1. Introduction
Neonatal jaundice is present in >50% of full-term
newborns and it is more serious in late-preterm infants (1,2).
Neonatal jaundice may be classed as physiological or pathological
(3,4) and is principally caused by an increase
in serum bilirubin during the neonatal period, which causes yellow
discoloration of the skin, mucous membranes and sclerae (5). Neonatal hyperbilirubinemia is caused
by an increase in blood bilirubin levels above the normal range. An
increase in the levels of unconjugated bilirubin is the most common
manifestation of neonatal jaundice.
Although there are several treatment schemes
available in the clinical setting, blue light is generally the
preferred choice for preventing the development of bilirubin
encephalopathy or nuclear jaundice caused by excessive accumulation
of unconjugated bilirubin (6,7). A
high serum level of free bilirubin may cause neurotoxicity, but
mildly elevated bilirubin concentrations are beneficial due to
their protective antioxidant role in cell membranes, while markedly
low concentrations may also be harmful (8,9).
However, during the treatment of neonatal hyperbilirubinemia, the
level of free bilirubin in the serum is decreased, which may weaken
the protective effect of bilirubin on the cell membrane, thus
making the cell membrane susceptible to injury and causing cell
apoptosis. This injury may be associated with the oxidative stress
induced by phototherapy, or with the upregulation of the BAX
gene, which promotes apoptosis induced by phototherapy (10).
Moreover, accumulating evidence in recent years has
indicated that phototherapy may elicit a series of adverse
reactions, including DNA damage (11), cancer risk (12) and mortality (13). Since the mechanism underlying
certain phototherapy-induced adverse effects remains unclear,
additional in-depth studies are required. Furthermore, novel
treatments must be designed to prevent serious harm to
newborns.
2. Neonatal hyperbilirubinemia
Etiology
During the neonatal period, several factors,
including preterm birth, exclusive breastfeeding, infection (such
as pulmonary and skin infections), hemolysis (due to blood type
incompatibility), internal bleeding (such as cranial hematoma),
hypoxia, acidosis, hypoglycemia and genetic factors (4), represent common causes of elevated
plasma bilirubin levels (14,15).
Pathogenesis
Aging red blood cells are recognized and
phagocytosed by mononuclear macrophages in the circulation, which
then release hemoglobin. The released hemoglobin is decomposed into
two major components, globin and heme; heme is catalyzed by heme
oxygenase to create biliverdin, which is then reduced to bilirubin
by biliverdin reductase (16). The
bilirubin produced during this process is referred to as
unconjugated bilirubin, and the majority of the bilirubin produced
daily in healthy individuals is derived from this pathway (17). Free bilirubin is first bound to
plasma albumin and then transported to the liver as a
bilirubin-albumin complex. Subsequently, bilirubin is first
separated from albumin and is then taken up by hepatocytes. It is
then combined with ligandins (Y and Z proteins), thus forming the
bilirubin-ligand complex, and is then transported to the smooth
endoplasmic reticulum of the hepatocyte, where it is conjugated
with glucuronic acid to form conjugated bilirubin (1). Conjugated bilirubin, which is
characterized by strong water solubility, is then secreted by
hepatocytes into the bile and drained into the small intestinal
lumen. Under the action of intestinal flora, conjugated bilirubin
is hydrolyzed and reduced to generate bilinogen, the majority of
which is excreted in the feces, while a small quantity of bilinogen
is reabsorbed into the circulation by intestinal mucosal cells. The
majority (~90%) of the reabsorbed bilinogen is discharged into the
intestinal cavity with bile, forming the enterohepatic circulation
of bilinogen (18), whereas only a
small amount (~10%) enters the systemic circulation, passes through
the kidneys and is excreted in the urine (Fig. 1).
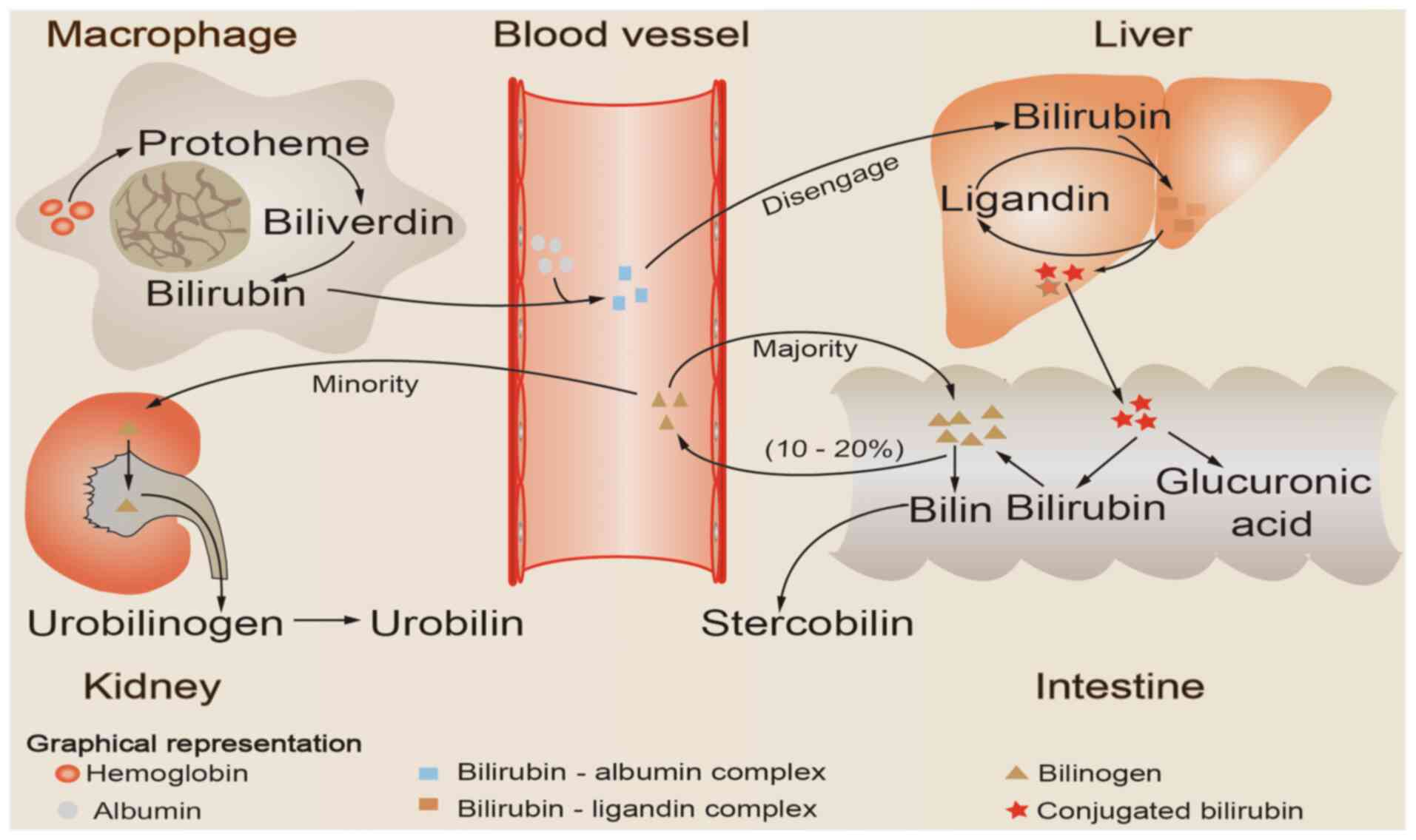 | Figure 1Schematic illustration of bilirubin
metabolism. Aging red blood cells are recognized and phagocytosed
by mononuclear macrophages in the circulation, which then release
hemoglobin. The released hemoglobin is catabolized to produce heme,
which is then reduced and oxidized to bilirubin. Bilirubin formed
by this process first binds to plasma albumin and is then
transported to the liver as a bilirubin-albumin complex. Next,
bilirubin is first separated from albumin and then taken up by
hepatocytes. Subsequently, it is combined with ligandins (Y and Z
proteins) to form a bilirubin-ligand complex, and is then
transported to the smooth endoplasmic reticulum of the hepatocyte,
where is conjugated with glucuronic acid to form conjugated
bilirubin. Conjugated bilirubin is then released into the intestine
with bile, hydrolyzed and reduced to generate bilinogen, the
majority of which is excreted with the feces, while a small
quantity of bilinogen is reabsorbed into the circulation by
intestinal mucosal cells. The majority (~90%) of the reabsorbed
bilinogen is discharged into the intestinal cavity with bile,
forming the enterohepatic circulation of bilinogen, whereas only a
small amount (~10%) enters the systemic circulation, passes through
the kidneys and is excreted in the urine. |
The number of bacteria in the infantile gut is very
low, and the bilirubin that enters the small intestine is not
metabolized by bacteria, but is rather excreted directly in the
stool; however, disruption in bilirubin liver uptake and/or binding
ability, or obstruction of bilirubin excretion (for example, due to
hepatitis or biliary atresia) during the neonatal period lead to an
excess of plasma bilirubin (19,20).
