Introduction
Stroke remains a major cause of morbidity and
disability (1). In the USA, stroke
is the most frequent cause of adult-onset disability and the cost
of care for stroke patients is among the fastest-growing expenses
for Medicare (2). Therapy can
improve recovery and reduce long-term disability in patients who
have suffered a stroke (3).
Post-stroke rehabilitation services are generally targeted to the
initial post-acute phase, predominantly the first several weeks or,
perhaps up to a few months. This limited period of intervention
derives from the perception of deficit permanence after a 3–6-month
‘critical window’ of enhanced neuroplasticity after stroke
(4), and thus, expectations of no
subsequent improvement. Remarkably, many studies have demonstrated
a gradient of rehabilitation therapy responsiveness in stroke
patients that extends beyond a year (5). New rehabilitation strategies enabled
by new technological developments have been shown to yield
performance gains in chronic stroke patients (6). Serious games (7,8) and
robotic devices (9) have emerged as
promising rehabilitation tools for the improvement of motor
function and quality of life.
Serious game-based interventions immerse patients in
enriched, stimulating computer simulated environments in which they
interact with virtual objects while performing functional
task-specific activities. Real-time feedback, predetermined task
goals, and avatars are used to increase a patient's motivation and
engagement. The amenability of such gaming interventions to being
accessed via commercially available game alleviates cost while
maximizing convenience in that rehabilitation protocols can be
followed at home. Several studies have shown modest advantages of
virtual reality-based interventions compared to traditional
repetitive task practice protocols (10).
Clinical studies have shown that robot-assisted
therapy can enhance neurological recovery (11). The main advantage of rehabilitation
robots is that they can deliver high-dosage, high-intensity
training, which is especially beneficial for patients working to
improve motor system dysfunction due to stroke or spinal cord
disease. Incremental improvements in clinically observed
performance following intensive robotic therapy, although small,
are statistically significant and promising for a variety of
patient population, including chronic stroke patients (12).
The aim of the present study was to examine whether
and to what extent the motor performance of chronic stroke patients
can be improved with a combination of a robot-assisted therapy and
serious gaming. Additionally, we assessed the potential utility of
robotic device output parameters and game performance metrics as
indirect indices of motor improvement.
Materials and methods
Subjects
The study cohort included eight stroke patients (5
women and 3 men) ranging in age from 39 to 60 years old who were
right-handed according to the Edinburgh Handedness Inventory
(13). They were recruited through
the registries of stroke survivors who agreed to be contacted for
stroke recovery studies that are maintained at Massachusetts
General Hospital. Each had suffered an ischemic stroke affecting
the left middle cerebral artery territory at least 6 months prior
to recruitment. According to their medical records, they presented
with acute unilateral loss of hand strength (Medical Research
Council scale score <4, on 0-5 scale in which 5 is normal) that
lasted for >48 h. They did not have hearing, vision, language,
or cognitive deficits. Institutional review board approval of the
study was granted by the Massachusetts General Hospital Human
Research Committee (protocol number 2005P000570) and all
participants provided written informed consent.
Data collection
We evaluated a total of 928 game rounds distributed
over 386 training sessions. Patients were trained with a robotic
hand rehabilitation system coupled to an interactive game for 45
min per day, 3 days per week, over a 10-week period. Each training
session consisted of four 8-minute-long scenarios separated by 1-
to 5-minute rest breaks. One session was conducted on each of three
training days per week. Training was conducted at the patients'
homes under supervision to ensure compliance. Motor performance was
assessed prior to rehabilitation training (Pre), after finishing
the treatment period (Post), and again 1 month after finishing the
treatment in a follow-up assessment (FU) intended to probe the
persistence of motor benefits over time. Motor performance was
assessed via four clinical motor scales: The Fugl-Meyer scale,
which we used to assess sensorimotor impairment (includes upper
extremity, wrist, hand, coordination, sensation, passive joint
motion, joint pain, and total scores) (14); the Action Research Arm Test (ARAT),
which includes grasp, grip, pinch, gross movement, and total scores
(15); the Modified Ashworth scale
to assess spasticity of the elbow, wrist, fingers, and thumb
(16); and the Box and Blocks test
of gross manual dexterity (17).
Rehabilitation system
Patients underwent training with a newly re-designed
robotic hand rehabilitation device called the Magnetic Resonance
compatible Hand-Induced RObotic Device, version 3 (MR_CHIROD v3)
(18-20).
It was engineered to provide adjustable levels of force for
handgrip exercising (Fig. 1).
Continuous, grip-opposing, restoring force was provided by a
low-friction glass cylinder/graphite piston assembly under the
control of an electronic pneumatic pressure regulator with a
portable air compressor that allowed the MR_CHIROD v3 to be used in
a home environment. The MR_CHIROD v3 device was linked to a
low-cost, Arduino-compatible microcontroller via a simple USB
serial interface (57.6-kbps data transfer rate) thereby allowing
pressure control, force/displacement acquisition, and interface
with a laptop computer.
