Introduction
Obstructive jaundice is a frequently observed
condition caused by biliary tract occlusion, which inevitably
causes liver injury (1). In biliary
obstruction, secondary gram-negative pathogen infection may occur,
causing acute obstructive cholangitis, which progresses rapidly and
has a poor prognosis (2,3). The liver is the first organ to become
involved and is severely injured during biliary obstruction. An
important factor affecting prognosis is the extent of liver injury.
Therefore, the identification of effective methods to alleviate
cholestasis-induced liver injury can improve the prognosis of this
disease.
Heat shock proteins (HSPs) are highly conserved
proteins that are induced by a wide variety of physiological and
environmental insults, such as heat, inflammation, toxic chemicals
and oxidative stress. HSP90 is an essential member of the HSP
family of chaperone proteins, and is upregulated in various
conditions, including inflammation, organ injury and cancer
(4-7).
According to its subcellular localization, HSP90 comprises
cytosolic HSP90 (HSP90AA1), endoplasmic reticulum-localized
chaperone HSP90 (HSP90B1, also known as gp96 or grp94) and the
mitochondrial member TRAP1 (HSP90L) (8). The potential therapeutic effects of
HSP90 inhibitors, such as
17-dimethylamino-ethylamino-17-demethoxygeldanamycin (17-DMAG),
have been the focus of a number of studies due to the important
roles of HSP90 (9-11).
Although studies have shown that 17-DMAG effectively prevents
lipopolysaccharide (LPS)- or alcohol-induced liver injury (11,12),
the applicability of HSP90 inhibitors to biliary
obstruction-induced liver injury remains unclear.
Toll-like receptors (TLRs) are a family of pattern
recognition receptors for pathogens with critical roles in innate
immunity (13). TLR9 is an
essential TLR and regulates pro-inflammatory cytokines, such as
interleukin (IL)-1β and IL-18 (14,15).
In addition, HSP90B1 is required for the folding of TLR9 and is
involved in the biogenesis of TLR9 (16,17).
TLR9 has been identified as a recipient protein of HSP90 and is
associated with certain refractory diseases, such as hepatotoxicity
(15).
In the present study, we hypothesized that HSP90
inhibition may reduce liver injury during biliary obstruction.
Therefore, 17-DMAG was used to evaluate the effects of HSP90
inhibition in an animal model of biliary obstruction.
Materials and methods
Animal model
Wistar rats weighing 300-350 g and aged 12 weeks
were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. All
procedures were approved by the Ethics Committee of Shaoxing
People's Hospital and conformed to the Guide for the Care and Use
of Laboratory Animals published by the US National Institutes of
Health (revised 2011). The rats had ad libitum access to
food and water and were maintained at 20˚C, with 50% humidity under
12-h light/dark cycles.
Rats were randomly grouped into sham, bile duct
ligation (BDL) and BDL + lipopolysaccharide (LPS) groups. The
animals were anesthetized with an intraperitoneal injection of 50
mg/kg pentobarbital. In the BDL and BDL + LPS groups, the distal
common bile ducts were dissociated and ligated with 6-0 silk, and
PE-10 polyethylene catheters long enough to reach the skin were
inserted into the proximal bile ducts as described in our previous
study (18,19). Intra-bile duct infusion was
performed by injecting 0.2 ml saline or LPS (2 mg/ml, purified from
Escherichia coli O111:B4; Sigma-Aldrich; Merck KGaA) into
the proximal bile ducts through the catheter. The catheter was
sealed with a sealing cap and the abdominal cavity was covered
using silk sutures following the introduction of 0.1 ml air. The
rats in the sham group underwent a sham procedure, which involved
dissecting bile duct carefully without injury and closing the
abdominal cavity. The rats in the sham, BDL and BDL + LPS groups
were further divided into three groups according to the
intraperitoneal injections they received after surgery. Briefly, in
each group, one-third of the rats were intraperitoneally injected
with normal saline (NS), another one-third were intraperitoneally
injected with 2 mg/kg 17-DMAG (MedChemExpress) and the remaining
one-third were intraperitoneally injected with 5 mg/kg 17-DMAG. The
intraperitoneal injections were performed daily. A total of 108
rats were randomly classified into the aforementioned three groups
according to the surgical procedure (n=36/group). Each of these
groups comprised rats treated with NS, 2 mg/kg 17-DMAG and 5 mg/kg
17-DMAG (n=12/treatment), respectively. At 24 and 72 h after the
surgery, a fraction (n=6/group/time point) of the rats in each
group were euthanized. Caspase-3 activity detection and pathology
experiments, including tissue staining and immunohistochemistry,
were performed on samples taken at 72 h. Other experiments were
performed on samples taken at 24 h. The rats were sacrificed via
cervical dislocation under 2.5% sevoflurane inhalation anesthesia.
The death of the animals was confirmed by examining the cessation
of vital signs. Blood and liver tissue samples were harvested from
the euthanized animals. Prior to analysis, blood samples were
centrifuged (1,000 x g for 5 min at 4˚C) and the serum was stored
at -80˚C. Liver tissues were snap-frozen in liquid nitrogen or
fixed with 10% formalin.
Clinical tissue samples
Clinical tissue samples were obtained from patients
who underwent hepatectomy at Shaoxing People's Hospital (Shaoxing,
China) from January 2014 to December 2017. Normal liver tissues
(n=5; age range, 42-63 years; inclusion criteria: Definite
pathological diagnosis of hemangioma; exclusion criteria: Liver
cirrhosis, fatty liver, hepatitis virus positive, liver tumor and
hepatolithiasis) were obtained from patients who underwent
hepatectomy to treat liver hemangioma; non-hemangioma tissues were
selected for pathological examination. Intrahepatic cholangitic
liver tissues (n=5; age range: 45-69 years; inclusion criteria:
Definite pathological diagnosis of hepatolithiasis; exclusion
criteria: Liver cirrhosis, fatty liver, hepatitis virus positive
and liver tumor, such as intrahepatic cholangiocarcinoma) were
obtained from patients who underwent hepatectomy to treat
intrahepatic stones and cholangitis. There were two male patients
and three female patients in the two groups, respectively. All
participants provided written informed consent, and Shaoxing
People's Hospital Institutional Review Board approved the tissue
acquisition protocol.
