Introduction
As a clinical ophthalmic disease that has an
extremely high incidence and causes great harm, glaucoma causes
severe damage to tissues of various parts in the eyeball due to the
imbalance of intraocular pressure (IOP) (1,2).
According to Garway-Heath et al, more than 900,000 people
newly developed glaucoma worldwide in 2015, and the disease may
occur in people of all ages (3).
According to Wang et al, the incidence of glaucoma has
increased by approximately 10-fold compared with that 10 years ago.
Thus, the incidence is predicted to continue to gradually increase,
which makes glaucoma a most significant and daunting problem in
clinical ophthalmology (4).
Glaucoma patients usually suffer from optic atrophy and visual
deterioration, and approximately 70% of them will go blind if they
are not treated in a proper or timely manner (5,6). Li
et al have predicted that by 2020, approximately 20 million
people worldwide will develop blindness caused by glaucoma
(7). Acute angle-closure glaucoma
(AACG) is an eye disease in which the IOP undergoes a sharp
increase due to the sudden closure of the anterior chamber angle.
As the most serious type of glaucoma, AACG progresses to complete
loss of sight within 24 to 48 h if not diagnosed and treated in
time (8). The disease has no
obvious and special symptoms, and some patients with strong pain
tolerance experience only eye discomfort, and this may lead to loss
of optimal treatment timing (9). At
present, diagnostic methods for AACG are complex, therefore the
disease requires evaluation and detection of various aspects
(10), which is not conducive to
its treatment because it has an extremely fast onset. AACG is
relatively complex in clinical practice, and it is impossible to
carry out large-scale clinical surveys and early screening. As the
challenges of glaucoma become more serious, it is urgent to find an
effective serum marker for the early screening or auxiliary
diagnosis of AACG.
Highly conserved in structure, heat shock proteins
(HSP) are a family of proteins that are produced by prokaryocytes
and eukaryocytes under high temperature or other stresses (11). HSP70 that can inhibit apoptosis is
widely distributed in all tissues and structures except
photoreceptors (11). According to
a previous study, it is a crucial gene locus for eye development,
and is aberrantly expressed in glaucoma (12). However, there is currently little
in-depth research on this protein in AACG. Therefore, whether this
protein has potential as a reliable marker for diagnosing and
treating AACG in the future remains to be confirmed. Thus, it is of
great significance to conduct research on HSP70 at home and abroad
to improve its clinical value in glaucoma and to confirm its
effects on AACG.
Materials and methods
General information
A prospective analysis was performed on 74 AACG
patients [enrolled as the study group (SG)] who were admitted to
Hebei Eye Hospital (Xingtai, China) from April 2017 to April 2019
and on 70 healthy people [enrolled as the control group (CG)] who
underwent physical examinations during the same period. The present
study was approved by the Ethics Committee of Hebei Eye Hospital.
All research subjects signed an informed consent form. General
characteristics of all participants are listed in Table I.
 | Table IComparison of general information [n
(%)]. |
Table I
Comparison of general information [n
(%)].
| Features | SG (n=74) | CG (n=70) | χ2 or
t | P-value |
|---|
| Age (years) | 52.8±10.4 | 53.6±11.6 | 0.436 | 0.663 |
| BMI
(kg/cm2) | 22.62±3.54 | 22.86±3.84 | 0.390 | 0.697 |
| Systolic blood
pressure (mmHg) | 116.62±10.98 | 117.24±11.24 | 0.335 | 0.738 |
| Diastolic blood
pressure (mmHg) | 74.62±7.85 | 75.14±8.06 | 0.392 | 0.696 |
| Sex | | | 0.355 | 0.551 |
|
Male | 30 (40.54) | 25 (35.71) | | |
|
Female | 44 (59.46) | 45 (64.29) | | |
| Dwelling
environment | | | 0.716 | 0.397 |
|
City | 65 (87.84) | 58 (82.86) | | |
|
Countryside | 9 (12.16) | 12 (17.14) | | |
| Educational
background | | | 0.298 | 0.585 |
|
<Senior
high school | 34 (45.95) | 29 (41.43) | | |
|
≥Senior high
school | 40 (54.05) | 41 (58.57) | | |
| Smoking | | | 0.598 | 0.440 |
|
Yes | 25 (33.78) | 28 (40.00) | | |
|
No | 49 (66.22) | 42 (60.00) | | |
| Alcohol drinking | | | 0.337 | 0.561 |
|
Yes | 20 (27.03) | 22 (31.43) | | |
|
No | 54 (72.97) | 48 (68.57) | | |
Inclusion and exclusion criteria
The inclusion criteria were as follows: i) patients
whose clinical manifestations were consistent with glaucoma
symptoms, and who were diagnosed with AACG at our hospital; ii)
patients who were operated on after diagnosis; iii) patients aged
20-60 years; and iv) patients with complete medical records.
