Introduction
High mobility group protein B1 (HMGB1) is a member
of a class of non-histone chromosomal binding proteins that is
highly conserved and primarily present in the nucleus (1,2).
Nuclear HMGB1 functions as a DNA chaperone and is involved in DNA
replication, maintenance of DNA structure, genetic recombination,
DNA repair and transcription (3).
By contrast, extranuclear HMGB1 functions as a classical
damage-associated molecular pattern (4). Inflammatory factors, including
infection, cell necrosis and drugs can stimulate the displacement
and release of HMGB1, which promotes autophagy and apoptosis
(4). HMGB1 is highly homologous and
human and rodent HMGB1 share >98% homology (5). HMGB1 has two main functional regions:
The A- and B-boxes (5). The B-box
has cytokine-like activity and the A-box inhibits the function of
the B-box (5). HMGB1 has multiple
receptors, including the receptor for advanced glycation
end-products (RAGE), toll-like receptors (TLRs) and multi-ligand
proteoglycans, including syndecan and plasminogen, although RAGE
and TLR4/TLR2 are its main receptors (6).
HMGB1 is a multifunctional protein that exerts
numerous biological functions in both normal and malignant tumor
cells (7). HMGB1 was demonstrated
to be involved in the development of various diseases, including
sepsis, arthritis and various tumors, and has been shown to be of
prognostic value (7). Additionally,
HMGB1 is involved in the regulation of autophagy and apoptosis in
tumor cells (4,8). HMGB1 is expressed in breast cancer
(8,9), colon cancer (10,11),
melanoma (12,13) and ovarian cancer (14). HMGB1 is associated with various
characteristics of tumor cells, including proliferation,
angiogenesis, escape from programmed cell death and resistance to
growth factor inhibitors and biological behavior of tumors,
including tissue invasion and distant spread (7,13).
Furthermore, evidence has demonstrated that HMGB1 may induce
tumorigenesis, metastasis and chemotherapy resistance and may be
useful as a novel treatment target for lung cancer (7).
MicroRNAs (miRs or miRNAs) are a class of non-coding
RNAs involved in post-transcriptional regulation of gene expression
(15). Numerous miRNAs have been
reported to be abnormally expressed in tumor cells (15). miRNAs are a class of evolutionarily
conserved small non-coding RNAs that regulate numerous genes during
tumorigenesis in humans and other mammals (15). Numerous long non-coding RNAs
(lncRNAs) and miRNAs have been reported to regulate HMGB1. For
example, the lncRNA metastasis-associated lung adenocarcinoma
transcript 1 (MALAT1) regulated HMGB1 by sponging miR-129-5p to
promote the proliferation of SW480 and HCT116 colon cancer cells
(15). Additionally, HMGB1 is a
target of miR-1284 and was demonstrated to reverse the effects of
miR-1284 on the proliferation and chemosensitivity of cervical
cancer cells (16). The lncRNA
urothelial carcinoma-associated 1 functions as an oncogene to
promote the progression of lung cancer and lung cancer cell
migration via the miRNA-193a/HMGB1 axis (17). In pancreatic cancer cells, the
lncRNA zinc finger E-box-binding homeobox 2/antisense RNA 1
increases the proliferation and invasion of cancer cells by
regulating the miR-204/HMGB1 axis (18). Additionally, miRNAs were reported to
regulate cancer progression, invasion and metastasis. For instance,
miR-216b expression decreased in colorectal cancer tissues compared
with levels in corresponding adjacent normal tissues and miR-216b
functioned as a tumor suppressor by inhibiting cancer development
and progression partially through the HMGB1-mediated JAK2/STAT3
pathway (19). In non-small cell
lung cancer cells, miR-449a inhibited the proliferation, migration
and invasion of lung cancer cells via the HMGB1-mediated NF-κB
signaling pathway (20).
However, the function and molecular mechanism of
HMGB1 in cervical cancer remains to be elucidated. It has been
demonstrated that miRNA-1284, acting as a regulator of HMGB1,
suppressed cell proliferation and migration in osteosarcoma
(21). Furthermore, the lncRNA
MALAT1 was shown to promote the proliferation of osteosarcoma cells
by inhibiting miR-142-3p or miR-129-5p and by targeting
HMGB1(22). The present study
investigated miRNAs that target HMGB1 in cervical cancer and
explored the underlying molecular mechanism involved in the
proliferation of cervical cancer cells, which may provide novel
insights into the tumorigenesis of cervical cancer.
Materials and methods
Cell lines and reagents
Primary cervical epithelial cells (cat. no.
ATCC® PCS-480-011™) were purchased from the American
Type Culture Collection. Cells were cultured and passaged in
complete expansion medium, which was prepared by adding the
contents of the Cervical Epithelial Cell Growth kit (cat. no.
ATCC® PCS-480-042) to one bottle of Cervical Epithelial
Cell Basal medium (cat. no. ATCC® PCS-480-032),
according to the manufacture's protocol. Human cervical cancer cell
lines HeLa (cat. no. CBP60232; Nanjing Cobioer Co., Ltd.) and CaSKi
(cat. no. CC-Y1086; EK Biosciences GmbH) cells were maintained and
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 1X streptomycin and penicillin (Gibco; Thermo Fisher
Scientific, Inc.) in a cell incubator under a humidified atmosphere
and 5% CO2 at 37˚C. MISSION® microRNA mimic
miR-142-3p (has-miR-142-3p mimic; cat. no. HMI0219) and
corresponding MISSION® miRNA, negative control 1
(negative control mimic; cat. no. HMC0002), MISSION®
Lenti microRNA inhibitor, human miR-142-3p (miR-142-3p inhibitor;
cat. no. HLTUD0219) and MISSION® Lenti microRNA
inhibitor (negative control inhibitor; cat. no. HLTUD001C) were
purchased from Sigma-Aldrich; Merck KGaA.
Specimens
Three pairs of clinical specimens (cervical cancer
tissues and surrounding non-cancerous tissues; distance between
tissues, 3 cm) were obtained from patients at Tianjin Central
Hospital of Gynecology and Obstetrics (Tianjin, China). Specimen 1
was collected on April 4th, 2017, specimen 2 on July 8th, 2017 and
specimen 3 on October 13th, 2017. The age distribution was 43-68
years old and the mean age was 6±2 years old.
