Introduction
Liver transplantation (LTx) is the most effective
treatment for patients with end-stage liver diseases, including
hepatic cirrhosis, hepatocellular carcinoma, hepatic hemangioma and
hepatic echinococcosis, which has been extensively applied in the
clinical setting for more than half a century (1-5).
The discrepancy between donor organ supply and demand continues to
increase with the growing number of potential candidates on the
transplant waiting list (6). Thus,
a series of strategies have been advocated to expand the donor pool
from the extended criteria donors (ECD), including donation after
cardiac death, age-related mortality, and malignancy and hepatic
steatosis (7-9).
However, liver grafts donated from ECD are associated with a higher
risk of primary non-function (PNF), early allograft dysfunction and
mortality (10).
Steatotic liver, which belongs to marginal liver
grafts (MLG), is the most common liver disorder in developed
countries, with a prevalence of 20-30% among the general population
(11-16).
Hepatic steatosis is typically characterized by the accumulation of
lipid droplets in hepatocytes and is commonly divided into two
subtypes, macrovescicular steatosis or microvescicular steatosis,
based on the size of the fat vacuole and the location of the
nucleus (17). Macrovesicular
steatosis is defined as the accumulation of large fat droplets in
the hepatocyte, displacing the nucleus to the edge of the cell,
whereas microvesicular steatosis is defined as the presence of tiny
lipid vesicles in the hepatocyte, without nuclear displacement
(17). In the clinical setting,
steatosis has been classified into mild (<30%), moderate
(30-60%) or severe (>60%) depending on the degree of the fatty
infiltration (18).
Hepatic steatosis, particularly that in the liver
with 30% macrosteatosis, is considered an independent risk factor
for PNF due to increased susceptibility to ischemia-reperfusion
injury (17,19-21).
The following mechanisms have been proposed for hepatic steatosis:
Liver microcirculation is hampered with excessive fat accumulation,
which leads to mitochondrial damage, and oxidative stress during
reperfusion, coupled with inflammatory response involving lipid
peroxidation and leukocyte adhesion may contribute to the graft
failure following transplantation (20,22,23).
Ploeg et al (18) reported
that PNF rates increase up to 80% in the severely steatotic liver.
In addition, previous studies have demonstrated that severely
steatotic graft is associated with high PNF rates (0-66%) and a
1-year graft with a survival rate of 25-90% (18,24-26).
Currently, macrovesicular steatosis of >30% is considered an
independent predictor for a reduction of PNF and 1-year graft
survival (27). Thus, the
evaluation and restoring of organs from ECD attract increasing
attention.
Due to difficulties and complexities in
microsurgical technology (28),
establishing a rat steatotic liver transplantation model can be
challenging. In addition, donor livers or grafts require multiple
biopsies at the point of procurement, during preservation and after
transplantation. When using liver transplantation models, a single
biopsy requires animals to be sacrificed, resulting in an increased
number of experimental animals, which is not conducive to animal
welfare and ethical principles. Thus, the present study established
a novel method to decrease the number of experimental animals. The
results of the present study suggest that liver tissues from
different parts at different time points may significantly decrease
the number of experimental animals used. In addition, the proposed
method did not affect post-operative animal mortality (papillary
process and quadrate lope excision: Partial liver transplantation
vs. whole liver transplantation survival; 5/5 vs. 5/5).
The steatotic liver animal model induced by a
high-fat diet (HFD) is commonly applied to assess hepatic steatosis
in vivo. This model can mimic the etiology of hepatic
steatosis in human beings (29).
The present study aimed to establish a stable and reproducible
steatotic liver model induced by a HFD that may be used to
investigate the efficacy of reduced size transplantation in MLG
research.
Materials and methods
Animals
A total of 90 male Sprague-Dawley rats (210±10 g),
aged 6-8 weeks, were obtained from Hubei Provincial Center for
Disease Control and Prevention in China. All animals were housed in
an environment with a temperature of 23±1˚C, relative humidity of
55±10%, air exchange 12-14 times/h, a light/dark cycle of 12/12 h,
and were provided with food and tap water ad libitum. The
present study was approved (IRB approval no. AF-177) by Wuhan
University Institutional Animal Care and Use Committee (Wuhan,
China).
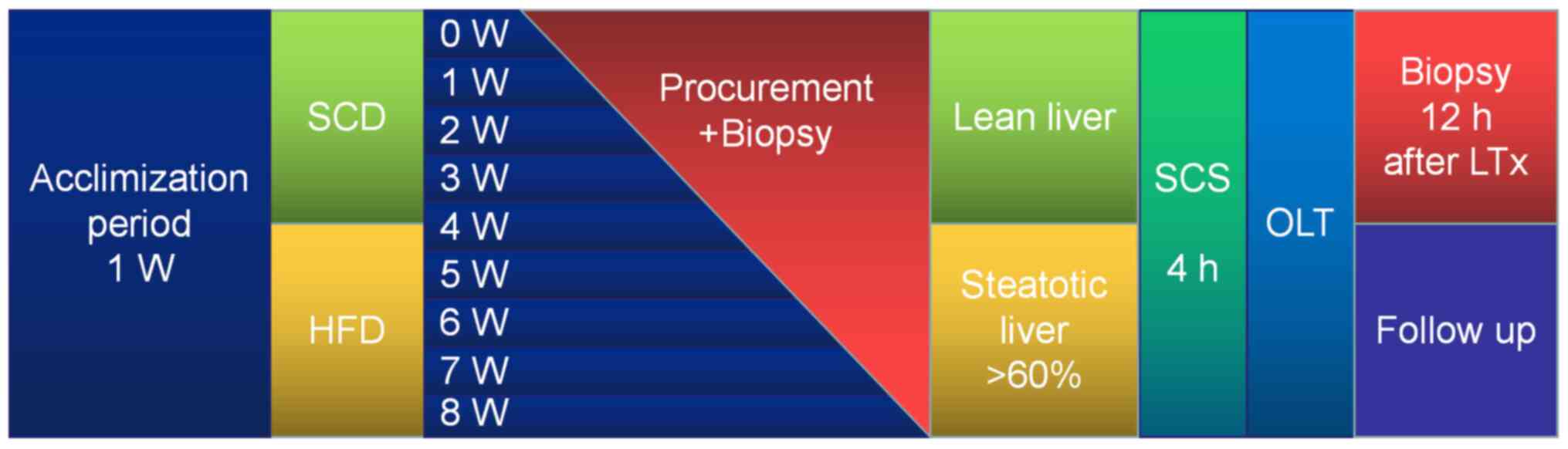
A total of 10 rats were procured after 7 days of
acclimation to provide baseline values. A total of five rats in the
HFD group or standard chow diet (SCD) group were samples each week
during 8 weeks of modeling. Rats were randomly divided into 8
groups (n=10). Body weight and food intake were monitored on a
daily basis. The time schedules for blood extraction in relation to
the frozen sections used for analysis are presented in Fig. 1.
Dietary interventions
The HFD consisted of 60% lipid, 20.6% carbohydrate
and 19.4% protein (kJ), and was provided in rods direct from the
manufacturer (Trophic Animal Feed High-Tech Co., Ltd.). The lipids
included in the HFD consisted of 90% lard and 10% soybean oil
(Trophic Animal Feed High-Tech Co., Ltd.). The SCD consisted of
usual pellet rat chow (Trophic Animal Feed High-Tech Co., Ltd.). In
order to avoid fatty diarrhea, rats in the HFD group were fed using
the following 6 day schedule: i) 2 days of 30% weight (wt) HFD and
70% wt SCD; ii) 2 days of 50% wt HFD and 50% wt SCD, and iii) 2
days of 70% wt HFD and 30% wt SCD.
