Introduction
Ovarian cancer is a common gynecological malignant
solid tumor, and it also exhibits the highest mortality of cancer
of the female reproductive tract (1-3).
The majority of patients are diagnosed at the advanced stage. With
the advancement of technology, the treatment of ovarian cancer has
been significantly improved in the past few years, however, the
5-year overall survival rate for patients with ovarian cancer is
still low (4,5). Therefore, it is important to identify
new targets for the treatment of ovarian cancer.
MicroRNAs (miRNAs) are highly conservative short
non-coding RNAs that are 20-24 nucleotides in length and can
post-transcriptionally regulate gene expression (6-9).
miRNAs can mediate degradation or inhibit translation of mRNAs by
directly binding to the 3'untranslated region (3'UTR) (10,11). A
growing number of studies have demonstrated that the abnormal
expression and function of miRNAs serves an important role in the
pathogenesis of malignant disease (12,13).
It has been reported that miR-205 can induce cell invasion by
inhibiting transcription factor 21 in human ovarian cancer
(14). Furthermore, miR-663 has
been demonstrated to promote cell proliferation, migration and
invasion by targeting TUSC2 in ovarian cancer (15). Cui et al (16) reported that miR-361-5p was
downregulated in hepatocellular carcinoma (HCC) and suppressed HCC
proliferation and invasion through targeting VEGFA. Ma et al
(17) demonstrated that miR-361-5p
expression was associated with prognosis in patients with breast
cancer. A recent study has indicated that miR-361-5p was
upregulated in serous ovarian carcinoma (18). However, another study reported that
miR-361-5p was downregulated in epithelial ovarian cancer (19). Therefore, the expression and role of
miR-361-5p in ovarian cancer is controversial, and further research
is required.
TNF receptor-associated factor 3 (TRAF3) is a member
of the TRAF family and is ubiquitously expressed in a number of
tissues and cells, including in the brain, lungs, heart, spleen and
liver (20,21). TRAF3 is currently considered to be a
central regulator of the ischemic signaling cascade, including
neuronal death, neuronal apoptosis, inflammation and oxidative
stress (22). Through
bioinformatics software analysis, the current study identified the
binding sites between miR-361-5p and TRAF3. Therefore, it was
hypothesized that miR-361-5p may affect ovarian cancer cell growth
through regulating the expression of TRAF3.
The aim of the present study was to explore the role
and mechanism of miR-361-5p in ovarian cancer cells.
Materials and methods
Clinical specimens collection
Human ovarian cancer tissues and adjacent normal
tissues were obtained from 30 patients with ovarian cancer from
Nantong Maternal and Child Health Care Hospital. These specimens
were stored in liquid nitrogen or at -80˚C for subsequent use. The
experiment was approved by the Ethics Committee of Nantong Maternal
and Child Health Care Hospital. All patients were notified that
their specimens would be used in the current research, and written
informed consent was obtained from every patient.
Cell culture and transfection
Human ovarian cancer cell lines, SKOV3 and ES-2 were
obtained from the American Type Culture Collection, and human
ovarian surface epithelial cells (HOSEpiC) were purchased from the
BeNa Cell Culture Collection (cat. no. BNCC340096). SKOV3 and ES-2
cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) at 37˚C with 5% CO2. HOSEpiC cells
were cultured in DMEM medium (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% FBS.
SKOV3 cells were transfected with inhibitor control,
miR-361-5p inhibitor, TRAF3-short-hairpin (sh)RNA, control-shRNA,
miR-361-5p inhibitor + control-shRNA or miR-361-5p inhibitor +
TRAF3-shRNA for 48 h using Lipofectamine 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. A period of 48 h after cell transfection, transfection
efficiency was detected using reverse transcription-quantitative
(RT-q)PCR.
RT-qPCR
Total RNA was acquired using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) in line with the
manufacturer's protocol, and RNA was stored at -80˚C. RNA was
subsequently reverse transcribed into complementary DNA using a
reverse transcription kit (Vazyme), according to the manufacturer's
protocol. RT-qPCR was performed using SYBR Green PCR Master Mix
(Vazyme) following the manufacturer's protocol. The reaction
conditions were as follows: 95˚C for 5 min, followed by 35 cycles
of amplification at 95˚C for 30 sec, 60˚C for 30 sec and 72˚C for
30 sec. U6 or GAPDH was used for normalization. The primer
sequences for qPCR were as follows: U6 forward,
5'-GCTTCGGCAGCACATATACTAAAAT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'; GAPDH forward,
5'-CTTTGGTATCGTGGAAGGACTC-3' and reverse
5'-GTAGAGGCAGGGATGATGTTCT-3'; miR-361-5p forward,
5'-ATAAAGRGCRGACAGTGCAGATAGTG-3' and reverse,
5'-TCAAGTACCCACAGTGCGGT-3'; TRAF3 forward, 5'-GGACCGCGAGATGAGGAA-3'
and reverse 5'-CGGTCAGTGTGCAGCTTTAG-3'. Additionally, gene
expression was quantified using the
2-ΔΔCq method. Each experiment was
performed in triplicate.
Western blot analysis
Following treatment, cells were washed three times
with ice-cold PBS and lysed with RIPA lysis solution (Beijing
Solarbio Science & Technology Co., Ltd.) supplemented with
protease inhibitors for 30 min. Cells were then centrifuged at 4˚C
and stored at -20˚C. Equal amounts of protein were separated using
12% SDS-PAGE and transferred to PVDF membranes (EMD Millipore). The
membranes were blocked using 5% skim milk in PBS containing 0.1%
Tween for 2 h. Membranes were incubated with primary antibodies at
4˚C overnight. Subsequently, the membranes were incubated with an
HRP-conjugated secondary antibody at room temperature for 2 h. The
protein band was visualized using the ECL method (EMD Millipore).