Patients were included into the current study if
they did not receive any cancer-related treatment prior to the
surgery. Patients who underwent chemotherapy or had diabetes were
excluded. The clinical specimens were immediately frozen in liquid
nitrogen and stored at -80˚C. All patients provided written
informed consent prior to enrollment. Studies involving humans were
conducted in accordance with the Declaration of Helsinki and were
approved by the Ethics Committee of Tianjin Central Hospital of
Gynecology and Obstetrics.
Cell transfection
HeLa cells (2x105 cells/48-well) were
transfected with the aforementioned has-miR-142-3p mimics, negative
control mimics, hsa-miR-142-3p inhibitors and negative control
inhibitors using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Briefly, a total of 0.5 µg has-miR-142-3p mimics, inhibitors,
negative control mimics or negative control inhibitors was diluted
in 100 µl Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.).
Opti-MEM was purchased from Thermo Fisher Scientific, Inc. A total
of 2 µl Lipofectamine® 2000 (Thermo Fisher Scientific,
Inc.) was diluted with 200 µl of Opti-MEM. Following 5 min at room
temperature, 100 µl of diluted DNA and 100 µl of diluted
Lipofectamine 2000® was mixed gently and incubated for
20 min at room temperature. The mixture was then added to the cells
with Opti-MEM and free FBS and transfected for 6 h at 37˚C.
Following transfection, Opti-MEM medium was changed into DMEM + FBS
+ antibiotics at 37˚C, as described above. Subsequently, HMGB1
expression was assessed and cell proliferation assays were
performed.
Additionally, HMGB1 shRNA(h) (cat. no. sc-37982-SH)
and control shRNA (cat. no. sc-108060) were purchased from Santa
Cruz Biotechnology, Inc. Transfection into HeLa cells was performed
as described above. The transfected cells were cultured for 24, 48
and 72 h for western blotting and MTT assays.
ELISA
A human HMGB1 ELISA kit (cat. no. JL13893-48T) was
purchased from Shanghai Jianglai Biotech Co., Ltd. ELISA was
performed to determine the concentration of HMGB1 in the cell
culture supernatant of miR-142-3p mimic-transfected, mimic negative
control-transfected and untreated HeLa cells. Cell culture
supernatants in each group were collected and the concentration of
HMGB1 was detected using the kit, according to the manufacturer's
protocol.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from transfected HeLa cells
using a PureLink RNA Mini kit (cat. no. 12183018A; Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. RNA was reverse transcribed into cDNA using a
High-Capacity RNA-to-cDNA kit (cat. no. 4387406; Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. The MystiCq® microRNA qPCR assay primer
for miR-142-3p (cat. no. MIRAP00174) was purchased from
Sigma-Aldrich; Merck KGaA. Multiplex qPCR assay was performed using
a TaqMan Gene Expression master mix (cat. no. 4369016; Invitrogen;
Thermo Fisher Scientific, Inc.). The sequences used were as
follows: U6 forward, CTCGCTTCGGCAGCACA and reverse,
AACGCTTCACGAATTTGCGT. miR-143-3p was commercially purchased from
Sigma-Aldrich; Merck KgaA and, therefore, the sequences are not
known. The thermocycling conditions were as follows for qPCR:
denaturation for 1 min at 95˚C, 40 cycles of 15 sec at 95˚C and 1
min at 60˚C. Samples were stored at 4˚C. U6 was used as the
reference gene. miR-142-3p levels were quantified according to the
2-ΔΔCq method (22).
MTT assay
HeLa cells (1.2x105 cells/well) were
plated into 48-well plates and incubated for 8 h at 37˚C.
Subsequently, cells were transfected with miR-142-3p mimics, mimic
negative controls, miR-142-3p inhibitors or inhibitor negative
controls for 24 and 48 h. Cell viability was determined using an
MTT assay, as previously described (23). For the assay, 20 µl MTT stock
solution (5 mg/ml) was added to the plate and incubated for 4 h. A
total of 100 µl DMSO(cat. no. D8418; Sigma-Aldrich; Merck KGaA) was
added to the culture medium and incubated for 10 min. The plates
were then read on a microplate reader at 490 nm and data were
analyzed.
Binding prediction of miRNAs and
HMGB1
To investigate how HMGB1 is regulated in cervical
cancer cells, the 3'-untranslated region (3'UTR) of HMGB1 was
scanned using the online search tool TargetScan 7.1 (www.targetscan.org/vert_72/) to search for
possible complementary sites to human miRNAs and predict the
possible miRNAs that may bind to and interact with HMGB1.
Isolation of nuclear and cytoplasmic
proteins
HeLa cells were transfected with miR-142-3p
inhibitors and inhibitor negative controls for 48 h. Following
transfection, total protein in the cytoplasm or nucleus of
transfected HeLa cells was extracted using a cell nuclear protein
and cytoplasmic protein extraction kit (cat. no. P0028; Beyotime
Institute of Biotechnology), according to the manufacturer's
protocol. Lamin B1 was used as a control for nuclear proteins and
α-tubulin was used as a control for cytoplasmic proteins.
Western blotting
HeLa cells were transfected with miR-142-3p mimics,
mimic negative controls, miR-142-3p inhibitors and inhibitor
negative controls and cultured for 24 or 48 h. Subsequently,
transfected cells were washed twice with ice-cold PBS and cell
lysates were prepared using RIPA lysis buffer (cat. no. P0013B;
Beyotime Institute of Biotechnology). Total protein concentration
of the prepared lysates was determined using a BCA kit. Next, 30 µg
of total protein was separated by 10% SDS-PAGE at 120 V for 2 h and
at 80 V for 15 min. The separated proteins were transferred to PVDF
membranes, blocked with 5% non-fat milk for 1 h at room temperature
and incubated with the following primary antibodies: Anti-HMGB1
(cat. no. ab79823; Abcam), anti-GAPDH (cat. no. ab70699; Abcam),
anti-lamin B1 (cat. no. 66095-1-Ig; ProteinTech Group, Inc.) and
rabbit monoclonal anti-α tubulin (cat. no. ab179513; Abcam) at 4˚C
for 8 h. Dilutions of all primary antibodies were 1:5,000.
