Introduction
Among all primary liver cancers, hepatocellular
carcinoma (HCC) accounts for 90% of cases, being the fourth leading
cause of cancer death worldwide in 2018, with 841,000 new cases and
782,000 deaths annually (1).
Diagnosis of HCC is not always easy on simple
hematoxylin and eosin (H&E) stain. The diagnostic problems
arise when a tumor shows pseudoglandular, trabecular, pleomorphic
or clear cell differentiation (2-4).
The histological diagnosis poses many challenges
particularly when dealing with liver biopsy specimens due to the
heterogeneity of HCC and the difficulty to confirm hepatocellular
differentiation in some instances (5).
The pleomorphism of cancer cells in HCC is caused by
instability and disorganization of the cytoskeleton system, with an
abnormal modulation of the intermediate filaments (such as
cytokeratins, particularly CK8 and CK18), as well as with a
deficiency of a cytoskeleton cross-linking protein. An unstable
cytoskeleton may play a role in tumor transformation and
progression, local invasion and distant metastasis (6).
Well-differentiated HCC may be differentiated from
large regenerative or low-grade dysplastic nodules and high-grade
dysplastic nodules by IHC features. A variety of antibodies were
proposed for positive and differential diagnosis, each of them with
its own limitation: α-fetoprotein (AFP), Hep Par-1, Glypican
(GPC-3), CK8, CK18, CK7, CK19, MOC-31, CD34, p-CEA, HSP70, glutamin
synthetase, or albumin (7).
On the background of constantly accumulating data
regarding human HCC, the aim of the study was to gain further
understanding of HCC diagnosis and behaviour.
Materials and methods
Case selection for human tissue
specimens
An extensive study was conducted on a batch of 42
cases with HCC, selected from a group of 72 patients with
hepatocellular lesions (hepatitis B and C, cirrhosis and hepatic
adenomas with or without dysplasia and malignant hepatic tumours,
especially hepatocarcinomas).
The study batch comprised 36 men and 6 women (sex
ratio 6:1), with age ranging from 10 to 77 years (m, 59, SD,
±13.2), in order to assess the tumour antigenic constellation in
HCC by means of IHC, along with microscopic analysis of peritumoral
stromal elements [such as tumour-infiltrating lymphocytes (TIL)]
and the association between tumour and stroma.
The study was performed according to the World
Medical Association Declaration of Helsinki and the tissue
specimens were collected according to national legislation.
Tissue sampling and stains
Tissue samples from surgically resected specimens of
HCC were taken for microscopic investigation. The selected tissue
samples were fixed in 10% neutral-buffered formalin (pH 7.0) for
24-48 h and paraffin embedded. Sections were cut at 5 µm and
stained with standard H&E.
Additional special stains such as PAS, Gomori silver
stain and van Gieson were carried out. Tissue samples were divided
into appropriate-sized (3-5 µm) sections for conventional
microscopy and immunohistochemistry.
Immunohistochemical analysis (IHC) was performed for
a vast panel of 13 antibodies, using sections displayed on slides
treated first with poly-L-lysine. The panel comprised the following
antibodies: CK8 (clone: B22.1, ready to use (RTU), Cell Marque),
CK18 (clone: B23.1, RTU, Cell Marque), CK7 (clone: OV-TL 12/30,
RTU, Cell Marque), CK19 (clone: A53-B/A2.26, RTU, Cell Marque), Hep
Par-1 (clone: OCH1E5, RTU, Cell Marque), CD34 (clone: QBend, RTU,
Cell Marque), CD68 (clone: KP-1, RTU, Cell Marque), Ki-67 (clone:
MIB-1, RTU, Cell Marque), PCNA (clone: PC10, 1:200, Dako),
α-fetoprotein (poly, RTU, Cell Marque), pre-albumin (PAB, poly,
1:75, Dako), albumin (ALB, poly, 1:5,000, Dako) and telomerase
(clone: NCL-hTERT, 1:30, Novocastra). IHC was performed on 3 µm
sections from formalin-fixed paraffin-embedded specimens.
