Introduction
Tonsillectomy is a common surgical intervention
(1-3).
It involves the complete or partial removal of the palatine tonsils
primarily to prevent recurring infections and inflammation
(4,5). However, the surgical site is a highly
vascularized zone and complications are frequent due to unintended
trauma during the procedure (6,7). For
instance, tonsillectomy is associated with large amounts of blood
loss during the operation (8,9) and
with subsequent inflammatory responses due to tissue trauma that
causes high levels of pain and post-operative morbidities (due to
the accumulation of local tissue exudates around the surgical site)
(10-12).
A high level of edema within 24 h of the operation in the uvula and
the palatopharyngeal and palatoglossal areas may interfere with
optimal healing and is associated with increased post-operative
morbidities (13). In addition,
tonsillectomy causes collateral damage to the pharyngeal muscles
and exposes nerve endings (tonsillar glossopharyngeal, maxillary
trigeminal and lesser palatine nerve branches) (14), leading to post-operative
complications such as severe pain, difficulty swallowing or
breathing, as well as vomiting and otalgia (13,15,16).
These complications may be reduced with local
anesthetic agents such as bupivacaine (17,18).
The application of bupivacaine may decrease the onset of
post-operative pain by blocking afferent nerve endings through
inhibition of voltage-gated Na+ channels (19). Furthermore, the anesthetic agent
inhibits synaptic N-methyl-D-aspartate receptors (19,20)
and has anti-inflammatory properties (21,22).
In 2013, Block et al (21)
also reported that bupivacaine reduces inflammatory activity by
inhibiting Ca2+ ion signaling and the release of
interleukin-1β in astrocytes, and by interacting with
5-hydroxytryptamine, opioid and glutamate receptors. Similarly, the
reduction of vascular permeability by bupivacaine has also been
reported to help reduce intra-operative and post-operative
complications (23,24). Bupivacaine has been deemed superior
to other anesthetic agents such as lidocaine and ropivacaine due to
its sustained effects, higher potency and lower toxicity profile
(25-27).
Despite the enhanced effectiveness demonstrated by
bupivacaine, there is still no consensus regarding its application
during tonsillectomy to reduce intra-operative and post-operative
complications. Certain studies have recommended the administration
of bupivacaine during rhinoplasty due to its ability to prevent
intra-operative and post-operative complications (28-31).
Previous studies, particularly meta-analyses (18), have failed to provide conclusive
evidence to support bupivacaine application during tonsillectomies.
This lack of agreement has delayed the adoption of a standard
protocol for optimal drug interventions during tonsillectomy. The
present review includes, for the first time, a detailed analysis of
the isolated efficacy of bupivacaine to improve intra-operative
factors associated with tonsillectomy such as the duration of the
operative procedure. Since the publication of the last
meta-analysis on this subject, several high-quality randomized
controlled trials (RCTs) have been published, evaluating the
efficacy of bupivacaine to improve intra-operative and
post-operative morbidities associated with tonsillectomy (28-34,34-36).
The present systematic review and meta-analysis
aimed to provide an updated evaluation of the effects of
bupivacaine on operative and post-operative outcomes associated
with tonsillectomy. Endpoints included the mean perceived level of
pain based on the visual analogue scale score, the mean operative
procedure duration and the incidence of post-operative morbidities.
The present results should help otolaryngologists to make optimal
decisions about the best approach to minimize morbidities
associated with tonsillectomy procedures.
Materials and methods
Data search strategy
The Preferred Reporting Items for Systematic reviews
and Meta-Analyses guidelines were followed (35). A total of four academic databases
(MEDLINE, CENTRAL, EMBASE and Scopus) were searched from inception
until April 2020 using the following MeSH key words:
‘Tonsillectomy', ‘Bupivacaine’,
‘1-Butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide’,
‘Bupivacain janapharm’, ‘Bupivacain-RPR’, ‘Bupivacaina braun’,
‘Bupivacaine anhydrous’, ‘Bupivacaine carbonate’, ‘Bupivacaine
hydrochloride’, ‘Bupivacaine monohydrochloride’, ‘Bupivacaine
monohydrate’, ‘Buvacaina’, ‘Carbostesin’, ‘Dolanaest’, ‘Marcain’,
‘Marcaine’, ‘Sensorcaine’, ‘Svedocain sin vasoconstr’,
‘anesthesia’, ‘anesthetics’, ‘visual analog scale’, ‘Children's
Hospital of Eastern Ontario pain scale', ‘morbidity’,
‘complications’, ‘blood loss’ and ‘post-operative morbidity’. The
bibliography of the included studies was subsequently searched for
any additional relevant studies. The inclusion criteria for studies
were defined as follows: a) Studies that assessed and stated
outcomes in a post-operative follow-up assessment; b) studies that
were either RCTs, quasi-RCTs, controlled clinical trials,
prospective observational trials with established control groups or
retrospective trials; c) studies in peer-reviewed scientific
journals or conference proceedings; d) studies written in English.
To avoid any bias, two reviewers (NW and FG) independently
replicated the selection process. The data extracted from the
selected studies included the authors' names, patient information
(age, sex), variables assessed, country, follow-up duration and
outcome measures. Appropriate attempts to contact corresponding
authors of relevant articles with incomplete quantitative outcome
data were made to obtain additional data.
Quality assessment
The Cochrane risk of bias assessment tool for RCTs
was used to evaluate the risk of bias (36). A total of two independent reviewers
(NW and FG) appraised the selected studies critically on the basis
of their methodology. The reviewers also analyzed each selected
study for inadequate randomizations, allocations, selection outcome
reports and distributions of allocation as a potential source of
bias (37). Data ambiguity was
resolved by discussions between the reviewers and a level of
evidence analysis was included based on the guidelines of the
Centre for Evidence-Based Medicine (38).
Data analysis
Statistical meta-analysis of the encompassed studies
was performed using the Comprehensive Meta-analysis version 2.0
software (39). Data for relevant
variables were extracted from the selected studies for analysis and
mean values were compared between the groups of patients treated
with either bupivacaine or normal saline. The statistical
meta-analysis was based on the random-effects model (40), in which effect sizes are reported as
weighted Hedge's g values. Results for weighted effect sizes were
categorized as small (≤0.2), medium (0.2-0.8) or large (≥0.8)
(41). I2 statistics
were computed to assess heterogeneity, which was classified as
either insignificant (0-25%), modest (25-75%) or considerable
(≥75%) (42). Sensitivity analysis
was performed for studies with considerable sources of
heterogeneity (43). Certain
results were excluded due to insufficient randomization methods in
the studies. For each statistic, 95% confidence intervals (CIs) and
the level of heterogeneity were calculated and Duval and Tweedie's
trim and fill process and the Egger's test of intercept were used
to assess publication bias (44).
The number of missing studies that may exist and the possible
consequences for the present meta-analysis were estimated.
Imputation of asymmetric studies was performed to define an
unbiased overall effect. Subsequently, these trimmed effects were
refilled in a plot and the combined effect was recalculated. The
alpha level was set at 5%.