Exclusion criteria were as follows: i) patients complicated with
other eye diseases; ii) iii) patients with severe cardiovascular
and cerebrovascular diseases; iv) patients with tumors; v) patients
complicated with other congenital immunodeficiency diseases or
infectious diseases; vi) patients with mental disorders; vii)
patients complicated with diabetes mellitus; viii) patients with
physical disabilities; ix) patients with a contraindication to
surgery; and x) patients who had transferred to other
hospitals.
Methods
Surgery for the patients was performed by senior
ophthalmologists at our hospital. Conventional treatment was as
follows: YAG laser peripheral iridectomy was conducted on patients
in the prodromal and intermittent stages. Positive rescue was
conducted on patients in the acute attack stage, and the anterior
chamber was opened as soon as possible to prevent permanent
goniosynechia. Drugs (alternative drugs included 2% pilocarpine
solution; acetazolamide; glycerol; 2% lidocaine; 20% mannitol) were
first used to reduce IOP, and then the patients were operated after
their blood pressure lowered and their congestive inflammation
subsided. According to the IOP and anterior chamber, filtration
surgery or peripheral iridectomy was selected, and the eyeball
would be removed if necessary. Fasting venous blood (4 ml) was
respectively drawn from the patients before and after the
treatment, allowed to stand at room temperature for 30 min, and
then centrifuged for 10 min (1,809 x g, at 4˚C), to obtain the
upper serum. The concentration of HSP70 in the serum was detected
by enzyme-linked immunosorbent assay (ELISA), and the kit (cat. no.
JLC11484) was purchased from Shanghai Jingkang Bioengineering Co.,
Ltd., with the steps strictly and sterilely carried out according
to the instructions of the kit.
Outcome measures
The concentration of serum HSP70 in the two groups;
the predictive value of HSP70 for AACG; the correlation of
pre-treatment HSP70 with IOP, central anterior chamber depth
(CACD), peripheral anterior chamber depth (PACD) and anterior angle
(AA); the predictive value of HSP70 for adverse reactions during
treatment; changes of HSP70 concentration before and after
treatment in SG.
Statistical methods
SPSS24.0 (IBM Corp.) was used to analyze and process
the data. GraphPad 8 (GraphPad Software, Inc.) was used to plot
figures. Count data were expressed by (rate), and Chi-square test
was used for their comparison between groups. Measurement data were
expressed by the mean ± standard deviation (SD), and independent
sample t-test was used for their comparison between groups.
Spearman Correlation Coefficient was used for correlation analysis.
Receiver operating characteristic (ROC) curves were plotted to
analyze the predictive value of HSP70. P<0.05 indicated a
statistically significant difference.
Results
Comparison of general information
There were no significant differences between the SG
and CG in terms of age, body mass index (BMI), blood pressure,
heart rate, sex, dwelling environment, educational background,
smoking and alcohol drinking habits (P>0.050; Table I).
