Introduction
Previous reports have revealed that thyroid hormone
deficiency may lead to functional injuries, including congenital
hypothyroidism (CH) (1,2). Several developmental processes may be
induced by CH, such as disrupted neurogenesis and abnormal
hippocampal neuron apoptosis (3-5).
It was previously demonstrated that the majority of newborn infants
with CH did not have significant clinical manifestations (6). It is widely accepted that the
hippocampus is involved in cognitive activities in humans.
Accumulating evidence has suggested that hypothyroidism promotes
hippocampal neuron apoptosis (4,7-9).
Moreover, a previous study suggested that perinatal hypothyroidism
affects behavioral development and may lead to a decrease in
spatial learning ability and memory, and these changes are closely
associated with the increasing number of apoptotic neurons in the
hippocampus (10). Therefore, early
diagnosis and timely treatment are crucial for patients with
CH.
MicroRNAs (miRNAs) are highly conserved small RNAs,
20-22 nucleotides in length, which modulate protein function via
directly binding to the 3'-untranslated region (3'-UTR) of their
target mRNAs (11). Recent studies
have demonstrated that changes in miRNA levels are involved in
numerous diseases, including myocardial ischemia (12), inflammation (13), diabetes (14) and CH (4,8,9). In
addition, miRNAs serve important functions in physiological
processes, such as post-transcriptional regulation, cell
proliferation and apoptosis. For example, You et al
(15) found that miR-498 suppressed
gastric cancer cell proliferation, migration and invasion via
targeting B lymphoma Mo-MLV insertion region 1 homolog and
inactivating the AKT pathway. Zhou et al (16) reported that miR-429 attenuated
neuroblastoma cell viability, migration and invasion via the
nuclear factor-κB pathway. Furthermore, it has been reported that
miR-489-3p serves key functions in several diseases. For example,
Chen et al (17)
demonstrated that increased expression of miR-489-3p and miR-630
inhibited oxaliplatin uptake in renal cell carcinoma via targeting
octamer-binding protein 2. Additionally, Kuppa et al
(18) reported that autotaxin
accelerated cancer development via enhancing the expression of
mitogen-activated protein kinase kinase 1 and overriding the role
of miR-489-3p. However, the role of miR-489-3p in neuronal cells
and CH remains elusive. Therefore, the present study was undertaken
to investigate the role of miR-489-3p in CH and elucidate the
potential underlying mechanisms.
Translationally controlled tumor protein 1 (TPT1), a
multifunctional protein, has been evidenced to be highly expressed
in various diseases, including polycystic ovary syndrome (19) and breast cancer (20), and is involved in cell
proliferation, invasion, cell cycle progression and apoptosis
(21). Furthermore, accumulating
evidence has suggested that miR-489-3p inhibits glioblastoma
progression via downregulating TPT1(22).
The aim of the present study was to investigate the
effects of miR-489-3p on hippocampal neuronal cell apoptosis in CH
in vivo and vitro and to determine the role of the
TPT1/Pim-3 signaling pathway in CH, thereby improving our
understanding of the molecular biology of the CH.
Materials and methods
Animals
A total of 50 female pregnant Sprague Dawley rats
(age, 4-6 weeks; weight, 200±5 g) were purchased from the
Experimental Animal Center of Shanghai and kept in a controlled
environment (temperature, 22±1˚C; humidity, 50-60%; 12-h light/dark
cycle). All animal experiments were carried out according to the
guidelines provided by the National Institutes of Health (NIH) for
the Care and Use of Laboratory Animals. The study protocol was
approved by the Animal Ethics Committee of the Experimental Animal
Center of Yancheng Maternal and Child Health Hospital.
Establishment of the CH model
To establish the CH rat model, pregnant rats were
intraperitoneally injected with propylthiouracil (50 mg/day)
starting on gestational day 15 and then daily thereafter until
parturition, in order to generate pups with CH (4,23). For
CH therapy, the newborn rats (12 days old) were anesthetized with
intraperitoneal injection of 2% pentobarbital sodium (40 mg/kg),
and then their skulls were opened as previously described (9). Subsequently, inhibitor control
(5'-CAGUACUUUUGUGUAGUACAA-3'; GenePharma Co., Ltd.), miR-489-3p
inhibitor (5'-GCUGCCGUAUAUGUGAUGUCAC-3'; GenePharma Co., Ltd.),
miR-489-3p inhibitor + control-shRNA (cat. no. sc-108060; Santa
Cruz Biotechnology, Inc.), or miR-489-3p inhibitor + TPT1-shRNA
(cat. no. sc-40675-SH; Santa Cruz Biotechnology, Inc.) were
injected into the left lateral ventricle of the 12-day-old rats
using micro-syringes. The newborn rats were divided into the
following six groups (n=8) as follows: Control; CH; inhibitor
control; miR-489-3p inhibitor; miR-489-3p inhibitor +
control-shRNA; and miR-489-3p inhibitor + TPT1-shRNA groups. At day
21 after birth, the rats (body weight <200 g) were anesthetized
by intraperitoneal injection of pentobarbital sodium (40 mg/kg) and
sacrificed by cervical dislocation (death was verified by cardiac
and respiratory arrest). Brain hippocampal tissues from different
groups were obtained following euthanasia. After anesthesia with
intraperitoneal injection of pentobarbital sodium (40 mg/kg), the
mother rats were also sacrificed by cervical dislocation, and death
was verified by cardiac and respiratory arrest. No rats died during
the experiment. The tests were terminated when the rats had lost
>15% of their body weight (body weight prior to injection).
Primary neuron cultures
After being deeply anaesthetized with 2% sevoflurane
inhalation, neurons were isolated from the hippocampal tissues of 3
normal male rats (postnatal day 0 rat pups from the control group;
weight, 5-6 g) and digested with trypsin for 1 h. Subsequently, the
neurons were harvested and cultivated in DMEM supplemented with 10%
FBS (both from Gibco; Thermo Fisher Scientific, Inc.) for 4-6 h.
Then, neurons were cultured in neural basal medium (Thermo Fisher
Scientific, Inc.) containing 2% B27, 100 U/ml
penicillin/streptomycin and 0.5 mmol/l glutamine (Gibco; Thermo
Fisher Scientific, Inc.) at 37˚C in a humidified 5% CO2
incubator.