Following primary antibody incubation, membranes were incubated
with goat anti-rabbit immunoglobulin G (IgG)-horseradish peroxidase
(HRP; cat. no. sc-2004; Santa Cruz Biotechnology, Inc.) and goat
anti-mouse IgG-HRP (cat. no. sc-2005; Santa Cruz Biotechnology,
Inc.) secondary antibodies at room temperature for 1 h. The
dilution of all secondary antibodies was 1:10,000. Bands were
visualized using a Pierce™ ECL Western Blotting Substrate (cat. no.
32209; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The densitometry of the bands was analyzed
by ImageJ software (version no. 1.51k; National Institutes of
Health).
Statistical analysis
Data were analyzed using SPSS software (version no.
20.0; IBM Corp.). Multiple comparisons were analyzed using ANOVA,
followed by Tukey's post-hoc test. An independent samples t-test
was used to analyze independent samples. A paired t-test was used
to analyze differences between paired experimental specimens. Data
are presented as the mean ± SEM. P<0.05 was considered to
indicate a statistically significant difference.
Results
HMGB1 levels are higher in cervical cancer cells
compared with normal cells. The expression levels of HMGB1 in human
primary cervical epithelial cells and the human cervical cancer
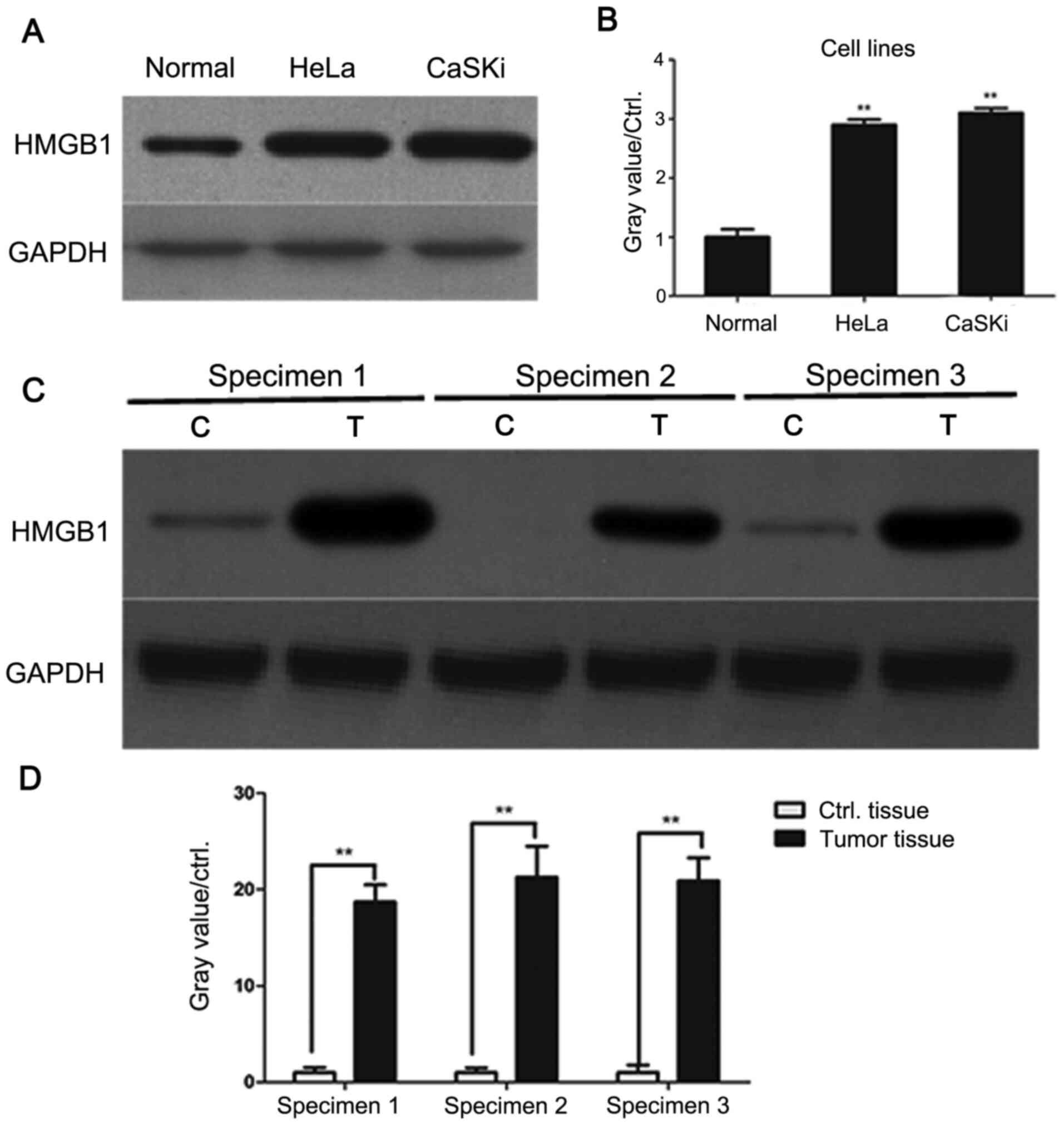
cell lines HeLa and CaSKi were examined. As presented in Fig. 1A and B, HMGB1 levels were significantly higher
in HeLa and CaSKi cells compared with primary cervical epithelial
cells, indicating that HMGB1 may be involved in cervical
cancer.
Subsequently, three pairs of cervical cancer and
paracancer tissues were collected. The levels of HMGB1 were
assessed by western blotting. As demonstrated by Fig. 1C and D, HMGB1 protein levels were significantly
higher in human cervical cancer tissues compared with paracancer
tissues. These data showed that HMGB1 may serve an oncogenic role
during the tumorigenesis of cervical cancer.