β-actin was used as the loading control for normalization.
The primary antibodies used were as follows:
Anti-TRAF3 (Cat. no. 61095; dilution rate: 1:1,000), anti-Bcl-2
(Cat. no. 4223; dilution rate: 1:1,000), anti-Bax (Cat. no. 5023;
dilution rate: 1:1,000), anti-p-p65 (Cat. no. 3033; dilution rate:
1:1,000), anti-β-actin (Cat. no. 4970; dilution rate: 1:1,000) and
anti-p65 (Cat. no. 8242; dilution rate: 1:1,000), and were
purchased from Cell Signaling Technology, Inc. The secondary
antibody: Horseradish peroxidase-conjugated anti-rabbit
Immunoglobulin G secondary antibody (Cat. no. 7074; dilution rate:
1:2,000) was also purchased from Cell Signaling Technology,
Inc.
MTT assay
MTT reagent (Beijing Solarbio Science &
Technology Co., Ltd.) was used to assess cell viability. Cells were
divided into five groups: Control (SKOV3 cells without any
treatment); inhibitor control (SKOV3 cells were transfected with
inhibitor control for 48 h); inhibitor (SKOV3 cells were
transfected with miR-361-5p inhibitor for 48 h); inhibitor +
control-shRNA (SKOV3 cells were co-transfected with miR-361-5p
inhibitor and control-shRNA for 48 h); inhibitor + TRAF3-shRNA
(SKOV3 cells were co-transfected with miR-361-5p inhibitor and
TRAF3-shRNA for 48 h). SKOV3 cells were seeded into 96-well plates
at a density of 2,000 cells/well and cultured for 48 h. Then, 20 µl
MTT reagent was added into each well for an additional incubation
for 4 h at 37˚C, and 150 µl DMSO was added into each well and
plates were shaken for 15 min. The optical density (OD) values were
read at 490 nm using a micro-plate reader. Cell viability (%) = (OD
value of experimental group-OD value of the blank group)/(OD value
of control group-OD value of the blank group) x100%.
Flow cytometry assay
Cell apoptosis was analyzed using an
Annexin-V/propidium iodide (PI) Apoptosis Detection kit. SKOV3
cells were plated in six-well plates at a density of 106
cells per well overnight. The next day, cells were transfected with
inhibitor control, miR-361-5p inhibitor, miR-361-5p inhibitor +
control-shRNA, or miR-361-5p inhibitor + TRAF3-shRNA. Following
transfection for 48 h, the cells were collected, centrifuged at a
low temperature and high speed, and re-suspended in 100 µl of
FITC-binding buffer. Subsequently, the buffer was added with ~5 µl
ready-to-use Annexin V-FITC (BD Bioscience) and 5 µl PI. In the
dark, cells were incubated for 30 min at room temperature. Annexin
V-FITC and PI fluorescence were assessed using a BD FACSCalibur
flow cytometer (BD Biosciences). The data was subsequently analyzed
using FlowJo software (version 7.2.4; FlowJo LLC).
Dual-luciferase reporter assay
The binding sites between TRAF3 and miR-361-5p were
predicted using TargetScan (http://www.targetscan.org/vert_72/). To confirm the
binding sites, a dual-luciferase reporter assay was performed.
Luciferase reporter plasmids (psi-CHECK2) containing the wild-type
3'UTRs of TRAF3 and mutant 3'UTRs of TRAF3 were manufactured by
TSINGKE BioTech. SKOV3 cells were co-transfected with the wild-type
(WT) or mutant (MUT) 3'UTR luciferase reporter plasmids, and the
miR-361-5p mimic or mimic control, respectively, using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
Cells were harvested after transfection for 48 h, and the
luciferase activities were measured using the dual-luciferase assay
system (Promega Corporation) following the manufacturer's protocol.
Firefly luciferase was used as the normalization control.
Statistical analysis
GraphPad Prism was used for statistical analysis.
