Introduction
Postoperative cognitive dysfunction (POCD)
represents a serious complication following anesthesia and surgical
procedures for patients undergoing surgical intervention (1). POCD is characterized by temporary or
permanent cognitive decline, memory impairment, deterioration in
language comprehension and social adaption ability, and
particularly affects elderly people (>65 years) (2). POCD can lead to increased mortality,
prolonged hospitalization, other complications such as Alzheimer's
disease and higher treatment costs (3). Although the pathogenic mechanisms for
POCD remain unknown, its risk factors comprise trauma surgery,
postoperative pain and neuronal apoptosis (4). Therefore, the development of POCD
prevention and treatment tools has become a focus of interest for
research.
Amyloid precursor protein (APP) is hydrolyzed in two
ways: i) Degradation by α-secretase during normal physiological
conditions; or ii) generation of soluble β-APP8 and C99 by β-site
APP-cleaving enzyme-1 (BACE-1), followed by C99 hydrolyzation by
γ-secretase to generate insoluble amyloid-β (Aβ) (5). APP is distributed in neuronal synapses
(6). Aβ, a 36-43-amino acid
peptide, is the main constituent of amyloid plaques in Alzheimer's
disease (AD) (7). It is widely
accepted that Aβ oligomers are causally associated with the
neurodegenerative processes accompanying AD (8). The most common isoforms of Aβ are Aβ42
and Aβ40(9), which serve important
roles in POCD (10). A previous
study revealed that POCD was associated with apoptosis of
hippocampal neurons in rats (11).
Therefore, effective inhibition of Aβ and apoptosis of hippocampal
neurons demonstrated potential for the prevention and treatment of
POCD.
Insulin-like growth factor I (IGF-I) serves critical
roles in regulating body growth and metabolism and affects multiple
cerebral functions (12). IGF-I
promotes brain development, neuronal excitability, myelin sheath
production, angiogenesis, synaptogenesis and neuronal survival,
growth and differentiation (13).
Additionally, IGF-I stimulates cell proliferation and survival in
multiple cell types (14-16)
and is considered a universal cytoprotective molecule, protecting
cells from free radicals and apoptosis (17). Notably, a reduction in the amount of
IGF-1 markedly contributed to age-associated cognitive impairment
(18). A previous study revealed
that IGF-1 expression was negatively associated with the
progression of cognitive impairment (19).
Therefore, the current study aimed to assess whether
IGF-I improved POCD by mediating apoptosis and Aβ production. The
present study studied cognitive function in aged rats following
surgery with or without IGF-I administration to investigate the
protective effects of IGF-I on splenectomy-induced POCD.
Materials and methods
Animals and groups
A total of 150 male Wistar rats (age, 16-18 months;
weight, 350-550 g) were purchased from the SPF Beijing
Biotechnology Co., Ltd. (license no. SCXK-2016-0002) and examined.
The animals were housed under a 12-hour light/dark cycle with free
access to water and rodent chow. All animal experiments followed
the Guidance Suggestions for the Care and Use of Laboratory Animals
by the Ministry of Science and Technology of the People's Republic
of China (20). Approval was
obtained from the Animal Ethics Committee of Qingdao Municipal
Hospital (Qingdao, China). Rats were housed under standard
conditions with food and water available ad libitum and were
allowed to acclimatize at 24-26˚C for 1 week prior to experiments.
The living environment of the rats was clean and tidy and suitable
for survival. The rats were randomized into five groups
(n=30/group), as follows: i) Control (C); ii) isoflurane (I); iii)
splenectomy (S); iv) S + normal saline (S + NS) and v) S + IGF-1 (S
+ IGF-1).
Surgery and injection
The control group underwent no treatment. Rats in
the I group were given continuous inhalation of 1.5-2% isoflurane
for intubation and given 1.5% isoflurane and mechanical ventilation
with 100% oxygen for anesthesia maintenance. This anesthetic
procedure was selected due to its clinical relevance; additionally,
anesthetics are considered to play a role in cognitive impairment
(21). Rats in Group S underwent
splenectomy following the same anesthesia as those in Group I.
Briefly, the animals were placed in a supine position followed by
skin shaving. After disinfection, an incision was made 1.5-2.0 cm
below the costal margin for spleen removal. The splenic artery and
vein were ligatured with silk threads. The abdominal cavity was
closed after hemostasis and bupivacaine (0.25%) was administered
subcutaneously prior to wound closure to ensure that the rats did
not undergo pain during and following the operation (22). IGF-1 (50 µg/kg; cat. no. 50437-MNAY;
Sino Biological) was diluted in NS and administered via abdominal
hypodermic injection every day from splenectomy to 7 days
post-surgery in the S + I GF-1 group. In the S + NS group, equal
volumes of NS were administered via abdominal hypodermic injection
every day from splenectomy to 7 days post-surgery.
Current POCD models are achieved by splenectomy
(23), partial hepatectomy
(24) and limb orthopedic surgery
(25). Splenectomy results in
postsurgical reversible learning and memory dysfunction,
subsequently inducing POCD (26).
Furthermore, splenectomy shares significant similarity with
clinical abdominal surgery (short surgery time, controllability,
high success rate and reduced mortality) (27). Therefore, splenectomy is often
performed to induce a model of POCD (28).
After the rats were anesthetized with 4-5%
isoflurane for 5-7 min, reflexes disappeared. When the monitor
indicated cardiac arrest, respiratory arrest and pupil dilation,
the rats were euthanized by cervical dislocation.
Morris Water Maze (MWM)
To assess learning and memory abilities, MWM tests
were performed as described previously (29). This assay can identify animals
presenting with sensorimotor dysfunction and was used to assess
cognitive function.
