Introduction
Heat shock protein 60 (HSP60) is an evolutionarily
conserved molecular chaperone protein that is abundantly expressed
in primary human tumors (1). HSP60
has been shown to possess anti-apoptotic properties and serves a
central role in tumor cell maintenance by stabilizing mitochondrial
survivin expression and restraining p53 function (2). At present, the majority of studies on
HSP60 focus on its intracellular anti-apoptotic functions in tumor
cells (3-5).
An increasing number of studies have shown that
endogenous HSP60 is upregulated in cells when cellular stress is
increased and is released into the extracellular environment to
induce an autoimmune response. This is particularly prevalent in
activated microglia and in the myocardium of failing heart
(6-10).
HSP60 expression in colorectal cancer (CRC) tissue and the serum
antibody titer to HSP60 is significantly higher in patients with
CRC compared with healthy subjects (11). Extracellular HSP60 is a target of
Toll-like receptor 4 (TLR-4), where the activation of which
stimulates downstream signaling molecules, such as myeloid
differentiation primary response 88 (MYD88) and NF-κB, resulting in
an increase in the release inflammatory factors, such as inducible
nitric oxide synthase (iNOS), IL-1β, TNF-α and IL-6(12). These inflammatory factors can
promote tumor growth (13).
Therefore, HSP60 inhibitors may serve as anticancer agents by
inhibiting tumor growth, invasion and infiltration.
Curcumin (CCM) is a natural polyphenolic compound
present in the rhizome of Curcuma longa and belongs to the
family Zingiberaceae (14). An
increasing number of experimental studies have revealed that CCM
exhibits multiple biological effects and may serve as a potential
protective factor of various diseases due to its anti-inflammatory,
anti-oxidant and anti-apoptotic properties, whilst also possessing
an excellent safety profile (15-17).
The anticancer effects of CCM manifest due to its ability to induce
growth arrest and apoptosis in various premalignant and malignant
cells (18).
Several studies have shown the effects and
mechanisms of CCM against human neuroglioma cells (19-21).
CCM was shown to induce G2/M cell cycle arrest and apoptosis in U87
cells by increasing forkhead box protein O1 expression (22), and was also reported to suppress
tumor growth and angiogenesis in human glioma cells through
modulation of VEGF/angiopoietin-2/thrombospondin-1 signaling
(23,24). Since inflammatory factors can
enhance glioma growth, and CCM is an effective anti-inflammatory
factor, it remains unknown whether CCM can exert its antitumor
effects by inhibiting the inflammatory HSP60/TLR-4 signaling
pathway.
The present study investigated the effects of CCM on
the viability and invasive ability of neuroglioma U87 cells and
determined whether the HSP60/TLR-4 signaling pathway is involved in
this effect. The results demonstrated that CCM can exert its
antitumor effects by inhibiting the inflammatory HSP60/TLR-4
signaling pathway. These findings suggested that CCM may be used as
a potential therapy for the treatment of human glioma.
Materials and methods
Chemicals
The U87 cell line (glioblastoma of unknown origin)
was purchased from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. CCM was purchased from Sigma-Aldrich;
Merck KGaA (cat. no. C1386). Antibodies against caspase-3 (cat. no.
9665), p53 (cat. no. 2524), MYD88 (cat. no. 4283) and TLR-4 (cat.
no. 2219) were purchased from Cell Signaling Technology, Inc;
anti-iNOS (cat. no. ab129372) and NF-κB (cat. no. ab31481)
antibodies were purchased from Abcam; antibodies against HSP60
(cat. no. API-SPA-901) and heat shock factor (HSF)-1 (cat. no.
API-SPA-806), and a HSP60 ELISA kit (cat. no. ADI-EKS-600) were
purchased from Enzo Life Sciences, Inc. and anti-β-actin antibody
(cat. no. TA-09) was purchased from OriGene Technologies, Inc. IL-6
(cat. no. NOV-NR-E10276-1x96T), IL-1β (cat. no. EHC002b.96.10) and
TNF-α (cat. no. ADI-901-099) ELISA kits were purchased from
Neobioscience Technology Co., Ltd. BCA kits (cat. no. 23225) and
ECL reagent (cat. no. 32106) were purchased from Thermo Fisher
Scientific, Inc. DMEM (cat. no. 220511) and FBS (cat. no. 16140071)
were purchased from Gibco (Thermo Fisher Scientific, Inc.). Cell
Counting Kit-8 (CCK-8; cat. no. BB-4202-1) was purchased from
BestBio Science.
Cell culture
U87 cells were cultured in DMEM supplemented with
10% FBS and maintained at 37˚C in a humidified incubator with 5%
CO2. CCM was dissolved in DMSO (1 mM). Preliminary
experiments were performed to determine the concentrations and time
points of CCM treatment (data not shown). Cells were treated with
different concentrations of CCM (10, 20, 40, 60 or 80 µM) for 24 h.
The supernatant of culture medium and protein lysate of cells
[lysed with 1 ml RIPA buffer (Solarbio Life Sciences, cat. no.
R0010)] with 1 µl protease inhibitor, 5 µl PMSF and 5 µl
phosphatase inhibitor) were collected for subsequent experiments.
The morphological features of cell growth were observed using an
inverted light microscope (magnification, x100 or 200).
Cell viability assay
A CCK-8 kit was used to assess the viability of
cells following various treatments. A total of 5x104
cells/well were seeded into 96-well microtiter plates and treated
with various concentrations of CCM. After 24 h, 10 µl CCK-8
solution was added to each well, and cells were cultured for a
further 2 h. Absorbance was measured at a wavelength of 450 nm with
an Immunoreader (Bio-Rad Laboratories, Inc.). Cell viability is
presented as the percentage of the control value.
ELISA
ELISA kits were used to determine the quantity of
TNF-α, IL-6, IL-1β and HSP60 in culture medium according to the
manufacturer's protocol. Absorbance was measured at a wavelength of
450 nm on a microplate reader (Bio-Rad Laboratories, Inc.).
Western blotting
A BCA kit was used to determine protein
concentration. Equal amounts of protein (20 µg) were resolved
electrophoretically using 10% SDS-PAGE and transferred to a 0.45-µm
polyvinylidene difluoride membrane. The membrane blots were blocked
with 5% milk in TBS-Tween-20 (0.1% TBS-T) at room temperature (RT)
for 1 h and incubated at 4˚C overnight with antibodies against iNOS
(1:200), TLR-4 (1:1,000), HSF-1 (1:1,000), NF-кB (1:1,000), HSP60
(1:1,000), p53 (1:1,000), Caspase-3 (1:2,000), MYD88 (1:1,000) or
β-actin (1:1,000). After thoroughly washing the membrane with TBS-T
buffer, they were incubated with HRP-conjugated anti-mouse (cat. no
ZB-2305; OriGene Technologies, Inc.) or anti-rabbit (cat. no
ZB-2301; OriGene Technologies, Inc.) secondary antibodies at RT for
2 h. Signals were visualized using an ECL kit and the membranes
were then exposed to X-ray films. The densitometry was analyzed by
Image-Pro Plus software version 6.0 (Media Cybernetics, Inc.).