Knockdown of HMGB1 suppresses the
viability of HeLa cells
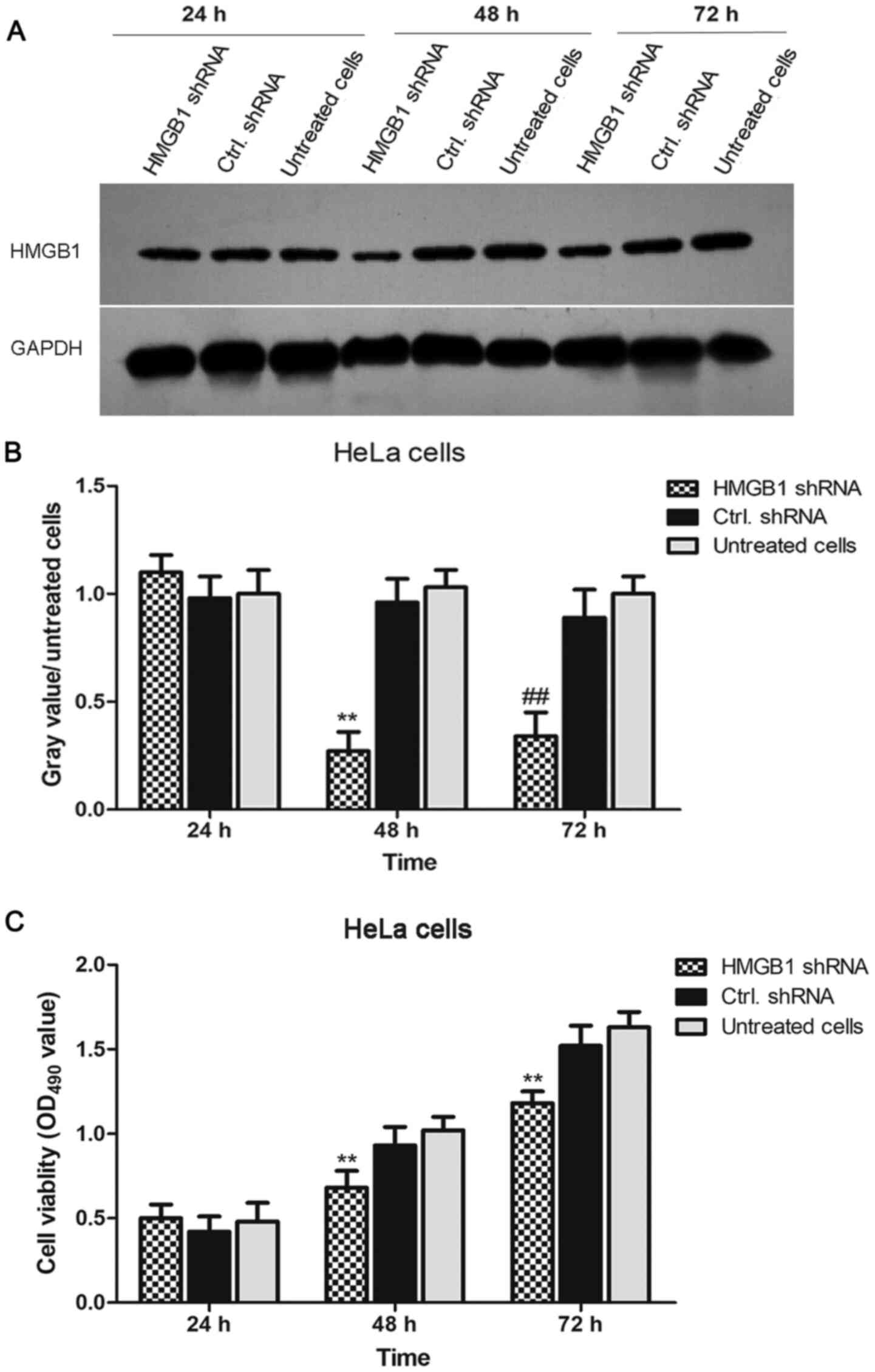
To investigate the function of HMGB1 in the
proliferation of cervical cancer cells, HMGB1 expression in HeLa
cells was knocked down by RNA interference. As shown in Fig. 2A and B, the expression levels of HMGB1 were
significantly decreased in HMGB1 shRNA-transfected HeLa cells
compared with the levels in negative control shRNA-transfected HeLa
cells at 24, 48 and 72 h. Subsequently, cell viability in HMGB1
shRNA-transfected, negative control shRNA-transfected and untreated
HeLa cells was determined using an MTT assay. The results
demonstrated that the viability of HMGB1 shRNA-transfected HeLa
cells was significantly lower compared with negative control
shRNA-transfected HeLa cells (Fig.
2C). These results indicated that HMGB1 shRNA effectively
decreased HMGB1 expression in HeLa cells and the cell viability of
HeLa cells was significantly decreased by knockdown of HMGB1.
