Introduction
Type 2 diabetes mellitus (T2DM) is characterized by
insulin resistance combined with progressive β-cell failure
(1). Despite advances in the
treatment of T2DM, devising an appropriate treatment strategy for
patients with newly diagnosed T2DM with severe hyperglycaemia
[glycosylated haemoglobin (HbA1c) >9%; fasting plasma glucose
(FPG) ≥11.1 mmol/l] provides a formidable challenge to physicians
(2). Glucose toxicity and relative
defects in insulin secretion make achieving glycaemic targets with
metformin monotherapy difficult for these individuals (3). Therefore, patients with newly
diagnosed severe hyperglycaemia require prompt and effective
glycaemic control.
Most current guidelines, such as those by the
American Diabetes Association (ADA) and American Association of
Clinical Endocrinologists, indicate that either initial insulin
treatment or oral antidiabetic drug treatment with metformin are
options for patients with severe hyperglycaemia (4,5). In
addition, the ADA guidelines recommend that combination insulin
treatment should be initiated when HbA1c is at ≥86-108 mmol/mol
(10-12%) (6). The ADA 2019
guidelines clearly mention that early introduction of insulin
should be considered when HbA1C or blood glucose levels are high
[>10% (86 mmol/mol) and ≥300 mg/dl (16.7 mmol/l), respectively]
(7). However, insulin treatment is
associated with an increased risk of adverse events, such as high
rates of severe hypoglycaemia, glycaemic variability and weight
gain (8,9). In addition, certain patients refuse
insulin injections, partly due to fear of adverse effects, but also
for other reasons (inconvenience, fear of injection and pain)
(10). Therefore, an alternative
should be developed for those patients.
Metformin, a classic drug used to treat T2DM, has
been applied in clinical practice for decades, achieving acceptable
therapeutic efficacy (11). It has
a role in insulin resistance (hyperinsulinemia), which is closely
related to the pathogenesis and mechanisms of T2DM (12). Sitagliptin, the first available
dipeptidyl peptidase-4 (DPP-4) inhibitors, was approved as a
treatment for T2DM by the US Food and Drug Administration in 2006,
and it has been widely used in clinical practice. Safety and
tolerability have been confirmed in clinical trials for up to 2
years, with a low risk of hypoglycaemia when administered as a
monotherapy or when used in combination with antihyperglycaemic
agents that are generally not known to cause hypoglycaemia
(13). DPP-4 inhibitors are a novel
group of medicines used for the treatment of T2DM, which improve
meal stimulated insulin secretions by protecting glucagon-like
peptide-1 (GLP-1) and glucose dependent insulinotropic polypeptide
from enzymatic degradation (14).
Sitagliptin is a well-tolerated monotherapy or add-on therapeutic
agent used in combination with other oral antidiabetic agents; it
has numerous favourable features, including once-daily
administration, potent glucose-lowering effects, a low risk of
hypoglycaemia and a neutral effect on body weight (15,16).
However, an optimal treatment strategy for patients
with T2DM with severe hyperglycaemia has remained to be provided.
The present study aimed to prove that initiation therapy in
patients with newly diagnosed T2DM presenting with severe
hyperglycaemia should also include noninsulin alternatives,
particularly for those patients who refuse to use insulin therapy.
A prospective observational study was therefore performed to
compare the efficacy and safety of sitagliptin combined with
metformin vs. insulin as an initial treatment for patients newly
diagnosed with T2DM with severe hyperglycaemia. In addition to
glycaemic control, the effects of these treatments on insulin
levels and β-cell insulin secretory capacity were investigated and
an attempt was made to establish an optimal therapeutic regimen for
these individuals.
Materials and methods
Study design
A prospective observational cohort study with a
non-randomized design comparing sitagliptin combined with metformin
and insulin therapy in patients with newly diagnosed T2DM with
severe hyperglycaemia was performed. From January 2014 to June
2019, 168 consecutive participants from the First Affiliated
Hospital of Xi'an JiaoTong University (Xi'an, China) in the
northwest region of China, who had been newly diagnosed with T2DM
according to the 1999 World Health Organization diagnostic
criteria, had severe hyperglycaemia [HbA1c >9% and FPG ≥200
mg/dl (11.1 mmol/l)] and were symptomatic (present with hallmark
symptoms of polyuria/polydipsia), were included in the study. An
additional cohort of 448 patients with T2DM was enrolled at the
outpatient service of the First Affiliated Hospital of Xi'an
JiaoTong University (Xi'an, China). These patients were made to
complete a Therapy Attitude Questionnaire to determine their
preferred treatment and the specific reasons for their choice
(10).
In the cohort of newly diagnosed patients, there
were 2 treatment regimen options used based on the real-world
settings. The option chosen was based on the clinician's choice,
considering age, body mass index (BMI), economic conditions, other
complications, HbA1c, fasting and postprandial plasma glucose of
patients. A combination of sitagliptin (100 mg/day) and metformin
(1,500 mg/day) in the Sig group, which were the recommended daily
dosages and invariable (17). In
the Ins group, three combinations of insulin therapy were used at
the start of treatment: i) Insulin glargine or detemir combined
with insulin for a total dosage of 0.3-0.4 U/kg/day; ii) low-dose
insulin glargine or detemir at a total dosage of 0.1-0.2 U/kg/day
combined with oral hypoglycaemic agents, including metformin and
acarbose, and excluding insulin secretagogues such as sulfonylureas
and DPP-4 inhibitors; iii) Novolin 30R insulin at a total dosage of
0.3-0.4 U/kg/day. The insulin regimens administered to the Ins
group were regarded as a low dose, which is suitable for patients
with newly diagnosed T2DM presenting with severe hyperglycaemia
(17). FPG levels of <7.0 mmol/l
and postprandial blood glucose of <10.0 mmol/l were considered
to indicate euglycaemia. After achieving euglycaemia (almost within
4 weeks), sitagliptin and insulin therapy were paused, while
lifestyle interventions and metformin therapy were continued. The
experimental protocol was approved by the Ethics Committee of the
First Affiliated Hospital of Xi'an JiaoTong University (Xi'an,
China; approval no. XJTU1AF2016LSL-048). Informed consent was
obtained from all participants.