Therefore, increased bilirubin levels caused by abnormal processing
by the liver and/or blocked excretion disrupt the balance between
the production and elimination of bilirubin, which may be key
factors causing neonatal hyperbilirubinemia (21). Furthermore, the pathogenesis of
neonatal hyperbilirubinemia may be associated with infection,
hypoxia leading to decreased glucuronic transferase activity,
congenital deficiency of glucose-6-phosphate dehydrogenase,
mutation of the uridine diphosphate glucuronic acid transferase 1A
gene and perinatal factors, such as preterm birth and/or the use of
oxytocin (22).
Neurotoxicity
As free bilirubin is lipid-soluble, it can cross the
blood-brain barrier (23). However,
the neonatal ability to take up, conjugate and excrete bilirubin is
poor, particularly in premature infants with an immature
blood-brain barrier. When excessive amounts of free bilirubin cross
the blood-brain barrier, they may form deposits in the globus
pallidus, cerebellum, thalamus, hippocampus and other parts of the
brain, thereby causing severe neurotoxicity (24,25),
acute bilirubin encephalopathy (26) or kernicterus, which may lead to
chronic and permanent damage of the nervous system (27-30).
Children with nuclear jaundice often suffer from permanent
neurological sequelae, such as paralysis, epilepsy, deafness,
speech and motor dysfunction (31-34),
potentially even leading to death (35,36).
Treatment
To prevent the serious damage to the nervous system
caused by free serum bilirubin, early detection and timely
intervention are crucial. In clinical practice, liver enzyme
inducers, albumin, intravenous immunoglobulin (37), phototherapy (38) and blood exchange therapy (39) are the treatments mainly used for
neonatal hyperbilirubinemia. Phototherapy is widely used, and blue
light therapy is currently employed to treat neonatal jaundice in
the majority of countries, including China. Blue light is employed
mainly because bilirubin absorbs light, particularly on the blue
part of the spectrum, near the main wavelength peak of 460 nm
(40,41); in addition, compared with green and
ultraviolet (UV) light, bilirubin molecules absorb blue light more
readily (3). Furthermore, although
UV light accounts for a small proportion (~0.3%) of the traditional
blue-green phototherapy, when the skin is exposed to UV light, the
pro-inflammatory pathway of the skin's immune system is activated,
which increases the production of inflammatory factors and triggers
an autoimmune response. In addition, genes can easily mutate upon
exposure to UV light, which may increase the risk of cancer
(42,43). Phototherapy is a simple, convenient,
non-invasive and effective method for scavenging unconjugated
plasma bilirubin. Exchange transfusion therapy, which is generally
performed after failure of phototherapy (44), may lead to severe complications,
such as embolism, sepsis, necrotizing enterocolitis or even death.
Therefore, phototherapy may alleviate the need for blood exchange
therapy, and is often the preferred treatment for neonatal
hyperbilirubinemia, which is mainly caused by increased levels of
unconjugated bilirubin (45).
Upon exposure to light, non-polar unconjugated
bilirubin (Z,Z-bilirubin) in the skin is converted to water-soluble
bilirubin isomers (Fig. 2),
including Z,E-bilirubin, E,Z-bilirubin, E,Z-cyclobilirubin and
E,E-cyclobilirubin; the former two are configurational isomers,
while the latter two are structural isomers (46,47).
Furthermore, certain photooxidation reactions occur (48), which convert free bilirubin into
colorless polar molecules. These water-soluble substances can be
directly excreted from the body in the bile or urine (47,49),
thereby attenuating the unconjugated plasma bilirubin to prevent
the development of bilirubin-induced encephalopathy (48).
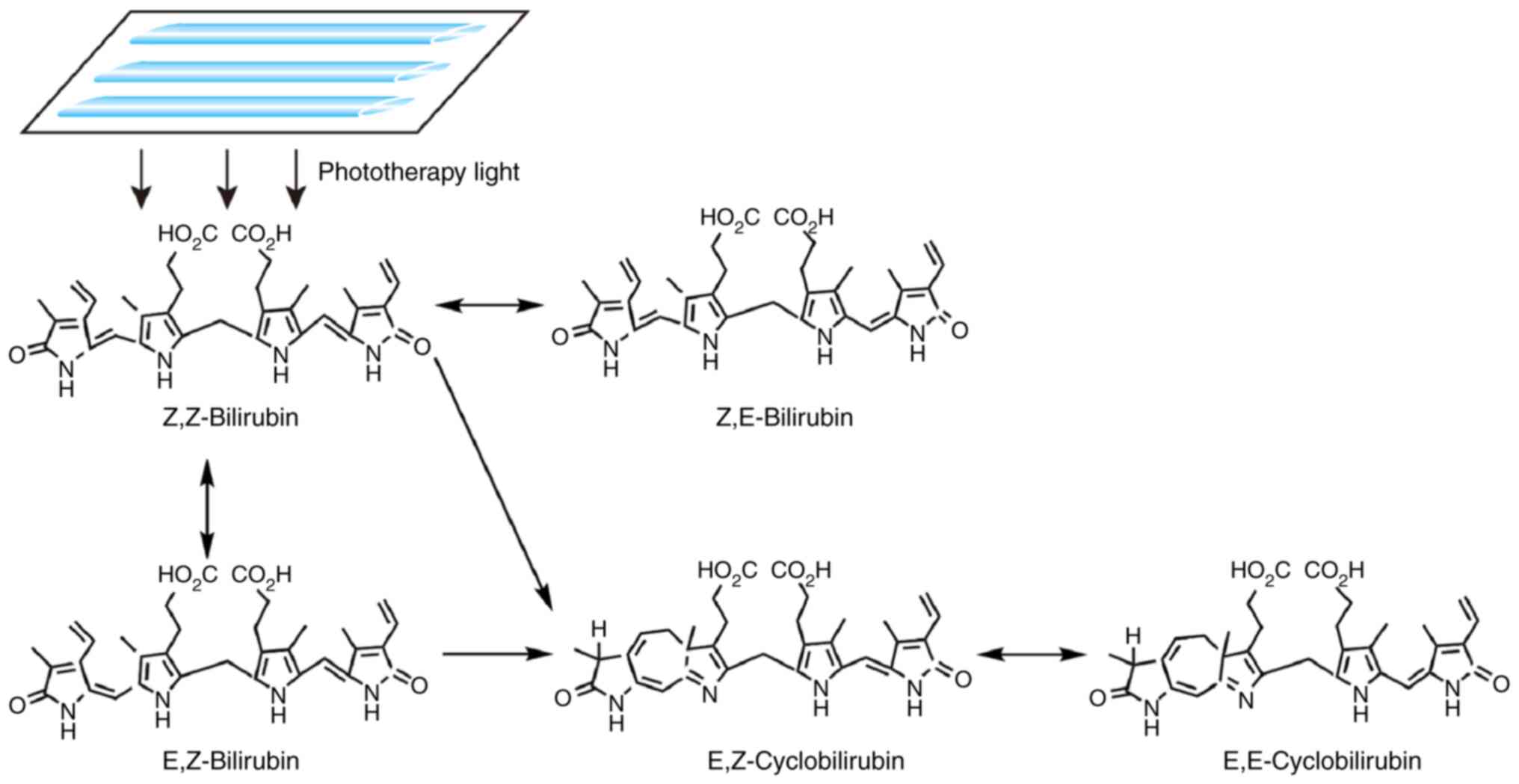 | Figure 2Mechanism of action of phototherapy
for neonatal hyperbilirubinemia. Upon exposure to light, non-polar
unconjugated bilirubin (Z,Z-bilirubin) in the skin is converted
into water-soluble bilirubin isomers, including Z,E-bilirubin,
E,Z-bilirubin, E,E-bilirubin, E,Z-cyclobilirubin and
E,E-cyclobilirubin. |
However, phototherapy used for neonatal
hyperbilirubinemia may compromise the protective barrier of the
skin. A series of studies have demonstrated that phototherapy also
evokes numerous serious adverse reactions in newborns (13,42,50,51).