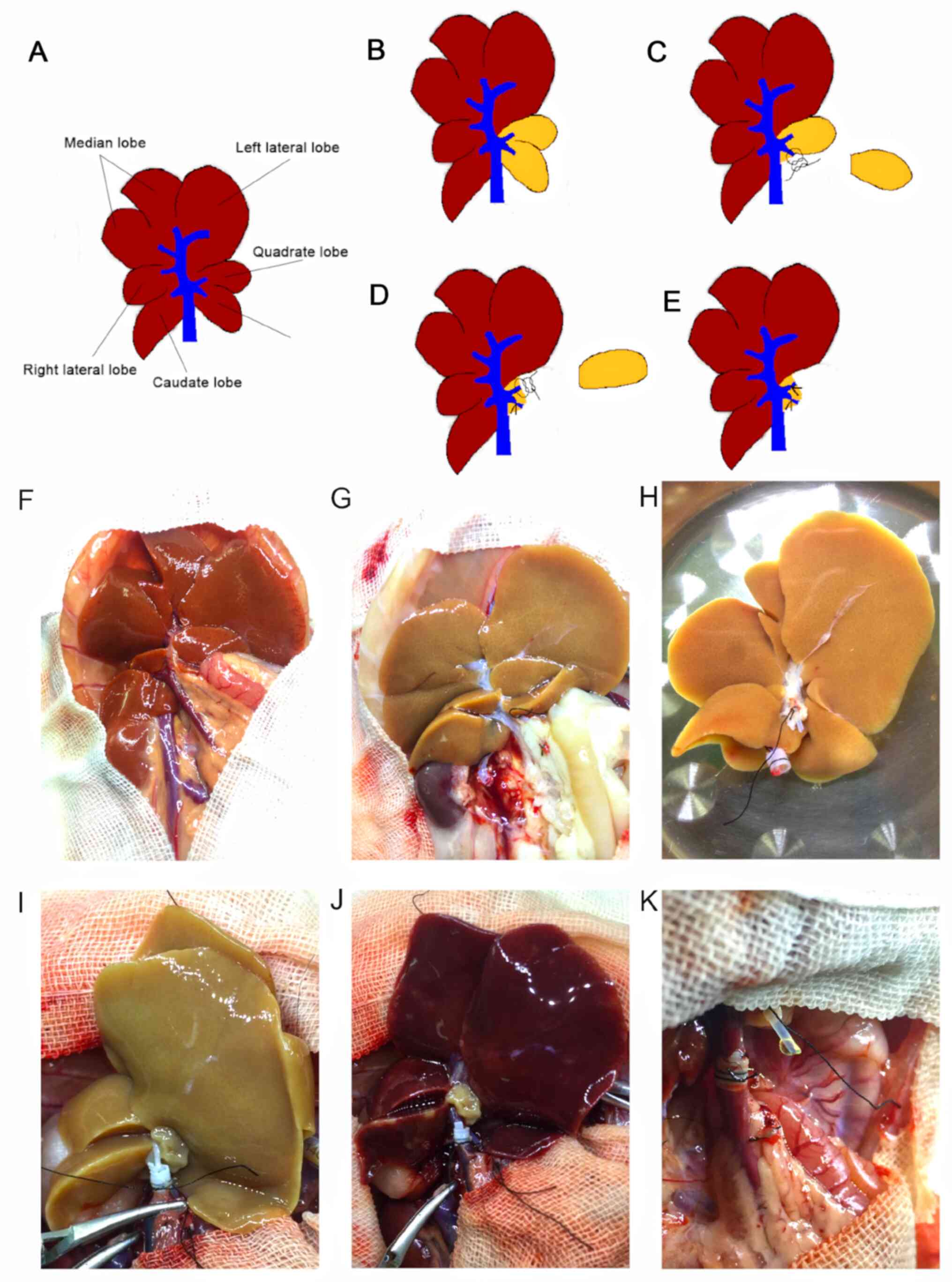
Graft procurement and reduced size
procedure
Anesthesia during liver procurement and
transplantation was maintained using isoflurane (cat. no.
R510-22-4; RWD Life Technology Co., Ltd.; 4% isoflurane for
induction and 2% isoflurane for maintenance). Briefly, heparin (100
IU) in 2 ml saline solution (cat. no. H8060-1g; Beijing Solarbio
Science & Technology Co., Ltd.) was injected into the penile
vein, and a 5 mm long stent prepared from polyethylene tube was
inserted into the common bile duct (CBD) and secured with 6-0
sutures. Livers were flushed in situ with 20 ml of
University of Wisconsin (UW) solution at 4˚C. A total of two
hepatic lobes, the papillary process and the quadrate lobe were
procured for assessment, using the liver volume reduction method
illustrated in Fig. 2. The
papillary process (Fig. 2C) was
removed to assess the extent of hepatic steatosis before cold
storage (CS) at 4˚C. The quadrate lobe (Fig. 2D) was cut following preservation
with UW solution at 2-4˚C for 4 h. Venous cuffs prepared from two
sizes of polyethylene tubes were subsequently placed in the portal
vein (PV) and intrahepatic inferior vena cava (IHVC). The
inside/outside diameters of polyethylene tube for CBD, PV and IHVC
were 0.6/1.0, 1.8/2.2 and 2.8/3.2 mm, respectively. Grafts were
stored in UW solution at 4˚C for 4 h prior to implantation
(Fig. 2E).
Serum analyses
Blood was drawn from the rats every week during the
modeling process to detect hepatocyte injury and serum lipid levels
via alanine aminotransaminase (ALT), aspartate aminotransferase
(AST), triglyceride (TG), total cholesterol (TC), free fatty acid,
high-density lipoprotein and low-density lipoprotein. These indices
were measured at the Institute for Clinical Biochemistry and
Diagnostics, Zhongnan Hospital of Wuhan University (Wuhan, China).
Lipids from rat livers were prepared using chloroform-methanol
extraction (30). Plasma glucose
concentration was detected using a glucose analyzer (590; Yuwell),
while insulin concentration was detected using commercially
available RIA kits (cat. no. E-EL-R3034; Elabscience Biotechnology
Co., Ltd.).
Histological assessment
The papillary process of liver tissues was obtained
and divided into two parts immediately after collection. One part
was fixed in a cold buffered 4% paraformaldehyde solution or cold
buffered 3% glutaraldehyde solution. Following fixation, the first
part of tissues in each group was embedded in olefin, cut into 4-mm
thick slices and stained with hematoxylin (10 min at room
temperature) and eosin (2 min at room temperature) or toluidine
blue (3 min at room temperature) (31). The second part of tissues was
treated with Oil Red O (ORO) for 8 min at room temperature to
assess the degree of steatosis (32), and for intraoperative assessment of
the steatotic extent to determine whether it can be used as a
donor. The degree of steatosis was estimated based on the
percentage of hepatocytes containing lipid droplets using Scoring
System Definitions (33) and the
following formula: Degree of steatotic change (%)=[(number of
hepatocytes with fatty droplets in the all microscopic
field)/(number of total hepatocytes in the all microscopic field)]
x100. The degree of steatotic change in all groups was determined
by calculating the percentage in 10 random areas. A total of two
pathologists (Zhongnan Hospital of Wuhan University), blindly
assessed and confirmed all biopsies. All liver sections were
observed under a light microscope (magnification, x200; TE2000-U;
Nikon Corporation) and the number and area of fat droplets in
hepatocytes were assessed using Image-Pro Plus (version 6.0; Media
Cybernetics Inc.).
Orthotopic liver transplantation
(OLT)
Rats in the HFD group (n=15; 6-8 weeks), with
hepatic macrovesicular steatosis >60%, were subjected to
intraoperative ORO staining. OLT was performed as previously
described by Kamada and Calne (28). Steatotic liver grafts or lean liver
grafts were procured and transplanted into healthy adult rat
recipients 4 h after preservation. For transplantation, the liver
of the recipient was removed after clamping the suprahepatic
inferior vena cava (SHVC), PV and the IHVC, and grafts were
transplanted by anastomosing the SHVC with 8-0 monofilament nylon
suture. The cuff was subsequently inserted into the corresponding
vessels and secured with 6-0 sutures. The bile duct was anastomosed
using an intraluminal stent. The macroscopic changes of liver
grafts after portal vein opening were recorded in different groups.
A total of five rats in each group were transplanted and observed
for 180 days, whereby the survival rate was calculated. For the
control group, five rats from the SCD group underwent the same
treatment. The transplanted rats were recovered at the Intensive
Care Unit Cage (Vetario S10; Brinsea Products Ltd.) and
subcutaneously injected with 0.1 mg/kg buprenorphine (Shanghai
Hengyuan Biotechnology Co., Ltd.) twice a day for 3 continuous days
post-surgery to relieve pain. To fulfill ethical obligations set by
the Swiss legislation, all rats were appropriately evaluated using
an animal suffering score, as previously described (34). All transplanted rats were
individually housed at a temperature of 23±1˚C, relative humidity
of 55±10%, air change 12-14 times/h, light/dark cycles of 12/12 h,
and had ad libitum access to food and tap water 6 h
post-surgery. The steatotic liver graft was procured from the
recipient for biopsy 1-year after LTx.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software (version 4.0; GraphPad Software, Inc.), the
non-parametric Mann-Whitney-Wilcoxon test or two-way analysis of
variance, followed by Bonferroni test for selected pairs of
columns. All data are presented as the mean ± standard deviation.
