Introduction
Cardiac arrest (CA) and subsequent cardiopulmonary
resuscitation (CPR) induces systemic organ tissue
ischemia/reperfusion (I/R) injury, particularly cerebral I/R injury
(CIRI), which directly affects the prognosis and quality of life of
patients. Numerous pathophysiological mechanisms of I/R injury,
including oxidative stress, amino acid toxicity, energy metabolism,
calcium overload, apoptosis and autophagy, have been targeted by
interventions for brain protection (1-3).
In addition, the pathological effects of NLR family pyrin
domain-containing 3 (NLRP3) inflammasome-induced inflammation and
pyroptosis exacerbate CIRI (4).
During I/R, excessive quantities of oxygen free radicals attack
tissues or cells and induce damage-associated molecular patterns
that are recognized by specific pattern recognition receptors,
thereby resulting in caspase-1 precursor (pro-caspase-1) activation
and cell death; this is the classical pathway underlying
caspase-1-dependent pyroptosis (5).
NLRP3 accompanied by pyroptosis is widely observed in neurons,
microglia and astrocytes and is associated with neurological
diseases (6). When
damage-associated molecular signaling occurs, NLRP3 interacts with
apoptosis-associated speck-like protein containing a CARD (ASC) and
recruits pro-caspase-1. Subsequently, pro-caspase-1 is cleaved to
form mature caspase-1p20, which cleaves pro-interleukin (IL)-1β and
gasdermin D (GSDMD) to form mature IL-1βp17 and the GSDMD-N domain
(GSDMD-N). The GSDMD-N protein has been demonstrated to execute
pyroptosis (7). The GSDMD-N domain
targets the plasma membrane and forms a pore of diameter 10-14 nm
that allows mature IL-1βp17 to leak out and ions and water to
enter, which causes inflammation and cell pyroptosis (8-11).
Therefore, the inhibition of NLRP3 inflammasome activation by
antioxidants may be clinically useful for the treatment of I/R
injury.
A previous study has demonstrated that, in most
organs, including the brain, heart, liver, lungs and intestines,
activation of the NLRP3 inflammasome aggravates CIRI (12). Although NLRP3-mediated CIRI has been
reported in the middle cerebral artery occlusion (MCAO) model
(1), it remains unclear whether the
global CIRI following CA/CPR, which causes more extensive critical
brain damage than MCAO, is associated with the NLRP3 inflammasome
and pyroptosis.
Pomelo (Citrus maxima), which is a citrus
fruit belonging to the genus Rutaceae, has high nutritional value
(13). Traditional Chinese medicine
considers pomelo to be beneficial for the prophylaxis and treatment
of nervous system disorders, peroxidation damage, cardiovascular
disease, bruising, hyperlipidemia, wounds, acne, osteoarthritis and
fatigue (14-18).
Pomelo peel contains numerous beneficial chemicals, including
flavonoids, coumarins, essential oils and limonoids. The essential
oils of pomelo peel, extracted using a cold-press method, were
found to contain 94.15% terpenes, comprising 55.92% limonene,
31.17% β-myrcene, 3.16% β-pinene, 1.42% ocimene and 1.24%
β-copaene, when analyzed using gas chromatography-mass spectrometry
(GC-MS) (19). Pomelo peel oils
(PPOs) have been demonstrated to exhibit strong antioxidant,
anti-inflammatory, antiviral and antibacterial activities (20-22).
Therefore, we hypothesize that PPO may inhibit the NLRP3
inflammasome-associated inflammation and pyroptosis induced by
CIRI. In the present study, the chemical composition of PPO was
analyzed. In addition, the effects of PPO on reactive oxygen
species (ROS) and the NLRP3 inflammasome, GSDMD, IL-1β and NF-κB
expression were examined in a rat model of CA/CPR in order to
elucidate the potential neuroprotective effect of PPO.
Materials and methods
PPO extract
Fresh and mature pomelo peels [C. maxima
(Burm.) Merr. cv. Shatian Yu, identified by the Institute of
Botany, Guangxi Zhuang Autonomous Region, Chinese Academy of
Science; specimen ID: (W.Y.Wang:201909001)] were obtained from the
village of Shatian in Rongxia, Guangxi, China. The steam
distillation method was used to extract the PPO (23). The outer layer of the pomelo peel
was washed and cut into ~5x5-mm pieces. The pieces of peel were
combined with distilled water (1:2) and put in a distiller, where
they were boiled for 1 h. The PPO was separated from the water
using a cooling condenser and stored at -4˚C.
GC-MS analysis
The compounds in the PPO were qualitatively analyzed
using GC-MS (TRACE™ 1300; Thermo Fisher Scientific, Inc.). Helium
was injected as a carrier gas at a flow rate of 1 ml/min. The
temperature was increased from 45 to 250˚C according to the
procedure. The heating program is maintained at 45˚C for 1 min,
then at 10˚C/min to 165˚C for 2 min, then at 1.5˚C/min to 180˚C for
2 min and finally at 10˚C/min to 250˚C for 2 min. The temperatures
of the injector and detector were set at 250˚C. Mass spectra were
scanned from m/z 41-400 amu. The electron impact ionization energy
was 70 eV. Identification of the detected compounds was performed
by comparing the mass spectra with published data. Samples were
identified using the Retention Time Locked database (NIST MS Search
2.3; http://www.Inchi-trust,org/download/105/licence.pdf)
with Deconvolution Reporting Software (MSD ChemStation F.
01.03.2357; 1989-2015; Agilent Technologies, Inc.).
CA/CPR animal model
In total, 60 male Sprague-Dawley (SD) rats (body
weight, 220-250 g; age 7-8 weeks) were provided by the Experimental
Animal Center of Guangxi Medical University (License number SYXK
Gui 2014-0003). All animals were handled in accordance with the
Guide for the Care and Use of Laboratory Animals of the National
Institutes of Health (24). The
study was approved by the Animal Care and Use Committee of Guangxi
Medical University. The rats were raised at 22±2˚C, humidity
50-60%, 12-h light/dark cycle, were free to eat and drink and
fasted 12 h before the operation. Prior to modeling, the rats were
anesthetized with an intraperitoneal injection of 2% pentobarbital
sodium (30 mg/kg). A PE50 tube was inserted into the left femoral
artery and connected to a physiological recorder (BL-420E; Chengdu
TME Technology Co., Ltd.) via a pressure converter to monitor the
blood pressure. Another PE50 tube was inserted into the femoral
vein for the injection of drugs.