An indirect tristadial Avidin-Biotin-Complex
technique was used together with a NovoLink Polymer detection
system which utilizes a novel control polymerization technology to
prepare polymeric HRP-linker antibody conjugates, according to the
manufacturer's specifications (Novocastra). Antigen retrieval
technique (enzymatic pre-treatment) was performed as per the
manufacturer specifications.
Molecular biology investigation was performed using
a chromogenic in situ hybridization technique for hepatic
albumin mRNA, using an oligonucleotidic cDNA probe with 51 base
pairs, complementary to mRNA sequence which encodes human
albumin.
Digital images obtained with an incorporated
software program were processed and analysed with Microsoft Office
Picture Manager (Washington, DC), running under Windows 10.
Statistical analysis
Statistical analysis was carried out using SPSS
version 20 (IBM Corp.). The Student's t-test was used to determine
the median, and mean ± standard deviation as well as association
between various parameters (monoclonal antibodies). P<0.05 was
considered statistically significant.
Results
The studied HCC occurred more frequently on
cirrhotic liver, all with an advanced degree (grade II and III
Edmondson in 62.5% of cases), with a trabecular-type predominance
and the tumour cells were hepatocyte-like and pleomorphic
types.
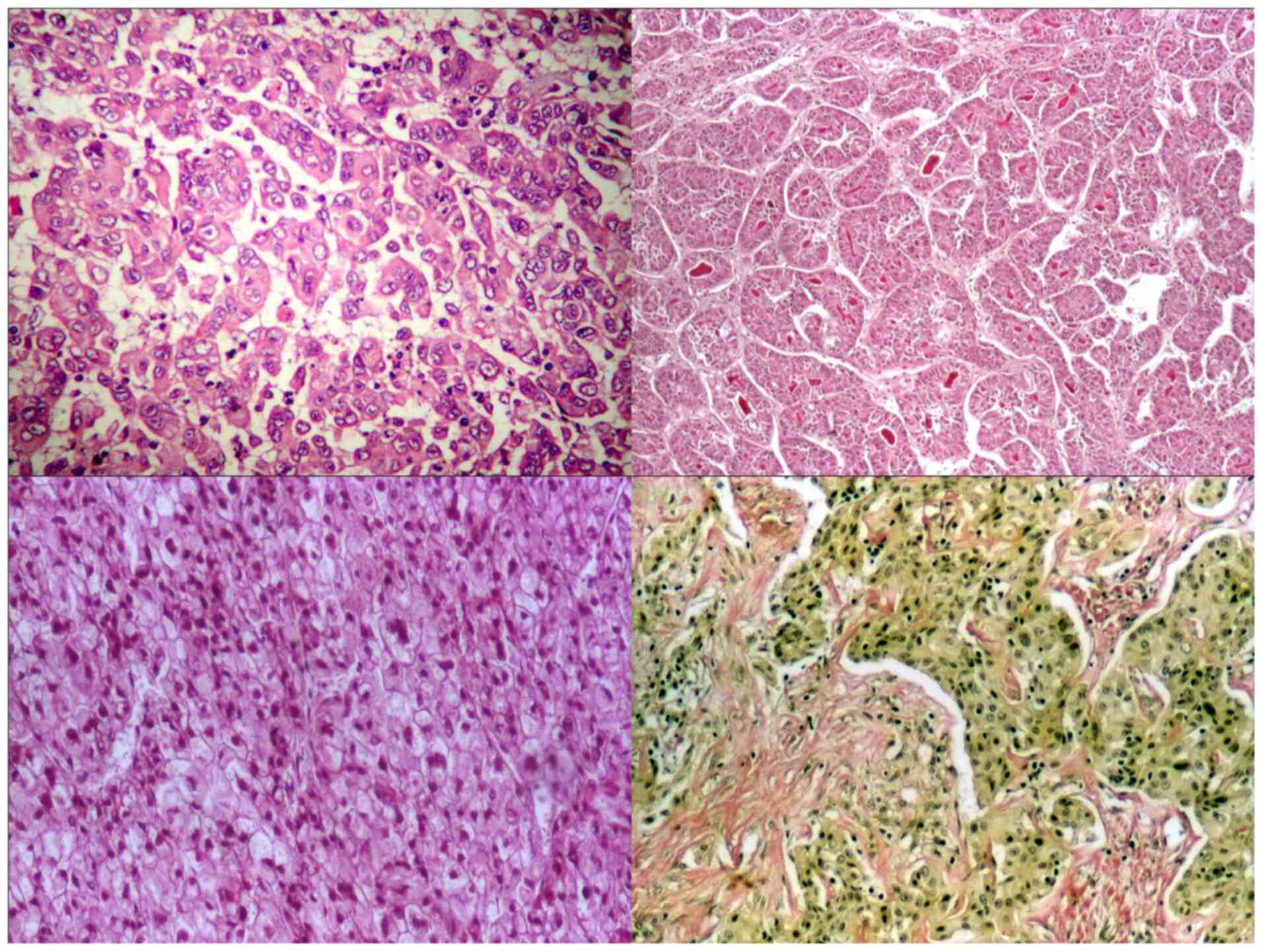
According to the recent WHO classification (8), 97% of tumours were HCC-NOS (with a
microscopic morphology of trabecular type in 69% of cases,
pseudo-glandular and compact types, in 15 and 13% of cases,
respectively) and 3% of tumours were HCC of scirrhous type, with
marked desmoplastic reaction (Fig.
