Introduction
Heart failure is a complex disorder characterized by
interstitial fibrosis, chamber remodeling and reduced ventricular
compliance. This is due to an excessive deposition of extracellular
matrix proteins by cardiac fibroblasts (1).
The cardiac fibroblast is an interstitial cell,
predominantly of embryonic epicardial and endothelial origins,
which is located within the myocardial interstitium, epicardial and
perivascular regions. Physiologically, it is responsible for
homeostasis of the extracellular matrix (2).
Fibroblasts are the main effector cells of cardiac
fibrosis. The adult mammalian heart contains abundant fibroblasts
that expand following injury and can produce large amounts of
extracellular matrix proteins. In the dynamic environment of the
ischemic heart, the fibroblasts undergo phenotypic changes, turning
into various subtypes with functional diversity, such as:
Inflammatory fibroblast which secretes cytokines (IL-1, TNF-α),
matrix-degrading fibroblast which secretes matrix-metal proteinases
(MMP), phagocytic fibroblast, angiogenic fibroblast which may turn
into pericyte, myofibroblast which secretes extra-cellular matrix
proteins (3,4).
The function of the fibroblast/myofibroblast is
modulated by cytokines, hormones and also by mechanical stretch and
changes in oxygen availability. In turn, the myofibroblast
responses includes changes in cell proliferation, cell migration,
extracellular matrix metabolism and secretion of different
bioactive molecules and growth factors (5).
In cardiac ischemia followed by myocardial
infarction, the fibroblast plays an active role through
communication with the cardiomyocytes by direct cell-cell
interactions and autocrine/paracrine-mediated pathways (6). Fibroblasts play central roles in two
types of fibrosis: Reparative and reactive. Reparative fibrosis is
a replacement fibrosis which accompanies cardiomyocyte death,
followed by scar formation. Reactive fibrosis is an interstitial
and perivascular fibrosis, which is indirectly associated with
cardiomyocyte death (6).
Fibroblasts are the main player in form of
mesenchymal cardiac cells, together with stem cells, endothelial
cells and vascular smooth muscle cells. Scientists have found
various stem cells with therapeutic potential. Stem cells have been
found in almost all tissues: In the skin (epidermal stem cells),
skeletal muscle (satellite stem cells), small bowel (intestinal
stem cells), and liver (oval cells). The mucosa of the small bowel
is one of the best examples of a rapidly self-renewing tissue
(7). Dedicated stem cells, located
at the crypt base represent the origin in the renewing process.
They provide, by differentiation, several functional mature cells,
by migrating from the base of the crypt toward the top of the
villi. All these cells are part of the bigger family of adult
mesenchymal and stem cells, also present in the gut and other adult
tissues (8,9).
The aim of the present study was to assess the
fibroblast involvement in cardiac repair under ischemic conditions
after myocardial infarction. The findings indicated that these
cells play a role in cardiac remodelling.
Materials and methods
Case selection for human tissue
specimens
A retrospective study was performed on a study batch
composed of 23 cases with various ischemic heart diseases. Some of
the cases had associated conditions: 4 cases with chronic cardiac
failure, 9 cases with coronary atherosclerosis, 1 case with
hypertrophic cardiomyopathy with left ventricular hypertrophy.
The cases, 20 males and 3 females (sex ratio ~5:1),
with ages ranging from 47 to 90 years (mean =67.7, SD =12.3), were
selected during an interval of 1 year. They were selected for
histopathology, in order to assess cardiac remodeling in hypoxic
ischemic micro-environment conditions. All of the patients
succumbed following long-term cardiac complications, secondary to
scarring myocardial infarctions.
The study was performed according to the World
Medical Association Declaration of Helsinki and the tissue
specimens were collected according to national legislation, using a
protocol approved by the local Bioethics Committee of ‘Sf.
Pantelimon’ Emergency Clinical Hospital (Bucharest, Romania). All
the patients provided signed informed consent regarding
hospitalization, treatment and the possible future publication of
data.