Evaluation of liver function
An Automated Chemical Analyzer (Dimension RxL Max
HM; Siemens AG) was used to quantify the levels of aspartate
aminotransferase (AST), alanine aminotransferase (ALT) and total
bilirubin (TBIL) in rat serum to determine the degree of liver
injury.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from liver tissue samples or
cells using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) and
reverse transcribed into cDNA using a PrimeScript™ RT reagent Kit
(cat. no. RR047A; Takara Bio, Inc.), according to the
manufacturer's protocols. Briefly, RT was conducted at 37˚C for 15
min and 85˚C for 5 sec, and the cDNA was stored at 4˚C until
further use. qPCR was performed using SYBR® Premix Ex
Taq™ II (cat. no. RR820A; Takara Bio, Inc.) and an ABI 7500
Real-time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. The primer
sequences used are shown in Table
I. β-actin was used as an endogenous control. The qPCR
conditions comprised 95˚C for 30 sec, and a total of 40 cycles at
95˚C for 5 sec and extension at 60˚C for 34 sec, followed by a
dissociation curve analysis. All assays were performed three times.
Relative expression levels were then determined using the
2-ΔΔCq method (20).
 | Table IPrimer sequences for quantitative
polymerase chain reaction. |
Table I
Primer sequences for quantitative
polymerase chain reaction.
| Gene | Forward
(5'-3') | Reverse
(5'-3') |
|---|
| β-actin |
ACACCCGCCACCAGTTCG |
CCCACGATGGAGGGGAAGA |
| Hsp90aa1 |
TCAGGCAGAAATTGCCCAGT |
ATCCAGAGCGTCTGAGGAGT |
| Hsp90b1 |
ACCGAAAAGGACTTGCGACT |
GCTCTCACAAACCCGAAGGT |
| Trap1 |
TGGCACCCGCAACATCTATT |
CGTAGCAGAAGAGCACCTCA |
| Hspa1b |
CTCCTTCGTTCGGTCTGCAA |
TGCAAAGACACACTCCCAGT |
| Hspa4 |
TCAGAGCTGCTATGTCGCTG |
GGCATTGGAAATTACCTGGCTC |
| Bax |
GGGATGGCCTCCTTTCCTAC |
CTTTCCCCGTTCCCCATTCA |
| Bcl2 |
AGCATGCGACCTCTGTTTGA |
TCACTTGTGGCCCAGGTATG |
| IL-1β |
CAGCTTTCGACAGTGAGGAGA |
TTGTCGAGATGCTGCTGTGA |
| IL-18 |
ACCGCAGTAATACGGAGCAT |
CTGGGATTCGTTGGCTGTTC |
Caspase-3 activity detection
A Caspase-3 Activity Assay Kit (cat. no. C1116;
Beyotime Institute of Biotechnology) was used according to the
manufacturer's protocol to detect the activity of caspase-3 in the
rat liver tissues. The absorbance was measured at 405 nm and the
relative caspase-3 activity to the activity of the sham group was
determined. Six samples from each group were analyzed.
Enzyme-linked immunosorbent assay
(ELISA)
The liver tissues were weighed and homogenized
immediately in 10 volumes of saline after washing in saline.
Supernatants were collected to perform ELISAs following
centrifugation (12,000 x g for 10 min at 4˚C). ELISA kits (cat.
nos. BMS630 and KRC2341, eBioscience; Thermo Fisher Scientific,
Inc.) were used according to the manufacturer's instructions to
measure the content of IL-1β and IL-18 in the liver samples. Each
sample was tested in triplicate.
Tissue staining
Liver tissue samples obtained from the rats were
fixed with 10% formalin. The formalin-fixed specimens (3 µm) were
stained using automatic H&E slide stainer (Leica ST5020
Multistainer; Leica Microsystems, Inc.) according to the
manufacturer's instructions for histological evaluation. The
stained liver tissues were examined for histopathological evidence
of pathological damage under light microscope (magnification,
x100).
Immunohistochemistry (IHC)
HSP90 expression in the rat liver tissues was
detected by IHC. Deparaffinization and rehydration of the sections
(3 µm thickness) were performed with gradient xylene and a
descending ethanol gradient. Antigen retrieval was performed with
Citrate Antigen Retrieval solution (1:50; cat. no. P0083; Beyotime
Institute of Biotechnology) at 95˚C for 20 min. Endogenous
peroxidase activity was quenched using 0.3% hydrogen peroxide
(25˚C, 10 min). The sections were blocked with 1% BSA (Beyotime
Institute of Biotechnology) for 2 h at room temperature. The
sections were then incubated with primary anti-HSP90 antibody
(1:100; cat. no. 13171-1-AP; ProteinTech Group, Inc.) overnight at
4˚C, followed by incubation with horseradish peroxidase-conjugated
secondary antibody (1:50; cat. no. A0208; Beyotime Institute of
Biotechnology) for 1.5 h at room temperature. After thoroughly
washing with washing buffer (cat. no. P0106L; Beyotime Institute of
Biotechnology), the sections were developed using a
3,3'-diaminobenzidine kit (cat. no. P0202; Beyotime Institute of
Biotechnology) and counterstained with hematoxylin staining
solution (cat. no. C0107; Beyotime Institute of Biotechnology) for
10 min at room temperature. Each stained sample was observed under
high power magnification using a light microscope (magnification,
x200; Leica Microsystems, Inc.).
‘Cholestatic culture’ assay
BDL and sham rat models were prepared using the
aforementioned procedure. After 1 week, cholestatic blood samples
were harvested from the rats and cholestatic serum was obtained
after centrifugation (2,000 x g for 10 min at 4˚C). Cholestatic
serum samples from all BDL model rats were mixed in one tube and
non-cholestatic serum samples from all sham model rats were mixed
in another tube. The sera were filtered using a 0.22-µm filter
(Millipore; Merck KGaA) for sterilization. The automated chemical
analyzer was used to examine the cholestatic and non-cholestatic
serum. The cholestatic serum contained 188.02 µmol/l total
bilirubin (TBIL) and 146.6 µmol/l direct bilirubin (DBIL), while
the non-cholestatic serum contained 2.3 µmol/l TBIL and 1.72 µmol/l
DBIL. The two types of serum were used in the subsequent
experiments.