1). No fibro-lamellar type was observed.
A part of hepatic tumours, especially the
well-differentiated types and those with clear cells kept the
capacity of glycogen synthesis, emphasized by PAS stain; >50% of
HCC presented a peri-acinar reticulin network. Bile (with intra- or
extra-cellular deposition) was also observed in 25% of cases.
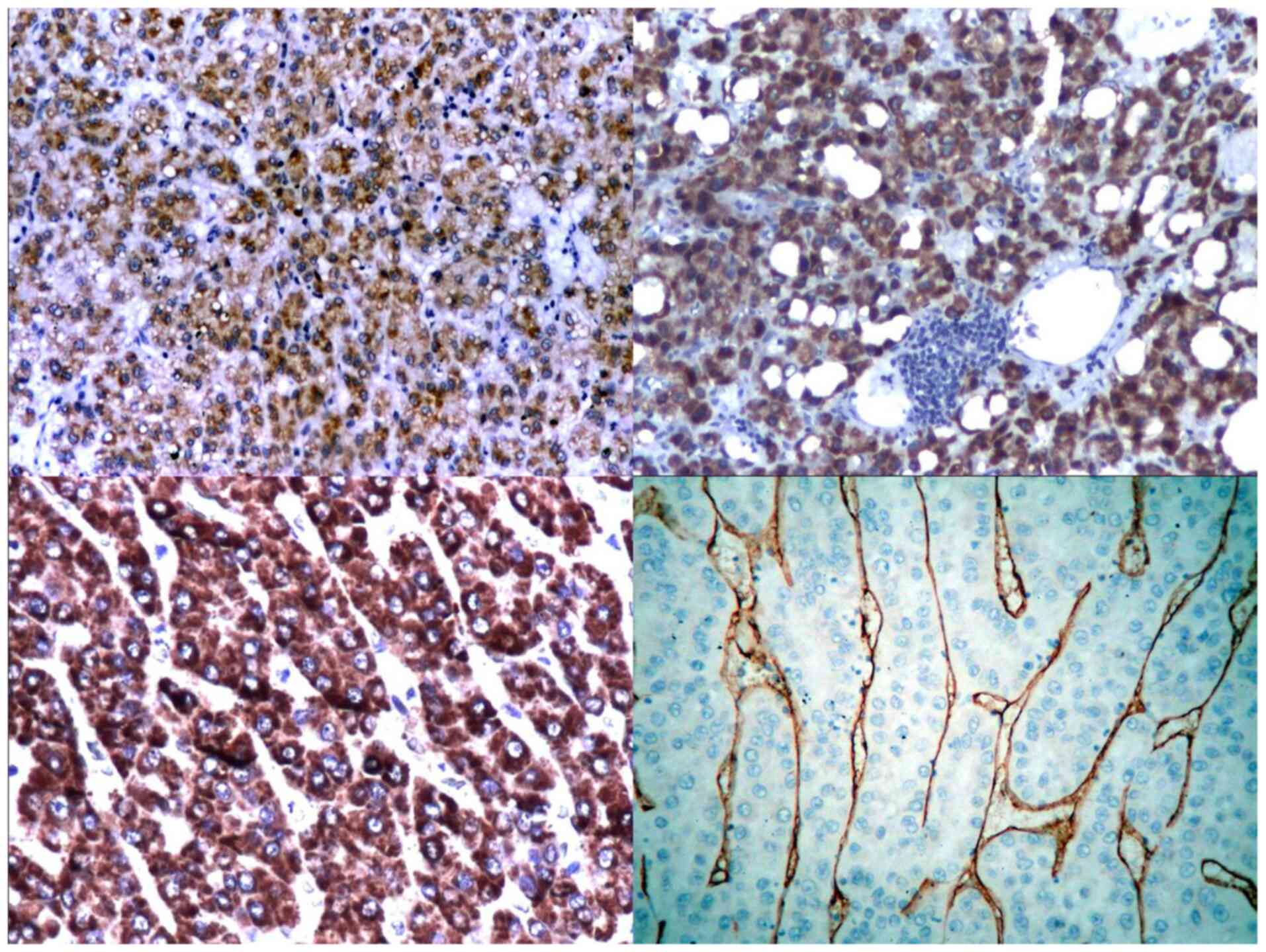
CK8 was positive in 54.54% of cases, while CK18 was
positive in 75.75% of cases. CK8 and 18 were better expressed by
well-differentiated HCC than low-differentiated HCC. CK18 appeared
to be more specific than CK8 for tumour hepatic tissue (Fig. 2).
Alpha-fetoprotein was expressed in 84.84% of cases,
while Hep Par-1 was positive in 75.75% of cases (with a tendency of
variation depending upon the degree of differentiation, but
retaining its capacity of staining the tumour cells even in
low-differentiated types). Hep Par-1 was diffusely expressed in the
cytoplasm of tumour cells, with a focal or diffuse granular pattern
(Fig. 2).
Well-differentiated tumours had a strong reaction to
CD34 (81.81% of cases), with a sinusoidal pattern (Fig. 2). Low-differentiated tumours with
compact pattern had a weak reaction to CD34 with random pattern or
were negative. Micro vascular density (MVD) was high in
well-differentiated HCC with trabecular pattern and in
low-differentiated HCC with pleomorphic cells; also, the increasing
of MVD was accompanied by the Kupffer cells hyperplasia in HCC.
The density of intra-tumoral Kupffer cells
infiltrate (assessed by CD68) was influenced directly by the
density of peritumoral Kupffer cells infiltrate. ITO cell
hyperplasia was independent of Kupffer cell hyperplasia and was
accompanied by a dense infiltration with tumour histiocytes, others
than Kupffer cells.
The density of tumour-infiltrating lymphocytes (TIL)
was independent from the density of macrophages. Kupffer cell
hyperplasia from the histiocytic infiltrate seemed to be more
important in anti-tumour immune defence than TIL, the histiocytes
coordinating as antigen presenting cells or the dynamic of the
local anti-tumour immune response. Peritumoral TIL density did not
influence the intra-tumoral TIL density.
