Introduction
The blood-brain barrier (BBB) consists of a highly
selective semipermeable border of endothelial cells that prevents
solutes in the circulating blood from crossing into the
extracellular fluid of the central nervous system where neurons
reside (1). BBB is comprised of
cerebral endothelial cells, astrocytes and pericytes. The tight
junctions (TJ) between adjacent endothelial cells are responsible
for the low paracellular permeability and high electrical
resistance of the BBB. They regulate the movement of polar solutes
and macromolecules across the barrier (2). Significant advances in BBB research
over the past decade has led to the discovery of an increasing
number of structural and regulatory proteins in TJ (3). TJ is comprised of a wide range of
different protein complexes, including occludin, claudin-5 and
ZO-1. Under normal circumstances, TJ can effectively prevent
harmful substances from entering the brain (4). However, during ischemia-reperfusion
injury, TJ become disrupted, causing the loss of BBB function and
subsequent aggravation of brain damage (5).
Ischemic stroke accounts for ~85% of all cases of
stroke and is one of the most common diseases worldwide with a high
disability and mortality rates (6).
During cerebral ischemia, pro-inflammatory factors, including tumor
necrosis factor-α (TNF-α), interleukin (IL)-1β IL-1β and IL-6, in
addition to the rate-limiting enzymes of nitric oxide (NO), become
activated (7). During the early
stages of cerebral ischemia, the PI3K-AKT signaling pathway is
immediately activated, where endothelial nitric oxide synthase
(eNOS) becomes phosphorylated downstream (8). Co-existence of inflammatory factors
and NO activates the matrix metalloproteinase (MMP) family
(9). MMP2/9 is the most important
matrix degrading enzyme in the body (10), which can degrade almost all
components of the extracellular matrix and basement membrane
(11). MMP2/9 activation promotes
the destruction of endothelial cells during blood reperfusion,
specifically the degradation of TJ proteins (12), which leads to cerebral hemorrhage
and cerebral edema. Eventually, the BBB is destroyed and the
barrier effect disappears. Therefore, targeting BBB integrity
appears to be a promising therapeutic approach for ischemic
stroke.
To the best of our knowledge, 4-methoxybenzyl
alcohol (4-MA) is one of the main active compounds that can be
isolated from Gastrodia elata Blume (GEB). Previous studies
revealed a significant protective effect of GEB on cerebral
ischemia/reperfusion injury (13,14).
In addition, 4-MA has been demonstrated previously to cross the BBB
freely in both healthy rats and rats with cerebral ischemia
reperfusion injury (MCAO/R), where it remained in the brain tissue
and cerebrospinal fluid for 30 min after MCAO/R induction (15). 4-MA has also been demonstrated to
protect the brain against ischemic injury by decreasing the
permeability of the BBB after MCAO/R, where the underlying
mechanism may be associated with the inhibition of NOS activity,
upregulation of TJ protein expression and downregulation of
aquaporin-4 protein expression (16). However, the potential effects of
4-MA on brain microvascular endothelial cells, especially their
barrier function during oxygen-glucose deprivation/reoxygenation
(OGD/R) insult and the underlying mechanisms remain poorly
elucidated.
In the present study, an in vitro
oxygen-glucose deprivation/reperfusion (OGD/Rep) model was
established to investigate the effects of 4-MA on BBB integrity and
activation of PI3K/AKT pathway following ischemic-reperfusion
injury. In addition, the expression levels of key components of the
PI3K/AKT pathway and TJs were determined, whilst cell viability,
lactate dehydrogenase (LDH) release, NO levels, pro-inflammatory
factors TNF-α, IL-1β and IL-6 were also measured to explore the
potential molecular mechanisms of 4-MA against ischemic-reperfusion
injury.
Materials and methods
Materials
Mouse brain microvascular endothelial cells (bEnd.3)
were obtained from the American Type Culture Collection. DMEM, FBS
(cat. no. 10437028) and penicillin/streptomycin (cat. no.
15140-122) were purchased from Gibco, Thermo Fisher Scientific,
Inc. Occludin (cat. no. sc-5562) and claudin-5 (cat. no. sc-28670)
primary antibodies were purchased from Santa Cruz Biotechnology,
Inc. FITC-conjugated secondary antibodies (cat. no. bs-0296R-FITC),
DAPI (cat. no. C02-04002) and β-actin primary antibody (cat. no.
bs-0061R) were purchased from BIOSS. LY294002 (cat. no. S1737) was
purchased from Beyotime Institute of Biotechnology. Primary
antibodies against eNOS (cat. no. 32027S), phosphorylated (p)-eNOS
(cat. no. 9571S), AKT (cat. no. 9272S) and p-AKT (cat. no. 4060S)
were purchased from Cell Signaling Technology Inc. Horseradish
peroxidase (HRP)-conjugated Affinipure Goat Anti-Rabbit IgG (cat.
no. SA00001-2) secondary antibody was obtained from Proteintech
Group, Inc. Additionally, 96-well and six-well plates were
purchased from Corning Inc. Bicinchoninic acid (BCA) protein assay
kit (cat. no. A045-4-2), lactate dehydrogenase assay kit (LDH; cat.
no. A020-2-2), nitric oxide (NO) assay kit (cat. no. A013-2-1), MTT
cell proliferation and cytotoxicity assay kit (cat. no. G020-1-1),
tumor necrosis factor-α (TNF-α; cat. no. H052), Interleukin (IL)-1β
(cat. no. H002) and IL-6 ELISA assay kits (cat. no. H007) were
purchased from Nanjing Jiancheng Bioengineering Institute. 4-MA
(cat. no. M107568) was obtained from Aladdin.
Cell culture and treatment
bEnd.3 cells were cultured in DMEM supplemented with
10% FBS and 1% penicillin/streptomycin and incubated at 37˚C under
5% CO2. A fresh medium was added every 2 days. Cell
growth curves of 1x103, 5x103,
1x104 cells/ml were produced. The OD value at 570 nm of
each density was measured daily for 6 days using MTT assay. To
select for the optimal conditions for OGD/Rep cell models, cell
viability after Oxygen-glucose (OGD) for 1 h/reperfusion (Rep) for
1 h, OGD for 2 h/Rep for 2 h, OGD for 3 h/Rep for 3 h, OGD for 4
h/Rep for 4 h and OGD for 6 h/Rep for 4 h were also determined by
MTT. Oxygen-glucose deprivation/reperfusion (OGD/Rep) in
vitro model was established for the treatment of bEnd.3 cells
as described previously (17).