Cell transfection
The inhibitor control (5'-CAGUACUUUUGUGUAGUACAA-3';
GenePharma Co., Ltd.), miR-489-3p inhibitor
(5'-GCUGCCGUAUAUGUGAUGUCAC-3'; GenePharma Co., Ltd.), control-shRNA
(cat. no. sc-108060; Santa Cruz Biotechnology, Inc.) and TPT1-shRNA
(cat. no. sc-40675-SH; Santa Cruz Biotechnology, Inc.) were
synthesized by Shanghai GenePharma Co., Ltd. and transfected into
neurons using the Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Following incubation for 48 h, the
transfection efficiency was confirmed using reverse
transcription-quantitative PCR (RT-qPCR) and western blot
analyses.
MTT assay
Cell proliferation was assessed using an MTT assay.
Briefly, neurons (5x104 cells per well) were seeded into
96-well plates in triplicate, transfected with inhibitor control,
miR-489-3p inhibitor, control-shRNA or TPT1-shRNA, and cultured at
37˚C for 48 h. Following transfection, each well was supplemented
with 20 µl MTT solution (Sigma-Aldrich; Merck KGaA) and the cells
were cultured for an additional 4 h. Subsequently, the medium was
discarded, and 100 µl DMSO was added to dissolve the formazan. The
absorbance at 490 nm was determined using a microplate reader
(BioTek Instruments, Inc.).
Cell apoptosis assay
Treated neurons were digested, cleaned and collected
at 4˚C overnight. For the detection of cell apoptosis, the Annexin
V-FITC/propidium iodide apoptosis detection kit (Beyotime Institute
of Biotechnology) was used according to the manufacturer's
protocol. Subsequently, apoptotic cells were identified using the
FACScan flow cytometry system (BD Biosciences), and their number
was measured using the FlowJo 7.6.1 software (BD Biosciences).
Dual luciferase reporter assay
TargetScan bioinformatics software (version 7.2;
http://www.targetscan.org/vert_72/)
was employed to identify the potential targets of miR-489-3p.
Subsequently, the 3'-UTR of TPT1 containing miR-489-3p-binding
sites was sub-cloned into pMIR vectors (Ambion; Thermo Fisher
Scientific, Inc.) to generate the TPT1 wild-type (TPT1-WT) and TPT1
mutant (TPT1-MUT) plasmids. TPT1 was identified as a possible
target of miR-489-3p. A QuikChange Site-Directed Mutagenesis Kit
(Stratagene; Agilent Technologies, Inc.) was applied according to
the manufacturer's instructions to point-mutate the
miR-489-3p-binding domain in the 3'-UTR of TPT1. For luciferase
reporter activity analysis, 293T cells were co-transfected with 1
ng TPT1-WT or 1 ng TPT1-MUT and 100 nM miR-489-3p mimic
(5'-GUGACAUCACAUAUACGGCAGC-3'; Suzhou GenePharma Co., Ltd.) or 100
nM mimic control (5'-UUGUCCGAACGUGUCACGUTT-3'; Suzhou GenePharma
Co., Ltd.) with Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. After 24 h, the luciferase activity was determined
using the Dual-Luciferase Reporter Assay System (Promega
Corporation) and normalized to Renilla luciferase
activity.
Behavioral tests Open-field test
(OFT)
This test was conducted to assess the anxiety and
physical activity of experimental rats (21 days old). OFT was
carried out into a 48x48x36 cm opaque apparatus partitioned into 16
equal squares by white lines, including center squares and a
peripheral area. Mice were left in the center squares for 5 min and
their activity was tracked using a digital camcorder (Ethovision
2.0; Noldus Information Technology). In this test, the following
four parameters were analyzed: Ambulation distance, referring to
the total distance of the grid lines crossed; center square
entries, referring to the frequency of squares crossed with all
four paws; center area duration, referring to the accumulated time
of rat in central square; and rearing, referring to the frequency
of each rat standing on the hind paws. The maze was wiped with 75%
ethanol prior to testing another rat.
Forced swimming test (FST)
This test was conducted to assess depression-like
behavior in rats (21 days old). Briefly, the rats were placed into
a transparent cylinder container (diameter, 22 cm; depth, 40 cm)
filled with water to a height of 30 cm at 25˚C for 6 min.
Subsequently, the swimming duration of each rat was recorded.
During this experiment, immobility time was defined as the time
during which the rats were floating motionless or keeping their
head above water for 4 min. Swimming activity was indicated as a
non-depressive behavior.
RT-qPCR analysis
Total RNA from cultured neurons or hippocampal
tissues was harvested using TRIzol® reagent (Takara
Biotechnology Co., Ltd.) according to the manufacturer's
instructions. Subsequently, RNA was reverse-transcribed into cDNA
using the cDNA Reverse Transcription kit (Takara Biotechnology Co.,
Ltd.) according to the manufacturer's protocol. The miR-489-3p and
TPT1 expression levels were determined using the SYBR Prime Script
RT-PCR Kit (Takara Biotechnology Co., Ltd.). GAPDH and U6 served as
the internal controls for mRNA and miRNA expression, respectively.
The primer sequences used were as follows: GAPDH, forward,
5'-ACGGATTTGGTCGTATTGG-3' and reverse, 5'-TCCCGTTCTCAGCCTTG-3'; U6,
forward, 5'-CCAAGCATCCATGTCTCAA-3' and reverse,
5'-TCCAGATTAACCCCATCC-3'; TPT1, forward,
5'-ATGATTATCTACCGGGACCTC-3' and reverse,
5'-TACATTTTTCCATTTCTAAACCATCC-3'; and miR-489-3p, forward
5'-GTGACATCACATATACGG-3' and reverse 5'-GAACATGTCTGCGTATCTC-3'.
Gene expression were analyzed using the 2-ΔΔCq method
(24).
Western blot analysis
Total proteins were extracted from neurons and
hippocampi using a lysis buffer (Beyotime Institute of
Biotechnology), and protein concentration was measured using a BCA
assay (Solarbio Science and Technology Co., Ltd.). Subsequently,
the protein samples were resolved on 10% SDS-PAGE and transferred
onto PVDF membranes. The membranes were blocked with 5% skimmed
milk in TBS containing 0.1% Tween at room temperature for 1.5 h.