HMGB1 is predicted to be a target of
miR-142-3p
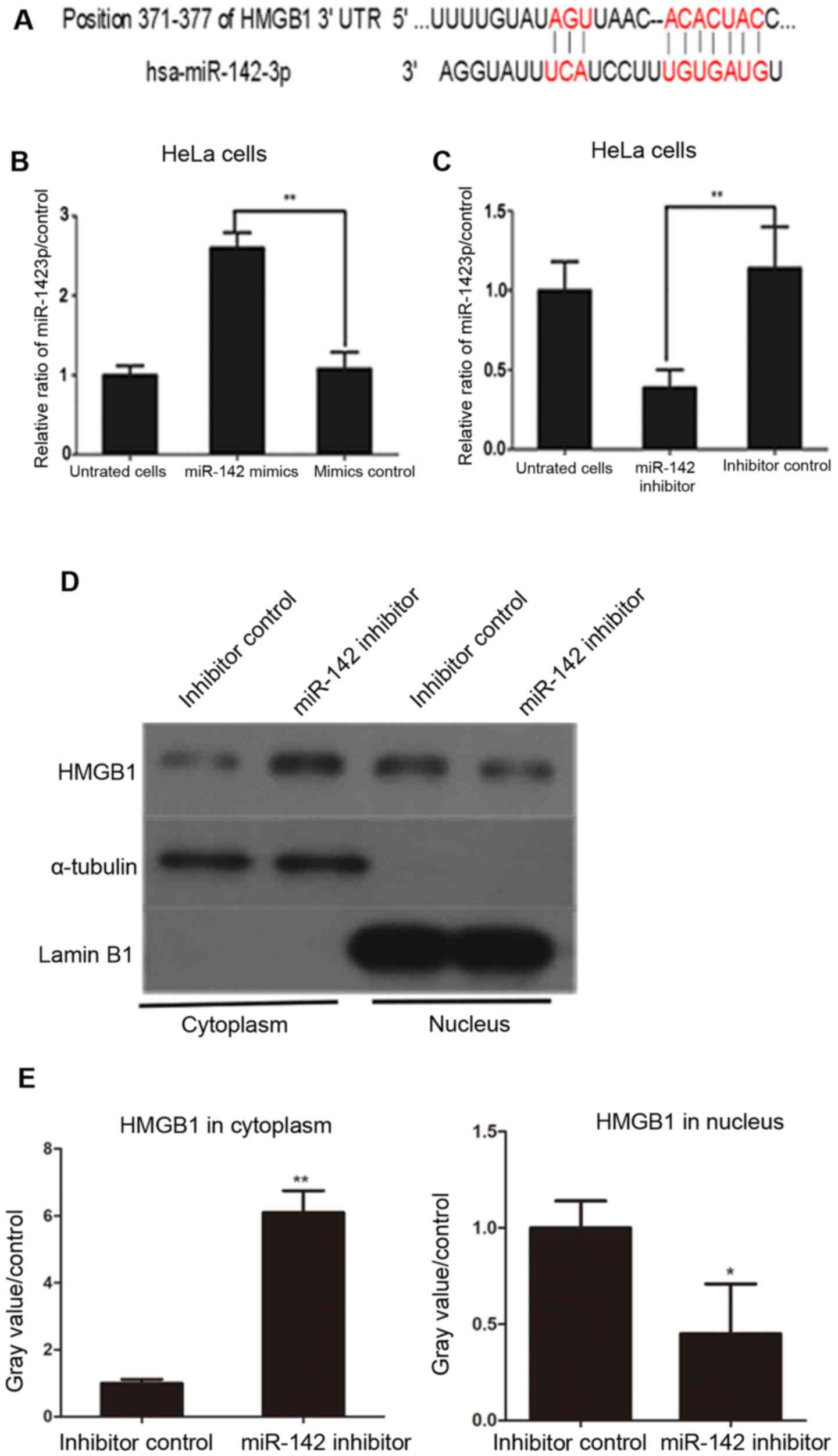
As presented in Fig.
3A, a possible miR-142-3p binding sequence was found at
position 371-377 of the HMGB1 3'UTR.
Transfection with miR-142-3p inhibitor
increases cytoplasmic HMGB1 expression in HeLa cells
Western blotting was used to investigate the
association between miR-142-3p and HMGB1 in human cervical cancer
cells. miR-142-3p mimics were transfected into HeLa cells and
cultured for 48 h. miR-142-3p expression significantly increased in
cells transfected with miR-142-3p mimics compared with cells
transfected with mimic negative controls (Fig. 3B). HeLa cells transfected with
miR-142-3p inhibitor exhibited decreased expression of miR-142-3p
compared with inhibitor control-transfected HeLa cells (Fig. 3C). Next, the effect of miR-142-3p on
the expression and distribution of HMGB1 in cervical cancer cells
was assessed.
HMGB1 protein levels in the cytoplasm and nucleus of
miR-142-3p inhibitor- and negative control inhibitor-transfected
HeLa cells were assessed at 48 h post-transfection. The results
demonstrated that transfection with miR-142-3p inhibitors
significantly increased cytoplasmic levels of HMGB1 in HeLa cells