Briefly, bEnd.3 cells were first incubated in glucose-free DMEM
(Thermo Fisher Scientific, Inc.) without FBS and subsequently
transferred into a Tri-Gas incubator (HF100; Heal Force
Bio-meditech Holdings, Ltd.) with 1% O2, 94%
N2, and 5% CO2 for 6 h at 37˚C. After OGD,
glucose-free DMEM was replaced with high-glucose DMEM (Thermo
Fisher Scientific, Inc.) with 10% FBS and the cells were incubated
under 95% O2, 5% CO2 and maintained 4 h at
37˚C. For cell experiments, the solvent used for drugs are 0.05%
DMSO. The control and OGD/Rep groups were treated with 0.05% DMSO.
To assess the safety of 4-MA, bEnd.3 cells were treated with 800,
400, 200, 100, 50 and 25 µM 4-MA for 36 h at 37˚C before cell
viability was determined using MTT assay. To deduce the optimal
dose of 4-MA, bEnd.3 cells were treated with 800, 400, 200, 100, 50
and 25 µM 4-MA for 36 h at 37˚C before OGD/Rep induction before
cell viability was determined using MTT assay.
Cell viability
In total, 1x104 bEnd.3 cells/ml were seeded into
96-well plates before cell viability was determined using MTT
assay. Firstly, 20 µl MTT solution was then added into each well so
that the final concentration of MTT per well is 0.5 mg/ml. The
plate was then incubated at 37˚C for 3 h. After incubation, 150 µl
DMSO was add into each well and the plate was wrapped in foil,
which was shaken on an orbital shaker for 10 min at 37˚C.
Absorbance at 570 nm was read in each well using a microplate
reader.
LDH release, NO levels and
pro-inflammatory factors
In total, 1x104 bEnd.3 cells/ml were
seeded into 96-well plates. The OGD/Rep-induced damage to cells
were then treated with 200, 100 and 50 µM 4-MA for 36 h at 37˚C. In
the media supernatant, LDH release and levels of NO were detected
by colorimetry whilst the levels of pro-inflammatory factors TNF-α,
IL-1β and IL-6 were measured using respective ELISA kits according
to the protocols from the respective manufacturers.
Immunofluorescence assay
bEnd.3 cells were seeded into six-well plates at a
density of 1x104 cells/ml. bEnd.3 cells that underwent
OGD/Rep were treated with 200 µM 4-MA for 36 h. The culture medium
was then discarded and the cells were washed three times with PBS
for 5 min each. The bEnd.3 cells were subsequently fixed in 4%
paraformaldehyde at room temperature for 1 h and washed with PBS
three times for 5 min each. Permeabilization was then performed
using 0.5% Triton X-100 for 30 min at room temperature and the
cells were washed with PBS again three time for 5 min each. A total
of 10% goat serum (Beijing Solarbio Science & Technology Co.,
Ltd.) was used for blocking at room temperature for 1 h.
Subsequently, 200 µl occludin (dilution, 1:100) and claudin-5
(dilution, 1:25) antibodies was added and the cells were incubated
at room temperature for 1 h at 4˚C. After washing three times with
PBS for 5 min each, 200 µl FITC-conjugated secondary antibody
(dilution, 1:200) was added and incubated at room temperature for 2
h. The cells were counterstained with 0.5 µg/ml DAPI (cat. no.
C02-04002) at room temperature for 5 min. In total, threes fields
of view were randomly selected per well before images were taken
using a fluorescence microscope (magnification, x100; Olympus
Corporation). ImageJ image analysis software (version 15.1P;
National Institutes of Health) was used to measure the integrated
optical density (IOD) and picture area (Area) of each field of view
and the mean optical density (MOD) was calculated using the
following formula: MOD = IOD/Area. The average value of MOD in four
fields of view is the result of immunofluorescence determination of
the sample, where the MOD represents the expression level of
occludin and claudin-5.
Western blot analysis
bEnd.3 cells were seeded into six-well plates at a
density of 1x104 cells/ml. bEnd.3 cells that underwent
OGD/Rep were treated with 200 µM 4-MA or 200 µM 4-MA + 50 µM
LY294002 for 36 h. The cells were then washed with ice-cold PBS
before ice-cold RIPA lysis buffer (Beyotime Institute of
Biotechnology) was added and the cell lysates were collected into a
pre-cooled microcentrifuge tube, which was maintained under
constant agitation for 30 min at 4˚C. Following centrifugation in a
microcentrifuge at 4˚C for 20 min at 14,006 x g, the supernatant
was collected and the pellet was discarded. After that, 60 µg
protein loaded per lane was separated by 10% SDS-PAGE and were
transferred onto PVDF membranes, membranes were blocked with 5%
skimmed milk powder at room temperature for 1 h. The membranes were
then incubated with eNOS (dilution, 1:1,000), p-eNOS (dilution,
1:1,000), Akt (dilution, 1:1,000) or p-Akt (dilution, 1:2,000)
antibodies for 2 h at room temperature. The membranes were washed
with TBS-0.1% Tween-20 three times for 8 min each and incubated
with HRP-conjugated Affinipure Goat Anti-Rabbit IgG secondary
antibody (dilution, 1:1,000) for 1 h at room temperature. The
membrane was developed using an ECL Detection Reagent (cat. no.
B500023; Proteintech Group, Inc.) and exposed using an Imaging
System (cat. no. EC3; UVP Inc.). Western blotting data was
quantified by ImageJ software (version no. 1.48; National
Institutes of Health).
Statistical analysis
Results were presented as the mean ± standard
deviation, representative of six experimental repeats. Differences
between groups were assessed using one-way ANOVA followed by
Tukey's post hoc test using GraphPad Prism 6.0 software (GraphPad
Software Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of 4-MA on cell viability, LDH
release and the levels of NO and pro-inflammatory factors
Cell growth curves after using the cell densities of
1x103, 5x103 and 1x104 cells/ml
were plotted. The cell growth rates of 1x104 cells/ml
density was found to be optimal (Fig.