Survival analysis was performed using the Kaplan Meier method and
log-rank test. P<0.05 was considered to indicate a statistically
significant difference.
Results
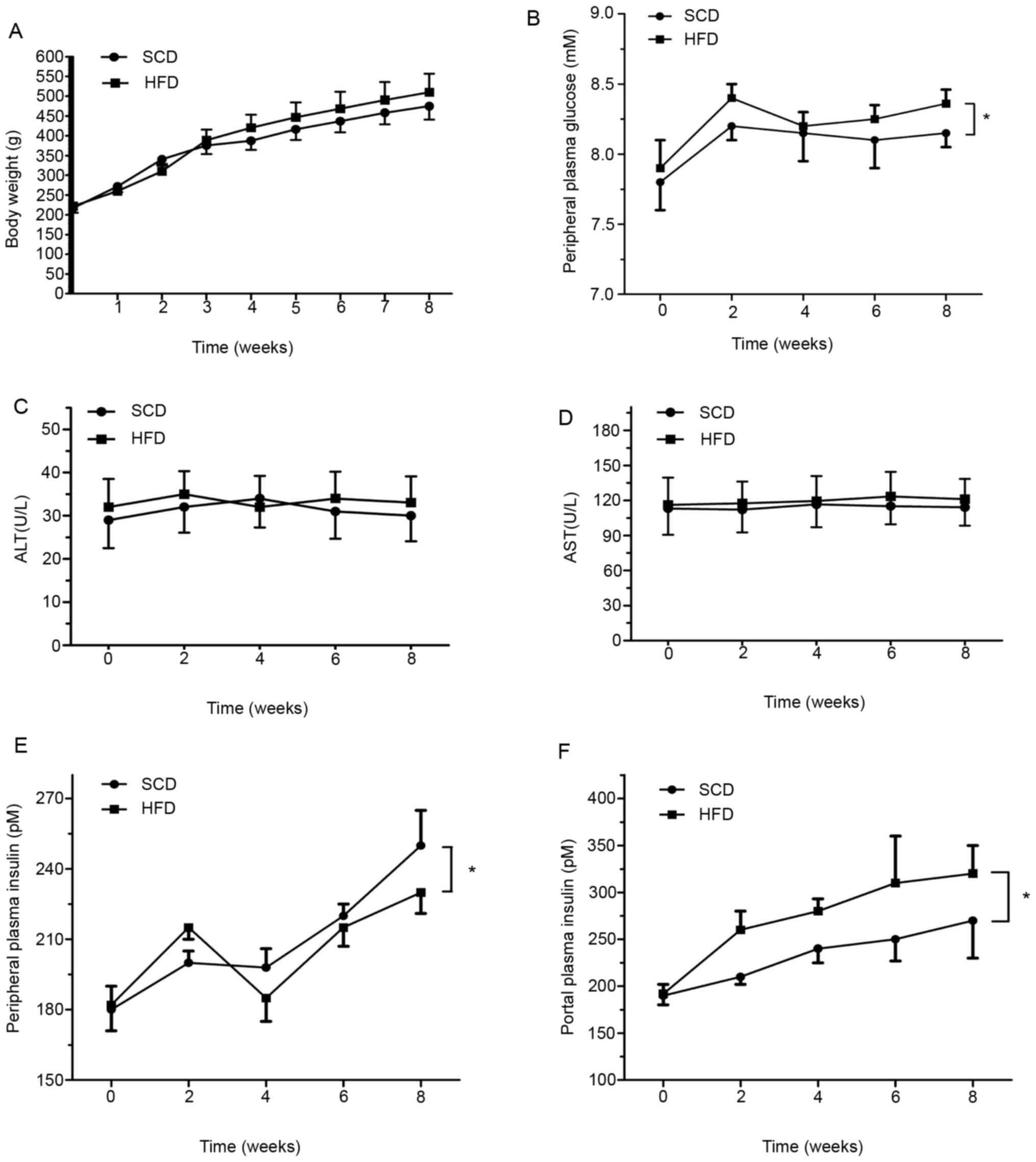
Body weight
The changes in body weight are presented in Fig. 3A. In weeks 2 and 3, lower body
weight was observed in the HFD group compared with the SCD group
(340.70±16.50, 310.20±18.24 g vs. 375.4±22.2, 388.8±26.7 g,
respectively). However, no significant differences were observed
between the groups (P>0.05).
HFD induces rats to develop
dyslipidemia and causes no inflammation
The results of the present study demonstrated
changes in ALT and AST expression in rats fed a HFD compared with
those fed a SCD; however, no statistical differences were observed
between the groups (P>0.05; Fig.
3C and D).
In addition, the level of plasma TG in the HFD group
increased by 1.76-fold at week 2, and progressively decreased to
baseline levels by week 8. Significantly higher levels of TG were
observed in the HFD group compared with the SCD group by week 2
(P<0.05; 1.548±0.172 mmol/l vs. 0.691±0.153 mmol/l). The peak TG
level was observed at week 4 in the SCD group (Fig. 4). Notably, the levels of plasma TG
in both the HFD and SCD groups were not time-dependent (P>0.05;
Fig. 4A).
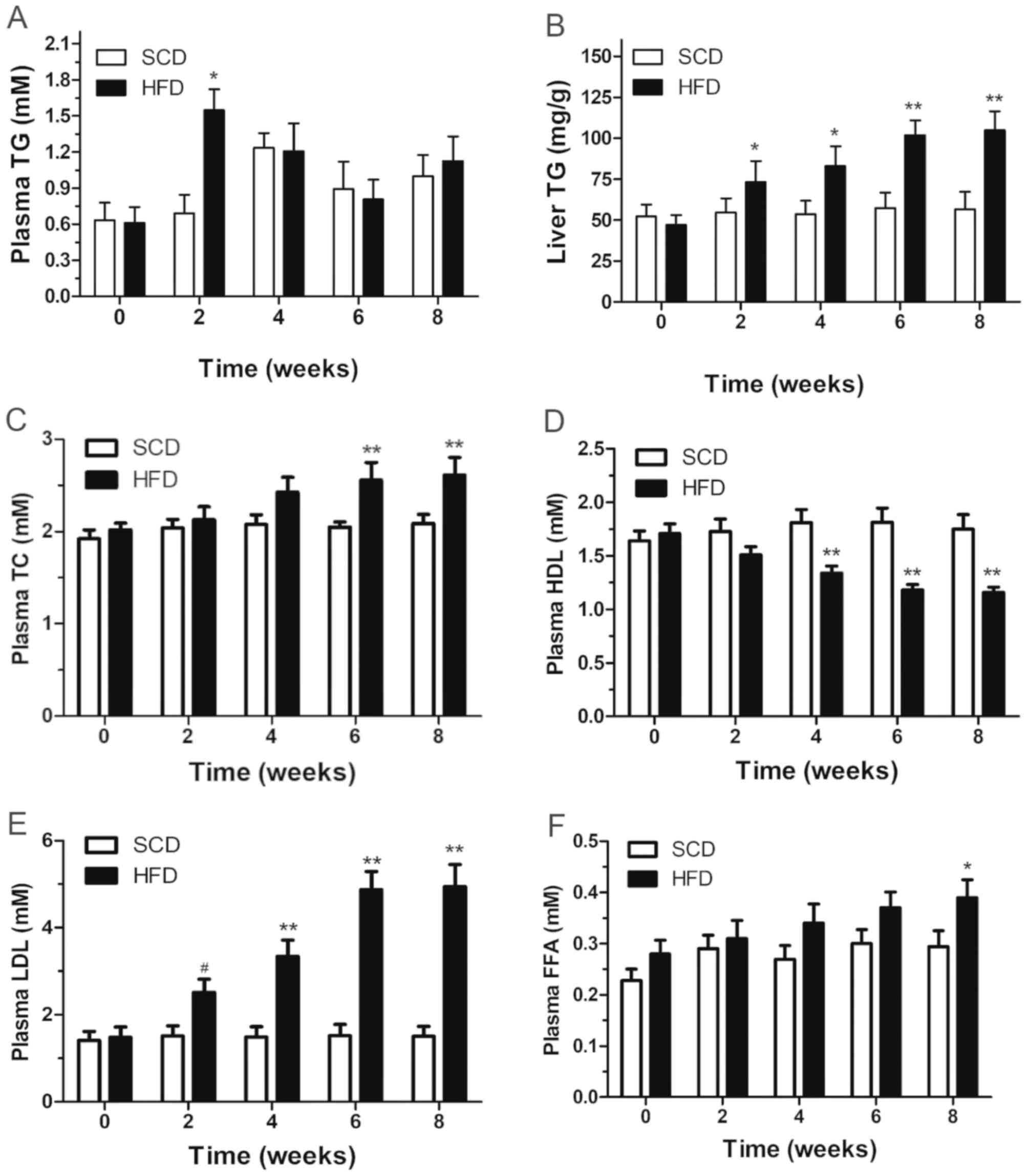 | Figure 4Plasma (A) TG, (B) liver TG, (C)
plasma TC, (D) HDL, (E) LDL and (F) FFA levels in rats fed a HFD or
SCD. Date are presented as the mean ± standard deviation. SCD
compared with HFD, *P<0.05 and **P<0.01
vs. SCD. TG, triglyceride; TC, cholesterol; HDL, high density
lipoprotein; LDL, low density lipoprotein; FFA, free fatty acid;
HFD, high-fat diet; SCD, standard chow diet. |
The HFD group exhibited small but significantly
higher plasma glucose values (P<0.05; Fig. 3B), accompanied by significantly
higher overall plasma insulin values in portal blood (P<0.05;
Fig. 3E) but not in peripheral
blood (Fig. 3F), compared with the
SCD group. Furthermore, the column demonstrated that liver TG
content ascended with time, with a statistically significant
difference between the two groups after week 2 (P<0.05; Fig. 4B).