compared with negative control inhibitor-transfected cells
(Fig. 3D and E). By contrast, nuclear levels of HMGB1 in
miR-142-3p inhibitor-transfected cells were lower compared with
control cells (Fig. 3D and E). These results suggested that
overexpression of miR-142-3p inhibited the expression of HMGB1 in
HeLa cells.
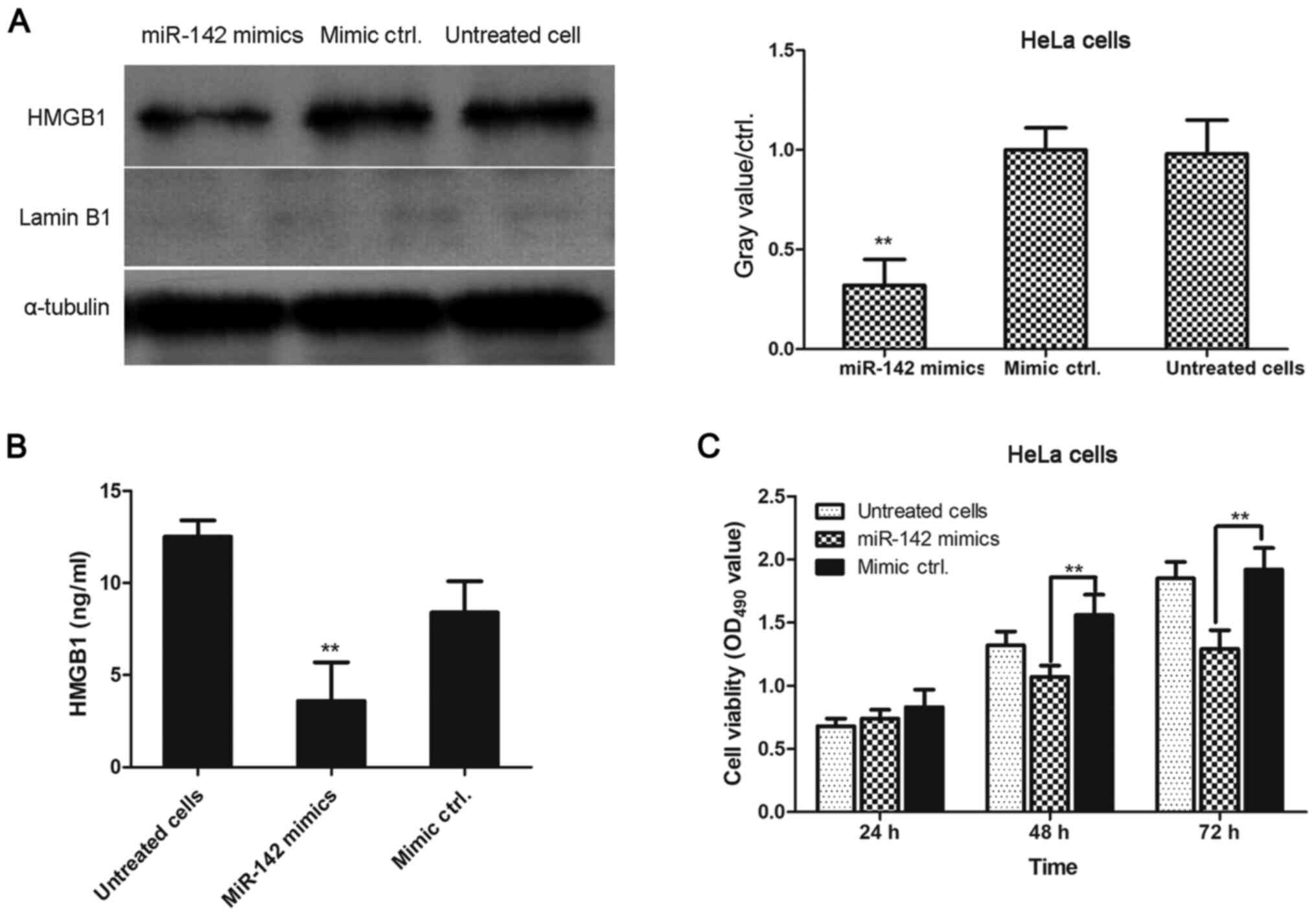
miR-142-3p negatively regulates the
levels of HMGB1 in cervical cancer cells
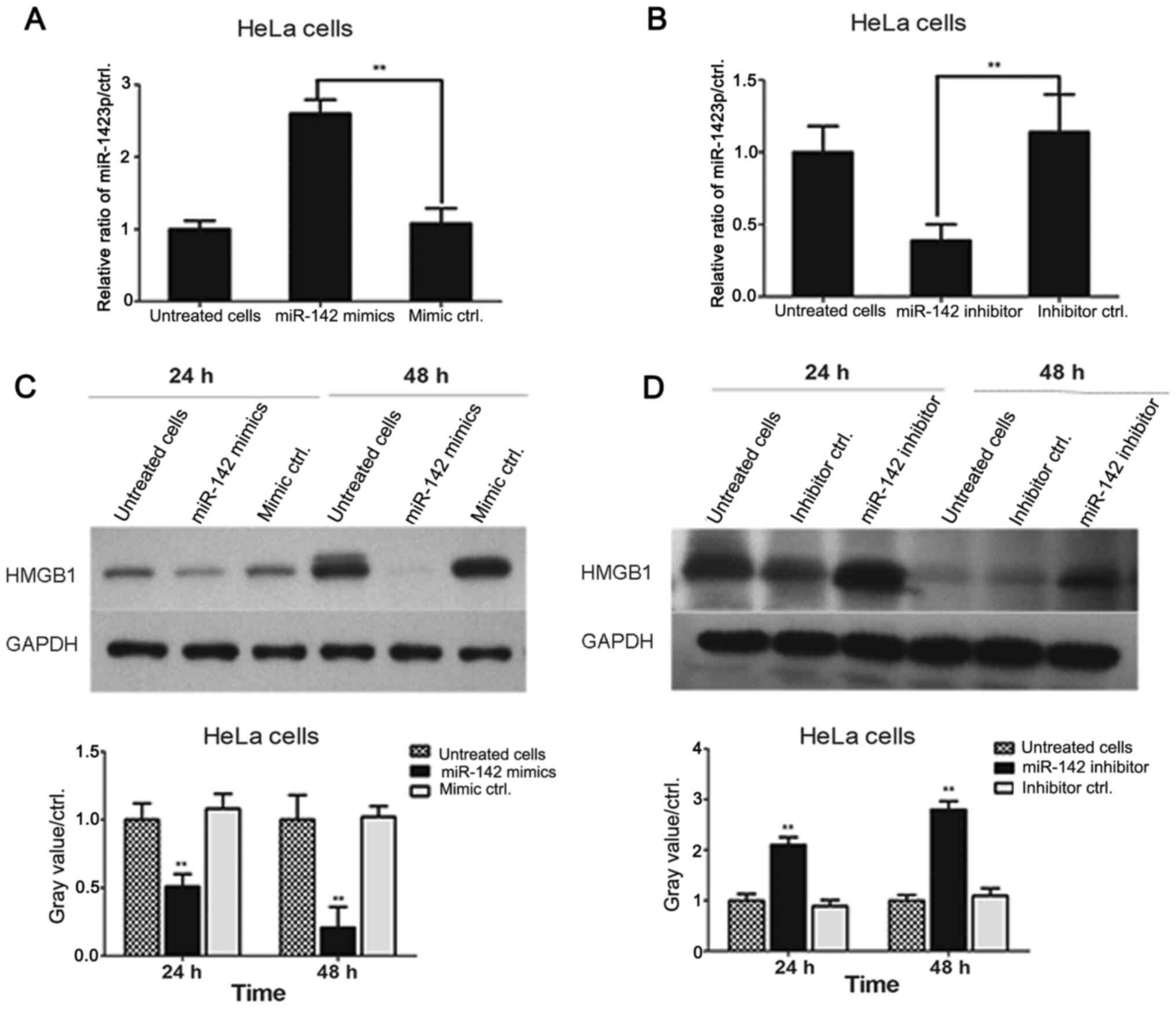
miR-142-3p mimics were transfected into HeLa cells
for 24 and 48 h. HMGB1 levels were assessed by western blotting. As
shown in Fig. 4A, transfection of
miR-142-3p mimics significantly downregulated HMGB1 expression
levels in HeLa cells compared with the levels in mimic negative
control-transfected cells at 24 and 48 h. Additionally, HeLa cells
were transfected with either miR-142-3p inhibitors or inhibitor
negative controls for 24 and 48 h, and the results demonstrated
that transfection with miR-142-3p inhibitor significantly increased
the levels of HMGB1 in cervical cancer cells compared with the
levels in inhibitor negative control-transfected cells (Fig. 4B). These data revealed that
miR-142-3p levels were negatively associated with HMGB1 expression
in HeLa cells.