1A). Compared with 100±13.87% in the control group, treatment
of cells with OGD/Rep for OGD 1 h/Rep 1 h, OGD 2 h/Rep 2 h, OGD 3
h/Rep 3 h, OGD 4 h/Rep 4 h, and OGD 6 h/Rep 4 h reduced cell
viability to 93.91±7.50, 85.06±4.57 (P<0.05), 77.02±4.28
(P<0.01), 69.21±4.22 (P<0.01) and 56.95±1.99% (P<0.01),
respectively (Fig. 1B). Since the
survival rate in the OGD 6 h/Rep 4 h model was found to be
56.95±1.99%, the OGD 6 h/Rep 4 h model was used for subsequent
experiments.
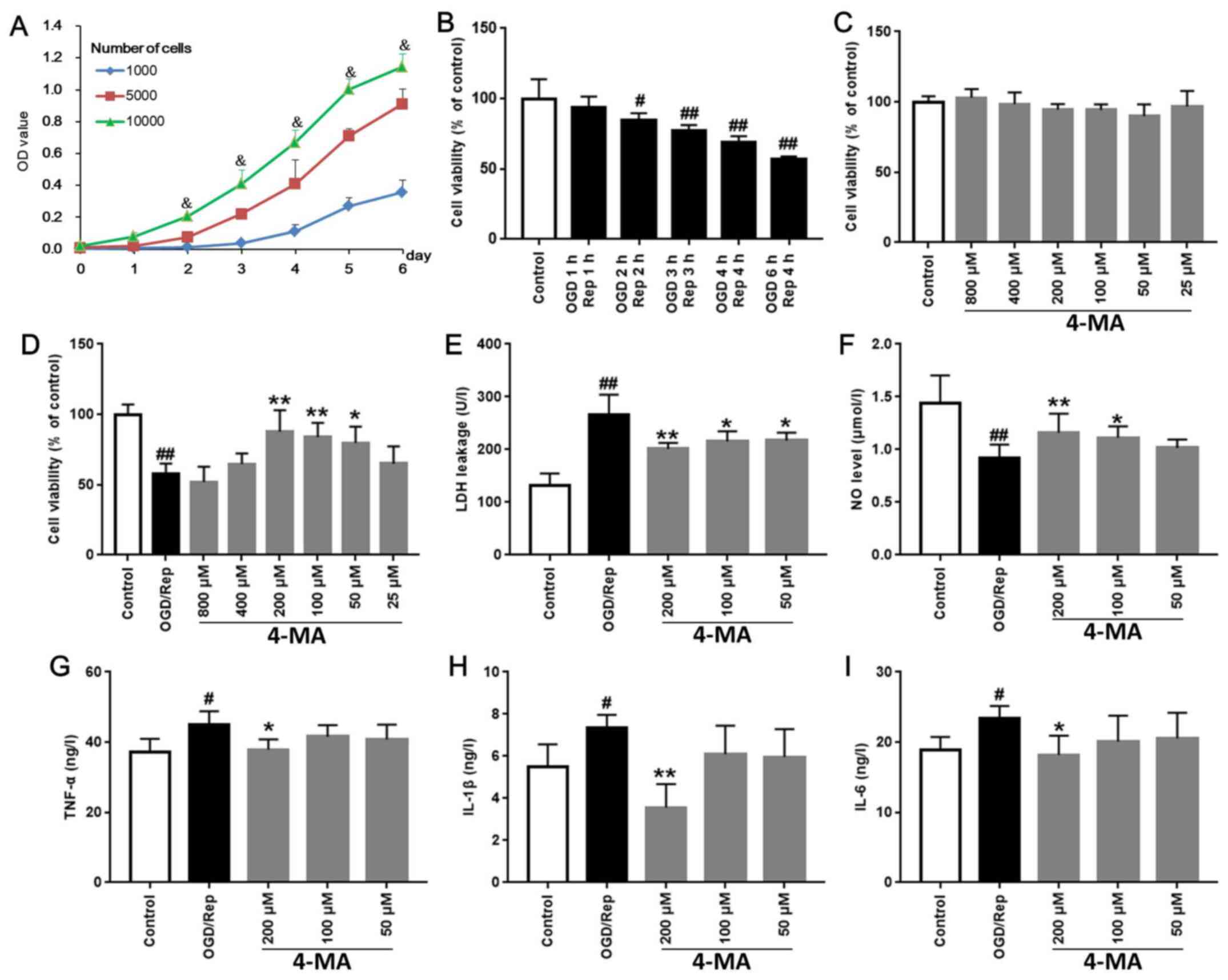 | Figure 1Effects of 4-MA on cell viability, LDH
release, NO levels and pro-inflammatory factors after OGD/Rep in
bEnd.3 cells. (A) Cell growth curves at seeding densities of
1x103, 5x103, 1x104 cells/ml were
measured daily for 6 days using MTT. (B) To select the conditions
for OGD/Rep cell models, cell viability after OGD 1 h/Rep 1 h, OGD
2 h/Rep 2 h, OGD 3 h/Rep 3 h, OGD 4 h/Rep 4 h and OGD 6 h/Rep 4 h
were determined by MTT. (C) To assess the safety of 4-MA, bEnd.3
cells were treated with 800, 400, 200, 100, 50 and 25 µM 4-MA for
36 h and cell viability was determined using MTT assay. (D) To
deduce the optimal dose of 4-MA, bEnd.3 cells were treated with
800, 400, 200, 100, 50 and 25 µM 4-MA for 36 h before OGD/Rep
induction, and cell viability was determined using MTT assay. (E)
LDH release and (F) NO levels were detected by colorimetry. The
levels of pro-inflammatory factors (G) TNF-α, (H) IL-1β and (I)
IL-6 were measured using respective ELISA kits.
&P<0.05 vs. 1x103 and 5x103
cells/ml; #P<0.05 and ##P<0.01 vs.
Control; *P<0.05 and **P<0.01 vs.
OGD/Rep. OD, optical density; TNF-α, tumor necrosis factor-α; IL,
interleukin; NO, nitric oxide; LDH, lactate dehydrogenase; OGD/Rep,
oxygen-glucose deprivation/reperfusion; 4-MA,
4-methoxybenzyl-alcohol. |
Treatment with 4-MA at concentrations of 800, 400,
200, 100, 50 and 25 µM exhibited little to no effects on bEnd.3
cells (Fig. 1C). Subsequently, it
was revealed that 4-MA with dose of 200 µM had the highest efficacy
on bEnd.3 cells after OGD/R (Fig.