The CA model was established according to the method
reported by Chen et al (25). A temporary pacemaker electrode
(Chengdu TME Technology Co., Ltd.) was inserted into the esophagus
of the rat at a depth of ~7 cm. Ventricular fibrillation was
induced by the application of 12 V of direct current for 1 min. The
criteria used to define CA were as follows: ECG exhibiting
ventricular fibrillation and a mean arterial pressure of <20
mmHg. Following 7 min of CA, CPR was initiated using a frequency of
mechanical chest compression of 180/min, with a depth of 25-30% of
the anterior posterior diameter of the chest. Ventilator-assisted
ventilation (DH-150; Zhejiang University Medical Instrument Co.,
Ltd.; https://med4868.yixie8.com/) was applied
immediately through endotracheal intubation after 1 min of CPR
(tidal volume, 6 ml/kg; respiration rate, 70 breaths/min; positive
end expiratory pressure, 0 cmH2O). In addition, 20 mg/kg
epinephrine was administered through the PE50 tube in the femoral
vein. The restoration of spontaneous circulation (ROSC) standard
was as follows: Supraventricular rhythm (sinus, atrial and
borderline heart rhythm) accompanied by a mean arterial pressure of
>60 mmHg lasting for >1 min. Surviving rats were randomly
divided into five groups: NS group (n=8; 0.9% physiological saline
200 µl), glycerin group (Gly group; n=8; 10% glycerin 200 µl),
PPO-low group (PPO-L group; n=8; 10 mg/kg PPO), PPO-medium group
(PPO-M group; n=8; 20 mg/kg PPO) and PPO-high group (PPO-H; n=8; 40
mg/kg PPO). The mass of 1 ml PPO, weighed using an electronic
balance (BSA223S; Sartorius AG), was 800 mg. In each group treated
with PPO, the PPO was combined with 10% glycerol to a total volume
of 200 µl. Following 1 min of ROSC, the rats were intravenously
injected with the respective treatment. In addition, 8 rats were
randomly selected as the sham group, which underwent exposure of
the left femoral artery and femoral vein followed by vascular
ligation without CA/CPR. All procedures were performed by two
skilled operators.
Preparation of brain tissues
All rats were anesthetized using pentobarbital (30
mg/kg) by intraperitoneal injection 24 h post reperfusion. Three
rats in each group were perfused with 4% paraformaldehyde for
hematoxylin and eosin (H&E) staining and immunofluorescence
experiments. Cerebral cortices were immediately harvested from the
remaining 5 rats in each group and stored at -80˚C for subsequent
western blot analysis.
ROS assay
Fresh cerebral cortex was homogenized with phosphate
buffer in a weight (g):volume (ml) ratio of 1:20. Following
centrifugation at 1,000 x g for 10 min, the supernatant was used
for the measurement of ROS and protein concentrations. Thereafter,
190 µl supernatant was mixed with 10 µl DCFH-DA from an ROS assay
kit (WLA070; Wanleibio Co., Ltd.) in a 96-well plate and incubated
at 37˚C in the dark for 30 min. ROS were detected via fluorescence
with an excitation wavelength of 500 nm and emission wavelength of
525 nm. The protein concentration was determined using the BCA
method (P0010; Beyotime Institute of Biotechnology) and the results
were expressed as fluorescence intensity/mg protein.
Histological assessment
The rat brains fixed with 4% paraformaldehyde were
embedded in paraffin and cut into 3-µm coronal sections. These
sections were then subjected to H&E staining. At room
temperature, the paraffin sections were dewaxed using xylene
followed by a descending ethanol gradient, rehydrated, stained with
hematoxylin for 5 min at 25˚C, reacted with hydrochloric acid
ethanol for 5 sec and stained with eosin for 1 min at 25˚C before
being finally dehydrated. The slices were observed under a light
microscope (Olympus, Japan) at a magnification of x400.
Immunofluorescence assessment
The dry 3-µm paraffin slices were subjected to
antigen retrieval by heating in a microwave oven at 65˚C with pH
8.0 EDTA antigen repair buffer (Beijing Solarbio Science &
Technology Co., Ltd.), then incubated in 0.01 M PBS (pH 7.4)
containing 0.3% Triton X-100 (TBST) for 20 min at 25˚C prior to
blocking in normal goat serum (OriGene Technologies, Inc.) for 30
min at 37˚C in BSA (OriGene Technologies, Inc.). The slices were
incubated with anti-NLRP3 primary antibodies (cat. no. wl03379;
1:100; Wanleibio Co., Ltd.) overnight at 4˚C, followed by
horseradish peroxidase (HRP)-labeled goat anti-rabbit secondary
antibodies (cat. nos. GB23303; 1:500; Wuhan Servicebio Technology
Co., Ltd.) for 50 min at 25˚C. FITC-Tyramide (cat. no. G1225-1;
1:1,000; Wuhan Servicebio Technology Co., Ltd.) was added for 10
min at 25˚C. The sections were then placed into citric acid (pH
6.0) antigen repair solution (Wuhan Servicebio Technology Co.,
Ltd.) and heated in a microwave oven at 65˚C for 5 min.
Anti-allograft inflammatory factor 1 (IBA-1) primary antibodies
(cat. no. ab153696; 1:1,000; Abcam) were added to the sections,
which were incubated overnight at 4˚C. A Cy3-conjugated fluorescent
secondary antibody (cat. no. G1225-2; 1:1,000; Wuhan Servicebio
Technology Co., Ltd.) was then added for 50 min at 25˚C, followed
by an autofluorescence quenchant (cat. no. G1221; Wuhan Servicebio
Technology Co., Ltd.) for 5 min. Immunoreactivity was visualized by
the fluorescence of the dye to which the secondary antibodies were
conjugated. DAPI dye (cat. no. G1012; Wuhan Servicebio Technology
Co., Ltd.) was added and the slices were incubated for 10 min at
25˚C in the absence of light. The slices were sealed with an
anti-fluorescence quenching sealant (cat. no. G1401; Wuhan
Servicebio Technology Co., Ltd.), and images were captured using a
fluorescence microscope (Olympus Corporation) with an excitation
wavelength of 465-495 nm for NLRP3 (green), 510-560 nm for IBA-1
(red) and 330-380 nm (blue) for DAPI staining. Three magnification
fields (x400) in the slices were randomly selected. The
NLRP3-positive area (%), Iba-1 positive area (%) and number of
microglia with co-localized NLRP3 and IBA-1 expression were
determined using ImageJ 6.0 software (National Institutes of
Health).
Western blot analysis of neuroenolase
(NSE), NF-κBp105/p50, NLRP3, ASC, caspase-1, IL-1β and GSDMD
Brain tissue samples (50 mg) were lysed by RIPA
Lysis Buffer (Wuhan Servicebio Technology Co, Ltd.) and centrifuged
at 12,000 x g for 15 min at 4˚C and the supernatant was collected.
The protein concentration was determined using a bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology). Protein
samples (40 µg/lane) were separated via SDS-PAGE (12 or 8%
separation gel) and then transferred to a PVDF membrane (Merck
KGaA). The membranes were incubated overnight at 4˚C with the
following primary antibodies: NSE (cat. no. ab53025; 1:1,000;
Abcam), NF-κBp105/p50 (cat. no. ab32360; 1:1,000; Abcam),
NFκBp105/p50 (phospho S337; cat. no. ab194729; 1:1,000; Abcam),
NLRP3 (cat. no. wl03379; 1:1,000; Wanleibio Co., Ltd.), ASC (cat.
no. wl02462; 1:500; Wanleibio Co., Ltd.), caspase-1 (cat. no.
wl03325; 1:500; Wanleibio Co., Ltd.), IL-1β (cat. no. ab9787;
1:1,000; Abcam) and GSDMD (cat. no. ab219800; 1:1,000; Abcam) and
GAPDH (cat. no. 5174, 1:1,000; Cell Signaling Technologies, Inc.).
After washing with TBST (0.1% tween), the membranes were incubated
with HRP-conjugated goat anti-rabbit secondary antibodies (cat. no.
sc-2004; 1:10,000; Santa Cruz Biotechnology, Inc.) for 1 h at 25˚C.
Proteins were detected using the Tanon™ High-sig ECL Western
Blotting Substrate (Guangzhou Yuwei Biotechnology Instrument Co.,
Ltd.). Image J 6.0 software was used to analyze the intensities of
the bands.