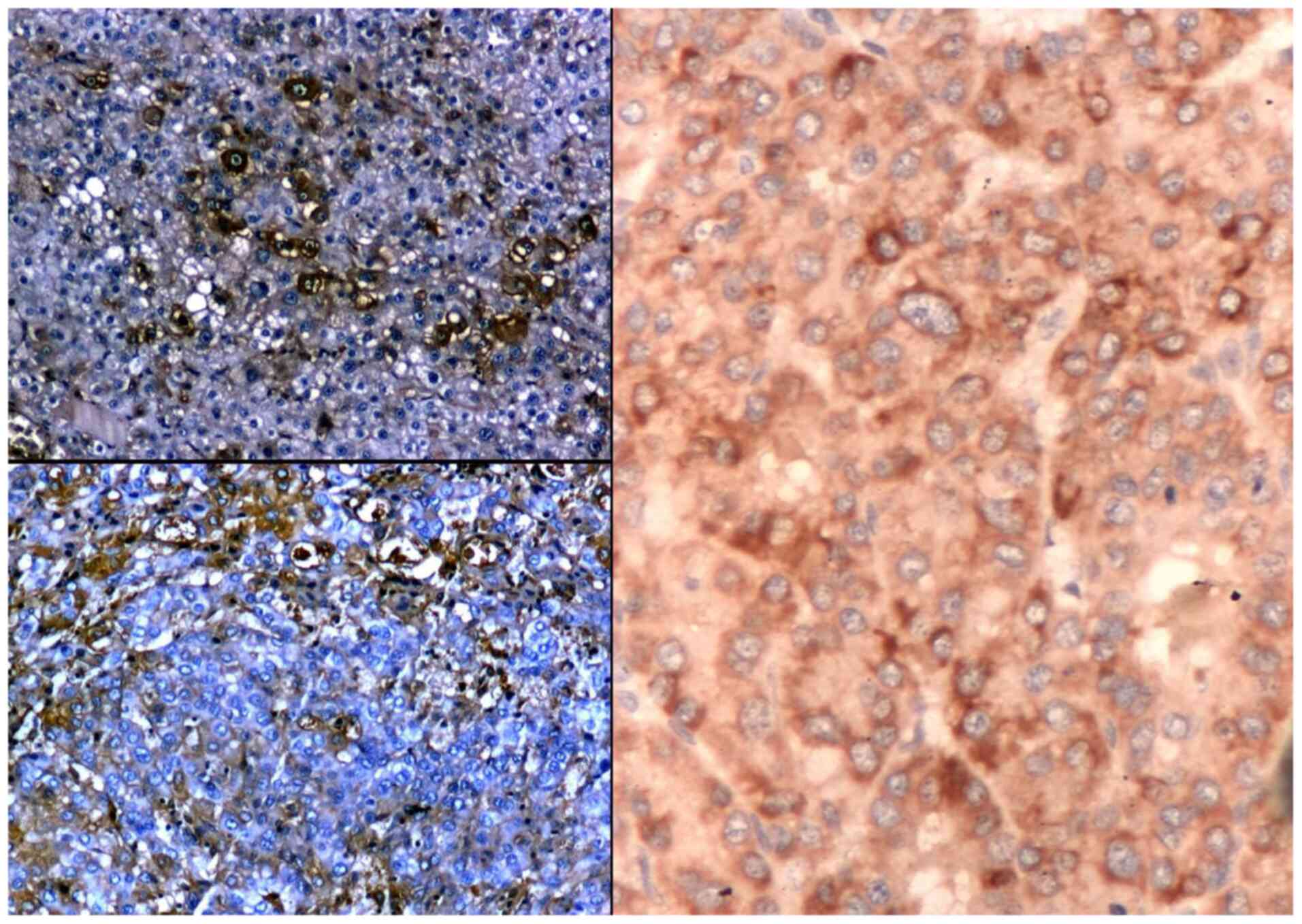
The immune reaction to albumin showed positivity in
trabecular type of HCC (56%) and was also positive in pleomorphic
cytology types (43%). Well-differentiated HCC expressed albumin
better than low-differentiated tumours (Fig. 3). There was a strong direct
relationship between the IHC expression of ALB and PAB in HCC.
The variability of ISH reaction in studied HCC was
high, recording extreme values (Fig.
3). There was also a slight reverse proportion between the
intensity of IHC reaction and the intensity of ISH signal of ALB in
HCC (r=-0.3, P=0.03).
IHC expression of PAB was independent of ISH
expression of albumin mRNA, but there was a statistical correlation
between PCNA and ISH for albumin (r=0.5); the decreasing IHC
expression of albumin was accompanied by an increasing of CK8
expression.
A cocktail of hepatic cytokeratins (CK8 and CK18)
combined with Hep Par-1 and associated to albumin proved to be more
powerful than albumin alone in differentiating HCC and increased
the value of tumour diagnosis.