The membranes were then incubated with primary antibodies against
TPT1 (cat. no. 5128; dilution, 1:1,000; Cell Signaling Technology,
Inc.), Pim-3 (cat. no. 4165; dilution, 1:1,000; Cell Signaling
Technology, Inc.), p-Bad (Ser112) (cat. no. 5284; dilution,
1:1,000; Cell Signaling Technology, Inc.), Bad (cat. no. 9292;
dilution, 1:1,000; Cell Signaling Technology, Inc.), Bcl-xL (cat.
no. 2764; dilution, 1:1,000; Cell Signaling Technology, Inc.) and
GAPDH (cat. no. 5174; dilution, 1:1,000; Cell Signaling Technology,
Inc.) at 4˚C overnight. After washing with PBST, the membranes were
incubated with corresponding secondary antibody (cat. no. 7074;
dilution, 1:1,000; Cell Signaling Technology, Inc.) for 2 h at room
temperature. Finally, the protein bands were quantified using an
ECL reagent (Cytiva) according to the manufacturer's
instructions.
Statistical analysis
Data are expressed as the mean ± standard deviation
from three independent experiments. GraphPad Prism 6 (GraphPad
Software, Inc.) and SPSS 21.0 software (IBM Corp.) were employed
for statistical analyses. The variables were analyzed using
Student's t-test or one-way ANOVA followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
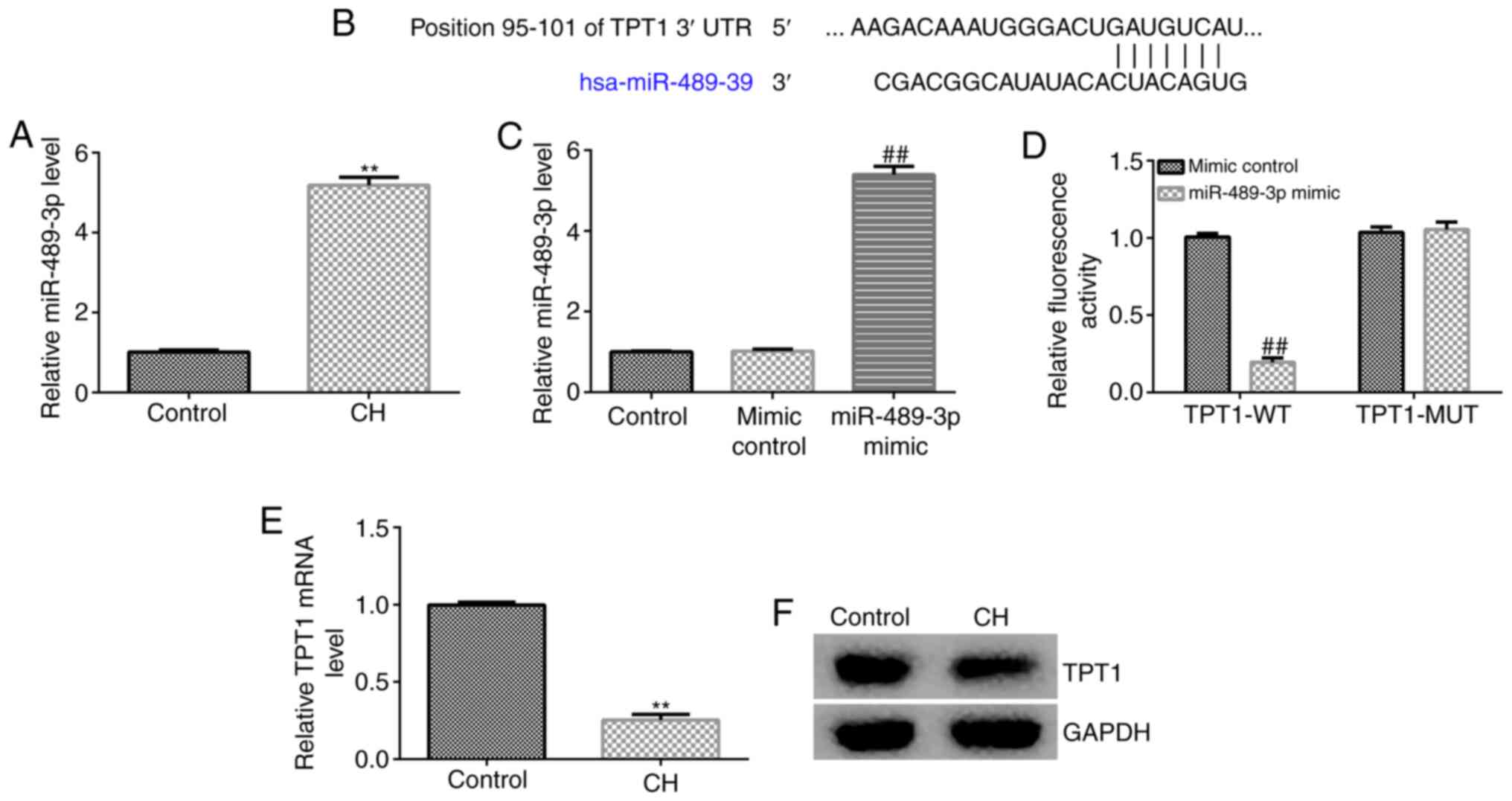
miR-489-3p is upregulated in
hippocampal tissues of CH rats via targeting TPT1
The expression of miR-489-3p in the hippocampal
tissue of CH rats was determined by RT-qPCR analysis. The results
revealed that the miR-489-3p expression levels were higher in the
hippocampal tissues of CH rats compared with those in the control
group (Fig. 1A). To verify the
mechanisms underlying the effects of miR-489-3p, bioinformatics
analysis was performed to predict its target genes. The analysis
identified a potential binding site of miR-489-3p on TPT1 3'-UTR
(Fig. 1B). Previous reports have
indicated that TPT1 is a target gene of miR-489-3p. Therefore, a
dual luciferase reporter assay was performed to verify the
association between miR-489-3p and TPT1. It was first confirmed
that, compared with the mimic control group, miR-489-3p mimic
significantly enhanced miR-489-3p expression in 293T cells
(Fig. 1C). As shown in Fig. 1D, miR-489-3p notably decreased
luciferase activity in the TPT1-WT group, but not in the TPT1-MUT
group, compared with the control group. These findings suggested
that miR-489-3p directly targets TPT1. Furthermore, RT-qPCR and
western blot assays were performed to assess whether miR-489-3p
regulates TPT1 expression. RT-qPCR analysis indicated that TPT1
mRNA was notably reduced in CH rat hippocampal tissues compared
with the control group (Fig. 1E).
