Introduction
Peritoneal dialysis (PD) is one of the most commonly
used dialysis methods in the clinic, and utilizes the nature of the
peritoneum as a semipermeable membrane to exchange water and toxic
solutes in the peritoneal cavity (1). Compared with hemodialysis, PD has
advantages such as stable hemodynamics, lower risk of death
(2) and better residual renal
function preservation (3). In
addition, studies have suggested that there is increased cognitive
ability (4) and relatively low
suicide rate (5) in patients on
long-term PD treatment in comparison with patients treated with
hemodialysis. Statistically, patients on PD treatment account for
about 11% of the dialysis population worldwide, especially in
developing countries (6). In the
Unites States, China, and Thailand, the use of this therapy is
increasing year on year (7).
Nevertheless, PD treatment is still associated with significant
adverse events. For example, long-term PD treatment can alter the
structure and function of the peritoneum, leading to
ultrafiltration failure and eventually withdrawal from PD (1). At present, risk factors in PD
treatment include biocompatibility of the peritoneal dialysate,
dialysis catheter factors and infection (8,9).
However, due to the limitation of human experiments imposed by
medical ethics, an in-depth understanding of PD is still needed. A
suitable experimental model could help people better study the
physiological and pathological changes in the peritoneum during PD
treatment. Therefore, establishing an experimental model similar to
human PD is of great significance for studying PD techniques,
improving dialysis efficacy and prolonging the survival rate of
patients.
2. Experimental animals
In recent years, scholars have studied and clarified
the principles and characteristics of different in vitro
experimental models using human tissues, and they have found that
mesenchymal transformation of peritoneal mesothelial cells is
closely related to peritoneal injury (10-12).
In vitro models are usually obtained by culturing peritoneal
mesothelial cells (13,14). This variety of model has advantages,
such as relatively low cost, clear target and less confounding
factors if a single cell type is used. Researchers can conduct an
in vitro test of biocompatibility of the peritoneal
dialysate through human peritoneal mesothelial cell culture
(15).
In terms of in vivo animal models,
researchers have attempted to use dogs, cats, rabbits, rats and
mice to establish PD models (16,17).
However, due to the influence of many factors, such as cost and
individual size of animals, different animals have specific
advantages and disadvantages (Table
I). From an economic cost perspective, rats and mice are
cheaper and faster to breed, while rabbits, dogs, and cats are
relatively expensive and less prone to reproduce. From the
perspective of surgical operation possibility, rats have a strong
peritoneal defense system, while rabbits are extremely prone to
peritoneal infection, although their peritoneum is the most similar
to the human peritoneum. In addition, PD requires catheter
insertion in the abdominal cavity, and one should note that,
catheter implantation is difficult in mice due to their small size.
Therefore, rats are currently considered the most suitable animal
PD model because of their low economic cost, the ease of performing
a surgical operation on them and as they are a relatively stable
model (16,18). However, in order to achieve better
experimental results, different experimental animals should be
selected according to different research purposes and actual
conditions.
 | Table IMain advantages and disadvantages of
animal experimental models commonly used in peritoneal dialysis
(16-18). |
Table I
Main advantages and disadvantages of
animal experimental models commonly used in peritoneal dialysis
(16-18).