Results
Study selection
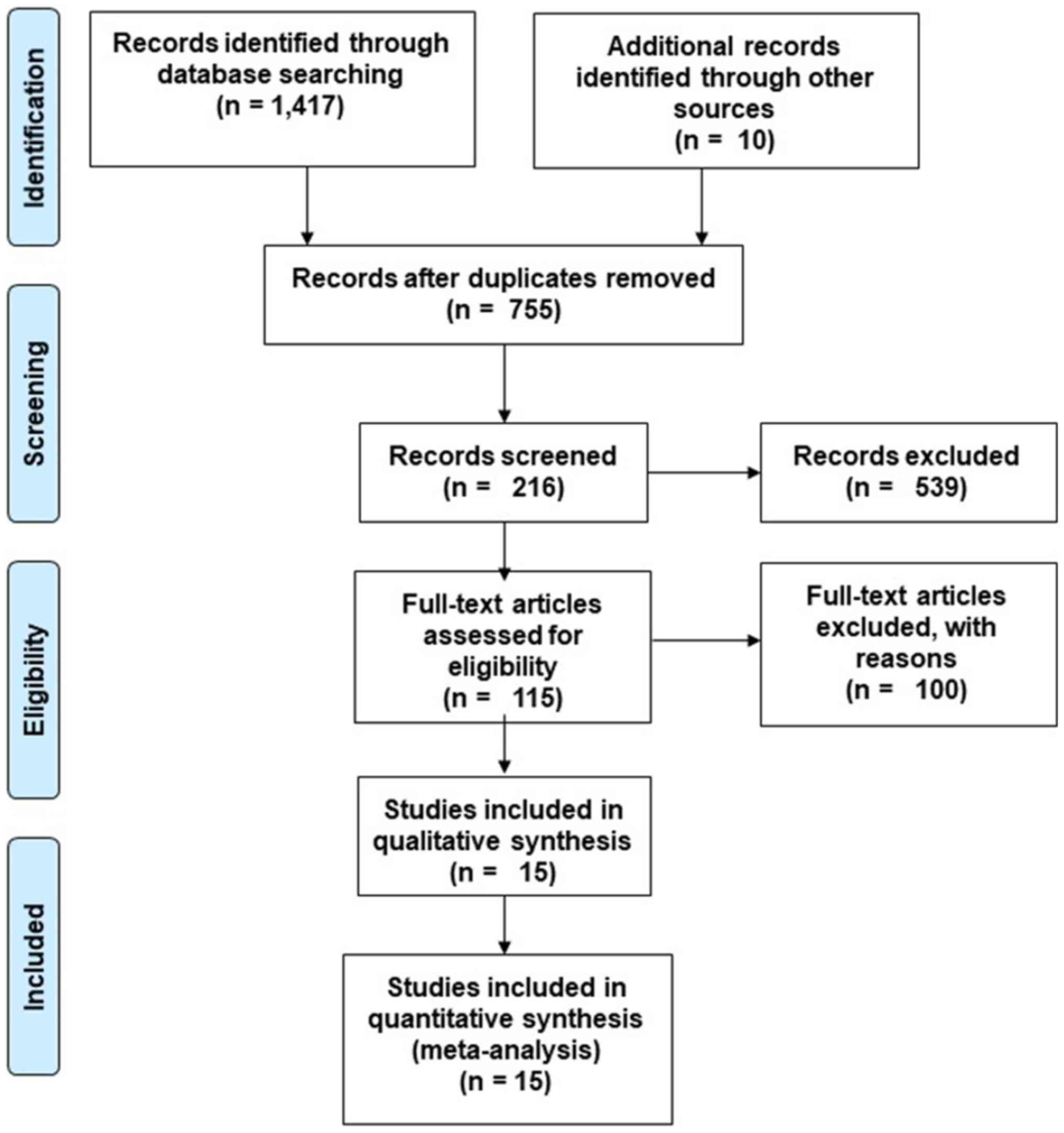
Initial examination of databases resulted in a total
of 1,417 studies. After review of the bibliography of relevant
studies, 10 additional studies were selected (Fig. 1). After removing duplicate
references and applying selection criteria, 15 RCT studies were
retained (17,28-34,45-51).
The included studies compared the perceived level of pain after
tonsillectomy between bupivacaine and normal saline groups
(14,17,28-34,45-47,49-51)
(12 studies evaluated the perceived level of pain with visual
analog scale scores (17,28-31,33,34,47-51)
and 2 with the Children's Hospital of Eastern Ontario pain scale
scores (45,46). In terms of pain levels, 9 studies
(17,28-34,46)
reported a significant (P<0.05) reduction and 2 (45,49) an
insignificant reduction (P>0.05) with the use of bupivacaine as
compared with the use of normal saline during tonsillectomy.
Furthermore, three studies reported no differences in the perceived
level of pain between the groups (47,50,51).
In addition, five of the included studies reported on the incidence
of post-operative morbidity after tonsillectomy in both groups, of
which 3 studies reported a significant (P<0.05) reduction
(33,46,49), 1
reported an insignificant (P>0.05) reduction (34) and one an insignificant increase in
the onset of post-tonsillectomy morbidity (for example, adverse
effects of the operative procedure) (32,33,45,46,49) in
patients receiving bupivacaine as compared to those in the placebo
group. Finally, four studies compared the mean duration of the
tonsillectomy procedure between the bupivacaine and normal saline
groups (17,29,31,34)
and reported a significant reduction in the mean duration of the
procedure for the group receiving bupivacaine as compared to the
placebo group.