The MWM system (Shanghai XinRuan Information
Technology Co., Ltd.) comprised an imaging device for swimming
tracking and a circular test pool (diameter, 120 cm; height, 40 cm)
with a cylindrical platform (diameter, 10 cm; height, 30 cm) 2 cm
below the water surface. For the swimming test, animals were
allowed to adapt to the platform for 30 sec before entering the
water from four different quadrants on the pool wall. The time
spent before finding the platform (escape latency) was recorded to
assess learning and memory abilities prior to surgery. Animals that
could not find the platform were guided within 60 sec to it and
allowed a 10-sec adaptation. The swimming test was performed 4
trials/day for 5 consecutive days starting from 1 week prior to
surgery. In the spatial test, the platform was removed prior to
animal placement in the water for 60 sec and the ratio of swimming
time in the target quadrant was evaluated. The spatial test was
performed on the day preceding the operation and at postoperative
days 1, 3 and 7. Escape latency and swimming distance in the target
quadrant were analyzed.
Western blotting
Western blotting was performed as previously
reported (30) to detect proteins.
The hippocampus was lysed in RIPA lysis buffer (Beyotime Institute
of Biotechnology) and protease inhibitors (PMSF; Beyotime Instittue
of Biotechnology), homogenized and placed in ice for full lysis for
40 min, followed by 20 min of centrifugation (1,600 x g; 4˚C).
Total protein was quantified using a bicinchoninic acid assay kit.
Proteins (30 µg/lane) were resolved by 10% SDS-PAGE and
electrotransferred onto polyvinylidene fluoride membranes (EMD
Millipore), which were blocked with 10% skimmed milk containing
TBS-Tween-20 (TBS-T; 0.1%) in ambient conditions for 1 h at room
temperature. This was followed by overnight incubation at 4˚C with
the following primary antibodies: Anti-β-actin (1:2,000; cat. no.
ab8226; Abcam), anti-APP (1:2,000; cat. no. ab32136; Abcam),
anti-BACE1 (1:1,000; cat. no. ab183612; Abcam), anti-caspase-3
(1:200; cat. no. ab13847; Abcam), anti-Bax (1:200; cat. no.
ab32503; Abcam), anti-Bcl2 (1:200; cat. no. ab32124; Abcam) and
anti-Aβ (1:2,000; cat. no. ab126649; Abcam). Following overnight
incubation, the membranes were washed with TBS-T three times (10
min each) and were further incubated with goat anti-mouse secondary
antibody (1:5,000; cat. no. SAA544Mu19; Cloud-Clone Corp.) and goat
anti-rabbit secondary antibody (1:5,000; cat. no. bs-0295G-HRP;
Bioss) for 1 h at room temperature. membranes were then washed with
TBS-T three times (10 min each). The generated immune complexes
were detected with an enhanced chemiluminescence detection system
(Amersham; Cytiva), and visualization was performed with the Sygene
Bio Image system (Vilber). Gray values for APP, BACE-1, Aβ,
caspase3, Bax and Bcl-2 were detected by ImageJ 1.8.0 (National
Institutes of Health). The ratio of the target protein to the
internal control, β-actin, was used in the final analysis.
TUNEL assay
TUNEL staining was performed according to a previous
study (31). Neuronal apoptosis in
hippocampal samples was analyzed by TUNEL assays. The samples were
fixed with 4% paraformaldehyde at 4˚C for 4 h. Following this,
samples were treated with 3% hydrogen peroxidase and incubated in a
labeling reaction mixture comprised of terminal deoxynucleotidyl
transferase and deoxynucleotides overnight at 4˚C. Sections were
then subjected to further incubation with horseradish peroxidase
(1:500; Shanghai Macklin Biochemical Co., Ltd.) for 30 min and
treatment with 3,3'-diaminobenzidine for 15 min at 37˚C in the
dark. Reactions were stopped with running water and counterstaining
was performed with hematoxylin at 37˚C for 10 min. Following
dehydration with a graded ethyl alcohol series and xylene
treatment, tissue samples were mounted on coverslips with neutral
gum. Apoptotic nuclei appeared as dark brown dots. Apoptotic cells
in the CA1 region were assessed by light microscopy (magnification,
x400) in a blinded manner in five random high-power fields.
Statistical analysis
Data are presented as the mean ± SD. All parameters
were assessed by one-way ANOVA followed by Tukey's post hoc test.