Similar findings were observed using western blot assay (Fig. 1F), as the TPT1 protein was found to
be markedly downregulated in the hippocampal tissues of CH rats.
These results indicated that miR-489-3p may be involved in CH
progression via negatively regulating TPT1 expression.
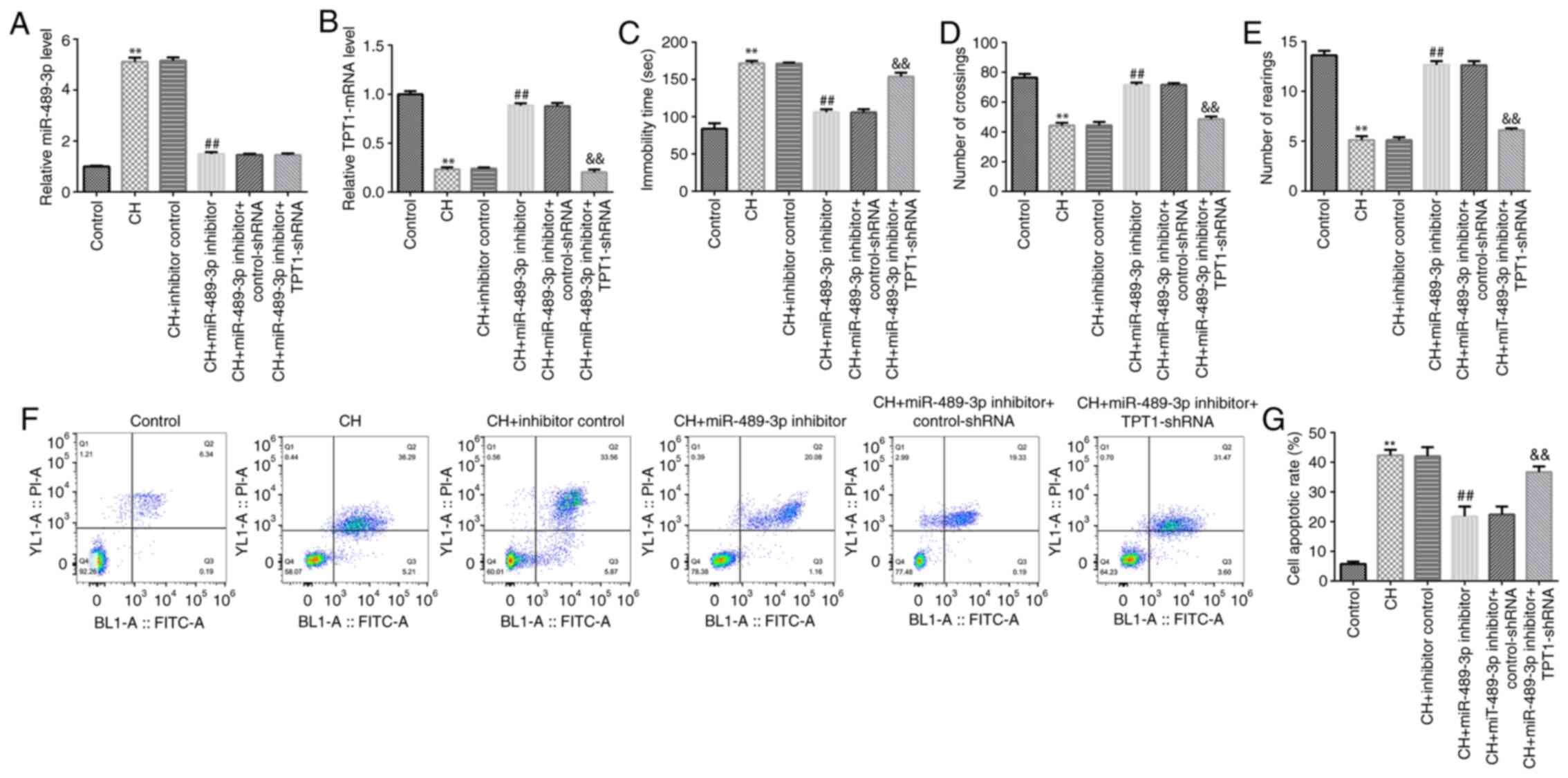
miR-489-3p inhibitor improves CH rat
behavior and reduces CH-mediated neuronal cell apoptosis
To determine whether miR-489-3p is involved in CH,
inhibitor control, miR-489-3p inhibitor, miR-489-3p inhibitor +
control-shRNA or miR-489-3p inhibitor + TPT1-shRNA were injected
into rats to establish CH rat models. The rats were divided into
the following six groups: Control; CH; inhibitor control;
miR-489-3p inhibitor; miR-489-3p inhibitor + control-shRNA; and
miR-489-3p inhibitor + TPT1-shRNA groups. First, RT-qPCR analysis
was performed to determine miR-489-3p and TPT1 expression in rat
hippocampal tissues in different groups. Compared with the control
group, miR-489-3p was upregulated in the model group, while this
increase was reversed following treatment with miR-489-3p inhibitor
(Fig. 2A). Furthermore, the
expression of TPT1 was lower in the model group compared with that
in the control group. In addition, compared to the CH + inhibitor
control group, TPT1 was upregulated in CH rats in the CH +
miR-489-3p inhibitor group, and this effect was significantly
reversed by TPT1-shRNA (Fig.
2B).
Subsequently, behavioral tests were performed to
evaluate the neuronal injury-induced behavioral variation following
treatment with miR-489-3p inhibitor. miR-489-3p inhibitor
attenuated rat anxiety- and depressive-like behavior, as observed
in the FST (Fig. 2C) and OFT
(Fig. 2D and E), respectively. These results indicated
that miR-489-3p inhibitor significantly improved the behavior of CH
rats. Furthermore, cell apoptosis assay was performed to assess the
effect of miR-489-3p inhibitor on neuronal cell apoptosis. The data
demonstrated that miR-489-3p inhibitor suppressed CH-induced
neuronal cell apoptosis, while this effect was reversed by
TPT1-shRNA (Fig. 2F and G). The aforementioned findings suggested
that miR-489-3p inhibitor may relieve CH via regulating neuronal
cell apoptosis.