To implement a reasonable and standard phototherapy strategy in the
clinical setting, the negative effects of phototherapy are
summarized in Tables I and II.
 | Table IAcute adverse reactions to
phototherapy in neonatal hyperbilirubinemia. |
Table I
Acute adverse reactions to
phototherapy in neonatal hyperbilirubinemia.
| Acute adverse
reactions | Underlying
mechanisms | Signal
molecules | Targets | Preventive
measures | (Refs.) |
|---|
| Interference with
mother-infant interaction | - | - | - | More contact
between the infant and the mother should be encouraged during the
phototherapy interval | (52-54) |
| Alteration of
circadian rhythm | Abnormal expression
of circadian rhythm genes; decreased plasma melatonin | Decreased Bmal1
expression; significantly increased Cry1 expression | - | The time of
phototherapy should be adjusted according to the individual
physiological characteristics of each patient | (55-58) |
| Dehydration | Increased bilirubin
decomposition products; alterations in intestinal transmembrane
potential | - | - | Water and
electrolytes should be replenished when necessary | (18,48,59,60) |
| Hypocalcemia | Increased urinary
calcium excretion; decreased plasma melatonin, enhanced bone
calcium absorption, reduce blood calcium levels | - | - | Blood calcium must
be closely monitored and supplementation administered if
necessary | (61-66) |
| Rash | - | - | - | Adjustment of light
exposure time, intensity and distance to avoid skin damage | (67-69) |
| Bronze baby
syndrome | May be associated
with the deposition of bilirubin photoisomers in vivo | - | - | No need for
preventive measures | (47,70,71) |
| Hemolysis | May be associated
with the oxidative stress of phototherapy | - | Erythrocyte
membrane | Intermittent
phototherapy can be used to reduce oxidative stress in non-severe
cases | (41,72-74) |
| Altered
hemodynamics | Increased NO
production and relaxed vascular smooth muscle relaxation through
the cGMP-protein kinase A pathway; plasma ET and NO levels increase
with the prolongation of phototherapy time, and the increase in NO
levels may cause vasodilation | The ratio of NO to
ET goes up. | Blood vessels | Blood pressure
should be closely monitored | (75-79) |
| Patent ductus
arteriosus |
Ca2+-dependent K+
channels are activated to relax the smooth muscle in the wall of
the great vessels | - | Smooth muscle of
the cardiovascular system | Adequate coverage
of the chest during phototherapy | (76,80-85) |
| Retinal injury | During
phototherapy, the absorption of photons by the retina is more
significant. | - | - | Protective
blindfold and appropriate Eye care | (86-89) |
 | Table IILate adverse reactions to
phototherapy in neonatal hyperbilirubinemia. |
Table II
Late adverse reactions to
phototherapy in neonatal hyperbilirubinemia.
| Late adverse
reactions | Underlying
mechanisms | Signal
molecules | Targets | Preventive
measures | (Refs.) |
|---|
| Phototherapy and
allergic diseases | Oxidative stress
induced by phototherapy damages the relevant regulatory genes of
Th2 to Th1 conversion, resulting in disrupted Th2 to Th1
conversion | - | Correlation
regulatory genes converted from Th2 to Th1 | Intermittent
phototherapy applied when possible | (8,42,90-100) |
| DNA damage by
phototherapy | May be associated
with production of oxygen free radicals, BCL2 downregulation
and BAX upregulation | BCL2 gene,
BAX gene | DNA of the
mitochondria and nucleus | Intermittent
phototherapy applied when possible to reduce oxidative stress | (10,11,41,50,100-106) |
| Phototherapy and
tumor | May be associated
with oxidative stress | - | - | Intermittent
phototherapy applied when possible to reduce oxidative stress
associated with tumorigenesis | (12,51,107-112) |
| Phototherapy and
infant mortality | May be associated
with oxidative stress | - | - | Intermittent
phototherapy applied when possible to shorten the duration of light
exposure | (13,113-115) |
3. Adverse reactions of phototherapy for
neonatal hyperbilirubinemia
Acute adverse reactions Interference
with mother-infant interaction
It was previously reported that mothers caressing
infants is a major factor contributing to their psychosomatic
development (52). Current
treatments for jaundice in the newborn usually require separating
the newborn from the mother. Apart from cases with severe jaundice,
phototherapy may generally be interrupted to allow breastfeeding or
parent visitations, so as to enable skin contact and mother-infant
interaction and reduce the anxiety of the parents (53). In addition, phototherapy may
temporarily affect the vision, hearing and alertness of the newborn
(54).
Alteration of circadian rhythm
It has been reported that brain and muscle
ARNT-like1 (Bmal1) and cryptochrome 1 (Cry1) are two major
circadian rhythm genes expressed in neonatal peripheral blood
monocytes (55). Chen et al
(56) reported that, after
phototherapy, the expression of Bmal1 decreased in peripheral
monocytes of neonates, whereas the expression of Cry1 was
significantly enhanced, whereas the plasma melatonin, which is
involved in the regulation of the circadian rhythm (57,58),
was downregulated. In addition, it has been reported that newborns
receiving phototherapy have more frequent crying episodes compared
to those receiving no therapy for clinical jaundice, which may be
associated with changes in the circadian rhythm during neonatal
phototherapy (56).
Dehydration
Dehydration may occur during phototherapy,
particularly in premature infants. By measuring the skin moisture
content of premature infants before and after phototherapy,
Maayan-Metzger et al (59)
found that the mean skin moisture loss increased by 26.4% during
phototherapy, with the most significant loss observed in the elbow
socket, groin and back. The warming effect of conventional
phototherapy increases water loss from the body surface, while
light-emitting diode (LED) phototherapy, which is currently widely
used, causes less water loss. Additionally, phototherapy may cause
excessive bilirubin decomposition, which is excreted through the
intestine, thus stimulating the intestinal wall and altering the
transmembrane potential difference across the epithelium (48), thereby causing diarrhea with
consequent loss of water, sodium and potassium (18,60).
Hypocalcemia
Following neonatal phototherapy, the serum level of
total free calcium is often diminished, leading to hypocalcemia,
the incidence of which is higher among premature infants compared
with that among full-term infants (61). The mechanism underlying the
development of hypocalcemia driven by phototherapy remains unclear,
but it may be associated with increased excretion of calcium in the
urine during phototherapy. It may also be caused by light passing
through the skull, which exerts an inhibitory effect on the pineal
gland, thus leading to decreased melatonin secretion, which in turn
leads to a decrease in cortisol secretion, thus enhancing the
absorption of calcium by bone tissue and causing a decrease in
blood calcium levels (62,63). Therefore, it has been hypothesized
that hypocalcemia may be alleviated by wearing protective headgear
during phototherapy (64,65). Moreover, to avoid severe
complications caused by hypocalcemia, such as convulsions,
laryngeal spasm or apnea (66),
blood calcium levels should be closely monitored during
phototherapy, and calcium supplements should be administered when
necessary.
Rash
Certain newborns develop petechiae and skin rashes
from phototherapy, which gradually fade when phototherapy is
discontinued. Petechiae may be associated with light-induced
thrombocytopenia (67); thus, the
platelet count should be closely monitored during phototherapy. A
small number of infants with cholestatic jaundice develop purpuric
rash and bullous eruptions after phototherapy, which may increase
the total circulating porphyrin levels (68). Since bilirubin may act as a
photosensitizer, children with congenital porphyria or a family
history of porphyria may develop severe blisters due to enhanced
photosensitivity; thus, phototherapy is an absolute
contraindication in these patients (69).
Bronze baby syndrome
The bronze baby syndrome is an irregular
pigmentation resulting from phototherapy in newborn infants with
neonatal jaundice that is mainly noticeable in the skin, mucous
membranes and urine, and generally occurs in neonates with elevated
serum conjugated bilirubin levels (70). However, the reason for this
phenomenon remains unclear; it may be associated with the
accumulation of photoisomers of bilirubin in the body (47) and pigmentation may be caused by the
deposition of biliverdin (71).
Obstructive jaundice and hepatic insufficiency are more likely to
predispose to this syndrome (47).
Hemolysis
The occurrence and aggravation of hemolysis may also
be associated with phototherapy. Since the neonatal antioxidant
capacity is weak and phototherapy may increase oxidative stress in
infants with neonatal jaundice, this may reduce the levels of
antioxidants, such as reduced glutathione and ascorbic acid
(72), leading to an imbalance of
the oxidative and antioxidant defense system in the neonatal body
(73,74), with alterations of the erythrocyte
membrane structure and ensuing hemolysis (41). Phototherapy leads to an increase in
lipid peroxidation of erythrocyte membranes, which can aggravate
hemolysis. Furthermore, the loss of riboflavin resulting from
phototherapy may reduce the activity of erythrocyte glutathione
reductase and induce hemolysis.