The data were presented as mean ± standard deviation from three
independent experiments in triplicate. Differences between two
groups were determined using a paired Student's t-test, and
comparisons between multiple groups were performed using a one-way
ANOVA followed by a Tukey's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of miR-361-5p in ovarian
cancer tissues and cells
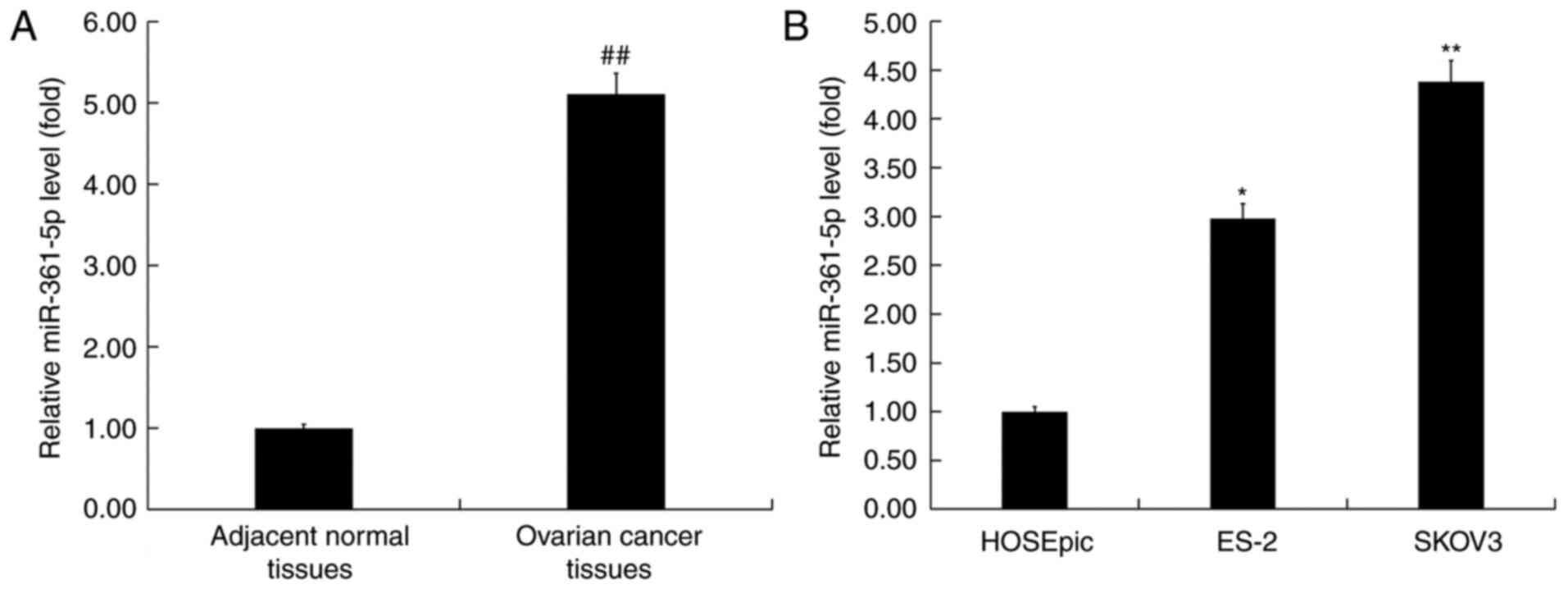
To explore the role of miR-361-5p in ovarian cancer,
the expression of miR-361-5p was determined in ovarian cancer
tissues and cancer cells using RT-qPCR. The RT-qPCR assay indicated
that miR-361-5p was highly expressed in ovarian cancer tissues
(Fig. 1A). The level of miR-361-5p
was detected in a human ovarian clear cell carcinoma cell line ES-2
and a human ovarian adenocarcinoma cell line SKOV3. The results
also demonstrated that compared with human ovarian epithelial cells
HOSEpiC, miR-361-5p expression was upregulated in ES-2 and SKOV3
cells, and miR-361-5p expression was indicated to be most prominent
in SKOV3 cells (Fig. 1B). SKOV3
cells were subsequently selected for further study.
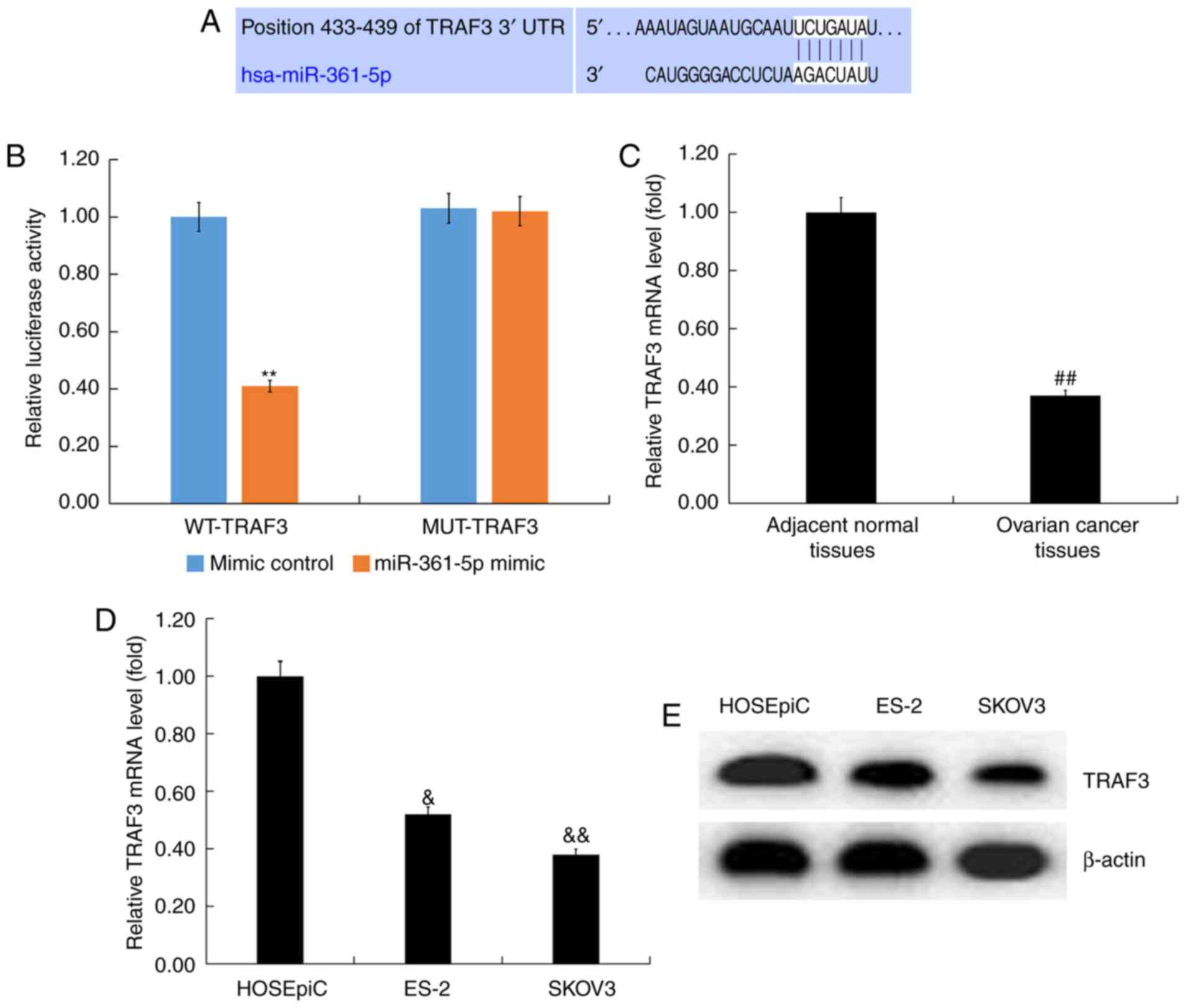
TRAF3 was indicated to be a direct
target of miR-361-5p
TargetScan was performed to analyze the potential
target genes of miR-361-5p, and the predicted sequence analysis
data indicated that TRAF3 was the target of miR-361-5p (Fig. 2A). To further verify this
prediction, a dual luciferase reporter gene assay was performed,
and the results demonstrated that miR-361-5p mimic significantly
inhibited the luciferase activity of cells co-transfected with
TRAF3-WT and miR-361-5p mimic. However, miR-361-5p mimic could not
inhibit the luciferase activity of cells co-transfected with
TRAF3-MUT and miR-361-5p mimic (Fig.