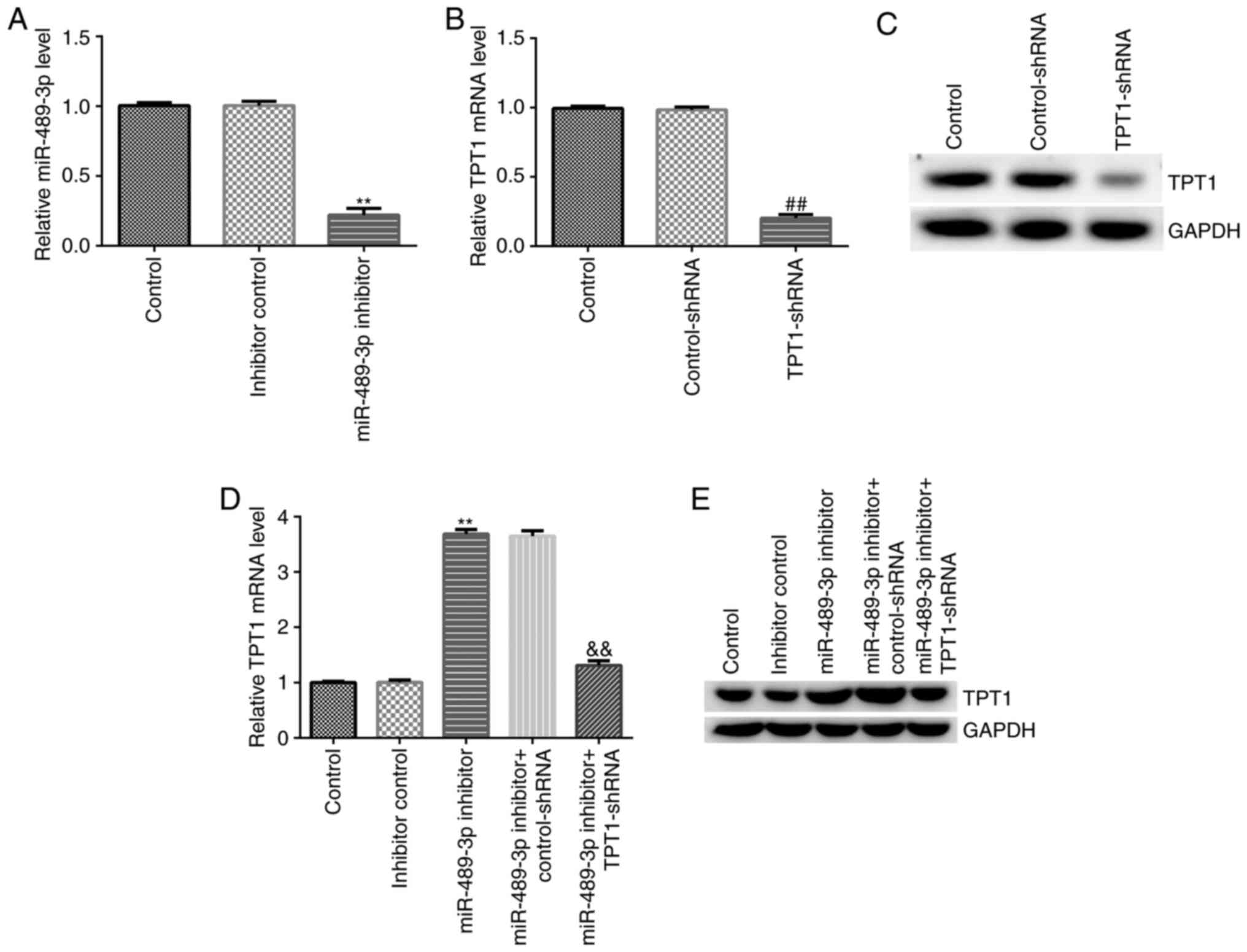
TPT1-shRNA reverses the effects of
miR-489-3p inhibitor on TPT1 expression in neuronal cells
Subsequently, the effect of miR-489-3p inhibitor on
TPT1 expression was explored in neuronal cells by knockdown assay.
miR-489-3p inhibitor, inhibitor control, miR-489-3p inhibitor +
control-shRNA or miR-489-3p inhibitor + TPT1-shRNA were transfected
into neuronal cells, and transfection efficiency was determined
using RT-qPCR and western blot assays. The RT-qPCR results
suggested that miR-489-3p inhibitor successfully downregulated
miR-489-3p expression in neuronal cells compared with the inhibitor
control group (Fig. 3A).
Furthermore, in neuronal cells, transfection with TPT1-shRNA
downregulated TPT1 expression at both the mRNA and protein levels
compared with the control-shRNA group (Fig. 3B and C). In addition, compared with the
inhibitor control group, miR-489-3p inhibitor upregulated TPT1 mRNA
and protein expression levels. On the contrary, the effects of
miR-489-3p inhibitor were reversed following transfection of
neuronal cells with TPT1-shRNA (Fig.
3D and E). Taken together,
these results confirmed that miR-489-3p negatively regulated TPT1
expression in neuronal cells.