Risk of bias
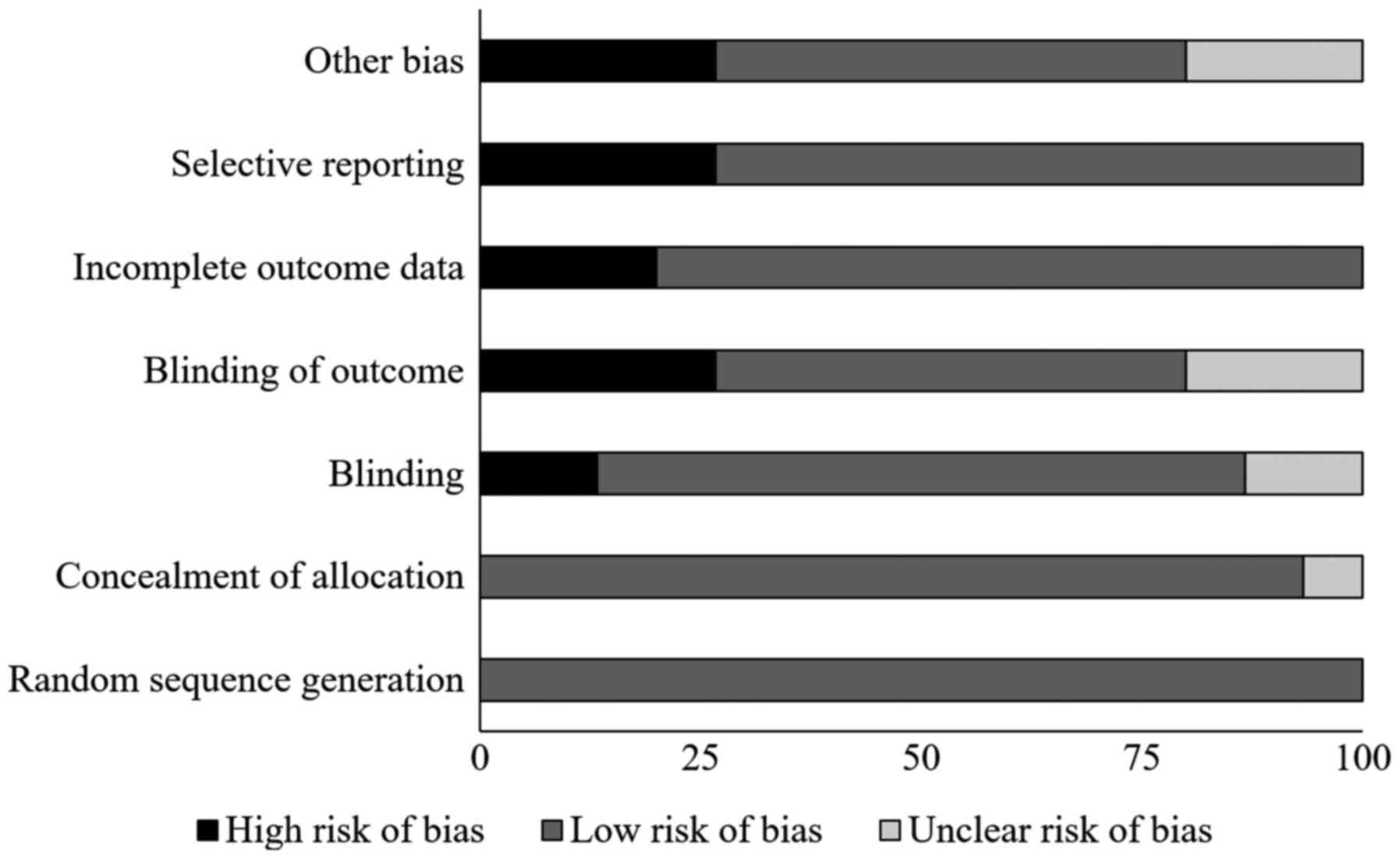
Table I and Fig. 2 present the results of Cochrane's
risk of bias assessment for the selected RCTs. The general bias
risk in the studies included was rated as low. The highest risk of
bias was due to selective reporting and insufficient blinding. A 1b
level of evidence was determined for all of the studies based on
their described experimental design.
 | Table IQuality of the analyzed studies
according to the Cochrane risk of bias assessment tool for
randomized controlled trials. |
Table I
Quality of the analyzed studies
according to the Cochrane risk of bias assessment tool for
randomized controlled trials.
| Study | Random sequence
generation | Concealment of
allocation | Blinding | Blinding of
outcome | Incomplete outcome
data | Selective
reporting | Other biases | Level of
evidence | Ref. |
|---|
| Junaid et al
(2020) | + | + | + | ? | - | + | - | 1b | (30) |
| Abdel Raheem and
Farouk (2019) | + | + | - | - | + | + | - | 1b | (28) |
| Tuhanioglu and
Erkan (2018) | + | + | + | + | - | - | ? | 1b | (34) |
| Haksever et
al (2014) | + | + | + | + | + | + | + | 1b | (32) |
| Ergil et al
(2012) | + | + | + | + | + | + | + | 1b | (29) |
| Özkiriş et
al (2012) | + | + | ? | - | + | + | + | 1b | (31) |
| Özmen and Özmen
(2011) | + | + | + | + | + | + | + | 1b | (33) |
| Nikandish et
al (2008) | + | + | + | + | + | + | + | 1b | (17) |
| Karaaslan et
al (2008) | + | + | + | - | + | + | + | 1b | (45) |
| Unal et al
(2007) | + | + | + | + | + | - | ? | 1b | (51) |
| Akoglu et al
(2006) | + | + | + | ? | + | + | + | 1b | (46) |
| Kaygusuz and
Susaman (2003) | + | + | - | - | + | + | - | 1b | (49) |
| Johansen et
al (1996) | + | + | + | + | + | + | - | 1b | (48) |
| Stuart et al
(1994) | + | + | + | + | + | - | + | 1b | (50) |
| Jebeles et
al (1992) | + | ? | ? | ? | - | - | ? | 1b | (47) |
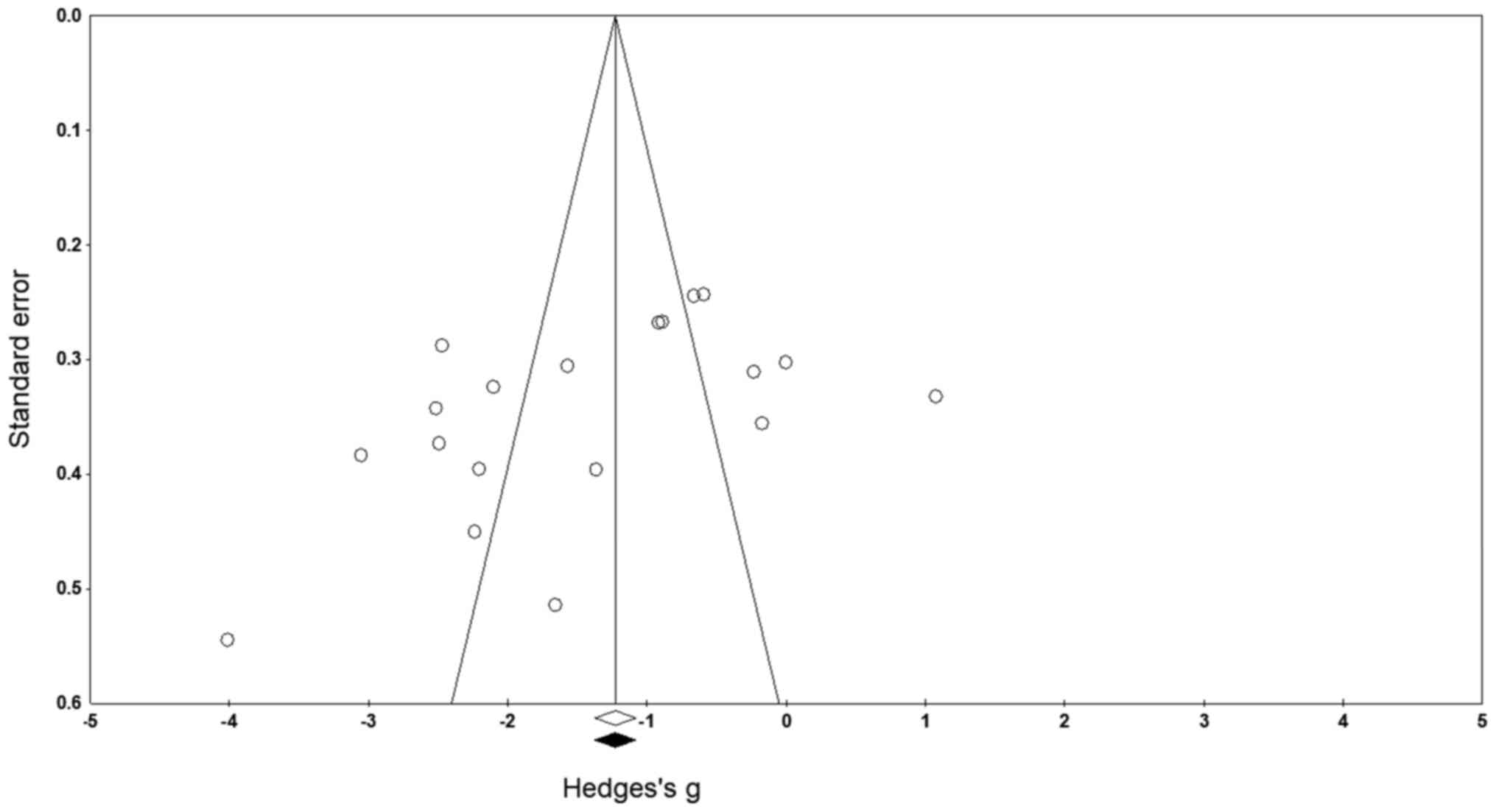
Publication bias
No missing studies on either side of the mean result
were identified after applying the trim and fill technique
(Fig. 3). The studies that fall out
of the funnel area indicate the possibility of bias. In this case,
it can be presumed that the asymmetry in our funnel plot is
possibly as a result of heterogeneity arising due to differences
between study results and methodology (52,53)
had earlier stated that heterogeneity can lead to funnel plot
asymmetry if it induces a correlation between study sizes and
intervention effects. Nevertheless, in the current analysis the
random-effects model indicated overall point estimates and 95% CIs
for the evaluated strictures as -1.44 (-1.95 to -0.93),
respectively. According to the trim and fill procedure report,
these values remained unchanged. These values represent the overall
effect size of all the parameters in the included studies before
the assessment of publication bias. Furthermore, the intercept,
i.e. the captured bias according to the Egger's test was B0: -7.46,
95% CI: -14.51 to -0.41 (P<0.05).