Statistical analysis
Data are presented as the mean ± SEM. The data were
analyzed using SPSS 19.0 (IBM Corp.). Statistical comparisons were
performed using paired Student's t-test or one-way ANOVA followed
by Dunnett's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Chemical structure of CCM and its
effects on U87 cell viability
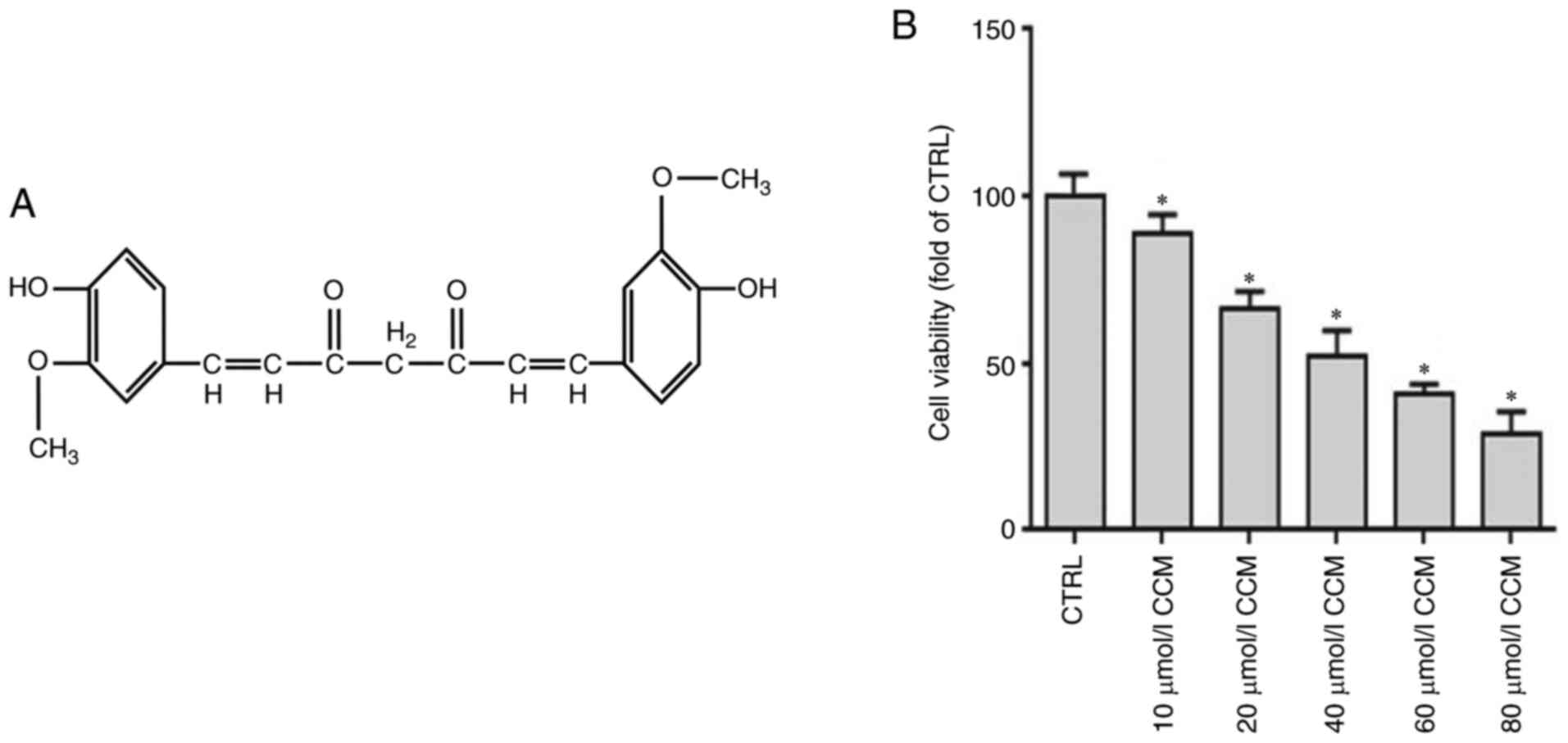
The chemical structure of CCM is
[1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3, 5-dione] and
is shown in Fig. 1A. To determine
the effects of CCM on U87 cell viability, cck-8 assays were
performed. Cells were treated with various concentrations of CCM
(10, 20, 40, 60 or 80 µM) for 24 h and cell viability was assessed.
The results showed that CCM treatment significantly inhibited the
viability of U87 cells compared with the control group (P<0.05;
Fig. 1B). Viability was normalized
to cells that were incubated in media without CCM. The cell
viability decreased with an increase in CCM concentration. An
IC50 of 48.77 µM was obtained by calculating the median
lethal concentration. In order to prevent false positive results
caused by low cell activity, 40 µM CCM was chosen as the follow-up
concentration for subsequent experiments.