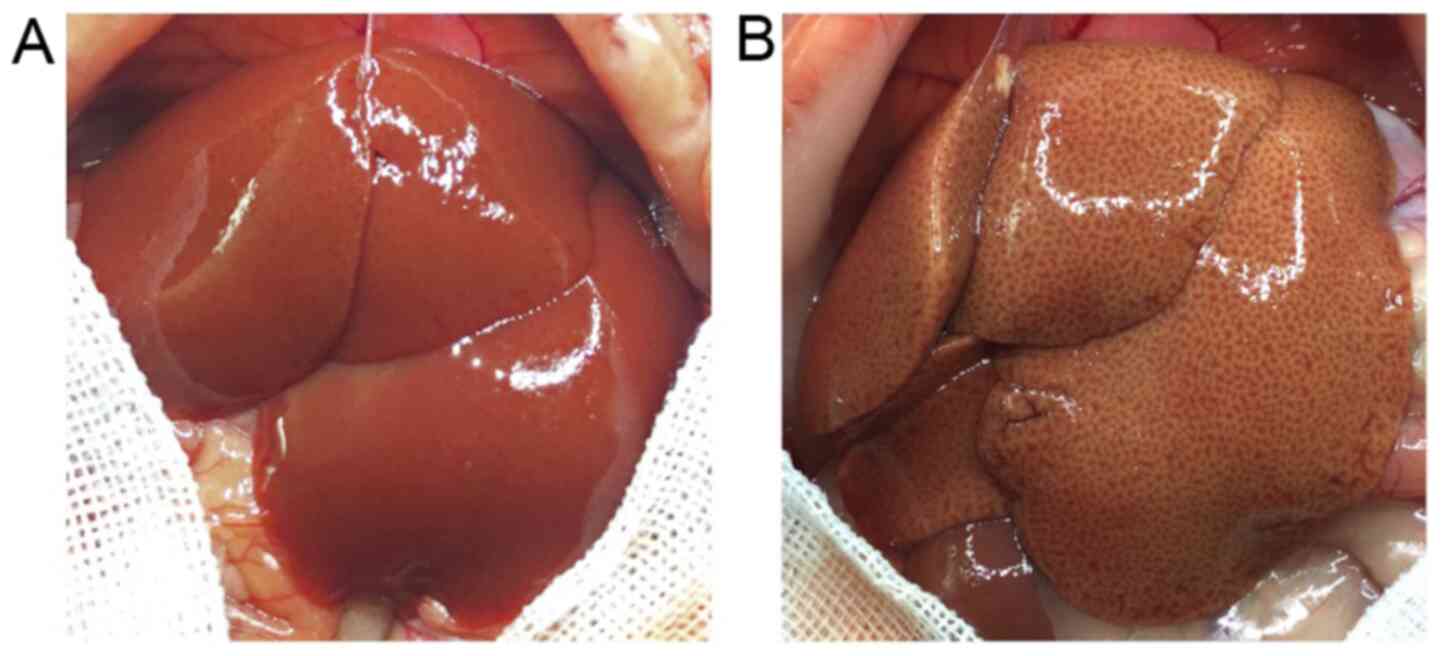
Morphological assessment
The rat liver receiving HFD was marginally obtuse
and became dark yellow in color compared with the SCD-fed rats. In
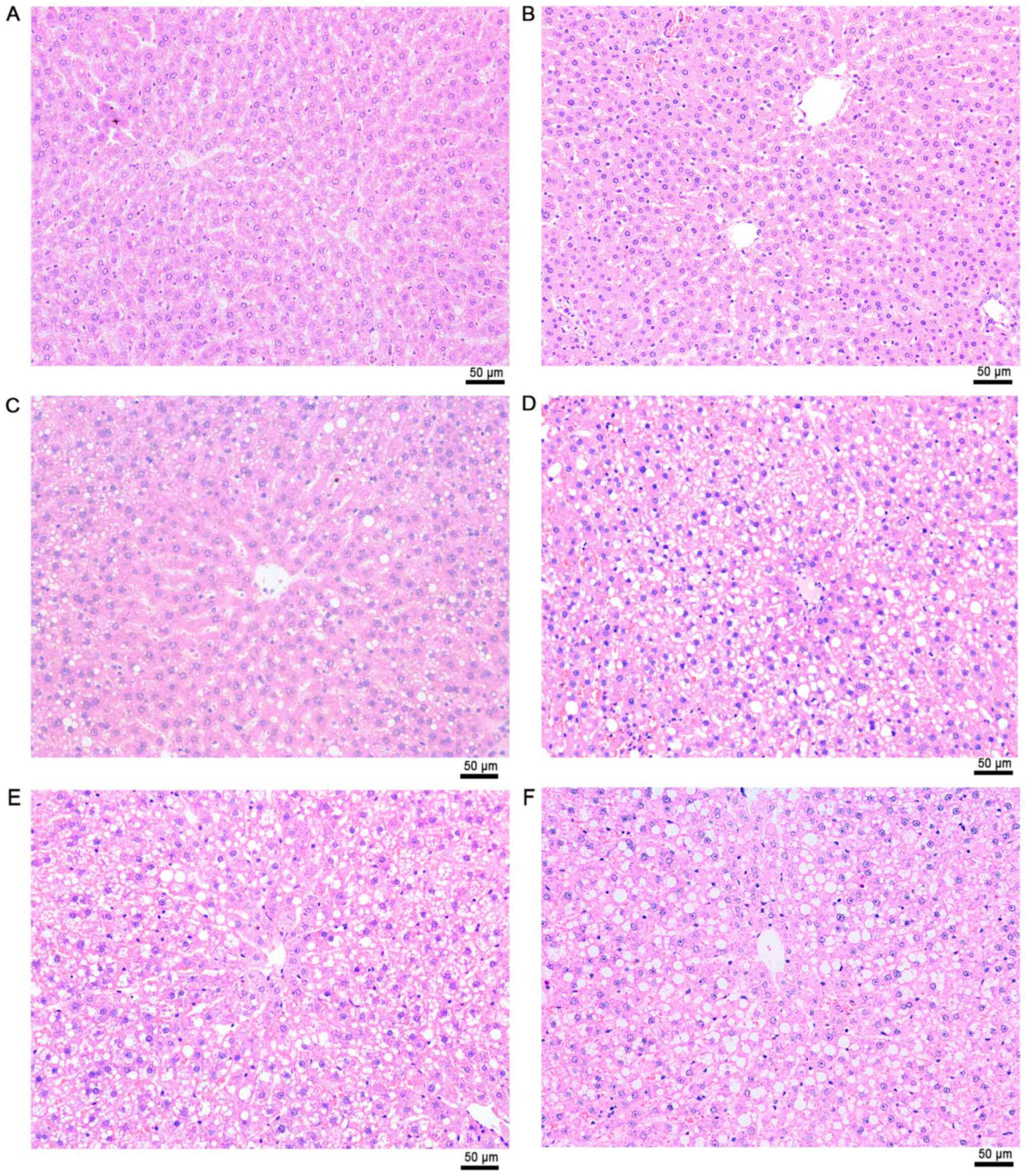
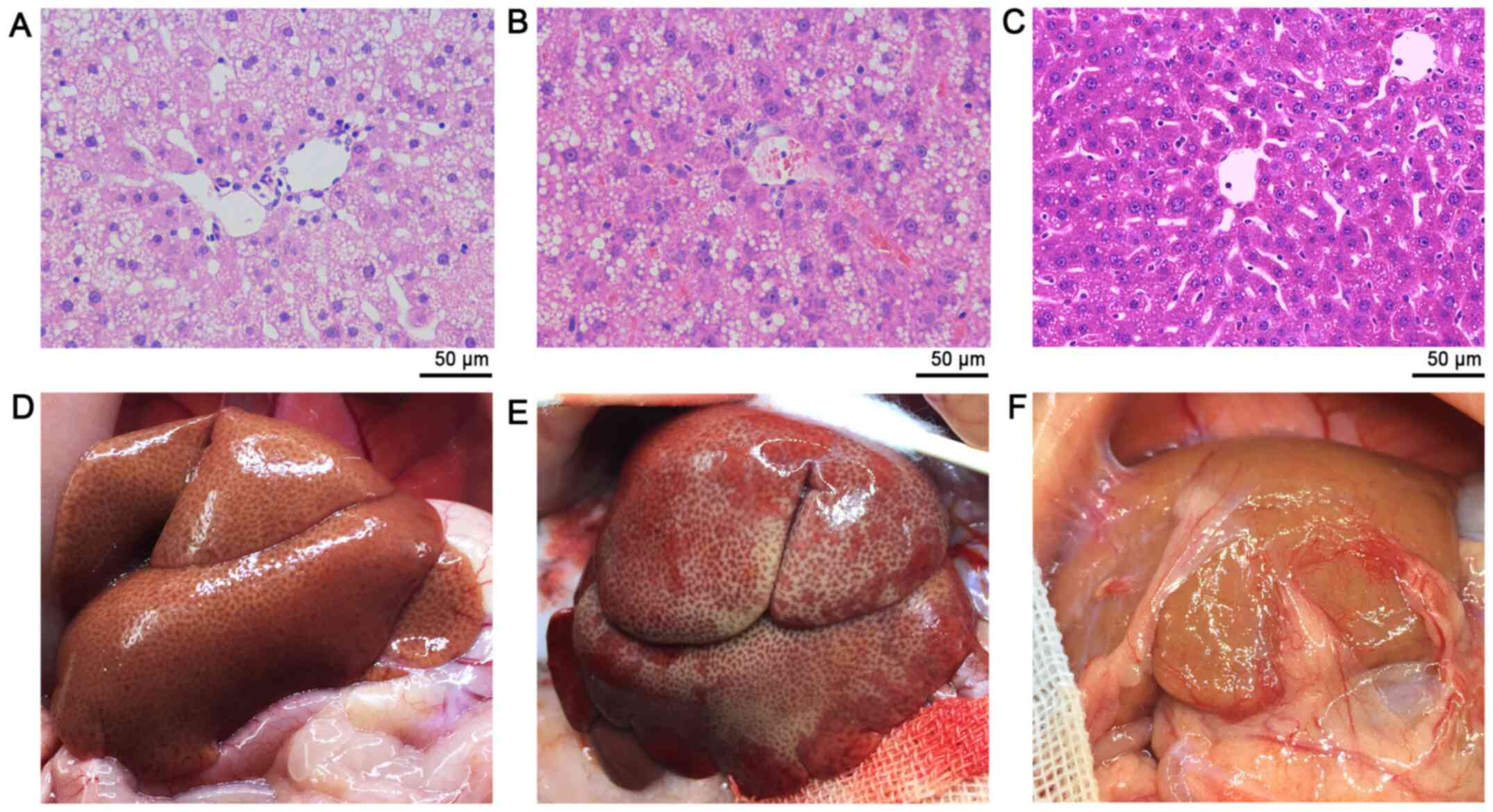
