rev Med
Pharmacol Sci. 22:8383–8390. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lu K, Wang J, Song Y, Zhao S, Liu H, Tang
D, Pan B, Zhao H and Zhang Q: miRNA-24-3p promotes cell
proliferation and inhibits apoptosis in human breast cancer by
targeting p27Kip1. Oncol Rep. 34:995–1002. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bai Y, Li JY, Li J, Liu YH and Zhang B:
miR-615 inhibited cell proliferation and cell cycle of human breast
cancer cells by suppressing of AKT2 expression. Int J Clin Exp Med.
8:3801–3808. 2015.PubMed/NCBI
|
|
8
|
Dong Y, Huo X, Sun R, Liu Z, Huang M and
Yang S: lncRNA Gm15290 promotes cell proliferation and invasion in
lung cancer through directly interacting with and suppressing the
tumor suppressor miR-615-5p. Biosci Rep. 38:
pii(BSR20181150)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sun Y, Zhang T, Wang C, Jin X, Jia C, Yu S
and Chen J: miRNA-615-5p functions as a tumor suppressor in
pancreatic ductal adenocarcinoma by targeting AKT2. PLoS One.
10(e0119783)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pu HY, Xu R, Zhang MY, Yuan LJ, Hu JY,
Huang GL and Wang HY: Identification of microRNA-615-3p as a novel
tumor suppressor in non-small cell lung cancer. Oncol Lett.
13:2403–2410. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Asano Y, Kawase T, Okabe A, Tsutsumi S,
Ichikawa H, Tatebe S, Kitabayashi I, Tashiro F, Namiki H, Kondo T,
et al: IER5 generates a novel hypo-phosphorylated active form of
HSF1 and contributes to tumorigenesis. Sci Rep.
6(19174)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gao W, Gu Y, Li Z, Cai H, Peng Q, Tu M,
Kondo Y, Shinjo K, Zhu Y, Zhang J, et al: miR-615-5p is
epigenetically inactivated and functions as a tumor suppressor in
pancreatic ductal adenocarcinoma. Oncogene. 34:1629–1640.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huang FY, Zhao HJ, Du ZJ and Jiang H:
miR-615 inhibits prostate cancer cell proliferation and invasion by
directly targeting Cyclin D2. Oncol Res. 27:293–299.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Naresh DJ and Nadav B: Reconstruction of
temporal activity of microRNAs from gene expression data in breast
cancer cell line. BMC Genomics. 16(1077)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fang F, Chang R and Yang L: Heat shock
factor 1 promotes invasion and metastasis of hepatocellular
carcinoma in vitro and in vivo. Cancer. 118:1782–1794.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cen H, Zheng S, Fang YM, Tang XP and Dong
Q: Induction of HSF1 expression is associated with sporadic
colorectal cancer. World J Gastroenterol. 10:3122–3126.
2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Santagata S, Hu R, Lin NU, Mendillo ML,
Collins LC, Hankinson SE, Schnitt SJ, Whitesell L, Tamimi RM,
Lindquist S and Ince TA: High levels of nuclear heat-shock factor 1
(HSF1) are associated with poor prognosis in breast cancer. Proc
Natl Acad Sci USA. 108:18378–18383. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen FY, Dong Z, Xia Y, Tang J, Peng L,
Wang S and Lai D: Nucleoside analog inhibits microRNA-214 through
targeting heat-shock factor 1 in human epithelial ovarian cancer.
Cancer Sci. 104:1683–1689. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dai C, Santagata S, Tang Z, Shi J, Cao J,
Kwon H, Bronson RT, Whitesell L and Lindquist S: Loss of tumor
suppressor NF1 activates HSF1 to promote carcinogenesis. J Clin
Invest. 122:3742–3745. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Heimberger T, Andrulis M, Riedel S,
Stühmer T, Schraud H, Beilhack A, Bumm T, Bogen B, Einsele H,
Bargou RC and Chatterjee M: The heat shock transcription factor 1
as a potential new therapeutic target in multiple myeloma. Br J
Haematol. 160:465–476. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ishiwata J, Kasamatsu A, Sakuma K, Iyoda
M, Yamatoji M, Usukura K, Ishige S, Shimizu T, Yamano Y, Ogawara K,
et al: State of heat shock factor 1 expression as a putative
diagnostic marker for oral squamous cell carcinoma. Int J Oncol.
40:47–52. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hoang AT, Huang J, Rudra-Ganguly N, Zheng
J, Powell WC, Rabindran SK, Wu C and Roy P: A novel association
between the human heat shock transcription factor 1 (HSF1) and
prostate adenocarcinoma. Am J Pathol. 156:857–864. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Miyachi K, Fritzler MJ and Tan EM:
Autoantibody to a nuclear antigen in proliferation cells. J
Immunol. 121:2228–2234. 1978.PubMed/NCBI
|
|
26
|
Liao XH, Lu DL, Wang N, Liu LY, Wang Y, Li
YQ, Yan TB, Sun XG, Hu P and Zhang TC: Estrogen receptor α mediates
proliferation of breast cancer MCF-7 cells via a
p21/PCNA/E2F1-dependent pathway. FEBS J. 281:927–942.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li T, Zhang C, Ding Y, Zhai W, Liu K, Bu
F, Tu T, Sun L, Zhu W, Zhou F, et al: Umbilical cord-derived
mesenchymal stem cells promote proliferation and migration in MCF-7
and MDA-MB-231 breast cancer cells through activation of the ERK
pathway. Oncol Rep. 34:1469–1477. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wu J, Li H, Wang X, Zhang X, Liu W, Wang
Y, Zhang Y, Pan H, Wang Q and Han Y: Effect of polysaccharide from
Undaria pinnatifida on proliferation, migration and apoptosis of
breast cancer cell MCF7. Int J Biol Macromol. 121:734–742.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Min JN, Huang L, Zimonjic DB, Moskophidis
D and Mivechi NF: Selective suppression of lymphomas by functional
loss of Hsf1 in a p53-deficient mouse model for spontaneous tumors.