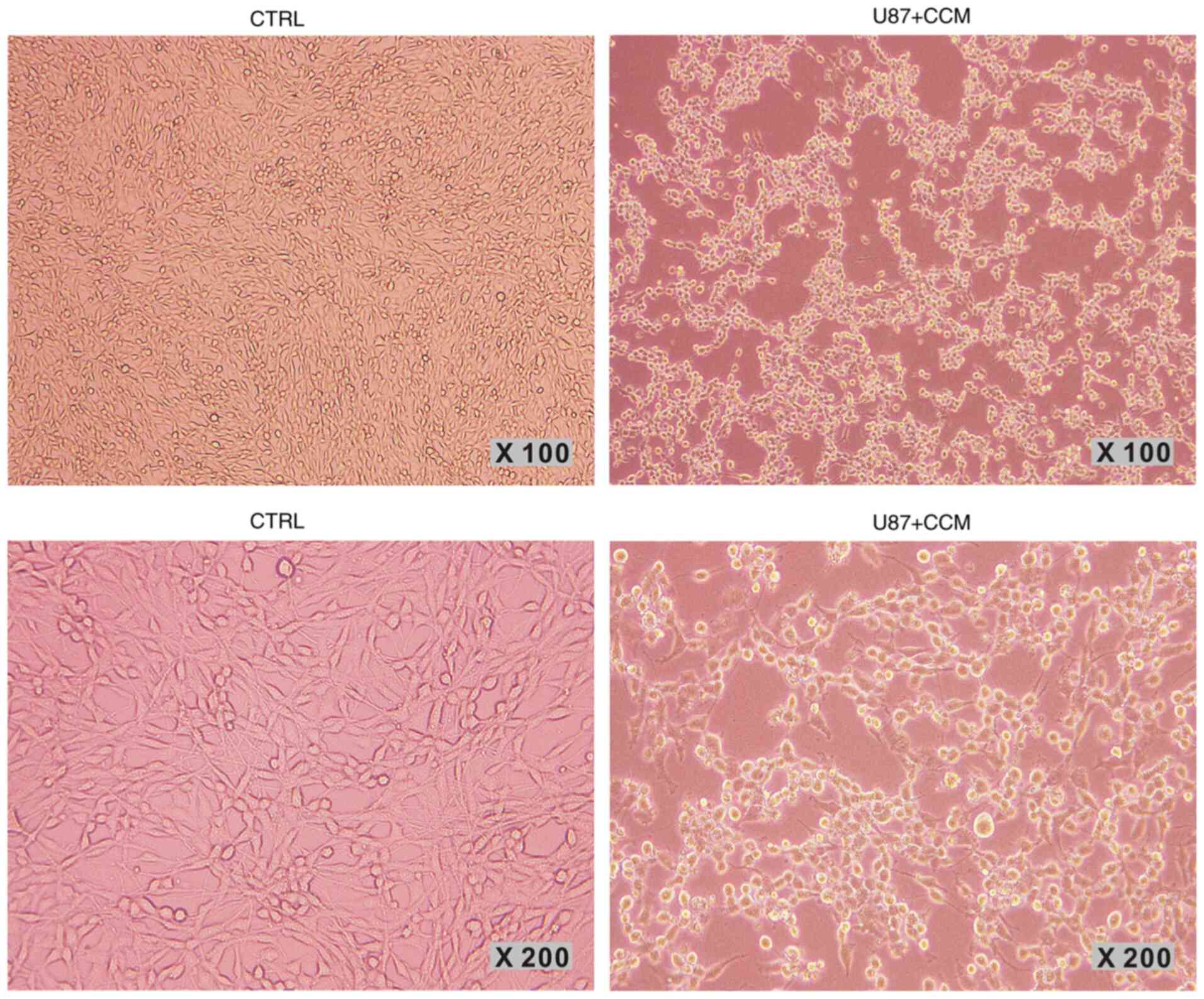
CCM treatment affects cell
morphology
The morphology of cells in the control group and CCM
group (40 µM for 24 h) was observed under an inverted microscope.
As shown in Fig. 2, U87 cells in
the control group (left panels) were polymorphous and the majority
of them exhibited long fusiform morphology. Cells were cross-linked
and had several slender dendrites. Cells treated with CCM are shown
in the right panels. Compared with the control group, the number of
cells was notably lower, and the majority of cells exhibited a
round morphology with shorter dendrites, showing signs of
apoptosis.
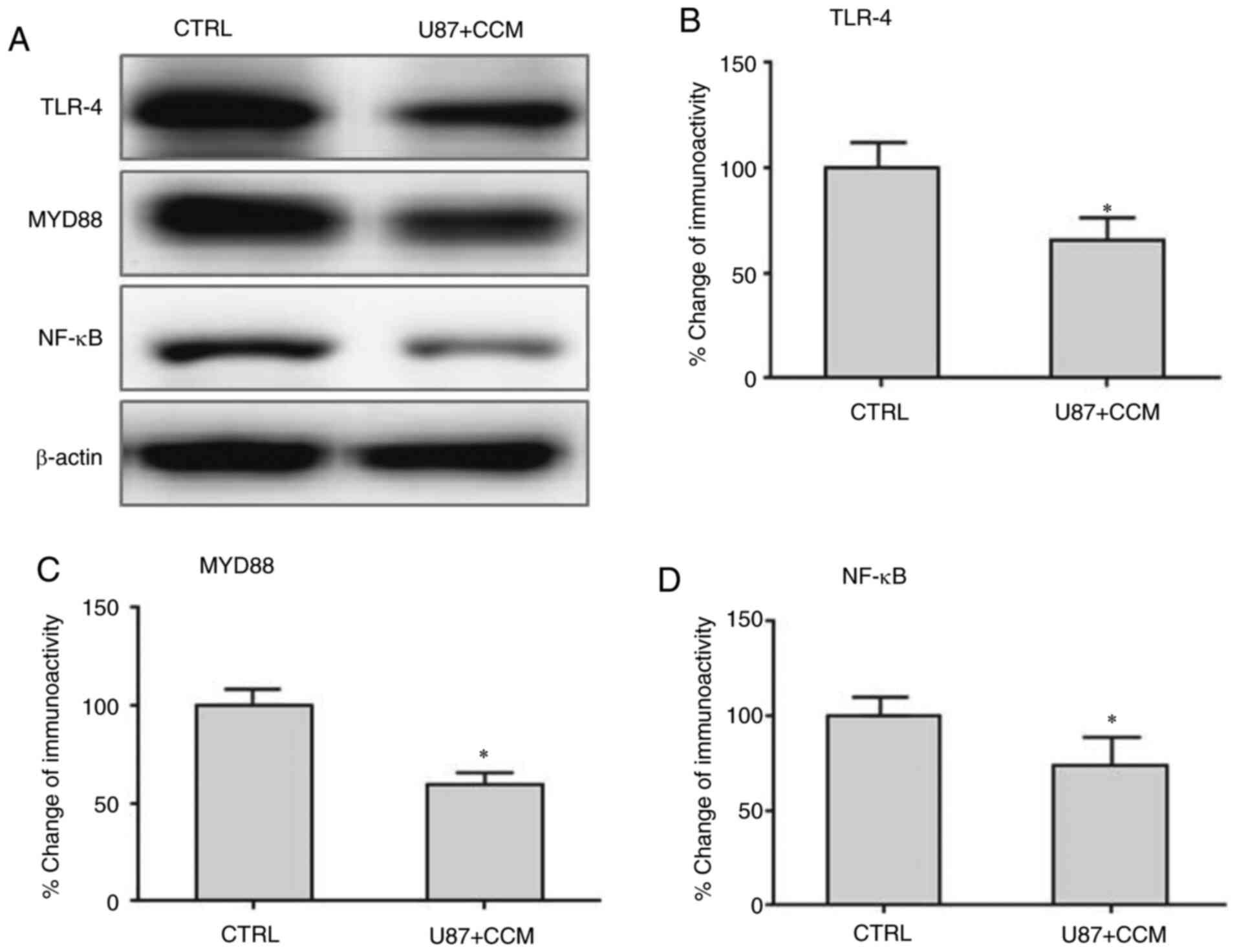
Effects of CCM on expression of TLR-4,
MYD88 and NF-κB in U87 cells
The expression levels of TLR-4, MYD88 and NF-κB were
detected using western blotting. As shown in Fig. 3, TLR-4, MYD88 and NF-κB protein
expression was significantly lower following CCM treatment compared
with the controls.