addition, a greasy surface was observed in fatty livers (Fig. 5). Histological analysis demonstrated
that the HFD group developed macrovesicular steatosis after 4 weeks
of receiving HFD (Fig. 6). The
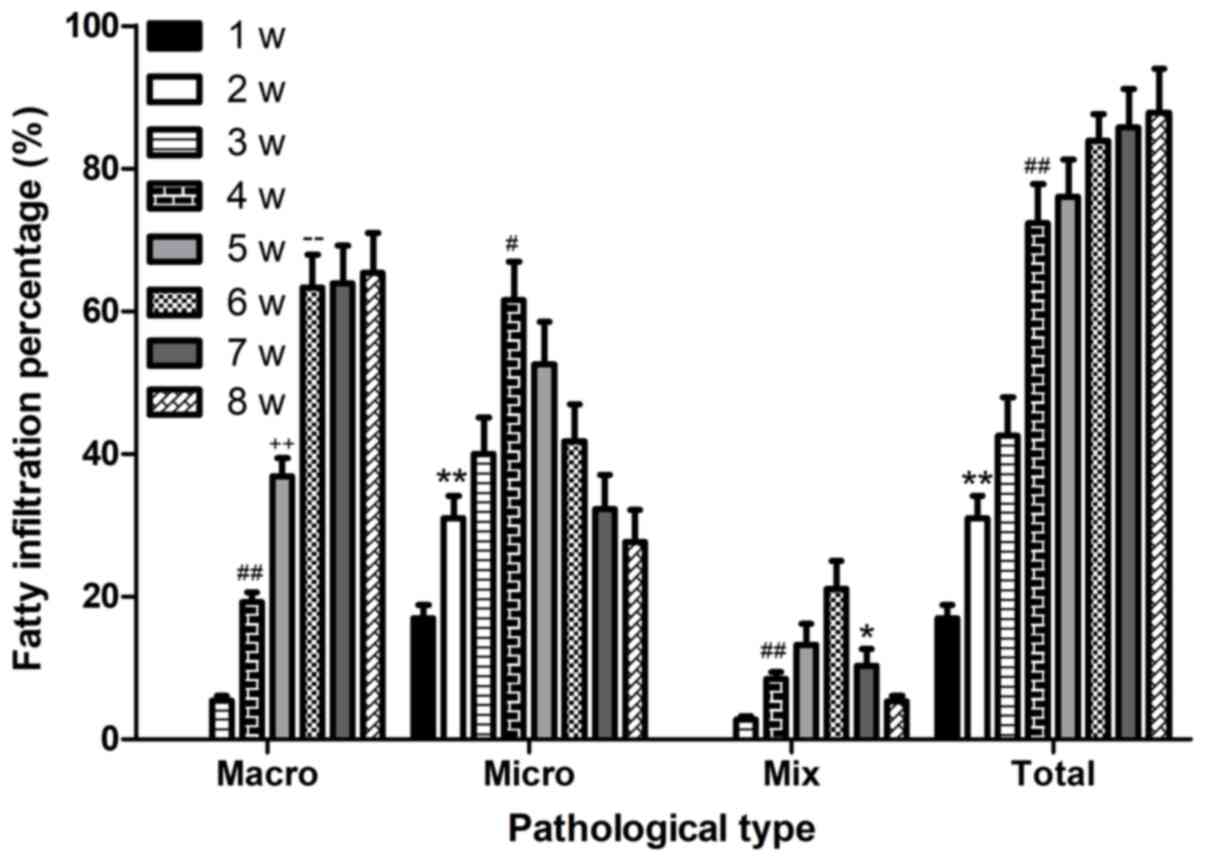
hepatic steatosis aggravated ~10% per week during the experimental
process (Fig. 7).