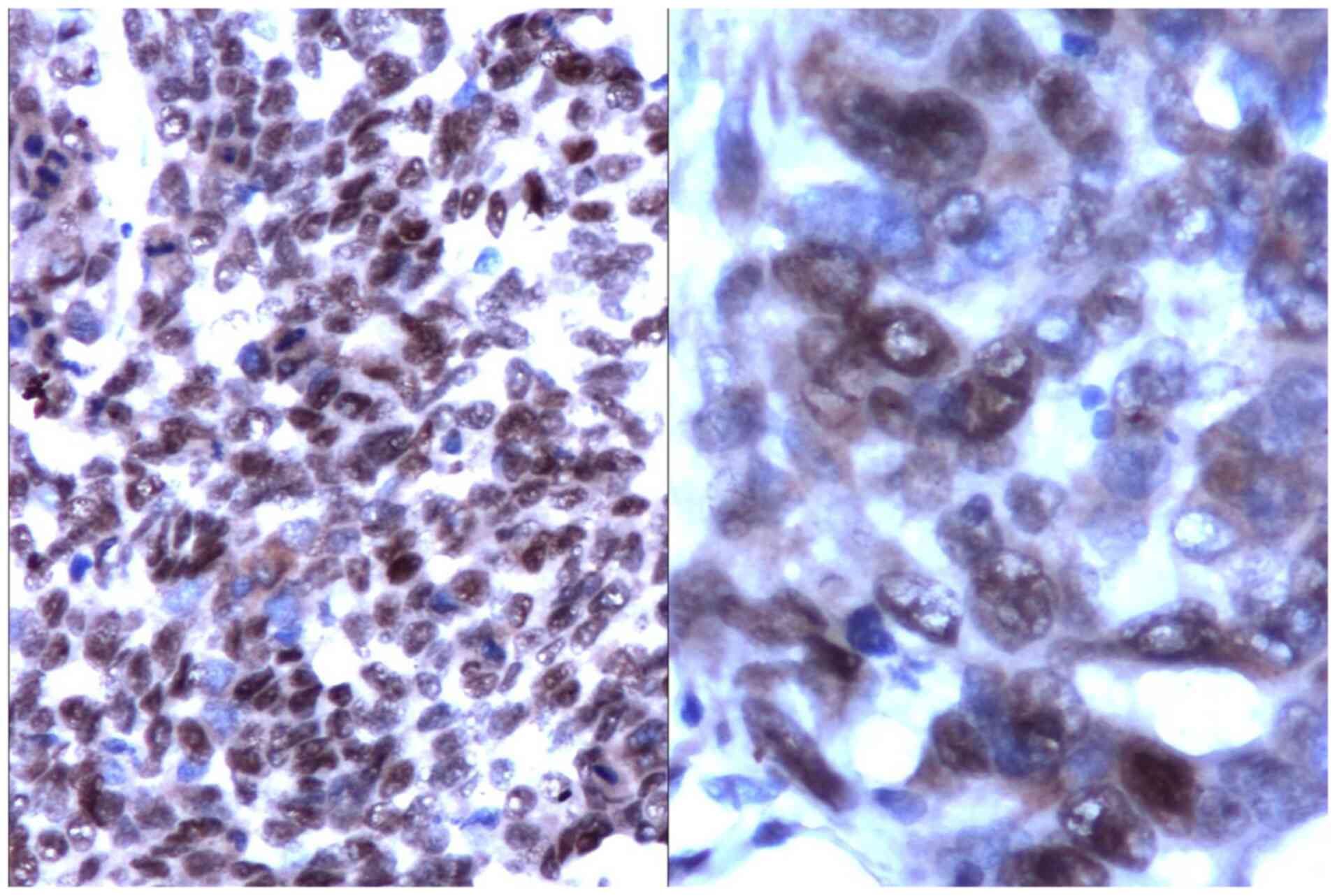
The catalytic subunit of the telomerase (hTERT)
showed a homogenous nuclear reaction or in clusters in all types of
HCC (Fig. 4).
hTERT expression was reverse proportional to the
tumour degree of differentiation (low-differentiated tumours had a
high level of expression and vice versa). Interestingly, the hTERT
expression was independent from the expression of tumoral
proliferating indexes (Ki-67 or PCNA).
There was a statistically significant association
between CD34 and hTERT (r=-0.4, P=0.0002), which suggests a reverse
proportion between telomerase activity and microvascular
density.