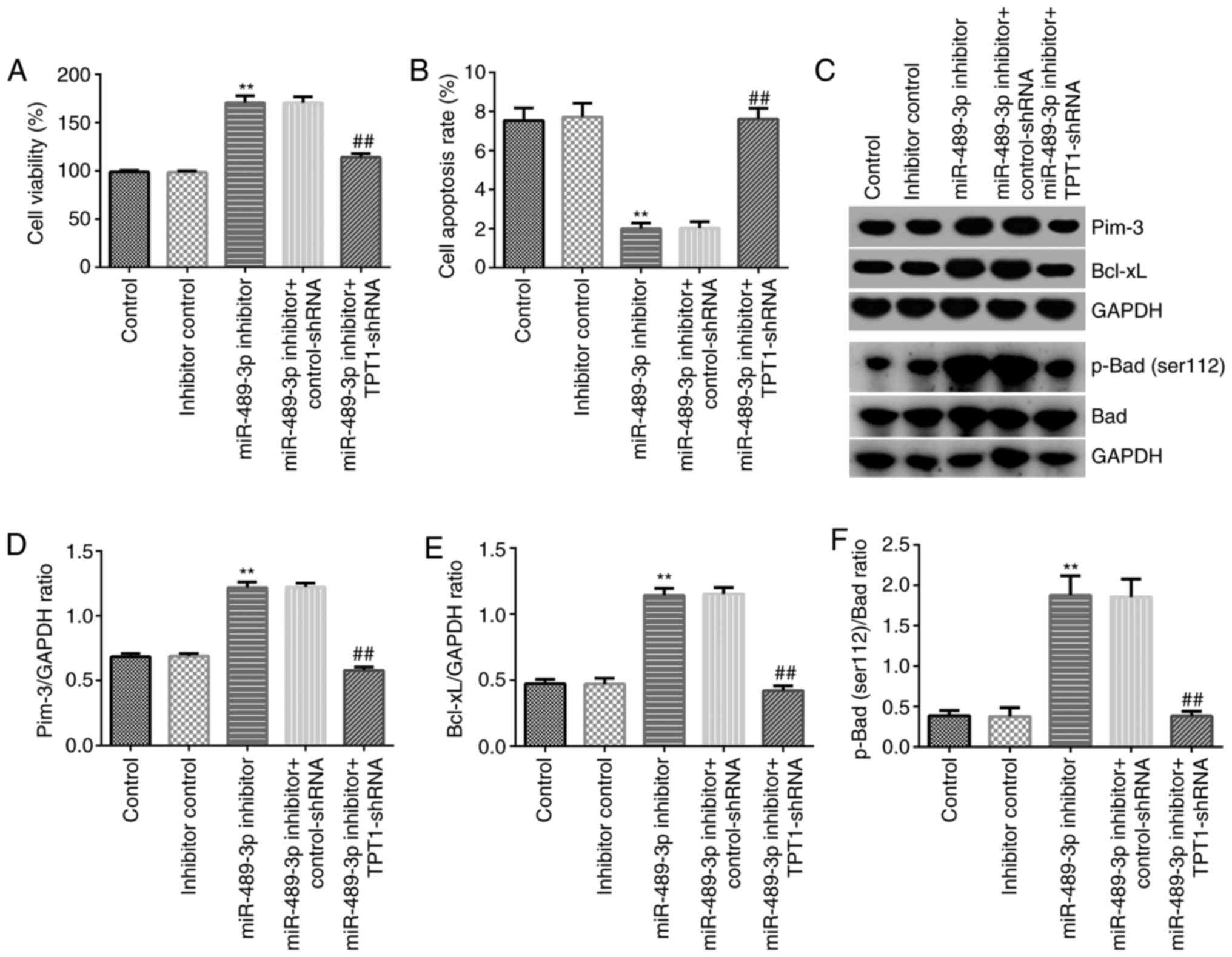
TPT1 silencing abolishes the effect of
miR-489-3p inhibitor on neuronal cell viability and apoptosis via
the TPT1/Pim-3 signaling pathway
To further explore the effect of miR-489-3p on CH,
MTT and flow cytometry assays were conducted to assess neuronal
cell viability and apoptosis, respectively. Neuronal cells were
transfected with miR-489-3p inhibitor, inhibitor control,
miR-489-3p inhibitor + control-shRNA or miR-489-3p inhibitor +
TPT1-shRNA. As shown in Fig. 4A,
miR-489-3p inhibitor markedly increased cell viability compared
with that observed in the inhibitor control group. However,
TPT1-shRNA reversed this effect and significantly inhibited cell
viability (Fig. 4A). In addition,
flow cytometry revealed that miR-489-3p inhibitor significantly
reduced cell apoptosis, while TPT1-shRNA rescued the inhibitory
effects of miR-489-3p inhibitor on neuronal cells (Fig. 4B).
Additionally, the mechanism underlying the effect of
the miR-489-3p inhibitor on the suppression of neuronal cell
apoptosis was investigated. The expression levels of the
apoptosis-related proteins Pim-3, Bcl-xL p-Bad (Ser112) and Bad
were detected using western blot assay. The results demonstrated
that downregulation of miR-489-3p increased the expression levels
of Pim-3, Bcl-xL, p-Bad (Ser112) and the ratio of p-Bad
(Ser112)/Bad in neuronal cells. However, this effect was reversed
following co-transfection with TPT1-shRNA (Fig. 4C-F). These findings suggested that
miR-489-3p may promote CH progression via mediating neuronal cell
apoptosis by targeting TPT1.
Discussion
CH, one of the most common preventable diseases, may
be associated with mental retardation, various developmental
barriers and high anxiety scores (25). Alcigir et al (5) revealed the neuroprotective activity of
cannabinoid receptor 2 against oxidative stress and apoptosis in
rat pups with experimentally induced CH. Li et al (9) reported that miR-124-3p attenuated the
progression of CH via targeting programmed cell death protein 6.
miRNAs are small non-coding molecules that play key roles in
myocardial diseases (26) and
diseases of the nervous system, such as Alzheimer's (27) and Parkinson's disease (28). In addition, miRNAs play critical
roles in the development of tumors, thus serving as diagnostic
markers (29). Several studies have
evidenced that numerous miRNAs are differentially expressed in CH
tissues and may be involved in the development of CH (4,8,9).
However, the expression and associated mechanisms of action of
miR-489-3p in CH remain elusive. Therefore, the present study was
undertaken to investigate the exact role of miR-489-3p in CH.
Propylthiouracil was injected into pregnant rats to
establish a CH model, and the results revealed that miR-489-3p was
markedly upregulated in the hippocampi of CH rats compared with the
control group. Furthermore, the potential targets of miR-489-3p
were predicted using bioinformatics assay. Therefore, a binding
site for miR-489-3p was identified in the TPT1 3'-UTR. The
association between miR-489-3p and TPT1 3'-UTR was confirmed by a
dual luciferase reporter assay. TPT1, a highly conserved protein,
has been reported to be combined with other proteins to alter
protein expression levels (30).
Our findings were consistent with those of previous reports
suggesting that TPT1 was directly targeted by miR-489-3p (22). The present study demonstrated that
TPT1 was downregulated in the hippocampal tissues of CH rats
compared with the control group. The aforementioned observations
indicated that miR-489-3p may be involved in CH via negatively
regulating TPT1 expression.
Dysregulation of miRNA expression has been
associated with several diseases. To identify whether miR-489-3p
affects the progression of CH, inhibitor control, miR-489-3p
inhibitor, miR-489-3p inhibitor + control-shRNA or miR-489-3p
inhibitor + TPT1-shRNA were injected into rats to establish CH
models. As dysregulation of miRNAs may alter behavioral cognition
(31), the present study further
evaluated the effects of miR-489-3p inhibitor and TPT1-shRNA on rat
behavior. Treatment with miR-489-3p inhibitor led to spatial memory
reconstruction, and decreased anxiety- and depressive-like
behaviors. It has been previously demonstrated that several miRNAs
may protect neurons against apoptosis in various diseases (32). The results of the present study
suggested that CH may promote neuronal apoptosis, whereas
transfection with miR-489-3p inhibitor attenuated this effect,
which was eliminated following treatment with TPT1-shRNA. These
results indicated that miR-489-3p inhibitor may relieve CH via
regulating neuronal cell apoptosis.