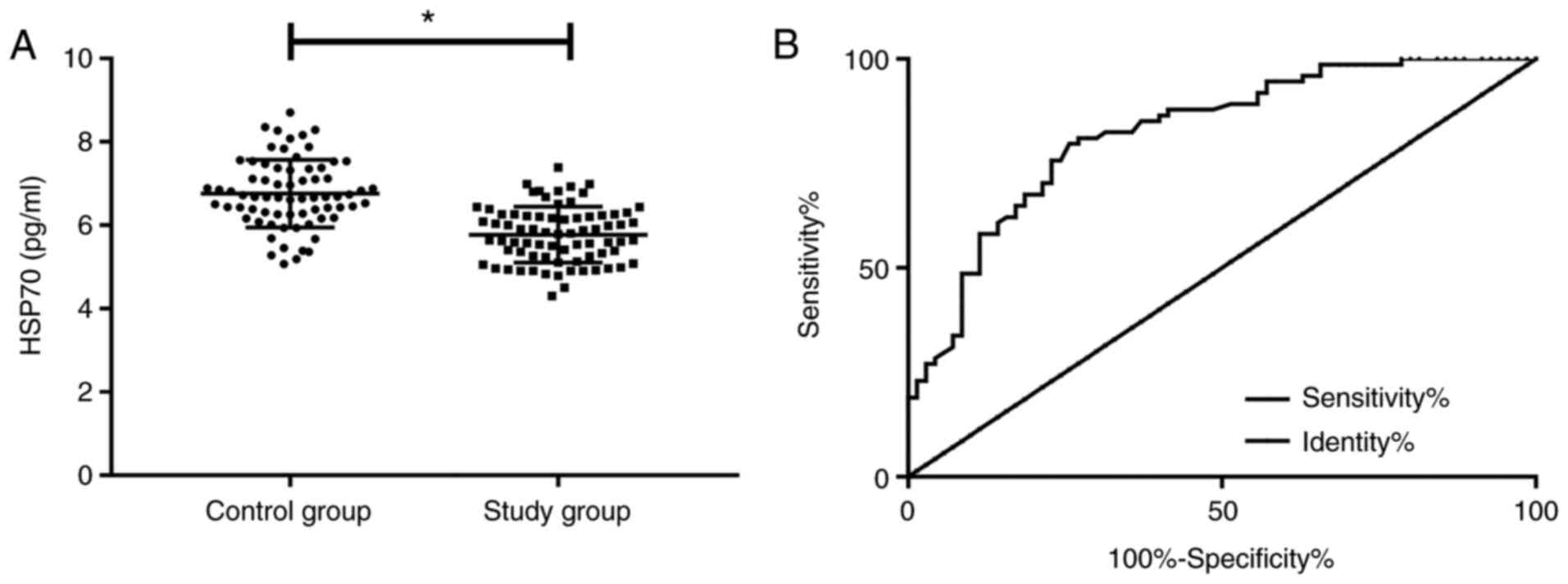
Comparison of HSP70 concentration
The concentration of HSP70 in the SG was
significantly lower than that in the CG before treatment
(P<0.05). According to the ROC curve, when the cut-off value was
6.27, the sensitivity of HSP70 for diagnosing AACG was 79.73%, the
specificity was 74.29%, the area under the curve (AUC) was 0.824,
and the 95% confidence interval (CI) was 0.757-0.891 (P<0.001;
Fig. 1).
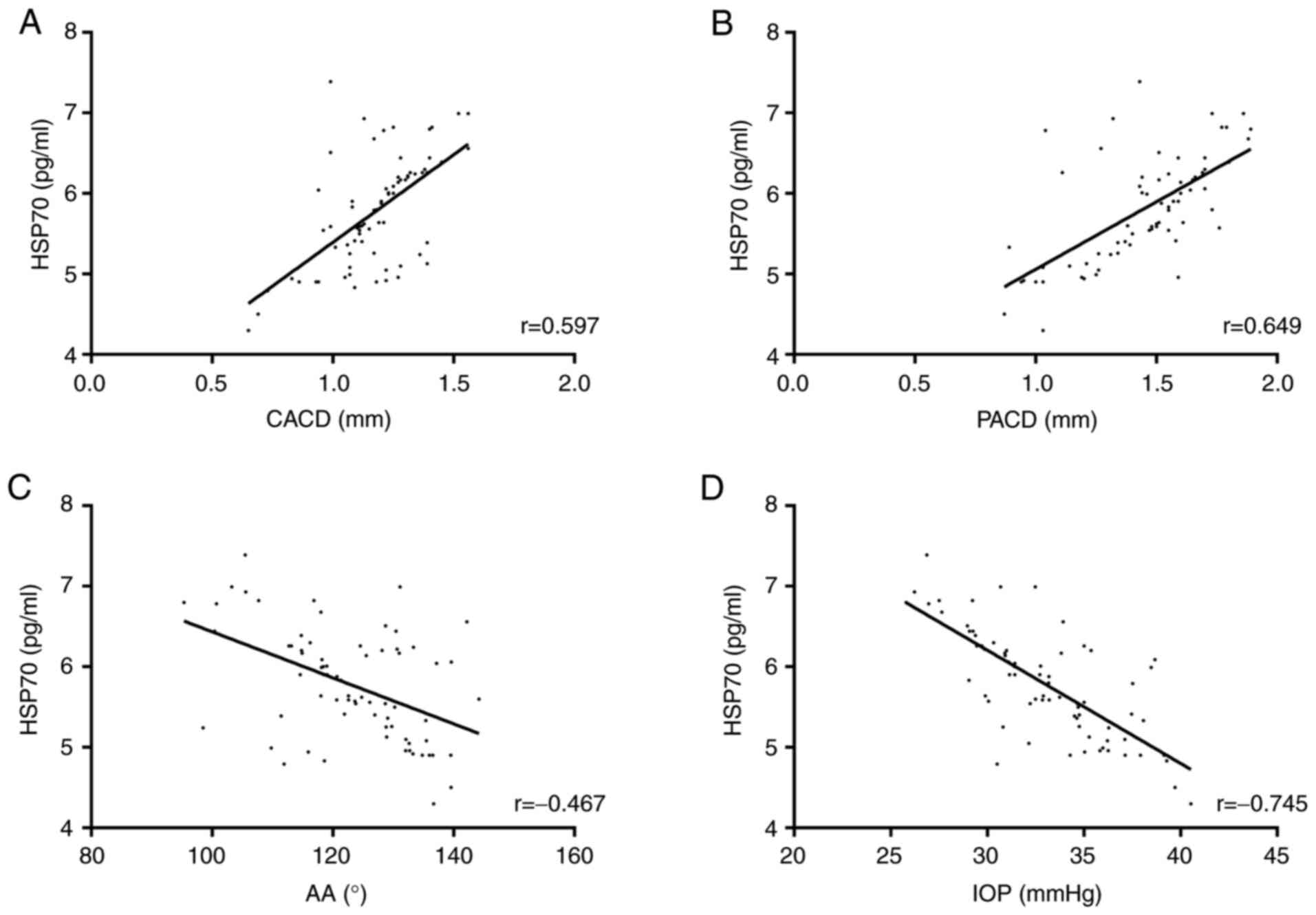
Correlation of HSP70 with indicators
related to the course of the disease
According to the Pearson correlation coefficient,