Inclusion criteria
i) Males or females with newly diagnosed T2DM; ii)
age, 18-70 years, FPG of ≥11.1 mmol/l, HbA1c of ≥9.0% and BMI of
18-28 kg/m2; iii) no previous treatment with
antidiabetic or antihyperlipidaemic medication.
Exclusion criteria
i) T1DM, gestational diabetes or diabetes with an
identifiable secondary cause; ii) evidence of elevated alanine or
aspartate aminotransferase or significant renal impairment
(estimated creatinine clearance of < ml/min); iii) severe
complications associated with diabetes or severe infection; iv)
history of organ transplantation, cancer, macrovascular disease,
autonomic neuropathy, proliferative retinopathy, scheduled surgery
or serious trauma; v) premenopausal females who were nursing,
pregnant or with child-bearing potential.
Data collection
Anthropometric and laboratory data were collected
prior to treatment and 1 and 3 months after treatment. An oral
glucose tolerance test (OGTT) and insulin release test were
conducted in a subgroup of participants 3 months after treatment
and β-cell function was also evaluated (18). To calculate the homeostasis model
assessment (HOMA) index, the following formulae were used:
HOMA-insulin resistance (IR)=fasting insulin (FINS)xFPG/22.5;
HOMA-β=20xFINS/(FPG-3.5) (18). The
β-cell response to the OGTT was also calculated as the area under
the curve (AUC) for plasma glucose (PG) and insulin (AUC-PG and
AUC-INS) at 0, 60, 120 and 180 min using the trapezoid rule:
AUC-PG=(PG0min+PG60min)/2+PG120min+PG180min;
AUC-INS=(INS0min+INS60min)/2+INS120min+INS180min
(19);
ΔI60min/ΔG60min=(INS60min-INS0min)/(PG60min-PG0min),
Ip/I0= peak insulin/fasting insulin (20); disposition index
(DI)=(ΔI60min/ΔG60min)/HOMA-IR; modified
β-cell function index
(MBCI)=(INS0minxPG0min)/(PG120min+PG60min-2xPG0min)
(21).
Data regarding hypoglycaemic events were collected
by asking participants and checking prescriptions. Hypoglycaemia
was defined as self-monitored blood glucose (SMBG) of <3.9
mmol/l with or without signs/symptoms of hypoglycaemia.
Participants were asked about symptoms of hypoglycaemia that
required medical or non-medical assistance, including heart
palpitations, sweating, confusion and weakness or dizziness.
Follow-up
Outpatient examinations were performed prior to the
beginning of the experiment and after 1 and 3 months. The 1-month
visit was a safety evaluation, which included a physical
examination, evaluation of glucose control (plasma FPG and SMBG
recorded in the diary), reinforcement of lifestyle advice and
completion of an adverse events record. An OGTT and insulin release
test were performed in a subgroup of participants 3 months after
treatment; demographic and biochemistry data were also
collected.
Outcome measures
The primary endpoints were changes in FPG and HbA1c
and assessment of islet β-cell function at the 3-month follow-up.
Secondary endpoints included glycaemic remission rate, time of
glycaemic remission, changes in body weight and BMI at the 1- and
3-month follow-ups, as well as hypoglycaemic episodes during the
study period. Hypoglycaemia was assessed using a questionnaire and
supplemented with SMBG values.
Data analysis and statistics
Noninferiority of the outcomes was assumed if the
upper limit of the 95% confidence interval (CI) for the difference
in the effects of the treatment was less than a 0.3% change in
HbA1c levels from baseline to month 3. With the assumption of a
standard deviation (SD) of 1.15%, 49 patients in the Sig group and
98 patients in the Ins group (a total of 147 patients) were
required to achieve 80% power for the analysis.
All statistical analyses were performed using SPSS
22.0 software (IBM Corp.). Normally distributed data are reported
as the mean ± SD and non-normally distributed data as the median
and interquartile range. Count data are expressed as n (%). To test
the differences between groups, a two-samples t-test was used if
the normality criteria were met and Wilcoxon's rank-sum test if
they were not. A paired t-test and repeated-measures ANOVA were
used to evaluate differences after vs. prior to the intervention.
For nominal parameters, differences among the groups were analysed
using the χ2 test. Subgroup analyses were conducted for
each stratification factor (HbA1c at baseline <10, 10-12 or
>12%) in the two groups. A two-sided P<0.05 was considered to
indicate a statistically significant difference.
Results
Baseline clinical characteristics of
the two treatment groups
Results of the Therapy Attitude Questionnaire,
suggested that the additional cohort of 448 patients with T2DM, who
had received numerous years of treatment for T2DM, preferred oral
treatment and refused to use insulin injections (Table SI and Fig. S1). Of these, 56.92% of all patients
were male and 58.04% were more than 55 years old. 40.63% received
oral treatment, 22.32% received both insulin and oral treatment and
37.05% received only insulin treatment (Table SI). However, only a small
proportion of patients (7.81%) preferred insulin as their
treatment, while most patients with T2DM (95.98%) rejected insulin
therapy mainly due to inconvenience and fear of injection, adverse
effects and pain (Fig. S1).
Furthermore, a total of 168 participants with newly
diagnosed T2DM were recruited, including 64 in the Sig group and
104 in the Ins group. None of the participants dropped out and no
serious adverse effects were observed during the intervention. The
ADA guidelines recommend initiating combination insulin therapy at
an HbA1c of ≥10-12% (86-108 mmol/mol) (2). Consequently, participants in each
group were stratified into three subgroups according to their HbA1c
levels. The baseline clinical characteristics of the participants
(age, sex, weight and BMI) were similar and there were no
statistically significant differences in glucose levels (HbA1c and
FPG) between the two groups at the beginning of the study
(P>0.05; Table I; the values for
the total Ins and Sig groups are shown in Table SII).
 | Table IBaseline demographic and clinical
characteristics. |
Table I
Baseline demographic and clinical
characteristics.