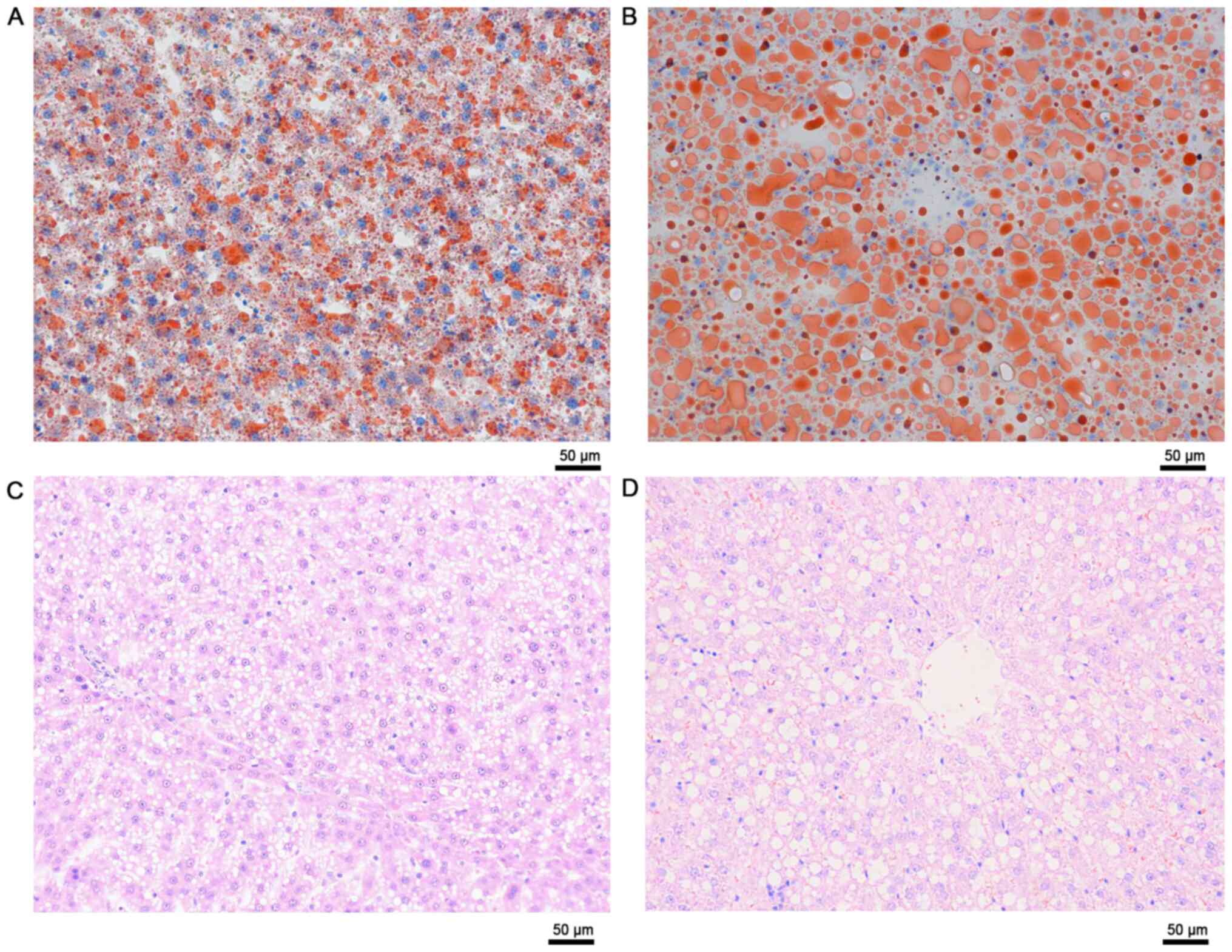
These results were validated via ORO staining and
quantification of TGs. The results demonstrated that
intracytoplasmic accumulation of TGs aggravated with time (Fig. 8). The cytoplasm was primarily
occupied by several microvesicular steatosis and small fat vacuoles
around the nucleus in week 3. However, macrovesicular steatosis was
observed in most of the sections in week 6. The size of the
vacuoles increased, pushing the nucleus to the periphery of the
cell (Fig. 8).
Post-transplantation graft function
and rat survival
Steatotic LTx displayed poor hepatic morphology
following reperfusion compared with the normal graft
transplantation (Video S1). The
reperfusion condition was better in the SCD group compared with the
HFD group (Video S1). Furthermore,
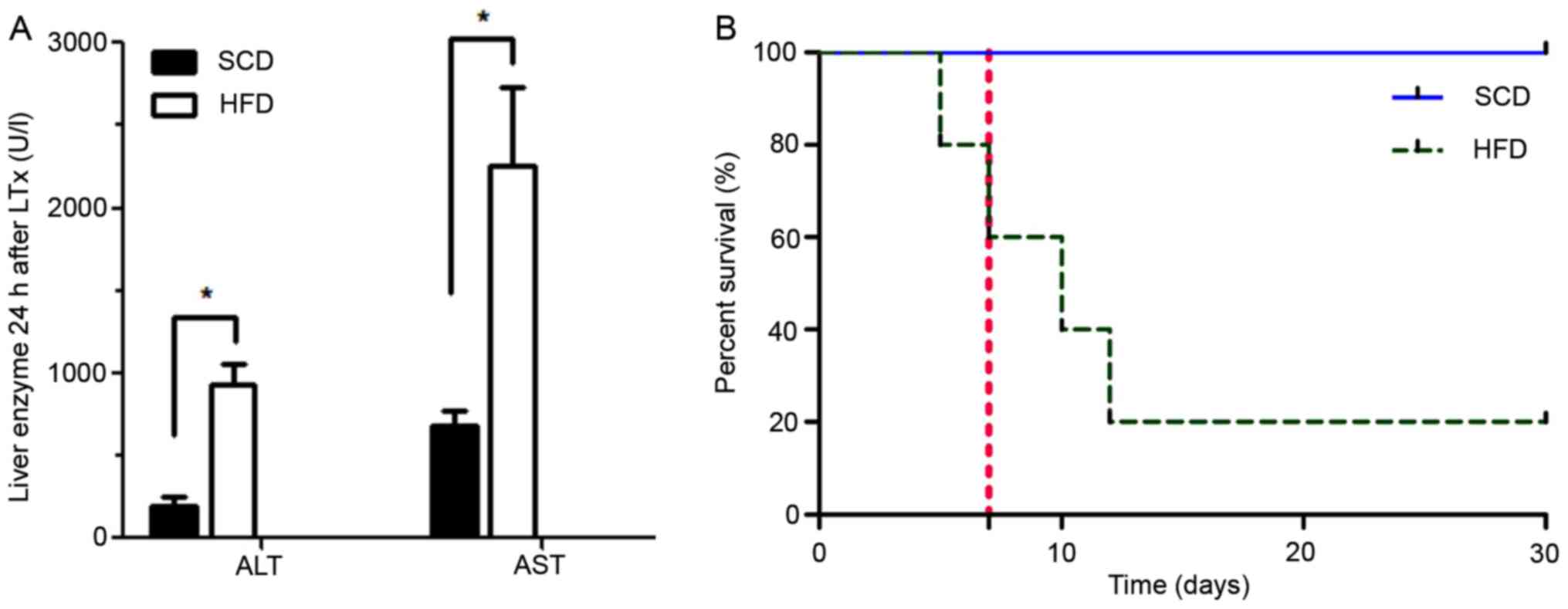
the liver enzymes were significantly elevated 24 h after LTx in the
HFD group compared with the SCD group (Fig. 9A). The early post-transplantation
survival rates on day 7, 30 and 365 were 80% (4/5), 20% (1/5) and
20% (1/5) in the HFD group, and 100% (5/5), 100% (5/5) and 100%
(5/5) in the SCD group (Fig. 9B).
Among the rats that accepted severely steatotic grafts, only one
rat survived, while the other four died 8 days post-surgery (8±2
days). The postmortem examination revealed massive ascites, and it
was concluded that the rats died from PNF (4/5). Macrovesicular
steatosis dissipated from severely steatotic liver graft 1-year
after LTx (Fig. 10).