the concentration of HSP70 in the SG was positively correlated with
CACD (r=0.597, P<0.001) and PACD (r=0.649, P<0.001), but
negatively correlated with AA (r=-0.467, P<0.001) and IOP
(r=-0.745, P<0.001) (Fig.
2).
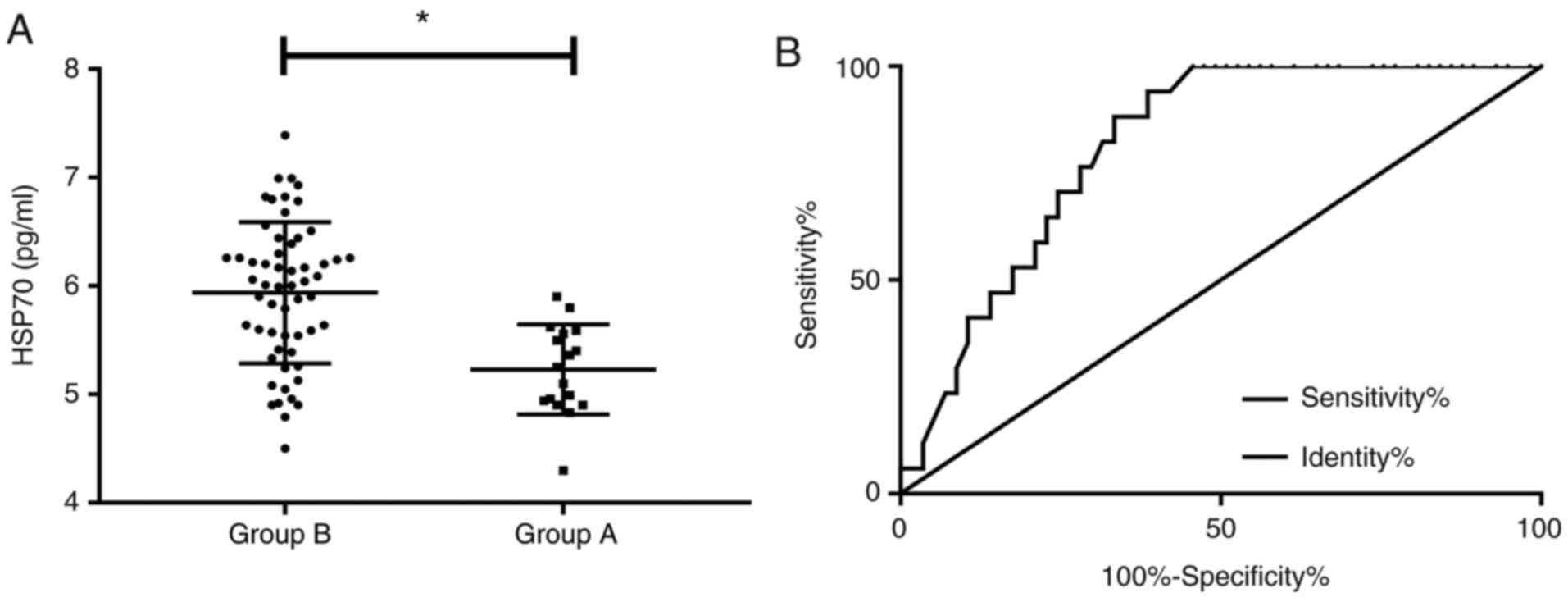
Predictive value of HSP70 for adverse
reactions
During treatment of all patients, there were 5 cases
of fibrinoid exudation, 3 cases of anterior chamber inflammation, 3
cases of choroidal detachment, 4 cases of corneal edema and 2 cases
of age-related macular degeneration, with an incidence of adverse
reactions at 22.97%. Patients who developed adverse reactions were