| | HbA1c<10% | | 10%≤HbA1c≤12% | | HbA1c>12% | |
|---|
| Item | Sig (n=29) | Ins (n=22) | P-value | Sig (n=27) | Ins (n=55) | P-value | Sig (n=8) | Ins (n=27) | P-value |
|---|
| Male, sex (%) | 79.31 | 63.64 | 0.214 | 74.07 | 83.64 | 0.304 | 75.00 | 74.07 | 0.958 |
| Age (years) | 49.62±9.45 | 46.09±13.98 | 0.288 | 48.48±12.48 | 44.98±11.95 | 0.224 | 47.63±14.01 | 45.44±10.98 | 0.646 |
| Height (cm) | 170.21±6.81 | 167.46±6.70 | 0.156 | 168.70±7.81 | 170.32±7.10 | 0.355 | 168.87±8.95 | 170.15±7.26 | 0.682 |
| Body weight
(kg) | 73.92±9.12 | 69.71±10.45 | 0.131 | 71.05±9.94 | 71.73±10.72 | 0.784 | 72.16±7.36 | 67.31±6.45 | 0.115 |
| BMI
(kg/m2) | 25.49±2.70 | 24.78±2.86 | 0.366 | 24.87±2.17 | 24.62±2.74 | 0.685 | 24.52±1.45 | 23.16±1.93 | 0.114 |
| FPG (mmol/l) | 11.86±0.87 | 12.88±1.93 | 0.074 | 12.43±1.14 | 13.16±1.34 | 0.062 | 14.19±1.75 | 14.86±1.83 | 0.370 |
| HbA1c (%) | 9.36±0.28 | 9.31±0.25 | 0.516 | 10.74±0.57 | 10.98±0.64 | 0.106 | 12.65±0.46 | 13.27±0.92 | 0.076 |
| TG (mmol/l) | 1.96±0.94 | 4.44±3.50 | 0.004 | 2.08±1.27 | 3.57±5.89 | 0.205 | 1.82±0.40 | 2.93±5.87 | 0.600 |
| Cho (mmol/l) | 4.55±0.86 | 5.27±1.51 | 0.057 | 4.92±0.98 | 5.08±1.21 | 0.571 | 5.55±0.82 | 5.04±0.95 | 0.181 |
| LDL-C (mmol/l) | 2.80±0.78 | 2.84±0.78 | 0.883 | 2.99±0.58 | 2.90±0.91 | 0.648 | 3.61±0.63 | 2.99±0.76 | 0.046 |
| HDL-C (mmol/l) | 1.02±0.15 | 1.04±0.26 | 0.633 | 1.04±1.99 | 1.05±0.23 | 0.910 | 1.20±0.21 | 1.11±0.20 | 0.279 |
| ALT (U/l) | 30.50±16.12 | 31.20±17.23 | 0.884 | 25.89±12.42 | 36.58±20.73 | 0.018 | 36.44±33.51 | 28.36±13.40 | 0.310 |
| AST (U/l) | 25.67±11.23 | 25.43±16.29 | 0.952 | 25.34±16.54 | 25.49±9.81 | 0.959 | 26.78±17.30 | 22.53±9.45 | 0.368 |
| BUN (mmol/l) | 4.89±0.99 | 8.49±15.51 | 0.225 | 5.30±1.24 | 5.14±1.15 | 0.557 | 4.73±0.59 | 5.21±1.33 | 0.333 |
| Cr (µmol/l) | 57.41±11.56 | 54.79±11.42 | 0.441 | 56.08±13.93 | 57.45±8.66 | 0.587 | 51.75±5.85 | 54.11±10.96 | 0.565 |
Insulin as well as sitagliptin
combined with metformin reduce hyperglycaemia in patients with
newly diagnosed T2DM and severe hyperglycaemia
First, it was examined whether sitagliptin combined
with metformin treatment was able to reduce hyperglycaemia in
patients newly diagnosed T2DM with severe hyperglycaemia. Paired
t-tests were used to evaluate differences in FPG and HbA1c after
the intervention vs. baseline. FPG was significantly decreased
after 1 month of intervention in both groups; thus, all
participants had significantly improved glycaemic control and
achieved euglycaemia within 1 month (all P<0.001; Table II). At the 3-month follow-up,
significant reductions in FPG and HbA1c were observed in these two
groups as compared with the baseline values and euglycaemia was
maintained (all P<0.001; Table
II).
 | Table IIComparison of glycaemic outcome
parameters following treatment in the stratified groups. |
Table II
Comparison of glycaemic outcome
parameters following treatment in the stratified groups.
| | HbA1c<10% | | 10%≤HbA1c≤12% | | HbA1c>12% | |
|---|
|
Time-point/parameter | Sig | Ins | P-value | Sig | Ins | P-value | Sig | Ins | P-value |
|---|
| At baseline | | | | | | | | | |
|
FPG
(mmol/l) | 11.86±0.87 | 12.88±1.93 | 0.074 | 12.43±1.14 | 13.16±1.34 | 0.062 | 14.19±1.75 | 14.86±1.83 | 0.370 |
|
HbA1c
(%) | 9.36±0.28 | 9.31±0.25 | 0.516 | 10.74±0.57 | 10.98±0.64 | 0.106 | 12.65±0.46 | 13.27±0.92 | 0.076 |
| At 1-month
follow-up | | | | | | | | | |
|
FPG
(mmol/l) |
6.72±0.77a |
6.39±1.07a | 0.217 |
6.49±0.88a |
6.74±0.62a | 0.156 |
6.55±0.91a |
6.64±0.91a | 0.798 |
|
ΔFPG
(mmol/l) | -5.11±1.16 | -4.45±4.44 | 0.502 | -6.65±2.25 | -7.23±3.73 | 0.463 | -7.65±1.32 | -8.46±2.25 | 0.338 |
| At 3-month
follow-up | | | | | | | | | |
|
FPG
(mmol/l) |
6.71±0.62a |
6.34±0.57a | 0.035 |
6.26±0.85a |
6.31±0.83a | 0.811 |
6.16±0.85a |
6.27±0.71a | 0.696 |
|
ΔFPG
(mmol/l) | -5.12±0.86 | -4.50±4.39 | 0.552 | -6.88±2.10 | -7.53±3.53 | 0.382 | -8.04±1.58 | -8.58±2.00 | 0.484 |
|
HbA1c
(%) |
6.43±0.42a |
6.19±0.55a | 0.095 |
6.36±0.66a |
6.44±0.60a | 0.597 |
6.61±0.51a |
6.42±0.56a | 0.397 |
|
ΔHbA1c
(%) | -2.93±0.49 | -3.11±0.56 | 0.225 | -4.61±1.38 | -4.54±0.80 | 0.748 | -6.04±0.72 | -6.85±1.04 | 0.050 |
Comparison of glycaemic outcome
parameters following treatment
Following stratification into the three subgroups,
no significant differences were observed in the baseline glucose
levels (HbA1c and FPG; Table I)
between the two groups and no significant differences were obtained
in the changes in FPG (ΔFPG) after the 1- or 3-month, and HbA1c
(ΔHbA1c) after the 3-month follow-up in either group (all
P>0.05; Table II).