| Animal | Advantages | Disadvantages |
|---|
| Rat | Easy operation, low
cost and easy reproduction | Short lifespan |
| Mouse | Low cost and easy
reproduction | Small size and
short lifespan |
| Rabbit | Peritoneum is
similar to humans and long lifespan | High price and not
easy to reproduce |
| Genetically
modified mouse | Multiple
possibilities of gene manipulation | Small size and
short lifespan |
| Dog | Long lifespan and
larger size | High cost and not
easy to reproduce |
| Sheep | Long lifespan and
larger size | High cost and not
easy to reproduce |
3. Classification of animal PD models
PD models can be applied for different research
purposes, including for the study of peritoneal fibrosis (19,20),
peritoneal sclerosis (21) and
angiogenesis (22,23), and they can be divided into a uremic
PD model and a non-uremic PD model. The uremic PD model refers to
catheter implantation under the condition of uremia, which is
achieved by nephrectomy or drug methods (24). If the creatinine and urea nitrogen
levels are 2-3 times higher than those in normal rats, it is
considered as successful establishment of an uremic animal model
(25,26). After the model is successfully
established, peritoneal dialysate can be infused directly or
through a dialysis catheter that is already implanted. There are
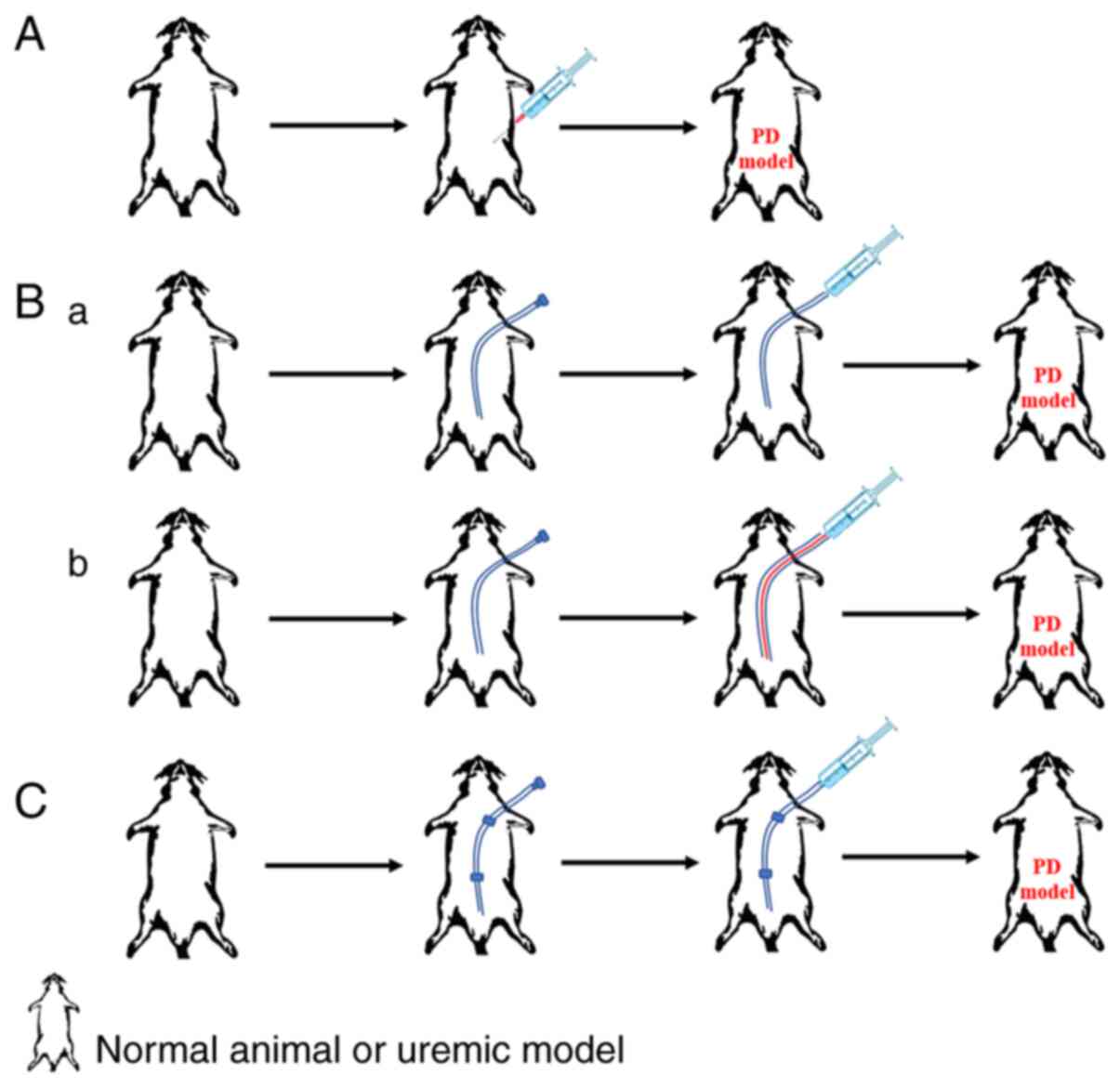
three main methods that are commonly used at present. The first
method is direct intraperitoneal injection, which can be with or
without anesthesia. However, this method can easily result in
unintentional injection into the abdominal wall (as opposed to the
intraperitoneal cavity) or even puncture of the blood vessels,
bladder or intestines (27,28); additionally, repeated injections
will increase the risk of infection (Fig. 1A). The second method is to establish
open access characterized by a peritoneal catheter inserted
subcutaneously into the peritoneal cavity of the animal then
leading from the neck. The peritoneal dialysate can be injected
directly into the catheter (Fig.