Effect of phototherapy on
hemodynamics
Phototherapy may also cause hemodynamic changes
(75). In response to phototherapy,
photoreceptors in blood vessels may promote the generation of
nitric oxide (NO), which can cause vascular smooth muscle
relaxation (76). NO can activate
guanylate cyclase (GC); when GC is activated, its catalytic
intracellular domain can catalyze the decomposition of guanosine
triphosphate, which leads to an increase in cyclic guanosine
monophosphate (cGMP) levels in the cytoplasm and causes vascular
smooth muscle relaxation through the cGMP-protein kinase A pathway
(77). Liu et al (78) reported that phototherapy may affect
the levels of endothelin (ET) and NO in the blood, thus altering
the hemodynamics of premature infants. However, hemodynamic
homeostasis is normally maintained by two important vasoactive
substances, ET and NO. When the NO:ET ratio increases, it causes
vasodilation and low blood pressure; when the arterial blood
pressure decreases, the body adjusts through the baroreceptor
reflex negative feedback loop, which weakens the reflex, thereby
increasing heart rate and blood pressure (79).
Patent ductus arteriosus
Functional closure of the ductus arteriosus usually
occurs immediately after birth. However, the ductus arteriosus may
fail to close in infants who receive phototherapy. A prospective
study investigating the association between phototherapy and patent
ductus arteriosus (80) reported
that phototherapy greatly increased the incidence of patent ductus
arteriosus among children with markedly low birth weight. In a
study by Benders et al (76), >50% of premature infants
receiving phototherapy were diagnosed with patent ductus
arteriosus, the re-opening of the ductus arteriosus may be evoked
by blue light penetrating the chest wall of the premature infant
and causing relaxation of the smooth muscle of the cardiovascular
system (such as the ascending aorta, left pulmonary artery and
ductus arteriosus) by activating the Ca2+-dependent
K+ channel (81). The
patent ductus arteriosus can shunt blood from the aorta to the
pulmonary artery, significantly increasing the blood volume in the
pulmonary circulation and increasing the load on the left heart,
thus leading to complications such as congestive heart failure or
even death (82,83). It has been reported in the
literature that appropriate shielding of the chest during
phototherapy may reduce the incidence of patent ductus arteriosus
induced by phototherapy (84).
However, some researchers disagree with this preventive measure
(85).
Retinal injury
Retinal damage represents another challenge
associated with phototherapy for neonatal jaundice (86). The light-sensitive retinas absorb
photons more readily when exposed to blue light, which is the most
effective at degrading bilirubin. Following continuous or stronger
blue light irradiation, the retinal function degenerates due to a
significant increase in retinal cell death rate (87,88).
However, a previous study reported no significant association
between phototherapy and permanent eye damage in children (89). The association between phototherapy
and retinal injury requires further investigation in the
future.
Late adverse reactions Phototherapy
and allergic diseases
Eosinophilic cationic protein (ECP) is the basic
protein released by eosinophilic granulocytes (90) and serves as an indicator of the
activation of eosinophilic granulocytes, which play a crucial role
in allergic and immune reactions. By measuring the serum ECP level
of neonates with hyperbilirubinemia before and after phototherapy,
a previous study revealed that the ECP level after receiving LED
phototherapy was significantly higher compared with that before
phototherapy [median, 37.5 vs. 16.3 ng/ml (range, 5.4-192.0 vs.
3.6-106.0 ng/ml), respectively; P=0.006], which suggested that
children with hyperbilirubinemia who had received phototherapy were
more likely to develop allergic conditions in the future (91). However, allergic diseases are often
associated with an abnormal immune system function, in which helper
T (Th) cells play a crucial role. Th cells are generally divided
into Th1 and Th2 cells. Th1 cells are mainly involved in delayed
hypersensitivity reactions and cellular immunity, while Th2 cells
are primarily involved in humoral immunity and facilitation of
antibody production by B cells. The immune system normally switches
from a Th2 to a Th1 immune response during the neonatal period
(92).
Glutathione is a known antioxidant that is
hydrophilic and prevents oxidation of water-soluble proteins,
thereby protecting substances in the cytoplasm (93). It was previously reported that
bilirubin has physiological properties that complement those of
glutathione in cellular defense by protecting the cell membrane
(94). By contrast, bilirubin is
lipophilic. Under physiological conditions, bilirubin is a natural
antioxidant and has cytoprotective properties (95). It can prevent lipid peroxidation of
cell membranes caused by hydroxyl radicals, thus maintaining cell
membrane stability and preventing cell apoptosis. It can also
remove reactive oxygen species (ROS) and reactive nitrogen species,
as well as prevent oxidative damage and diseases associated with
oxidative stress. In addition, it can promote the transformation of
a Th2 immune response into a Th1 immune response (96). A previous in vitro study
demonstrated that bilirubin also plays an important role in
inhibiting tumor cell proliferation and promoting tumor cell
apoptosis, which may be associated with the extracellular
signal-regulated kinase pathway (97). When the bilirubin concentration is
excessive, it acts as a pro-oxidant, causing irreversible nerve
damage (98). It was previously
demonstrated that unbound bilirubin may stimulate the production of
endogenous ROS in platelets, thereby inducing platelet apoptosis,
which is a process mediated mainly by the p38 mitogen activated
protein kinase pathway; in addition, bilirubin may damage
mitochondria, inhibiting the energy metabolic process of the cell
and causing cell apoptosis (99).
Low levels of bilirubin may also be harmful (8). Phototherapy may disrupt the protective
role of the cell membrane by lowering serum bilirubin, causing
production of free oxygen radicals; it may also damage lymphocyte
DNA and cause a disturbance of the skin cytokine environment, which
disrupts the Th2 to Th1 conversion, thus leading to allergic
diseases such as asthma and allergic rhinitis (42,100).
DNA damage by phototherapy
Whether phototherapy can damage DNA remains a
controversial topic. Although it has been suggested that
phototherapy cannot trigger DNA damage (101), other studies have suggested that
phototherapy may damage the DNA of neonatal peripheral blood
lymphocytes (11,50). Under the influence of visible light,
cellular DNA may be mutated and the DNA chain may be broken, with
consequent sister chromatid exchange. Previous studies have
proposed that phototherapy can damage the DNA of neonates and
induce apoptosis of peripheral blood lymphocytes, while neonatal
hyperbilirubinemia per se does not cause DNA damage. Aycicek
et al (100) concluded that
endogenous mononuclear leukocyte DNA was damaged in infants with
jaundice treated with traditional and intensive phototherapy. Tatli
et al (102) also confirmed
that phototherapy was associated with DNA damage in neonates. The
main mechanism through which phototherapy removes unconjugated
bilirubin from the body is by isomerization. As the newborn's
antioxidant mechanisms are immature and the skin has low
antioxidant capacity, blue light directly induces the generation of
free oxygen radicals, which may cause DNA damage in the
mitochondria and nucleus of the cell (41,103).
p53 is a tumor suppressor gene that is mainly
involved in maintaining normal cell growth and inhibiting malignant
proliferation, and participates in the process of DNA replication
and repair (104). If the repair
mechanism fails, p53 induces cell apoptosis to prevent malignant
transformation (105). Under
normal conditions, the content of p53 in cells is markedly low and
has a short half-life, but its content can significantly increase
during cell proliferation. After exploring the genotoxicity and
apoptosis induced by phototherapy in peripheral blood lymphocytes
of full-term infants, Yahia et al (106) reported that the DNA damage markers
(tail DNA% and tail moment) and the p53 levels of newborns with
hyperbilirubinemia subjected to phototherapy were significantly
higher compared with those prior to phototherapy (all P<0.0001).
Furthermore, it has been proposed that the DNA damage and cell
apoptosis caused by bilirubin and phototherapy in newborns with
hyperbilirubinemia may be associated with the downregulation of the
BCL-2 gene (which can inhibit apoptosis) and the
upregulation of the BAX gene (which can promote apoptosis)
(10).
Phototherapy and tumors
Phototherapy may increase the risk of cancer in
children (107). A large
epidemiological study found that newborns treated with phototherapy
may be at a higher risk of developing cancer later in life
(51). During an 11-year follow-up
of ~800,000 infants after birth, the study suggested that children
treated with phototherapy were more than twice as likely to develop
solid tumors after 4 years of age compared with those who did not
receive phototherapy, but the potential cancer risk of bilirubin
could not be ruled out (108).
Another large retrospective cohort study reported that children who
received phototherapy had a higher incidence of cancer compared
with those who did not receive phototherapy, with a ratio of ~1.4
(P=0.01); in particular, the incidence of non-lymphocytic leukemia
increased significantly (12).