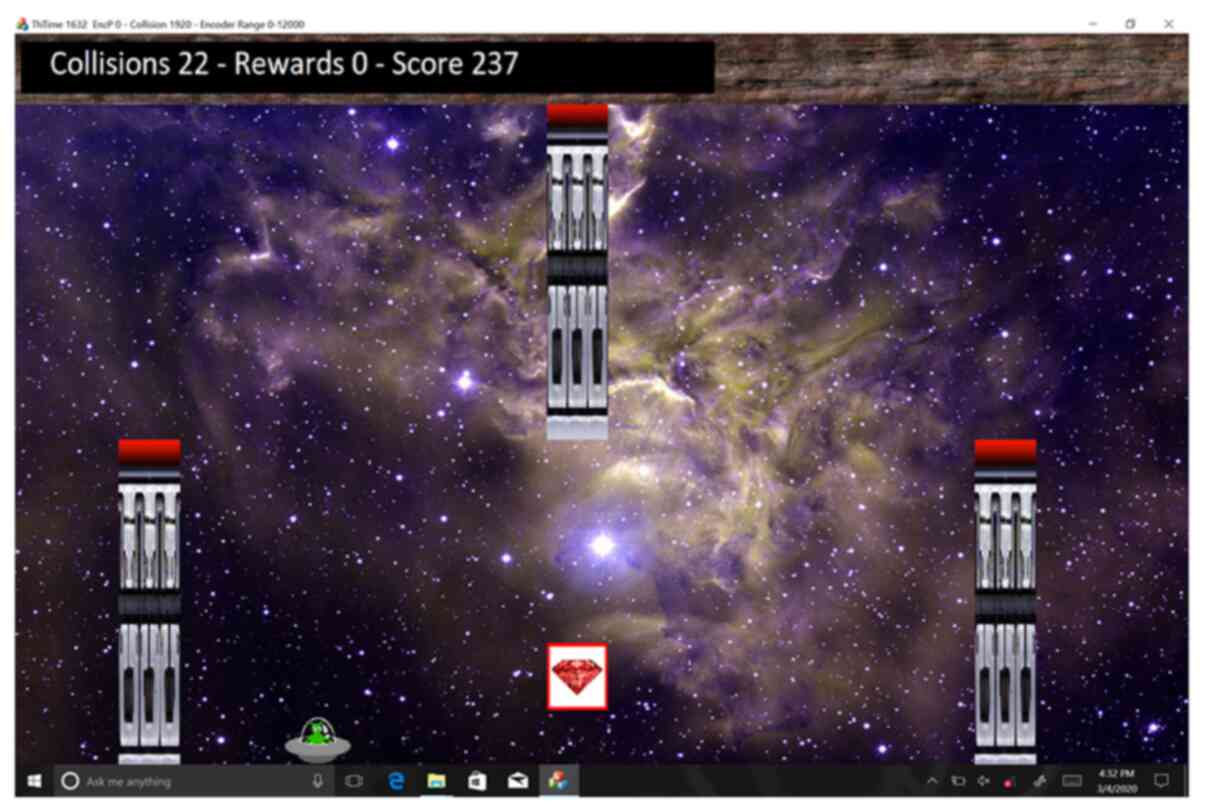
Game software
A serious game was developed in Microsoft Visual C++
for use on a Microsoft Surface (Microsoft Corp., Redmond, WA). The
user plays the game via an avatar, namely a small green alien in a
flying saucer that travels through a linear scrolling labyrinth
(right to left scrolling). The speed of the saucer starts slow and
increases linearly to a final level for each stage. The saucer
flies through a scene, earning points for collision-free flight.
The scene is populated with obstacles and rewards, collisions with
which contribute negatively and positively to the user's score,
respectively. During the game, the patient uses the MR_CHIROD
handle to control the altitude of the saucer such that the handle
position controls saucer height on the screen directly, with the
fully open and fully closed positions corresponding to the lowest
and highest screen positions. The goal of the game is to maximize
one's overall score by avoiding obstacles and collecting rewards
(Fig. 2). To increase user
involvement, a soundtrack is looped and several sound effects are
played upon collision with obstacles and rewards.
The game scenario was parametrized through an
Extensible Markup Language (XML) script (‘stage script’) that
specifies what the game engine loads in the form of sprites
(graphic elements such as the avatar, obstacles, and rewards), the
background picture, and game information. The stage script
specifies obstacle and reward point values as well as all game
components and mechanisms, including speed, sounds, and supportive
visuals. Obstacles can be avoided with large, low accuracy motions
to reach a safe position, whereas reward contact requires fine
position control within a small range of motion. However, for a
greater challenge in high-level play, users face more
closely-spaced obstacles that leave narrow passages that require
fine control to negotiate. The combination of obstacles and rewards
allows the design of precise and complex trajectories for training
gross motor and fine grip motions.
Prior to the commencement of each stage of the game,
the prescribed pressure command is sent to the MR_CHIROD v3. When
the game starts, the game engine polls the MR_CHIROD interface to
retrieve the handle position in encoder counts and normalizes the
position based on the range of motion set by end-stops on the
device appropriate to each patient, and then updates the vertical
and horizontal position coordinates of the avatar.
Game scores are calculated continuously based on the
following formula:
where the instantaneous score S(t) is equal to the
previous score S(t - 1) plus any reward collected, R(t), minus any
penalty for hitting an obstacle, O(t), plus the constant g, which
is the incremental score for each time interval. The scoring
algorithm was tuned by adjusting R, O, and g in the stage script.
The game was programmed to save each user's position trajectory,
events at each time interval, score, handle pressure, and timestamp
into a secondary XML script, which we used for offline analysis of
raw and processed information. The stage script was individualized
such that the handle force setting was set to ~75% of the user's
maximal grip strength, thereby requiring our patients to exert
effort and generate correspondingly large motor cortex activation,
while not being so high as to impede completion of the training
sessions.