Participant information
Data from 710 patients from the studies included in
the present analysis were assessed (Table II). Of these, 379 patients (122
females and 167 males) received bupivacaine and 331 patients (130
females and 141 males) received normal saline (control group).
Furthermore, two studies did not define or mention the patients'
sex distributions (28,30). The mean age of all the patients was
9.1±5.6 years. The mean age of the patients in the bupivacaine and
the control group was 9.1±6.4 and 8.8±4.9 years, respectively.
However, two studies reported only the overall mean age of their
sample (28,30).
 | Table IICharacteristics of the studies
included. |
Table II
Characteristics of the studies
included.
| Author, year,
(ref.) | Patient age
(years) | Sample size
(n) | Country | Assessment | Follow-up
(hours) | Outcomes |
|---|
| Junaid et
al, 2020(30) | 14.5±7.4 | 180 (83 F, 97
M) | Pakistan | Visual analog
scale | 4, 8, 12, 16 | Significant
reduction in visual analog scale scores in BP as compared to
NS. |
| BP: NA | BP: 60 (F, M) |
| NS: NA | NS: 30 (F, M) |
| Abdel and Farouk
2019(28) | Mean: 8.7 | 60 (31 F, 29
M) | Egypt | Visual analog
scale | 1, 4, 8, 24 | Significant
reduction in visual analog scale scores in BP as compared to
NS. |
| BP: NA | BP: 30 (F, M) |
| NS: NA | NS: 30 (F, M) |
| Tuhanioglu and
Erkan, 2018(34) | BP: 30±6 | BP: 15 (9 F, 6
M) | Pakistan | Visual analog
scale, operating time | 0.25, 6, 12, 24,
168 | Significant
reductions in visual analog scale scores and operating time in BP
as compared to NS. |
| NS: 25±6 | NS: 15 (8 F, 7
M) |
| Haksever et
al, 2014(32) | BP: 6.0±2.9 | BP: 40 (20 F, 20
M) | Turkey | Visual analog
scale, postoperative morbidity | 1, 5, 13, 17, 21,
24, 48, 72, 96, 120, 144 | Significant
reduction in visual analog scale scores in BP as compared to NS.
Reduced morbidities related to BP as compared to NS. |
| NS: 6.7±3.6 | NS: 20 (10 F, 10
M) |
| Ergil et al,
2012(29) | BP: 6±2 | BP: 30 (14 F, 16
M) | Turkey | Visual analog
scale, operation duration | 0, 0.15, 0.5, 6,
12, 24 | Significant
reductions in visual analog scale scores and operating time scores
in BP as compared to NS. |
| NS: 6±2 | NS: 30 (15 F, 15
M) |
| Özkiriş et
al, 2012(31) | BP: 8.1±4.2 | BP: 29 (13 F, 16
M) | Turkey | Visual analog
scale, operation duration | 1, 4, 8, 16, 24,
48, 72, 96, 120, 144, 168 | Significant
reduction in visual analog scale scores and operating time scores
in BP as compared to NS. |
| NS: 8.1±4.2 | NS: 29 (12 F, 17
M) |
| Özmen and Özmen,
2011(33) | BP: 6.0±3.7 | BP: 20 (10 F, 10
M) | Turkey | Visual analog
scale, post-operative morbidity | 1, 5, 13, 17, 21,
24, 48, 72, 96, 120, 144 | Significant
reduction in visual analog scale scores and comorbidities in BP as
compared to NS. |
| NS: 6.7±3.6 | NS: 20 (11 F, 9
M) |
| Nikandish et
al, 2008(17) | BP: 10±2.4 | BP: 33 (14 F,