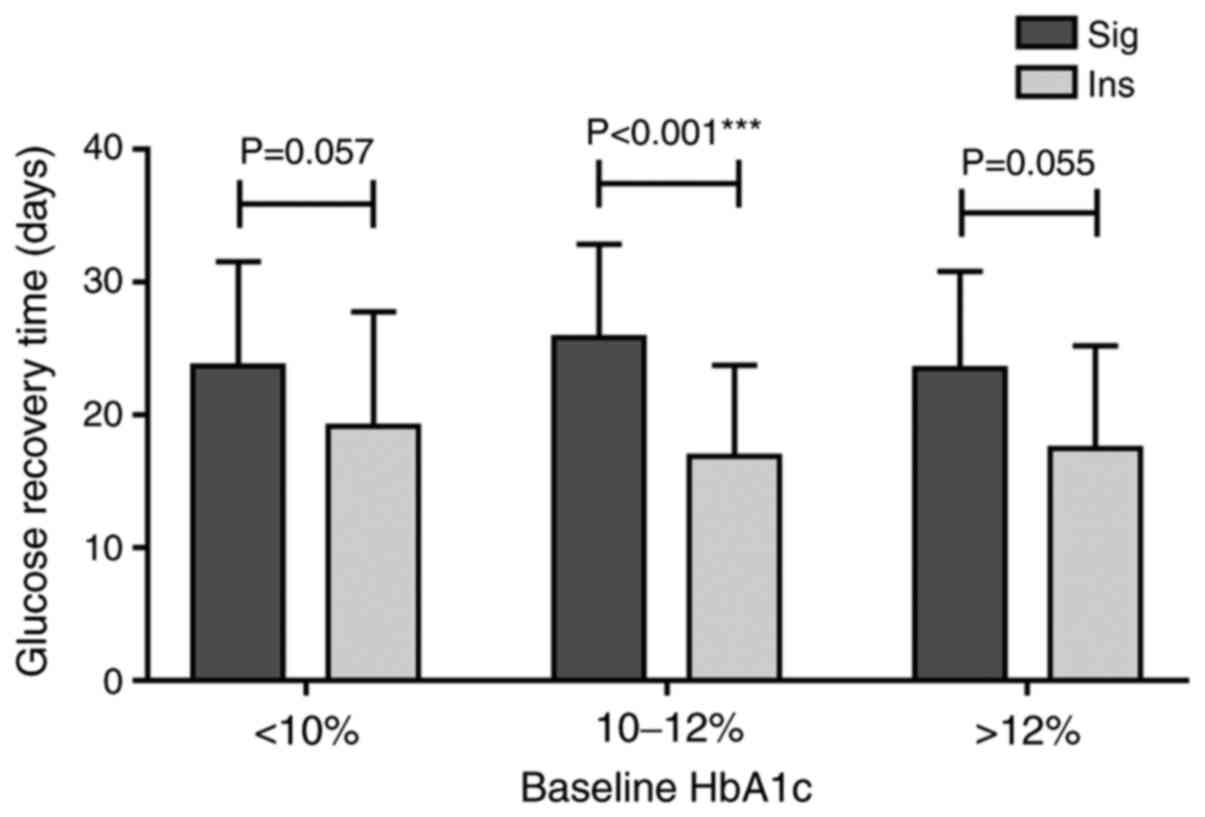
Although both insulin and sitagliptin combined with
metformin were able to reduce hyperglycaemia and achieve similar
glycaemic outcomes, patients in the Ins group were able to achieve
euglycaemia in a significantly shorter time than those in the
10%≥HbA1c≤12% subgroup of the Sig group, with the average time to
achieve euglycaemia being significantly shorter in the Ins than in
the Sig group (P<0.001; Fig 1).
The same trend was observed but no significant difference was
observed in the other two subgroups (P>0.05; Fig. 1).
Adverse events in the two groups
following treatment
No severe hypoglycaemic events or other adverse
events were reported in either group. Significant differences were
observed in the changes in body weight between the Sig and Ins
groups (Table III). Of note,
participants in the Ins group exhibited body weight gain and those
in the Sig group body-weight loss, with significant differences
(Δbody weight and ΔBMI between the two groups) becoming evident
after 1 month. The mean change in BMI from baseline to 1 month
was-0.28±0.61 kg/m2 in the Sig group and 0.26±0.36
kg/m2 in the HbA1c <10% subgroup of the Ins group
(P<0.001; Table III). At the
3-month follow-up, those subgroups exhibited no significant
differences in Δbody weight and ΔBMI between the two groups
(P>0.05; Table III).
 | Table IIIBody weight and BMI changes in the
two groups following treatment. |
Table III
Body weight and BMI changes in the
two groups following treatment.