2B). These results indicated that TRAF3 was a direct target of
miR-361-5p.
To determine the role of TRAF3 in ovarian cancer, a
RT-qPCR assay was performed to detect TRAF3 mRNA expression in
ovarian cancer tissues and adjacent normal tissues. A RT-qPCR assay
indicated that TRAF3 was significantly downregulated in ovarian
cancer tissues (Fig. 2C). Compared
with HOSEpiC cells, TRAF3 expression was downregulated in ovarian
cancer ES-2 and SKOV3 cells and TRAF3 expression was minimal in
SKOV3 cells (Fig. 2D and E).
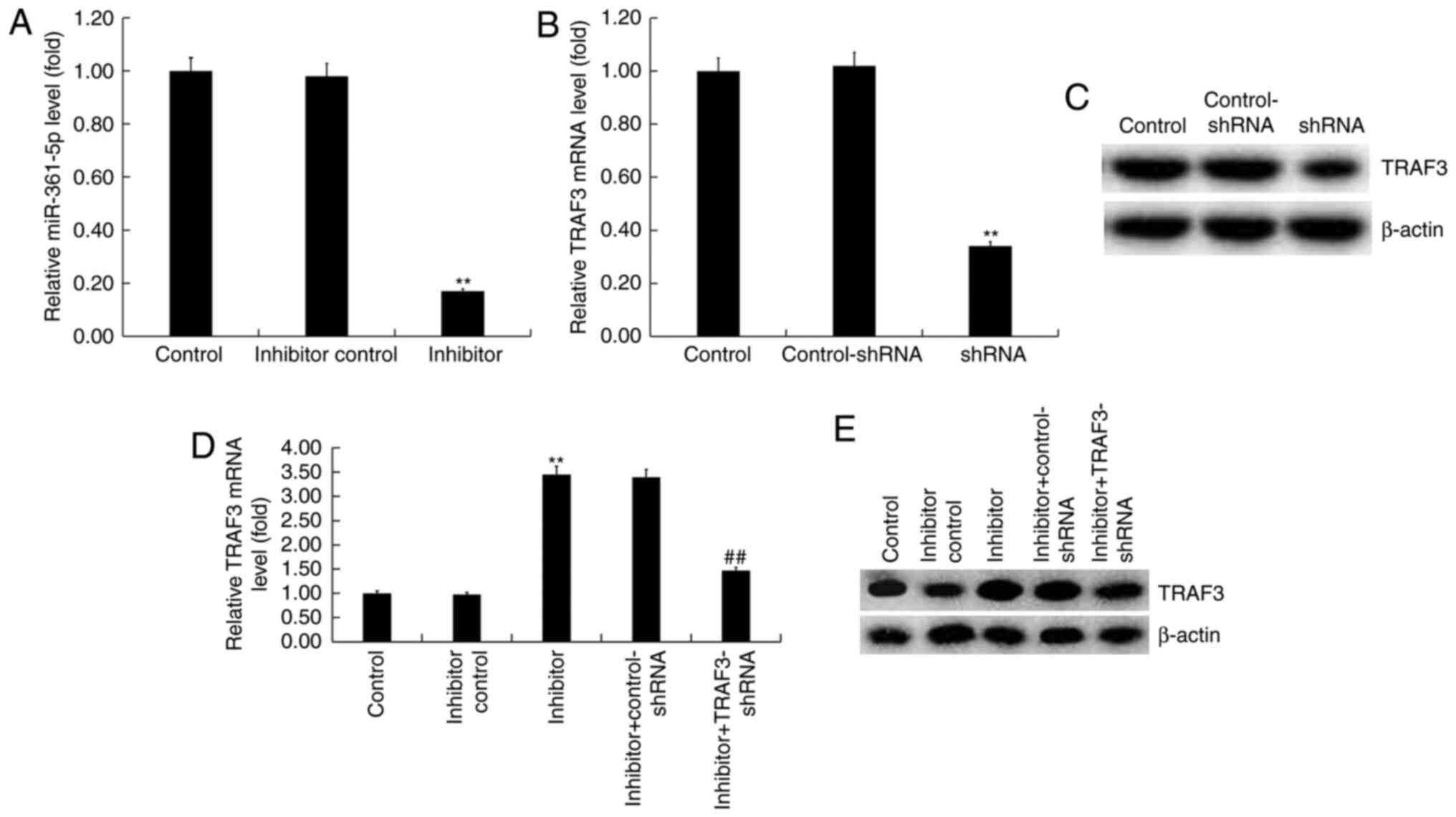
miR-361-5p negatively regulated TRAF3
expression in SKOV3 cells
SKOV3 cells were transfected with an inhibitor
control, miR-361-5p inhibitor, TRAF3-shRNA, control-shRNA,
miR-361-5p inhibitor + control-shRNA, or miR-361-5p inhibitor +
TRAF3-for 48 h. A RT-qPCR assay indicated that compared with the
inhibitor control group, miR-361-5p inhibitor significantly
decreased the expression of miR-361-5p in SKOV3 cells (Fig. 3A). The RT-qPCR assay and western
blot analysis indicated that compared with the control-shRNA group,
TRAF3-shRNA reduced the expression of TRAF3 at the mRNA and protein
level in SKOV3 cells (Fig. 3B and
C). Additionally, compared with the
inhibitor control group, miR-361-5p inhibitor promoted the
expression of TRAF3 in SKOV3 cells, and this increase was reversed
by TRAF3-shRNA (Fig. 3D and
E).