1D). Compared with that in the OGD/Rep group (57.97±7.20), 36 h
treatment with 4-MA at doses of 200, 100 and 50 µM significantly
increased cell viability to 87.77±15.26 (P<0.01), 83.90±10.04
(P<0.01) and 79.74±11.69% (P<0.05), respectively (Fig. 1D).
LDH release from the cells into the medium has been
frequently used as an indicator of cell injury (18). Compared with that in the control
group (131.44±23.56), LDH release was found to be significantly
increased in the OGD/Rep group to 266.87±37.75 U/l (P<0.01).
Compared with that in the OGD/Rep group (266.87±37.35), 36 h
treatment with 200, 100 and 50 µM 4-MA significantly reduced LDH
release in cells that underwent OGD/Rep to 201.09±11.58
(P<0.01), 215.45±19.42 (P<0.05) and 217.00±15.14 U/l
(P<0.05), respectively (Fig.
1E).
Activation of eNOS has been previously reported to
mediate protection from stroke by preserving cerebral blood flow
and inhibiting inflammation, platelet aggregation, thrombosis and
apoptosis (19). Compared with that
in the control group (1.44±0.27), NO level was significantly
reduced in OGD/Rep group (0.92±0.13 µM/l; P<0.01; Fig. 1F). Compared with that in the OGD/Rep
group, 36 h treatment with 200 and 100 µM 4-MA significantly
increased NO levels, to 1.16±0.18 µM/l (P<0.01) and 1.11±0.11
µM/l (P<0.05), respectively (Fig.
1F).
Compared with those in the control group
(37.33±3.65, 5.52±1.05, 18.91±1.86 for TNF-α, IL-1β and IL-6,
respectively), the levels of TNF-α, IL-1β and IL-6 were
significantly elevated in OGD/Rep group to 45.09±3.71 (P<0.01),
7.37±0.60 (P<0.01) and 23.44±1.73 ng/l (P<0.01), respectively
(Fig. 1G-I). Compared with those in
the OGD/Rep group, 36 h treatment with 200 µM 4-MA significantly
reduced the levels of TNF-α, IL-1β and IL-6 to 37.89±2.93
(P<0.05), 3.57±1.11 (P<0.01) and 18.20±2.73 ng/l (P<0.05),
respectively (Fig. 1G-I).
Data from the cell viability and LDH release assays,
in addition to measurements of NO and pro-inflammatory factor
levels suggest that 36 h treatment with 200 µM 4-MA exhibited the
strongest effect on protecting bEnd.3 cells against OGD/Rep-induced
injury.
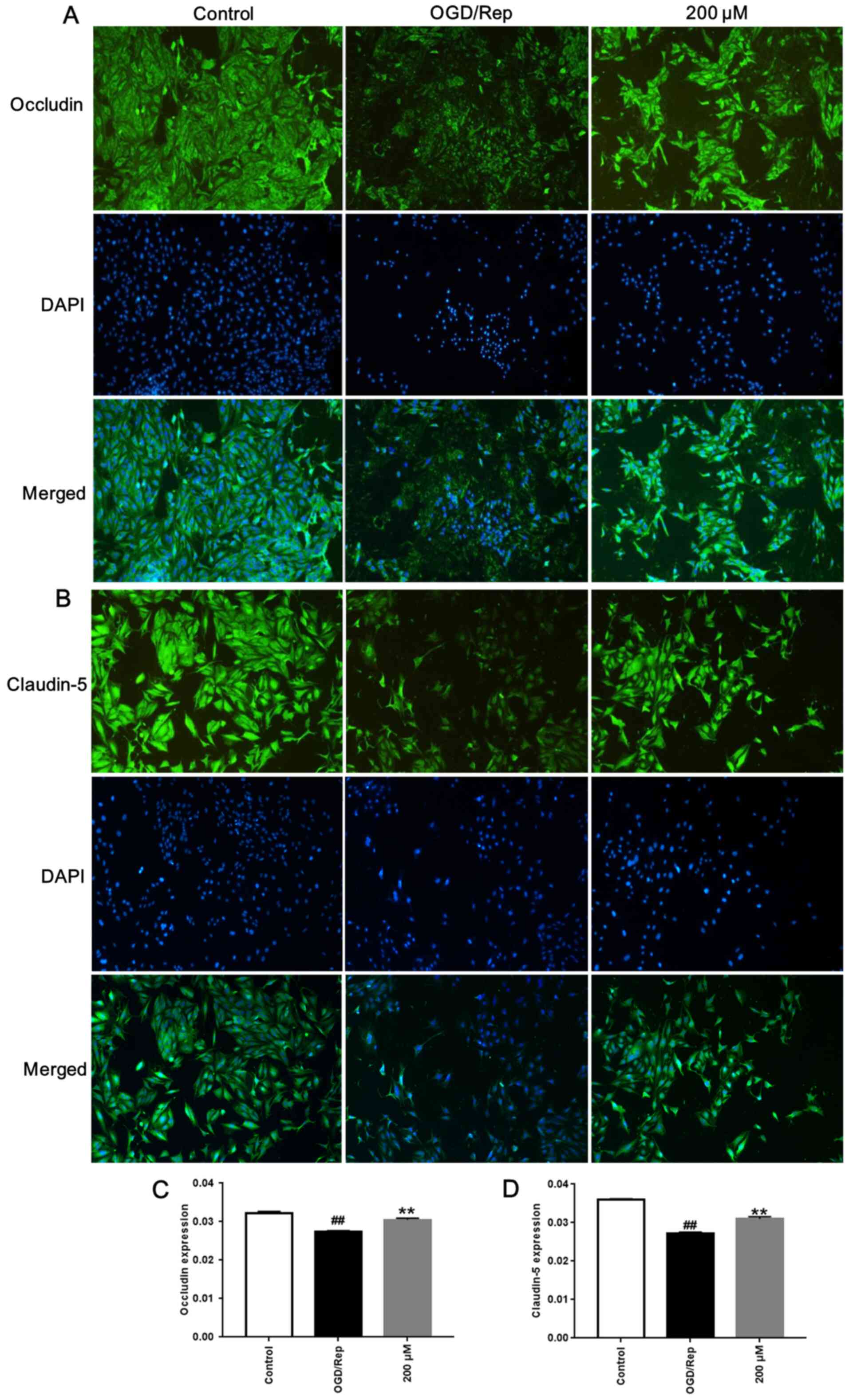
4-MA enhances occludin and claudin-5
expression
Compared with those in control group, OGD/Rep
significantly reduced the average optical densities of claudin-5
and occluding in bEnd.3 cells (P<0.01; Fig. 2). However, treatment with 200 µM
4-MA significantly increased the average optical densities of
claudin-5 and occludin in bEnd.3 cells compared with those in the
OGD/Rep group (P<0.01; Fig.