Data analysis
For the purposes of the present study analyses,
motor impairment was classified according to a three-level
Fugl-Meyer upper extremity (FM UE) scale scheme (21), in which an FM UE score <20 was
classified as severe, a score in the range of 20-40 was classified
as moderate, and a score >40 was classified as mild. Improvement
of at least 4.25 on the FM UE scale (22) and/or a smallest real difference of
at least 5.5 blocks/min on the Box and Blocks test (23) were considered signs of clinically
meaningful improvement in grip ability.
The mean force and the Pearson correlation
coefficient between handle position and force (an indirect measure
of grip control) were calculated for each round. These device
metrics and the game metrics (final score, collisions, and rewards)
for each of four scenarios were averaged for each session.
Associations of device and game metrics with clinical motor scale
scores were assessed with the Spearman ρ correlation coefficient
after Bonferroni correction of multiple comparisons. Stepwise
linear regression analysis was used to assess which metrics were
significant predictors of motor performance, as indexed by FM UE,
ARAT grip, and Box and Blocks scores. All statistical analyses were
performed in SPSS version 23.0 (IBM, Inc.). Analyses with
two-tailed P-values <0.05 were regarded as statistically
significant.
Results
Log files from a total of 928 game rounds were
processed offline. Of 389 training sessions, 6 early data sets were
discarded due to equipment malfunction, affecting 2 of the 8
patients' results. All 8 patients had significant motor impairments
before they began training (Table
I). Specifically, according to our three-level FM UE
scale-based classification scheme, two patients (#4, #5) had severe
(score <20) impairment while the other four had moderate (score,
20-40) motor impairment prior to starting the training program.
ARAT grip and Box and Blocks scale scores also indicated that