| | HbA1c<10% | | 10%≤HbA1c≤12% | | HbA1c>12% | |
|---|
|
Time-point/parameter | Sig | Ins | P-value | Sig | Ins | P-value | Sig | Ins | P-value |
|---|
| At baseline | | | | | | | | | |
|
Body weight
(kg) | 73.92±9.12 | 69.71±10.45 | 0.131 | 71.05±9.94 | 71.73±10.72 | 0.784 | 72.16±7.36 | 67.31±6.45 | 0.115 |
|
BMI
(kg/m2) | 25.49±2.70 | 24.78±2.86 | 0.366 | 24.87±2.17 | 24.62±2.74 | 0.685 | 24.52±1.45 | 23.16±1.93 | 0.114 |
| At 1-month
follow-up | | | | | | | | | |
|
Body weight
(kg) |
72.98±8.57a | 70.14±9.87 | 0.277 |
70.67±9.79a | 71.83±10.17 | 0.624 | 73.38±7.33 |
67.57±6.34a | 0.035 |
|
ΔBody weight
(kg) | -1.00±2.00 | 0.75±1.00 | <0.001 | -0.39±0.95 | 0.10±1.09 | 0.051 | -0.13±0.64 | 0.35±0.73 | 0.108 |
|
BMI
(kg/m2) |
25.17±2.48a | 24.94±2.68 | 0.754 |
24.73±2.12a | 24.66±2.57 | 0.902 | 25.82±2.99 |
23.36±1.95a | 0.009 |
|
ΔBMI
(kg/m2) | -0.28±0.61 | 0.26±0.36 | <0.001 | -0.14±0.33 | 0.04±0.38 | 0.043 | -0.04±0.23 | 0.12±0.26 | 0.129 |
| At 3-month
follow-up | | | | | | | | | |
|
Body weight
(kg) |
72.76±8.48a | 69.17±9.81 | 0.243 | 70.57±11.27 | 72.91±10.42 | 0.446 | 73.14±8.28 | 67.64±4.84 | 0.139 |
|
ΔBody weight
(kg) | -0.79±1.16 | -0.10±1.15 | 0.085 | -0.32±2.45 | -0.21±1.30 | 0.845 | -0.28±1.11 | 0.47±1.80 | 0.313 |
|
BMI
(kg/m2) |
25.11±2.83a | 24.65±2.81 | 0.629 | 24.40±2.20 | 25.07±2.59 | 0.335 | 25.99±3.52 | 23.28±2.16 | 0.094 |
|
ΔBMI
(kg/m2) | -0.26±0.37 | -0.03±0.41 | 0.081 | -0.12±0.85 | -0.08±0.45 | 0.832 | -0.07±0.37 | 0.20±0.68 | 0.332 |
In the present study, hypoglycaemia [≤70 mg/dl (3.9
mmol/l)] was reported by 6.25% of patients in the Sig group (4/64)
and 6.73% of those in the Ins group (7/104), with no significant
difference between groups (Table
IV). No severe hypoglycaemia events were reported in the two
groups.
 | Table IVHypoglycaemia events in the two
groups following treatment. |
Table IV
Hypoglycaemia events in the two
groups following treatment.
| Item | Sig (n=64) | Ins (n=104) | P-value |
|---|
| Hypoglycaemic
subjects, % | 6.25 | 6.73 | 0.903 |
| HbA1c <10%,
% | 6.90 | 9.09 | 0.773 |
| 10%≤HbA1c≤12%,
% | 3.70 | 5.45 | 0.729 |
| HbA1c >12%,
% | 1.25 | 7.41 | 0.651 |
Comparative effects of sitagliptin
combined with metformin and insulin therapy on islet β-cell
function
Glucose levels, total insulin response and
amelioration of β-cell function between these two treatment groups
were compared and no significant differences in HOMA-IR, HOMA-β,
Ip/I0, ΔI60/ΔG60, DI or MBCI were identified (all P>0.05;
Table V). The glucose and insulin
responses expressed as AUC (AUC-Ins, AUC-PG and AUC-PG/AUC-Ins) for
a period of 180 min during the OGTT after 3 months of treatment
were not significantly different between the two groups (all
P>0.05; Table V).
 | Table VComparative effects of the two
treatments on islet β-cell function. |
Table V
Comparative effects of the two
treatments on islet β-cell function.
| | HbA1c<10% | | 10%≤HbA1c≤12% | | HbA1c>12% | |
|---|
| Item | Sig | Ins | P-value | Sig | Ins | P-value | Sig | Ins | P-value |
|---|
| HOMA-IR | 3.90±1.35 | 4.92±2.06 | 0.359 | 3.09±1.39 | 5.84±3.29 | 0.068 | 2.47±0.34 | 3.69±1.53 | 0.327 |
| HOMA-β | 72.17±30.90 | 124.18±86.32 | 0.231 | 144.66±116.25 | 178.58±196.41 | 0.662 | 154.20±25.67 | 124.97±130.02 | 0.774 |
| AUC-PG | 40.35±21.64 | 27.21±6.98 | 0.159 | 29.02±8.07 | 33.17±8.27 | 0.380 | 21.69±5.36 | 28.28±3.78 | 0.096 |
| AUC-INS | 99.08±44.20 | 182.18±81.71 | 0.067 | 156.44±103.24 | 213.88±144.59 | 0.336 | 70.45±5.65 | 252.75±229.07 | 0.327 |
|
ΔI60min/ΔG60min | 6.02±6.38 | 8.27±6.54 | 0.567 | 14.59±15.68 | 15.39±19.37 | 0.922 | 2.55±1.02 | 9.65±5.71 | 0.147 |
| Ip/I0 | 3.42±2.68 | 5.86±2.11 | 0.108 | 6.57±3.90 | 6.82±7.24 | 0.927 | 3.37±0.11 | 9.73±8.47 | 0.353 |
| DI | 2.21±3.19 | 1.78±1.23 | 0.749 | 5.18±5.41 | 6.71±14.19 | 0.774 | 1.07±0.56 | 2.59±1.13 | 0.129 |
| MBCI | 6.87±3.88 | 10.56±4.39 | 0.163 | 8.88±6.67 | 11.10±11.31 | 0.620 | 5.83±0.62 | 7.05±2.93 | 0.597 |
Discussion
Glucotoxicity in patients with newly diagnosed T2DM
with severe hyperglycaemia presents considerable challenges for
physicians in the quest to improve the current therapeutic regimen
to meet individualized needs (22,23).
The present study assessed the use of the oral antidiabetic agent
sitagliptin combined with metformin and insulin injection therapy
in patients with newly diagnosed T2DM with severe hyperglycaemia by
evaluating clinical efficacy, including the reduction in glucose
excursions and differences in glucose amelioration and β-cell
function.