Transfection with miR-142-3p mimics
decreases the levels of cytoplasmic HMGB1 in HeLa cells
As presented in Fig.
5A, HMGB1 expression was significantly lower in miR-142-3p
mimic-transfected cells compared with mimic negative
control-transfected cells. ELISA was performed to determine the
concentration of HMGB1 in the cell culture supernatant of
miR-142-3p mimic-transfected, mimic negative control-transfected
and untreated HeLa cells. As demonstrated by Fig. 5B, miR-142-3p mimic-transfected cells
secreted significantly lower levels of HMGB1 into the cell culture
supernatant compared with mimic negative control-transfected cells.
Additionally, the viability of transfected cells was assessed using
an MTT assay. As shown in Fig. 5C,
cell viability was significantly inhibited in miR-142-3p
mimic-transfected HeLa cells compared with mimic negative
control-transfected HeLa cells at 48 and 72 h. Cell viability was
not significantly different between groups at 24 h.
Discussion
HMGB1 is a highly conserved non-histone DNA-binding
protein that is abnormally expressed in numerous types of human
cancer (7,8). The present study revealed that HMGB1
levels were significantly higher in the two tested cervical cancer
cell lines, HeLa and CaSKi, compared with primary cervical
epithelial cells. This is consistent with previous findings that
have demonstrated that HMGB1 is involved in cell proliferation and
migration, and alters the protein expression of
epithelial-mesenchymal transition-associated genes (7). These results indicated that HMGB1 may
function as an oncogene in the progression of cervical cancer.
Subsequently, possible miRNAs involved in regulating
the expression of HMGB1 were predicted using TargetScan. The
results demonstrated that miR-142-3p may bind to position 371-377
of the HMGB1 3'UTR. Transfection with miR-142-3p mimics
significantly decreased the levels of HMGB1 and the proliferation
of miR-142-3p mimic-transfected HeLa cells was significantly
decreased compared with mimic negative control-transfected HeLa
cells. Conversely, transfection with miR-142-3p inhibitor increased
the expression of HMGB1 in cervical cancer cells. This was
consistent with the results reported in a study by Liu et al
(24). Two candidate miRNAs,
miR-142-3p and miR-129-5p, were screened and the results
demonstrated that MALAT1 promoted the development of osteosarcoma
by association with HMGB1 via both miR-142-3p and miR-129-5p.
Additionally, previous studies have reported that miR-1284(21) and miR-22 (25,26)
regulated HMGB1 and were involved in the regulation of cell
proliferation and migration in cervical cancer.
Higher HMGB1 expression induced the proliferation
and invasion of cervical cancer cells, which was consistent with
the findings of Jiang et al (27). This previous study reported that
HMGB1 suppressed the progression and development of cervical
cancer. However, there were certain differences between the
findings by Jiang et al (27) and the present study. While this
previous study focused on the effects of miR-142 on the
proliferation and invasiveness of cervical cancer cells, the
present study demonstrated that miR-142 affected the subcellular
distribution of HMGB1 in HeLa cells.
The molecular mechanism underlying the effects of
HMGB1 on the proliferation of cervical cancer cells was explored.
Western blotting showed that transfection with miR-142-3p inhibitor
increased cytoplasmic HMGB1 expression in HeLa cells, while
transfection with miR-142-3p mimics decreased cytoplasmic HMGB1
levels in HeLa cells. It has been reported that cytoplasmic HMGB1
translocation and HMGB1-induced cell autophagy contributed to
cisplatin resistance by inhibiting the apoptosis of cervical cancer
cells (28). Additionally,
cytoplasmic HMGB1 expression was reported to be associated with the
levels of tumor-infiltrating lymphocytes in breast cancer (29) and induced acute liver failure
(30). These data indicated that
the cytoplasmic translocation of HMGB1 likely promoted cancer
progression. The present study demonstrated that miR-142-3p
affected the subcellular distribution of HMGB1, as it inhibited the
translocation of HMGB1 from the nucleus into the cytoplasm.
Since the current experiments were primarily
performed in HeLa cells, future experiments will be performed in
several other cervical cancer cell lines to support the results
obtained. The levels of HMGB1 in three pairs of cervical cancer
tissues and paracancer tissues were detected. The 5-year overall
survival and disease-free survival rates of patients with different
expression levels of HMGB1 and miR-142-3p have not been analyzed in
the current study and will be investigated in future research.
In conclusion, the present results demonstrated that
miR-142-3p negatively regulated the cytoplasmic localization of
HMGB1, which inhibited the proliferation of human cervical cancer
cells. Therefore, miR-142-3p may be a novel therapeutic target for
human cervical cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HD designed the current study and experiments,
analyzed data and wrote the manuscript. JS performed the
experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Studies involving humans were conducted in
accordance with the Declaration of Helsinki and were approved by
the Ethics Committee of Tianjin Central Hospital of Gynecology and
Obstetrics, Tianjin, China.
Patient consent for publication
Written informed consent was obtained from all
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang K, Shan S, Wang S, Gu X, Zhou X and
Ren T: HMGB1-containing nucleosome mediates chemotherapy-induced
metastasis of human lung cancer. Biochem Biophys Res Commun.
500:758–764. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kumari T and Kumar B: High-mobility group
box 1 protein (HMGB1) gene polymorphisms and cancer susceptibility:
A comprehensive meta-analysis. Clin Chim Acta. 483:170–182.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Osmanov T, Ugrinova I and Pasheva E: The
chaperone like function of the nonhistone protein HMGB1. Biochem
Biophys Res Commun. 432:231–235. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kang R, Xie Y, Zhang Q, Hou W, Jiang Q,
Zhu S, Liu J, Zeng D, Wang H, Bartlett DL, et al: Intracellular
HMGB1 as a novel tumor suppressor of pancreatic cancer. Cell Res.