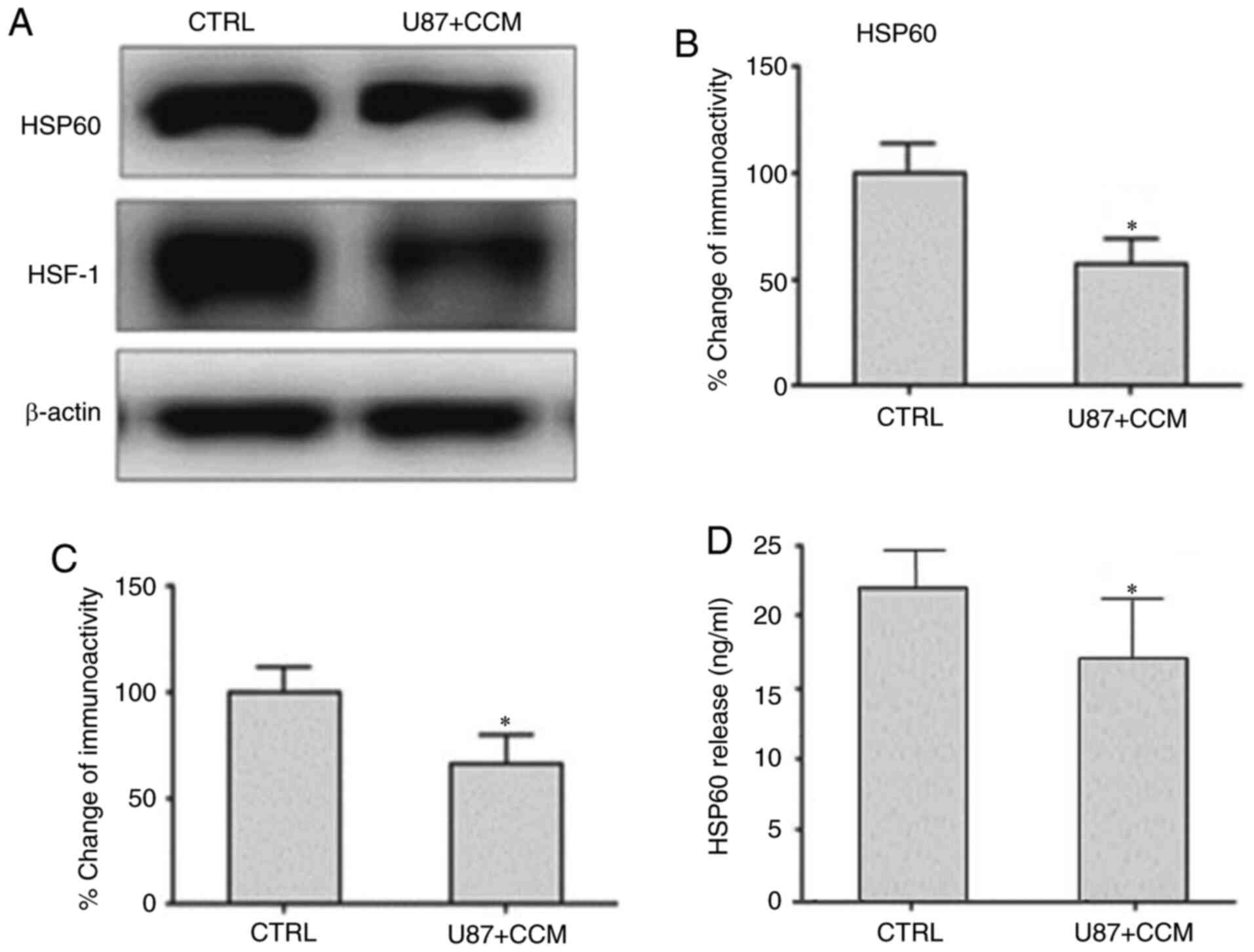
CCM reduces the expression of HSP60
and HSF-1 and reduces HSP60 release in U87 cells
The expression levels of HSP60 and HSF-1, which can
induce HSP60 expression, was detected by western blotting. As shown
in Fig. 4, the levels of HSP60 and
HSF-1 were significantly decreased in U87 cells treated with CCM
compared with the control group. Extracellular HSP60 was measured
by ELISA, and its release was significantly reduced in cells
treated with CCM compared with controls.
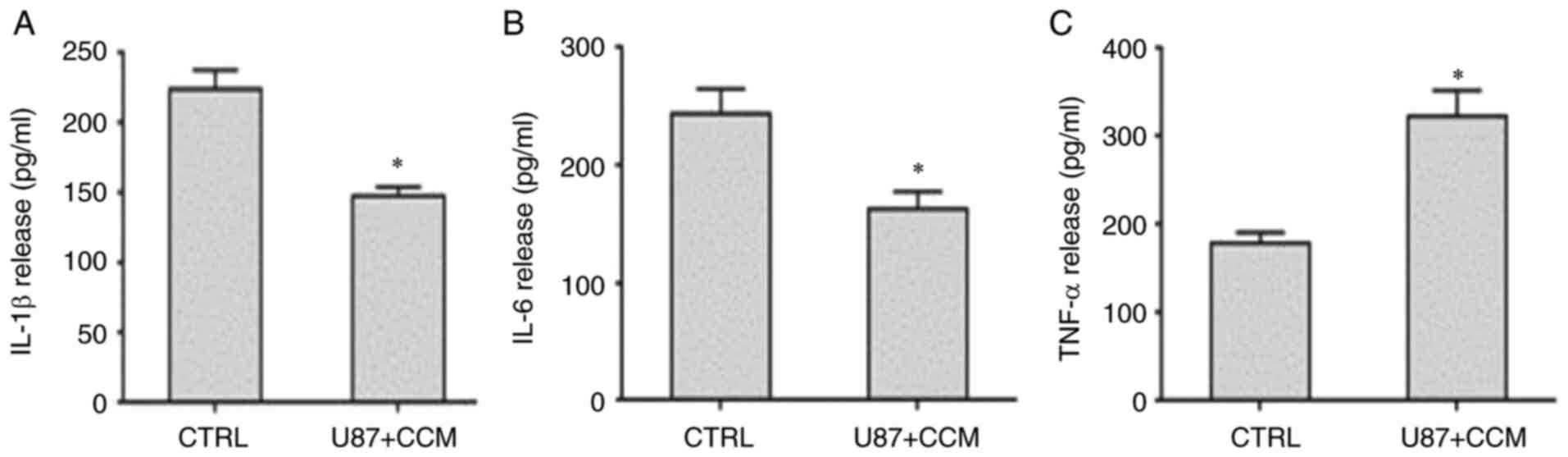
Effects of CCM on inflammatory
cytokine production in U87 cells
IL-1β and IL-6 secretion promotes tumor
proliferation (25), whereas TNF-α
can cause tumor cell apoptosis (26). Therefore, the levels of IL-1β, IL-6
and TNF-α in culture medium were measured using ELISA. As shown in
Fig. 5, the levels of IL-1β and
IL-6 significantly decreased in U87 cells treated with CCM, whereas
TNF-α expression significantly increased compared with the control
group. Thus, CCM may inhibit tumor cell proliferation by blocking
IL-1β and IL-6 release and promote tumor cell apoptosis by
increasing TNF-α secretion.