Glucotoxicity was assessed and it was indicated that
FPG decreased rapidly in both groups within the first month, even
in severe glucotoxic states (HbA1c>12%). Significant reductions
in FPG and HbA1c from baseline were observed in the two groups and
euglycaemia was maintained after the 3-month follow-up, suggesting
that both treatments were efficacious. However, the average
intervention time to achieve euglycaemia in the Sig group was
significantly longer than that in the 10%≤HbA1c≤12% subgroup of the
Ins group and more participants in the Ins group achieved the
glycaemic control goal earlier than participants in the Sig group.
The results were consistent with those of a previous study
(24).
A patient-centred approach should be used to guide
the choice of pharmacological agents. Considerations include
efficacy, risk of hypoglycaemia, impact on body weight, potential
side effects, cost and individual preferences (4). These possible adverse events may cause
concern among patients with T2DM and physicians, possibly
constituting major barriers to initiating and maintaining adherence
to insulin treatment. The results obtained with the Therapy
Attitude Questionnaire indicated that most patients preferred oral
treatment and refused to receive insulin injections, mainly due to
inconvenience, fear of injection, adverse effects and pain.
The UK Prospective Diabetes Study highlighted the
importance of preserving β-cell function and insulin sensitivity in
the management of T2DM (25,26).
Accumulating evidence suggests that DPP-4 inhibitors preserve
pancreatic β-cell function (25,27).
In the real-world clinical setting, endocrinologists refuse to
perform OGTT for newly diagnosed patients with T2DM and severe
hyperglycaemia, since oral glucose administration to patients with
glucotoxicity may result in diabetic ketoacidosis, hyperosmolar
hyperglycaemic syndrome, dehydration, shock and other emergencies.
Therefore, the assessment of β-cell function and insulin
sensitivity were usually performed at 3 months, when PG control was
stable, instead of the baseline. Therefore, in the present study,
the amelioration of β-cell function was compared between the
sitagliptin combined with metformin and insulin groups and no
significant differences in HOMA-IR, HOMA-β, AUC-Ins, AUC-PG,
AUC-PG/AUC-Ins, Ip/I0, ΔI60/ΔG60, DI and MBCI were identified.
Likewise, concomitant with the glucose-lowering effects of
treatment, no differences in β-cell function or insulin resistance
indexes were observed between the two groups, suggesting that early
sitagliptin combined with metformin therapy in patients with newly
diagnosed T2DM with severe hyperglycaemia leads to similar outcomes
in terms of the recovery of β-cell function to those of insulin
treatment. The present results regarding the effects of sitagliptin
combined with metformin treatment on β-cell function were largely
consistent with those from large trials investigating sitagliptin
as an add-on therapy agent and mixed studies involving multiple
trials (28,29).
Of note, trials involving sitagliptin for
non-alcoholic fatty liver disease (NAFLD) have yielded promising
results. NAFLD may deteriorate abnormal glucose and lipid
metabolism, as well as increase potential risks of cardiovascular,
cerebrovascular and peripheral vascular events for patients with
T2DM (30). Iwasaki et al
(31) demonstrated the efficacy of
sitagliptin in patients with NAFLD and T2DM, with not only the
parameters of diabetes improving, but also those of liver tests,
following treatment with sitagliptin. Recent international
guidelines universally emphasize the need for an individualized
stepwise approach to pharmacotherapy for the management of T2DM and
the choice of pharmacotherapy depends on numerous factors,
including patient attributes and drug characteristics (e.g., route
of administration, drug-drug interactions, safety profile and
cost). As part of the present study, the cost of sitagliptin
combined with metformin and insulin therapy was calculated and
compared and no significant difference was observed in the cost of
these two treatments (results not shown). In addition, in a
real-world study (32), insulin
therapy was associated with an increased risk of adverse events,
such as a high rate of severe hypoglycaemia, glycaemic variability
and weight gain, while sitagliptin was generally well tolerated,
with most adverse events being of mild to moderate intensity and
relatively few patients discontinuing treatment due to these
events. Safety concerns have been raised regarding the potential
risk of rare cases of pancreatitis and pancreatic cancer following
the long-term use of DPP-4 inhibitors. However, no causal link
between sitagliptin and these events has been established to date.
With its convenient once-daily oral regimen, low potential for
pharmacokinetic drug-drug interactions, as well as good efficacy
and safety profiles, sitagliptin remains an important option for
the management of patients with T2D.
Moreover, sitagliptin is approved for use in
combination with insulin. Sitagliptin improves postprandial
glycaemic control by stabilizing the active forms of endogenous
incretins that are released following the ingestion of a meal, and
thus, it is well suited as a supplement to insulin therapy. A
comparison of benefits and drawbacks between sitagliptin combined
with insulin and metformin combined with insulin is provided in
Table SIII. Based on previous
research, sitagliptin is able to stimulate the sarcolemmal
translocation of the glucose transporter-4, in detriment of the
fatty acyl translocase/CD36, and thus improve hyperglycemia,
insulin resistance and GLP-1 levels (33). In addition, combining insulin with
sitagliptin improved glycemic control and ameliorated oxidative
stress and inflammation and thus induced significantly greater
glucose-lowering effects than either drug alone (34).
Increases in body weight associated with specific
antidiabetic agents are an undesired side effect in patients with
T2DM. As indicated in previous studies, sitagliptin is thought to
have a neutral effect on body weight, whereas insulin is associated
with a slight weight gain in overweight and obese individuals with
T2DM (35). However, the present
study indicated that sitagliptin combined with metformin was
associated with a considerable improvement in glycaemic control and
slight weight reduction within the 1- and 3-month follow-up
periods. A possible reason for this may be the role of metformin in
weight loss and another may be the sitagliptin treatment-induced
increase in GLP-1 levels. However, the underlying mechanisms of
weight reduction by sitagliptin and their clinical relevance
require to be further examined.
In the present study, both treatment regimens
featured relatively low risks of hypoglycaemia. In the sitagliptin
group, sitagliptin combined with metformin produced
glucose-lowering effects that occurred in a glucose-dependent
manner, indicating a low risk of hypoglycaemia. In the insulin
group, insulin requirements were able to be estimated based on
weight, with typical doses of 0.4-1.0 U/kg/day, according to the
2019 ADA recommendations (7). In
the present study, low-dose insulin was used at a total dosage of
0.1-0.4 U/kg/day and resulted in a low risk of hypoglycaemia.