Oncogene. 26:5086–5097. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Diehl JA: Cycling to cancer with cyclin
D1. Cancer Biol Ther. 1:226–231. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
He Y, Liu Z, Qiao C, Xu M, Yu J and Li G:
Expression and significance of Wnt signaling components and their
target genes in breast carcinoma. Mol Med Rep. 9:137–143.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Qin H and Liu W: MicroRNA-99a-5p
suppresses breast cancer progression and cell-cycle pathway through
downregulating CDC25A. J Cell Physiol. 234:3526–3537.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Song X, Wu JQ, Yu XF, Yang XS and Yang Y:
Trichostatin A inhibits proliferation of triple negative breast
cancer cells by inducing cell cycle arrest and apoptosis.
Neoplasma. 65:898–906. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chi Y, Xu H, Wang F, Chen X, Shan Z, Sun Y
and Fan Q: ZKSCAN3 promotes breast cancer cell proliferation,
migration and invasion. Biochem Biophys Res Commun. 503:2583–2589.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sawai M, Ishikawa Y, Ota A and Sakurai H:
The proto-oncogene JUN is a target of the heat shock transcription
factor HSF1. FEBS J. 280:6672–6680. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Escórcio-Dourado CS, Martins LM,
Simplício-Revoredo CM, Sampaio FA, Tavares CB, da Silva-Sampaio JP,
Borges US, Alves-Ribeiro FA, Lopes-Costa PV, Lima-Dourado JC and da
Silva BB: Bcl-2 antigen expression in luminal A and triple-negative
breast cancer. Med Oncol. 34(161)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kallel-Bayoundh I, Hassen HB, Khabir A,
Boujelbene N, Daoud J, Frikha M, Sallemi-Boundawara T, Aifa S and
Rebai A: Bcl-2 expression and triple negative profile in breast
carcinoma. Med Oncol. 28 (Suppl 1):S55–S61. 2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
de Ruijter TC, Veeck J, de Hoon JP, van
Engeland M and Tjan-Heijnen VC: Characteristics of triple-negative
breast cancer. J Cancer Res Clin Oncol. 137:183–192.
2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang X, Zhang D, Cao M, Ba J, Wu B, Liu T
and Nie C: A study on the biological function of heat shock factor
1 proteins in breast cancer. Oncol Lett. 16:3821–3825.
2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Du L, Fei Z, Song S and Wei N: Antitumor
activity of Lobaplatin against esophageal squamous cell carcinoma
through caspase-dependent apoptosis and increasing the Bax/Bcl-2
ratio. Biomed Pharmacother. 95:447–452. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yang F, Yu Y, Lei Q, Zeng A, Li Y, Xie Y,
Ye T and Wei Y: Lobaplatin arrests cell cycle progression, induces
apoptosis and impairs migration and invasion in B16-F10 melanoma
cell line in vitro. Biomed Pharmacother. 69:402–408.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lapierre M, Linares A, Dalvai M,
Duraffourd C, Bonnet S, Boulahtouf A, Rodriguez C, Jalaquier S,
Assou S, Orsetti B, et al: Histone deacetylase 9 regulates breast
cancer cell proliferation and the response to histone deacetylase
inhibitors. Oncotarget. 7:19693–19708. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Quisbert-Valenzuela EO and Calaf GM:
Apoptotic effect of noscapine in breast cancer cell lines. Int J
Oncol. 48:2666–2674. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lou Q, Hu Y, Ma Y and Dong Z: Heat shock
factor 1 induces crystallin-αB to protect against cisplatin
nephrotoxicity. Am J Physiol Renal Physiol. 311:F94–F102.
2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Jiang Y, Zhang Y, Li F, Du X and Zhang J:
CDX2 inhibits pancreatic adenocarcinoma cell proliferation via
promoting tumor suppressor miR-615-5p. Tumor Biol. 37:1041–1049.
2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Song LJ, Zhang WJ, Chang ZW, Pan YF, Zong
H, Fan QX and Wang LX: PU. 1 is identified as a novel metastasis
suppressor in hepatocellular carcinoma regulating the
miR-615-5p/IGF2 axis. Asian Pac J Cancer Prev. 16:3667–3671.
2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Shetty PJ, Movva S, Pasupuleti N,
Vedicherlla B, Vattam KK, Venkatasubramaniam S, Ahuja YR and Hasan
Q: Regulation of IGF2 transcript and protein expression by altered
methylation in breast cancer. J Cancer Res Clin Oncol. 137:339–345.
2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Schulz R, Streller F, Scheel AH, Rüschoff
J, Reinert MC, Dobbelstein M, Marchenko ND and Moll UM: HER2/ErbB2
activates HSF1 and thereby controls HSP90 clients including MIF in
HER2-overexpressing breast cancer. Cell Death Dis.
5(e980)2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Guettouche T, Boellmann F, Lane WS and
Voellmy R: Analysis of phosphorylation of human heat shock factor 1
in cells experiencing a stress. BMC Biochem. 6(4)2005.PubMed/NCBI View Article : Google Scholar
|