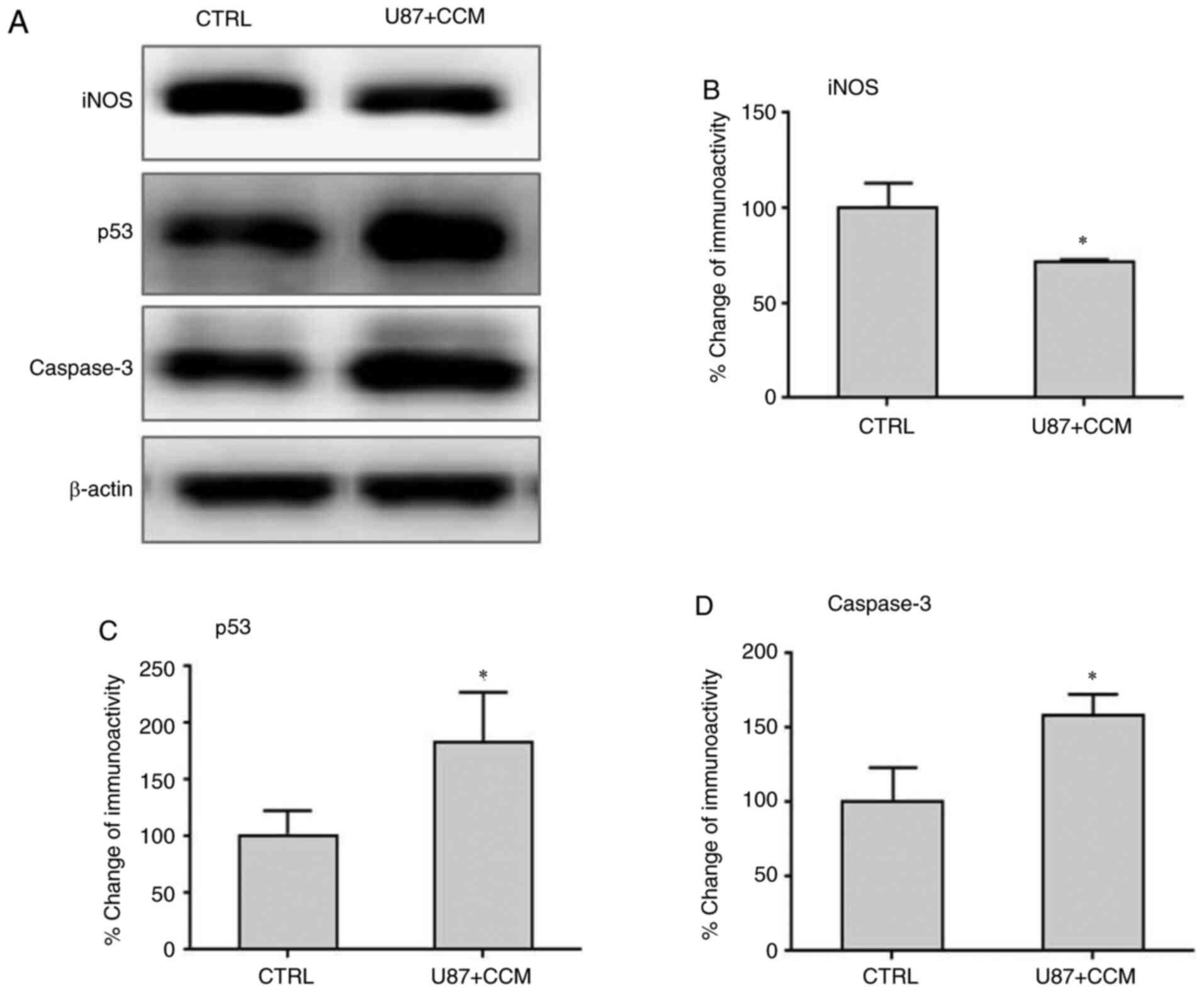
Effects of CCM on the expression of
iNOS, p53 and caspase-3 in U87 cells
The expression levels of iNOS, caspase-3 and p53
were detected by western blotting. The results showed that the
levels of iNOS, which may promote malignant cell transformation and
proliferation, was significantly reduced following treatment with
CCM compared with controls (Fig. 6A
and B). However, the expression
levels of caspase-3 and p53, which can induce tumor apoptosis,
significantly increased following CCM treatment compared with
controls.
Discussion
The present study showed that treatment with 40 µM
CCM effectively inhibited U87 glioma cell proliferation and
invasion (data not shown) and induced U87 apoptosis. CCM reduced
the expression of HSP60, HSF-1, TLR-4, MYD88 and NF-κB, and
increased the expression of the apoptosis-related proteins
caspase-3 and tumor suppressor gene p53 in U87 cells. Additionally,
expression of the proinflammatory factors IL-6, IL-1β and iNOS was
decreased, and expression of TNF-α was increased. To the best of
our knowledge, the present study is the first to report that HSP60
participated in the anticancer activities of CCM in neuroglioma U87
cells via the HSP60/TLR-4/MYD88/NF-κB signaling pathway.
CCM is known to possess antitumor properties in
several tumor cell lines and animal model experiments such as
gastric carcinoma, tongue squamous cell carcinoma (27-29).
The present study showed that CCM could significantly reduce the
growth and invasion of U87 glioma cells. CCM inhibited the
proliferation, invasion, angiogenesis and metastasis of different
types of cancer via interactions with multiple cell signaling
proteins, which differ based on the type of cancer (30). However, it remains unknown whether
HSP60 is involved in the anticancer properties of CCM.