Tissue sampling and stains
Tissue samples from the heart were taken for
microscopy investigation. The fragments were harvested from
different parts of the anterior, lateral and posterior wall of the
left ventricle.
The selected tissue samples were fixed in 10%
neutral-buffered formalin (pH 7.0) for 24-48 h and paraffin
embedded. Sections were cut at 5 µm and stained with standard
hematoxylin and eosin (H&E).
Additional special stains such as Gomori silver
stain (for reticulin fibers), elastic van Gieson and Masson's
trichrome (for collagen fibers) were carried out. Tissue samples
were divided into appropriate-sized slices (3-5 µm) for
conventional microscopy and immunohistochemistry. Multiple series
of histological sections were also performed and examined.
Immunohistochemical analysis (IHC) was performed for
vimentin (clone: V9, RTU, Novocastra) and smooth muscle actin
(clone: 1a4, RTU, Abcam), using sections displayed on slides
treated first with poly-L-lysine. IHC was performed on 3 µm
sections from formalin-fixed paraffin-embedded specimens. The
method used was an indirect tristadial Avidin-Biotin-Complex
technique, with a NovoLink Polymer detection system which utilizes
a novel control polymerization technology to prepare polymeric
HRP-linker antibody conjugates, according to the manufacturer's
specifications (Novocastra, UK). Antigen retrieval technique
(enzymatic pre-treatment) was carried out, according to the
producer's specifications.
All the slides were examined and photographed on an
AccuScope Imager microscope (AccuScope®). Digital images
obtained with an incorporated software program were processed and
analyzed with Microsoft Office Picture Manager (Washington, DC),
running under Windows 10.
Statistical analysis
Statistical analysis was carried out using SPSS
version 20 (IBM). Data were analyzed using the Student's t-test, to
determine the median, as well as mean ± standard deviation (SD).
P<0.05 was considered statistically significant.
Results
In the cases from the study batch with previous
pathological conditions of myocardial infarction, all tissues had
collagen scars, ranging from granulation tissue in various stages
of evolution to heavy sclerosis with hyalin deposition, located
mainly as intramural or trans-mural. These lesions were accompanied
by compensatory hypertrophy of the residual ‘stunned’ hibernating
peri-necrotic cardiomyocytes.
There were also associated lesions in all cases from
the study batch, such as variable degrees of interstitial and
perivascular fibrosis, ranging from mild collagen deposition to
severe sclerosis, with disorganized myocardial fibers (which
sustains a variable ischemic cardiac disease), interstitial
vasodilation with hyperemia (which sustains various degrees of
cardiac failure) and hypoxic myocardial lesions, in form of ‘wavy
fibers’ cardiomyocytes, along with interstitial lipomatosis and
focal degeneration of myocardial fibers of granular vacuolar
type.
There was an interstitial deposition in the residual
myocardium, first of collagen type III (reticulin fibers) which is
brittle, then it was replaced in time with collagen type I or II,
which is more sturdy. This led to substitution fibrosis and
replacement myocardial fibrosis with scar formation, depending on
the circumstances.