1Bb) and the catheter can be used as a fixed channel. During
each exchange, a new sterile catheter is inserted for dialysate
infusion. Upon completion, the sterile catheter is removed and the
channel is sealed (Fig. 1Bb).
Establishing open access does not require anesthesia, but the
incidence of infection is high, and catheter failure frequently
occurs due to hardening or adhesion of the omentum (27). The third method is to establish
closed access. In this method, an incision is made under the skin
of the neck, the catheter is permanently retained and connected to
the peritoneal cavity through a subcutaneous tunnel from the neck,
and the dialysate is retained in the abdominal cavity until it is
completely absorbed (Fig. 1C). This
method reduces the incidence of infection. However, catheter
blockage is still a problem (29).
The non-uremic model is a PD model which is directly
established in normal animals. Establishment methods can be further
divided into the following two types: i) Intraperitoneal injection
of the peritoneal dialysate alone; and ii) clinical simulation of
an indwelling peritoneal dialysis catheter (29). Both methods have their advantages
and disadvantages. Compared with catheter implantation, direct
intraperitoneal injection is easy to perform and can avoid damage
to the PD device caused by rats biting the catheter. However,
repeated puncture will cause mechanical peritoneal damage and
ultimately affect the experimental results (30). The catheter implantation model can
be further divided into the large omentum intact and the large
omentum resection models (27). The
omentum is an organ with defensive function. In a normal rat PD
model, keeping the large omentum intact during the PD process can
reduce the incidence of infection, however, the incidence of
catheter blockage is increased (31). Goh (32) proposed that peritoneal folding is a
safe and effective technique to solve the issue of catheter
occlusion.
4. Peritoneal function assessment
PD models are primarily used to study structural and
functional changes in peritoneal tissues after long-term exposure
to the peritoneal dialysate (33).
Therefore, the success of an animal PD model is crucial for
subsequent research. In addition, the success or failure of the
model is assessed by evaluation of the functional changes in the
peritoneum. At present, the commonly used method is the peritoneal
equilibrium test. The main parameters include ultrafiltration
volume, creatinine, urea nitrogen, and 24 h urine protein. The most
commonly used parameters for evaluating peritoneal transport
function are ultrafiltration volume and glucose transport volume,
which are detected after the dialysate is left in the peritoneal
cavity for four hours (34,35). Of note, is that uremia itself,
following nephrectomy, also affects the peritoneal structure and
permeability (25,36), which should receive comprehensive
consideration in specific studies.