HSP60 is a molecular chaperone. In addition to its
chaperone role in assisting protein folding, HSP60 contributes to
regulation of apoptosis and modulation of immune system activity,
such as microglial activation-induced inflammation in the central
nervous system (31,32). Upregulated HSP60 expression has been
detected in several malignant tumors, and its high expression has
been shown to enhance cell survival by exhibiting an anti-apoptotic
effect and through maintaining tumor cell growth (2). Therefore, inhibiting HSP60 expression
may be a potential strategy for suppressing tumor growth. In the
present study, HSP60 and its transcription factor HSF-1, which
regulates the expression of HSP60 by binding to its promoter, were
shown to be expressed in U87 cells. HSP60 was classically regarded
as an intracellular protein, but in the last few years,
considerable evidence has shown that it is expressed pericellularly
and extracellularly (33,34).
Our previous studies showed that intracellular HSP60
is released into the extracellular space, where it acts as a ligand
for TLR-4 (6,7). TLR4 is expressed on a variety of
immune and tumor cells, but its activation can have opposing
effects, as it can either promote antitumor immunity or result in
increased tumor growth and immunosuppression (35).
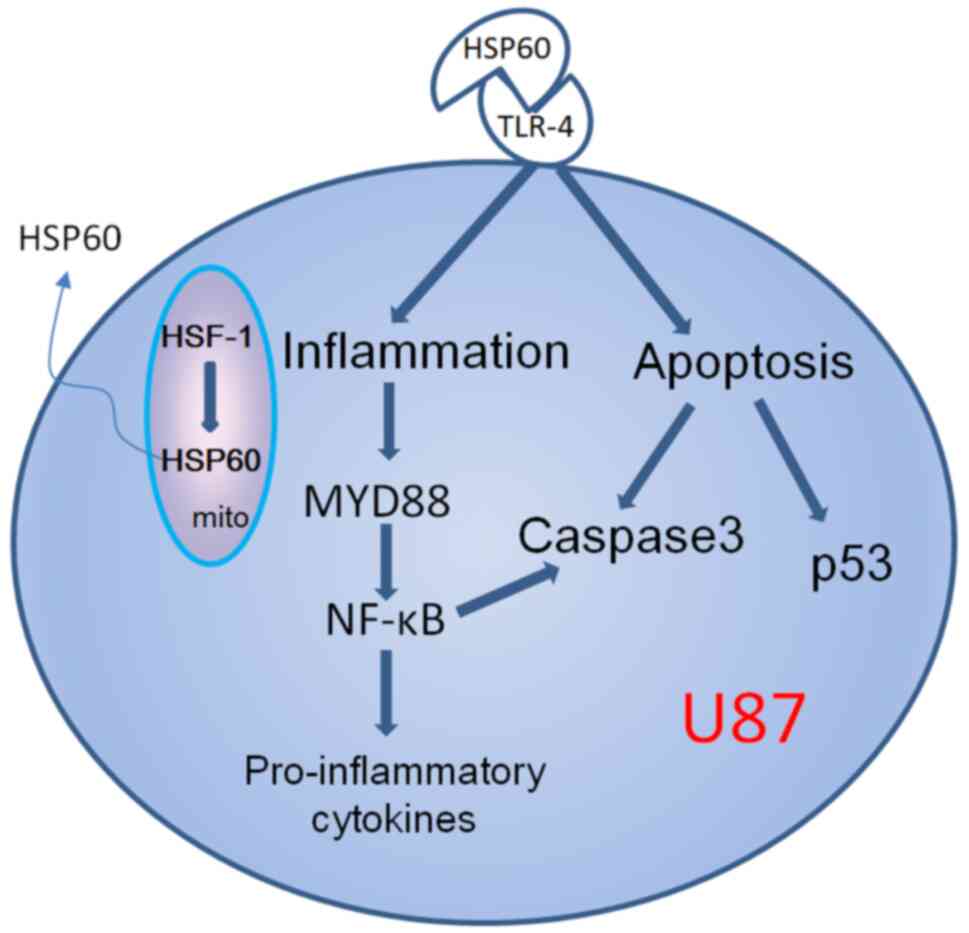
The present study hypothesized that CCM may directly
inhibit the expression of HSP60 or through inhibiting its
transcription factor HSF-1 to suppress HSP60 gene expression,
thereby decreasing HSP60 protein. As a mitochondrial protein, upon
inflammation or stress, HSP60 can translocate to the cytosol and
released to the extracellular space (9-10). Extracellular
HSP60 is a ligand of TLR-4, where binding of HSP60 to TLR-4 can
result in the activation of one of two potential signaling
pathways, which are either MYD88-dependent or MYD88-independent
(Fig. 7). The present study showed
that both TLR-4 and MYD88 were highly expressed in U87 cells, and
their expression was reduced by CCM, indicating that inhibition of
HSP60 expression by CCM resulted in downregulation of TLR-4
signaling via a MYD88-dependent pathway. TLR4/MYD88 expression
levels were shown to be positively correlated with the metastasis
of breast cancer cells (36).
Highly expressed TLR4/MYD88 may be useful as a novel biomarker to
evaluate the prognosis and treatment of patients with cancer.
NF-κB as a downstream signaling factor of TLR4/MYD88
and is a key factor in tumorigenesis, given its ability to regulate
the expression and function of a number of genes involved in these
processes (37). Constitutive
activation of NF-κB is a common feature of several human tumors,
including gastric cancer, breast cancer and lung cancer, amongst
others (38,39). Aberrant NF-κB activity was also
detected in human malignant astrocytoma cells (40). Therefore, inhibiting NF-κB should be
effective in the prevention and treatment of cancer. In the present
study, it was shown that curcumin could significantly decrease
NF-κB expression in U87 cells, suggesting that curcumin may
suppress U87 cell growth by inhibiting NF-κB activity.
When NF-κB is activated, the downstream
corresponding proinflammatory cytokines IL-1β and IL-6 are
activated and secreted out of the cells, which can promote the
proliferation of tumor cells (41).
TLR-signaling and proinflammatory cytokines have been shown to act
as drivers of tumorigenesis (42).
CCM was shown to decrease IL-1β and IL-6 levels in the present
study. TNF-α is a member of the TNF/TNFR cytokine superfamily and
serves an inhibitory role in the formation and growth of tumor
cells (43). The results of the
present study showed that TNF-α was upregulated by CCM, and this
may contribute to the reduction in malignant progression of
U87.
Apoptosis is a form of programmed cell death that
results in the orderly and efficient removal of damaged cells, such
as those resulting from DNA damage or during development. However,
too little apoptosis occurs in cancers, resulting in malignant
cells that will not die (44). The
p53 tumor suppressor gene is a pivotal molecule mediating cell
cycle arrest and apoptosis (45).
Caspase-3 is a major mediator of apoptosis that is activated during
cellular exposure to cytotoxic drugs, radiotherapy or
immunotherapy, and is frequently used as a marker to assess the
efficacy of cancer therapy (46).