SPSS (version no. 20.0; IBM Corp.) was used for data analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cognitive function declines in aged
rats following splenectomy
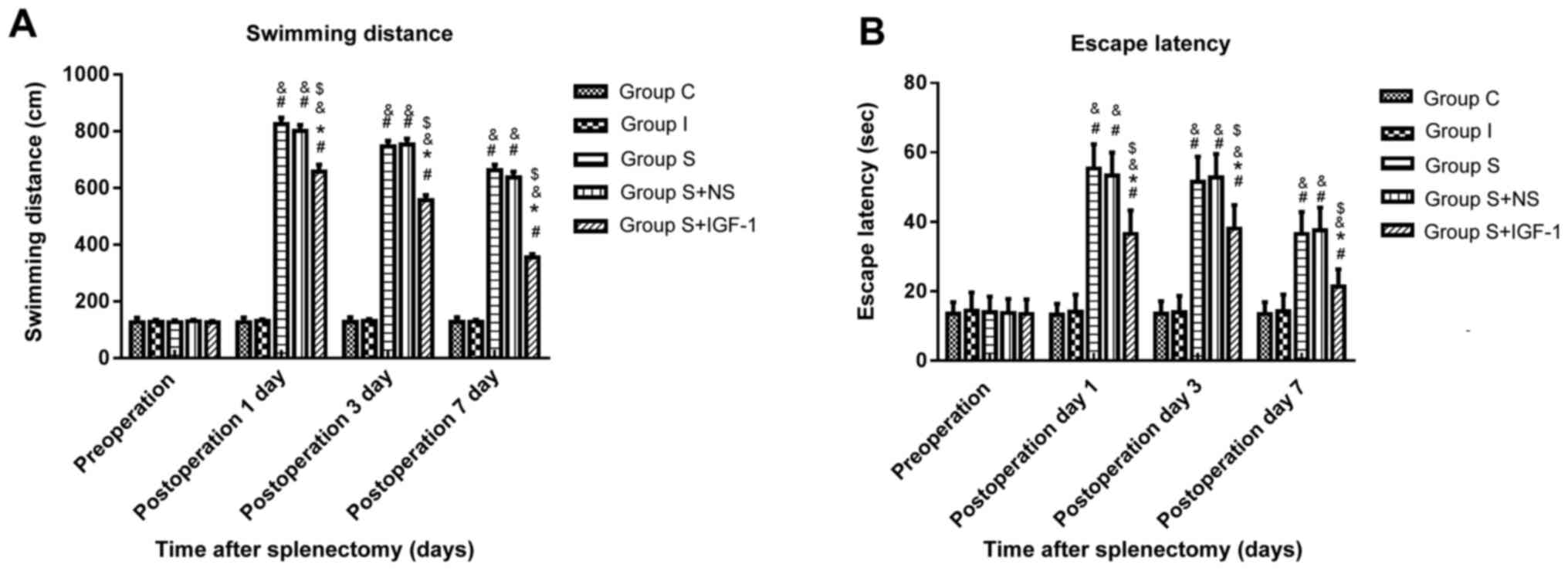
The MWM was performed to assess spatial learning and
memory abilities. In Group S, the swimming distance (Fig. 1A) and escape latency (Fig. 1B) were significantly longer at days
1, 3 and 7 post-surgery compared with the C and I groups. The C and
I groups presented similar values for swimming distance and escape
latency throughout the experiment. These results indicated that
surgery aggravated cognitive impairment. Swimming distance and
escape latency in the S + IGF-1 group were significantly shorter at
days 1, 3 and 7 post-surgery compared with the S and S + NS groups,
indicating that IGF-1 improved cognitive function following
splenectomy.
IGF-1 decreases Aβ protein production
in the hippocampus of aged rats following splenectomy
Protein levels of APP, BACE-1 and Aβ were assessed
in hippocampal specimens following surgery by immunoblotting.
Splenectomy significantly upregulated APP, BACE-1 and Aβ expression
at the protein level in hippocampal samples from aged rats at days
1, 3 and 7 compared with the C and I groups (Fig. 2). IGF-1 administration following
splenectomy significantly decreased the protein amounts of APP,