2).
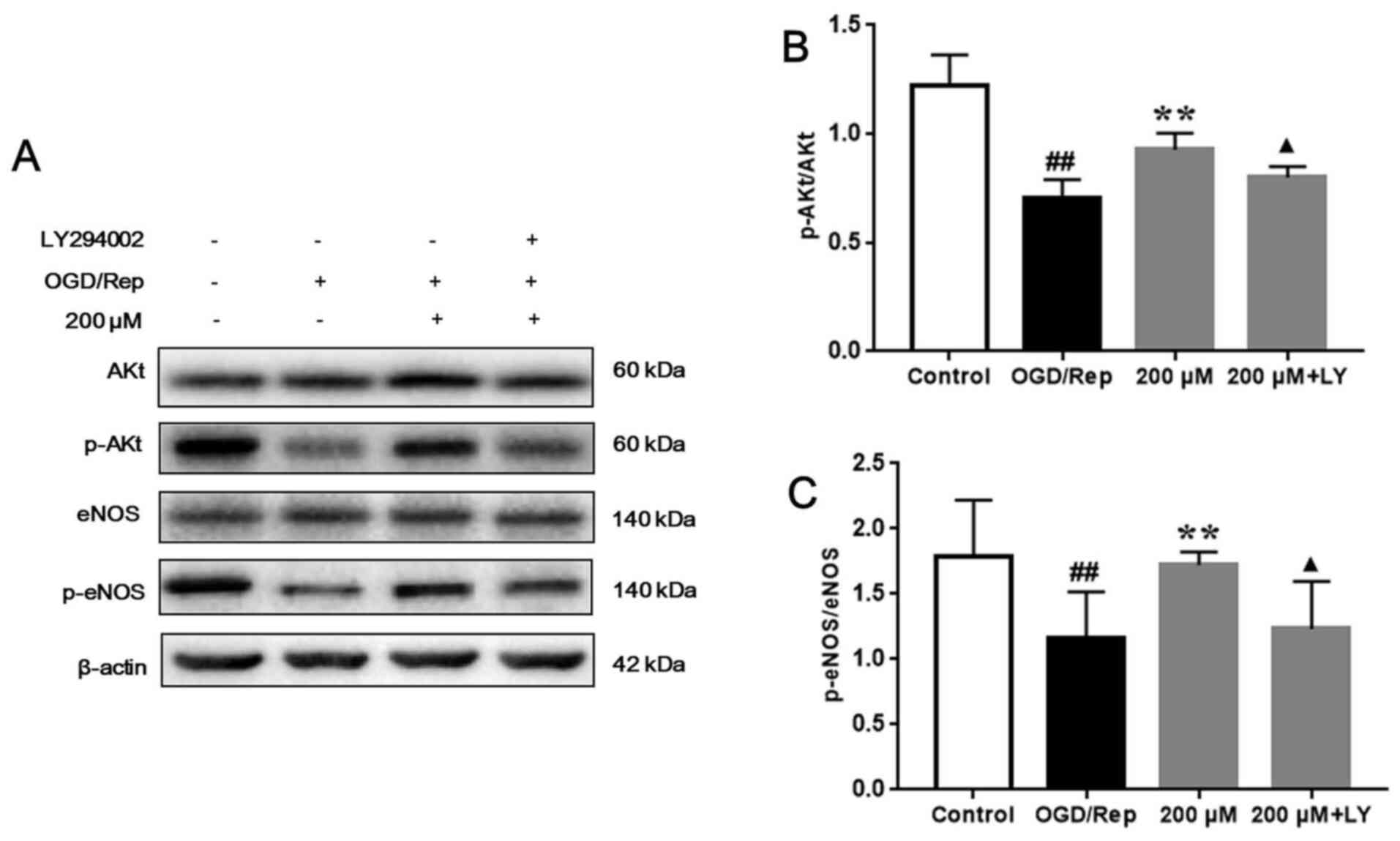
4-MA activates the PI3K/AKT signaling
pathway
Compared with that in the control group, AKT and
eNOS phosphorylation were significantly downregulated in the
OGD/Rep group (P<0.01). After treatment, 200 µM 4-MA
significantly upregulated AKT and eNOS phosphorylation (P<0.01),
which was abolished by LY294002 (P<0.01; Fig. 3A, B
and C). These results suggest that
4-MA can activate the PI3K/AKT signaling pathway in bEnd.3 cells
after OGD/Rep-induced injury and that PI3K/Akt signaling pathway
could be inhibited by LY294002.
Discussion
After ischemic stroke, the integrity of the
blood-brain barrier is compromised. The BBB serves to restrict the
passage of pathogens, restrict the diffusion of solutes and large
or hydrophilic molecules, whilst allowing the diffusion of
hydrophobic molecules, including O2, CO2,
hormones and small polar molecules, from the blood circulation into
the cerebrospinal fluid (20).
Brain microvascular endothelial cells differ from peripheral
endothelial cells with regards to the expression of specific ion
transporters and receptors, where they contain fewer gap and
pinocytotic vesicles (21). There
are several factors that can cause BBB destruction, including MMPs,
inflammatory factors, trauma and tumor-infiltrating immune cells
(22). Damage can be caused by
reductions in TJ protein expression and function (23), degradation of the basement membrane,
increased permeability and the entry of harmful substances such as
free radicals into the brain (24).
Therefore, protection of BBB integrity serves a vital role in the
prevention and treatment of ischemic stroke.