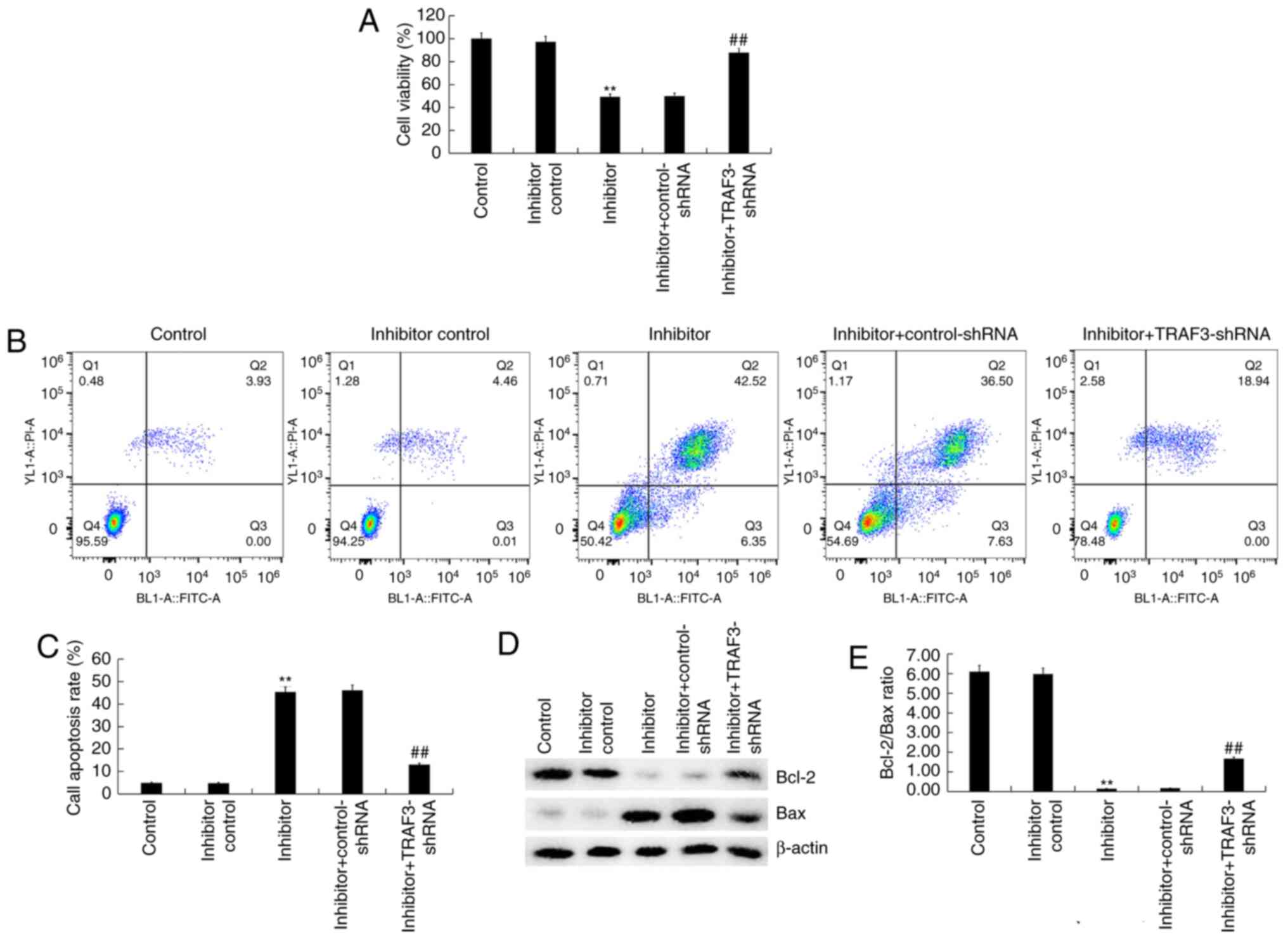
Effect of miR-361-5p inhibitor on
viability and apoptosis of SKOV3 cells
To further explore the effect of miR-361-5p
inhibitor on cell viability and apoptosis of SKOV3 cells, flow
cytometry and MTT assay were performed. MTT assay indicated that
compared with the inhibitor control group, miR-361-5p inhibitor
significantly reduced the viability of SKOV3 cells (Fig. 4A). The flow cytometry assay
indicated that miR-361-5p inhibitor significantly induced SKOV3
cell apoptosis (Fig. 4B and
C). Additionally, miR-361-5p
inhibitor decreased Bcl-2 expression, increased Bax expression
(Fig. 4D), and decreased Bcl2/Bax
ratio (Fig. 4E). All of these
changes were reversed using TRAF3-shRNA. The results demonstrated
that miR-361-5p inhibitor decreased SKOV3 cell viability and
promoted cell apoptosis.