Apoptosis is regulated by p53-dependent signaling pathways, which
regulate caspase-3 expression (47). The results of the present study
showed that CCM significantly upregulated p53 and caspase-3
levels.
Proinflammatory factors that are secreted by tumor
cells and the tumor microenvironment, whose expression may be
driven by TLR4-mediated signals, induce angiogenesis and metastasis
and promote tumor growth (48).
They also provide signals necessary for the survival, accumulation
and function of cancer cells (49).
Therefore, the use of inhibitors of proinflammatory factors may
prove beneficial for tumor therapy. TNF-α is a multifunctional
cytokine, which serves key roles in apoptosis and cell survival as
well as in inflammation and immunity, indicating its role as a
double-edged sword (50). In the
present study, CCM treatment increased TNF-α levels and decreased
the levels of the proinflammatory cytokines IL-6, IL-1β and iNOS in
U87 cells. This suggested that curcumin may inhibit tumor cell
proliferation by blocking IL-1β and IL-6 release and promote tumor
cell apoptosis by increasing TNF-α secretion.
Taken together, CCM may inhibit the invasion and
growth of neuroglioma via the HSP60/TLR-4/MYD88/NF-κB signaling
pathway. Therefore, HSP60 may be a potential therapeutic target for
treating neuroglioma. However, the lack of a proper control cell
line is a limitation of the present study, and further studies
should be performed to confirm these results.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Ningxia
Natural Science Foundation (grant nos. 2020AAC03143, 2020AAC03150),
National Natural Science Foundation of China (grant nos. 82060223,
81571098 and 31560273) and Undergraduate Innovation and
Entrepreneurship Training Program (S202010752032 and
S202010752039).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FB and JW performed most of the experiments. XZ
conducted some of the cell culture experiments. JX performed some
of the molecular experiments. JG and ZM contributed to conception
of the study, performed part of experiments and directed graduate
students. JL and CZ contributed to acquisition and interpretation
of data and revised figures. YZ acquired funding, performed some
experiments, analyzed certain data, revised the manuscript and
created Fig. 7. YW designed the
project and gave final approval of the version to be published. YL
designed the study, wrote the manuscript and analyzed part of data.
All authors read and approved the final manuscript. FB and YW
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ghosh AC, Dohi T, Kang BH and Altieri DC:
Hsp60 regulation of tumor cell apoptosis. J Biol Chem.
283:5188–5194. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Huang YH and Yeh CT: Functional
compartmentalization of HSP60-survivin interaction between
mitochondria and cytosol in cancer Cells. Cells.
9(23)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li XS, Xu Q, Fu XY and Luo WS: Heat shock
protein 60 overexpression is associated with the progression and
prognosis in gastric cancer. PLoS One. 9(e107507)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Desmetz C, Bibeau F, Boissière F, Bellet
V, Rouanet P, Maudelonde T, Mangé A and Solassol J:
Proteomics-based identification of HSP60 as a tumor-associated
antigen in early stage breast cancer and ductal carcinoma in situ.
J Proteome Res. 7:3830–3837. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tsai YP, Yang MH, Huang CH, Chang SY, Chen
PM, Liu CJ, Teng SC and Wu KJ: Interaction between HSP60 and
beta-catenin promotes metastasis. Carcinogenesis. 30:1049–1057.
2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang R, Li YH, Hou XL, Miao ZH and Wang
Y: Neuroprotective effect of heat shock protein 60 on
matrine-suppressed microglial activation. Exp Ther Med.
14:1832–1836. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ding FJ, Li F, Li YH, Hou XL, Ma Y, Zhang
N, Ma J, Zhang R, Lang B, Wang HY and Wang Y: HSP60 involved in
neuroprotective effects of curcumin by suppressing microglia
activation. Exp Ther Med. 12:823–828. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cheng WJ, Li YH, Qi Q, Wang L, Ding FJ, Li
F, Miao ZH, Yang SQ, Li GH, Wang J, et al: HSP60 is involved in the
neuroprotective effects of Naloxone. Mol Med Rep. 10:2172–2176.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang Y, Chen L, Hagiwara N and Knowlton
AA: Regulation of heat shock protein 60 and 72 expression in the
failing heart. J Mol Cell Cardiol. 48:360–366. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lin L, Kim SC, Wang Y, Gupta S, Davis B,
Simon SI, Torre-Amione G and Knowlton AA: HSP60 in heart failure:
Abnormal distribution and localization to lipid rafts. Am J Physiol
Heart Circ Physiol. 293:H2238–H2247. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vocka M, Langer D, Fryba V, Petrtyl J,
Hanus T, Kalousova M, Zima T and Petruzelka L: Novel serum markers
HSP60, CHI3L1, and IGFBP-2 in metastatic colorectal cancer. Oncol
Lett. 18:6284–6292. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ding F, Li Y, Hou X, Zhang R, Hu S and
Wang Y: Oxymatrine inhibits microglia activation via HSP60-TLR4
signaling. Biomed Rep. 5:623–628. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Korniluk A, Koper O, Kemona H and
Dymicka-Piekarska V: From inflammation to cancer. Ir J Med Sci.
186:57–62. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kocaadam B and Şanlier N: Curcumin, an
active component of turmeric (Curcuma longa), and its effects on
health. Crit Rev Food Sci Nutr. 57:2889–2895. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pluta R, Ułamek-Kozioł M and Czuczwar SJ:
Neuroprotective and neurological/cognitive enhancement effects of
curcumin after brain ischemia injury with Alzheimer's disease
phenotype. Int J Mol Sci. 19(4002)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rashid K, Chowdhury S, Ghosh S and Sil PC:
Curcumin attenuates oxidative stress induced NFκB mediated
inflammation and endoplasmic reticulum dependent apoptosis of
splenocytes in diabetes. Biochem Pharmacol. 143:140–155.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Patel SS, Acharya A, Ray RS, Agrawal R,
Raghuwanshi R and Jain P: Cellular and molecular mechanisms of
curcumin in prevention and treatment of disease. Crit Rev Food Sci
Nutr. 60:887–939. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Devassy JG, Nwachukwu ID and Jones PJ:
Curcumin and cancer: Barriers to obtaining a health claim. Nutr
Rev. 73:155–165. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Panchal HD, Vranizan K, Lee CY, Ho J, Ngai
J and Timiras PS: Early anti-oxidative and anti-proliferative
curcumin effects on neuroglioma cells suggest therapeutic targets.