patients #4 and #5 had severe motor impairment (Table I). Patient #5 presented with
spasticity (Modified Ashworth scale scores: Elbow, 3; wrist, 4;
fingers, 3; and thumb, 3). Patients #1 and #5 showed marginal
progress during the rehabilitation period, as indicated by
attending a clinically important difference for grip ability of
≥4.25 on the FM UE scale. According to the criterion of a smallest
real difference of 5.5 blocks/min in the Box and Blocks test,
patient #3 showed significant improvement.
 | Table IDemographic characteristics of and
motor assessment scores for each patient. |
Table I
Demographic characteristics of and
motor assessment scores for each patient.
| | FM UE | ARAT grip | Box and Blocks |
|---|
| Patient no. | Age, years | Gender | Pre | Post | FU | Pre | Post | FU | Pre | Post | FU |
|---|
| 1 | 39 | Female | 27 | 31 | 32 | 12 | 12 | 12 | 43 | 45 | 47 |
| 2 | 64 | Male | 36 | 36 | 36 | 12 | 12 | 12 | 54 | 56 | 57 |
| 3 | 50 | Male | 36 | 36 | 36 | 12 | 12 | 12 | 69 | 82 | 92 |
| 4 | 59 | Female | 18 | 21 | 21 | 0 | 0 | 0 | 0 | 0 | 2 |
| 5 | 60 | Female | 15 | 21 | 17 | 3 | 6 | 6 | 0 | 0 | 0 |
| 6 | 46 | Female | 28 | 29 | 28 | 12 | 12 | 12 | 13 | 13 | 14 |
| 7 | 40 | Male | 32 | 32 | 32 | 12 | 12 | 12 | 46 | 44 | 45 |
| 8 | 33 | Female | 32 | 3 | 32 | 12 | 12 | 12 | 51 | 56 | 56 |
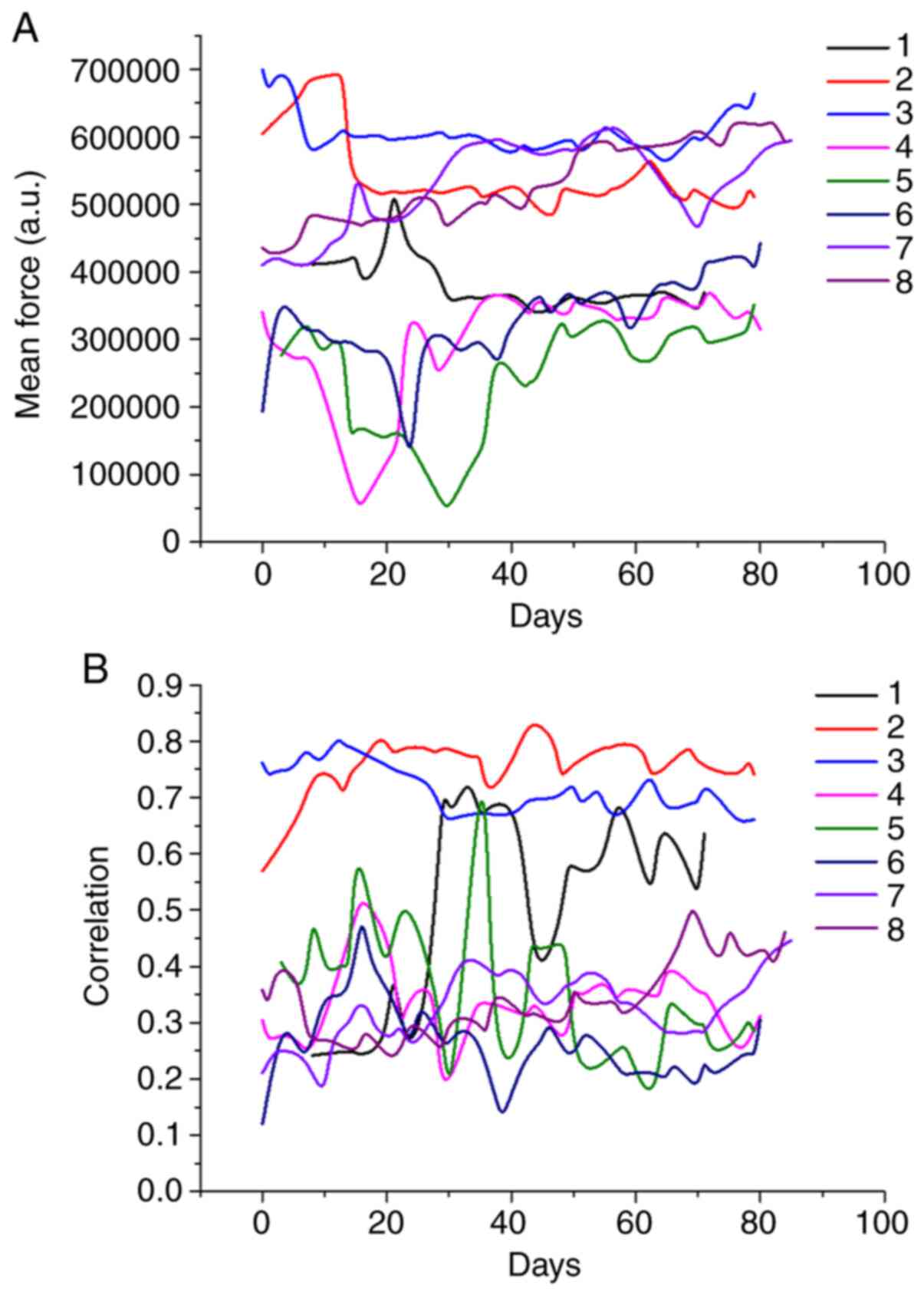
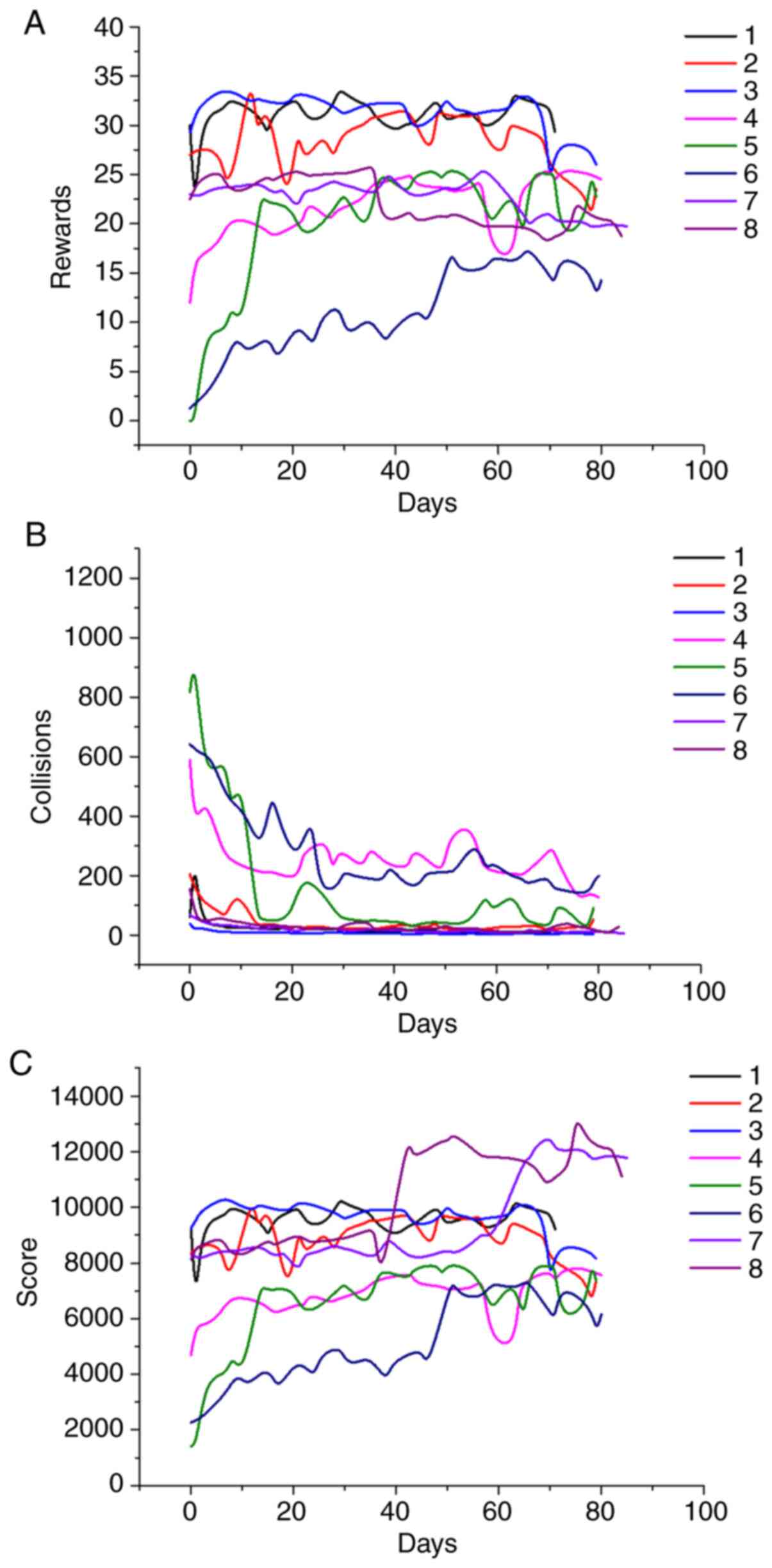
In most cases, the evolution of robotic device
metrics (Fig. 3) and game metrics
(Fig. 4) followed discernable
patterns. For example, the mean forces exerted by subjects #2, #3,
#7 and #8 were higher than those of the other patients and remained
at similar levels throughout rehabilitation (Fig. 3), corresponding with the FM UE
assessments of their motor functions. The same subjects had the
highest force-position correlations. Subject #1 showed a
discernable increase, nearly reaching the same correlation value by
the end of training (Fig. 3).