5. Choice of peritoneal dialysis
catheter
Establishing smooth PD access is an essential step
for successful PD treatment. PD catheter-related factors are the
crux to establish this access. PD therapy is often withdrawn early
due to catheter dysfunction related to catheter displacement,
occlusion (32,37) and corrosion (38). Similar problems may also be
encountered during the preparation of animal models. Moreover, the
biomaterial present in the catheter can also affect the peritoneal
structure (39). Consequently, the
selection of higher quality PD catheters and appropriate
implantation position can effectively reduce the occurrence of
catheterization-related complications, such as exit site infection,
poor peritoneal dialysate outflow, or leakage. Ross et al
(40) found a significant reduction
in tissue inflammatory cells occurred when coating the peritoneal
dialysis catheter with a bioactive glass, which proved to have
important research value and application prospects in preventing
tunnel infection caused by the peritoneal catheter. In another
experimental study of non-uremic peritoneal dialysis rats (41), improvement of the material and
insertion method of the PD catheter were described. The catheter
was made of a silicone tube, and there was an iodophor cap on the
external catheter branch that could easily be replaced. In the
anesthetized rats, the back of the neck between ears and scapulae,
as well as the right-side and left-side of the back under the arcus
costarum were shaved. A longitudinal incision of 2 cm was made over
the skin of the left or right back, at a distance of 1 cm below the
arcus costarum and 1 cm from the lateral side to the spine. This
type of catheter insertion rarely causes complications such as
infection and catheter dysfunction. Additionally, it has
advantages, such as convenient operation, cost and practicality,
which make it worthy to use (41,42).
6. Potential complications in the
preparation of a PD model
Selection of a non-uremic model or a
uremic model
Non-uremic models can avoid complications caused by
nephrectomy and the development of uremia in animals (41). Compared with non-uremic experimental
models, uremic models can better simulate the clinical peritoneal
dialysis process. However, the model preparation period is long,
and hemorrhage, postoperative infection, and even death may occur
during this preparation process (43). Therefore, different models should be
selected according to different experimental purposes and actual
conditions. Commonly used methods to induce uremia include
nephrectomy and drug administration (44). Nephrectomy includes double
nephrectomy (43), 5/6 nephrectomy
and bilateral ureter ligation. Considering the technical aspects of
the experiment, preparation cycle of the model and
hemorrhage/infection risk during the preparation process, 5/6
nephrectomy is often the most suitable and is frequently used
(25,29,45,46).
Commonly used drug methods include adenine infusion (47,48)
and adriamycin infusion (49). It
is worth noting that different animal species have differences in
model establishment. Specifically, 5/6 nephrectomy, adenine
infusion or adriamycin infusion are usually used in rats, while
bilateral nephrectomy and bilateral ureter ligation are typically
used in rabbits (16,44).
Infection
Among the various complications of PD, the incidence
of infection is relatively high and possible infections include
peritonitis, subcutaneous tunnel infection and exit site infection.
Subcutaneous tunnel infection and exit site infection are common
causes of catheter extraction (50), while peritonitis is the most
frequent and serious complication. As described by Tăranu et
al (51), changes in peritoneal
morphology usually occur 3-4 years after starting PD, and they
progressively aggravate with passage of time on PD. When infection
occurs near the PD catheter site, pus secretion, erythema, pain or
swelling and other characteristics are often evident (52). Ordinary preventive measures against
infection are administration of antibiotics, such as cefazolin and
penicillin. In addition, injection of heparin on a regular basis
was found to have a positive effect on prevention and treatment of
catheter blockage (26,45). If infection occurs, antibiotics
should be actively used once the infection is confirmed. PD should
be restarted after the infection is treated. In severe cases, it
may be necessary to arrange for catheter replacement or even PD
termination (53).
Difference in peritoneal function
between animals and humans
Knowledge of the differences between the animal
peritoneum and human peritoneum in terms of morphology and
functions is significant. The main feature of human peritoneum is
that the peritoneal area is larger than the surface area of
glomerular capillaries of both kidneys, which is conducive to
peritoneal solute clearance (16,29).
Differences in peritoneal morphology between humans and animals
will inevitably lead to differences in physiological and
pathological mechanisms of peritoneal transport. Therefore,
interspecific differences in peritoneal morphology should be
considered when the results of animal experimental studies are
extended to clinical research and application (54). Peritoneal surface area has an
important effect on dialysis adequacy. Different animal models,
with their different peritoneal surface area/body surface area
ratios, will affect the dialysis efficiency (55). Another study found that the
proportion of parietal peritoneal area in rats is larger than that
in humans, that surface area of the peritoneum in lighter animals
is also larger and that the surface area increases with aging in
rats (56). Consequently, changes
in the experimental results due to animal age and weight need to be
fully considered in experimental research.