The effects of miR-489 on cell viability and
apoptosis have been previously reported. Wu et al (33) demonstrated that miR-489 suppressed
multiple myeloma cell proliferation via inhibiting lactate
dehydrogenase A-mediated aerobic glycolysis. Consistent with this
finding, Gao et al (34)
reported that miR-489 may suppress tumor growth and invasion via
targeting histone deacetylase 7 in colorectal cancer. In addition,
it has been demonstrated that TPT1 promotes cell proliferation and
attenuates apoptosis (21).
Hippocampal neuronal cell apoptosis is considered as the key
characteristic of CH (35).
Therefore, the effect of miR-489-3p on hippocampal neuronal cell
apoptosis was explored. The in vitro results indicated that
miR-489-3p inhibitor enhanced neuronal cell viability and inhibited
apoptosis, which was alleviated by TPT1-shRNA. It has been reported
that Pim-3, a member of the proto-oncogene Pim family, regulates
cell viability during cancer development (36). For example, Fan et al
(37) revealed that Pim-3 reduced
cell proliferation and apoptosis in A549 lung adenocarcinoma cells.
Additionally, Pim-3 enhanced melanoma cell migration and invasion
via promoting signal transducer and activator of transcription 3
phosphorylation (38). Therefore,
the expression of apoptosis-related proteins was further assessed
using western blot analysis. The data demonstrated that the
expression levels of Pim-3, p-Bad (Ser112) and Bcl-xL were
significantly increased by miR-489-3p inhibitor in hippocampal
neurons. Furthermore, transfection with TPT1-shRNA abolished the
miR-489-3p inhibitor-mediated upregulation of Pim-3, p-Bad (Ser112)
and Bcl-xL.
A limitation of the present study was that it did
not include a group treated exclusively with sh-TPT1, which would
further improve our understanding of the regulatory association
between miR-489-3p and TPT1.
In conclusion, the results of the present study
suggested that the miR-489-3p inhibitor may relieve CH-associated
neurological damage via regulating the TPT1/Pim-3 signaling
pathway. The findings of the study may contribute to the better
understanding of the molecular biology of CH.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL and YL designed the current study, collected the
data, performed statistical analysis and interpretation, and
prepared the manuscript. YZ collected and analyzed the data. All
authors read and approved the final manuscript. QL and YZ confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
All animal experiments were carried out according to
the guidelines provided by the National Institutes of Health (NIH)
for the Care and Use of Laboratory Animals. The study protocol was
approved by the Animal Ethics Committee of the Experimental Animal
Center of Yancheng Maternal and Child Health Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rousseau JP, Buteau-Poulin A and Kinkead
R: Maternal thyroid hormone deficiency and cardiorespiratory
disorder in rat pups. Exp Neurol. 320(112960)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tiosano D, Pannain S, Vassart G, Parma J,
Gershoni-Baruch R, Mandel H, Lotan R, Zaharan Y, Pery M, Weiss R,
et al: The hypothyroidism in an inbred kindred with congenital
thyroid hormone and glucocorticoid deficiency is due to a mutation
producing a truncated thyrotropin receptor. Thyroid. 9:887–894.
1999.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang L, Blomgren K, Kuhn HG and
Cooper-Kuhn CM: Effects of postnatal thyroid hormone deficiency on
neurogenesis in the juvenile and adult rat. Neurobiol Dis.
34:366–374. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Meng T, Shen S, Li C and Liu X:
MicroRNA-1236-3p/translationally controlled tumor protein (TPT1)
axis participates in congenital hypothyroidism progression by
regulating neuronal apoptosis. Exp Ther Med. 19:459–466.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Alcigir ME, Dogan HO, Atalay VS and Yilmaz
FM: Neuroprotective activity of cannabinoid receptor-2 against
oxidative stress and apoptosis in rat pups having
experimentally-induced congenital hypothyroidism. Dev Neurobiol.
77:1334–1347. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Akin MA, Aydogan S, Gunes T, Artis AS,
Karakukcu M and Kurtoglu S: Changes of red blood cell rheology in
newborns with congenital hypothyroidism during treatment. J Matern
Fetal Neonatal Med. 26:1532–1536. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vetrovoy O, Sarieva K, Lomert E,
Nimiritsky P, Eschenko N, Galkina O, Lyanguzov A, Tyulkova E and
Rybnikova E: Pharmacological HIF1 inhibition eliminates
down-regulation of the pentose phosphate pathway and prevents
neuronal apoptosis in rat hippocampus caused by severe hypoxia. J
Mol Neurosci. 70:635–646. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shao Q, Jiang W and Jin Y: MiR-124 effect
in neurons apoptosis in newborn rat with thyroid hypofunction. Int
J Clin Exp Pathol. 8:14465–14471. 2015.PubMed/NCBI
|
|
9
|
Li W, Song D, Sun Y, Lv Y and Lv J:
microRNA-124-3p inhibits the progression of congenital
hypothyroidism via targeting programmed cell death protein 6. Exp
Ther Med. 15:5001–5006. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huang XW, Yin HM, Ji C, Qin YF, Yang RW
and Zhao ZY: Effects of perinatal hypothyroidism on rat behavior
and its relation with apoptosis of hippocampus neurons. J
Endocrinol Invest. 31:8–15. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Schira-Heinen J, Czapla A, Hendricks M,
Kloetgen A, Wruck W, Adjaye J, Kögler G, Werner Müller H, Stühler K
and Trompeter HI: Functional omics analyses reveal only minor
effects of microRNAs on human somatic stem cell differentiation.
Sci Rep. 10(3284)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Omidkhoda N, Wallace HA, Reiter RJ and
Karimi G: The role of microRNAs on endoplasmic reticulum stress in
myocardial ischemia and cardiac hypertrophy. Pharmacol Res.