Statistical analysis
All data are expressed as mean ± standard error of
the mean (SEM). Statistical analyses were performed using GraphPad
Prism 7 (GraphPad Software, Inc.). The Shapiro-Wilk test was used
to validate assumptions of normality. One-way ANOVA followed by a
Tukey's multiple comparison test was employed to analyze
differences among groups. The Kruskal-Wallis test was used to
evaluate non-normally distributed data, with Dunn's test for
intergroup comparisons. P<0.05 was considered to indicate a
statistically significant differences.
Results
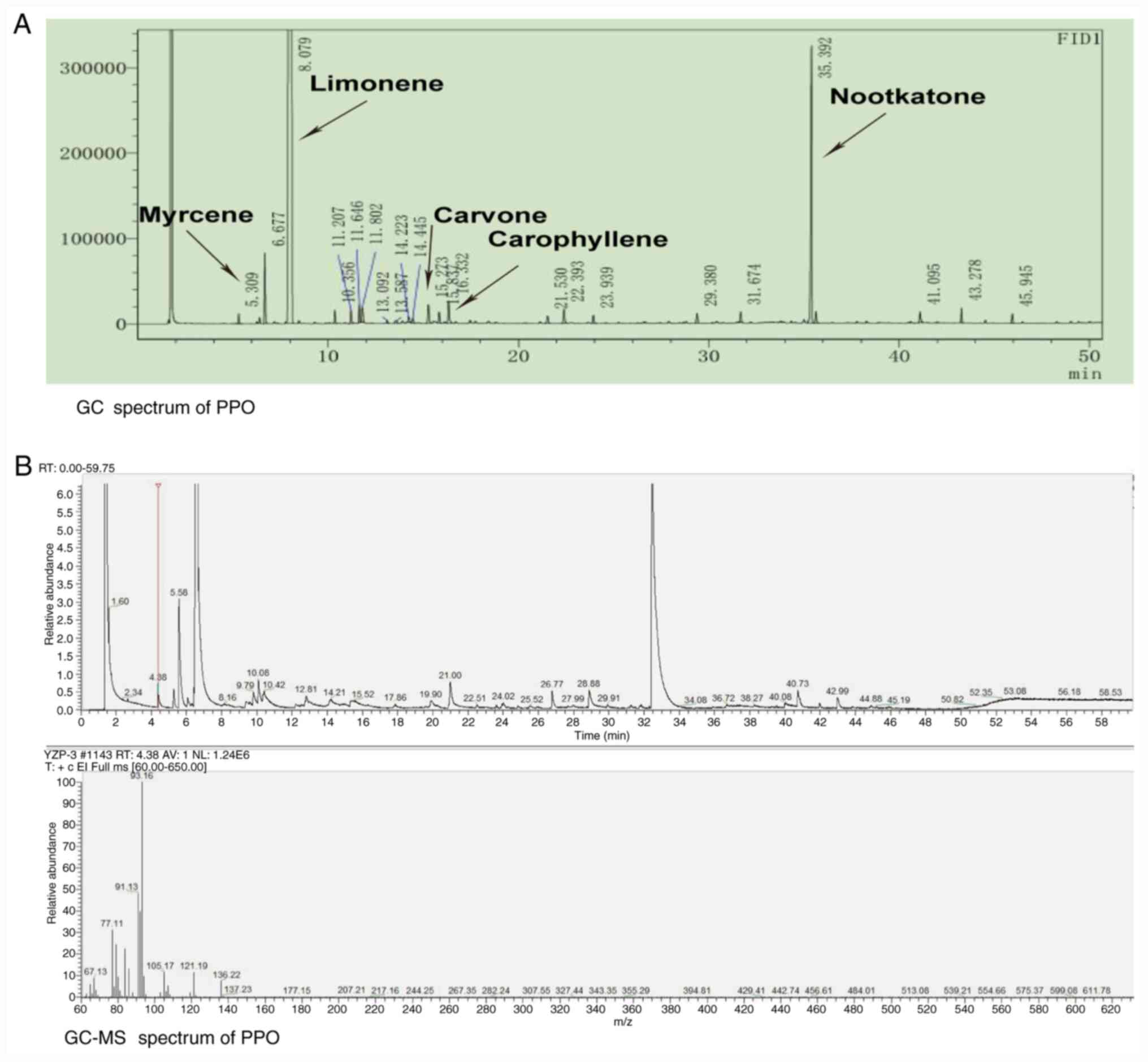
Chemical composition of PPO
The extraction yield of PPO was 0.2%. The PPO was
obtained as a faint yellow, transparent liquid. The total ion
chromatogram obtained from the GC-MS analysis of the PPO is shown
in Fig. 1. A total of 17 compounds
were detected (Table I). The most
abundant compound in the PPO was limonene at 88.683%, followed by
nootkatone (5.732%) and myrcene (1.027%; Table I).
 | Table IChemical components of pomelo peel
oil. |
Table I
Chemical components of pomelo peel
oil.
| No. | Compound | Molecular
formula | Retention time
(min) | Peak area (%) |
|---|
| 1 | α-pinene |
C10H16 | 5.309 | 0.127 |
| 2 | Myrcene |
C10H16 | 6.677 | 1.027 |
| 3 | Limonene |
C10H16 | 8.079 | 88.683 |
| 4 | γ-terpinene |
C10H16 | 10.356 | 0.250 |
| 5 | Limonene oxide |
C10H16O | 11.207 | 0.270 |
| 6 | α-terpineol |
C10H18O | 14.223 | 0.149 |
| 7 | Carvone |
C10H14O | 15.273 | 0.403 |
| 8 | Geranyl
acetate |
C12H20O2 | 15.837 | 0.234 |
| 9 | Carophyllene |
C15H24 | 16.332 | 0.505 |
| 10 |
α-caryophyllene |
C15H24 | 21.530 | 0.193 |
| 11 | α-gurjunene |
C15H24 | 22.393 | 0.351 |
| 12 | 8-cedren-13-ol |
C15H24O | 23.939 | 0.183 |
| 13 | Nerolidol |
C15H26O | 29.380 | 0.208 |
| 14 | Globulol |
C15H26O | 31.674 | 0.184 |
| 15 | Nootkatone |
C15H22O | 35.392 | 5.732 |
| 16 | Osthole |
C15H16O3 | 41.095 | 0.196 |
| 17 | Eicosane |
C20H42 | 43.278 | 0.197 |
| - | Total | - | - | 98.89 |
Antioxidant activities of PPO
The antioxidant activities of PPO in the cerebral
cortex tissues of the CIRI model rats are shown in Fig. 2B. In comparison with the ROS content
in the sham group, the levels of ROS were significantly increased
in the NS, Gly and PPO-L groups. However, in the PPO-H group, the
PPO treatment significantly decreased the levels of ROS compared
with those in the NS and Gly groups.