One strength of the present study was that it was
performed in a real-world setting. Sitagliptin combined with
metformin was well-tolerated, significantly improved hyperglycaemia
in a clinically challenging population with severe hyperglycaemia
and led to a recovered β-cell function as compared with insulin
treatment. However, there were several limitations to the present
study. First, the follow-up duration was only 3 months. The
longer-term effects of sitagliptin combined with metformin
treatment on β-cell function warrant further investigation.
Furthermore, the present study was a small-sample study, partly due
to the uncertain safety of oral antidiabetic drugs in patients with
glucotoxicity. Further studies with larger samples are required. In
addition, each patient was given an SMBG record book and
hypoglycaemic events were documented by the patients themselves, so
there may be omissions. Another possible drawback was that,
although there was no gender bias in the prevalence of T2DM, most
participants were males. This gender imbalance may reflect better
health care utilization by females (36), leading to reduced outpatient
department use due to severe hyperglycaemia. A similar prevalence
of male patients was also observed in other studies on severe
hyperglycaemia (37).
In conclusion, the present results suggested that
sitagliptin combined with metformin is a well-tolerated and
effective treatment for improving early glycaemic excursions and
β-cell function, with reduced hypoglycaemia and no weight gain.
These results confirmed the efficacy and safety of sitagliptin
combined with metformin in patients with newly diagnosed T2DM,
suggesting that this combination is also beneficial as a first-line
treatment in this patient population. Further larger and
longer-term clinical studies are required to confirm the present
results.
The results of the present and previous studies
suggested that future guidelines regarding initiation therapy in
newly diagnosed patients with T2DM and severe hyperglycaemia should
also include non-insulin alternatives and a patient-centred
approach should be used to guide the choice of pharmacological
agents.
Supplementary Material
Reasons for rejecting insulin therapy.
Results of the Therapy Attitude Questionnaire, which was completed
by 448 patients with T2DM. T2DM, type 2 diabetes mellitus.
Demographic and therapy demand data of
patients who completed the questionnaire.
Baseline demographic and clinical
characteristics in total groups.
Benefits and drawbacks of sitagliptin
combined with insulin and metformin combined with insulin.
Acknowledgements
Not applicable.
Funding
This study was funded by the Award of the First
Affiliated Hospital of Xi'an Jiaotong University, China (grant no.
2013YK20) and the Clinical Research Award of the First Affiliated
Hospital of Xi'an JiaoTong University (Xi'an, China; grant no.
XJTU1AF-CRF-2016-016).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JS and BS conceived the study and participated in
its design. MD, PF, YW, XZ and YH collected the questionnaires and
performed the statistical analyses. JS and MH confirmed the
authenticity of the raw data and drafted the manuscript. JS, MH and
JW interpreted the data. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the First Affiliated Hospital of Xi'an JiaoTong University (Xi'an,
China; no. XJTU1AF2016LSL-048). Informed consent was obtained from
all participants. This study was registered with ClinicalTrials.gov under no. NCT03180281.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pratley RE and Weyer C: The role of
impaired early insulin secretion in the pathogenesis of type II
diabetes mellitus. Diabetologia. 44(929)2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
American Diabetes Association. 15.
Diabetes care in the hospital: Standards of medical care in
diabetes-2020. Diabetes Care. 43 (Suppl 1):S193–S202.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Haak T: Initial combination with
linagliptin and metformin in newly diagnosed type 2 diabetes and
severe hyperglycemia. Adv Ther. 29:1005–1015. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
American Diabetes Association. 5.
Lifestyle management: Standards of medical care in diabetes-2019.
Diabetes Care. 42 (Suppl 1):S46–S60. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Garber AJ, Abrahamson MJ, Barzilay JI,
Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, DeFronzo RA,
Einhorn D, Fonseca VA, et al: Consensus statement by the American
association of clinical endocrinologists and American college of
endocrinology on the comprehensive type 2 diabetes management
algorithm-2017 executive summary. Endocr Pract. 23:207–238.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
American Diabetes Association Approaches
to glycemic treatment. Sec. 7. In standards of medical care in
diabetes-2016. Diabetes Care. 39 (Suppl 1):S52–S59. 2016.
|
|
7
|
American Diabetes Association. 9.
Pharmacologic approaches to glycemic treatment: Standards of
medical care in diabetes-2019. Diabetes Care. 42 (Suppl
1):S90–S102. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pastores SM: ACP journal club. Review:
Intensive insulin therapy does not reduce mortality but increases
severe hypoglycemia in hospitalized patients. Ann Intern Med.
155:JC1–JC12. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nyenwe E: Intensive insulin therapy in
hospitalised patients increases the risk of hypoglycaemia and has
no effect on mortality, infection risk or length of stay. Evid
Based Med. 17:8–9. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Aleali AM, Payami SP, Latifi SM,
Yazdanpanah L, Hesam S and Khajeddin N: Evaluation of psychological
resistance to insulin treatment in type II diabetic patients.
Diabetes Metab Syndr. 12:929–932. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sanchez-Rangel E and Inzucchi SE:
Metformin: Clinical use in type 2 diabetes. Diabetologia.
60:1586–1593. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zinman B, Aroda VR, Buse JB, Cariou B,
Harris SB, Hoff ST, Pedersen KB, Tarp-Johansen MJ and Araki E:
PIONEER 8 Investigators. Efficacy, safety, and tolerability of oral
semaglutide versus placebo added to insulin with or without
metformin in patients with type 2 diabetes: The PIONEER 8 trial.
Diabetes Care. 42:2262–2271. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Scott R, Morgan J, Zimmer Z, Lam RLH,
O'Neill EA, Kaufman KD, Engel SS and Raji A: A randomized clinical
trial of the efficacy and safety of sitagliptin compared with
dapagliflozin in patients with type 2 diabetes mellitus and mild
renal insufficiency: The CompoSIT-R study. Diabetes Obes Metab.
20:2876–2884. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Thornberry NA and Gallwitz B: Mechanism of
action of inhibitors of dipeptidyl-peptidase-4 (DPP-4). Best Pract
Res Clin Endocrinol Metab. 23:479–486. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ahrén B, Landin-Olsson M, Jansson PA,
Svensson M, Holmes D and Schweizer A: Inhibition of dipeptidyl
peptidase-4 reduces glycemia, sustains insulin levels, and reduces
glucagon levels in type 2 diabetes. J Clin Endocrinol Metab.