7. Conclusion
A successful PD model should simulate the clinical
PD process and have characteristics including good reproducibility,
feasibility and economic value. Additionally, it should also help
in the process of deeply understanding the etiology and
pathogenesis of PD-related diseases, providing guidance for
clinical treatment and prolonging the duration where PD can be
utilized as a dialysis method. There is currently no recognized
standard model for studying PD, and there are great differences in
the selection of animal models, administration routes, modeling
methods and model evaluation methods. Moreover, due to the
complexity and diversity of influencing factors in the PD process,
it is often not possible to use a single experimental model for all
aspects of the study. Therefore, future research is needed to
establish a relatively standardized experimental PD model that is
more in accordance with the clinical situation.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 81774065).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BY, MMW and XT wrote and revised the manuscript. GA,
LS and HTY reviewed and edited the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bajo MA, Del Peso G and Teitelbaum I:
Peritoneal membrane preservation. Semin Nephrol. 37:77–92.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mehrotra R, Devuyst O, Davies SJ and
Johnson DW: The Current state of peritoneal dialysis. J Am Soc
Nephrol. 27:3238–3252. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Krediet RT: Preservation of residual
kidney function and urine volume in patients on dialysis. Clin J Am
Soc Nephrol. 12:377–379. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Neumann D, Mau W, Wienke A and Girndt M:
Peritoneal dialysis is associated with better cognitive function
than hemodialysis over a one-year course. Kidney Int. 93:430–438.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen IM, Lin PH, Wu VC, Wu CS, Shan JC,
Chang SS and Liao SC: Suicide deaths among patients with end-stage
renal disease receiving dialysis: A population-based retrospective
cohort study of 64,000 patients in Taiwan. J Affect Disord.
227:7–10. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jain AK, Blake P, Cordy P and Garg AX:
Global trends in rates of peritoneal dialysis. J Am Soc Nephrol.
23:533–544. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li PK, Chow KM, Van de Luijtgaarden MW,
Johnson DW, Jager KJ, Mehrotra R, Naicker S, Pecoits-Filho R, Yu XQ
and Lameire N: Changes in the worldwide epidemiology of peritoneal
dialysis. Nat Rev Nephrol. 13:90–103. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kitterer D, Biegger D, Segerer S, Braun N,
Alscher MD and Latus J: Alteration of membrane complement
regulators is associated with transporter status in patients on
peritoneal dialysis. PLoS One. 12(e0177487)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cho Y and Johnson DW: Peritoneal
dialysis-related peritonitis: Towards improving evidence,
practices, and outcomes. Am J Kidney Dis. 64:278–289.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Aroeira LS, Aguilera A, Selgas R,
Ramírez-Huesca M, Pérez-Lozano ML, Cirugeda A, Bajo MA, del Peso G,
Sánchez-Tomero JA, Jiménez-Heffernan JA, et al: Mesenchymal
conversion of mesothelial cells as a mechanism responsible for high
solute transport rate in peritoneal dialysis: Role of vascular
endothelial growth factor. Am J Kidney Dis. 46:938–948.
2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
López-Cabrera M, Aguilera A, Aroeira LS,
Ramírez-Huesca M, Pérez-Lozano ML, Jiménez-Heffernan JA, Bajo MA,
del Peso G, Sánchez-Tomero JA and Selgas R: Ex vivo analysis of
dialysis effluent-derived mesothelial cells as an approach to
unveiling the mechanism of peritoneal membrane failure. Perit Dial
Int. 26:26–34. 2006.PubMed/NCBI
|
|
12
|
Kim YL: Update on mechanisms of
ultrafiltration failure. Perit Dial Int. 29 (Suppl 2):S123–S127.