The BRL rat hepatic cell line was obtained from the
Shanghai Cell Bank of the Chinese Academy of Sciences. Liver
sinusoidal endothelial cells (LSECs) were isolated from the rat
liver tissues with modifications to the previously described
protocol (21). Briefly, the rat
liver tissue was perfused with collagenase (Invitrogen; Thermo
Fisher Scientific, Inc.). The single-cell suspension was subjected
to velocity and density centrifugations (50 x g for 2 min at 25˚C)
in Percoll gradients to separate hepatocyte and nonparenchymal cell
suspensions. The nonparenchymal cell suspension was incubated with
CD146 (LSEC)-biotin antibodies (1:50; cat. no. 130-111-844;
Miltenyi Biotec, Inc.) for cell surface staining (30 min at room
temperature). The LSEC cells were positively selected by magnetic
separation using Anti-Biotin MicroBeads (Miltenyi Biotec, Inc.)
according to the manufacturer's instructions.
Prior to stimulation with serum, BRL cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.), and LSECs were
cultured with a medium comprising 40% MCDB 131 (Gibco; Thermo
Fisher Scientific, Inc.), 40% RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc.) and 20% FBS. The ‘cholestatic culture’ was
initiated when the FBS was replaced with the cholestatic serum or
non-cholestatic serum obtained from the rats.
Cell Counting Kit-8 (CCK-8) assay
BRL cells were seeded into a 96-well plate at a
density of 2x103 cells/well and allowed to attach
overnight at 37˚C. The cells were then cultured in a medium
containing non-cholestatic serum or cholestatic serum, with or
without 17-DMAG (0.5 µM) for 48 h. Cell proliferation was
determined using the CCK-8 assay (MedChemExpress). Briefly, a 1/10
volume of CCK-8 solution was added to each well and the cells were
cultured for 2 h. Cell viability was then determined by measuring
the absorbance at 450 nm using a plate reader (BioTek Instruments,
Inc.). Six repeated wells for each cell condition were
analyzed.
Flow cytometric analysis
BRL cells were cultured in a cholestatic medium with
or without 17-DMAG (0.5 µM) at 37˚C. After 24 h, the cells were
collected and stained using Annexin V-FITC and propidium iodide
(Becton, Dickinson and Company) according to the manufacturer's
instructions. Apoptotic cells were quantified by flow cytometry
using a Flow Cytometer (Cytomics FC500; Beckman Coulter, Inc.). The
data was analyzed with CXP software version 1.0 (Beckman Coulter).
The experiments were performed in triplicates.
Statistical analysis
Data are presented as the mean ± SD. The statistical
significance of a difference between two groups was determined
using the Student's t-test. Two-way ANOVA followed by Bonferroni's
adjustment were used to evaluate differences among multiple groups.
P<0.05 was considered to indicate a statistically significant
result. All statistical analyses were conducted using SPSS 13.0
software (SPSS, Inc.).
Results
HSP90 expression is upregulated during
cholestatic liver injury
Serum levels of ALT, AST and TBIL were examined to
confirm the successful establishment of the animal model. The
significantly increased ALT, AST and TBIL levels in the BDL group
compared with sham group were indicative of substantial cholestasis
and liver injury in the BDL group (Fig.
1A). In addition to simple biliary obstruction, the
gram-negative pathogen LPS is another factor that aggravates
biliary obstruction-induced liver injury (22). Therefore, a BDL + LPS rat model was
established to simulate frequently occurring clinical cases. As
shown in Fig. 1A, LPS
administration further aggravated the BDL-induced liver injury.
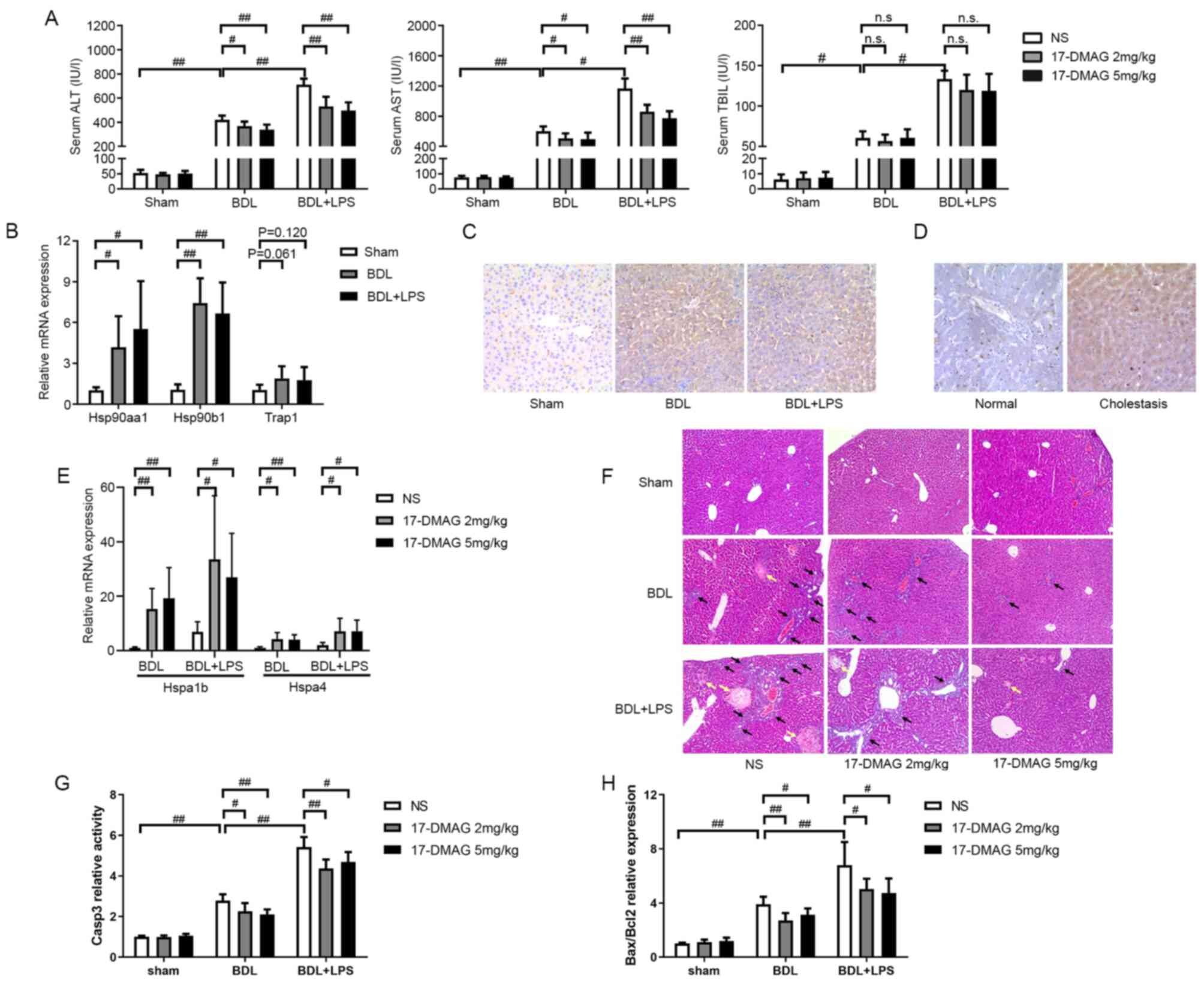 | Figure 117-DMAG alleviates cholestatic liver
injury in vivo. (A) ALT, AST and TBIL were examined in rat
serum 24 h after BDL or sham surgery (n=6/group). The rapidly
increased ALT, AST and TBIL levels demonstrated that
cholestasis-induced liver injury occurred in the BDL and BDL + LPS
groups. (B) Relative expression of HSP90 mRNA in liver tissues from
three rat models (n=6/group). Hsp90aa1 and Hsp90b1 mRNA were
upregulated after biliary obstruction. (C) IHC staining shows that
HSP90 protein was upregulated 72 h after biliary obstruction
(images are representative of 6 rats/group; magnification, x200).