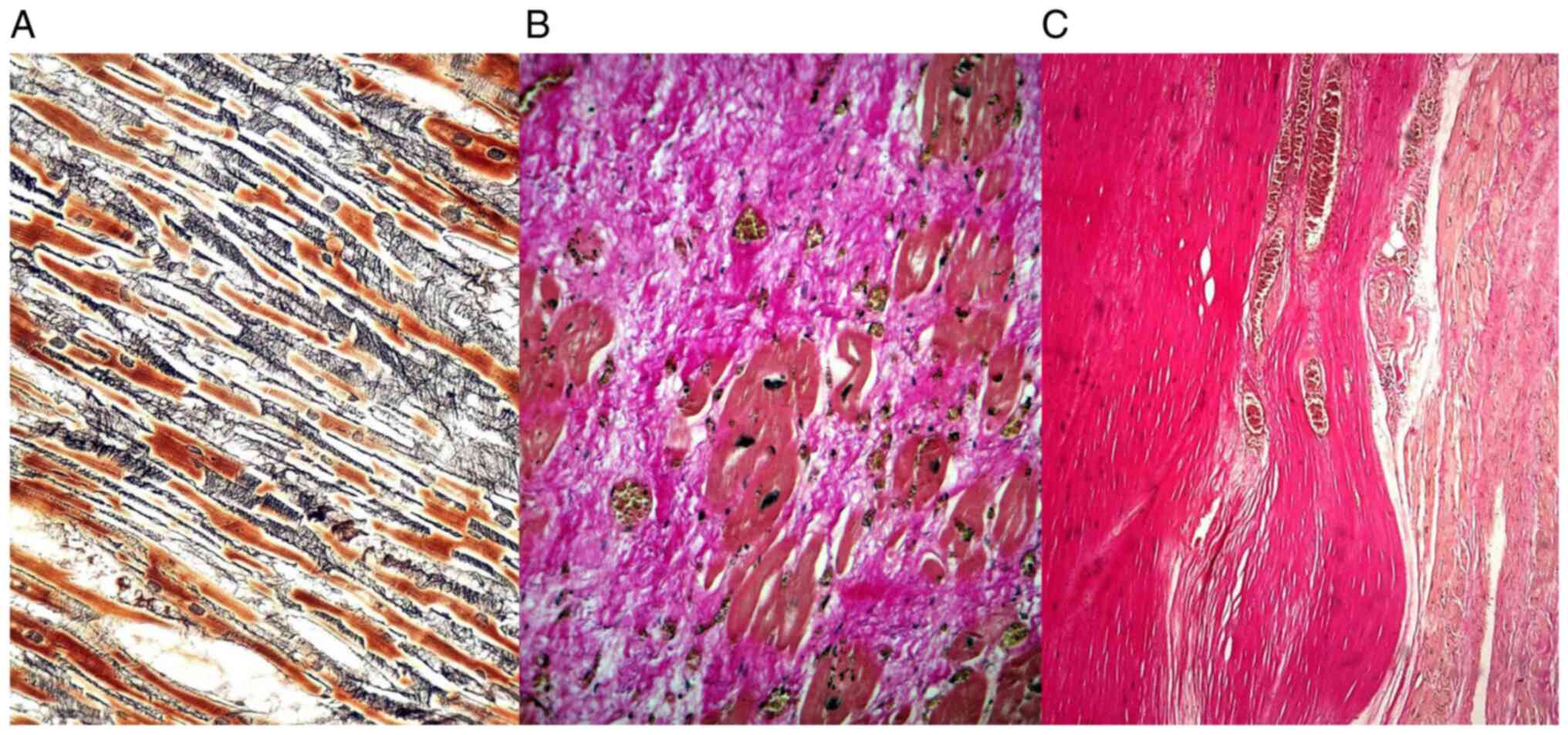
The reticulin fibers constituted an interstitial
continuous loose network perpendicular on the myocardial cells,
while the collagen fibers were more densely, compactly distributed,
parallel with the cardiomyocytes (Fig.
1).