BACE-1 and Aβ in the hippocampus of aged rats at days 1, 3 and 7
compared with the S + NS group.
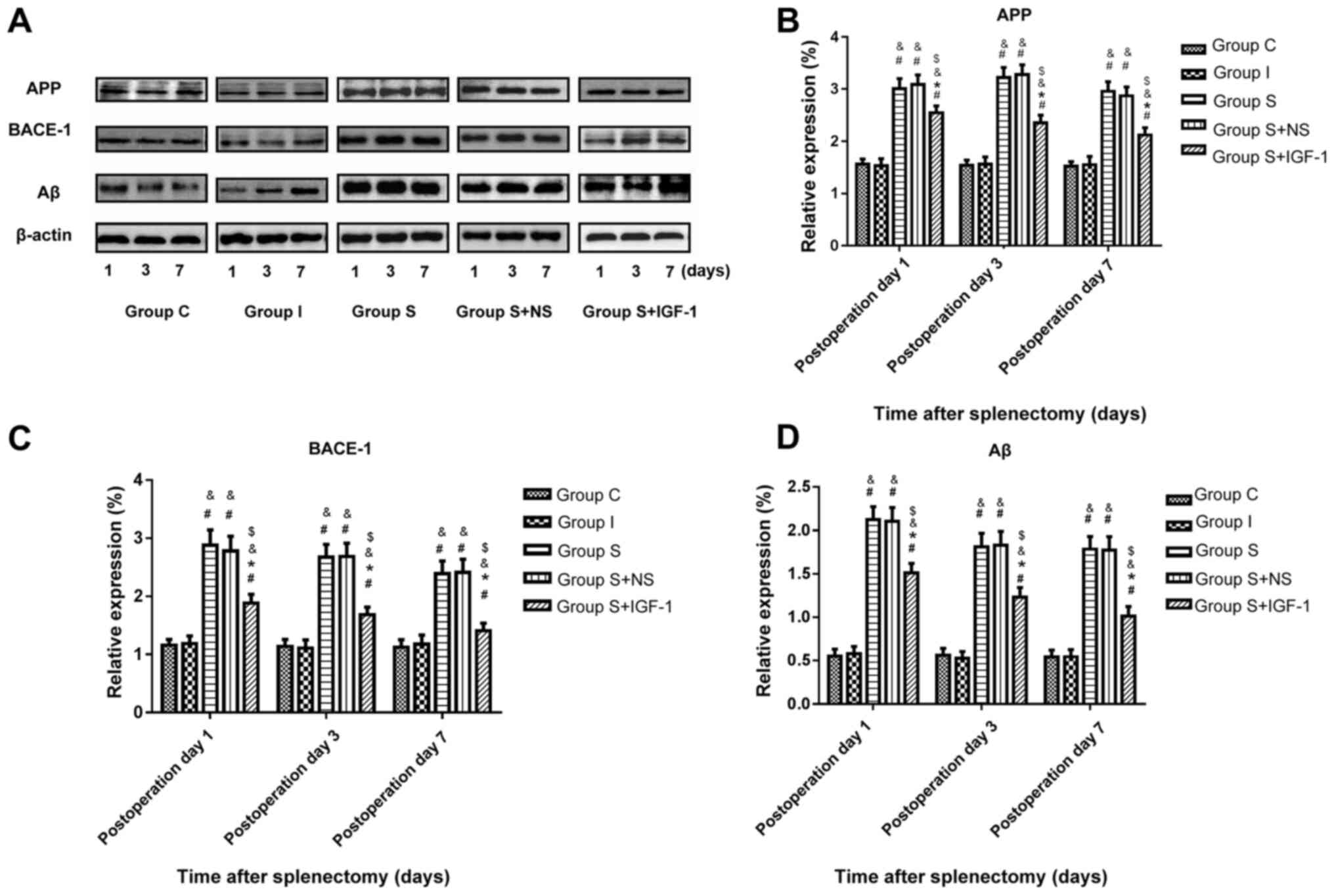 | Figure 2Hippocampal levels of APP, BACE-1 and
Aβ after splenectomy at different timepoints. (A) IGF reduces the
levels of (B) APP, (C) BACE-1 and (D) Aβ in the hippocampus of aged
rats at days 1, 3 and 7 post-surgery. #P<0.05 vs. C
and &P<0.05 vs. I. *P<0.05 vs. S
and $P<0.05 vs. S + NS. IGF-1, insulin-like growth
factor-1; APP, amyloid precursor protein; BACE-1, β-site
APP-cleaving enzyme-1; Aβ, amyloid-β; C, control; I, isoflurane; S,
splenectomy; NS, normal saline. |
IGF-1 inhibits the apoptosis of
neurons in the hippocampal CA1 region in aged rats following
splenectomy
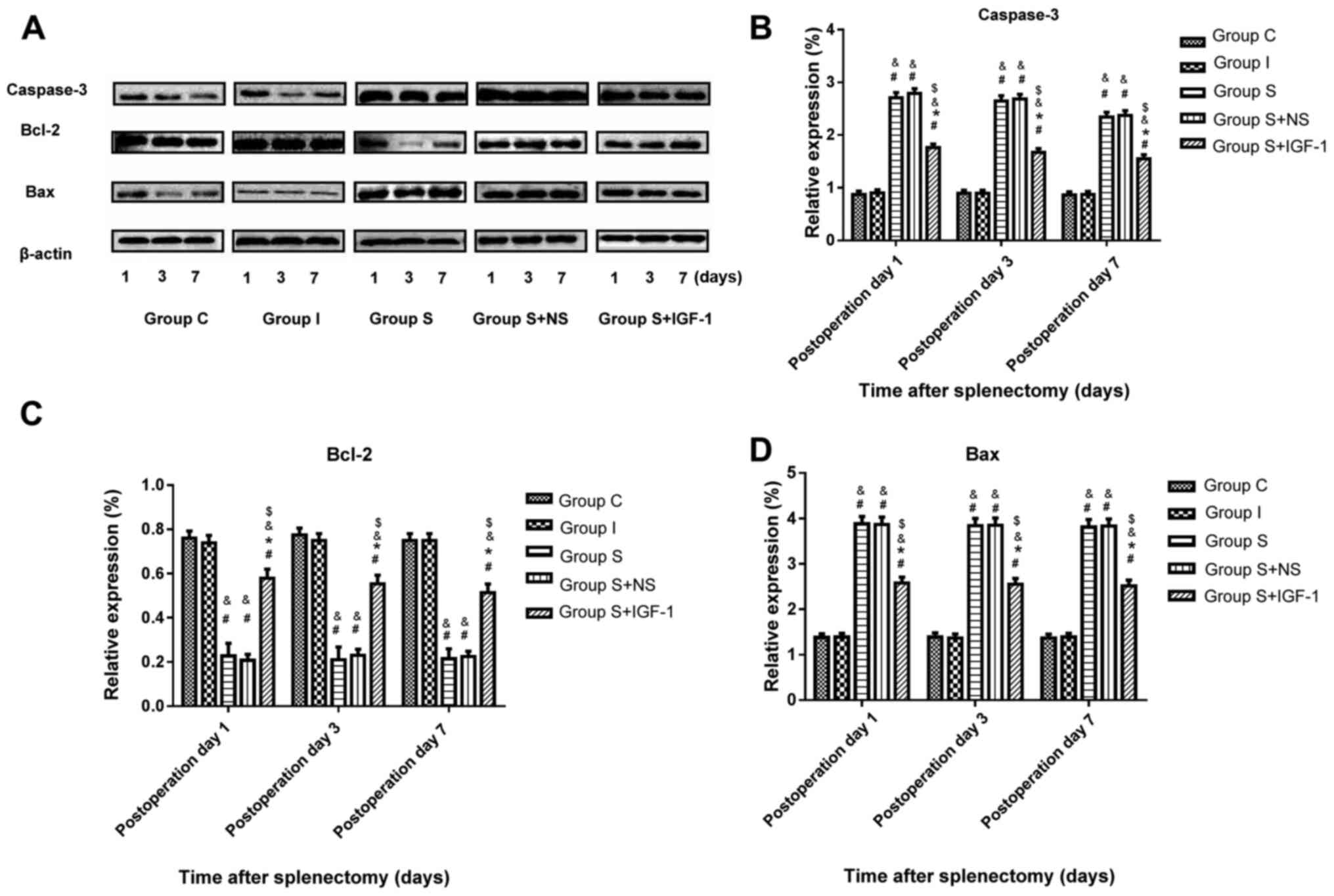
Immunoblotting was performed to assess the protein
levels of capase-3, Bax and Bcl2 in rat hippocampi following
surgery. The results demonstrated that splenectomy significantly
upregulated caspase-3 and Bax expression, and significantly
downregulated Bcl2 expression in the hippocampal CA1 region of aged
rats at days 1, 3 and 7 post-surgery compared with the C and I
groups (Fig. 3). Furthermore, IGF-1
administration following splenectomy significantly reduced capase-3
and Bax protein levels, and significantly increased Bcl2 levels in
hippocampal samples in aged rats at days 1, 3 and 7 post-surgery
compared with the S and S + NS groups.