(D) IHC staining shows that HSP90 expression was upregulated in
patients with intrahepatic cholangitis compared with (images are
representative of five rats/group; magnification, x200). (E)
Relative mRNA expression levels of HSP70 family members Hspa1b and
Hspa4 in rat liver tissues (n=6/group). Elevated mRNA levels of
HSP70 indicate that the activity of HSP90 was effectively
inhibited. (F) Histological examination (magnification, x100), (G)
the activity of caspase-3 and (H) the ratio of Bax to Bcl2
expression levels show that 17-DMAG alleviated cholestasis-induced
liver injury (n=6/group). Black arrows indicate inflammatory cell
infiltration and yellow arrows indicate necrosis.
#P<0.05; ##P<0.01. 17-DMAG,
17-dimethylamino-ethylamino-17-demethoxygeldanamycin; ALT, alanine
aminotransferase; AST, aspartate aminotransferase; TBIL, total
bilirubin; NS, normal saline; HSP90, heat shock protein 90;
Hsp90aa1, mitochondrial HSP90; Hsp90b1, cytosolic HSP90; Trap1,
endoplasmic reticulum-localized HSP90; IHC, immunohistochemistry;
BDL, bile duct ligation; LPS, lipopolysaccharide; HSP70, heat shock
protein 70; Hspa1b, HSP70 member 1b; Hspa4, HSP70 member 4; casp3,
caspase-3; n.s., not significant. |
The mRNA expression levels of three HSP90 family
members in the liver tissues of the BDL and BDL + LPS rat models
were compared with those in the sham group to clarify the
association between HSP90 expression and cholestatic liver injury.
In the BDL and BDL + LPS groups, the results showed that Hsp90aa1
and Hsp90b1 mRNA levels were significantly upregulated compared
with those in the sham group (Fig.
1B). IHC showed that the HSP90 protein expression was also
upregulated in the liver tissues of rats in the BDL and BDL + LPS
groups (Fig. 1C). Whether a similar
phenomenon exists in human liver tissues was also examined. Liver
specimens obtained from patients with hepatolithiasis or
intrahepatic cholangitis who had undergone hepatectomy were
analyzed using IHC. The results demonstrated that HSP90 expression
was upregulated in the patients with intrahepatic cholangitis
(Fig. 1D). These results imply that
aberrant HSP90 expression is associated with cholestasis and
cholangitis.
HSP90 inhibitor 17-DMAG alleviates
cholestasis-induced liver injury
Previous studies have supported the potential of
17-DMAG, a water-soluble HSP90 inhibitor, as a therapeutic agent
(23,24). The effect of 17-DMAG on
cholestasis-induced liver injury was therefore investigated in
vivo to identify whether the inhibition of HSP90 enhances the
transcription of HSP70, as indicated by previous studies (25-27).
The elevated mRNA levels of HSP70 members 1b and 4 (Hspa1b and
Hspa4, respectively) in the rats following 17-DMAG administration
indicated that the activity of HSP90 was effectively inhibited
(Fig. 1E).
Following the administration of 17-DMAG or NS to the
rats, the serum levels of ALT and AST were assessed. ALT and AST
levels in the BDL and BDL + LPS groups exhibited significant
reductions upon treatment with 17-DMAG compared with those in the
NS-treated rats (Fig. 1A). However,
17-DMAG administration did not significantly mitigate the increase
of TBIL levels after the onset of cholestasis (Fig. 1A).
A morphological assessment was performed to evaluate
the degree of injury exhibited by the rat liver tissue.
Pathological examination revealed dilation of the bile capillaries
and increased local necrosis in the livers of rats with BDL-induced
cholestasis (Fig. 1F). In addition,
following LPS administration, large areas of necrosis and increased
inflammatory cell infiltration were observed (Fig. 1F). However, compared with NS
treatment, 17-DMAG treatment reduced local necrosis and
inflammatory cell infiltration during cholestasis with or without
LPS (Fig. 1F).
Caspase-3 activity and the ratio of Bax to Bcl2 are
often used to evaluate the apoptotic response (28). Notably, caspase-3 activity increased
significantly in the liver tissues of the BDL and BDL + LPS groups
compared with the sham group. However, treatment with 17-DMAG
effectively attenuated this change (Fig. 1G). Furthermore, the Bax to Bcl2
ratio exhibited similar changes (Fig.
1H). These results indicate that 17-DMAG effectively alleviated
cholestasis-induced liver injury.