Discussion
Currently, several fatty liver animal models have
been developed, including rats, mice, the sand rat, rabbit, duck,
geese and miniature swine (35-40).
The rat model, which is the most commonly used fatty liver animal
model, can be classified into three types: Congenital, genetically
modified and food/drug-induced model (35-40).
However, the former two are not in conformity with the pathogenesis
of the human disease. In the present study, food-induction was used
to induce hepatic steatosis and simultaneously exclude other
interference factors (41). Volume
reduction was implemented as a self-control method, making the data
in the control group more accurate without enlarging samples, which
is in line with the 3Rs concept of animal experiments (replacement,
reduction and refinement) (42).
OLT was performed using normal grafts, with or without a 20% volume
reduction to verify the safety of this method. Post-surgery
survival rates were assessed within the two groups (100 vs.
100%).
Regarding body weight, lower body weight was
observed in the HFD group compared with the SCD group; however, no
significant differences were observed between the two groups. The
lower body weight may be attributed to a time period of adaptation
for HFD rats to their diet. In addition, the diameter of PV, SHVC,
IHVC and bile duct was narrower in rats fed with HFD compared with
those fed with SCD.
The pathogenesis of fatty liver remains unclear;
however, it is speculated that its formation is associated with fat
metabolism disorders, increase in fat synthesis and
oxidation-reduction, as well as the imbalance in synthesis or
discharge of TG and LDL (12). The
results of the present study demonstrated no significant
differences in ALT and AST levels between the HFD and SCD groups,
which indicated the absence of additional steatotic liver injury.
In addition, the enzymes did not increase with the aggravation of
hepatic steatosis.
Notably, TC was not consistent with the degree of
hepatic steatosis, as speculated. Conversely, no linear association
was observed between TG levels in serum and liver. As an integral
part of the metabolic syndrome, hepatic steatosis is associated
with the development of insulin resistance (43,44).
In the present study, the HFD group was significantly associated
with higher plasma glucose levels and higher portal insulin levels.
These higher portal insulin levels in HFD rats may participate in
the rapid development of hepatic steatosis. This may be one reason
as to why some steatotic grafts reach full recovery following
implantation in recipients with no insulin resistance.
In the present study, the steatotic liver was larger
in size, has a fatty surface, obtuse margin and was a dark yellow
color compared with the healthy liver (SCD group). In addition, no
signs of fibrosis were observed in any of the groups.
Histopathological analyses demonstrated that the hepatocytes of HFD
rats had little lipid droplets 1 week after the model was
established, and the number of hepatocytes increased in a
time-dependent manner. After 2 weeks, the little lipid drops
demonstrated a mutual-fusion-trend, indicating microvesicular
steatosis. A portion of microvesicular lipid droplets started to
fuse into macrovesicular fat vacuoles at week 3, and notable
macrovesicular steatosis was observed at week 4, which
progressively decreased up to week 6. In addition, fat vacuoles
were observed in the hepatocytes, and their nuclei were squeezed to
one side, revealing the irregular shape of hepatocytes and hepatic
sinusoid constriction, while the field of vision was filled with
macro fat vacuoles. The hepatocytes in the previous 3 weeks
predominantly revealed microvesicular steatosis, while they
primarily revealed macrovesicular steatosis in the latter weeks. A
more significant difference was observed between images at weeks 3
and 6 via H&E and ORO staining.
Based on this model, strategies such as, ischemic
preconditioning, pharmacological preconditioning and machine
perfusion may be used to enhance graft quality (6,34).
Although the steatotic liver model was stabilized by two
preliminary experiments, the results depended on the energy intake
and individual differences. Regrouping would be more accurate based
on TG content in tissues, combined with the degree of hepatic
steatosis estimated by ORO staining.
LTx was performed using steatotic grafts or normal
liver. Clinically, hepatic macrovesicular steatosis >60% is
considered a contraindication for transplantation due to the high
morbidity of PNF (10). With
reference to long-term survival, rats receiving SCD survived longer
than those fed a HFD; however, no statistically significant
differences were observed in short-term survival between the two
groups. Restoring the rat intestinal blood flow, relieving
congestion and stabilizing the hemodynamics are critical features
for improving animal survival (28). In the present study, an hepatic
phase was limited between 20-22 min, including the time for
suturing the SHVC, cuffing and opening the PV, outflowing and
clamping the IHVC, removing the SHVC clamp and letting
approximately 0.2 ml blood outflow from the IHVC in case thrombus
or other impurities circulated into the blood when opening PV. The
results of the present study demonstrated that fatty LTx displayed
poor hepatic morphology following PV reperfusion compared with the
normal graft transplantation. All of the steatotic grafts appeared
to be male-reperfusion during the transplantation. The liver
surface appeared to be piebald due to the uneven blood reperfusion.
In addition, combined with the post-surgery survival, the
intraoperative reperfusion situation of the liver did not have a
decisive role in predicting rat prognosis, it was not possible to
determine whether the rats would achieve a good outcome just based
on the reperfusion morphology. In one case of fatty LTx in the
present study, nearly no blood reperfusion was observed on the
liver surface except in the portal vein and its branches. However,
despite the poor performance, this was the only case that survived
more than 6 months, longer than the others that had even better
reperfusion. As a result, the prognosis could not be predicted only
by intraoperative morphology in steatotic LT.
The steatotic liver is vulnerable to ischemic injury
(21). PNF was commonly observed in
the severely steatotic liver group that had sustained 4 h CS. The
post-surgery rats only survived if the liver function was restored,
usually within 2 weeks. In the present study, except for one rat in
the HFD group that survived, four rats died 8±2 days after the
surgery. In total, 80% of rats did not recover from PNF until their
death. As steatotic liver leads to poor prognosis, the authors aim
to use machine perfusion in future studies to improve the survival
outcomes.
In conclusion, the results of the present study
suggest that HFD may be used to induce stable and rapid hepatic
steatosis in rats without inducing inflammation. In addition, the
lipid droplets accumulate into fat vacuoles over time, from
microvesicular steatosis into macrovesicular steatosis.
Furthermore, volume reduction, as a self-control method, may be
applied in steatotic LTx without increasing the number of animals
being used, thus promoting animal welfare. However, therapeutic
approaches or machine perfusion need to be implemented in steatotic
grafts to improve the quality of donated organs and improve the
post-transplantation survival.
Supplementary Material
Steatotic liver transplantation
displayed poor hepatic morphology following reperfusion compared
with the normal graft transplantation.
Supplementary Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China, (grant no. 81970548), the Medical
Science Advancement Program (Youth Scholars) of Wuhan University
(grant no. TFZZ2018035) and the Zhongnan Hospital of Wuhan
University Science, Technology and Innovation Seed Fund (grant no.
ZNPY2018010).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LF, ZF and QY contributed to the conception and
design. LF, ZF, YX and SY performed the experiments. YX, SY, YW and
GP contributed to data acquisition and analysis. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved (IRB approval no.
AF-177) by Wuhan University Institutional Animal Care and Use
Committee (Wuhan, China) and all animal experiments were performed
in accordance with the Association for the Assessment and
Accreditation of Laboratory Animal Care and Institutional Animal
Care and Use Committee regulations and guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Starzl TE, Marchioro TL, Von Kaulla KN,
Hermann G, Brittain RS and Waddell WR: Homotransplantatin of the
liver in humans. Surg Gynecol Obstet. 117:659–676. 1963.PubMed/NCBI
|
|
2
|
Wolfe RA, Merion RM, Roys EC and Port FK:
Trends in organ donation and transplantation in the United States,
1998-2007. Am J Transplant. 9:869–878. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wertheim JA, Petrowsky H, Saab S,
Kupiec-Weglinski JW and Busuttil RW: Major challenges limiting
liver transplantation in the United States. Am J Transplant.
11:1773–1784. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Durand F, Renz JF, Alkofer B, Burra P,
Clavien PA, Porte RJ, Freeman RB and Belghiti J: Report of the
Paris consensus meeting on expanded criteria donors in liver
transplantation. Liver Transpl. 14:1694–1707. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Berg CL, Steffick DE, Edwards EB, Edwards
EB, Heimbach JK, Magee JC, Washburn WK and Mazariegos GV: Liver and
intestine transplantation in the United States 1998-2007. Am J
Transplant. 9:907–931. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Henry SD and Guarrera JV: Protective
effects of hypothermic ex vivo perfusion on ischemia/reperfusion
injury and transplant outcomes. Transplant Rev (Orlando).