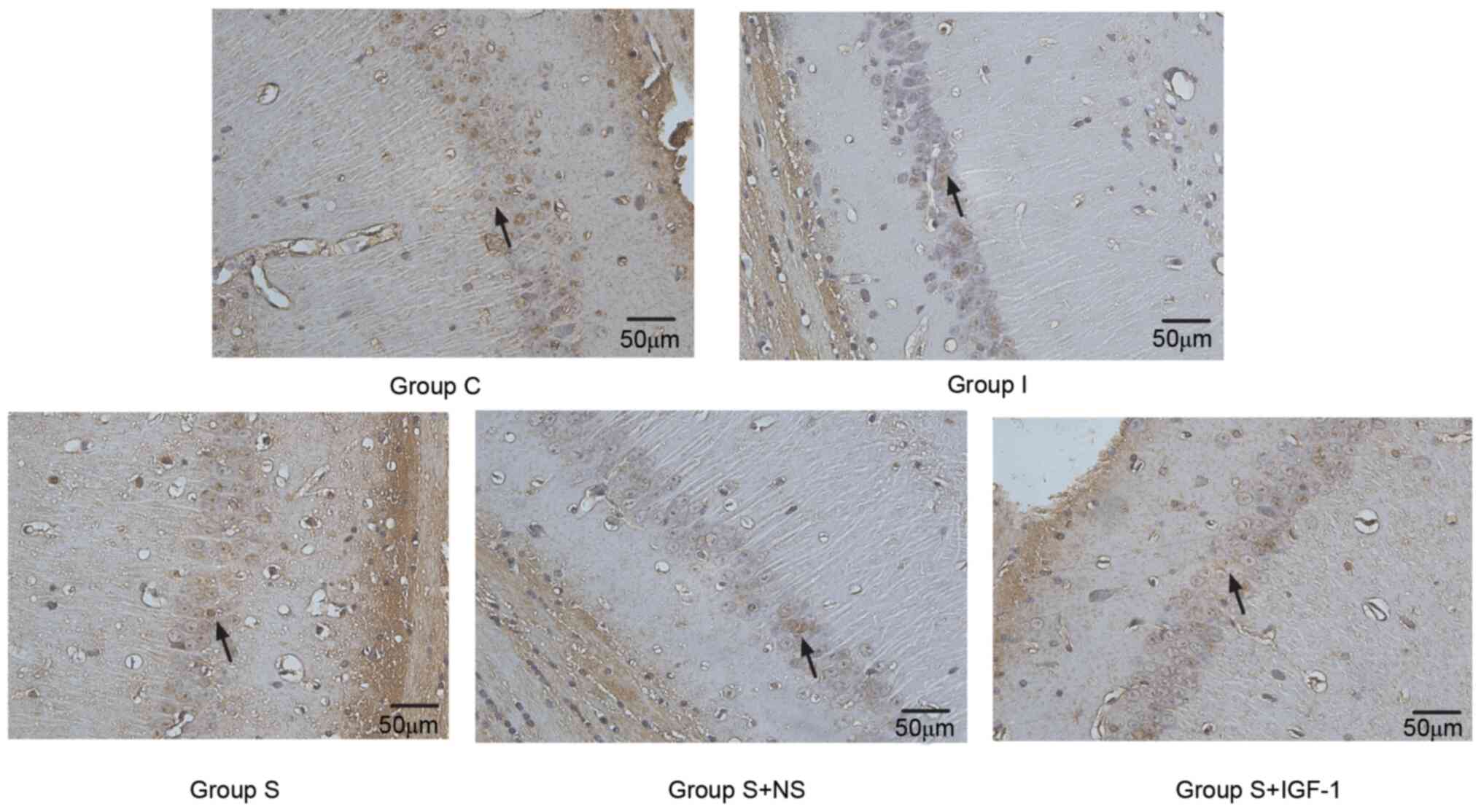
The TUNEL assay revealed that IGF-1 administration
markedly reduced neuronal apoptosis associated with surgery in the
hippocampal CA1 region of animals in the S + IGF-1 group compared
with the S and S + NS groups (Fig.
4).
Discussion
The current study demonstrated that swimming
distance and escape latency increased post-operatively, indicating
splenectomy induced POCD in aged rats. This was consistent with
previous studies which indicated markedly aggravated cognitive
dysfunction in rats that underwent splenectomy (27,32).
Furthermore, the results of the current study revealed that
splenectomy induced the overexpression of APP, BACE-1 and Aβ in the
hippocampus. This indicated that changes in Aβ-protein may be
associated with early POCD, which is consistent with findings
published by Canet et al (33). Furthermore, IGF-1, a multifunctional
polypeptide essential for normal growth and development (12), inhibited the production of upstream
proteins APP and BACE-1, attenuated Aβ production and improved
surgery-induced POCD. These results indicated IGF-1 had a
protective role in POCD by attenuating Aβ production in aged rats.
Cognitive dysfunction persists transiently due to the acute
production of APP and Aβ (34).