Effect of miR-361-5p inhibitor on
NF-kB pathway in SKOV3 cells
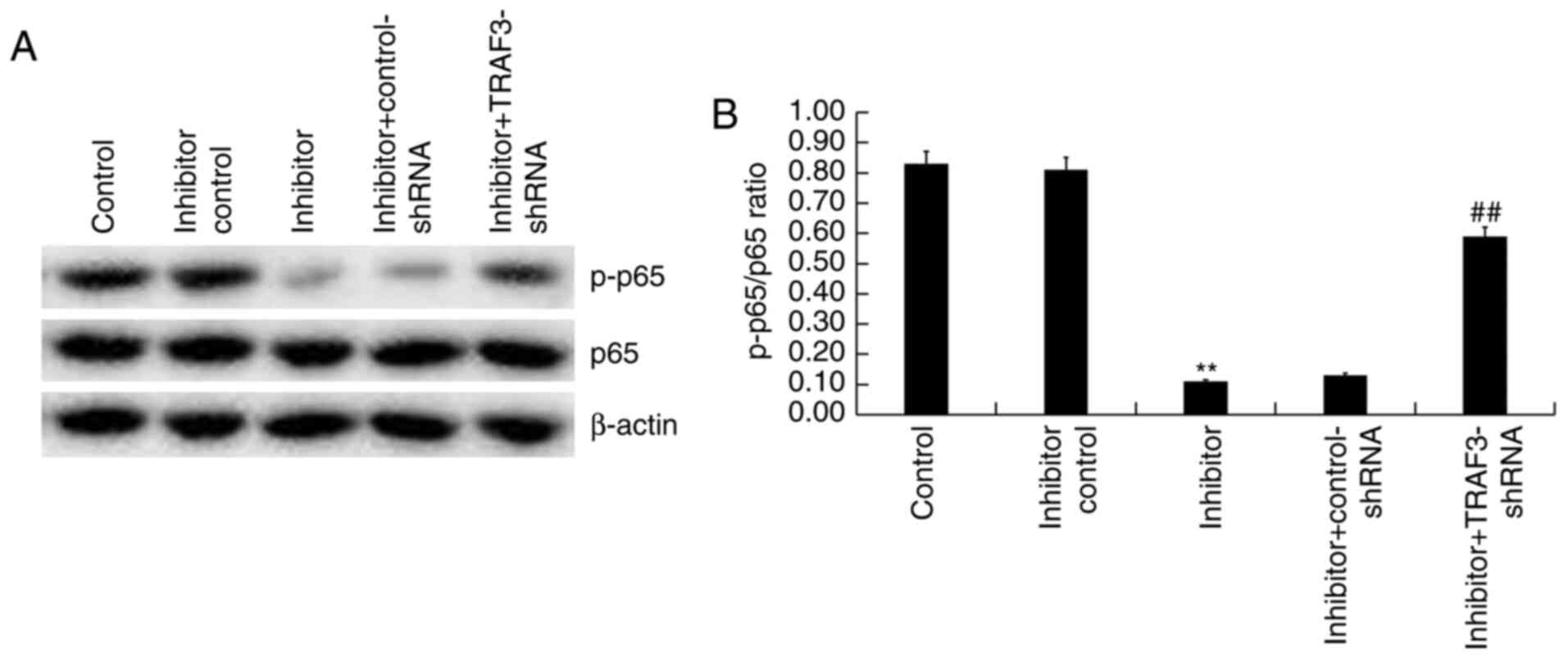
Western blot analysis revealed the expression of p65
and p-p65 in SKOV3 cells. Compared with the inhibitor control
group, miR-361-5p inhibitor markedly decreased the expression of
p-p65 protein and significantly reduced p-p65/p65 ratio in SKOV3
cells, and these changes were reversed using TRAF3-shRNA (Fig. 5A and B). Therefore, the role of miR-361-5p
inhibitor in ovarian cancer cells may be associated with the NF-kB
signaling pathway.
Discussion
It has previously been demonstrated that miRNAs can
serve as either an oncogene or tumor suppressor by directly or
indirectly modulating cancer genes. In the current study, it was
revealed that miR-361-5p was highly expressed in ovarian cancer
tissues and cancer cell lines. The results of TargetScan and dual
luciferase reporter gene assay identified TRAF3 as a direct target
of miR-361-5p. Additionally, it was demonstrated that TRAF3 was
downregulated in ovarian cancer tissues and cancer cell lines. To
further determine the role of miR-361-5p and TRAF3 in ovarian
cancer, SK-OV-3 cells were transfected with miR-361-5p inhibitor
or/and TRAF3-shRNA for 48 h. The results indicated that miR-361-5p
inhibitor significantly suppressed SK-OV-3 cell viability and
induced cell apoptosis. These changes were reversed by
TRAF3-shRNA.
Ovarian cancer exhibits one of the highest mortality
rates of all gynecologic malignancies. Currently, the combination
of surgery and chemotherapy has improved the treatment of ovarian
cancer. However, the successful complete cure rate of this disease
is only 30% (23). The mechanism of
occurrence and development of ovarian cancer remain largely
undetermined, and consequently, the identification of a new target
is required to treat ovarian cancer. There is increasing evidence
that miRNAs serve an important role in the early diagnosis,
prognosis, prevention and treatment of cancer (24-26).