However, the association was weakened due to the large and
uncontrollable confounders during the study period. A previous
retrospective cohort study on the risk of skin cancer from neonatal
jaundice phototherapy did not confirm that phototherapy
significantly increases the risk of skin cancer (109). However, due to the confounding
variables and limited statistical power (follow-up was interrupted
for some members of the cohort as they emigrated), this possibility
cannot be excluded. In addition, a previous study demonstrated that
phototherapy can increase the incidence of neonatal nevi, and
melanocytic nevi are the most important risk factor for the
occurrence of skin melanoma (110). Therefore, when children receive
phototherapy, the nevi should be closely monitored to prevent the
development of melanoma. It has also been suggested that neonatal
phototherapy may increase the risk of hemangioma in infants, which
may be associated with oxidative stress caused by phototherapy,
which can damage vascular endothelial cells and stimulate the
formation of new blood vessels (111). The increased risk of cancer in
children treated with phototherapy may be associated with
hyperbilirubinemia per se, phototherapy or a combination of
the two (112).
Phototherapy and infant mortality
Phototherapy is widely used in the treatment of
neonatal jaundice, and it can indeed prevent the neurotoxicity
caused by hyperbilirubinemia (113). In an attempt to elucidate the
effect of phototherapy on infant mortality, Morris et al
(13) conducted a large multicenter
randomized controlled trial, and the results demonstrated that
infant mortality did not differ significantly between infants with
jaundice who received aggressive or conservative phototherapy [24
vs. 23%, respectively; 95% confidence interval (CI): 0.90-1.22],
and the possibility of neural development disorders was reduced by
26 and 30%, respectively (95% CI, 0.74-0.99). However, for infants
whose birth weight ranged between 501 and 750 g, the mortality rate
was higher in the aggressive phototherapy group compared with that
in the conservative group (39 vs. 34%, respectively). Therefore,
attention should be paid to the increasing mortality rate possibly
associated with phototherapy. Prolonged duration of phototherapy
(114) and increased oxidative
stress (115) may be relevant
factors associated with increased mortality.
Other adverse reactions
When the jaundiced newborn is treated with blue
phototherapy, apart from the areas protected by the black blindfold
and the diaper, all other areas are exposed to illumination. As a
result, neonates with jaundice treated with blue light often
experience alterations in body temperature (60). Since the wavelength of absorption of
blue light by riboflavin is similar to that of bilirubin, both
riboflavin and bilirubin will decompose at the same time when a
newborn with jaundice receives blue light therapy, leading to the
loss of riboflavin in the body. The riboflavin deficiency will
reduce the synthesis of active riboflavin adenine dinucleotide,
impair the hydrogen delivery of erythrocytes, reduce glutathione
reductase and weaken the activity of erythrocyte glutathione
reductase (116), thus aggravating
hemolysis. In addition, the risk of secondary intestinal
obstruction may increase after phototherapy. The velocity of blood
flow in the upper mesenteric artery at the end of the diastolic
period is accelerated post-phototherapy, indicating that the
mesenteric vascular smooth muscle may undergo diastolic changes
during phototherapy, leading to mesenteric ischemia, which may be
one of the causes of intestinal obstruction in premature infants
(117). A retrospective study
reported that phototherapy is associated with the incidence of
intestinal obstruction in infants with markedly low birth weight
(118). In addition, blue light
treatment during the neonatal period may also be associated with
subsequent diseases, such as diabetes, autism and epilepsy
(119,120).
4. Preventive measures for adverse reactions
caused by phototherapy of neonatal hyperbilirubinemia
Intermittent phototherapy may be used in non-severe
cases, which may increase the contact time between the infant and
the mother. The efficacy of intermittent phototherapy is comparable
to that of continuous phototherapy, in addition to increasing the
interaction time of the mother and the infant, intermittent
phototherapy may also reduce the adverse reactions caused by
continuous phototherapy (3),
including oxidative stress, hemolysis, allergic reactions, DNA
damage, cancer and increased mortality. Due to the effect of
phototherapy on the circadian rhythm of children, the time of light
exposure can be adjusted according to the physiological
characteristics of the infants. The intensity and distance of
illumination should also be adjusted, and the duration of
illumination should be controlled. Close monitoring of the
temperature of both the infant and the incubator is also important.
To prevent the loss of water and electrolytes (such as
Na+, K+ and Ca2+) caused by
phototherapy, water and electrolytes must be replenished when
necessary. To address the loss of riboflavin caused by
phototherapy, vitamin B2 supplementation should be routine
practice. Appropriate light exposure time, intensity and distance
should be used to avoid skin damage. As regards the rash caused by
phototherapy, no treatment is generally required. If the skin
becomes cracked or infected, active skin care, such as disinfection
with iodophor, should be administered. Blood pressure should be
closely monitored to prevent hemodynamic changes caused by
phototherapy. Adequate coverage of the chest during phototherapy
can reduce the incidence of patent ductus arteriosus. To prevent
direct exposure of the eye to blue light and minimize the risk of
damage to the retina, an appropriate blindfold should be applied
and secured in place during phototherapy. However, as the incidence
of conjunctivitis is increased among children receiving
phototherapy who wear eye masks over prolonged periods of time,
thorough eye care, such as cleaning eye secretions and surrounding
skin with normal saline cotton balls, must be applied.
5. Conclusions
It is generally acknowledged that blue phototherapy
is a simple, effective and safe treatment for neonatal
hyperbilirubinemia. However, the possible adverse reactions of
phototherapy, including hemolysis, allergic disease, DNA damage and
cancer, must be taken into consideration. To avoid serious harm to
the infant's health, it is necessary to standardize, rationalize
and normalize phototherapy in the clinical setting. More in-depth
studies are also required to shed light on the mechanism underlying
adverse reactions to phototherapy in infants, and to explore and
optimize novel therapeutic schemes in the future. Due to the
potentially toxic effects of blue light therapy, green LED light
may also be used to reduce total serum bilirubin levels (121), as it may cause fewer adverse
reactions compared with blue light. Therefore, green light therapy
must be more extensively investigated in the future.
Acknowledgements
Not applicable.
Funding
The present study was partly supported by grants
from the National Natural Science Foundation of China (grant nos.
31700736 and 81872412), the Hubei Province Scientific and
Technological Research Project (grant no. D20201306), the Hubei
Province Health Research Project (WJ2019-01), the Leading Talent
Program of Yangtze Talent Project, Hubei Medical Youth Tip-Top
Talent, Leading Talent Program of Yangtze Talent Project, and the
College Students Innovative Entrepreneurial Training Program in
Yangtze University (grant nos. 2018184, 2019372 and Yz2020334).
Availability of data and materials
Not applicable.
Authors' contributions
JW, GG, AL and XW wrote the manuscript. JW and WQC
performed the literature search and produced the figures and
tables. XW conceived and reviewed the original manuscript. JW and
XW confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mitra S and Rennie J: Neonatal jaundice:
Aetiology, diagnosis and treatment. Br J Hosp Med (Lond).
78:699–704. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Greco C, Arnolda G, Boo NY, Iskander IF,
Okolo AA, Rohsiswatmo R, Shapiro SM, Watchko J, Wennberg RP,
Tiribelli C and Coda Zabetta CD: Neonatal jaundice in low- and
middle-income countries: Lessons and future directions from the
2015 don ostrow trieste yellow retreat. Neonatology. 110:172–180.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhou S, Wu X, Ma A, Zhang M and Liu Y:
Analysis of therapeutic effect of intermittent and continuous
phototherapy on neonatal hemolytic jaundice. Exp Ther Med.
17:4007–4012. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Deshmukh J, Deshmukh M and Patole S:
Probiotics for the management of neonatal hyperbilirubinemia: A
systematic review of randomized controlled trials. J Matern Fetal
Neonatal Med. 32:154–163. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mojtahedi SY, Izadi A, Seirafi G, Khedmat
L and Tavakolizadeh R: Risk factors associated with neonatal
jaundice: A cross-sectional study from Iran. Open Access Maced J
Med Sci. 6:1387–1393. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jiao Y, Jin Y, Meng H and Wen M: An
analysis on treatment effect of blue light phototherapy combined
with Bifico in treating neonatal hemolytic jaundice. Exp Ther Med.
16:1360–1364. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ebbesen F, Hansen TWR and Maisels MJ:
Update on phototherapy in jaundiced neonates. Curr Pediatr Rev.