17-DMAG protects hepatocytes against
cholestasis-induced cell damage in vitro
A ‘cholestatic culture’ experiment was conducted to
further clarify the function of 17-DMAG during cholestasis-induced
cell damage in vitro. Cholestatic serum from BDL model rats
was added to the culture medium of BRL cells to simulate the
cholestatic microenvironment in vitro. CCK-8 assays were
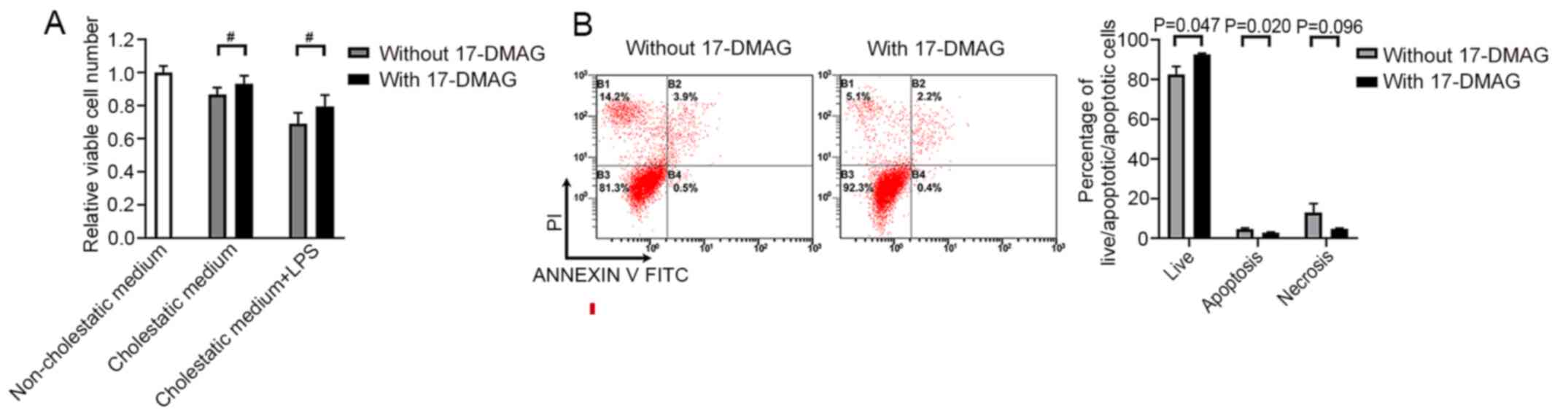
then performed to evaluate cell viability. Moderate cytotoxicity
was observed when BRL cells were treated with cholestatic serum
(Fig. 2A). However, 17-DMAG
administration significantly alleviated this cytotoxicity, whether
or not LPS was present in vitro (Fig. 2A). In addition, the flow cytometric
analysis of cell apoptosis indicated that 17-DMAG administration
promoted the survival of BRL cells and reduced the number of
apoptotic cells in the cholestatic serum-containing medium
(Fig. 2B).
17-DMAG administration prevents
cholestasis-induced liver injury by decreasing the expression of
IL-1β and IL-18 in vivo
Pro-inflammatory cytokines induced by bile acid play
an important role in cholestasis-induced liver injury (29,30).
IL-1β and IL-18 are important cytokines, as they have a role in the
induction of apoptosis during liver injury (15,31).
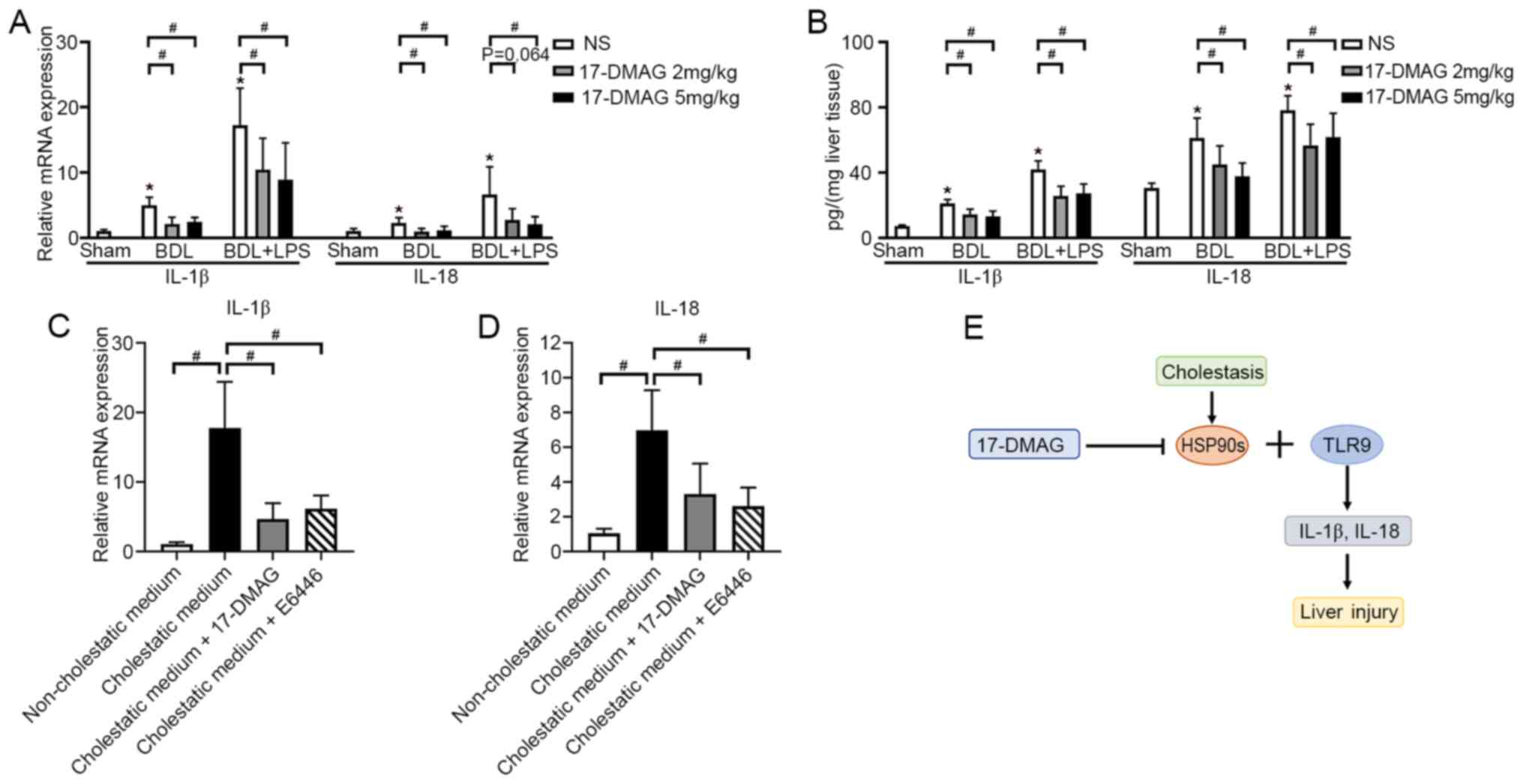
Therefore, they were analyzed in the present study. The results
showed that the mRNA expression levels of IL-1β and IL-18 increased
significantly during cholestasis-induced liver injury, and
treatment with 17-DMAG substantially attenuated these changes
(Fig. 3A). ELISAs of the liver
tissues also demonstrated that the cholestasis-induced increases in
IL-1β and IL-18 levels were significantly attenuated following
treatment with 17-DMAG (Fig.