89:2078–2084. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dicker D: DPP-4 inhibitors: Impact on
glycemic control and cardiovascular risk factors. Diabetes Care. 34
(Suppl 2):S276–S278. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
American Diabetes Association. Standards
of medical care in diabetes-2013. Diabetes Care. 36 (Suppl
1):S11–S66. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Matthews DR, Hosker JP, Rudenski AS,
Naylor BA, Treacher DF and Turner RC: Homeostasis model assessment:
Insulin resistance and beta-cell function from fasting plasma
glucose and insulin concentrations in man. Diabetologia.
28:412–419. 1985.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Burden RL and Faires JD: Numerical
analysis. J R Statist Soc. 71:48–50. 2005.
|
|
20
|
Li Q, Wang L, Xiao L, Wang Z, Wang F, Yu
X, Yan S and Wang Y: Effect of intensive insulin therapy on
first-phase insulin secretion in newly diagnosed type 2 diabetic
patients with a family history of the disease. Exp Ther Med.
9:612–618. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li G, Yang W and Jiang Y: The possibility
of (FINSxFPG)/(PG2h+PG1h-2FPG) being taken as an index of
pancreatic β cell insulin secretion in a population based study.
Chin J Intern Med. 13:191–196. 2000.
|
|
22
|
Kang X, Wang C, Chen D, Lv L, Liu G, Xiao
J, Yang Y, He L, Chen L, Li X, et al: Contributions of basal
glucose and postprandial glucose concentrations to hemoglobin A1c
in the newly diagnosed patients with type 2 diabetes-the
preliminary study. Diabetes Technol Ther. 17:445–448.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Weir GC, Marselli L, Marchetti P, Katsuta
H, Jung MH and Bonner-Weir S: Towards better understanding of the
contributions of overwork and glucotoxicity to the beta-cell
inadequacy of type 2 diabetes. Diabetes Obes Metab. 11 (Suppl
4):82–90. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Defronzo RA, Eldor R and Abdul-Ghani M:
Pathophysiologic approach to therapy in patients with newly
diagnosed type 2 diabetes. Diabetes Care. 36 (Suppl 2):S127–S138.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lyu X, Zhu X, Zhao B, Du L, Chen D, Wang
C, Liu G and Ran X: Effects of dipeptidyl peptidase-4 inhibitors on
beta-cell function and insulin resistance in type 2 diabetes:
Meta-analysis of randomized controlled trials. Sci Rep.
7(44865)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cnop M, Vidal J, Hull RL, Utzschneider KM,
Carr DB, Schraw T, Scherer PE, Boyko EJ, Fujimoto WY and Kahn SE:
Progressive loss of beta-cell function leads to worsening glucose
tolerance in first-degree relatives of subjects with type 2
diabetes. Diabetes Care. 30:677–382. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mu J, Petrov A, Eiermann GJ, Woods J, Zhou
YP, Li Z, Zycband E, Feng Y, Zhu L, Roy RS, et al: Inhibition of
DPP-4 with sitagliptin improves glycemic control and restores islet
cell mass and function in a rodent model of type 2 diabetes. Eur J
Pharmacol. 623:148–154. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Heise T, Larbig M, Patel S, Seck T, Hehnke
U, Woerle HJ and Dugi K: The dipeptidyl peptidase-4 inhibitor
linagliptin lowers postprandial glucose and improves measures of
β-cell function in type 2 diabetes. Diabetes Obes Metab.
16:1036–1039. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Leibowitz G, Cahn A, Bhatt DL, Hirshberg
B, Mosenzon O, Wei C, Jermendy G, Sheu WH, Sendon JL, Im K, et al:
Impact of treatment with saxagliptin on glycaemic stability and
β-cell function in the SAVOR-TIMI 53 study. Diabetes Obes Metab.
17:487–494. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tarantino G, Citro V and Capone D:
Nonalcoholic fatty liver disease: A challenge from mechanisms to
therapy. J Clin Med. 9(15)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Iwasaki T, Yoneda M, Inamori M, Shirakawa
J, Higurashi T, Maeda S, Terauchi Y and Nakajima A: Sitagliptin as
a novel treatment agent for non-alcoholic fatty liver disease
patients with type 2 diabetes mellitus. Hepatogastroenterology.
58:2103–2105. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhou FL, Ye F, Berhanu P, Gupta VE, Gupta
RA, Sung J, Westerbacka J, Bailey TS and Blonde L: Real-world
evidence concerning clinical and economic outcomes of switching to
insulin glargine 300 U/ml vs other basal insulins in patients with
type 2 diabetes using basal insulin. Diabetes Obes Metab.
20:1293–1297. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kelany ME, Hakami TM, Omar AH and Abdallah
MA: Combination of sitagliptin and insulin against type 2 diabetes
mellitus with neuropathy in rats: Neuroprotection and role of
oxidative and inflammation stress. Pharmacology. 98:242–250.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ramírez E, Picatoste B, González-Bris A,
Oteo M, Cruz F, Caro-Vadillo A, Egido J, Tuñón J, Morcillo MA and
Lorenzo Ó: Sitagliptin improved glucose assimilation in detriment
of fatty-acid utilization in experimental type-II diabetes: Role of
GLP-1 isoforms in Glut4 receptor trafficking. Cardiovasc Diabetol.
17(12)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
American Diabetes Association. 4.
Lifestyle management: Standards of medical care in diabetes-2018.
Diabetes Care. 41 (Suppl 1):S38–S50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bertakis KD, Azari R, Helms LJ, Callahan
EJ and Robbins JA: Gender differences in the utilization of health
care services. J Fam Pract. 49:147–452. 2000.PubMed/NCBI
|
|
37
|
Babu A, Mehta A, Guerrero P, Chen Z, Meyer
PM, Koh CK, Roberts R, Schaider J and Fogelfeld L: Safe and simple
emergency department discharge therapy for patients with type 2
diabetes mellitus and severe hyperglycemia. Endocr Pract.
15:696–704. 2009.PubMed/NCBI View Article : Google Scholar
|