Neuroinflammation associated with Aβ aggregation is an essential
factor in cognitive dysfunction (35). Cleavage of APP by BACE-1 produced
soluble β-APP8 and C99, and C99 is hydrolyzed by γ-secretase to
produce insoluble Aβ (36).
Research has demonstrated that Aβ is located in the brain as
metastable monomeric Aβ is constantly produced by APP under
conditions of catalysis by secretases (37). AD and POCD have similar
neuropathogenesis (38). Given that
cognitive function is unavoidably impaired by major surgeries in
aged patients, developing efficient therapeutic tools is of high
significance (39). The present
work recommended IGF-1 as a novel potent drug as it improved POCD
by reducing the generation of Aβ. A previous study reported that
IGF-1 inhibited JNK activity (40)
and enhanced APP phosphorylation at Thr668 in rat hippocampal
tissue (41). Furthermore, IGF-1
increased α-secretase expression in the hippocampus and lowered the
levels of BACE1 and γ-secretase, thereby reducing the levels and
deposition of Aβ in hippocampal tissue (42).
Additionally, the results of the current study
revealed that IGF-1 downregulated caspase-3 and Bax, and
upregulated Bcl2 following splenectomy in hippocampal samples at
days 1, 3 and 7 post-surgery. IGF-1 administration markedly reduced
neuronal apoptosis associated with surgery in the hippocampal CA1
region of rats. Apoptosis is another important mechanism for POCD
development (43). The present
study demonstrated that in the hippocampus of aged rats following
splenectomy, caspase-3 and Bax were significantly increased, while
Bcl-2 was significantly decreased. The Bcl-2 family protein is
located upstream of the mitochondria and is an important regulator
of mitochondrial membrane permeability, which controls the release
of cytochrome c and activates downstream caspase-3 proteases,
mediating cell survival or death (44,45).
Under the condition of apoptosis inducer signals, caspases are
activated by the combination of specific cofactors (46). Once caspases are activated,
degradation of cellular proteins occurs, eventually causing
irreversible cell death (47).
Bcl-2 localization in the outer membrane of mitochondria is
mediated by the indirect action of the caspases (48). Apoptotic protease activating factor
1 is targeted to the mitochondrial membrane by Bcl-2, which blocks
the activation of the apoptotic protease by regulating its
structure and regulates the action of cry-e (49). IGF-1 prevents apoptosis by inducing
the signaling pathway mediated by PI3K and its downstream target
Akt (50). The interaction between
IGF-1 and the IGF-1 receptor phosphorylates tyrosine kinase or
activates PI3K by activating the insulin receptor substrate
(51). When activated, PI3K
subunits phosphorylate phosphoinositide-dependent protein kinases
and activate gene expression and protein translation of downstream
target Akt. When the upstream signal activates the main target
enzyme Akt, anti-apoptotic genes are upregulated, and Akt regulates
Bcl-2 protein expression and enhances Bcl-2 activity (52). Peruzzi et al (53) demonstrated that PI3K inhibitors
prevented IGF-1 from upregulating Bax and downregulating Bcl-2.
Therefore, IGF-1 inhibited the apoptotic pathway (54).
Certain limitations of the current study must be
discussed. Firstly, the number of animal experiments was limited.
Sample sizes will be increased in future experiments. Secondly, the
behavioral memory of rats can be measured comprehensively using
behavior tests, including contextual fear conditioning test and the
elevated plus maze test. Thirdly, since animal models cannot
completely reproduce complex clinical situations, it remains
essential to confirm whether similar changes occur in patients
following surgery.
Overall, the current study reported a
neuroprotective role for IGF-1 for POCD in aged rats. The mechanism
involved decreased Aβ-protein production and inhibited neuronal
apoptosis in the hippocampus. The present results indicated the use
of IGF-1 for preventing POCD in aged patients.
Acknowledgements
Not applicable.
Funding
The current study was funded by the Qingdao Medical
Research Guidance Program, Qingdao, China (grant no.
2018-WJZD011),
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YB and MW conceived and designed the current study
and drafted the manuscript. CX, CL and JZ performed the experiments
at the physical laboratory of Qingdao University, China. RD, XL and
XS analyzed data. GZ and BW performed the experiments and wrote and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics and approval and consent to
participate
The present study followed the recommendations of
the National Institute of Health guidelines for the care and use of
laboratory animals and obtained approval from the Clinical Trial
Ethics Committee of Qingdao Municipal Hospital, Qingdao, China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Safavynia SA and Goldstein PA: The role of
neuroinflammation in postoperative cognitive dysfunction: Moving
from hypothesis to treatment. Front Psychiatry.
9(752)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ramaiah R and Lam AM: Postoperative
cognitive dysfunction in the elderly. Anesthesiol Clin. 27:485–496.
2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Steinmetz J, Christensen KB, Lund T, Lohse
N and Rasmussen LS: ISPOCD Group. Long-term consequences of
postoperative cognitive dysfunction. Anesthesiology. 110:548–555.
2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wuri G, Wang DX, Zhou Y and Zhu SN:
Effects of surgical stress on long-term memory function in mice of
different ages. Acta Anaesthesiol Scand. 55:474–485.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Songjiang Z and Lixiang W: Amyloid-beta
associated with chitosan nano-carrier has favorable immunogenicity
and permeates the BBB. AAPS PharmSciTech. 10:900–905.
2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hoe HS, Lee HK and Pak DT: The upside of
APP at synapses. CNS Neurosci Ther. 18:47–56. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Poulsen SA, Watson AA, Fairlie DP and
Craik DJ: Solution structures in aqueous SDS micelles of two
amyloid beta peptides of A beta(1-28) mutated at the
alpha-secretase cleavage site (K16E, K16F). J Struct Biol.