Regarding game performance, the subjects could be divided into
three types: i) good performers (#1, #2 and #3), with relatively
high scores, a large number of rewards, and few collisions, albeit
with limited improvement; ii) patients with (at least initially)
low scores who achieved marked improvement (#4, #5, #6, and #7);
and iii) one poor performer (#8) whose scores started and remained
low. The middle group patients tended to show rapid improvement
initially followed by a gradual improvement such that their metrics
approached the levels of the first group.
We observed strong correlations of robotic device
metrics and game metrics with motor ability scale indices (Table II). Of the five device and game
metrics included in our correlational analyses, the robotic device
metric mean force had the strongest correlations with motor ability
scale indices. Among the three game metrics, number of collisions
had the strongest correlation with motor scale scores. Stepwise
linear regression analysis indicated that device and game metrics
were indeed predictive of motor scale scores. Specifically,
independent predictors of FM UE score were mean force (standardized
Beta=0.662; P<0.001) and collisions (standardized Beta=-0.273;
P<0.039). Meanwhile, independent predictors of ARAT grip score
were mean force (standardized Beta=0.336; P=0.049) and collisions
(standardized Beta=-0.471; P=0.007). Finally, independent
predictors of Box and Blocks scores were mean force (standardized
Beta=0.585; P<0.001), collisions (standardized Beta=-0.289;
P=0.007), and correlation (standardized Beta=0.223; P=0.033). In
order of most to least predictable, Box and Blocks scale
(R2=0.821), FM UE scale (R2=0.700), and ARAT
grip scale (R2=0.499) scores all correlated very
significantly with metrics (all 3, P<0.001).
 | Table IIAnalysis of robotic system and game
metrics correlations with motor assessment scale scores. |
Table II
Analysis of robotic system and game
metrics correlations with motor assessment scale scores.
| | FM UE | ARAT grip | Box and Blocks |
|---|
| Parameter | ρ | P-value | ρ | P-value | Ρ | P-value |
|---|
| Robotic system | | | | | | |
|
Mean
force | 0.869 | <0.001 | 0.677 | <0.001 | 0.865 | <0.001 |
|
Position-force
correlation | 0.651 | <0.001 | 0.228 | 0.234 | 0.613 | <0.001 |
| Game | | | | | | |
|
Rewards | 0.528 | 0.002 | 0.330 | 0.070 | 0.488 | 0.005 |
|
Collisions | -0.642 | <0.001 | -0.585 | 0.001 | -0.670 | <0.001 |
|
Score | 0.590 | <0.001 | 0.602 | <0.001 | 0.636 | <0.001 |
Discussion
In this study, we examined the utility of a hand
rehabilitation system for stroke patients consisting of a serious
game coupled to a magnetic resonance (MR)-compatible robotic
device. Combining robotic technology with serious gaming has the
potential to achieve good patient engagement, immersion, and
motivation and thus to produce motor skill-relearning associated
neuronal activation that can, in principle, promote neuroplasticity
(24). We observed large game
metric improvements in the early days of training, suggesting that
more measurements should be monitored over the initial weeks of
training to establish stronger conclusions. These preliminary
results hint that finer-grained temporal analyses will show that
such data would be helpful for building a motor ability improvement
prediction model.
Very few studies (25,26)
have attempted to assess the additional benefits of this
combination of technologies. Patients #1 and #5 in our study showed
marginal motor improvements on the FM UE scale (27,28).
Compared with conventional therapy, these improvements appear to be
similar to or greater than is achieved with conventional therapy
(28). Nevertheless, the
combination of robotics with serious gaming addresses known
limitations (29) of conventional
post-stroke rehabilitation including limited availability of
specialized facilities, transportation challenges, patient
non-compliance, and costs.
Previous attempts to predict motor outcomes have
been hindered by methodological flaws and a lack of external
validation (30,31). The inclusion of neuroimaging and
neurophysiological indices in clinical outcome predictive models
could improve their accuracy (30,31).
These additional data become more important when clinical status is
determined based on self-reported assessments, a common practice
for chronic stroke patients who often follow prescribed, but
unsupervised, rehabilitation protocols at home. Self-reported
clinical monitoring becomes even less reliable when patients have
cognitive deficits. The strong correlations of our device and game
metrics with motor scale scores suggests that our hand
rehabilitation system can provide indirect but objective
assessments of motor improvement. Moreover, MR compatibility allows
state of the art neuroimaging during game play to provide insights
into underlying structural remodeling and reorganization processes
of functional recovery in the brain during the performance of
rehabilitation tasks.