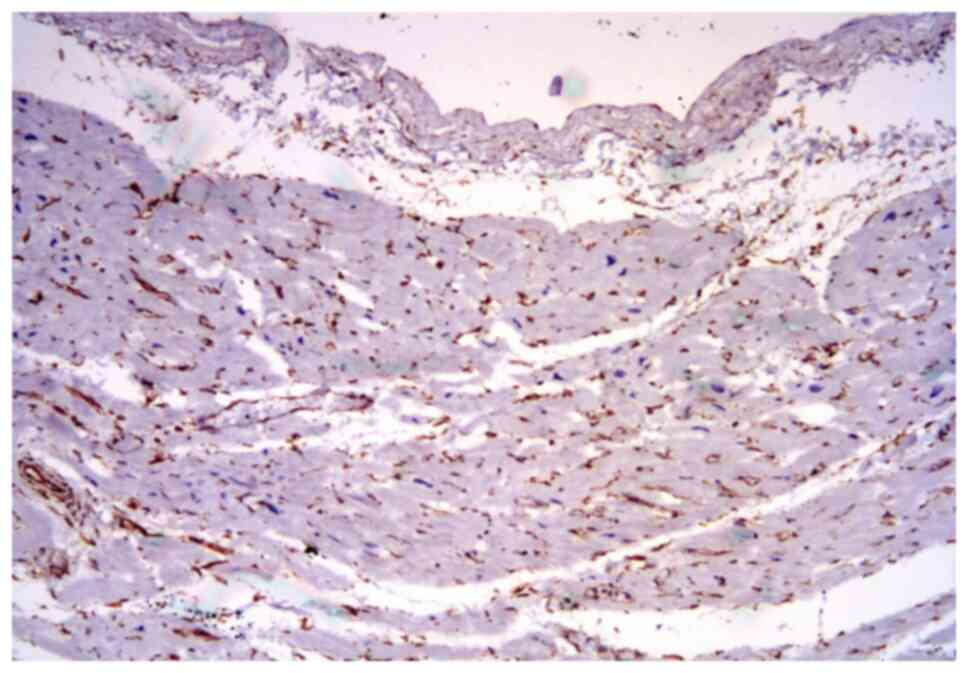
The universal marker for fibroblast is vimentin,
which showed an unusual expression in the tissue specimens.
Vimentin was variably expressed in the interstitial stroma and
capillary vessels (Fig. 2),
depending on the age of the collagenous scar or the long-term
hypoxia.
On the other hand, vimentin was almost completely
negative in the fibrous areas or with a mild deposition next to the
scar lesions in the extracellular matrix and in the
fibroblast/myofibroblast cells.
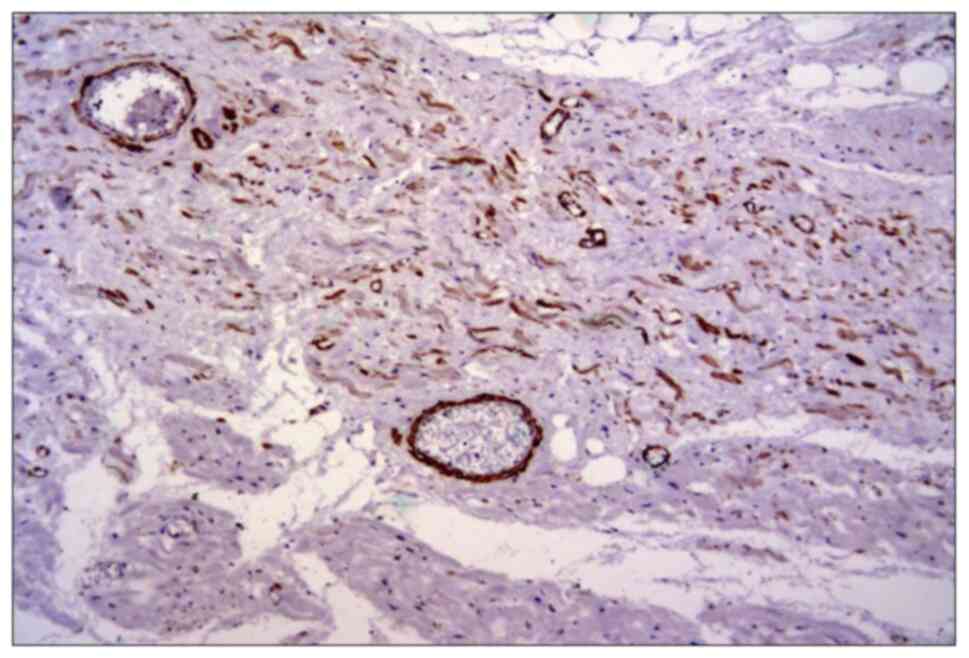
The smooth muscle actin was also frequently
expressed in perivascular myofibroblasts among the residual cardiac
fibers and in small vessels (Fig.