3B).
17-DMAG administration decreases the
expression of IL-1β and IL-18 by LSECs in vitro
LSECs serve an important role in the immune response
and liver injury (32,33). LSECs were isolated from rats for
in vitro analyses to study the underlying mechanism. The
results showed that the addition of cholestatic medium significantly
increased the expression of IL-1β and IL-18 by LSECs, compared with
non-cholestatic medium (Fig. 3C and
D). Notably, the administration of
17-DMAG significantly attenuated the increase in the expression of
IL-1β and IL-18 induced by cholestatic medium (Fig. 3C and D).
Mammalian TLRs detect microbial infection and
regulate the cell death-associated cellular response during stress.
The HSP90 family, particularly HSP90B1, have been identified as TLR
chaperones that inhibit TLR signaling and the biogenesis of
downstream pro-inflammatory cytokines (16,17).
TLR9 has been reported to induce liver injury via the regulation of
IL-1β and IL-18 (15,34). Therefore, the TLR9 inhibitor E6446
dihydrochloride was used to treat the LSECs. The administration of
E6446 also reversed the cholestatic medium-induced expression of
IL-1β and IL-18, in a comparable manner to 17-DMAG (Fig. 3C and D). These results suggest a potential
association between the protective effect of HSP90 inhibitors on
the liver and the regulation of pro-inflammatory cytokine
biogenesis by TLR9.
Discussion
Biliary obstruction is a common clinical symptom in
various benign or malignant diseases and often causes severe liver
injury. HSP90 inhibitors have been described as protective drugs
for various organs due to their anti-inflammatory effects (11,12,35-37).
Given that the present study observed that HSP90 expression
increased significantly in cholestatic human and rat livers, the
inhibition of HSP90 may be a potential method for the alleviation
of liver injury during biliary obstruction. Both simple biliary
obstruction and biliary obstruction with bacterial infection cause
liver injury in clinical cases. Given that gram-negative bacteria
are the most common pathogens associated with biliary infection and
LPS is the most important pathogenic factor of gram-negative
bacteria, a BDL + LPS group was employed in the present study to
simulate biliary obstruction with secondary infection, in addition
to a BDL group. The results demonstrated that the HSP90 inhibitor
17-DMAG significantly decreased the levels of ALT and AST and
alleviated the pathological changes caused by cholestasis with or
without LPS. These results strongly imply that HSP90 inhibitors can
protect the liver from cholestatic injury.
A novel cell experiment named ‘cholestatic culture’
was designed and performed in the present study to clarify the
protective function of 17-DMAG against cholestasis-induced cell
damage in vitro. In a preliminary experiment, the
dissolution of bilirubin in DMEM was attempted in order to simulate
the microenvironment of hepatocytes after biliary obstruction.
Although the addition of dimethyl sulfoxide improved the solubility
of bilirubin, this method only provided a culture medium containing
unconjugated bilirubin. However, following biliary obstruction, the
increase in serum bilirubin is often characterized by a sharp
increase in conjugated bilirubin rather than unconjugated bilirubin
(1). Therefore, since both BRL
cells and LSECs are rat cells, cholestatic serum from BDL rats was
used instead. Whether the rat serum influenced the growth of BRL
cells in vitro was examined using CCK-8 assays. The BRL
cells grew faster in medium supplemented with rat serum than in the
medium supplemented with FBS (data not shown). This experiment
provides a viable experimental model for the simulation of the
cholestatic microenvironment in vitro.
Bile acid and severe liver injury during cholestasis
can trigger an innate immune response (29,38).
Secondary infection aggravates cholestasis-induced liver injury and
further activates the immune response. Several studies have shown
that IL-1β and IL-18 can trigger a specific pro-inflammatory type
of cell death during an immune response that may be damaging to
various organs (39-41).
The accumulation of IL-1β and IL-18 has been shown to aggravate
alcohol-mediated and acetaminophen-induced liver hepatotoxicity
during liver injury (15,34,42,43).
However, TLR9 is able to regulate the accumulation of IL-1β and
IL-18 (15,42). The present study revealed that IL-1β
and IL-18 accumulated in liver tissue while the inhibition of TLR9
attenuated their accumulation during cholestasis. These findings
indicate that IL-1β and IL-18 play a key role in
cholestasis-induced hepatotoxicity, which is in line with previous
findings.
Several studies have demonstrated that the
TLR-mediated innate immune response is associated with liver damage
and inflammation. In particular, TLR9 has a pathological role in
infection-induced liver injury (44-46),
as well as in alcohol- or acetaminophen-induced liver injury
(15,34). The present study found that HSP90B1
was upregulated in cholestatic liver tissues. A previous study
reported the binding affinity of 17-DMAG and HSP90B1(27). HSP90B1 has been identified as an
essential driver for TLR signaling, including TLR9 signaling
(17). The present study found that
the administration of 17-DMAG decreased IL-1β and IL-18 expression
during cholestasis-induced liver injury in vivo and in
vitro. Previous studies have also demonstrated that IL-1β and
IL-18 facilitate the apoptosis of hepatocytes during liver injury,
and that their biogenesis can be regulated by TLR9 signaling
(15,31,34).
These findings suggest that 17-DMAG alleviates cholestasis-induced
liver injury via the inhibition of HSP90s and TLR9 signaling and
the subsequent reduction of IL-1β and IL-18 expression, as
demonstrated by the schematic in Fig.
3E.
As a critical chaperone, HSP90 helps to regulate the
folding, maturation, stabilization and activation of >300 client
proteins (47). 17-DMAG is a
semi-synthetic HSP90 inhibitor that has several advantages over
other geldanamycin derivatives, such as higher water solubility,
good bioavailability, reduced metabolism and greater antitumor
effects (23). 17-DMAG has been
studied in preclinical and clinical trials for certain refractory
diseases, including cancer and inflammatory diseases (23). However, some dose-limiting
toxicities have been observed during treatment with 17-DMAG,
including fatigue, nausea, vomiting, diarrhea and anorexia
(48-51).
Despite significant improvements, the safety and efficacy of HSP90
inhibitors require further study prior to their clinical
translation.
In summary, the results of the present study showed
that the HSP90 inhibitor 17-DMAG protected against
cholestasis-induced liver injury in rats, via a mechanism involving
the reduction of IL-1β and IL-18 expression.