26:163–175. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Briceño J, Marchal T, Padillo J, Solórzano
G and Pera C: Influence of marginal donors on liver preservation
injury. Transplantation. 74:522–526. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Salizzoni M, Franchello A, Zamboni F,
Ricchiuti A, Cocchis D, Fop F, Brunati A and Cerutti E: Marginal
grafts: Finding the correct treatment for fatty livers. Transpl
Int. 16:486–493. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tekin K, Imber CJ, Atli M, Gunson BK,
Bramhall SR, Mayer D, Buckels JA, McMaster P and Mirza DF: A simple
scoring system to evaluate the effects of cold ischemia on marginal
liver donors. Transplantation. 77:411–416. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cameron A and Busuttil RW: AASLD/ILTS
transplant course: Is there an extended donor suitable for
everyone? Liver Transpl. 11 (suppl 2):S2–S5. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Shaker M, Tabbaa A, Albeldawi M and
Alkhouri N: Liver transplantation for nonalcoholic fatty liver
disease: New challenges and new opportunities. World J
Gastroenterol. 20:5320–5330. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rinella ME: Nonalcoholic fatty liver
disease: A systematic review. JAMA. 313:2263–2273. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Browning JD, Szczepaniak LS, Dobbins R,
Nuremberg P, Horton JD, Cohen JC, Grundy SM and Hobbs HH:
Prevalence of hepatic steatosis in an urban population in the
United States: Impact of ethnicity. Hepatology. 40:1387–1395.
2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Neuschwander-Tetri BA and Caldwell SH:
Nonalcoholic steatohepatitis: Summary of an AASLD Single topic
conference. Hepatology. 37:1202–1219. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ruhl CE and Everhart JE: Fatty liver
indices in the multiethnic united states national health and
nutrition examination survey. Aliment Pharmacol Ther. 41:65–76.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sanyal AJ: American Gastroenterological
Association. AGA technical review on nonalcoholic fatty liver
disease. Gastroenterology. 123:1705–1725. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
McCormack L, Dutkowski P, El-Badry AM and
Clavien PA: Liver transplantation using fatty livers: Always
feasible? J Hepatol. 54:1055–1062. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ploeg RJ, D'Alessandro AM, Knechtle SJ,
Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer
FO and Kalayoglu M: Risk factors for primary dysfunction after
liver transplantation-a multivariate analysis. Transplantation.
55:807–813. 1993.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Busuttil RW and Tanaka K: The utility of
marginal donors in liver transplantation. Liver Transpl. 9:651–663.
2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Selzner M and Clavien PA: Fatty liver in
liver transplantation and surgery. Semin Liver Dis. 21:105–113.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sun CK, Zhang XY, Zimmermann A, Davis G
and Wheatley AM: Effect of ischemia-reperfusion injury on the
microcirculation of the steatotic liver of the Zucker rat.
Transplantation. 72:1625–1631. 2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Imber CJ, St Peter SD, Lopez I, Guiver L
and Friend PJ: Current practice regarding the use of fatty livers:
A trans- Atlantic survey. Liver Transpl. 8:545–549. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
El-Badry AM, Moritz W, Contaldo C, Tian Y,
Graf R and Clavien PA: Prevention of reperfusion injury and
microcirculatory failure in macrosteatotic mouse liver by omega-3
fatty acids. Hepatology. 45:855–863. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Todo S, Demetris AJ, Makowka L, Teperman
L, Podesta L, Shaver T, Tzakis A and Starzl TE: Primary nonfunction
of hepatic allografts with preexisting fatty infiltration.
Transplantation. 47:903–905. 1989.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Verran D, Kusyk T, Painter D, Fisher J,
Koorey D, Strasser S, Stewart G and McCaughan G: Clinical
experience gained from the use of 120 steatotic donor livers for
orthotopic liver transplantation. Liver Transpl. 9:500–505.
2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gabrielli M, Moisan F, Vidal M, Duarte I,
Jiménez M, Izquierdo G, Domínguez P, Méndez J, Soza A, Benitez C,
et al: Steatotic livers. Can we use them in OLTX? Outcome data from
a prospective baseline liver biopsy study. Ann Hepatol. 11:891–898.
2012.PubMed/NCBI
|
|
27
|
Spitzer AL, Lao OB, Dick AA,
Bakthavatsalam R, Halldorson JB, Yeh MM, Upton MP, Reyes JD and
Perkins JD: The biopsied donor liver: Incorporating macrosteatosis
into high-risk donor assessment. Liver Transpl. 16:874–884.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Kamada N and Calne RY: Orthotopic liver
transplantation in the rat. Technique using cuff for portal vein
anastomosis and biliary drainage. Transplantation. 28:47–50.
1979.PubMed/NCBI
|
|
29
|
Hijona E, Hijona L, Arenas JI and Bujanda
L: Inflammatory mediators of hepatic steatosis. Mediators Inflamm.
2010(837419)2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bligh EG and Dyer WJ: A rapid method of
total lipid extraction and purification. Can J Biochem Physiol.
37:911–917. 1959.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Lattouf R, Younes R, Lutomski D, Naaman N,
Godeau G, Senni K and Changotade S: Picrosirius red staining: A
useful tool to appraise collagen networks in normal and
pathological tissues. J Histochem Cytochem. 62:751–758.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mehlem A, Hagberg CE, Muhl L, Eriksson U
and Falkevall A: Imaging of neutral lipids by oil red O for
analyzing the metabolic status in health and disease. Nat Protoc.
8:1149–1154. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kleiner DE, Brunt EM, Van Natta M, Behling
C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS,
Unalp-Arida A, et al: Design and validation of a histological
scoring system for nonalcoholic fatty liver disease. Hepatology.
41:1313–1321. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
de Rougemont O, Breitenstein S, Leskosek
B, Weber A, Graf R, Clavien PA and Dutkowski P: One hour
hypothermic oxygenated perfusion (HOPE) protects nonviable liver
allografts donated after cardiac death. Ann Surg. 250:674–683.
2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Corominas J, Marchesi JA, Puig-Oliveras A,
Revilla M, Estellé J, Alves E, Folch JM and Ballester M: Epigenetic
regulation of the ELOVL6 gene is associated with a major QTL effect
on fatty acid composition in pigs. Genet Sel Evol.
47(20)2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tuvdendorj D, Zhang XJ, Chinkes DL, Wang
L, Wu Z, Rodriguez NA, Herndon DN and Wolfe RR: Triglycerides
produced in the livers of fasting rabbits are predominantly stored
as opposed to secreted into the plasma. Metabolism. 64:580–587.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Katz DL: Ducks, geese, faith, and fatty
livers. Child Obes. 10:373–374. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Awde S, Marty-Gasset N, Prahkarnkaeo K and
Rémignon H: Relationship between proteolytic activities and cooking
loss variability in liver issued from force-fed mule ducks. J Agric
Food Chem. 62:3262–3268. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Spolding B, Connor T, Wittmer C, Abreu LL,
Kaspi A, Ziemann M, Kaur G, Cooper A, Morrison S, Lee S, et al:
Rapid development of non-alcoholic steatohepatitis in Psammomys
obesus (Israeli sand rat). PLoS One. 9(e92656)2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gauthier MS, Favier R and Lavoie JM: Time
course of the development of non-alcoholic hepatic steatosis in
response to high-fat diet-induced obesity in rats. Br J Nutr.
95:273–281. 2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Matteoni CA, Younossi ZM, Gramlieh T,
Boparai N, Liu YC and McCullough AJ: Nonalcoholic fatty liver
disease: A spectrum of clinical and pathological severity.
Gastroenterology. 116:1413–1419. 1999.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Flecknell P: Replacement, reduction and
refinement. ALTEX. 19:73–78. 2002.PubMed/NCBI
|
|
43
|
Saltiel AR and Kahn CR: Insulin signalling
and the regulation of glucose and lipid metabolism. Nature.
414:799–806. 2001.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S,
Befroy D, Romanelli AJ and Shulman GI: Mechanism of hepatic insulin
resistance in non-alcoholic fatty liver disease. J Biol Chem.
279:32345–32353. 2004.PubMed/NCBI View Article : Google Scholar
|