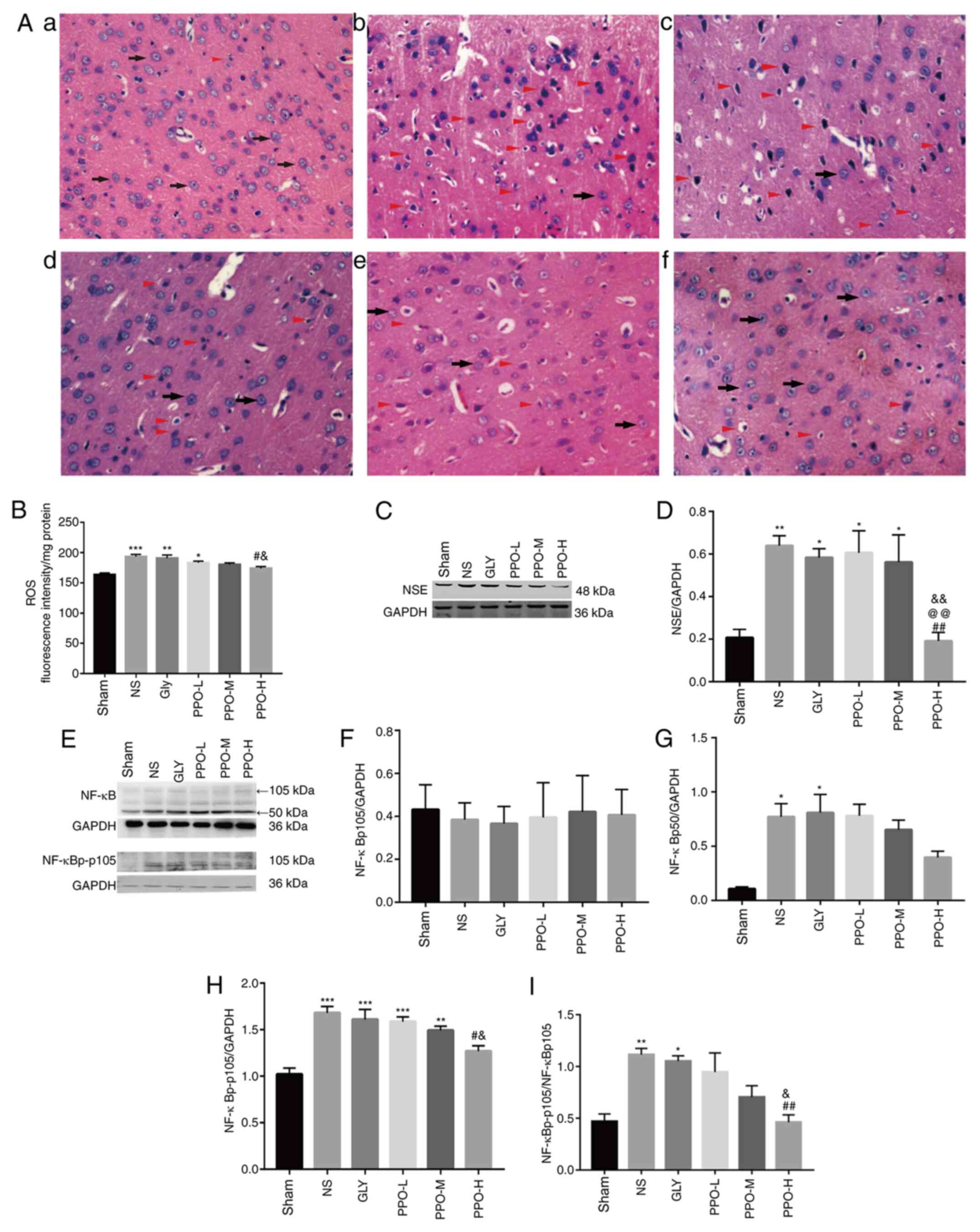 | Figure 2PPO improves cell morphology, reduces
ROS levels and attenuates the expression of NSE and NF-κB in a
cardiopulmonary resuscitation rat model. (A) Hematoxylin and eosin
staining of brain tissue from rats in the (A-a) sham, (A-b) NS,
(A-c) Gly, (A-d) PPO-L, (A-e) PPO-M and (A-f) PPO-H groups. A large
number of cerebral cortex cells with normal morphology were
observed in the sham group (indicated by black arrows). However,
numerous abnormal cerebral cortex cells exhibiting nuclear
pyknosis, intense staining, vacuolation, swelling and necrosis
(indicated by red triangles) were visible in the NS and Gly groups.
Cell morphology was ameliorated in the PPO groups. Magnification,
x400. (B) Antioxidant activity of PPO determined by an ROS assay.
Representative western blots of NSE (C) and (D) quantified results.
(E) Representative western blots of NF-κB and (F-I) quantified
results. Band intensities were quantified using densitometric
software. The band intensities of (F) NF-κBp105, (G) NF-κBp50 and
(H) NF-κBp-p105 were normalized to that of GAPDH, and (I) the
NF-κBp-p105/NF-κBp105 ratio was calculated. Data are presented as
the mean ± SEM of two independent experiments (n=3).
*P<0.05, **P<0.01 and
***P<0.001 vs. the sham group; #P<0.05
and ##P<0.01 vs. the NS group;
&P<0.05 and &&P<0.01 vs.
the Gly group; @@P<0.01 vs. the PPO-L group (n=5).
PPO, pomelo peel oil; ROS, reactive oxygen species; NSE,
neuroenolase; NS, physiological saline; Gly, glycerin; PPO-L, 10
mg/kg PPO; PPO-M, 20 mg/kg PPO; PPO-H, 40 mg/kg PPO; NF-κBp-p105,
phosphorylated NF-κBp105. |
PPO ameliorates cerebral cell
morphological changes and reduces the expression of NSE
To investigate the effects of PPO on CIRI, cerebral
cell morphology was evaluated with H&E staining (Fig. 2A). In the sham group, the cell
structures were complete and the tissue structure was tightly
packed, with uniform staining and clearly visible nuclei. By
contrast, the cerebral tissues from the NS and Gly groups exhibited
fewer intact cells; cells were disordered, with intense staining
and vacuoles clearly evident. The tissues treated with PPO
exhibited improved cell morphology compared with those in the NS
and Gly groups. NSE has been widely studied as a brain injury
marker (21,26). Therefore, the expression of NSE was
analyzed by western blotting (Fig.
2C). Compared with that in the sham group, the expression of
NSE was significantly increased in the NS, Gly, PPO-L and PPO-M
groups (Fig. 2D). However, the
expression of NSE in the PPO-H group was significantly lower than
that in the NS, Gly and PPO-L groups (Fig. 2D). These results indicate that is
able to PPO ameliorate cerebral injury in CA/CPR model rats.
PPO inhibits the expression of
NF-κB
When NF-κBp105 is lysed, it forms NF-κBp50 and
thereby serves pro-inflammatory functions (27). No significant differences in the
expression of NF-κBp105 were detected among the groups (Fig. 2E and F). However, in comparison with the sham
group, the NS and Gly groups exhibited significant increases in
NF-κBp50 and phosphorylated NF-κBp105 (NF-κBp-p105) levels
(Fig. 2G-I). Following treatment
with PPO, the levels of NF-κBp50 and NF-κBp-p105 decreased in a
dose-dependent manner, and the level of NF-κBp-p105 was
significantly decreased in the PPO-H group compared with the NS and
Gly groups (Fig. 2H). The
NF-κBp-p105/NF-κBp105 ratio also exhibited a significant reduction
in the PPO-H group compared with the NS and Gly groups after PPO
treatment (Fig. 2I). These results
suggest that PPO partially inhibits NF-κB-mediated
inflammation.