considered as Group A (n=17), while those without adverse reactions
were considered as Group B (n=57). The concentration of HSP70 in
Group A was significantly lower than that in Group B (P<0.05).
According to the ROC curve, when the cut-off value was 5.815, the
sensitivity of HSP70 for diagnosing AACG was 94.12%, the
specificity was 61.40%, the AUC was 0.813, and the 95% CI was
0.717-0.909 (P<0.001; Fig.
3).
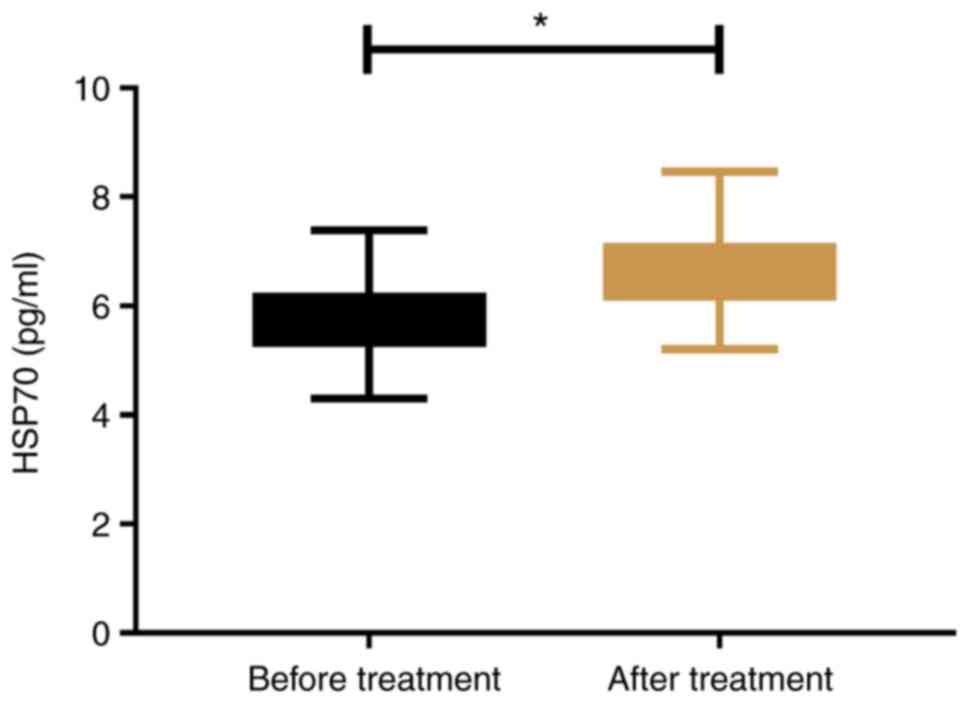
Changes of HSP70 concentration before
and after treatment
In the SG, the concentration of HSP70 after
treatment was significantly higher than that before treatment
(P<0.05; Fig. 4).