19M) | Iran | Visual analog
scale, operation duration | 1, 2, 4, 6, 8,
12 | Significant
reduction in visual analog scale scores, operating time score in BP
as compared to NS. |
| NS: 10±2.3 | NS: 36 (17 F, 19
M) |
| Karaaslan et
al, 2008(45) | BP: 7.0±0.5 | BP: 25 (11 F,
14M) | Turkey | Children's Hospital
of Eastern Ontario pain scale, post-operative morbidity | 0.25, 1, 4, 8, 16,
24 | Reduction in
Children's Hospital of Eastern Ontario pain scale scores in BP as
compared to NS. Increased co-morbidities in BP as compared to
NS. |
| NS: 7.4±0.5 | NS: 25 (17 F, 8
M) |
| Unal et al,
2007(51) | BP: 7.5±3.1 | BP: 20 (2 F, 18
M) | Turkey | Visual analog
scale | 0, 0.08, 0.16,
0.25, 0.5, 1, 2, 6, 12, 24 | No difference in
visual analog scale score between BP and NS. |
| NS: 8.2±2.9 | NS: 20 (4 F, 16
M) |
| Akoglu et
al, 2006(46) | BP: 6.0±2.5 | BP: 16 (6 F,
10M) | Turkey | Children's Hospital
of Eastern Ontario pain scale, post-operative morbidity | 0.25, 1, 4, 12, 16,
24 | Significant
reduction in Children's Hospital of Eastern Ontario pain scale
scores and comorbidities in BP as compared to NS. |
| NS: 6.1±1.6 | NS: 15 (6 F, 9
M) |
| Kaygusuz and
Susaman, 2003(49) | BP: 9±2.7 | BP: 20 (7 F, 13
M) | Turkey | Visual analogscale,
post-operative morbidity | 1, 3, 7 | Reductions in
visual analog scale scores and morbidities in BP as compared to
NS. |
| NS: 8±2.6 | NS: 20 (9 F, 11
M) |
| Johansen et
al, 1996(48) | BP: 25 | BP: 9 (5 F, 4
M) | Denmark | Visual analog
scale | 0, 24, 48, 72, 96,
120, 144, 168, 192, 226, 240 | Significant
reduction in visual analog scale scores in BP as compared to
NS. |
| NS: 23 | NS: 10 (6 F, 4
M) |
| Stuart et
al, 1994(50) | BP: 6.4 | BP: 21 (9 F, 12
M) | UK | Visual analog
scale | 0, 0.16, 1, 4,
24 | No difference in
visual analog scale scores between BP and NS. |
| NS: 6.0 | NS: 21 (12 F, 9
M) |
| Jebeles et
al, 1992(47) | BP: 7.5±3.1 | BP: 20 (7 F, 13
M) | USA | Visual analog
scale | 0, 24, 48, 72, 96,
120 | No difference in
visual analog scale scores between BP and NS. |
| NS: 8.2±2.9 | NS: 20 (9 F, 11
M) |
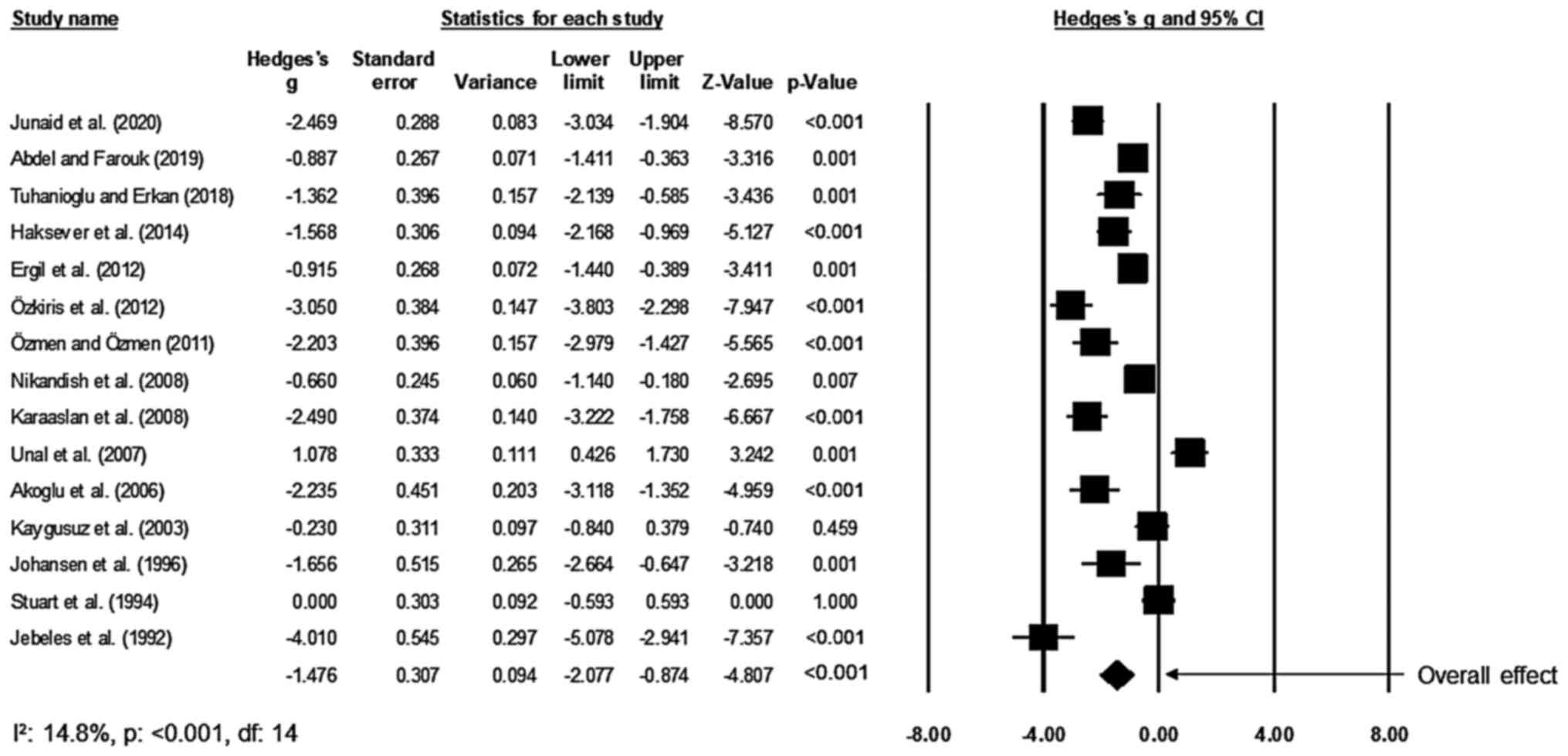
Post-operative pain
Of the included studies, 15 studies compared the
effects on the perceived levels of pain of patients after receiving
tonsillectomy between the bupivacaine and the placebo groups
(17,28-34,45-51).
An across-group random-effects analysis (Fig. 4) revealed a large negative and
significant effect of bupivacaine to reduce the perceived level of
pain after tonsillectomy as compared to the effect of normal saline
(g, -1.48; 95% CI, -2.08 to -0.87; P<0.01) with negligible
heterogeneity (I2, 14.8%).
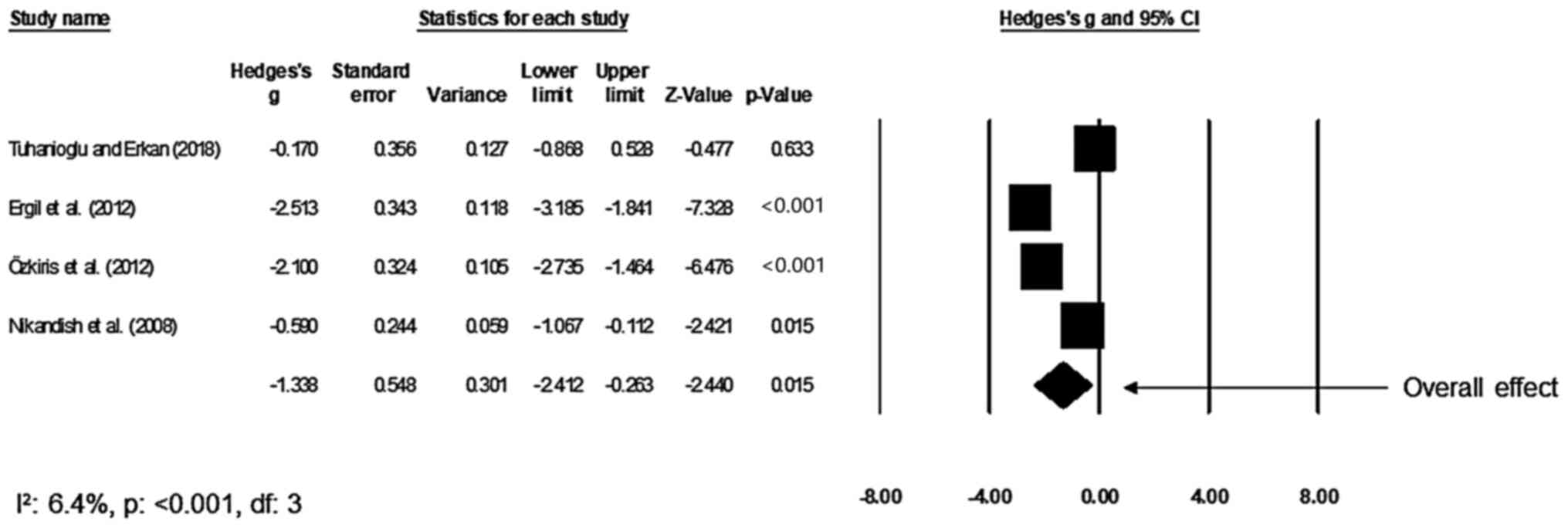
Duration of operation
A total of four studies compared the mean duration
of the tonsillectomy procedure between the bupivacaine and control
groups (17,29,31,34).