PPO decreases the expression of NLRP3
in the cerebral cortex
To determine whether PPO is able to inhibit NLRP3
activation in the microglia, the expression and co-expression of
NLRP3 and IBA-1 were detected using immunofluorescence double
staining. The expression of NLRP3 and IBA-1, and the co-expression
of NLRP3 + IBA-1 increased markedly in the microglia following
CR/CPR (Fig. 3A). The expression of
NLRP3, IBA-1 and NLRP3 + IBA-1 was significantly increased in the
NS and Gly groups compared with the sham group. After treatment
with PPO, the expression levels of NLRP3, IBA-1 and NLRP3 + IBA-1
in the PPO-H group were significantly lower than those in the NS
group, indicating that activation of microglia was inhibited
(Fig. 3B). The results indicate
that PPO decreased the expression of NLRP3 and activation of the
microglia.
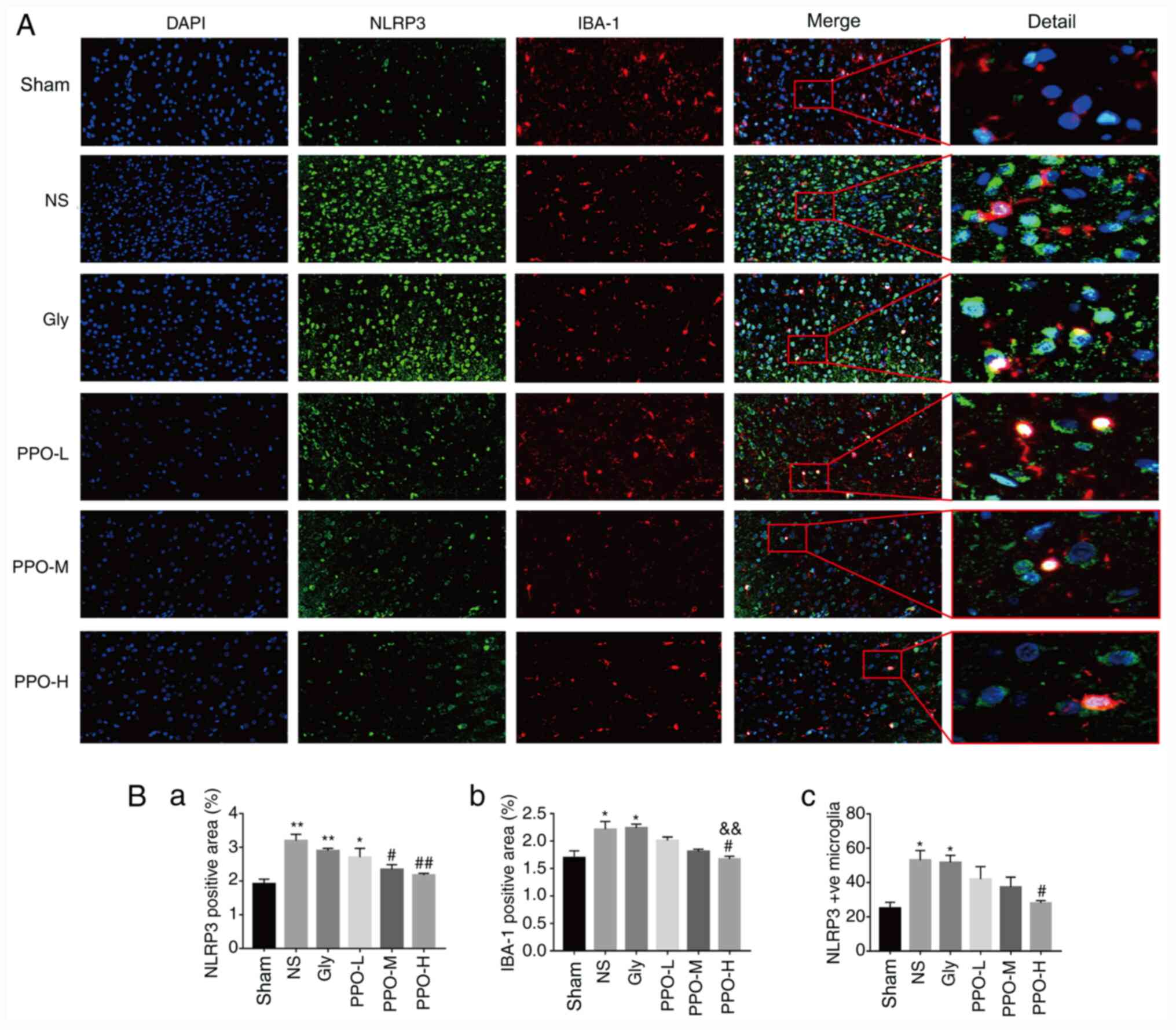 | Figure 3PPO decreases the expression of NLRP3
in the microglia. (A) Immunofluorescence staining of rat brain
slices. Green staining indicates NLRP3 protein expression, red
staining indicates IBA-1 protein expression and DAPI staining
indicates nuclei (blue). Magnification, x400 and x2,000. (B) Column
charts showing the (B-a) NLRP3-positive area (%), (B-b)
IBA-1-positive area (%) and (B-c) the amount of NLRP3 expression
co-localized with IBA-1-positive microglia in each group quantified
using image analysis software. All data are presented as the mean ±
SEM (n=3). *P<0.05 and **P<0.01 vs. the
sham group; #P<0.05 and ##P<0.01 vs.
the NS group; &&P<0.01 vs. the Gly group.
PPO, pomelo peel oil; NLRP3, NLR family pyrin domain-containing 3;
IBA-1, allograft inflammatory factor 1; Gly, glycerin; PPO-L, 10
mg/kg PPO; PPO-M, 20 mg/kg PPO; PPO-H, 40 mg/kg PPO. |
PPO downregulates the expression of
pyroptosis-associated proteins NLRP3, ASC, caspase-1, IL-1β and
GSDMD
To investigate the effects of PPO on the
inflammatory response and pyroptosis-associated proteins in the
CA/CPR model rats, the levels of NLRP3, ASC, caspase-1, IL-1β and
GSDMD were analyzed using western blotting (Fig. 4). Pro-caspase-1, pro-IL-1β and GSDMD
were activated to form caspase-1p20, IL-1βp17 and GSDMD-N,
respectively. The expression levels of NLRP3, ASC, caspase-1p20,
IL-1βp17 and GSDMD-N in the NS group were significantly higher than
those in the sham group. Following treatment with PPO, the levels
of these proteins were significantly lower than those in the NS
group. These data indicate that PPO reduced the
pyroptosis-associated protein cascade.