Discussion
In modern society, the incidence of eye diseases has
been annually increasing as the population ages (13). As the most serious type of glaucoma,
AACG has no obvious symptoms in its early stage, and causes great
damage to the eyeball and surrounding tissues by increasing IOP.
Therefore, the improper or untimely treatment of the disease easily
causes irreversible damage to the visual system of the patient
(14,15). Therefore, finding effective
examination indices to increase the early diagnostic rate of AACG
is the focus and difficulty of clinical research (16). In the present study, through the
investigation of HSP70 in the blood of AACG patients, it was
confirmed through experimental analysis whether HSP70 has the
potential to be a diagnostic and therapeutic marker for AACG, in
order to provide a reliable theoretical basis for conducting
large-scale and early screening and for assessing the progression
of the disease in the future.
In the present study, HSP70 concentration was
significantly reduced in the serum of the patients, suggesting that
this protein may be involved in the development or progression of
AACG. Waugh et al also studied HSP70 in patients with eye
diseases (17), and their findings
are consistent with the present experimental results and support
our conclusions. The aforementioned study detected the expression
of multiple genes and proteins in glaucoma, and determined that
HSP70 was aberrantly expressed in the disease. In the present
research, the expression of HSP70 in AACG was analyzed. Compared
with previous studies, our research is more detailed on the types
of targeted diseases, which can greatly reduce the contingency of
experimental results. According to previous studies, the
development of glaucoma is closely related to the death of retinal
ganglion cells, and the death mechanism is associated with the
increase in the apoptotic rate. The increase can be caused by
blocked retrograde transport, lack of nutritional factors and
excitatory amino acids (18-20).
HSP70 has an anti-apoptotic effect on cells, and induces HSP to
produce endogenous protection in neuronal cells (21). One of its forms of gene induction is
HSPA1B (22), which is a risk
factor for glaucoma according to Salehi et al (23). Therefore, it is inferred that the
mechanism of action of HSP70 in AACG may be correlated with its
differentiation into HSPA1B. However, since basic experiments were
not carried out, it is impossible to determine the exact mechanism
of action of HSP70 on AACG, which will be fully investigated in
future research. According to the ROC curve, the sensitivity and
specificity of HSP70 for predicting AACG were 79.79 and 74.29%,
respectively, indicating that this protein can be used as a
clinical screening index that may aid doctors in diagnosing AACG in
the future. Serum markers are superior to conventional diagnostic
methods for AACG in terms of the convenient acquisition of test
samples, the longer storage time of blood samples, and convenient
reexamination. Moreover, the detection results of the markers are
objective and do not rely on human subjective consciousness to
assess the disease, which reduces man-made misdiagnosis or missed
diagnosis. In the present study, the concentration of HSP70 was
significantly correlated with IOP, CACD, PACD and AA. This further
confirms the close relationship between HSP70 and the progression
of AACG, and is similar to the inference aforementioned. AACG is a
retinopathy during whose progression the normal activity of retinal
neuron cells plays an important role (24). HSP70 is a protective factor of
neuronal cells. The occurrence of AACG greatly reduces it and then
aggravates neuronal damage. As a result, the disease more markedly
deteriorates. According to Wang et al, the lesion of AACG is
closely related to neuronal damage (25). In the present study, HSP70 had a
relatively satisfactory predictive value for adverse reactions
during treatment, and its concentration significantly increased
after treatment, which demonstrated that HSP70 may be used for the
diagnosis and treatment of AACG in the future. In addition, if the
mechanism of HSP70 on the protection of neuronal cells and the
relationship between HSP70 and glaucoma are fully elucidated, then,
HSP70 may become a therapeutic target for glaucoma and even various
eye diseases in the future, thus rendering it a great scientific
application prospect.