In the present study, an OGD/Rep in vitro
model was established to mimic ischemic stroke (25), a cell model with a survival rate of
50-60% is the most suitable. If the survival rate of the model
group is too high, it will cause no difference compared with the
control group. If the survival rate of the model group is too low,
it is difficult for the drug to have a prevention and treatment
effects. LDH is rapidly released into the cell culture supernatant
when the plasma membrane is damaged, which is a key feature of
cells undergoing apoptosis, necrosis or other forms of cellular
damage (26). Results from the
present study showed that 4-MA can reduce LDH levels after OGD/Rep
induction. A number of pathogenic factors, including infection,
tissue injury or cardiac infarction, can induce inflammation to
mediate tissue damage (27). The
etiologies of inflammation can be either infectious or
non-infectious (28). Immune cells
produce and release a large number of cytokines, including IL-1β,
IL-6 and TNF-α and chemotactic factors. After ischemic stroke,
pro-inflammatory cytokines including IL-1β, IL-6 and TNF-α can
induce changes in the integrity of the BBB, which increases
permeability (29). This
pro-inflammatory environment can lead to a more robust release of
other inflammatory mediators, such as the chemokine MCP-1(30). Due to the excessive stimulation of
immune cells, large quantities of leukocyte adhesion to endothelial
cells occurs, resulting in the excessive activation of the
anti-infection response (31). Data
from the present study suggest that 4-MA can inhibit the release of
pro-inflammatory factors following OGD/Rep injury.
TJ membrane proteins were first identified in
retinal ECs, which include occludin, claudin-1, claudin-2,
claudin-5 and junctional adhesion molecule-A (32). Occludin is not essential for the
formation of TJ, since occludin-deficient embryonic stem cells can
differentiate into epithelial cells and can form well-developed
tight junction structures (33).
Claudin-5 is specifically expressed in the brain tissue, where it
regulates the integrity and permeability of the BBB (34). The results from the present study
demonstrated that 4-MA can enhance TJ protein expression following
OGD/Rep injury.
The PI3K/AKT signaling pathway is involved in
multiple biological processes, including cell proliferation,
survival and apoptosis (35,36).
PI3K is a heterodimer composed of a p85 regulatory subunit and a
p110 catalytic subunit that regulates a variety of cellular
responses (37). Activation of
class I PI3Ks generates phosphatidylinositol-3,4,5-trisphosphate,
as a second messenger that serve as a docking platform. At the
plasma membrane, AKT is phosphorylated at Thr-308 by
3-phosphoinositide-dependent kinase 1 whereas
3-phosphoinositide-dependent kinase 2 has also been suggested to
phosphorylate AKT (38). BBB damage
is the critical pathological process of ischemic stroke. PI3K/Akt
pathway was involved in altering BBB permeability in various
cerebral pathological conditions, where the activation of PI3K/Akt
pathway can result in neuroprotection in cerebral ischemia
(39,40). The results of the present study
suggest that 4-MA can activate the PI3K/AKT signaling pathway in
bEnd.3 cells after OGD/Rep-induced injury.
In summary, the present study demonstrates that 4-MA
can protect against OGD/Rep-induced cerebral microvascular
endothelial cell injury and the disruption of BBB integrity in
vitro. 4-MA can attenuate the production of inflammatory
mediators and activate the PI3K/AKT signaling pathway. These
findings suggest that 4-MA may have the potential for treating
cerebral ischemic events (Fig.
4).
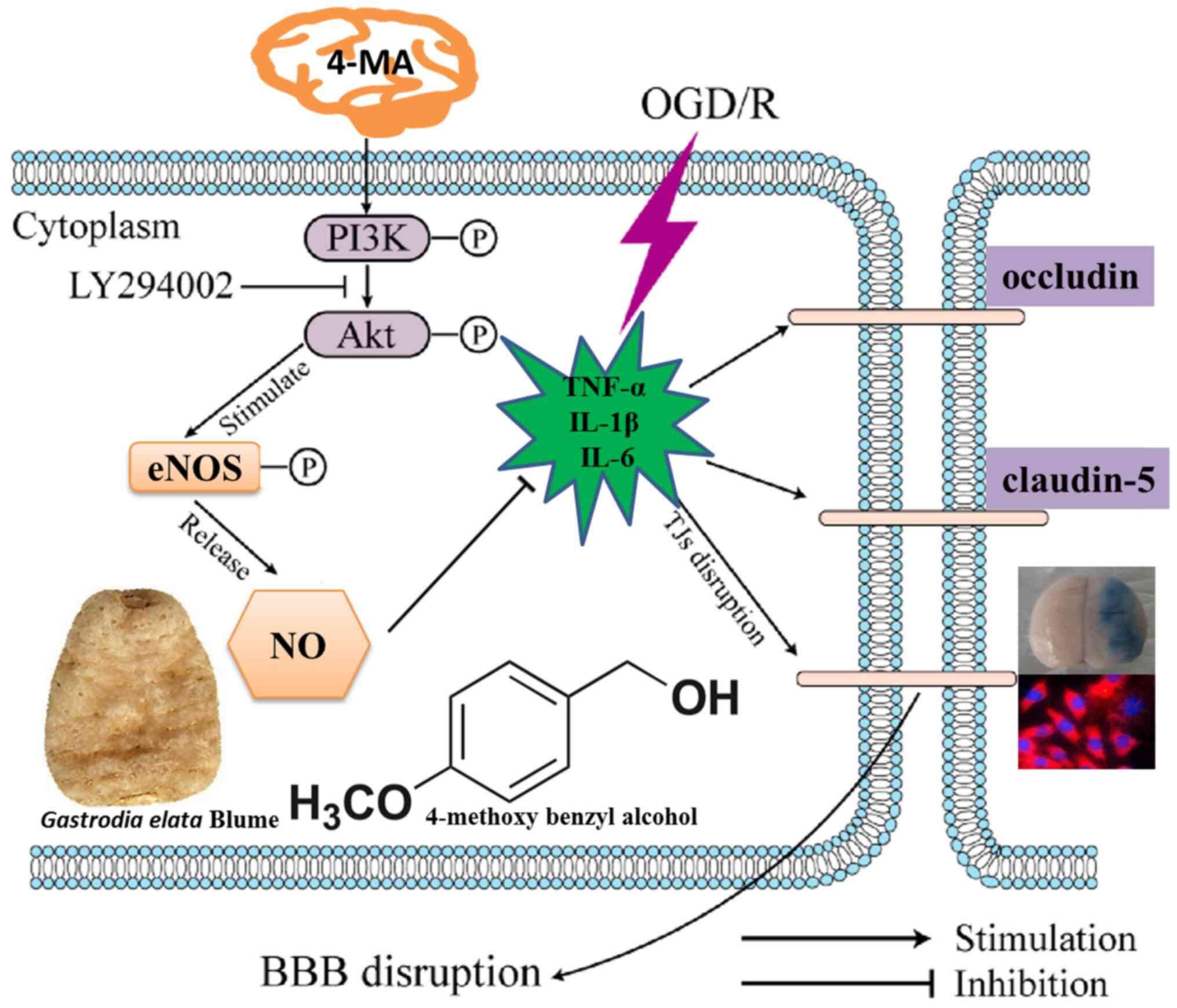 | Figure 4Proposed protective effects of 4-MA
against OGD/Rep-induced injury and associated mechanism. 4-MA
inhibits the production of inflammatory factors (TNF-α, IL-1β,
IL-6) and increased the expression of TJ proteins occluding and
claudin-5, ameliorates OGD/Rep-induced brain microvascular
endothelial barrier dysfunction through activating the PI3K/AKT
signaling pathway. After the PI3K/AKT signaling pathway is
activated, downstream eNOS is phosphorylated to produce a large
amount of NO. NO can expand blood vessels, increase blood supply in
ischemic areas and have a protective effect on the brain. BBB,
blood-brain barrier; TNF-α, tumor necrosis factor-α; IL,
interleukin; NO, nitric oxide; eNOS, endothelial nitric oxide
synthase; LDH, lactate dehydrogenase; OGD/R, oxygen-glucose
deprivation/reperfusion; 4-MA, 4-methoxybenzyl-alcohol; TJ, tight
junction; p-, phosphorylated. |
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81960733 and
81560664).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL and LY designed the study. WW performed all the