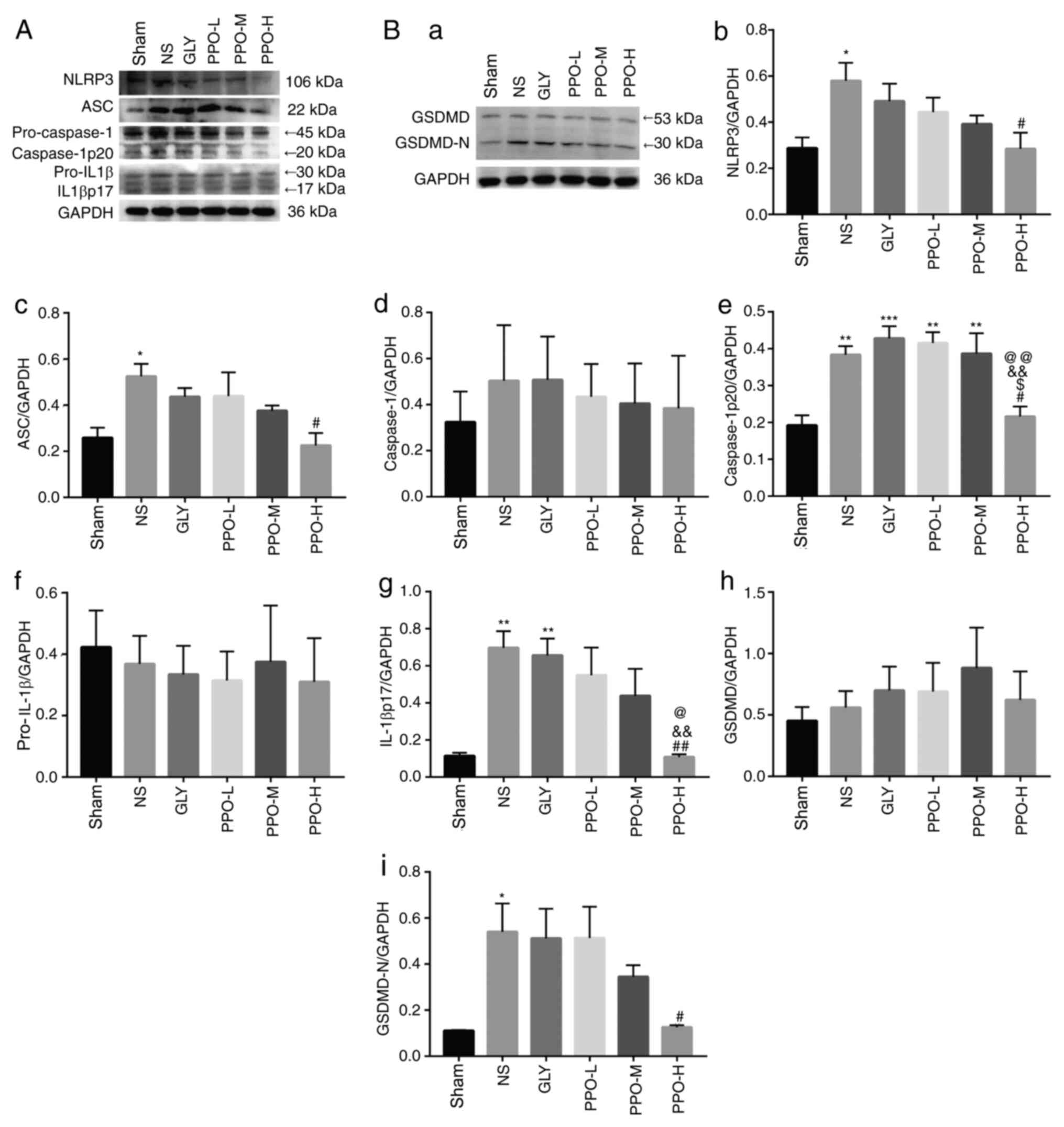 | Figure 4Effect of PPO on NLRP3, ASC,
caspase-1, IL-1β, and GSDMD in rat brain tissue. Representative
western blots of (A) NLRP3, ASC, caspase-1, IL-1β and (B-a) GSDMD.
The column charts shows the levels of (B-b) NLRP3, (B-c) ASC, (B-d)
caspase-1, (B-e) caspase-1p20, (B-f) pro-IL-1β, (B-g) IL-1βp17,
(B-h) GSDMD and (B-i) GSDMD-N in the various treatment groups. Band
intensity was normalized to GAPDH and quantified using image
analysis software. Data are presented as the mean ± SEM (n=5).
*P<0.05, **P<0.01 and
***P<0.001 vs. the sham group; #P<0.05
and ##P<0.01 vs. the NS group;
&&P<0.01 vs. the Gly group;
@P<0.05 and @@P<0.01 vs. the PPO-L
group; $P<0.05 vs. the PPO-M group. PPO, pomelo peel
oil; NLRP3, NLR family pyrin domain-containing 3; ASC,
apoptosis-associated speck-like protein containing a CARD; IL-1b,
interleukin-1b; GSDMD, gasdermin D; GSDMD-N, GSDMD N-domain; Gly,
glycerin; PPO-L, 10 mg/kg PPO; PPO-M, 20 mg/kg PPO; PPO-H, 40 mg/kg
PPO. |
Discussion
In the present study, PPO was obtained from fresh
and mature Shatian pomelo peels. The main ingredient of PPO was
found to be limonene (88.683%), followed by nootkatone (5.732%) and
myrcene (1.027%). The results of the in vivo experiments
revealed that PPO reduced the production of ROS induced by global
CIRI in rats following CA/CPR and ameliorated the pathological
injury of the cerebral cortex. The effects of PPO on the NLRP3
inflammasome response and pyroptosis were also demonstrated in this
model. Furthermore, PPO downregulated pyroptosis-associated protein
levels.
The proportions of limonene and other components
detected in the present study differ from those in other studies
(19,28); this may be due to different
extraction methods, growth environments, isolation conditions and
provenance. However, consistent with other studies (19,29),
limonene was the main component of the PPO extracted via
distillation in the present study. PPO has been reported to contain
94.15% terpenes (19), with
structures that include phenolic hydroxyl groups and unsaturated
double bonds, thereby providing electrons that are able to reduce
free radicals to form less active substances and chelate metal ions
to prevent the generation of free radicals (30). PPO has also been shown to exert an
antioxidant effect against superoxide anion radical formation
(19). Previous studies have
demonstrated that the overproduction of ROS is associated with CIRI
(31,32), which is consistent with the results
of the present study. Furthermore, the results indicate that PPO
inhibited the production of ROS associated with global CIRI. It has
been reported that limonene has antioxidant activities (33). Therefore, we hypothesize that the
antioxidant activity of PPO is associated with the limonene it
contains.
The generation of excessive ROS leads to cellular
damage and triggers the activation of microglia and immune pathways
(34). The present study
demonstrated that CIRI upregulated the expression of NLRP3 in the
microglia, and treatment with PPO reduced CIRI-induced activation
of the microglia and the expression of NLRP3. The microglia are the
resident immune cells in the brain. Following activation, microglia
switch their phenotypes between two polarizations, M1 and M2, which
can release large quantities of pro-inflammatory and
anti-inflammatory cytokines, respectively. In a previous study,
NLRP3 immunoreactivity was detected in the IBA-1-labeled microglia
of mice with CIRI following transient MCAO, which induced the
overproduction of the M1 microglia-regulated pro-inflammatory
cytokine IL-1β (35). Therefore,
the phenotypic changes occurring in the microglia following
activation require identification.
NLRP3, a specific pattern recognition receptor,
initiates cell death processes (pyroptosis) as soon as it is
activated by factors induced by pathological damage. It promotes
the pyroptosis-associated protein cascade and causes inflammation
and pyroptosis (36). The
examination of factors in this cascade in the present study
revealed that the upregulation of NLRP3, ASC, caspase-1p20,
IL-1βp17 and GSDMD-N induced in rats by CA/CPR was attenuated by
PPO treatment, suggesting that it targets NLRP3 inflammasome
induced inflammatory responses and pyroptosis, and serves as an
intervention against CIRI.
The control of NLRP3 activity can be divided into
two processes, namely priming and activation. Priming prepares
NLRP3 for subsequent activation (37) and may occur by an ROS-mediated
non-transcriptional pathway (38,39).
ROS released into the cytoplasm by damaged mitochondria also
promote activation of the NLRP3 inflammasome (40,41).
Since excess ROS production occurs during CIRI, antioxidants are
able block pyroptosis-associated NLRP3 inflammasomes. Therefore, we
hypothesize that the antioxidant activity of PPO resulted in the
levels of NLRP3 inflammasome being low in the early stages of
CIRI.