The purpose of the present study was to explore the
effects of HSP70 on AACG patients. Previous studies have suggested
that HSP and glaucoma are related (26-28),
but the present study focused on the analysis of AACG in glaucoma
and explored the significance of HSP as a diagnostic marker in
AACG, to the best of our knowledge, for the first time. However,
there are still numerous shortcomings due to the limited
experimental conditions. For example, the exact mechanism of action
of HSP70 on AACG was not determined due to the failure to conduct
basic experiments. The effects of HSP70 on the long-term prognosis
of patients remain unclear due to the short experimental period. In
addition, the present study focused and analyzed mainly AACG, thus
HSP70 may be aberrantly expressed in other pathological types of
glaucoma, yet to be determined. In the present study, HSP70 was
only detected in the blood of the patients, and its expression has
not been evaluated in glaucoma-related cells. In the future, more
detailed and in-depth experiments, analyses and discussions will be
conducted, to address these deficiencies and obtain more definitive
results.
In summary, HSP70 concentration was markedly reduced
in AACG patients, and its detection had a relatively satisfactory
predictive value for AACG, and thus HSP70 may be a potential and
effective indicator for diagnosing and treating glaucoma in the
future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HC wrote the manuscript. HC, AT and YW conceived and
designed the study. RL and RH were responsible for the collection
and analysis of the experimental data. XX and SC interpreted the
data and drafted the manuscript. HC and AT revised the manuscript
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Hebei Eye Hospital (Xingtai, China). Patients who
participated in this research, signed the informed consent and had
complete clinical data. Signed written informed consents were
obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu L, Jia Y, Takusagawa HL, Pechauer AD,
Edmunds B, Lombardi L, Davis E, Morrison JC and Huang D: Optical
coherence tomography angiography of the peripapillary retina in
glaucoma. JAMA Ophthalmol. 133:1045–1052. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Haffner DS, Smedley GT, Tu H and Burns TW:
Devices and methods for glaucoma treatment: U.S. Patent 9,597,230.
Filed August 8, 2007; issued March 21, 2017.
|
|
3
|
Garway-Heath DF, Crabb DP, Bunce C,
Lascaratos G, Amalfitano F, Anand N, Azuara-Blanco A, Bourne RR,
Broadway DC, Cunliffe IA, et al: Latanoprost for open-angle
glaucoma (UKGTS): A randomised, multicentre, placebo-controlled
trial. Lancet. 385:1295–1304. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang X, Jiang C, Ko T, Kong X, Yu X, Min
W, Shi G and Sun X: Correlation between optic disc perfusion and
glaucomatous severity in patients with open-angle glaucoma: An
optical coherence tomography angiography study. Graefes Arch Clin
Exp Ophthalmol. 253:1557–1564. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yarmohammadi A, Zangwill LM, Diniz-Filho
A, Suh MH, Manalastas PI, Fatehee N, Yousefi S, Belghith A,
Saunders LJ, Medeiros FA, et al: Optical coherence tomography
angiography vessel density in healthy, glaucoma suspect, and
glaucoma eyes. Invest Ophthalmol Vis Sci. 57:OCT451–OCT459.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pfeiffer N, Garcia-Feijoo J,
Martinez-De-La-Casa JM, Larrosa JM, Fea A, Lemij H, Gandolfi S,
Schwenn O, Lorenz K and Samuelson TW: A randomized trial of a
Schlemm's canal microstent with phacoemulsification for reducing
intraocular pressure in open-angle glaucoma. Ophthalmology.
122:1283–1293. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li Z, Allingham RR, Nakano M, Jia L, Chen
Y, Ikeda Y, Mani B, Chen LJ, Kee C, Garway-Heath DF, et al: A
common variant near TGFBR3 is associated with primary open angle
glaucoma. Hum Mol Genet. 24:3880–3892. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lai J, Choy BN and Shum JW: Management of
primary angle-closure glaucoma. Asia Pac J Ophthalmol (Phila).
5:59–62. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bansal S, Balakrishnan SA, Blachley T,
Weizer JS, Lee PP and Stein JD: Subsequent receipt of interventions
for glaucoma among a nationwide sample of patients who underwent
laser peripheral iridotomy. Am J Ophthalmol. 160:275–282.e4.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wright C, Tawfik MA, Waisbourd M and Katz
LJ: Primary angle-closure glaucoma: An update. Acta Ophthalmol.