Acknowledgements
Not applicable.
Funding
The research was supported by Zhejiang Provincial
Natural Science Foundation of China under grant no. LY19H160016,
National Natural Science Foundation of China (NSFC; grant no.
81602044), Zhejiang Provincial Public Welfare Technology
Application Research Projects (grant no. LGF18H030008) and Research
Foundation of Health Bureau of Zhejiang Province (grant nos.
2020RC127, 2019ZD057, 2018KY836 and 2018RC077).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY and BL designed the present study. CT, JL, WL, WC
and JY performed the experiments. WZ and ZZ analyzed the data. CT,
JL, JY and BL drafted and revised the paper. JY and BL can
authenticate the raw data in this study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shaoxing People's Hospital (Shaoxing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Addley J and Mitchell RM: Advances in the
investigation of obstructive jaundice. Curr Gastroenterol Rep.
14:511–519. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mosler P: Diagnosis and management of
acute cholangitis. Curr Gastroenterol Rep. 13:166–172.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lazaridis KN and LaRusso NF: The
cholangiopathies. Mayo Clin Proc. 90:791–800. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
O'Neill S, Ross JA, Wigmore SJ and
Harrison EM: The role of heat shock protein 90 in modulating
ischemia-reperfusion injury in the kidney. Expert Opin Investig
Drugs. 21:1535–1548. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tukaj S and Wegrzyn G: Anti-Hsp90 therapy
in autoimmune and inflammatory diseases: A review of preclinical
studies. Cell Stress Chaperones. 21:213–218. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Schopf FH, Biebl MM and Buchner J: The
HSP90 chaperone machinery. Nat Rev Mol Cell Biol. 18:345–360.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu J, Liu T, Rios Z, Mei Q, Lin X and Cao
S: Heat shock proteins and cancer. Trends Pharmacol Sci.
38:226–256. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Usmani SZ, Bona RD, Chiosis G and Li Z:
The anti-myeloma activity of a novel purine scaffold HSP90
inhibitor PU-H71 is via inhibition of both HSP90A and HSP90B1. J
Hematol Oncol. 3(40)2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Haque A, Alam Q, Alam MZ, Azhar EI, Sait
KH, Anfinan N, Mushtaq G, Kamal MA and Rasool M: Current
understanding of HSP90 as a novel therapeutic target: An emerging
approach for the treatment of cancer. Curr Pharm Des. 22:2947–2959.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fuhrmann-Stroissnigg H, Ling YY, Zhao J,
McGowan SJ, Zhu Y, Brooks RW, Grassi D, Gregg SQ, Stripay JL,
Dorronsoro A, et al: Identification of HSP90 inhibitors as a novel
class of senolytics. Nat Commun. 8(422)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ambade A, Catalano D, Lim A and Mandrekar
P: Inhibition of heat shock protein (molecular weight 90 kDa)
attenuates proinflammatory cytokines and prevents
lipopolysaccharide-induced liver injury in mice. Hepatology.
55:1585–1595. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ambade A, Catalano D, Lim A, Kopoyan A,
Shaffer SA and Mandrekar P: Inhibition of heat shock protein 90
alleviates steatosis and macrophage activation in murine alcoholic
liver injury. J Hepatol. 61:903–911. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Medzhitov R: Toll-like receptors and
innate immunity. Nat Rev Immunol. 1:135–145. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Khalafalla MG, Woods LT, Camden JM, Khan
AA, Limesand KH, Petris MJ, Erb L and Weisman GA: P2X7 receptor
antagonism prevents IL-1β release from salivary epithelial cells
and reduces inflammation in a mouse model of autoimmune
exocrinopathy. J Biol Chem. 292:16626–16637. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Teratani T, Tomita K, Suzuki T, Furuhashi
H, Irie R, Hida S, Okada Y, Kurihara C, Ebinuma H, Nakamoto N, et
al: Free cholesterol accumulation in liver sinusoidal endothelial
cells exacerbates acetaminophen hepatotoxicity via TLR9 signaling.
J Hepatol. 67:780–790. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu B, Yang Y, Qiu Z, Staron M, Hong F, Li
Y, Wu S, Li Y, Hao B, Bona R, et al: Folding of Toll-like receptors
by the HSP90 paralogue gp96 requires a substrate-specific
cochaperone. Nat Commun. 1(79)2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang J, Grishin AV and Ford HR:
Experimental anti-inflammatory drug semapimod inhibits TLR
signaling by targeting the TLR chaperone gp96. J Immunol.
196:5130–5137. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yu J, Zhang W, Qian H, Tang H, Lin W and
Lu B: SOCS1 regulates hepatic regenerative response and provides
prognostic makers for acute obstructive cholangitis. Sci Rep.
7(9482)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang J and Lu B: Establishment of a novel
rat model of severe acute cholangitis. Iran J Basic Med Sci.
18:1124–1129. 2015.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Braet F, De Zanger R, Sasaoki T, Baekeland
M, Janssens P, Smedsrød B and Wisse E: Assessment of a method of
isolation, purification, and cultivation of rat liver sinusoidal
endothelial cells. Lab Invest. 70:944–952. 1994.PubMed/NCBI
|
|
22
|
Navaneethan U, Jayanthi V and Mohan P:
Pathogenesis of cholangitis in obstructive jaundice-revisited.
Minerva Gastroenterol Dietol. 57:97–104. 2011.PubMed/NCBI
|
|
23
|
Mellatyar H, Talaei S,
Pilehvar-Soltanahmadi Y, Barzegar A, Akbarzadeh A, Shahabi A,
Barekati-Mowahed M and Zarghami N: Targeted cancer therapy through
17-DMAG as an Hsp90 inhibitor: Overview and current state of the
art. Biomed Pharmacother. 102:608–617. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Holzbeierlein JM, Windsperger A and
Vielhauer G: Hsp90: A drug target? Curr Oncol Rep. 12:95–101.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kim HR, Kang HS and Kim HD: Geldanamycin
induces heat shock protein expression through activation of HSF1 in
K562 erythroleukemic cells. IUBMB Life. 48:429–433. 1999.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang YL, Shen HH, Cheng PY, Chu YJ, Hwang
HR, Lam KK and Lee YM: 17-DMAG, an HSP90 inhibitor, ameliorates
multiple organ dysfunction syndrome via induction of HSP70 in
endotoxemic rats. PLoS One. 11(e0155583)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ge J, Normant E, Porter JR, Ali JA,
Dembski MS, Gao Y, Georges AT, Grenier L, Pak RH, Patterson J, et
al: Design, synthesis, and biological evaluation of hydroquinone
derivatives of 17-amino-17-demethoxygeldanamycin as potent,
water-soluble inhibitors of Hsp90. J Med Chem. 49:4606–4615.