experiments. QL and XD interpreted the data and drafted the
manuscript. All authors read and approved the final version of the
manuscript. XD can authenticate the raw data in this study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang F, Cao Y, Ma L, Pei H, Rausch WD and
Li H: Dysfunction of cerebrovascular endothelial cells: Prelude to
vascular dementia. Front Aging Neurosci. 10:376. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jiang X, Andjelkovic AV, Zhu L, Yang T,
Bennett MVL, Chen J, Keep RF and Shi Y: Blood-brain barrier
dysfunction and recovery after ischemic stroke. Prog Neurobiol.
163-164:144–171. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Greene C and Campbell M: Tight junction
modulation of the blood brain barrier: CNS delivery of small
molecules. Tissue Barriers. 4(e1138017)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Abdullahi W, Tripathi D and Ronaldson PT:
Blood-brain barrier dysfunction in ischemic stroke: Targeting tight
junctions and transporters for vascular protection. Am J Physiol
Cell Physiol. 315:C343–C356. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang H, Park JH, Maharjan S, Park JA,
Choi KS, Park H, Jeong Y, Ahn JH, Kim IH, Lee JC, et al: Sac-1004,
a vascular leakage blocker, reduces cerebral ischemia-reperfusion
injury by suppressing blood-brain barrier disruption and
inflammation. J Neuroinflammation. 14(122)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhu S, Tang S and Su F: Dioscin inhibits
ischemic stroke induced inflammation through inhibition of the
TLR4/MyD88/NF-κB signaling pathway in a rat model. Mol Med Rep.
17:660–666. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Alblihed MA: Astragalin attenuates
oxidative stress and acute inflammatory responses in
carrageenan-induced paw edema in mice. Mol Biol Rep. 47:6611–6620.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu J, Weaver J, Jin X, Zhang Y, Xu J, Liu
KJ, Li W and Liu W: Nitric oxide interacts with caveolin-1 to
facilitate autophagy-lysosome-mediated claudin-5 degradation in
oxygen-glucose deprivatio-treated endothelial cells. Mol Neurobiol.
53:5935–5947. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang QY, Wang ZJ, Sun DM, Wang Y, Xu P,
Wu WJ, Liu XH and Zhu YZ: Novel therapeutic effects of leonurine on
ischemic stroke: New mechanisms of BBB integrity. Oxid Med Cell
Longev. 2017:7150376. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kamat PK, Kalani A, Tyagi SC and Tyagi N:
Hydrogen sulfide epigenetically attenuates homocysteine-induced
mitochondrial toxicity mediated through NMDA receptor in mouse
brain endothelial (bEnd3) cells. J Cell Physiol. 230:378–394.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jing Y, Zhang L, Xu Z, Chen H, Ju S, Ding
J, Guo Y and Tian H: Phosphatase actin regulator-1 (PHACTR-1)
knockdown suppresses cell proliferation and migration and promotes
cell apoptosis in the bEnd.3 mouse brain capillary endothelial cell
line. Med Sci Monit. 25:1291–1300. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Paul D, Baena V, Ge S, Jiang X, Jellison
ER, Kiprono T, Agalliu D and Pachter JS: Appearance of
claudin-5+ leukocytes in the central nervous system
during neuroinflammation: A novel role for endothelial-derived
extracellular vesicles. J Neuroinflammation. 13:292.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Duan X, Wang W, Liu X, Yan H, Dai R and
Lin Q: Neuroprotective effect of ethyl acetate extract from
gastrodia elata against transient focal cerebral ischemia in rats
induced by middle cerebral artery occlusion. J Tradit Chin Med.
35:671–678. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
He F, Duan X, Dai R, Wang W, Yang C and
Lin Q: Protective effects of ethyl acetate extraction from
gastrodia elata blume on blood-brain barrier in focal cerebral
ischemia reperfusion. Afr J Tradit Complement Altern Med.
13:199–209. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Duan X, Wang W, Wu S, Yan H, Liu L and Un
Q: Detection of methoxybenzyl alcohol content in brain tissue and
cerebrospinal fluid of rats by HPLC. Med Plant. 5(29)2014.
|
|
16
|
He F, Duan X, Dai R, Li Y and Lin Q:
Protective effect of 4-methoxy benzyl alcohol on the blood-brain
barrier after cerebral ischemia reperfusion injury. J Stroke
Cerebrovasc Dis. 26:1258–1265. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xu T, Sun R, Wei G and Kong S: The
protective effect of safinamide in ischemic stroke mice and a brain
endothelial cell line. Neurotox Res. 38:733–740. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhao D, Sun X, Lv S, Sun M, Guo H, Zhai Y,
Wang Z, Dai P, Zheng L, Ye M, et al: Salidroside attenuates
oxidized low density lipoprotein induced endothelial cell injury
via promotion of the AMPK/SIRT1 pathway. Int J Mol Med.