94:217–225. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shevtsov M and Multhoff G: Heat shock
protein-peptide and HSP-based immunotherapies for the treatment of
cancer. Front Immunol. 7(171)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shi H, Zhang J, Zhu R, Hu N, Lu H, Yang M,
Qin B, Shi J and Guan H: Primary angle closure and sequence
variants within MicroRNA binding sites of genes involved in eye
development. PLoS One. 11(e0166055)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Uhr JH, Mishra K, Wei C and Wu AY:
Awareness and knowledge of emergent ophthalmic disease among
patients in an internal medicine clinic. JAMA Ophthalmol.
134:424–431. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Stegmann RC, Grieshaber MC and Grieshaber
HR: Method and device for the treatment of glaucoma: U.S. Patent
8,951,221. Filed August 20, 2009; issued February 10, 2015.
|
|
15
|
Lévêque PM, Zéboulon P, Brasnu E, Baudouin
C and Labbé A: Optic disc vascularization in glaucoma: Value of
spectral-domain optical coherence tomography angiography. J
Ophthalmol. 2016(6956717)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Waisbourd M, Pruzan NL, Johnson D, Ugorets
A, Crews JE, Saaddine JB, Henderer JD, Hark LA and Katz LJ: The
Philadelphia glaucoma detection and treatment project: Detection
rates and initial management. Ophthalmology. 123:1667–1674.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Waugh DT: The contribution of fluoride to
the pathogenesis of eye diseases: Molecular mechanisms and
implications for public health. Int J Environ Res Public Health.
16(856)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li CP, Wang SH, Wang WQ, Song SG and Liu
XM: Long noncoding RNA-Sox2OT knockdown alleviates diabetes
mellitus-induced retinal ganglion cell (RGC) injury. Cell Mol
Neurobiol. 37:361–369. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yadav KS, Sharma S and Londhe VY:
Bio-tactics for neuroprotection of retinal ganglion cells in the
treatment of glaucoma. Life Sci. 243(117303)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Almasieh M, Wilson AM, Morquette B, Cueva
Vargas JL and Di Polo A: The molecular basis of retinal ganglion
cell death in glaucoma. Prog Retin Eye Res. 31:152–181.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang MH, Zhou XM, Cui JZ, Wang KJ, Feng Y
and Zhang HA: Neuroprotective effects of dexmedetomidine on
traumatic brain injury: Involvement of neuronal apoptosis and HSP70
expression. Mol Med Rep. 17:8079–8086. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fang CT, Kuo HH, Pan TS, Yu FC and Yih LH:
HSP70 regulates the function of mitotic centrosomes. Cell Mol Life
Sci. 73:3949–3960. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Salehi Z, Gholaminia M, Gholaminia Z,
Panjtanpanah M and Qazvini MG: The GG genotype of the HSPA1B gene
is associated with increased risk of glaucoma in northern Iran. Mol
Biol (Mosk). 51:31–36. 2017.PubMed/NCBI View Article : Google Scholar : (In Russian).
|
|
24
|
Della Santina L and Ou Y: Who's lost
first? Susceptibility of retinal ganglion cell types in
experimental glaucoma. Exp Eye Res. 158:43–50. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang X, Jiang C, Kong X, Yu X and Sun X:
Peripapillary retinal vessel density in eyes with acute primary
angle closure: An optical coherence tomography angiography study.
Graefes Arch Clin Exp Ophthalmol. 255:1013–1018. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ayub H, Khan MI, Micheal S, Akhtar F,
Ajmal M, Shafique S, Ali SH, den Hollander AI, Ahmed A and Qamar R:
Association of eNOS and HSP70 gene polymorphisms with glaucoma in
Pakistani cohorts. Mol Vis. 6:18–25. 2010.PubMed/NCBI
|
|
27
|
Nowak A, Majsterek I, Przybyłowska-Sygut
K, Pytel D, Szymanek K, Szaflik J and Szaflik JP: Analysis of the
expression and polymorphism of APOE, HSP, BDNF, and GRIN2B genes
associated with the neurodegeneration process in the pathogenesis
of primary open angle glaucoma. Biomed Res Int.
2015(258281)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nowak A, Szaflik JP, Gacek M,
Przybylowska-Sygut K, Kamińska A, Szaflik J and Majsterek I: BDNF
and HSP gene polymorphisms and their influence on the progression
of primary open-angle glaucoma in a Polish population. Arch Med
Sci. 10:1206–1213. 2014.PubMed/NCBI View Article : Google Scholar
|