It has been reported that miR-145 serves as a tumor suppressor and
as a potential diagnostic target in ovarian cancer (27,28).
Salem et al (29) indicated
that miR-590-3p promoted ovarian cancer development by targeting
Cyclin G2 and FOXO3. Zhou et al (30) demonstrated that miR-183 was
associated with the pathogenesis of OC. However, the role of
miR-361-5p in ovarian cancer remains largely unknown.
A previous study indicated that miR-361-5p serves as
a tumor suppressor, and was revealed to suppress breast cancer cell
aerobic glycolysis and proliferation (17). Kanitz et al (31) demonstrated that miR-361-5p served a
crucial role in human cutaneous squamous cell carcinoma. miR-361-5p
has also been reported to inhibit prostate cancer cell
proliferation by targeting STAT6(32). Chen et al (18) indicated that miR-361-5p was
upregulated in serous ovarian carcinoma. However, Ma et al
(19) reported that miR-361-5p was
downregulated in epithelial ovarian cancer. In the current study,
it was demonstrated that miR-361-5p was upregulated in ovarian
cancer tissues and cancer cell lines.
In the present study, it was indicated that TRAF3
was a direct target of miR-361-5p. It has been previously reported
that TRAF3 is responsible for encoding the TRAF protein (33). Additionally, TRAF3 is an important
part in the TLR and RLH pathways and exerts a key role in IRF3
activation (34). TRAF3 is also
associated with a number of other pathways (35). Rehei et al (36) demonstrated that TRAF3 was a target
of microRNA-214 in human osteosarcoma. In the present study, it was
indicated that TRAF3 was downregulated in ovarian cancer tissues
and cancer cell lines. miR-361-5p inhibitor significantly reduced
the viability of SKOV3 cells and induced apoptosis. The results of
the current study also demonstrated that miR-361-5p inhibitor could
inhibit the NF-kB signaling pathway in ovarian cancer cells, and
this result suggested that miR-361-5p may be associated with the
NF-kB signaling pathway in ovarian cancer. All the effects of
miR-361-5p inhibitor on SKOV3 cells were reversed by TRAF3
silencing.
In conclusion, miR-361-5p regulated the
proliferation and apoptosis of ovarian cancer cells by targeting
TRAF3 and may be a new potential therapeutic target for ovarian
cancer. The current study is a preliminary study of the role of
miR-361-5p in ovarian cancer. To clarify the role of miR-361-5p in
ovarian cancer, the role of miR-361-5p in other ovarian cancer cell
lines required determination. The effect of miR-361-5p/TRAF3 on
ovarian cancer also required investigation in vivo.
Furthermore, the relationship between the expression of
miR-361-5p/TRAF3 in patients with ovarian cancer and the clinical
pathological features of the patients requires investigation in
future studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL contributed to data collection, statistical
analysis, data interpretation and manuscript preparation. PH
contributed to data collection and data interpretation. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The experiment was approved by the Ethics Committee
of Nantong Maternal and Child Health Care Hospital. All patients
were notified that their specimens would be used in the current
research, and written informed consent was obtained from every
patient.
Patient consent for publication
All patients consented to the publication of the
data in this manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coleman RL, Monk BJ, Sood AK and Herzog
TJ: Latest research and treatment of advanced-stage epithelial
ovarian cancer. Nat Rev Clin Oncol. 10:211–224. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bearfoot JL, Choong DY, Gorringe KL and
Campbell IG: Genetic analysis of cancer-implicated MicroRNA in
ovarian cancer. Clin Cancer Res. 14:7246–7250. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dahiya N, Sherman-Baust CA, Wang TL,
Davidson B, Shih IeM, Zhang Y, Wood W III, Becker KG and Morin PJ:
MicroRNA expression and identification of putative miRNA targets in
ovarian cancer. PLoS One. 3(e2436)2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Guo LM, Pu Y, Han Z, Liu T, Li YX, Liu M,
Li X and Tang H: MicroRNA-9 inhibits ovarian cancer cell growth
through regulation of NF-kappaB1. FEBS J. 276:5537–5546.
2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lu L, Schwartz P, Scarampi L, Rutherford
T, Canuto EM, Yu H and Katsaros D: MicroRNA let-7a: A potential
marker for selection of paclitaxel in ovarian cancer management.
Gynecol Oncol. 122:366–371. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Poy MN, Eliasson L, Krutzfeldt J, Kuwajima
S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P
and Stoffel M: A pancreatic islet-specifc microRNA regulates
insulin secretion. Nature. 432:226–230. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang Q, Liu N, Yang X, Tu L and Zhang X:
Small RNA-mediated responses to low- and high-temperature stresses
in cotton. Sci Rep. 6:35558–35571. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang Y, Chen F, Zhao M, Yang Z, Zhang S,
Ye L, Gao H and Zhang X: MiR-107 suppresses proliferation of
hepatoma cells through targeting HMGA2 mRNA 3'UTR. Biochem Biophys
Res Commun. 480:455–460. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhu Q, Gong L, Wang J, Tu Q, Yao L, Zhang
JR, Han XJ, Zhu SJ, Wang SM, Li YH and Zhang W: miR-10b exerts
oncogenic activity in human hepatocellular carcinoma cells by
targeting expression of CUB and sushi multiple domains 1 (CSMD1).