2009.PubMed/NCBI
|
|
13
|
Fan YP, Hsia CC, Tseng KW, Liao CK, Fu TW,
Ko TL, Chiu MM, Shih YH, Huang PY, Chiang YC, et al: The
therapeutic potential of human umbilical mesenchymal stem cells
from Wharton's jelly in the treatment of rat peritoneal
dialysis-induced fibrosis. Stem Cells Transl Med. 5:235–247.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Büchel J, Bartosova M, Eich G,
Wittenberger T, Klein-Hitpass L, Steppan S, Hackert T, Schaefer F,
Passlick-Deetjen J and Schmitt CP: Interference of peritoneal
dialysis fluids with cell cycle mechanisms. Perit Dial Int.
35:259–274. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
ter Wee PM, Beelen RH and van den Born J:
The application of animal models to study the biocompatibility of
bicarbonate-buffered peritoneal dialysis solutions. Kidney Int
Suppl. 88:S75–S83. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nikitidou O, Peppa VI, Leivaditis K,
Eleftheriadis T, Zarogiannis SG and Liakopoulos V: Animal models in
peritoneal dialysis. Front Physiol. 6(244)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ni J, Cnops Y, Debaix H, Boisdé I,
Verbavatz JM and Devuyst O: Functional and molecular
characterization of a peritoneal dialysis model in the C57BL/6J
mouse. Kidney Int. 67:2021–2031. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Van Biesen W, Vanholder R and Lameire N:
Animal models in peritoneal dialysis: A story of kangaroos and
ostriches. Perit Dial Int. 26:571–573. 2006.PubMed/NCBI
|
|
19
|
González-Mateo GT, Fernández-Míllara V,
Bellón T, Liappas G, Ruiz-Ortega M, López-Cabrera M, Selgas R and
Aroeira LS: Paricalcitol reduces peritoneal fibrosis in mice
through the activation of regulatory T cells and reduction in IL-17
production. PLoS One. 9(e108477)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xiang S, Li M, Xie X, Xie Z, Zhou Q, Tian
Y, Lin W, Zhang X, Jiang H, Shou Z, et al: Rapamycin inhibits
epithelial-to-mesenchymal transition of peritoneal mesothelium
cells through regulation of Rho GTPases. FEBS J. 283:2309–2325.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hirahara I, Sato H, Imai T, Onishi A,
Morishita Y, Muto S, Kusano E and Nagata D: Methylglyoxal induced
basophilic spindle cells with podoplanin at the surface of
peritoneum in rat peritoneal dialysis model. BioMed Res Int.
2015(289751)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li Z, Yan H, Yuan J, Cao L, Lin A, Dai H,
Ni Z, Qian J and Fang W: Pharmacological inhibition of
heparin-binding EGF-like growth factor promotes peritoneal
angiogenesis in a peritoneal dialysis rat model. Clin Exp Nephrol.
22:257–265. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Guo J, Xiao J, Gao H, Jin Y and Zhao Z,
Jiao W, Liu Z and Zhao Z: Cyclooxygenase-2 and vascular endothelial
growth factor expressions are involved in ultrafiltration failure.
J Surg Res. 188:527–536.e2. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Miller TE, Findon G and Rowe L:
Characterization of an animal model of continuous peritoneal
dialysis in chronic renal impairment. Clin Nephrol. 37:42–47.
1992.PubMed/NCBI
|
|
25
|
Ferrantelli E, Liappas G, Keuning ED, Vila
Cuenca M, González-Mateo G, Verkaik M, López-Cabrera M and Beelen
RH: A novel mouse model of peritoneal dialysis: combination of
uraemia and long-term exposure to PD fluid. BioMed Res Int.
2015(106902)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zareie M, De Vriese AS, Hekking LH, ter
Wee PM, Schalkwijk CG, Driesprong BA, Schadee-Eestermans IL, Beelen
RH, Lameire N and van den Born J: Immunopathological changes in a
uraemic rat model for peritoneal dialysis. Nephrol Dial Transplant.