3). There was also a direct correlation between vimentin and
smooth muscle actin expression and collagen deposition.
Discussion
In adult humans, the cell populations of the heart
are represented by myocytes (~75% of the total cardiac volume, but
only 30% of the total number of cells), fibroblasts (5% of the
cardiac volume, but 70% of the total number) and other cells
(endothelial cells, smooth muscle cells, vegetative nervous cells
and epicardial cells) (10).
Fibroblasts are diffusely located in the normal
heart, surrounding the myocytes, playing a role in collagen
production and deposition. In addition, they contribute to cardiac
development and structural modeling, cell signaling and electric
and mechanic functions of the normal and pathological myocardium
(11).
Concerning fibroblasts, vimentin is strongly
expressed in the fetal heart, but decreases in intensity during
life time (accompanied by a constantly increasing expression of
desmin). The cardiac fibroblasts are in contact with collagen,
other fibroblasts, and myocytes forming a mechanosensitive
three-dimensional arrangement (11).
Fibrosis with cardiac remodeling is based on
maintaining the proliferation capacity of the fibroblast and its
capacity of protein synthesis in the extracellular matrix. Under
hypoxic ischemic micro-environment conditions, the resident cardiac
fibroblasts become activated and produce collagen fibers with
subsequent cardiac remodeling. The cardiac remodeling occurs in the
presence of matrix metal proteinases (MMP), particularly MMP-9.
Angiogenic proliferation occurs simultaneously with high
micro-vascular density and with an increased variability of
immunohistochemical expression of certain adhesion molecules in the
extracellular matrix (such as ICAM-1/CD54 or tenascin-X) (12).
Cardiac repair following myocardial infarction may
be divided in three distinct, but overlapping phases: The
inflammatory, proliferative, and the maturation phase (13).
In the inflammatory phase, the fibroblasts secrete
large amounts of pro-inflammatory mediators and proteases (14); in the proliferative phase they
undergo myofibroblast trans-differentiation (expressing SMA) and
secrete extracellular matrix proteins (15); and in the maturation phase they
deactivate, become quiescent and undergo apoptosis.
Cardiac fibrosis is mainly categorized into four
types, based on the location and cause (16).
The first type is interstitial fibrosis, which
mainly describes the expansion of endomysium and perimysium, and is
caused by progressive deposition of extracellular proteins in the
interstitial space, leading to cardiomyocyte death (17).
The second type is replacement fibrosis, which
occurs due to necrosis of cardiomyocytes and is associated with
systolic ventricular dysfunction, hypertrophic cardiomyopathy and
myocarditis (17).
A third category is infiltrative fibrosis, which
shows infiltration of inflammatory cells in right ventricles of
systemic sclerosis-associated pulmonary arterial hypertension
(18).
The fourth type, termed endomyocardial fibrosis, is
a primary cause of congestive heart failure in children under 2
years of age with idiopathic etiology (although the literature
describes infections, autoimmunity, genetic factors, and
nutritional deficiencies) (19).
In the light of the aforementioned allegations, the
cardiac fibroblast may be a therapeutic target in myocardial
remodeling, with certain anti-fibrotic therapies directed against
it, such as: ACE inhibitors and angiotensin receptor antagonists,
aldosterone antagonists, endothelin receptor antagonists, statins,
anticytokine therapies (TNFα, IFN-γ, and TGF-β), inhibitors of
extracellular matrix metabolism involving collagen synthesis,
matrix metalloproteinases, and plasmin systems as well as novel
antifibrotic/antiinflammatory agents and nuclear receptor ligands
(20,21).
Anticoagulant therapy plays a crucial role in
preventing ischemic conditions (22-24).