An across-group random-effect analysis (Fig. 5) revealed a large negative and
significant effect of bupivacaine to reduce the mean duration of
the tonsillectomy procedure as compared to the effect of normal
saline (g, -1.36; 95% CI, -2.44 to -0.27; P=0.01) with negligible
heterogeneity (I2, 6.4%).
Post-operative morbidity
A total of 5 studies compared post-operative
morbidity of patients receiving tonsillectomy between the
bupivacaine and control groups (32,33,45,46,49).
An across-group random-effects analysis (Fig. 6) revealed a small negative and
insignificant effect of bupivacaine to reduce post-operative
morbidity as compared with that in the normal saline group (g,
-0.23; 95% CI, -0.65 to 0.19; P=0.3) with no heterogeneity
(I2, 0%).
Discussion
The present review provided a comprehensive update
on intra-operative and post-operative outcomes after administration
of bupivacaine. It was demonstrated that the bupivacaine
administration during tonsillectomy was associated with a reduction
of the perceived level of pain, shorter mean duration of the
operation and a decrease in the incidence of post-operative
morbidities as compared to the effects of normal saline
application.
Due to the complex anatomy of the pharyngeal
segments and their prominent vasculature, tonsillectomy represents
a challenge for otolaryngologists worldwide (54,55).
The traumatic nature of the procedure increases the likelihood of
widespread intra-operative and post-operative morbidities (18,30,31,33).
To counteract these side effects, bupivacaine has been increasingly
recommended during tonsillectomies due to its superior
antinociceptive properties and its ability to reduce vascular
permeability and inflammation (22-24,48).
A review from 1978 by Babst and Gilling (56) suggested that bupivacaine has a high
affinity towards neural tissue and acts by inhibiting the onset of
action potentials by obstructing Na+ ion transmission
through the neural membrane, and by binding Ca2+ ion
sites in the external lipid layer to interfere with the mobility of
phosphate groups. Other studies have indicated that the vascular
permeability reduction and vasodilation properties of bupivacaine
may help prevent the post-operative morbidity associated with
tonsillectomy (57,58). In agreement with these studies, the
present meta-analysis also suggested that bupivacaine use is
associated with beneficial effects after tonsillectomy. Haksever
et al (32) compared the
effects of adjunct administration of 0.5% topical bupivacaine
hydrochloride with the administration of normal saline during
tonsillectomy and observed a significant reduction in the perceived
level of pain from five hours post-surgery till the sixth day of
follow-up, and a substantial reduction in the incidence of
post-operative morbidities such as trismus, nausea, vomiting and
otalgia until the fourth day of follow-up post-tonsillectomy.
Similarly, another study reported a significant reduction in the
levels of post-tonsillectomy morbidity, including halitosis, fever,
nausea, vomiting and otalgia, for the group receiving bupivacaine
hydrochloride as compared to the rates in the placebo group
(33). The study also indicated
that post-operative morbidities were significantly lower during the
first, second and the fourth days of follow-up, and a significant
reduction in the perceived level of pain between groups from five
hours post-tonsillectomy until the sixth day of follow-up was
achieved (33). Comparison of the
efficacy of bupivacaine and lidocaine suggested that bupivacaine
was associated with a significant reduction in post-tonsillectomy
morbidities (33). In accordance
with the above, the results of the present meta-analysis further
confirmed the efficacy of bupivacaine, and a large and significant
effect size reduction in the perceived level of post-tonsillectomy
pain (Hedge's g, -1.48) was determined in the bupivacaine vs. the
placebo group. Furthermore, a small effect size reduction in the
onset of post-operative morbidities (-0.23) was obtained in the
bupivacaine vs. the control group.
The present analysis also indicated that the use of
bupivacaine reduced the incidence of intra-operative complications
associated with tonsillectomy. A large effect size reduction in the
mean duration of the tonsillectomy operation (-1.36) was observed
with the use of bupivacaine as compared to the use of normal
saline. In the published literature, a proportional association
exists between the duration of an operative procedure and the
amount of intra-operative blood loss (59,60),
which eventually leads to post-operative morbidities and prolongs
the post-surgery recovery period. Collateral incisional damage to
palatine, pharyngeal and tonsillar branches of the facial arteries
are common during tonsillectomies due to the vascular organization
of the tonsillar and the peri-tonsillar arch (61). These accidental incisions may
increase the volume of intra-operative hemorrhages, prolong the
recovery time after tonsillectomy and result in post-operative
complications (61,62). Under such circumstances, the use of
bupivacaine may limit intra-operative blood loss, thereby reducing
the onset of post-operative morbidities.
Previous studies reported that the use of
bupivacaine was associated with an improvement of the hematological
outcomes, such as hemoglobin levels. A study included in our review
reported an elevation in the level of hemoglobin in a pediatric
population undergoing tonsillectomy with bupivacaine (12.1±0.6
mg/dl) as compared to the level in the placebo group (10.8±0.6
mg/dl) (29). The authors concluded
that the improvement of hematological outcomes reflects the
vasoconstrictive properties of bupivacaine, and at the same time,
provides a prophylactic benefit against intra-operative and
post-operative hemorrhagic complications, which may require blood
transfusions in the pediatric population (63).
Of note, the present review and meta-analysis had
certain limitations. First, the systematic review was not
registered in a prospective registry such as PROSPERO and this may
raise questions regarding the validity of the review (64). Furthermore, no subgroup analyses
were performed on the specific doses of bupivacaine used in the
studies, which may potentially impact the development of efficient
otolaryngologic care guidelines for the optimal use of bupivacaine
during tonsillectomies. Future studies should address this issue by
performing meta regression-based analyses associating the effects
of different dosages of bupivacaine during tonsillectomies. In
addition, due to the paucity of the available data, the
effectiveness of bupivacaine was not compared between pediatric and
adult populations and the results cannot provide recommendations
for specific populations. For the same reason, the patients'
well-being outcomes, such as patient comfort and quality of life,
were not assessed. Hence, further studies addressing these issues
are required. The results will assist in developing robust
decision-making models for otolaryngologists to be able to choose
ideal interventions and provide high-quality care for their
patients.
In conclusion, the present systematic review and
meta-analysis provided evidence at the level 1b supporting the use
of bupivacaine during tonsillectomy to shorten the duration of the
procedure. In addition, bupivacaine reduced the level of
post-operative pain and the incidence of associated morbidities.