150(104516)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wu S, Wang J, Li J and Li F: microRNA-21
aggravates lipopolysaccharide-induced inflammation in MH7A cells
through targeting SNF5. Inflammation. 43:441–454. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sohrabifar N, Ghaderian S, Vakili H,
Ghaedi H, Rouhani B, Jafari H and Heidari L: MicroRNA-copy number
variations in coronary artery disease patients with or without type
2 diabetes mellitus. Arch Physiol Biochem. 1–7. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
You D, Wang D, Liu P, Chu Y, Zhang X, Ding
X, Li X, Mao T, Jing X, Tian Z and Pan Y: MicroRNA-498 inhibits the
proliferation, migration and invasion of gastric cancer through
targeting BMI-1 and suppressing AKT pathway. Hum Cell. 33:366–376.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhou X, Lu H, Li F, Hao X, Han L, Dong Q
and Chen X: MicroRNA-429 inhibits neuroblastoma cell proliferation,
migration and invasion via the NF-κB pathway. Cell Mol Biol Lett.
25(5)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen L, Chen L, Qin Z, Lei J, Ye S, Zeng
K, Wang H, Ying M, Gao J, Zeng S and Yu L: Up-regulation of
miR-489-3p and miR-630 inhibits oxaliplatin uptake in renal cell
carcinoma by targeting OCT2. Acta Pharm Sin B. 9:1008–1020.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kuppa SS, Jia W, Liu S, Nguyen H, Smyth
SS, Mills GB, Dobbin KK, Hardman WJ and Murph MM: Autotaxin
exacerbates tumor progression by enhancing MEK1 and overriding the
function of miR-489-3p. Cancer Lett. 432:84–92. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sun X, Su S, Zhang G, Zhang H and Yu X:
MiR-204 suppresses cell proliferation and promotes apoptosis in
ovarian granulosa cells via targeting TPT1 in polycystic ovary
syndrome. Biochem Cell Biol. 97:554–562. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Neuhäuser K, Küper L, Christiansen H and
Bogdanova N: Assessment of the role of translationally controlled
tumor protein 1 (TPT1/TCTP) in breast cancer susceptibility and ATM
signaling. Clin Transl Radiat Oncol. 15:99–107. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li R, Zhu H, Yang D, Xia J and Zheng Z:
Long noncoding RNA lncBRM promotes proliferation and invasion of
colorectal cancer by sponging miR-204-3p and upregulating TPT1.
Biochem Biophys Res Commun. 508:1259–1263. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang L, Wang Q, Wang F, Zhang X, Zhang L,
Tang Y and Wang S: LncRNA LINC01446 promotes glioblastoma
progression by modulating miR-489-3p/TPT1 axis. Biochem Biophys Res
Commun. 503:1484–1490. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fabian ID, Rosner M, Fabian I,
Vishnevskia-Dai V, Zloto O, Shinderman Maman E, Cohen K, Ellis M,
Lin HY, Hercbergs A, et al: Low thyroid hormone levels improve
survival in murine model for ocular melanoma. Oncotarget.
6:11038–11046. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sidibé el H: Reflections on mental
retardation and congenital hypothyroidism: Effects of trace mineral
deficiencies. Sante. 17:41–50. 2007.PubMed/NCBI(In French).
|
|
26
|
Boen J, Gevaert AB, De Keulenaer GW, Van
Craenenbroeck EM and Segers V: The role of endothelial miRNAs in
myocardial biology and disease. J Mol Cell Cardiol. 138:75–87.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Segaran RC, Chan LY, Wang H, Sethi G and
Tang FR: Neuronal development-related miRNAs as biomarkers for
Alzheimer's disease, Depression, Schizophrenia and Ionizing
Radiation Exposure. Curr Med Chem: Jan 21, 2020 (Epub ahead of
print) doi: 10.2174/092986732766620012112291.
|
|
28
|
Sadlon A, Takousis P, Alexopoulos P,
Evangelou E, Prokopenko I and Perneczky R: miRNAs identify shared
pathways in Alzheimer's and Parkinson's diseases. Trends Mol Med.
25:662–672. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Condrat CE, Thompson DC, Barbu MG, Bugnar
OL, Boboc A, Cretoiu D, Suciu N, Cretoiu SM and Voinea SC: miRNAs
as biomarkers in disease: Latest findings regarding their role in
diagnosis and prognosis. Cells. 9(276)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jian M, Du Q, Zhu D, Mao Z, Wang X, Feng
Y, Xiao Z, Wang H and Zhu Y: Tumor suppressor miR-145-5p sensitizes
prolactinoma to bromocriptine by downregulating TPT1. J Endocrinol
Invest. 42:639–652. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dong J, Liu Y, Zhan Z and Wang X:
MicroRNA-132 is associated with the cognition improvement following
voluntary exercise in SAMP8 mice. Brain Res Bull. 140:80–87.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li LM, Luo FJ and Song X: MicroRNA-370-3p
inhibits cell proliferation and induces chronic myelogenous
leukaemia cell apoptosis by suppressing PDLIM1/Wnt/β-catenin
signalling. Neoplasma. 67:509–518. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wu H, Wang X, Wu T and Yang S: miR-489
suppresses multiple myeloma cells growth through inhibition of
LDHA-mediated aerobic glycolysis. Genes Genomics. 42:291–297.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gao S, Liu H, Hou S, Wu L, Yang Z, Shen J,
Zhou L, Zheng SS and Jiang B: MiR-489 suppresses tumor growth and
invasion by targeting HDAC7 in colorectal cancer. Clin Transl
Oncol. 20:703–712. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
You Y, Tan J, Gong Y, Dai H, Chen H, Xu X,
Yang A, Zhang Y and Bie P: MicroRNA-216b-5p functions as a
tumor-suppressive RNA by targeting TPT1 in pancreatic cancer cells.
J Cancer. 8:2854–2865. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zan T, Piao L, Yang X, Gu Y and Liu B:
Down-regulation of microRNA-124 prevents the development of acute
liver failure through the upregulation of PIM-3. Exp Physiol.
105:108–119. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Fan X, Xie Y, Zhang L, Gao X, Han J, Chen
Y, Yang J and Li S: Effect of Pim-3 down-regulation on
proliferation and apoptosis in lung adenocarcinoma A549 cells. Ann
Clin Lab Sci. 49:770–776. 2019.PubMed/NCBI
|
|
38
|
Liu J, Qu X, Shao L, Hu Y, Yu X, Lan P,
Guo Q, Han Q, Zhang J and Zhang C: Pim-3 enhances melanoma cell
migration and invasion by promoting STAT3 phosphorylation. Cancer
Biol Ther. 19:160–168. 2018.PubMed/NCBI View Article : Google Scholar
|