Although gaming in post-stroke rehabilitation has
been considered a promising therapeutic resource, there has been
limited examination of clinical efficacy. Therapeutic outcomes were
evaluated in only about half of 31 reviewed studies and no
relationship was found between game score and clinical findings in
the remaining studies (32). Even
so, correlation between game score and clinical tests have the
potential to guide the development of biomedical systems that aid
in treatment and evaluation (32).
In our game, the score element ‘number of collisions’ had stronger
associations with clinical motor scale scores than ‘number of
rewards’ or ‘total score’. Hence, our results suggest that game
metrics could be used for clinical evaluation, but also that
different weighting of score elements would be appropriate
indicated.
Regarding gender, our sample was too small to allow
any meaningful analysis of gender differences. It is evident that,
in our sample, the three male patients started the rehabilitation
period with generally better clinical scores, and thus less severe
stroke-related impairment, than the five female patients; the
highest FM UE pre-rehabilitation period score observed among the
woman was the same as the lowest observed among the men. Both
patients that showed marginal improvement, according to FM UE
scores, were women. Thus, it may be that our protocol was
particularly beneficial to patients with relatively more severe
impairment.
Although the introduction of computer gaming into
rehabilitation is quite new, robotics have been used extensively
for motor performance assessments during rehabilitation (33). For example, the InMotion2 robot
(Bionik Laboratories, Corp., Toronto, Canada), a commercial version
of the MIT-Manus, provides automated estimates upper extremity
clinical scores for chronic stroke patients based on multiple
regression models of kinetic and kinematic macro-metrics (34). In agreement with this approach, we
observed strong correlations between robotic device metrics and
clinical scores. Importantly, our stepwise regression modeling
demonstrated that the inclusion of gaming metrics increased the
predictive power of clinical score models.
System advantages
Notably, the MR_CHIROD v3 system was designed with
features that facilitate its use and dissemination. The system's
USB serial connectivity and available WiFi compatibility allow it
to be used with multiple commercial operating systems and game
consoles. The master-side of the polling-based serial communication
protocol can also be implemented in different programs. To enable
ease of replication and wider use of the system, it has multiple
3D-printable and uncomplicated machinable components as well as
low-cost electronic and pneumatic components.
The strong magnetic fields characteristic of the
modern MR machines limit the range of actuators, sensors, and
materials that can be used in an MR-compatible rehabilitation
devices. Ferrous materials, electromagnetic actuators, and
unshielded conductors maybe forbidden for use in MR scanners
altogether for safety reasons or poorly suited due to imaging data
distortion. The MR-compatibility of the MR_CHIROD v3 robotic
device-made possible by plastic parts (3D-printed and machined),
shielded sensors/cables, and MR-compatible ball bearings and other
components-mean that patients brain activation can be analyzed
during its use. Generally, upper-extremity rehabilitation devices
are not made to MR-compatible (35); for exceptions see (36). In addition to being MR-compatible,
the currently used MR_CHIROD version 3 (manuscript describing the
design and testing is in preparation) is portable and suitable for
standardized use across clinical and home environments (18). Whereas the high cost of fabricating
our prior design of the MR_CHIROD device (version 2) was limiting
(19), the version 3 redesign
yielded fabrication cost reduction as well as overall
simplification of the fabrication process.
Finally, it is our view that the combination of
robotic technologies with serious gaming can be applied as a
rehabilitation tool as well as in multiparametric modeling of motor
performance and/or of the rehabilitation outcome. Whereas device
metrics are strongly linked to motor skill rehabilitation per se,
serious gaming metrics can reflect cognitive factors, such as
attention, working memory, and decision-making processes. Although
such cognitive factors are not typically regarded as relevant for
understanding motor performance, cognitive abilities represent
fundamental constraints on learning and execution of movements
(37,38).
Study limitations
This study has a couple of notable limitations.
Firstly, the small number of participants was the most important
major limitation of this pilot study. Studies with larger cohorts
are required to validate our preliminary results and allow clinical
trials focused on new rehabilitation approaches. Secondly, the
applicability of the MR_CHIROD v3 is limited by the simplicity of
its grip functions and the lack of multiple degrees of freedom.
Notwithstanding, the device's grip functions are critical for
stroke patients given that, from a neurology point of view, grip
rehabilitation is the most important goal for achieving
independence in daily life, including the ability to reliability
grasp and hold onto objects without dropping them. In this context,
it is important to note that although robotic therapy has been
shown to improve arm motor function after stroke (39-43),
apart from a few reports (43-45),
these efforts have not focused on the hand (46) as the presently examined system
does.
Conclusion
The present results demonstrate that MR_CHIROD v3
device output parameters and linked serious game score elements can
be used as indices of hand motor function during post-stroke hand
rehabilitation training. These robot and game metrics showed
especially marked improvements during the initial 2 weeks of
rehabilitative training in the present study sample. Clinical motor
scale scores were found to associate very strongly with these
indices, with the best correlated metric being average force. Game
score elements and device output parameters can be combined to
build a more reliable predictive model of clinical motor
performance and, potentially, replace commonly used unreliable
self-assessment methods. Larger studies are needed to inform the
development of models that can be used reliably for remote
monitoring and patient prognosis applications.
Acknowledgements
We wish to thank Dr Bruce R. Rosen, M.D., Ph.D.
(Director of the Athinoula A. Martinos Center for Biomedical
Imaging), for his support in the implementation of the imaging
studies.