20:1350–1361. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Peng WX, Guo QY, Liu SM, Liu CZ, Lindholm
B and Wang T: Comparison of three chronic dialysis models. Adv
Perit Dial. 16:51–54. 2000.PubMed/NCBI
|
|
28
|
van Westrhenen R, Westra WM, van den Born
J, Krediet RT, Keuning ED, Hiralall J, Dragt C and Hekking LH:
Alpha-2-macroglobulin and albumin are useful serum proteins to
detect subclinical peritonitis in the rat. Perit Dial Int.
26:101–107. 2006.PubMed/NCBI
|
|
29
|
Pawlaczyk K, Baum E, Schwermer K, Hoppe K,
Lindholm B and Breborowicz A: Animal models of peritoneal dialysis:
thirty years of our own experience. BioMed Res Int.
2015(261813)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang J, Liu S, Li H, Sun J, Zhang S, Xu X,
Liu Y, Wang Y and Miao L: A review of rodent models of peritoneal
dialysis and its complications. Int Urol Nephrol. 47:209–215.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
De Vriese AS, Mortier S, Cornelissen M,
Palmans E, Vanacker NJ, Leyssens A, Faict D, De Ridder L and
Lameire NH: The effects of heparin administration in an animal
model of chronic peritoneal dialysate exposure. Perit Dial Int.
22:566–572. 2002.PubMed/NCBI
|
|
32
|
Goh YH: Omental folding: A novel
laparoscopic technique for salvaging peritoneal dialysis catheters.
Perit Dial Int. 28:626–631. 2008.PubMed/NCBI
|
|
33
|
Fabbrini P, Zareie M, Ter Wee PM, Keuning
ED, Beelen RH and van den Born J: Peritoneal exposure model in the
rat as a tool to unravel bio(in)compatibility of PDF. Nephrol Dial
Transplant. 21 (Suppl 2):ii8–ii11. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yu D, Cai Y, Qin R, Tian X, Xiao J and
Zhao Z: Dialysate Creatinine Response Patterns During Peritoneal
Equilibration Test and the Association Between Cardiovascular
Mortality: Findings from a Prospective Cohort Study. Kidney Blood
Press Res. 43:162–169. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sun Y, Zhu F, Yu X, Nie J, Huang F, Li X,
Luo N, Lan HY and Wang Y: Treatment of established peritoneal
fibrosis by gene transfer of Smad7 in a rat model of peritoneal
dialysis. Am J Nephrol. 30:84–94. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Combet S, Ferrier ML, Van Landschoot M,
Stoenoiu M, Moulin P, Miyata T, Lameire N and Devuyst O: Chronic
uremia induces permeability changes, increased nitric oxide
synthase expression, and structural modifications in the
peritoneum. J Am Soc Nephrol. 12:2146–2157. 2001.PubMed/NCBI
|
|
37
|
Lee M and Donovan JF: Laparoscopic
omentectomy for salvage of peritoneal dialysis catheters. J
Endourol. 16:241–244. 2002.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kouri AM, Wilson AC and Nailescu C: A
malfunctioning peritoneal dialysis catheter: Answers. Pediatr
Nephrol. 32:441–442. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Guo W, Willén R, Andersson R, Pärsson H,
Liu X, Johansson K and Bengmark S: Morphological response of the
peritoneum and spleen to intraperitoneal biomaterials. Int J Artif
Organs. 16:276–284. 1993.PubMed/NCBI
|
|
40
|
Ross EA, Batich CD, Clapp WL, Sallustio JE
and Lee NC: Tissue adhesion to bioactive glass-coated silicone
tubing in a rat model of peritoneal dialysis catheters and catheter
tunnels. Kidney Int. 63:702–708. 2003.PubMed/NCBI
|
|
41
|
Peng YM, Shu ZJ, Xiao L, Sun L, Tang WB,
Huang Y, Liu YH, Li J, Ling GH, Xu XQ, et al: A new non-uremic rat
model of long-term peritoneal dialysis. Physiol Res. 60:157–164.
2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhao JL, Zhang T, Shao X, Zhu JJ and Guo
MZ: Curcumin ameliorates peritoneal fibrosis via inhibition of
transforming growth factor-activated kinase 1 (TAK1) pathway in a
rat model of peritoneal dialysis. BMC Complement Altern Med.