43:2279–2290. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhu W, Qiu W and Lu A: Cryptotanshinone
exhibits therapeutical effects on cerebral stroke through the
PI3K/AKT eNOS signaling pathway. Mol Med Rep. 16:9361–9366.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sadeghian N, Shadman J, Moradi A, Ghasem
Golmohammadi M and Panahpour H: Calcitriol protects the Blood-Brain
Barrier integrity against ischemic stroke and reduces vasogenic
brain edema via antioxidant and antiapoptotic actions in rats.
Brain Res Bull. 150:281–289. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hu X, De Silva TM, Chen J and Faraci FM:
Cerebral vascular disease and neurovascular injury in ischemic
stroke. Circ Res. 120:449–471. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jian Z, Liu R, Zhu X, Smerin D, Zhong Y,
Gu L, Fang W and Xiong X: The involvement and therapy target of
immune cells after ischemic stroke. Front Immunol.
10(2167)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang S, Mei S, Jin H, Zhu B, Tian Y, Huo
J, Cui X, Guo A and Zhao Z: Identification of two immortalized cell
lines, ECV304 and bEnd3, for in vitro permeability studies of
blood-brain barrier. PLoS One. 12:e0187017. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sweeney MD, Zhao Z, Montagne A, Nelson AR
and Zlokovic BV: Blood-brain barrier: From physiology to disease
and back. Physiol Rev. 99:21–78. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cao GS, Chen HL, Zhang YY, Li F, Liu CH,
Xiang X, Qi J, Chai CZ, Kou JP and Yu BY: YiQiFuMai powder
injection ameliorates the oxygen-glucose deprivation-induced brain
microvascular endothelial barrier dysfunction associated with the
NF-κB and ROCK1/MLC signaling pathways. J Ethnopharmacol.
183:18–28. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Deng F, Wang S, Cai S, Hu Z, Xu R, Wang J,
Feng D and Zhang L: Inhibition of caveolae contributes to propofol
preconditioning-suppressed microvesicles release and cell injury by
hypoxia-reoxygenation. Oxid Med Cell Longev.
2017(3542149)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng
J, Li Y, Wang X and Zhao L: Inflammatory responses and
inflammation-associated diseases in organs. Oncotarget.
9:7204–7218. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Guo T, Wang Y, Guo Y, Wu S, Chen W, Liu N,
Wang Y and Geng D: 1,25-D3 protects from cerebral ischemia by
maintaining BBB permeability via PPAR-γ activation. Front Cell
Neurosci. 12:480. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Michels M, Ávila P, Pescador B, Vieira A,
Abatti M, Cucker L, Borges H, Goulart AI, Junior CC, Barichello T,
et al: Microglial cells depletion increases inflammation and
modifies microglial phenotypes in an animal model of severe sepsis.
Mol Neurobiol. 56:7296–7304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gu M, Mei XL and Zhao YN: Sepsis and
cerebral dysfunction: BBB damage, neuroinflammation, oxidative
stress, apoptosis and autophagy as key mediators and the potential
therapeutic approaches. Neurotox Res: Sep 2, 2020 (Epub ahead of
print). doi: 10.1007/s12640-020-00270-5.
|
|
31
|
Cao G, Jiang N, Hu Y, Zhang Y, Wang G, Yin
M, Ma X, Zhou K, Qi J, Yu B, et al: Ruscogenin attenuates cerebral
ischemia-induced blood-brain barrier dysfunction by suppressing
TXNIP/NLRP3 inflammasome activation and the MAPK pathway. Int J Mol
Sci. 17(1418)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Puscas I, Bernard-Patrzynski F, Jutras M,
Lécuyer MA, Bourbonnière L, Prat A, Leclair G and Roullin VG: IVIVC
assessment of two mouse brain endothelial cell models for drug
screening. Pharmaceutics. 11(587)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rempe RG, Hartz AMS, Soldner ELB, Sokola
BS, Alluri SR, Abner EL, Kryscio RJ, Pekcec A, Schlichtiger J and
Bauer B: Matrix metalloproteinase-mediated blood-brain barrier
dysfunction in epilepsy. J Neurosci. 38:4301–4315. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lv J, Hu W, Yang Z, Li T, Jiang S, Ma Z,
Chen F and Yang Y: Focusing on claudin-5: A promising candidate in
the regulation of BBB to treat ischemic stroke. Prog Neurobiol.
161:79–96. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tian J, Zhang X, Wu H, Liu C, Li Z, Hu X,
Su S, Wang LF and Qu L: Blocking the PI3K/AKT pathway enhances
mammalian reovirus replication by repressing IFN-stimulated genes.
Front Microbiol. 6:886. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang SW, Deng LX, Chen HY, Su ZQ, Ye SL
and Xu WY: MiR-124 affects the apoptosis of brain vascular
endothelial cells and ROS production through regulating PI3K/AKT
signaling pathway. Eur Rev Med Pharmacol Sci. 22:498–505.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lv X, Xu T, Wu Q, Zhou Y, Huang G, Xu Y
and Zhong G: 6-Gingerol activates PI3K/Akt and inhibits apoptosis
to attenuate myocardial ischemia/reperfusion injury. Evid Based
Complement Alternat Med. 2018(9024034)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Song J, Kang SM, Lee WT, Park KA, Lee KM
and Lee JE: The beneficial effect of melatonin in brain endothelial
cells against oxygen-glucose deprivation followed by
reperfusion-induced injury. Oxid Med Cell Longev.
2014(639531)2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chi OZ, Mellender SJ, Kiss GK, Liu X and
Weiss HR: Blood-brain barrier disruption was less under isoflurane
than pentobarbital anesthesia via a PI3K/Akt pathway in early
cerebral ischemia. Brain Res Bull. 131:1–6. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hu S, Wu Y, Zhao B, Hu H, Zhu B, Sun Z, Li
P and Du S: Panax notoginseng saponins protect cerebral
microvascular endothelial cells against oxygen-glucose
deprivation/reperfusion-induced barrier dysfunction via activation
of PI3K/Akt/Nrf2 antioxidant signaling pathway. Molecules.
23(2781)2018.PubMed/NCBI View Article : Google Scholar
|