Funding
This work was supported by a grant from the National
Institute of Neurological Disorders and Stroke (grant no.
1R01NS105875-01A1) of the National Institutes of Health.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LGA analyzed the data and prepared the manuscript.
GDN designed and created the computer game. MPO designed,
manufactured and tested the robotic device and edited the
manuscript. CP and SL acquired the raw data and assessed their
authenticity. MAM provided data interpretation and manuscript
editing. AAT designed and supervised the study. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Institutional review board approval of the study was
granted by the Partners Human Research Committee (protocol no.
2005P000570) and all participants provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Virani SS, Alonso A, Benjamin EJ,
Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR,
Cheng S, Delling F, et al: Heart disease and stroke statistics-2020
update: A report from the American Heart Association. Circulation.
141:e139–e596. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Centers for Disease C and Prevention
(CDC). Public health and aging: Hospitalizations for stroke among
adults aged >/=65 years-United States, 2000. MMWR Morb Mortal
Wkly Rep. 52:586–589. 2003.PubMed/NCBI
|
|
3
|
Indredavik B, Slordahl SA, Bakke F,
Rokseth R and Haheim LL: Stroke unit treatment. Long-term effects.
Stroke. 28:1861–1866. 1997.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kwakkel G, Kollen B and Lindeman E:
Understanding the pattern of functional recovery after stroke:
Facts and theories. Restor Neurol Neurosci. 22:281–299.
2004.PubMed/NCBI
|
|
5
|
Ballester BR, Maier M, Duff A, Cameirão M,
Bermúdez S, Duarte E, Cuxart A, Rodríguez S, San Segundo Mozo RM
and Verschure PFMJ: A critical time window for recovery extends
beyond one-year post-stroke. J Neurophysiol. 122:350–357.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dobkin BH and Dorsch A: New evidence for
therapies in stroke rehabilitation. Curr Atheroscler Rep.
15(331)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Friedrich R, Hiesel P, Peters S, Siewiorek
DP, Smailagic A and Brugge B: Serious games for home-based stroke
rehabilitation. Stud Health Technol Inform. 213:157–160.
2015.PubMed/NCBI
|
|
8
|
Tamayo-Serrano P, Garbaya S and Blazevic
P: Gamified in-home rehabilitation for stroke survivors: Analytical
review. Int J Ser Games. 5:2018.
|
|
9
|
Fazekas G and Tavaszi I: The future role
of robots in neuro-rehabilitation. Expert Rev Neurother.
19:471–473. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee HS, Park YJ and Park SW: The effects
of virtual reality training on function in chronic stroke patients:
A systematic review and meta-analysis. Biomed Res Int.
2019(7595639)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chang WH and Kim YH: Robot-assisted
therapy in stroke rehabilitation. J Stroke. 15:174–181.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cho KH, Hong MR and Song WK: Upper limb
robotic rehabilitation for chronic stroke survivors: A single-group
preliminary study. J Phys Ther Sci. 30:580–583. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Oldfield RC: The assessment and analysis
of handedness: The Edinburgh inventory. Neuropsychologia. 9:97–113.
1971.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fugl-Meyer AR, Jääskö L, Leyman I, Olsson
S and Steglind S: The post-stroke hemiplegic patient. 1. a method
for evaluation of physical performance. Scand J Rehabil Med.
7:13–31. 1975.PubMed/NCBI
|
|
15
|
Lyle RC: A performance test for assessment
of upper limb function in physical rehabilitation treatment and
research. Int J Rehabil Res. 4:483–492. 1981.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ansari NN, Naghdi S, Arab TK and Jalaie S:
The interrater and intrarater reliability of the Modified Ashworth
Scale in the assessment of muscle spasticity: Limb and muscle group
effect. NeuroRehabilitation. 23:231–237. 2008.PubMed/NCBI
|
|
17
|
Mathiowetz V, Volland G, Kashman N and
Weber K: Adult norms for the Box and Block Test of manual
dexterity. Am J Occup Ther. 39:386–391. 1985.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ottensmeyer MP, Li S, De Novi G and Tzika
AA: Functional MRI in conjunction with a novel MRI-compatible
hand-induced robotic device to evaluate rehabilitation of
individuals recovering from hand grip deficits. J Vis Exp:
10.3791/59420, 2019.
|
|
19
|
Khanicheh A, Mintzopoulos D, Weinberg B,
Tzika AA and Mavroidis C: MR_CHIROD v.2: Magnetic resonance
compatible smart hand rehabilitation device for brain imaging. IEEE
Trans Neural Syst Rehabil Eng. 16:91–98. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Khanicheh A, Muto A, Triantafyllou C,
Weinberg B, Astrakas L, Tzika A and Mavroidis C: fMRI-compatible
rehabilitation hand device. J Neuroeng Rehabil.
3(24)2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Woytowicz EJ, Rietschel JC, Goodman RN,
Conroy SS, Sorkin JD, Whitall J and McCombe Waller S: Determining
levels of upper extremity movement impairment by applying a cluster
analysis to the fugl-meyer assessment of the upper extremity in
chronic stroke. Arch Phys Med Rehabil. 98:456–462. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Page SJ, Fulk GD and Boyne P: Clinically
important differences for the upper-extremity Fugl-Meyer Scale in
people with minimal to moderate impairment due to chronic stroke.