The present results have implications for developing best-practice
otolaryngologic care strategies for performing tonsillectomy
operations.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JW conceived and designed the study. NW and FG
collected the data and performed the analysis and the
interpretation of the data. JW was involved in the writing of the
manuscript. All authors approved the final manuscript for
publication, and agreed to be accountable for all aspects of the
work and ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Archer NM, Forbes PW, Dargie J, Manganella
J, Licameli GR, Kenna MA and Brugnara C: Association of blood type
with postsurgical mucosal bleeding in pediatric patients undergoing
tonsillectomy with or without adenoidectomy. JAMA Netw Open.
3(e201804)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Materia E, Di Domenicantonio R, Baglio G,
Marchisio P, Perletti L, Lispi L, Mele A and Guasticchi G:
Epidemiology of tonsillectomy and/or adenoidectomy in Italy.
Pediatr Med Chir. 26:179–186. 2004.PubMed/NCBI
|
|
3
|
Roushdy MM, Abdel-Ghaffar HS, Mohammed MA
and Khalifa AH: Comparative study between the effect of Diclofenac
and Ketorolac in post-tonsillectomy pain management. Egypt J Neck
Surg Otorhinolaryngol. 6:43–53. 2020.
|
|
4
|
Di Luca M, Iannella G, Montevecchi F,
Magliulo G, De Vito A, Cocuzza S, Maniaci A, Meccariello G,
Cammaroto G, Sgarzani R, et al: Use of the transoral robotic
surgery to treat patients with recurrent lingual tonsillitis. Int J
Med Robot. 16(e2106)2020.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Soaper AL, Richardson ZL, Chen JL and
Gerber ME: Pediatric tonsillectomy: A short-term and long-term
comparison of intracapsular versus extracapsular techniques. Int J
Pediatr Otorhinolaryngol. 133(109970)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Joseph M, Reardon E and Goodman M: Lingual
tonsillectomy: A treatment for inflammatory lesions of the lingual
tonsil. Laryngoscope. 94:179–184. 1984.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Krishna P and Lee D: Post-tonsillectomy
bleeding: A meta-analysis. Laryngoscope. 111:1358–1361.
2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Broadman LM, Patel RI, Feldman BA, Sellman
GL, Milmoe G and Camilon F: The effects of peritonsillar
infiltration on the reduction of intraoperative blood loss and
post-tonsillectomy pain in children. Laryngoscope. 99:578–581.
1989.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Teker AM, Korkut AY, Gedikli O and Kahya
V: Prospective, controlled clinical trial of Ankaferd Blood Stopper
in children undergoing tonsillectomy. Int J Pediatr
Otorhinolaryngol. 73:1742–1745. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kargi E, Hoşnuter M, Babucçu O, Altunkaya
H and Altinyazar C: Effect of steroids on edema, ecchymosis, and
intraoperative bleeding in rhinoplasty. Ann Plast Surg. 51:570–574.
2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wohlgemuth PR and O'Brien GR:
Postoperative edema in maxillofacial surgery; prevention and
treatment with promethazine. Am J Surg. 94:537–541. 1957.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xu F, Zeng W, Mao X and Fan GK: The
efficacy of melilotus extract in the management of postoperative
ecchymosis and edema after simultaneous rhinoplasty and
blepharoplasty. Aesthetic Plast Surg. 32:599–603. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Davidoss NH, Eikelboom R, Friedland PL and
Santa Maria PL: Wound healing after tonsillectomy - a review of the
literature. J Laryngol Otol. 132:764–770. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Deutsch ES and Isaacson GC: Tonsils and
adenoids: An update. Pediatr Rev. 16:17–21. 1995.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Linden BE, Gross CW, Long TE and Lazar RH:
Morbidity in pediatric tonsillectomy. Laryngoscope. 100:120–124.
1990.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Odhagen E, Stalfors J and Sunnergren O:
Morbidity after pediatric tonsillotomy versus tonsillectomy: A
population-based cohort study. Laryngoscope. 129:2619–2626.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nikandish R, Maghsoodi B, Khademi S,
Motazedian S and Kaboodkhani R: Peritonsillar infiltration with
bupivacaine and pethidine for relief of post-tonsillectomy pain: A
randomised double-blind study. Anaesthesia. 63:20–25.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sun J, Wu X, Meng Y and Jin L: Bupivacaine
versus normal saline for relief of post-adenotonsillectomy pain in
children: A meta-analysis. Int J Pediatr Otorhinolaryngol.
74:369–373. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Furutani K, Ikoma M, Ishii H, Baba H and
Kohno T: Bupivacaine inhibits glutamatergic transmission in spinal
dorsal horn neurons. Anesthesiology. 112:138–143. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Paganelli MA and Popescu GK: Actions of
bupivacaine, a widely used local anesthetic, on NMDA receptor
responses. J Neurosci. 35:831–842. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Block L, Jörneberg P, Björklund U,
Westerlund A, Biber B and Hansson E: Ultralow concentrations of
bupivacaine exert anti-inflammatory effects on
inflammation-reactive astrocytes. Eur J Neurosci. 38:3669–3678.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cassuto J, Sinclair R and Bonderovic M:
Anti-inflammatory properties of local anesthetics and their present
and potential clinical implications. Acta Anaesthesiol Scand.
50:265–282. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Anderson LA, Engel GM, Bruckner JD,
Stoddard GJ and Peters CL: Reduced blood loss after total knee
arthroplasty with local injection of bupivacaine and epinephrine. J
Knee Surg. 22:130–136. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Metaxotos NG, Asplund O and Hayes M: The
efficacy of bupivacaine with adrenaline in reducing pain and
bleeding associated with breast reduction: A prospective trial. Br
J Plast Surg. 52:290–293. 1999.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kuthiala G and Chaudhary G: Ropivacaine: A
review of its pharmacology and clinical use. Indian J Anaesth.
55:104–110. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Polley LS, Columb MO, Naughton NN, Wagner
DS and van de Ven CJ: Relative analgesic potencies of ropivacaine
and bupivacaine for epidural analgesia in labor: Implications for
therapeutic indexes. Anesthesiology. 90:944–950. 1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Spivey WH, McNamara RM, MacKenzie RS, Bhat
S and Burdick WP: A clinical comparison of lidocaine and
bupivacaine. Ann Emerg Med. 16:752–757. 1987.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Abdel Raheem AG and Farouk ZM: Effect of
preincisional peritonsillar infiltration of bupivacaine on
post-tonsillectomy pain. Egypt J Neck Surg Otorhinolaryngol.
5:40–46. 2019.
|
|
29
|
Ergil J, Akkaya T, Gozaydin O, Gunsoy B,
Alicura S, Aladag E, Gumus H and Akin I: Vasoconstrictive and
analgesic efficacy of locally infiltrated levobupivacaine in
tonsillectomy patients. Int J Pediatr Otorhinolaryngol.
76:1429–1433. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Junaid M, Halim MS, Onali MA, Qadeer S,
Khan HU and Ali NS: Intraoperative use of analgesics in tonsillar
fossa and postoperative evaluation with visual analogue scale
scores-A prospective, randomized, placebo-controlled, double-blind
clinical trial. Int Arch Otorhinolaryngol. 24:e62–e67.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ozkiriş M, Kapusuz Z and Saydam L:
Comparison of ropivacaine, bupivacaine and lidocaine in the
management of post-tonsillectomy pain. Int J Pediatr
Otorhinolaryngol. 76:1831–1834. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Haksever M, Özmen S, Akduman D and Solmaz
F: Topical bupivacaine compared to bupivacaine infiltration for
post-tonsillectomy pain relief in children: A prospective
randomized controlled clinical study. Eur Arch Otorhinolaryngol.