13:176–180. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Arnold C, Tyson JE, Pedroza C, Carlo WA,
Stevenson DK, Wong R, Dempsey A, Khan A, Fonseca R, Wyckoff M, et
al: Cycled phototherapy dose-finding study for extremely
low-birth-weight infants: A randomized clinical trial. JAMA
Pediatr. 174:649–656. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Roll EB, Christensen T and Gederaas OA:
Effects of bilirubin and phototherapy on osmotic fragility and
haematoporphyrin-induced photohaemolysis of normal erythrocytes and
spherocytes. Acta Paediatr. 94:1443–1447. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
El-Abdin MYZ, El-Salam MA, Ibrhim MY,
Koraa SSM and Mahmoud E: Phototherapy and DNA changes in full term
neonates with hyperbilirubinemia. Egypt J Med Hum Genet. 13:29–35.
2012.
|
|
11
|
Mesbah-Namin SA, Shahidi M and Nakhshab M:
An increased genotoxic risk in lymphocytes from
phototherapy-treated hyperbilirubinemic neonates. Iran Biomed J.
21:182–189. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Newman TB, Wickremasinghe AC, Walsh EM,
Grimes BA, McCulloch CE and Kuzniewicz MW: Retrospective cohort
study of phototherapy and childhood cancer in northern california.
Pediatrics. 137(e20151354)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Morris BH, Oh W, Tyson JE, Stevenson DK,
Phelps DL, O'Shea TM, McDavid GE, Perritt RL, Van Meurs KP, Vohr
BR, et al: Aggressive vs conservative phototherapy for infants with
extremely low birth weight. N Engl J Med. 359:1885–1896.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Das S and van Landeghem FKH:
Clinicopathological spectrum of bilirubin
encephalopathy/kernicterus. Diagnostics (Basel).
9(24)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Alizadeh Taheri P, Sadeghi M and Sajjadian
N: Severe neonatal hyperbilirubinemia leading to exchange
transfusion. Med J Islam Repub Iran. 28(64)2014.PubMed/NCBI
|
|
16
|
Ansong-Assoku B and Ankola PA: Neonatal
jaundice. In: StatPearls [Internet]. StatPearls Publishing,
Treasure Island (FL), 2020. PMID: 30422525.
|
|
17
|
Kalakonda A, Jenkins BA and John S:
Physiology, bilirubin. In: StatPearls [Internet]. StatPearls
Publishing, Treasure Island (FL), 2020.
|
|
18
|
Stokowski LA: Fundamentals of phototherapy
for neonatal jaundice. Adv Neonatal Care. 6:303–312.
2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tabrizi SO, Mirghafourvand M, Dost AJ,
Mohammad-Alizadeh-Charandabi S, Javadzadeh Y and Seyedi R: Effect
of metoclopramide administration to mothers on neonatal bilirubin
and maternal prolactin: A randomized, controlled, clinical trial.
World J Pediatr. 15:135–142. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Olusanya BO, Kaplan M and Hansen TWR:
Neonatal hyperbilirubinaemia: A global perspective. Lancet Child
Adolesc Health. 2:610–620. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hansen TWR, Wong RJ and Stevenson DK:
Molecular physiology and pathophysiology of bilirubin handling by
the blood, liver, intestine, and brain in the newborn. Physiol Rev.
100:1291–1346. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhou YY, Lee LY, Ng SY, Hia CP, Low KT,
Chong YS and Goh DL: UGT1A1 haplotype mutation among Asians in
Singapore. Neonatology. 96:150–155. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Allen D: Neonatal jaundice. Nurs Child
Young People. 28(11)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Amin SB and Wang H: Unbound unconjugated
hyperbilirubinemia is associated with central apnea in premature
infants. J Pediatr. 166:571–575. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Spoorthi SM, Dandinavar SF, Ratageri VH
and Wari PK: Prediction of neonatal hyperbilirubinemia using 1st
day serum bilirubin levels. Indian J Pediatr. 86:174–176.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mir SE, van der Geest BAM and Been JV:
Management of neonatal jaundice in low- and lower-middle-income
countries. BMJ Paediatr Open. 3(e000408)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Alkén J, Håkansson S, Ekéus C, Gustafson P
and Norman M: Rates of extreme neonatal hyperbilirubinemia and
kernicterus in children and adherence to national guidelines for
screening, diagnosis, and treatment in Sweden. JAMA Netw Open.
2(e190858)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Aprillia Z, Gayatri D and Waluyanti FT:
Sensitivity, specificity, and accuracy of kramer examination of
neonatal jaundice: Comparison with total bilirubin serum. Compr
Child Adolesc Nurs. 40:88–94. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
van der Schoor LWE, van Faassen M, Kema I,
Baptist DH, Olthuis AJ, Jonker JW, Verkade HJ, Groen H and Hulzebos
CV: Blue LED phototherapy in preterm infants: Effects on an
oxidative marker of DNA damage. Arch Dis Child Fetal Neonatal Ed.
105:628–633. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Karimzadeh P, Fallahi M, Kazemian M,
Taslimi Taleghani N, Nouripour S and Radfar M: Bilirubin induced
encephalopathy. Iran J Child Neurol. 14:7–19. 2020.PubMed/NCBI
|
|
31
|
Rennie JM, Beer J and Upton M: Learning
from claims: Hyperbilirubinaemia and kernicterus. Arch Dis Child
Fetal Neonatal Ed. 104:F202–F204. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Le Pichon JB, Riordan SM, Watchko J and
Shapiro SM: The Neurological sequelae of neonatal
hyperbilirubinemia: Definitions, diagnosis and treatment of the
kernicterus spectrum disorders (KSDs). Curr Pediatr Rev.
13:199–209. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee BK, Le Ray I, Sun JY, Wikman A, Reilly
M and Johansson S: Haemolytic and nonhaemolytic neonatal jaundice
have different risk factor profiles. Acta Paediatr. 105:1444–1450.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Dean E: Neonatal jaundice. Nurs Stand.
30(15)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Slusher TM, Zamora TG, Appiah D, Stanke
JU, Strand MA, Lee BW, Richardson SB, Keating EM, Siddappa AM and
Olusanya BO: Burden of severe neonatal jaundice: A systematic
review and meta-analysis. BMJ Paediatr Open.
1(e000105)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bahr TM, Christensen RD, Agarwal AM,
George TI and Bhutani VK: The neonatal acute bilirubin
encephalopathy registry (NABER): Background, aims, and protocol.
Neonatology. 115:242–246. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zheng J, Wei C, Zhao M and Zhao D:
Phototherapy is associated with the decrease in serum globulin
levels in neonatal hyperbilirubinemia. Biomed Rep. 10:63–69.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Woodgate P and Jardine LA: Neonatal
jaundice: Phototherapy. BMJ Clin Evid. 2015(0319)2015.PubMed/NCBI
|
|
39
|
Slusher TM, Zipursky A and Bhutani VK: A
global need for affordable neonatal jaundice technologies. Semin
Perinatol. 35:185–191. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cai A, Qi S, Su Z, Shen H, Yang Y, Cai W
and Dai Y: A pilot metabolic profiling study of patients with
neonatal jaundice and response to phototherapy. Clin Transl Sci.
9:216–220. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kale Y, Aydemir O, Celik Ü, Kavurt S,
Isikoglu S, Bas AY and Demirel N: Effects of phototherapy using
different light sources on oxidant and antioxidant status of
neonates with jaundice. Early Hum Dev. 89:957–960. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tham EH, Loo EXL, Goh A, Teoh OH, Yap F,
Tan KH, Godfrey KM, Van Bever H, Lee BW, Chong YS and Shek LP:
Phototherapy for neonatal hyperbilirubinemia and childhood eczema,
rhinitis and wheeze. Pediatr Neonatol. 60:28–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Slominski AT, Zmijewski MA, Plonka PM,
Szaflarski JP and Paus R: How UV light touches the brain and
endocrine system through skin, and why. Endocrinology.
159:1992–2007. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Waterham M, Bhatia R, Donath S, Molesworth
C, Tan K and Stewart M: Phototherapy in transport for neonates with
unconjugated hyperbilirubinaemia. J Paediatr Child Health.
52:67–71. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Donneborg ML, Vandborg PK, Hansen BM,
Rodrigo-Domingo M and Ebbesen F: Double versus single intensive
phototherapy with LEDs in treatment of neonatal hyperbilirubinemia.
J Perinatol. 38:154–158. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ebbesen F, Madsen PH, Vandborg PK,
Jakobsen LH, Trydal T and Vreman HJ: Bilirubin isomer distribution
in jaundiced neonates during phototherapy with LED light centered
at 497 nm (turquoise) vs 459 nm (blue). Pediatr Res. 80:511–515.
2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Itoh S, Okada H, Kuboi T and Kusaka T:
Phototherapy for neonatal hyperbilirubinemia. Pediatr Int.
59:959–966. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Faulhaber FRS, Procianoy RS and Silveira
RC: Side effects of phototherapy on neonates. Am J Perinatol.