Phys Ther. 92:791–798. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen HM, Chen CC, Hsueh IP, Huang SL and
Hsieh CL: Test-retest reproducibility and smallest real difference
of 5 hand function tests in patients with stroke. Neurorehabil
Neural Repair. 23:435–440. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Comani S, Velluto L, Schinaia L, Cerroni
G, Serio A, Buzzelli S, Sorbi S and Guarnieri B: Monitoring
neuro-motor recovery from stroke with high-resolution EEG, robotics
and virtual reality: A proof of concept. IEEE Trans Neural Syst
Rehabil Eng. 23:1106–1116. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Clark WE, Sivan M and O'Connor RJ:
Evaluating the use of robotic and virtual reality rehabilitation
technologies to improve function in stroke survivors: A narrative
review. J Rehabil Assist Technol Eng.
6(2055668319863557)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mubin O, Alnajjar F, Jishtu N, Alsinglawi
B and Al Mahmud A: Exoskeletons with virtual reality, augmented
reality, and gamification for stroke patients' rehabilitation:
Systematic review. JMIR Rehabil Assist Technols.
6(e12010)2019.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Byl NN, Abrams GM, Pitsch E, Fedulow I,
Kim H, Simkins M, Nagarajan S and Rosen J: Chronic stroke survivors
achieve comparable outcomes following virtual task specific
repetitive training guided by a wearable robotic orthosis (UL-EXO7)
and actual task specific repetitive training guided by a physical
therapist. J Hand Ther. 26:343–352; quiz 352. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Klamroth-Marganska V, Blanco J, Campen K,
Curt A, Dietz V, Ettlin T, Felder M, Fellinghauer B, Guidali M,
Kollmar A, et al: Three-dimensional, task-specific robot therapy of
the arm after stroke: A multicentre, parallel-group randomised
trial. Lancet Neurol. 13:159–166. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Saposnik G, Teasell R, Mamdani M, Hall J,
McIlroy W, Cheung D, Thorpe KE, Cohen LG and Bayley M: Stroke
Outcome Research Canada (SORCan) Working Group. Effectiveness of
virtual reality using Wii gaming technology in stroke
rehabilitation: A pilot randomized clinical trial and proof of
principle. Stroke. 41:1477–1484. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Heiss WD: Contribution of neuro-imaging
for prediction of functional recovery after ischemic stroke.
Cerebrovasc Dis. 44:266–276. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kwah LK and Herbert RD: Prediction of
walking and arm recovery after stroke: A critical review. Brain
Sci. 6(53)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Noveletto F, Hounsell MDS, Soares AV,
Eichinger FLF, Sagawa Y and Bertemes Filho P: Stronger: A serious
game framework for post-stroke rehabilitation. Ann Phys Rehabil
Med. 61(e487)2018.
|
|
33
|
Oña ED, Cano-de la Cuerda R,
Sánchez-Herrera P, Balaguer C and Jardón A: A review of robotics in
neurorehabilitation: Towards an automated process for upper limb. J
Healthc Eng. 2018(9758939)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bosecker C, Dipietro L, Volpe B and Krebs
HI: Kinematic robot-based evaluation scales and clinical
counterparts to measure upper limb motor performance in patients
with chronic stroke. Neurorehabil Neural Repair. 24:62–69.
2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chu CY and Patterson RM: Soft robotic
devices for hand rehabilitation and assistance: A narrative review.
J Neuroeng Rehabil. 15(9)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hartwig V, Carbonaro N, Tognetti A and
Vanello N: Systematic review of fMRI compatible devices: Design and
testing criteria. Ann Biomed Eng. 45:1819–1835. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gallivan JP, Chapman CS, Wolpert DM and
Flanagan JR: Decision-making in sensorimotor control. Nat Rev
Neurosci. 19:519–534. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Song JH: The role of attention in motor
control and learning. Curr Opin Psychol. 29:261–265.
2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jack D, Boian R, Merians AS, Tremaine M,
Burdea GC, Adamovich SV, Recce M and Poizner H: Virtual
reality-enhanced stroke rehabilitation. IEEE Trans Neural Syst
Rehabil Eng. 9:308–318. 2001.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hesse S, Schulte-Tigges G, Konrad M,
Bardeleben A and Werner C: Robot-assisted arm trainer for the
passive and active practice of bilateral forearm and wrist
movements in hemiparetic subjects. Arch Phys Med Rehabil.
84:915–920. 2003.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Huang VS and Krakauer JW: Robotic
neurorehabilitation: A computational motor learning perspective. J
Neuroeng Rehabil. 6(5)2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Pignolo L: Robotics in
neuro-rehabilitation. J Rehabil Med. 41:955–960. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Duret C and Hutin E: Effects of prolonged
robot-assisted training on upper limb motor recovery in subacute
stroke. NeuroRehabilitation. 33:41–48. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Balasubramanian S, Klein J and Burdet E:
Robot-assisted rehabilitation of hand function. Curr Opin Neurol.
23:661–670. 2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Metzger JC, Lambercy O, Califfi A, Dinacci
D, Petrillo C, Rossi P, Conti FM and Gassert R: Assessment-driven
selection and adaptation of exercise difficulty in robot-assisted
therapy: A pilot study with a hand rehabilitation robot. J Neuroeng
Rehabil. 11(154)2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Prange GB, Jannink MJ, Groothuis-Oudshoorn
CG, Hermens HJ and Ijzerman MJ: Systematic review of the effect of
robot-aided therapy on recovery of the hemiparetic arm after
stroke. J Rehabil Res Dev. 43:171–184. 2006.PubMed/NCBI View Article : Google Scholar
|