Discussion
Trabecular and pseudo-glandular types are
well-differentiated forms of HCC that must be distinguished from
cirrhosis with atypia, atypical adenomatous hyperplasia and
high-grade dysplastic nodule, which only by morphological means,
makes the differential diagnosis very difficult. The identification
of stromal invasion can be a clue for HCC, in contrast to the other
aforementioned lesions, which do not invade the surrounding
tissues.
Adenomatous hyperplasia (originally coined by
Edmondson) is divided into ‘ordinary’ (common) or atypical types.
The first one does not have a neoplastic nature (representing a
macro-regenerative nodule in cirrhosis), while the second one is a
pre-neoplastic lesion, with various degrees of dysplasia, based on
cytological and architectural changes (9).
There is a considerable overlap in microscopic
features in well-differentiated hepatocellular carcinoma and other
hepatic non-neoplastic lesions (such as the distinction of
well-differentiated HCC and hepatic adenoma in non-cirrhotic liver
or distinguishing between early HCC from high-grade dysplastic
nodule, in a cirrhotic liver), requiring the use of
immunohistochemistry and other techniques for diagnosis (10-12).
Normal and neoplastic hepatocytes express CK8 and
CK18 and are generally negative for CK7, CK19, and CK20(13). However, in our study, normal
peritumoral hepatocytes did not express CK7 and 19, but tumour
hepatocytes expressed these types of cytokeratin in ~25% of cases,
sometimes simultaneously with CK8 and 18. This may suggest the
possibility of immunophenotype change of tumour hepatocytes during
the progression of HCC.
Therefore, the cytokeratin set (CK8, 18, 7, 19)
along with Hep Par-1 expressed by HCC in our studied cases showed
the possibility of the existence of an antigenic mosaic, which can
be expressed as synchronous or metachronous, depending on tumour
differentiation degree.
In an exhaustive study on 799 patients with a large
panel of antibodies (α-fetoprotein, CD34, CK7, CK19, glypican-3,
Ki-67, glutamine synthetase and β-catenin), it was found that
immunohistochemical expression of these markers in HCC in a
non-cirrhotic and cirrhotic liver was comparable, having limited
additional value to characterise HCC in non-cirrhotic livers.
Additionally, none of the immunohistochemical stains were
associated with a worse overall survival (14).
Hep Par 1 is a monoclonal antibody that was
developed using formalin-fixed tissue from failed allograft liver,
which turned to be a sensitive and specific marker for HCC
(15).
In a recent comparative cross-sectional study, Hep
Par-1 proved useful in differentiating hepatocellular carcinoma
from metastatic carcinoma, taking histopathology as a gold standard
(16).
According to a study conducted by Kakar et al
(17), Hep Par 1 and polyclonal
carcinoembryonic antigen are the most reliable markers for
hepatocellular differentiation, but they have low sensitivity for
poorly differentiated cases, requiring additional markers such as
glypican-3 or different types of cytokeratin.
Glypican-3 (GPC-3) is a membrane-anchored
proteoglycan and was designated as an oncofetal protein, which was
normally expressed in fetal liver, but not in normal adult liver.
Certain studies showed that GPC-3 was expressed in ~72% of HCCs,
but not in normal liver or hepatic adenoma (18).
GPC-3 seems to be a relatively sensitive and
specific marker in the positive diagnosis of HCC, and when it is
coupled with other markers such as CD34, CD10 and AFP is useful in
differentiating HCC from dysplastic nodules, cirrhotic regenerative
nodules, focal nodular hyperplasia and hepatocellular adenoma
(19).
In combination with a complete CD34 immunostaining
pattern, GPC-3 greatly improves the accuracy of distinguishing
between malignant hepatic lesions and benign lesions (20). As HCC is a highly vascularized
tumour, angiogenesis plays a fundamental role in progression of
hepatocellular carcinoma (21,22).
In a relative recent study, microvascular density determined by
CD34 and CD105 (endoglin) expression proved to be useful as an
additional parameter to distinguish between benign and malignant
hepatic nodules, underlining that CD34 had higher average
microvascular density scores than CD105 in HCC, with a more uniform
positivity pattern (23).