Against all odds and adverse outcomes, cardiac
fibroblasts play a main role in the physiological turnover of the
extracellular matrix, as well as its pathological remodeling.
Although these cells are often associated with cardiac fibrosis, it
is important to note that the primary function of fibroblasts is
tissue repair-which in cases such as myocardial infarction is in
fact beneficial, and its interruption would have undesirable
outcomes (25-28).
In summary, under hypoxic ischemic conditions,
followed by myocardial infarction, the fibroblast switches
phenotype transdifferentiate into myofibroblast, contributing to
the healing by secreting extracellular matrix proteins and collagen
deposition, with subsequent cardiac remodeling and regulation of
the micro-environment metabolism.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
ZC, MCo and MCe performed the histological
examinations and IHC, and had major contributions in writing the
manuscript. BS, DP, DS, CGS, IP, HHA analyzed and interpreted the
patient data. MCTD, BS, FJA, CC, LP, AMD searched the literature
for similar work and articles and contributed to writing the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was conducted according to the World
Medical Association Declaration of Helsinki, using a protocol
approved by the local Bioethics Committee from ‘Sf. Pantelimon’
Emergency Clinical Hospital (Bucharest, Romania). All patients had
previously signed an informed written consent about
hospitalization, treatment and a possible future publication of
data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no conflict or competing
interests.
References
|
1
|
Travers JG, Kamal FA, Robbins J, Yutzey KE
and Blaxall BC: Cardiac fibrosis: The fibroblast awakens. Circ Res.
118:1021–1040. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Krenning G, Zeisberg EM and Kalluri R: The
origin of fibroblasts and mechanism of cardiac fibrosis. J Cell
Physiol. 225:631–637. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Humeres C and Frangogiannis NG:
Fibroblasts in the infarcted, remodeling and failing heart. JACC
Basic Transl Sci. 4:449–467. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Iorga RA, Bacalbasa N, Carsote M, Bratu
OG, Stanescu AMA, Bungau S, Pantis C and Diaconu CC: Metabolic and
cardiovascular benefits of GLP1 agonists, besides the hypoglycemic
effect (Review). Exp Ther Med. 20:2396–2400. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Porter KE and Turner NA: Cardiac
fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther.
123:255–278. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Takeda N and Manabe I: Cellular interplay
between cardiomyocytes and nonmyocytes in cardiac remodeling. Int J
Inflam. 2011(535241)2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tan DW and Barker N: Intestinal stem cells
and their defining niche. Curr Top Dev Biol. 107:77–107.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mehedintu C, Brinduse LA, Bratila E,
Monroc M, Lemercier E, Suaud O, Collet-Savoye C and Roman H: Does
computed tomography-based virtual colonoscopy improve the accuracy
of preoperative assessment based on magnetic resonance imaging in
women managed for colorectal endometriosis? J Minim Invasive
Gynecol. 25:1009–1017. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bratila E, Comandasu D, Coroleuca CA,
Cirstoiu M, Bohiltea R, Mehedintu C, Vladareanu S and Berceanu C:
Gastrointestinal symptoms in endometriosis correlated with the
disease stage, ISI Proceedings, XXXVIth National Congress of
Gastroenterology, Hepatology and Digestive Endoscopy, Filodiritto
Editore, 67, 2016.
|
|
10
|
Camelliti P, Borg TK and Kohl P:
Structural and functional characterization of cardiac fibroblasts.
Cardiovasc Res. 65:40–51. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Souders CA, Bowers SL and Baudino TA:
Cardiac fibroblast: The renaissance cell. Circ Res. 105:1164–1176.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sivestre JS, Levy BI and Tedgui A:
Mechanisms of angiogenesis and remodelling of the microvasculature.