2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vaughan AT, Betti CJ and Villalobos MJ:
Surviving apoptosis. Apoptosis. 7:173–177. 2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li M, Cai SY and Boyer JL: Mechanisms of
bile acid mediated inflammation in the liver. Mol Aspects Med.
56:45–53. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tanasescu C: Correlation between
cholestasis and infection. Rom J Gastroenterol. 13:23–27.
2004.PubMed/NCBI
|
|
31
|
Luan J and Ju D: Inflammasome: A
double-edged sword in liver diseases. Front Immunol.
9(2201)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shetty S, Lalor PF and Adams DH: Liver
sinusoidal endothelial cells-gatekeepers of hepatic immunity. Nat
Rev Gastroenterol Hepatol. 15:555–567. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cai J, Zhang XJ and Li H: The role of
innate immune cells in nonalcoholic steatohepatitis. Hepatology.
70:1026–1037. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Roh YS, Zhang B, Loomba R and Seki E: TLR2
and TLR9 contribute to alcohol-mediated liver injury through
induction of CXCL1 and neutrophil infiltration. Am J Physiol
Gastrointest Liver Physiol. 309:G30–G41. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Madrigal-Matute J, Fernandez-Garcia CE,
Gomez-Guerrero C, Lopez-Franco O, Muñoz-Garcia B, Egido J,
Blanco-Colio LM and Martin-Ventura JL: HSP90 inhibition by 17-DMAG
attenuates oxidative stress in experimental atherosclerosis.
Cardiovasc Res. 95:116–123. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Qi J, Liu Y, Yang P, Chen T, Liu XZ, Yin
Y, Zhang J and Wang F: Heat shock protein 90 inhibition by
17-dimethylaminoethylamino-17-demethoxygeldanamycin protects
blood-brain barrier integrity in cerebral ischemic stroke. Am J
Transl Res. 7:1826–1837. 2015.PubMed/NCBI
|
|
37
|
Leung AM, Redlak MJ and Miller TA: Role of
heat shock proteins in oxygen radical-induced gastric apoptosis. J
Surg Res. 193:135–144. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Arab JP, Cabrera D and Arrese M: Bile
acids in cholestasis and its treatment. Ann Hepatol. 16 (Suppl 1:
S3-S105):S53–S57. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liu C, Chen J, Liu B, Yuan S, Shou D, Wen
L, Wu X and Gong W: Role of IL-18 in transplant biology. Eur
Cytokine Netw. 29:48–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bortolotti P, Faure E and Kipnis E:
Inflammasomes in tissue damages and immune disorders after trauma.
Front Immunol. 9(1900)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mende R, Vincent FB, Kandane-Rathnayake R,
Koelmeyer R, Lin E, Chang J, Hoi AY, Morand EF, Harris J and Lang
T: Analysis of serum interleukin (IL)-1β and IL-18 in systemic
lupus erythematosus. Front Immunol. 9(1250)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Imaeda AB, Watanabe A, Sohail MA, Mahmood
S, Mohamadnejad M, Sutterwala FS, Flavell RA and Mehal WZ:
Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9
and the Nalp3 inflammasome. J Clin Invest. 119:305–314.
2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Khanova E, Wu R, Wang W, Yan R, Chen Y,
French SW, Llorente C, Pan SQ, Yang Q, Li Y, et al: Pyroptosis by
caspase11/4-gasdermin-D pathway in alcoholic hepatitis in mice and
patients. Hepatology. 67:1737–1753. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kader M, Alaoui-El-Azher M, Vorhauer J,
Kode BB, Wells JZ, Stolz D, Michalopoulos G, Wells A, Scott M and
Ismail N: MyD88-dependent inflammasome activation and autophagy
inhibition contributes to Ehrlichia-induced liver injury and toxic
shock. PLoS Pathog. 13(e1006644)2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hackstein CP, Assmus LM, Welz M, Klein S,
Schwandt T, Schultze J, Förster I, Gondorf F, Beyer M, Kroy D, et
al: Gut microbial translocation corrupts myeloid cell function to
control bacterial infection during liver cirrhosis. Gut.
66:507–518. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mridha AR, Haczeyni F, Yeh MM, Haigh WG,
Ioannou GN, Barn V, Ajamieh H, Adams L, Hamdorf JM, Teoh NC and
Farrell GC: TLR9 is up-regulated in human and murine NASH: Pivotal
role in inflammatory recruitment and cell survival. Clin Sci
(Lond). 131:2145–2159. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li L, Wang L, You QD and Xu XL: Heat shock
protein 90 inhibitors: An update on achievements, challenges, and
future directions. J Med Chem. 63:1798–1822. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ramanathan RK, Egorin MJ, Erlichman C,
Remick SC, Ramalingam SS, Naret C, Holleran JL, TenEyck CJ, Ivy SP
and Belani CP: Phase I pharmacokinetic and pharmacodynamic study of
17-dimethylaminoethylamino-17-demethoxygeldanamycin, an inhibitor
of heat-shock protein 90, in patients with advanced solid tumors. J
Clin Oncol. 28:1520–1526. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lancet JE, Gojo I, Burton M, Quinn M,
Tighe SM, Kersey K, Zhong Z, Albitar MX, Bhalla K, Hannah AL and
Baer MR: Phase I study of the heat shock protein 90 inhibitor
alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice
weekly to patients with acute myeloid leukemia. Leukemia.
24:699–705. 2010.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kummar S, Gutierrez ME, Gardner ER, Chen
X, Figg WD, Zajac-Kaye M, Chen M, Steinberg SM, Muir CA, Yancey MA,
et al: Phase I trial of
17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), a
heat shock protein inhibitor, administered twice weekly in patients
with advanced malignancies. Eur J Cancer. 46:340–347.
2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Jhaveri K, Miller K, Rosen L, Schneider B,
Chap L, Hannah A, Zhong Z, Ma W, Hudis C and Modi S: A phase I
dose-escalation trial of trastuzumab and alvespimycin hydrochloride
(KOS-1022; 17 DMAG) in the treatment of advanced solid tumors. Clin
Cancer Res. 18:5090–5098. 2012.PubMed/NCBI View Article : Google Scholar
|