19(280)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kajbafzadeh AM, Sabetkish N and Sabetkish
S: Establishment of colonic dialysis model in uremic rats by right
nephrectomy and left partial nephrectomy. J Pediatr Urol.
14:159.e1–159.e8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bao YW, Yuan Y, Chen JH and Lin WQ: Kidney
disease models: Tools to identify mechanisms and potential
therapeutic targets. Zool Res. 39:72–86. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kakuta T, Tanaka R, Satoh Y, Izuhara Y,
Inagi R, Nangaku M, Saito A and Miyata T: Pyridoxamine improves
functional, structural, and biochemical alterations of peritoneal
membranes in uremic peritoneal dialysis rats. Kidney Int.
68:1326–1336. 2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gotloib L, Crassweller P, Rodella H,
Oreopoulos DG, Zellerman G, Ogilvie R, Husdan H, Brandes L and Vas
S: Experimental model for studies of continuous peritoneal'dialysis
in uremic rabbits. Nephron. 31:254–259. 1982.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Silva FMO, Costalonga EC, Silva C,
Carreira ACO, Gomes SA, Sogayar MC, Fanelli C and Noronha IL:
Tamoxifen and bone morphogenic protein-7 modulate fibrosis and
inflammation in the peritoneal fibrosis model developed in uremic
rats. Mol Med. 25(41)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Santana AC, Degaspari S, Catanozi S, Dellê
H, de Sá Lima L, Silva C, Blanco P, Solez K, Scavone C and Noronha
IL: Thalidomide suppresses inflammation in adenine-induced CKD with
uraemia in mice. Nephrol Dial Transplant. 28:1140–1149.
2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yasuda K, Park HC, Ratliff B, Addabbo F,
Hatzopoulos AK, Chander P and Goligorsky MS: Adriamycin
nephropathy: A failure of endothelial progenitor cell-induced
repair. Am J Pathol. 176:1685–1695. 2010.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Liakopoulos V, Nikitidou O, Kalathas T,
Roumeliotis S, Salmas M and Eleftheriadis T: Peritoneal
dialysis-related infections recommendations: 2016 update What is
new? Int Urol Nephrol. 49:2177–2184. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Tăranu T, Florea L, Păduraru D, Georgescu
SO, Frâncu LL and Stan CI: Morphological changes of the peritoneal
membrane in patients with long-term dialysis. Rom J Morphol
Embryol. 55:927–932. 2014.PubMed/NCBI
|
|
52
|
Siddiqui M, Bradford L, Kaley J, Johnson
G, Kim KH, Addis K and Singh M: Noninfectious peritoneal dialysis
exit site rash-an unusual case report and review of the literature.
Kidney Int Rep. 3:11–13. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Choi J, Credit K, Henderson K, Deverkadra
R, Vanpelt HM, He Z and Flessner MF: Antibiotic prophylaxis in an
animal model of chronic peritoneal exposure. Perit Dial Int.
26:249–258. 2006.PubMed/NCBI
|
|
54
|
Pawlaczyk K, Kuzlan M, Wieczorowska-Tobis
K, Pawlik-Juzków H, Breborowicz A, Knapowski J and Oreopoulos DG:
Species-dependent topography of the peritoneum. Adv Perit Dial.
12:3–6. 1996.PubMed/NCBI
|
|
55
|
Fischbach M, Dheu C, Helms P, Terzic J,
Michallat AC, Laugel V, Wolff-Danner S and Haraldsson B: The
influence of peritoneal surface area on dialysis adequacy. Perit
Dial Int. 25 (Suppl 3):S137–S140. 2005.PubMed/NCBI
|
|
56
|
Kuzlan M, Pawlaczyk K, Wieczorowska-Tobis
K, Korybalska K, Breborowicz A and Oreopoulos DG: Peritoneal
surface area and its permeability in rats. Perit Dial Int.
17:295–300. 1997.PubMed/NCBI
|