27:916–932. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yang L, Wang F, Yang L, Yuan Y, Chen Y,
Zhang G and Fan Z: HMGB1 a-box reverses brain edema and
deterioration of neurological function in a traumatic brain injury
mouse model. Cell Physiol Biochem. 46:2532–2542. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ying S, Xiao X, Chen T and Lou J: PPAR
ligands function as suppressors that target biological actions of
HMGB1. PPAR Res. 2016(2612743)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu L and Yang L: The function and
mechanism of HMGB1 in lung cancer and its potential therapeutic
implications. Oncol Lett. 15:6799–6805. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Huang BF, Tzeng HE, Chen PC, Wang CQ, Su
CM, Wang Y, Hu GN, Zhao YM, Wang Q and Tang CH: HMGB1 genetic
polymorphisms are biomarkers for the development and progression of
breast cancer. Int J Med Sci. 15:580–586. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Exner R, Sachet M, Arnold T,
Zinn-Zinnenburg M, Michlmayr A, Dubsky P, Bartsch R, Steger G,
Gnant M, Bergmann M, et al: Prognostic value of HMGB1 in early
breast cancer patients under neoadjuvant chemotherapy. Cancer Med.
5:2350–2358. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Zhang CC, Gdynia G, Ehemann V and Roth W:
The HMGB1 protein sensitizes colon carcinoma cells to cell death
triggered by pro-apoptotic agents. Int J Oncol. 46:667–676.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yadav SS, Kumar M, Varshney A and Yadava
PK: KLF4 sensitizes the colon cancer cell HCT-15 to cisplatin by
altering the expression of HMGB1 and hTERT. Life Sci. 220:169–176.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xie B, Cao K, Li J, Chen J, Tang J, Chen
X, Xia K, Zhou X, Cheng Y, Zhou J, et al: Hmgb1 inhibits Klotho
expression and malignant phenotype in melanoma cells by activating
NF-κB. Oncotarget. 7:80765–80782. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Parodi M, Pedrazzi M, Cantoni C, Averna M,
Patrone M, Cavaletto M, Spertino S, Pende D, Balsamo M, Pietra G,
et al: Natural killer (NK)/melanoma cell interaction induces
NK-mediated release of chemotactic high mobility group box-1
(HMGB1) capable of amplifying NK cell recruitment. OncoImmunology.
4(e1052353)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhou LY, Shi LY and Xiao Y: Changes of
HMGB1 expression on angiogenesis of ovarian cancer and its
mechanism. J Biol Regul Homeost Agents. 30:233–238. 2016.PubMed/NCBI
|
|
15
|
Wu Q, Meng WY, Jie Y and Zhao H: LncRNA
MALAT1 induces colon cancer development by regulating
miR-129-5p/HMGB1 axis. J Cell Physiol. 233:6750–6757.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen J and Li G: MiR-1284 enhances
sensitivity of cervical cancer cells to cisplatin via
downregulating HMGB1. Biomed Pharmacother. 107:997–1003.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wu H and Zhou C: Long non-coding RNA UCA1
promotes lung cancer cell proliferation and migration via
microRNA-193a/HMGB1 axis. Biochem Biophys Res Commun. 496:738–745.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gao H, Gong N, Ma Z, Miao X, Chen J, Cao Y
and Zhang G: LncRNA ZEB2-AS1 promotes pancreatic cancer cell growth
and invasion through regulating the miR-204/HMGB1 axis. Int J Biol
Macromol. 116:545–551. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen X, Liu X, He B, Pan Y, Sun H, Xu T,
Hu X and Wang S: MiR-216b functions as a tumor suppressor by
targeting HMGB1-mediated JAK2/STAT3 signaling way in colorectal
cancer. Am J Cancer Res. 7:2051–2069. 2017.PubMed/NCBI
|
|
20
|
Wu D, Liu J, Chen J, He H, Ma H and Lv X:
miR-449a suppresses tumor growth, migration, and invasion in
non-small cell lung cancer by targeting a HMGB1-mediated NF-κB
signaling pathway. Oncol Res. 27:227–235. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lv S and Guan M: miRNA-1284, a regulator
of HMGB1, inhibits cell proliferation and migration in
osteosarcoma. Biosci Rep. 38(BSR20171675)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
He R, Yang L, Lin X, Chen X, Lin X, Wei F,
Liang X, Luo Y, Wu Y, Gan T, et al: MiR-30a-5p suppresses cell
growth and enhances apoptosis of hepatocellular carcinoma cells via
targeting AEG-1. Int J Clin Exp Pathol. 8:15632–15641.
2015.PubMed/NCBI
|
|
24
|
Liu K, Huang J, Ni J, Song D, Ding M, Wang
J, Huang X and Li W: MALAT1 promotes osteosarcoma development by
regulation of HMGB1 via miR-142-3p and miR-129-5p. Cell Cycle.
16:578–587. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li X, Wang S, Chen Y, Liu G and Yang X:
miR-22 targets the 3'UTR of HMGB1 and inhibits the HMGB1-associated
autophagy in osteosarcoma cells during chemotherapy. Tumour Biol.
35:6021–6028. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guo S, Bai R, Liu W, Zhao A, Zhao Z, Wang
Y, Wang Y, Zhao W and Wang W: miR-22 inhibits osteosarcoma cell
proliferation and migration by targeting HMGB1 and inhibiting
HMGB1-mediated autophagy. Tumour Biol. 35:7025–7034.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jiang D, Wang H, Li Z, Li Z, Chen X and
Cai H: MiR-142 inhibits the development of cervical cancer by
targeting HMGB1. Oncotarget. 8:4001–4007. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xia J, Yu X, Song X, Li G, Mao X and Zhang
Y: Inhibiting the cytoplasmic location of HMGB1 reverses cisplatin
resistance in human cervical cancer cells. Mol Med Rep. 15:488–494.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lee HJ, Kim A, Song IH, Park IA, Yu JH,
Ahn JH and Gong G: Cytoplasmic expression of high mobility group B1
(HMGB1) is associated with tumor-infiltrating lymphocytes (TILs) in
breast cancer. Pathol Int. 66:202–209. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhou RR, Zhao SS, Zou MX, Zhang P, Zhang
BX, Dai XH, Li N, Liu HB, Wang H and Fan XG: HMGB1 cytoplasmic
translocation in patients with acute liver failure. BMC
Gastroenterol. 11(21)2011.PubMed/NCBI View Article : Google Scholar
|