130:142–152. 2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Garcez ML, Mina F, Bellettini-Santos T, da
Luz AP, Schiavo GL, Macieski JMC, Medeiros EB, Marques AO, Magnus
NQ and Budni J: The involvement of NLRP3 on the effects of
minocycline in an AD-like pathology induced by β-amyloid oligomers
administered to mice. Mol Neurobiol. 56:2606–2617. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ghahghaei A, Bathaie SZ, Kheirkhah H and
Bahraminejad E: The protective effect of crocin on the amyloid
fibril formation of Aβ42 peptide in vitro. Cell Mol Biol Lett.
18:328–339. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wu Z, Zhang M, Zhang Z, Dong W, Wang Q and
Ren J: Ratio of β-amyloid protein (Aβ) and Tau predicts the
postoperative cognitive dysfunction on patients undergoing total
hip/knee replacement surgery. Exp Ther Med. 15:878–884.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang X, Dong H, Li N, Zhang S, Sun J,
Zhang S and Qian Y: Activated brain mast cells contribute to
postoperative cognitive dysfunction by evoking microglia activation
and neuronal apoptosis. J Neuroinflammation. 13(127)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vassilakos G and Barton ER: Insulin-like
growth factor I regulation and its actions in skeletal muscle.
Compr Physiol. 9:413–438. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
D'Ercole AJ, Ye P and O'Kusky JR: Mutant
mouse models of insulin-like growth factor actions in the central
nervous system. Neuropeptides. 36:209–220. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mishra N, Lata S, Deshmukh P, Kamat K,
Surolia A and Banerjee T: Insulin signaling pathway protects
neuronal cell lines by Sirt3 mediated IRS2 activation. Biofactors.
44:224–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liao R, Yan F, Zeng Z, Farhan M, Little P,
Quirion R, Srivastava LK and Zheng W: Amiodarone-Induced Retinal
Neuronal Cell Apoptosis Attenuated by IGF-1 via Counter Regulation
of the PI3k/Akt/FoxO3a Pathway. Mol Neurobiol. 54:6931–6943.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nakao Y, Otani H, Yamamura T, Hattori R,
Osako M and Imamura H: Insulin-like growth factor 1 prevents
neuronal cell death and paraplegia in the rabbit model of spinal
cord ischemia. J Thorac Cardiovasc Surg. 122:136–143.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lackey BR, Gray SL and Henricks DM:
Actions and interactions of the IGF system in Alzheimer's disease:
Review and hypotheses. Growth Horm IGF Res. 10:1–13.
2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sonntag WE1. Deak F, Ashpole N, Toth P,
Csiszar A, Freeman W and Ungvari Z: Insulin-like growth factor-1 in
CNS and cerebrovascular aging. Front Aging Neurosci.
5(27)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Angelini A, Bendini C, Neviani F,
Bergamini L, Manni B, Trenti T, Rovati R and Neri M: Insulin-like
growth factor-1 (IGF-1): Relation with cognitive functioning and
neuroimaging marker of brain damage in a sample of hypertensive
elderly subjects. Arch Gerontol Geriatr. 49 (Suppl 1):5–12.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Guidance Suggestions for the Care and Use
of Laboratory Animals 9: 30, 2006.
|
|
21
|
Umholtz M and Nader ND: Anesthetic
immunomodulation of the neuroinflammation in postoperative
cognitive dysfunction. Immunol Invest. 46:805–815. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Newman S, Stygall J, Hirani S, Shaefi S,
Maze M and Warltier DC: Postoperative cognitive dysfunction after
noncardiac surgery: A systematic review. Anesthesiology.
106:572–590. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wan Y, Xu J, Ma D, Zeng Y, Cibelli M and
Maze M: Postoperative impairment of cognitive function in rats: A
possible role for cytokine-mediated inflammation in the
hippocampus. Anesthesiology. 106:436–443. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li M, Yong-Zhe L, Ya-Qun M, Sheng-Suo Z,
Li-Tao Z and Ning-Ling P: Ulinastatin alleviates neuroinflammation
but fails to improve cognitive function in aged rats following
partial hepatectomy. Neurochem Res. 38:1070–1077. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cibelli M, Fidalgo AR, Terrando N, Ma D,
Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS,
et al: Role of interleukin-1beta in postoperative cognitive
dysfunction. Ann Neurol. 68:360–368. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang B, Li S, Cao X, Dou X, Li J, Wang L,
Wang M and Bi Y: Blood-brain barrier disruption leads to
postoperative cognitive dysfunction. Curr Neurovasc Res.
14:359–367. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yu L, Sun L and Chen S: Protective effect
of senegenin on splenectomy-induced postoperative cognitive
dysfunction in elderly rats. Exp Ther Med. 7:821–826.
2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kamer AR, Galoyan SM, Haile M, Kline R,
Boutajangout A, Li YS and Bekker A: Meloxicam improves object
recognition memory and modulates glial activation after splenectomy
in mice. Eur J Anaesthesiol. 29:332–337. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Weitzner DS, Engler-Chiurazzi EB,
Kotilinek LA, Ashe KH and Reed MN: Morris water maze test:
Optimization for mouse strain and testing environment. J Vis Exp.
22(e52706)2015.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Jawa RS, Anillo S, Huntoon K, Baumann H
and Kulaylat M: Interleukin-6 in surgery, trauma, and critical care
part II: Clinical implications. J Intensive Care Med. 26:73–87.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pang H, Huang T, Song J, Li D, Zhao Y and
Ma X: Inhibiting HMGB1 with glycyrrhizic acid protects brain injury
after DAI via its anti-inflammatory effect. Mediators Inflamm.