IL-1β is produced during CIRI; it induces
cerebrovascular endothelial cells to express adhesion molecules,
which mediate interaction between the endothelial cells and
leukocytes, thereby promoting the infiltration of leukocytes into
the brain tissues. Leukocyte infiltration activates microglial
cells, which causes them to secrete inflammatory factors and
aggravates brain injury. In addition, leukocytes adhere and
accumulate in microvessels, causing blockages and reducing cerebral
blood flow, which also aggravates brain injury. These pathological
changes occur at a late stage after CIRI (42-45).
We hypothesize that the antioxidant activity of PPO reduces the
secretion of IL-1β from cells and thus attenuates of the
IL-1β-mediated inflammatory response.
An increase in ROS levels can stimulate the
phosphorylation of IκB by casein kinase II or tyrosine kinases, and
thereby induce NF-κB gene transcription (46). NF-κB mediates the priming of NLRP3
via the transcriptional pathway (39). NF-κBp105/p50 is a member of the
NF-κB family, and NF-κBp50 is produced from NF-κBp105 by
ubiquitin-dependent limited proteolysis (27). The present study found that PPO
significantly downregulated the phosphorylation of NF-κB. The
phosphorylation of NF-κBp105 directly affects the formation of
NF-κBp50(47). NF-κBp50 has been
demonstrated to be involved in pyroptosis as an inflammasome
promoter (48). Furthermore, a
recent study indicated that the brain damage in MCAO rat models of
CIRI may be associated with increased levels of NF-κBp50(49). The results of the present study are
consistent with this, and showed that the levels of NF-κBp50
increased in a global CIRI model. PPO downregulated the formation
of NF-κBp50 in a dose-dependent manner; the antipyroptotic
properties of PPO may be partly due to its antioxidant activity.
Since significant differences were found, the results suggest that
the transcriptional pathway of NF-κB-mediated NLRP3 activation may
be one of the pathway in the present model.
In conclusion, the present study indicated that the
NLRP3 inflammasome, which is associated with pyroptosis, is
involved in CA/CPR-induced CIRI. Furthermore, PPO treatment
downregulated the expression of multiple factors in the NLRP3
inflammasome and ameliorated brain cell death. PPO, as a mixture,
may inhibit the inflammation and pyroptosis caused by activation of
the NLRP3 inflammasome through its antioxidant capacity. However,
further research is required to identify the active components and
the underlying mechanism.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81660312 and
81360286).
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
XSZ and LX contributed equally to this work, in
terms of developing the concept and study design, acquiring,
analyzing and interpreting the data, authenticate the raw data and
drafting the manuscript. WYW, GYZ and XYT performed the experiments
and analyzed the data. MHC designed and led the study. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Animal Care and Use
Committee of Guangxi Medical University (Nanning, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
He Q, Li Z, Wang Y, Hou Y, Li L and Zhao
J: Resveratrol alleviates cerebral ischemia/reperfusion injury in
rats by inhibiting NLRP3 inflammasome activation through
Sirt1-dependent autophagy induction. Int Immunopharmacol.
50:208–215. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chumboatong W, Thummayot S, Govitrapong P,
Tocharus C, Jittiwat J and Tocharus J: Neuroprotection of
agomelatine against cerebral ischemia/reperfusion injury through an
antiapoptotic pathway in rat. Neurochem Int. 102:114–122.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Buckley KM, Hess DL, Sazonova IY,
Periyasamy-Thandavan S, Barrett JR, Kirks R, Grace H, Kondrikova G,
Johnson MH, Hess DC, et al: Rapamycin up-regulation of autophagy
reduces infarct size and improves outcomes in both permanent MCAL,
and embolic MCAO, murine models of stroke. Exp Transl Stroke Med.
6(8)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ito M, Shichita T, Okada M, Komine R,
Noguchi Y, Yoshimura A and Morita R: Bruton's tyrosine kinase is
essential for NLRP3 inflammasome activation and contributes to
ischaemic brain injury. Nat Commun. 6(7360)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bi F, Ma H, Ji C, Chang C, Liu W and Xie
K: Rhein protects against neurological deficits after traumatic
brain injury in mice via inhibiting neuronal pyroptosis. Front
Pharmacol. 11(564367)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Albornoz E, Woodruff T and Gordon R:
Inflammasomes in CNS diseases. Exp Suppl. 108:41–60.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ding J, Wang K, Liu W, She Y, Sun Q, Shi
J, Sun H, Wang DC and Shao F: Pore-forming activity and structural
autoinhibition of the gasdermin family. Nature. 535:111–116.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fink SL and Cookson BT:
Caspase-1-dependent pore formation during pyroptosis leads to
osmotic lysis of infected host macrophages. Cell Microbiol.
8:1812–1825. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tsuchiya K and Hara H: The inflammasome
and its regulation. Crit Rev Immunol. 34:41–80. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Agostini L, Martinon F, Burns K, McDermott
MF, Hawkins PN and Tschopp J: NALP3 forms an IL-1beta-processing
inflammasome with increased activity in Muckle-Wells
autoinflammatory disorder. Immunity. 20:319–325. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Minutoli L, Puzzolo D, Rinaldi M, Irrera
N, Marini H, Arcoraci V, Bitto A, Crea G, Pisani A, Squadrito F, et
al: ROS-mediated NLRP3 inflammasome activation in brain, heart,
kidney, and testis ischemia/reperfusion injury. Oxid Med Cell
Longev. 2016(2183026)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu Y, Liu A, Ibrahim SA, Yang H and Huang
W: Isolation and characterization of microcrystalline cellulose
from pomelo peel. Int J Biol Macromo. 111:717–721. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Aumeeruddy-Elalfi Z, Gurib-Fakim A and
Mahomoodally MF: Kinetic studies of tyrosinase inhibitory activity
of 19 essential oils extracted from endemic and exotic medicinal
plants. South Afr J Botany. 103:89–94. 2016.
|
|
15
|
Kim GS, Park HJ, Woo JH, Kim MK, Koh PO,
Min W, Ko YG, Kim CH, Won CK and Cho JH: Citrus aurantium
flavonoids inhibit adipogenesis through the Akt signaling pathway
in 3T3-L1 cells. BMC Complement Altern Med. 12(31)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu Y, Heying E and Tanumihardjo SA:
History, global distribution, and nutritional importance of citrus
fruits. Compre Rev Food Sci Food Safety. 11:530–545. 2012.
|
|
17
|
Mulero J, Bernabé J, Cerdá B,
García-Viguera C, Moreno DA, Albaladejo MD, Avilés F, Parra S,
Abellán J and Zafrilla P: Variations on cardiovascular risk factors
in metabolic syndrome after consume of a citrus-based juice. Clin
Nutr. 31:372–377. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hwang SL, Shih PH and Yen GC:
Neuroprotective effects of citrus flavonoids. J Agric Food Chem.