36:252–257. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Altuntas N, Dogan OC and Kislal FM: Effect
of phototherapy on neutrophil VCS parameters and white blood cells.
J Coll Physicians Surg Pak. 29:453–455. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ramy N, Ghany EA, Alsharany W, Nada A,
Darwish RK, Rabie WA and Aly H: Jaundice, phototherapy and DNA
damage in full-term neonates. J Perinatol. 36:132–136.
2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wickremasinghe AC, Kuzniewicz MW, Grimes
BA, McCulloch CE and Newman TB: Neonatal phototherapy and infantile
cancer. Pediatrics. 137(e20151353)2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Abedi F, Mirbagher Ajorpaz N, Esalatmanesh
S, Rahemi Z, Gilasi HR, Kafaei Atrian M and Hosseinian M: The
effect of tactile-kinesthetic stimulation on growth indices of
healthy neonates. J Bodyw Mov Ther. 22:308–312. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Dalili H, Sheikhi S, Shariat M and
Haghnazarian E: Effects of baby massage on neonatal jaundice in
healthy Iranian infants: A pilot study. Infant Behav Dev. 42:22–26.
2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ju SH and Lin CH: The effect of moderate
non-hemolytic jaundice and phototherapy on newborn behavior.
Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 32:31–41.
1991.PubMed/NCBI
|
|
55
|
Kavcic P, Rojc B, Dolenc-Groselj L,
Claustrat B, Fujs K and Poljak M: The impact of sleep deprivation
and nighttime light exposure on clock gene expression in humans.
Croat Med J. 52:594–603. 2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Chen A, Du L, Xu Y, Chen L and Wu Y: The
effect of blue light exposure on the expression of circadian genes:
Bmal1 and cryptochrome 1 in peripheral blood mononuclear cells of
jaundiced neonates. Pediatr Res. 58:1180–1184. 2005.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Yeganeh Salehpour M, Mollica A, Momtaz S,
Sanadgol N and Farzaei MH: Melatonin and multiple sclerosis: From
plausible neuropharmacological mechanisms of action to experimental
and clinical evidence. Clin Drug Investig. 39:607–624.
2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Tarocco A, Caroccia N, Morciano G,
Wieckowski MR, Ancora G, Garani G and Pinton P: Melatonin as a
master regulator of cell death and inflammation: molecular
mechanisms and clinical implications for newborn care. Cell Death
Dis. 10(317)2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Maayan-Metzger A, Yosipovitch G, Hadad E
and Sirota L: Transepidermal water loss and skin hydration in
preterm infants during phototherapy. Am J Perinatol. 18:393–396.
2001.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Kumar P, Murki S, Malik GK, Chawla D,
Deorari AK, Karthi N, Subramanian S, Sravanthi J, Gaddam P and
Singh SN: Light emitting diodes versus compact fluorescent tubes
for phototherapy in neonatal jaundice: A multi center randomized
controlled trial. Indian Pediatr. 47:131–137. 2010.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Asghar I, Khan IA and Hassan F: Effect of
head covering on phototherapy induced hypocalcemia in term neonates
with hyperbilirubinemia: A randomised controlled study. J Neonatal
Perinatal Med: October 10, 2020 (Online ahead of print).
|
|
62
|
Khan M, Malik KA and Bai R: Hypocalcemia
in jaundiced neonates receiving phototherapy. Pak J Med Sci.
32:1449–1452. 2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Gheshmi AN, Naderi S, Homayrani E and
Safari B: Prevalence of hypocalcemia after phototherapy among
neonates who underwent phototherapy in Koodakan Hospital in Bandar
Abbas in 2013. Electron Physician. 7:1387–1390. 2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Barekatain B, Badiea Z and Hoseini N: The
effect of head covering in prevention of phototherapy-induced
hypocalcemia in icterus newborns with gestational age less than 35
weeks. Adv Biomed Res. 5(176)2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Kargar M, Jamshidi Z, Beheshtipour N,
Pishva N and Jamali M: Effect of head covering on
phototherapy-induced hypocalcaemia in icterus newborns; a
randomized controlled trial. Int J Community Based Nurs Midwifery.
2:121–126. 2014.PubMed/NCBI
|
|
66
|
Shahriarpanah S, Haji Ebrahim Tehrani F,
Davati A and Ansari I: Effect of phototherapy on serum level of
calcium, magnesium and vitamin D in infants with
hyperbilirubinemia. Iran J Pathol. 13:357–362. 2018.PubMed/NCBI
|
|
67
|
Khera S and Gupta R: Incidence of
thrombocytopenia following phototherapy in hyperbilirubinemic
neonates. Med J Armed Forces India. 67:329–332. 2011.PubMed/NCBI View Article : Google Scholar
|
|
68
|
LaRusso J, Wilson J and Ceilley R:
Phototherapy-induced purpuric eruption in a neonate. J Clin Aesthet
Dermatol. 8:46–48. 2015.PubMed/NCBI
|
|
69
|
Jeffrey Maisels M: Phototherapy and skin
rashes. Pediatr Dermatol. 30:636–637. 2013.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Le TN and Reese J: Bronze baby syndrome. J
Pediatr. 188:301–301.e1. 2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Kar S, Mohankar A and Krishnan A: Bronze
baby syndrome. Indian Pediatr. 50(624)2013.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Ayyappan S, Philip S, Bharathy N, Ramesh
V, Kumar CN, Swathi S and Kumar AA: Antioxidant status in neonatal
jaundice before and after phototherapy. J Pharm Bioallied Sci. 7
(Suppl 1):S16–S21. 2015.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Demirel G, Uras N, Celik IH, Aksoy HT,
Oguz SS, Erdeve O, Erel O and Dilmen U: Comparison of total
oxidant/antioxidant status in unconjugated hyperbilirubinemia of
newborn before and after conventional and LED phototherapy: A
prospective randomized controlled trial. Clin Invest Med.
33:335–341. 2010.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Suzen S, Gurer-Orhan H and Saso L:
Detection of reactive oxygen and nitrogen species by electron
paramagnetic resonance (EPR) technique. Molecules.
22(181)2017.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Uhrikova Z, Zibolen M, Javorka K,
Chladekova L and Javorka M: Hyperbilirubinemia and phototherapy in
newborns: Effects on cardiac autonomic control. Early Hum Dev.
91:351–356. 2015.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Benders MJ, Van Bel F and Van de Bor M:
Cardiac output and ductal reopening during phototherapy in preterm
infants. Acta Paediatr. 88:1014–1019. 1999.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Behrendt D and Ganz P: Endothelial
function. From vascular biology to clinical applications. Am J
Cardiol. 90:40L–48L. 2002.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Liu GS, Wu H, Wu BQ, Huang RZ, Zhao LH and
Wen Y: Effect of phototherapy on blood endothelin and nitric oxide
levels: Clinical significance in preterm infants. World J Pediatr.
4:31–35. 2008.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Benders MJ, van Bel F and van de Bor M:
Haemodynamic consequences of phototherapy in term infants. Eur J
Pediatr. 158:323–328. 1999.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Barefield ES, Dwyer MD and Cassady G:
Association of patent ductus arteriosus and phototherapy in infants
weighing less than 1000 g. J Perinatol. 13:376–380. 1993.PubMed/NCBI
|
|
81
|
Batenburg WW, Kappers MH, Eikmann MJ,
Ramzan SN, de Vries R and Danser AH: Light-induced vs
bradykinin-induced relaxation of coronary arteries: Do
S-nitrosothiols act as endothelium-derived hyperpolarizing factors?
J Hypertens. 27:1631–1640. 2009.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Bhola K, Foster JP and Osborn DA: Chest
shielding for prevention of a haemodynamically significant patent
ductus arteriosus in preterm infants receiving phototherapy.
Cochrane Database Syst Rev. (CD009816)2015.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Hamrick SE and Hansmann G: Patent ductus
arteriosus of the preterm infant. Pediatrics. 125:1020–1030.
2010.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Mannan J and Amin SB: Meta-analysis of the
effect of chest shielding on preventing patent ductus arteriosus in
premature infants. Am J Perinatol. 34:359–363. 2017.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Travadi J, Simmer K, Ramsay J, Doherty D
and Hagan R: Patent ductus arteriosus in extremely preterm infants
receiving phototherapy: Does shielding the chest make a difference?
A randomized, controlled trial. Acta Paediatr. 95:1418–1423.