BMC Cancer. 16(806)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wei J, Zhang L, Li J, Zhu S, Tai M, Mason
CW, Chapman JA, Reynolds EA, Weiner CP and Zhou HH: MicroRNA-205
promotes cell invasion by repressing TCF21 in human ovarian cancer.
J Ovarian Res. 10(33)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xie HH, Huan WT, Han JQ, Ren WR and Yang
LH: MicroRNA-663 facilitates the growth, migration and invasion of
ovarian cancer cell by inhibiting TUSC2. Biol Res. 52:18–27.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cui W, Li Y, Xu K, Chen G, Lu X, Duan Q
and Kang Z: miR-361-5p inhibits hepatocellular carcinoma cell
proliferation and invasion by targeting VEGFA. Biochem Biophys Res
Commun. 479:901–906. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma F, Zhang L, Ma L, Zhang Y, Zhang J and
Guo B: MiR-361-5p inhibits glycolytic metabolism, proliferation and
invasion of breast cancer by targeting FGFR1 and MMP-1. J Exp Clin
Cancer Res. 36(158)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen SF, Liu Z, Chaurasiya S, Dellinger
TH, Lu J, Wu X, Qin H, Wang J, Fong Y and Yuan YC: Identification
of core aberrantly expressed microRNAs in serous ovarian carcinoma.
Oncotarget. 9:20451–20466. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ma J, Jing XT, Chen Z, Duan Z and Zhang Y:
MiR-361-5p decreases the tumorigenicity of epithelial ovarian
cancer cells by targeting at RPL22L1 and c-Met signaling. Int J
Clin Exp Pathol. 11:2588–2596. 2018.PubMed/NCBI
|
|
20
|
Häcker H, Tseng PH and Karin M: Expanding
TRAF function: TRAF3 as a tri-faced immune regulator. Nat Rev
Immunol. 11:457–468. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Yi Z, Lin WW, Stunz LL and Bishop GA:
Roles for TNF-receptor associated factor 3 (TRAF3) in lymphocyte
functions. Cytokine Growth Factor Rev. 25:147–156. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gong J, Li ZZ, Guo S, Zhang XJ, Zhang P,
Zhao GN, Gao L, Zhang Y, Zheng A, Zhang XF, et al: Neuron-specifc
tumor necrosis factor receptor-associated factor 3 is a central
regulator of neuronal death in acute ischemic stroke. Hypertension.
66:604–616. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhu CL and Gao GS: MiR-200a overexpression
in advanced ovarian carcinomas as a prognostic indicator. Asian Pac
J Cancer Prev. 15:8595–8601. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhu L and Fang J: The structure and
clinical roles of MicroRNA in colorectal cancer. Gastroenterol Res
Pract. 2016(1360348)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shukla KK, Misra S, Pareek P, Mishra V,
Singhal B and Sharma P: Recent scenario of microRNA as diagnostic
and prognostic biomarkers of prostate cancer. Urol Oncol.
35:92–101. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shah MY, Ferrajoli A, Sood AK,
Lopez-Berestein G and Calin GA: microRNA therapeutics in cancer-an
emerging concept. EBioMedicine. 12:34–42. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhu X, Li Y, Xie C, Yin X, Liu Y, Cao Y,
Fang Y, Lin X, Xu Y, Xu W, et al: miR-145 sensitizes ovarian cancer
cells to paclitaxel by targeting Sp1 and Cdk6. Int J Cancer.
135:1286–1296. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gadducci A, Sergiampietri C, Lanfredini N
and Guiggi I: Micro-RNAs and ovarian cancer: The state of art and
perspectives of clinical research. Gynecol Endocrinol. 30:266–271.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Salem M, Shan Y, Bernaudo S and Peng C:
miR-590-3p targets cyclin G2 and FOXO3 to promote ovarian cancer
cell proliferation, invasion, and spheroid formation. Int J Mol
Sci. 20(E1810)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhou J, Zhang C, Zhou B and Jiang D:
miR-183 modulated cell proliferation and apoptosis in ovarian
cancer through the TGF-β/Smad4 signaling pathway. Int J Mol Med.
43:1734–1746. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kanitz A, Imig J, Dziunycz PJ, Primorac A,
Galgano A, Hofbauer GF, Gerber AP and Detmar M: The expression
levels of MicroRNA-361-5p and its target VEGFA are inversely
correlated in human cutaneous squamous cell carcinoma. PLoS One.
7(e49568)2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu D, Tao T, Xu B, Chen S, Liu C, Zhang
L, Lu K, Huang Y, Jiang L, Zhang X, et al: MiR-361-5p acts as a
tumor suppressor in prostate cancer by targeting signal transducer
and activator of transcription-6 (STAT6). Biochem Biophys Res
Commun. 445:151–156. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Häcker H, Redecke V, Blagoev B,
Kratchmarova I, Hsu LC, Wang GG, Kamps MP, Raz E, Wagner H, Häcker
G, et al: Specificity in Toll-like receptor signalling through
distinct effector functions of TRAF3 and TRAF6. Nature.
439:204–207. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Oganesyan G, Saha SK, Guo B, He JQ,
Shahangian A, Zarnegar B, Perry A and Cheng G: Critical role of
TRAF3 in the Toll-like receptor-dependent and -independent
antiviral response. Nature. 439:208–211. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xie P: TRAF molecules in cell signaling
and in human diseases. J Mol Signal. 8(7)2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rehei AL, Zhang L, Fu YX, Mu WB, Yang DS,
Liu Y, Zhou SJ and Younusi A: MicroRNA-214 functions as an oncogene
in human osteosarcoma by targeting TRAF3. Eur Rev Med Pharmacol
Sci. 22:5156–5164. 2018.PubMed/NCBI View Article : Google Scholar
|