2016(4569521)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lu SM, Gui B, Dong HQ, Zhang X, Zhang SS,
Hu LQ, Liu HL, Sun J and Qian YN: Prophylactic lithium alleviates
splenectomy-induced cognitive dysfunction possibly by inhibiting
hippocampal TLR4 activation in aged rats. Brain Res Bull.
114:31–41. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Canet J, Raeder J, Rasmussen LS, Enlund M,
Kuipers HM, Hanning CD, Jolles J, Korttila K, Siersma VD, Dodds C,
et al: ISPOCD2 investigators. Cognitive dysfunction after minor
surgery in the elderly. Acta Anaesthesiol Scand. 47:1204–1210.
2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Roher AE, Kokjohn TA, Clarke SG, Sierks
MR, Maarouf CL, Serrano GE, Sabbagh MS and Beach TG: APP/Aβ
structural diversity and Alzheimer's disease pathogenesis.
Neurochem Int. 110:1–13. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhu D, Yang N, Liu YY, Zheng J, Ji C and
Zuo PP: M2 Macrophage transplantation ameliorates cognitive
dysfunction in amyloid-β-treated rats through regulation of
microglial polarization. J Alzheimers Dis. 52:483–495.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Masters CL and Selkoe DJ: Biochemistry of
amyloid β-protein and amyloid deposits in Alzheimer disease. Cold
Spring Harb Perspect Med. 2(a006262)2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kulic L, McAfoose J, Welt T, Tackenberg C,
Späni C, Wirth F, Finder V, Konietzko U, Giese M, Eckert A, et al:
Early accumulation of intracellular fibrillar oligomers and late
congophilic amyloid angiopathy in mice expressing the Osaka
intra-Aβ APP mutation. Transl Psychiatry. 2(e183)2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xie Z and Tanzi RE: XieZ. Alzheimer's
disease and post-operative cognitive dysfunction. Exp Gerontol.
41:346–359. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Qian XL, Zhang W, Liu MZ, Zhou YB, Zhang
JM, Han L, Peng YM, Jiang JH and Wang QD: Dexmedetomidine improves
early postoperative cognitive dysfunction in aged mice. Eur J
Pharmacol. 746:206–212. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Teng JA, Wu SG, Chen JX, Li Q, Peng F, Zhu
Z, Qin J and He ZY: The activation of ERK1/2 and JNK MAPK signaling
by insulin/IGF-1 is responsible for the development of colon cancer
with type 2 diabetes mellitus. PLoS One.
11(e0149822)2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Araki W, Kume H, Oda A, Tamaoka A and
Kametani F: IGF-1 promotes beta-amyloid production by a
secretase-independent mechanism. Biochem Biophys Res Commun.
380:111–114. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhou Q, Wang M, Du Y, Zhang W, Bai M,
Zhang Z, Li Z and Miao J: Inhibition of c-Jun N-terminal kinase
activation reverses alzheimer disease phenotypes in APPswe/PS1d E9
mice. Ann Neurol. 77:637–654. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang Q, Li Y, Bao Y, Yin C, Xin X, Guo Y,
Gao F, Huo S, Wang X and Wang Q: Pretreatment with nimodipine
reduces incidence of POCD by decreasing calcineurin mediated
hippocampal neuroapoptosis in aged rats. BMC Anesthesiol.
18(42)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lin HH, Chen JH, Huang CC and Wang CJ:
Apoptotic effect of 3,4-dihydroxybenzoic acid on human gastric
carcinoma cells involving JNK/p38 MAPK signaling activation. Int J
Cancer. 120:2306–2316. 2007.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Scorrano L and Korsmeyer SJ: Mechanisms of
cytochrome c release by proapoptotic BCL-2 family members. Biochem
Biophys Res Commun. 304:437–444. 2003.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kopeina GS, Prokhorova EA, Lavrik IN and
Zhivotovsky B: Alterations in the nucleocytoplasmic transport in
apoptosis: Caspases lead the way. Cell Prolif.
51(e12467)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Shi Y: Mechanisms of caspase activation
and inhibition during apoptosis. Mol Cell. 9:459–470.
2002.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Peña-Blanco A and García-Sáez AJ: Bax, Bak
and beyond - mitochondrial performance in apoptosis. FEBS J.
285:416–431. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Festjens N, van Gurp M, van Loo G, Saelens
X and Vandenabeele P: Bcl-2 family members as sentinels of cellular
integrity and role of mitochondrial intermembrane space proteins in
apoptotic cell death. Acta Haematol. 111:7–27. 2004.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yang L, Wang H, Liu L and Xie A: The role
of insulin/IGF-1/PI3K/Akt/GSK3β signaling in Parkinson's disease
dementia. Front Neurosci. 12(73)2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ueki K, Fruman DA, Brachmann SM, Tseng YH,
Cantley LC and Kahn CR: Molecular balance between the regulatory
and catalytic subunits of phosphoinositide 3-kinase regulates cell
signaling and survival. Mol Cell Biol. 22:965–977. 2002.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Dufour C, Holy X and Marie PJ:
Transforming growth factor-beta prevents osteoblast apoptosis
induced by skeletal unloading via PI3K/Akt, Bcl-2, and phospho-Bad
signaling. Am J Physiol Endocrinol Metab. 294:E794–E801.
2008.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Peruzzi F, Prisco M, Dews M, Salomoni P,
Grassilli E, Romano G, Calabretta B and Baserga R: Multiple
signaling pathways of the insulin-like growth factor 1receptor in
protection from apoptosis. Mol Cell Biol. 19:7203–7215.
1999.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wymann MP and Pirola L: Structure and
function of phosphoinositide 3-kinases. Biochim Biophys Acta.
1436:127–150. 1998.PubMed/NCBI View Article : Google Scholar
|