271:2555–2559. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Özmen OA and Özmen S: Topical bupivacaine
compared to lidocaine with epinephrine for post-tonsillectomy pain
relief in children: A randomized controlled study. Int J Pediatr
Otorhinolaryngol. 75:77–80. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tuhanioglu B and Erkan SO: Tonsillectomy
pain control with IV dexamethasone, infiltrated dexamethasone and
infiltrated bupivacaine; a randomised, double-blind, placebo
controlled, prospective clinical trial. J Pak Med Assoc.
68:1002–1008. 2018.PubMed/NCBI
|
|
35
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group. Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. PLoS Med.
6(e1000097)2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jørgensen L, Paludan-Müller AS, Laursen
DR, Savović J, Boutron I, Sterne JA, Higgins JP and Hróbjartsson A:
Evaluation of the Cochrane tool for assessing risk of bias in
randomized clinical trials: Overview of published comments and
analysis of user practice in Cochrane and non-Cochrane reviews.
Syst Rev. 5(80)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Viswanathan M, Ansari MT, Berkman ND,
Chang S, Hartling L, McPheeters M, Santaguida PL, Shamliyan T,
Singh K, Tsertsvadze A, et al: Assessing the risk of bias of
individual studies in systematic reviews of health care
interventions. In: Methods Guide for Effectiveness and Comparative
Effectiveness Reviews. Agency for Healthcare Research and Quality
(US), Rockville, MD, 2008.
|
|
38
|
Burns PB, Rohrich RJ and Chung KC: The
levels of evidence and their role in evidence-based medicine. Plast
Reconstr Surg. 128:305–310. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bax L, Yu LM, Ikeda N and Moons KG: A
systematic comparison of software dedicated to meta-analysis of
causal studies. BMC Med Res Methodol. 7(40)2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Higgins JP, Thompson SG and Spiegelhalter
DJ: A re-evaluation of random-effects meta-analysis. J R Stat Soc
Ser A Stat Soc. 172:137–159. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rosenthal R: Parametric measures of effect
size. In: The Handbook of Research Synthesis. Russell Sage
Foundation, New York, NY, pp231-244, 1994.
|
|
42
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558.
2002.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Petitti DB: Approaches to heterogeneity in
meta-analysis. Stat Med. 20:3625–3633. 2001.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Duval S and Tweedie R: Trim and fill: A
simple funnel-plot-based method of testing and adjusting for
publication bias in meta-analysis. Biometrics. 56:455–463.
2000.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Karaaslan K, Yilmaz F, Gulcu N, Sarpkaya
A, Colak C and Kocoglu H: The effects of levobupivacaine versus
levobupivacaine plus magnesium infiltration on postoperative
analgesia and laryngospasm in pediatric tonsillectomy patients. Int
J Pediatr Otorhinolaryngol. 72:675–681. 2008.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Akoglu E, Akkurt BC, Inanoglu K, Okuyucu S
and Dagli S: Ropivacaine compared to bupivacaine for
post-tonsillectomy pain relief in children: A randomized controlled
study. Int J Pediatr Otorhinolaryngol. 70:1169–1173.
2006.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jebeles JA, Reilly JS, Gutierrez JF,
Bradley EL Jr and Kissin I: The effect of pre-incisional
infiltration of tonsils with bupivacaine on the pain following
tonsillectomy under general anesthesia. Pain. 47:305–308.
1991.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Johansen M, Harbo G and Illum P:
Preincisional infiltration with bupivacaine in tonsillectomy. Arch
Otolaryngol Head Neck Surg. 122:261–263. 1996.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kaygusuz I and Susaman N: The effects of
dexamethasone, bupivacaine and topical lidocaine spray on pain
after tonsillectomy. Int J Pediatr Otorhinolaryngol. 67:737–742.
2003.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Stuart JC, MacGregor FB, Cairns CS and
Chandrachud HR: Peritonsillar infiltration with bupivacaine for
paediatric tonsillectomy. Anaesth Intensive Care. 22:679–682.
1994.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Unal Y, Pampal K, Korkmaz S, Arslan M,
Zengin A and Kurtipek O: Comparison of bupivacaine and ropivacaine
on postoperative pain after tonsillectomy in paediatric patients.
Int J Pediatr Otorhinolaryngol. 71:83–87. 2007.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sedgwick P: Meta-analyses: How to read a
funnel plot. BMJ. 346(f1342)2013.
|
|
53
|
Terrin N, Schmid CH and Lau J: In an
empirical evaluation of the funnel plot, researchers could not
visually identify publication bias. J Clin Epidemiol. 58:894–901.
2005.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Bangera A: Anaesthesia for
adenotonsillectomy: An update. Indian J Anaesth. 61:103–109.
2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Verma R and Verma RR and Verma RR:
Tonsillectomy-comparative study of various techniques and changing
trend. Indian J Otolaryngol Head Neck Surg. 69:549–558.
2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Babst CR and Gilling BN: Bupivacaine: A
review. Anesth Prog. 25:87–91. 1978.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Iida H, Watanabe Y, Dohi S and Ishiyama T:
Direct effects of ropivacaine and bupivacaine on spinal pial
vessels in canine. Assessment with closed spinal window technique.
Anesthesiology. 87:75–81. 1997.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Newton DJ, McLeod GA, Khan F and Belch JJ:
Vasoactive characteristics of bupivacaine and levobupivacaine with
and without adjuvant epinephrine in peripheral human skin. Br J
Anaesth. 94:662–667. 2005.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Prasad KC and Prasad SC: Assessment of
operative blood loss and the factors affecting it in tonsillectomy
and adenotonsillectomy. Indian J Otolaryngol Head Neck Surg.
63:343–348. 2011.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Yu CN, Chow TK, Kwan AS, Wong SL and Fung
SC: Intra-operative blood loss and operating time in orthognathic
surgery using induced hypotensive general anaesthesia: Prospective
study. Hong Kong Med J. 6:307–311. 2000.PubMed/NCBI
|
|
61
|
Nosulia EV: Peculiarities of blood supply
of palatal tonsils and the potential risk of hemorrhage during
tonsillectomy: The literature review and case report. Vestn
Otorinolaringol. 1:75–78. 2014.PubMed/NCBI(In Russian).
|
|
62
|
Liu JH, Anderson KE, Willging JP, Myer CM
III, Shott SR, Bratcher GO and Cotton RT: Posttonsillectomy
hemorrhage: What is it and what should be recorded? Arch
Otolaryngol Head Neck Surg. 127:1271–1275. 2001.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Mutz I and Simon H: Hemorrhagic
complications after tonsillectomy and adenoidectomy. Experiences
with 7,743 operations in 14 years. Wien Klin Wochenschr.
105:520–522. 1993.PubMed/NCBI(In German).
|
|
64
|
PLoS Medicine Editors. Best practice in
systematic reviews: The importance of protocols and registration.
PLoS Med. 8(e1001009)2011.PubMed/NCBI View Article : Google Scholar
|