60:877–885. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
He W, Li X, Peng Y, He X and Pan S:
Anti-oxidant and anti-melanogenic properties of essential oil from
peel of pomelo cv. Guan Xi. Molecules. 24(242)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hosni K, Zahed N, Chrif R, Abid I, MedfeiW
Kallel M, Kallel NB and Sebei H: Composition of peel essential oils
from four selected Tunisian Citrus species: Evidence for the
genotypic influence. Food Chemistry. 123:1098–1104. 2010.
|
|
21
|
Wu F, Jin Y, Xu X and Yang N:
Electrofluidic pretreatment for enhancing essential oil extraction
from citrus fruit peel waste. J Clean Prod. 159:85–94. 2017.
|
|
22
|
Zhao YL, Yang XW, Wu BF, Shang JH, Liu YP,
Zhi-Dai and Luo XD: Anti-inflammatory effect of Pomelo Peel
and its Bioactive Coumarins. J Agric Food Chem. 67:8810–8818.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fadel H, Marx F, El-Sawy A and El-Ghorab
A: Effect of extraction techniques on the chemical composition and
antioxidant activity of Eucalyptus camaldulensis var. brevirostris
leaf oils. Zeitschrift für Lebensmitteluntersuchung und -Forschung
A. 208:212–216. 1999.
|
|
24
|
National Research Council (US): Institute
for Laboratory Animal Research. Guide for the Care and Use of
Laboratory Animals. National Academies Press (US), Washington, DC,
1996.
|
|
25
|
Chen MH, Liu TW, Xie L, Song FQ, He T,
Zeng ZY and Mo SR: A simpler cardiac arrest model in rats. Am J
Emerg Med. 25:623–630. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Abdel-Magied N, Shedid SM and Ahmed AG:
Mitigating effect of biotin against irradiation-induced cerebral
cortical and hippocampal damage in the rat brain tissue. Environ
Sci Pollut Res Int. 26:13441–13452. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cartwright T, Perkins ND and L Wilson C:
NFKB1: A suppressor of inflammation, ageing and cancer. FEBS J.
283:1812–1822. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ali MY, Rumpa NN, Paul S, Hossen MS,
Tanvir EM, Hossan T, Saha M, Alam N, Karim N, Khalil MI and Gan S:
Antioxidant potential, subacute toxicity, and beneficiary effects
of methanolic extract of pomelo (Citrus grandis L. Osbeck)
in long evan rats. J Toxicol. 2019(2529569)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen GW, Lin YH, Lin CH and Jen HC:
Antibacterial activity of emulsified pomelo (Citrus grandis
Osbeck) peel oil and water-soluble chitosan on Staphylococcus
aureus and Escherichia coli. Molecules. 23(840)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Koleva II, van Beek TA, Linssen JP, de
Groot A and Evstatieva LN: Screening of plant extracts for
antioxidant activity: A comparative study on three testing methods.
Phytochem Anal. 13:8–17. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Nguyen Thi PA, Chen MH, Li N, Zhuo XJ and
Xie L: PD98059 protects brain against cells death resulting from
ROS/ERK activation in a cardiac arrest rat model. Oxid Med Cell
Longev. 2016(3723762)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu D, Wang H, Zhang Y and Zhang Z:
Protective effects of chlorogenic acid on cerebral
ischemia/reperfusion injury rats by regulating oxidative
stress-related Nrf2 pathway. Drug Des Devel Ther. 14:51–60.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Roberto D, Micucci P, Sebastian T,
Graciela F and Anesini C: Antioxidant activity of limonene on
normal murine lymphocytes relation to H2O2
modulation and cell proliferation. Basic Clin Pharmacol Toxicol.
106:38–44. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liao S, Wu J, Liu R, Wang S, Luo J, Yang
Y, Qin Y, Li T, Zheng X, Song J, et al: A novel compound DBZ
ameliorates neuroinflammation in LPS-stimulated microglia and
ischemic stroke rats: Role of Akt(Ser473)/GSK3beta(Ser9)-mediated
Nrf2 activation. Redox Biol. 36(101644)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhao J, Piao X, Wu Y, Liang S, Han F,
Liang Q, Shao S and Zhao D: Cepharanthine attenuates cerebral
ischemia/reperfusion injury by reducing NLRP3 inflammasome-induced
inflammation and oxidative stress via inhibiting 12/15-LOX
signaling. Biomed Pharmacother. 127(110151)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Swanson KV, Deng M and Ting JP: The NLRP3
inflammasome: Molecular activation and regulation to therapeutics.
Nat Rev Immunol. 19:477–489. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhou R, Yazdi AS, Menu P and Tschopp J: A
role for mitochondria in NLRP3 inflammasome activation. Nature.
469:221–225. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Dostert C, Pétrilli V, Van Bruggen R,
Steele C, Mossman BT and Tschopp J: Innate immune activation
through Nalp3 inflammasome sensing of asbestos and silica. Science.
320:674–677. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Groslambert M and Py BF: Spotlight on the
NLRP3 inflammasome pathway. J Inflamm Res. 11:359–374.
2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nakahira K, Haspel JA, Rathinam VA, Lee
SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim
HP, et al: Autophagy proteins regulate innate immune responses by
inhibiting the release of mitochondrial DNA mediated by the NALP3
inflammasome. Nat Immunol. 12:222–230. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
41
|
Shimada K, Crother TR, Karlin J, Dagvadorj
J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, et
al: Oxidized mitochondrial DNA activates the NLRP3 inflammasome
during apoptosis. Immunity. 36:401–414. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Parada E, Casas AI, Palomino-Antolin A,
Gómez-Rangel V, Rubio-Navarro A, Farré-Alins V, Narros-Fernandez P,
Guerrero-Hue M, Moreno JA, Rosa JM, et al: Early toll-like receptor
4 blockade reduces ROS and inflammation triggered by microglial
pro-inflammatory phenotype in rodent and human brain ischaemia
models. Br J Pharmacol. 176:2764–2779. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Meng H, Zhao H, Cao X, Hao J, Zhang H, Liu
Y, Zhu MS, Fan L, Weng L, Qian L, et al: Double-negative T cells
remarkably promote neuroinflammation after ischemic stroke. Proc
Natl Acad Sci USA. 116:5558–5563. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen L, Kong L, Wei X, Wang Y, Wang B,
Zhang X, Sun J and Liu H: β-arrestin 2 negatively regulates NOD2
signalling pathway through association with TRAF6 in microglia
after cerebral ischaemia/reperfusion injury. J Cell Mol Med.
23:3325–3335. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wang Y, Li SY, Shen S and Wang J:
Protecting neurons from cerebral ischemia/reperfusion injury via
nanoparticle-mediated delivery of an siRNA to inhibit microglial
neurotoxicity. Biomaterials. 161:95–105. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bauernfeind F, Bartok E, Rieger A, Franchi
L, Núñez G and Hornung V: Cutting edge: Reactive oxygen species
inhibitors block priming, but not activation, of the NLRP3
inflammasome. J Immunol. 187:613–617. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Mandola AB, Sharfe N, Nagdi Z, Dadi H,
Vong L, Merico D, Ngan B, Reid B and Roifman CM: Combined
immunodeficiency caused by a novel homozygous NFKB1 mutation. J
Allergy Clin Immunol: Sep 25, 2020 (Epub ahead of print). doi:
10.1016/j.jaci.2020.08.040.
|
|
48
|
Yi H, Peng R, Zhang LY, Sun Y, Peng HM,
Liu HD, Yu LJ, Li AL, Zhang YJ, Jiang WH and Zhang Z:
LincRNA-Gm4419 knockdown ameliorates NF-κB/NLRP3
inflammasome-mediated inflammation in diabetic nephropathy. Cell
Death Dis. 8(e2583)2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chen J, Yang C, Xu X, Yang Y and Xu B: The
effect of focal cerebral ischemia-reperfusion injury on TLR4 and
NF-κB signaling pathway. Exp Ther Med. 15:897–903. 2018.PubMed/NCBI View Article : Google Scholar
|