Cardiovasc Res. 78:201–202. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Frangogiannis NG: Regulation of the
inflammatory response in cardiac repair. Circ Res. 110:159–73.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Saxena A, Chen W, Su Y, Rai V, Uche OU, Li
N and Frangogiannis NG: IL-1induces proinflammatory leukocyte
infiltration and regulates fibroblast phenotype in the infarcted
myocardium. J Immunol. 191:4838–4848. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shinde AV, Humeres C and Frangogiannis NG:
The role of α-smooth muscle actin in fibroblast-mediated matrix
contraction and remodeling. Biochim Biophys Acta. 1863:298–309.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ranjan P, Kumari R and Verma SK: Cardiac
fibroblasts and cardiac fibrosis: Precise role of exosomes. Front
Cell Dev Biol. 7(318)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Frangogiannis NG: Cardiac fibrosis: Cell
biological mechanisms, molecular pathways and therapeutic
opportunities. Mol. Aspects Med. 65:70–99. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Overbeek MJ, Mouchaers KT, Niessen HM,
Hadi AM, Kupreishvili K, Boonstra A, Voskuyl AE, Belien JA, Smit
EF, Dijkmans BC, et al: Characteristics of interstitial fibrosis
and inflammatory cell infiltration in right ventricles of systemic
sclerosis-associated pulmonary arterial hypertension. Int J
Rheumatol. 2010(604615)2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Duraes AR, de Souza Lima Bitar Y, Roever L
and Neto MG: Endomyocardial fibrosis: Past, present, and future.
Heart Fail Rev. 25:725–730. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Brown RD, Ambler SK, Mitchell MD and Long
CS: The cardiac fibroblast: Therapeutic target in myocardial
remodeling and failure. Annu Rev Pharmacol Toxicol. 45:657–687.
2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Socea B, Socea LI, Diaconu CC, Stanescu
AM, Grajdeanu IV, Iancu MA, Pantea Stoian A and Bratu OG:
Correlation of oral vitamin D administration with the severity of
psoriasis and the presence of metabolic syndrome. Rev Chim
(Bucharest). 69:1668–1672. 2018.
|
|
22
|
Laslo CL, Bacalbasa N, Stanescu AMA,
Carsote M, Bungau S, Rus M, Bratu OG and Diaconu CC: New oral
anticoagulants possible extension to other indications (Review).
Exp Ther Med. 20:2401–2405. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Iorga RA, Bratu OG, Marcu RD, Constantin
T, Mischianu DLD, Socea B, Gaman MA and Diaconu CC: Venous
thromboembolism in cancer patients: Still looking for answers. Exp
Ther Med. 18:5026–5032. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Vladu IM, Radu L, Girgavu SR, Baleanu V,
Clenciu D, Ene CG, Socea B, Mazen E, Cristea OM, Mota M and Tenea
Cojan TS: An easy way to detect cardiovascular risk. Rev Chim
(Bucharest). 69:4229–4232. 2018.
|
|
25
|
Fan D, Takawale A, Lee J and Kassiri Z:
Cardiac fibroblasts, fibrosis and extracellular matrix remodeling
in heart disease. Fibrogenesis Tissue Repair. 5(15)2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tica OA, Tica O, Antal L, Hatos A, Popescu
MI, Pantea Stoian A, Bratu OG, Gaman MA, Pituru SM and Diaconu CC:
Modern oral anticoagulant treatment in patients with atrial
fibrillation and heart failure: Insights from the clinical
practice. Farmacia. 66:972–976. 2018.
|
|
27
|
Laslo C, Pantea Stoian A, Socea B,
Paduraru D, Bodean O, Socea L, Neagu TP, Stanescu AMA, Marcu D and
Diaconu C: New oral anticoagulants and their reversal agents. J
Mind Medical Sci. 5:195–201. 2018.
|
|
28
|
Diaconu C: Treatment of diabetes in
patients with heart failure. In: The 3rd International conference
on interdisciplinary management of diabetes mellitus and its
complications - diabetes mellitus in internal medicine, INTERDIAB
2017 Proceedings. Serafinceanu C, Negoita O and Elian V (eds.).
Bucharest, pp170-177, 2017.
|