2006.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Nakanishi-Ueda T, Majima HJ, Watanabe K,
Ueda T, Indo HP, Suenaga S, Hisamitsu T, Ozawa T, Yasuhara H and
Koide R: Blue LED light exposure develops intracellular reactive
oxygen species, lipid peroxidation, and subsequent cellular
injuries in cultured bovine retinal pigment epithelial cells. Free
Radic Res. 47:774–780. 2013.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Grimm C, Wenzel A, Williams T, Rol P,
Hafezi F and Remé C: Rhodopsin-mediated blue-light damage to the
rat retina: Effect of photoreversal of bleaching. Invest Ophthalmol
Vis Sci. 42:497–505. 2001.PubMed/NCBI
|
|
88
|
Chen P, Lai Z, Wu Y, Xu L, Cai X, Qiu J,
Yang P, Yang M, Zhou P, Zhuang J, et al: Retinal neuron is more
sensitive to blue light-induced damage than glia cell due to DNA
double-strand breaks. Cells. 8(68)2019.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Kara S, Yalniz-Akkaya Z, Yeniaras A, Örnek
F and Bilge YD: Ocular findings on follow-up in children who
received phototherapy for neonatal jaundice. J Chin Med Assoc.
80:729–732. 2017.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Lin L, Chen Z, Tang X, Dai F, Wei J and
Sun G: 5-Oxo-ETE from nasal epithelial cells upregulates eosinophil
cation protein by eosinophils in nasal polyps in vitro. Int Arch
Allergy Immunol. 177:107–115. 2018.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Beken S, Aydin B, Zenciroğğlu A, Dilli D,
Özkan E, Dursun A and Okumus N: The effects of phototherapy on
eosinophil and eosinophilic cationic protein in newborns with
hyperbilirubinemia. Fetal Pediatr Pathol. 33:151–156.
2014.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Aspberg S, Dahlquist G, Kahan T and Källén
B: Confirmed association between neonatal phototherapy or neonatal
icterus and risk of childhood asthma. Pediatr Allergy Immunol.
21:e733–e739. 2010.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Magi S, Piccirillo S, Amoroso S and
Lariccia V: Excitatory amino acid transporters (EAATs): Glutamate
transport and beyond. Int J Mol Sci. 20(5674)2019.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Sedlak TW, Saleh M, Higginson DS, Paul BD,
Juluri KR and Snyder SH: Bilirubin and glutathione have
complementary antioxidant and cytoprotective roles. Proc Natl Acad
Sci USA. 106:5171–5176. 2009.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Karadag F, Sengul CB, Enli Y, Karakulah K,
Alacam H, Kaptanoglu B, Kalkanci O and Herken H: Relationship
between serum bilirubin levels and metabolic syndrome in patients
with schizophrenia spectrum disorders. Clin Psychopharmacol
Neurosci. 15:153–162. 2017.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Gloria-Bottini F and Bottini E: Is there a
role of early neonatal events in susceptibility to allergy? Int J
Biomed Sci. 6:8–12. 2010.PubMed/NCBI
|
|
97
|
Ollinger R, Kogler P, Troppmair J, Hermann
M, Wurm M, Drasche A, Königsrainer I, Amberger A, Weiss H, Ofner D,
et al: Bilirubin inhibits tumor cell growth via activation of ERK.
Cell Cycle. 6:3078–3085. 2007.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Rawat V, Bortolussi G, Gazzin S, Tiribelli
C and Muro AF: Bilirubin-induced oxidative stress leads to DNA
damage in the cerebellum of hyperbilirubinemic neonatal mice and
activates DNA double-strand break repair pathways in human cells.
Oxid Med Cell Longev. 2018(1801243)2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
NaveenKumar SK, Thushara RM, Sundaram MS,
Hemshekhar M, Paul M, Thirunavukkarasu C, Basappa Nagaraju G,
Raghavan SC, Girish KS, et al: Unconjugated bilirubin exerts
pro-apoptotic effect on platelets via p38-MAPK activation. Sci Rep.
5(15045)2015.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Aycicek A, Kocyigit A, Erel O and Senturk
H: Phototherapy causes DNA damage in peripheral mononuclear
leukocytes in term infants. J Pediatr (Rio J). 84:141–146.
2008.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Gómez-Meda BC, Barros-Hernández A,
Guzmán-Bárcenas J, Lemus-Varela Mde L, Zamora-Perez AL,
Torres-Mendoza BM, Gallegos-Arreola MP, Armendáriz-Borunda J and
Zúñiga-González GM: Effects of blue light phototherapy on DNA
integrity in preterm newborns. J Photochem Photobiol B.
141:283–287. 2014.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Tatli MM, Minnet C, Kocyigit A and Karadag
A: Phototherapy increases DNA damage in lymphocytes of
hyperbilirubinemic neonates. Mutat Res. 654:93–95. 2008.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Bulut O, Erek A and Duruyen S: Effects of
hyperbilirubinemia on markers of genotoxicity and total oxidant and
antioxidant status in newborns. Drug Chem Toxicol. 1–5.
2020.PubMed/NCBI View Article : Google Scholar : (Online ahead of
print).
|
|
104
|
Hong B, van den Heuvel AP, Prabhu VV,
Zhang S and El-Deiry WS: Targeting tumor suppressor p53 for cancer
therapy: Strategies, challenges and opportunities. Curr Drug
Targets. 15:80–89. 2014.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Kanapathipillai M: Treating p53 mutant
aggregation-associated cancer. Cancers (Basel).
10(154)2018.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Yahia S, Shabaan A, Gouida M, El-Ghanam D,
Eldegla H, El-Bakary A and Abdel-Hady H: Influence of
hyperbilirubinemia and phototherapy on markers of genotoxicity and
apoptosis in full-term infants. Eur J Pediatr. 174:459–464.
2015.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Tyson JE and Miller CC: Whether neonatal
phototherapy increases the risk of cancer in children is a
disturbing unresolved issue. Evid Based Med. 22:39–40.
2017.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Auger N, Laverdiere C, Ayoub A, Lo E and
Luu TM: Neonatal phototherapy and future risk of childhood cancer.
Int J Cancer. 145:2061–2069. 2019.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Brewster DH, Tucker JS, Fleming M, Morris
C, Stockton DL, Lloyd DJ, Bhattacharya S and Chalmers JW: Risk of
skin cancer after neonatal phototherapy: Retrospective cohort
study. Arch Dis Child. 95:826–831. 2010.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Matichard E, Le Hénanff A, Sanders A,
Leguyadec J, Crickx B and Descamps V: Effect of neonatal
phototherapy on melanocytic nevus count in children. Arch Dermatol.
142:1599–1604. 2006.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Auger N, Ayoub A, Lo E and Luu TM:
Increased risk of hemangioma after exposure to neonatal
phototherapy in infants with predisposing risk factors. Acta
Paediatr. 108:1447–1452. 2019.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Kanmaz HG, Okur N, Dilli D, Yeşilyurt A
and Oğuz ŞS: The effect of phototherapy on sister chromatid
exchange with different light density in newborn
hyperbilirubinemia. Turk Pediatri Ars. 52:202–207. 2017.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Arnold C, Pedroza C and Tyson JE:
Phototherapy in ELBW newborns: Does it work? Is it safe? The
evidence from randomized clinical trials. Semin Perinatol.
38:452–464. 2014.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Hansen TW: Let there be light-but should
there be less? J Perinatol. 32:649–651. 2012.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Lamola AA: A pharmacologic view of
phototherapy. Clin Perinatol. 43:259–276. 2016.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Sisson TR: Photodegradation of riboflavin
in neonates. Fed Proc. 46:1883–1885. 1987.PubMed/NCBI
|
|
117
|
Kadalraja R, Patole SK, Muller R and
Whitehall JS: Is mesenteric blood flow compromised during
phototherapy in preterm neonates? Arch Dis Child Fetal Neonatal Ed.
89(F564)2004.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Raghavan K, Thomas E, Patole S and Muller
R: Is phototherapy a risk factor for ileus in high-risk neonates? J
Matern Fetal Neonatal Med. 18:129–131. 2005.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Rosenberg K and Mechcatie E: Increased
seizure risk after phototherapy for jaundice. Am J Nurs. 119:50–51.
2019.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Newman TB, Wu YW, Kuzniewicz MW, Grimes BA
and McCulloch CE: Childhood seizures after phototherapy.
Pediatrics. 142(e20180648)2018.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Kuboi T, Kusaka T, Okada H, Arioka M, Nii
K, Takahashi M, Yamato S, Sadamura T, Jinnai W, Nakano A and Itoh
S: Green light-emitting diode phototherapy for neonatal
hyperbilirubinemia: Randomized controlled trial. Pediatr Int.
61:465–470. 2019.PubMed/NCBI View Article : Google Scholar
|
