Neurochem Res. 33:1701–1710. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang Y, Tu L, Zhou X and Li B:
Curcumin-mediated induction of apoptosis in human glioma CHME
cells. Med Sci Monit Basic Res. 24:216–224. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Seyithanoğlu MH, Abdallah A, Kitiş S,
Güler EM, Koçyiğit A, Dündar TT and Gündağ Papaker M: Investigation
of cytotoxic, genotoxic, and apoptotic effects of curcumin on
glioma cells. Cell Mol Biol (Noisy-le-grand). 65:101–108.
2019.PubMed/NCBI
|
|
22
|
Cheng C, Jiao JT, Qian Y, Guo XY, Huang J,
Dai MC, Zhang L, Ding XP, Zong D and Shao JF: Curcumin induces G2/M
arrest and triggers apoptosis via FoxO1 signaling in U87 human
glioma cells. Mol Med Rep. 13:3763–3770. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang Z, Li C, Tan Q, Xie CJ, Yang YY,
Zhan WG, Han F, Sharma SH and Sharma A: Curcumin suppresses tumor
growth and angiogenesis in human glioma cells through modulation of
vascular endothelial growth factor/angiopoietin-2/thrombospondin-1
signaling. CNS Neurol Disord Drug Targets. 16:346–350.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Perry MC, Demeule M, Régina A, Moumdjian R
and Béliveau R: Curcumin inhibits tumor growth and angiogenesis in
glioblastoma xenografts. Mol Nutr Food Res. 54:1192–1201.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cui G, Yuan A, Sun Z, Zheng W and Pang Z:
IL-1β/IL-6 network in the tumor microenvironment of human
colorectal cancer. Pathol Res Pract. 214:986–992. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
van Horssen R, Ten Hagen TL and Eggermont
AM: TNF-alpha in cancer treatment: Molecular insights, antitumor
effects, and clinical utility. Oncologist. 11:397–408.
2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Deguchi A: Curcumin targets in
inflammation and cancer. Endocr Metab Immune Disord Drug Targets.
15:88–96. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang XP, Wang QX, Lin HP and Chang N:
Anti-tumor bioactivities of curcumin on mice loaded with gastric
carcinoma. Food Funct. 8:3319–3326. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liao F, Liu L, Luo E and Hu J: Curcumin
enhances anti-tumor immune response in tongue squamous cell
carcinoma. Arch Oral Biol. 92:32–37. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kunnumakkara AB, Bordoloi D, Harsha C,
Banik K, Gupta SC and Aggarwal BB: Curcumin mediates anticancer
effects by modulating multiple cell signaling pathways. Clin Sci
(Lond). 131:1781–1799. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sun Y, Zheng J, Xu Y and Zhang X:
Paraquat-induced inflammatory response of microglia through
HSP60/TLR4 signaling. Hum Exp Toxicol. 37:1161–1168.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jakic B, Buszko M, Cappellano G and Wick
G: . Elevated sodium leads to the increased expression of HSP60 and
induces apoptosis in HUVECs. PLoS One. 12(e0179383)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Juwono J and Martinus RD: Does Hsp60
provide a link between mitochondrial stress and inflammation in
diabetes mellitus? J Diabetes Res. 2016(8017571)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Caruso Bavisotto C, Cappello F, Macario
AJL, Macario de EC, Logozzi M, Fais S and Campanella C: Exosomal
HSP60: A potentially useful biomarker for diagnosis, assessing
prognosis, and monitoring response to treatment. Expert Rev Mol
Diagn. 17:815–822. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shetab Boushehri MA and Lamprecht A:
TLR4-based immunotherapeutics in cancer: A review of the
achievements and shortcomings. Mol Pharm. 15:4777–4800.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen X, Zhao F, Zhang H, Zhu Y, Wu K and
Tan G: Significance of TLR4/MYD88 expression in breast cancer. Int
J Clin Exp Pathol. 8:7034–7039. 2015.PubMed/NCBI
|
|
37
|
Jing X, Tian Z, Gao P, Xiao H, Qi X, Yu Y,
Ding X, Yang L and Zong L: HBsAg/β2GPI activates the NF-κB pathway
via the TLR4/MyD88/IκBα axis in hepatocellular carcinoma. Oncol
Rep. 40:1035–1045. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sokolova O and Naumann M: NF-κB signaling
in gastric cancer. Toxins (Basel). 9(119)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liu B, Sun L, Liu Q, Gong C, Yao YD, Lv
XB, Lin L, Yao HR, Su FX, Li DS, et al: A cytoplasmic NF-κB
interacting long noncoding RNA blocks IκB phosphorylation and
suppresses breast cancer metastasis. Cancer Cell. 27:370–381.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Friedmann-Morvinski D, Narasimamurthy R,
Xia Y, Myskiw C, Soda Y and Verma IM: Targeting NF-κB in
glioblastoma: A therapeutic approach. Sci Adv.
2(e1501292)2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Martins GR, Gelaleti GB, Moschetta MG,
Maschio-Signorini LB and Zuccari DA: Proinflammatory and
anti-inflammatory cytokines mediated by NF-κB factor as prognostic
markers in mammary tumors. Mediators Inflamm.
2016(9512743)2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Das S, Shapiro B, Vucic EA, Vogt S and
Bar-Sagi D: Tumor cell-derived il1β promotes desmoplasia and immune
suppression in pancreatic cancer. Cancer Res. 80:1088–1101.
2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Hehlgans T and Männel DN: The TNF-TNF
receptor system. Biol Chem. 383:1581–1585. 2002.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Roos WP, Thomas AD and Kaina B: DNA damage
and the balance between survival and death in cancer biology. Nat
Rev Cancer. 16:20–33. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chen J: The cell-cycle arrest and
apoptotic functions of p53 in tumor initiation and progression.
Cold Spring Harb Perspect Med. 6(a026104)2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Galluzzi L, Kepp O and Kroemer G:
Caspase-3 and prostaglandins signal for tumor regrowth in cancer
therapy. Oncogene. 31:2805–2808. 2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sam MR and Pourpak RS: Regulation of p53
and survivin by prodigiosin compound derived from Serratia
marcescens contribute to caspase-3-dependent apoptosis in acute
lymphoblastic leukemia cells. Hum Exp Toxicol. 37:608–617.
2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Oblak A and Jerala R: Toll-like receptor 4
activation in cancer progression and therapy. Clin Dev Immunol.
2011(609579)2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sawa-Wejksza K and Kandefer-Szerszeń M:
Tumor-associated macrophages as target for antitumor therapy. Arch
Immunol Ther Exp (Warsz). 66:97–111. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lai WY, Wang JW, Huang BT, Lin EP and Yang
PC: A novel TNF-α-targeting aptamer for TNF-α-mediated acute lung
injury and acute liver failure. Theranostics. 9:1741–1751.
2019.PubMed/NCBI View Article : Google Scholar
|