Alpha-fetoprotein (AFP) is an oncofetal protein; its
expression in a tumor is relatively specific for hepatocellular
differentiation, if germ cell tumors can be excluded. The IHC
sensitivity is about 40%, but serum AFP levels are helpful in the
diagnosis of HCC and surveilling response to therapy for HCC
(24).
A clinic-pathological analysis of 375 cases revealed
that besides tumour size, tumour differentiation and vessel
invasion (as important factors which affect the prognosis of
patients with HCC), Hep Par-1 and AFP have predictive significance
in HCC, along with GPC-3 and CD34(25).
In patients with serum negative alpha-fetoprotein,
hepatocellular carcinoma with focal nodular hyperplasia showed high
CD34 and CK19, and low PCNA level (26).
Albumin in situ hybridization (ISH) is
specific for hepatocellular differentiation and has a high
sensitivity (~90%). Controversial findings however have been
reported (27,28).
In one study, even if ISH for albumin mRNA was
expressed in all HCCs from the study batch, it was also positive in
intrahepatic cholangiocarcinoma and focally positive in gallbladder
adenocarcinoma and a subset of other neoplasms, which could be a
potential pitfall (29).
On the other hand, in another study, mRNA albumin
ISH showed high correlation with Hep Par 1 immunoreactivity; their
combined use for diagnosis of HCC had a sensitivity of 100% in this
population (30).
Overall, branched chain ISH performed on manual and
automated mode is a sturdy assay for detecting albumin with
reliable sensitivity for poorly differentiated HCCs. When
interpreted in combination with Hep Par-1 and Arginase-1, ISH for
mRNA albumin offers a high level of sensitivity and specificity
(31).
Human telomerase has 3 elements: A template, an
associated protein and a catalytic subunit (hTERT). Telomerase
activity depends mainly on telomerase reverse transcriptase (hTERT)
in hepatocellular carcinoma.
The correlation of the expressions of hTERT, c-myc
and Ki-67 in HCC were closely associated, according to a study from
2009(32), the overexpression of
these three factors playing a vital role in the progress of
HCC.
In our study, the hTERT expression was independent
from the expression of tumoral proliferating indexes, such as Ki-67
or PCNA, but its expression was higher in low-differentiated
tumours than in high-differentiated tumours.
In another study meant to assess the correlation
between hTERT and PTEN expression in HCC, it was found that PTEN
and hTERT have different roles in the development of HCC (33). Thus, authors of that study showed
that a significantly negative correlation between PTEN and hTERT
gene expression indicates that hTERT activation and upregulation
may be conferred by the loss of PTEN gene expression in HCC. The
combined detection of PTEN and hTERT, however, may provide critical
clinical evidence for the diagnosis and biological behaviour of HCC
(33).
In conclusion, the heterogeneity of the antigenic
constellation in hepatocellular carcinoma suggests an antigenic
mosaicism, which can be expressed as synchronous or metachronous,
depending on the tumor degree of differentiation, and a targeted
use of a cytokeratin ‘cocktail’ (CK8 and CK18, CK7 and CK19),
combined with Hep Par-1, Glypican and CD34, along with albumin
detection (by both means of IHC and ISH) increases the value of the
positive and differential diagnosis of HCC.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MC and ZC performed the histological examinations
and IHC, designed the study and had major contributions in writing
the manuscript. BS, DS, CGS, DP and NB analyzed and interpreted the
patient data. DS, IS, AT and LA searched the literature for similar
work and articles, analyzed the data and had major contributtions
in writing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was conducted according to the World
Medical Association Declaration of Helsinki, using a protocol
approved by the local Bioethics Committee from ‘St. Pantelimon’
Emergency Clinical Hospital (Bucharest, Romania). All patients have
previously signed an informed written consent about
hospitalization, treatment and a possible future publication of
data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no conflict or competing
interests.
References
|
1
|
Jiang Y, Han QJ and Zhang J:
Hepatocellular carcinoma: Mechanisms of progression and
immunotherapy. World J Gastroenterol. 25:3151–3167. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Amarapurkar AD, Rege JD, Joshi AS, Vaiphei
K and Amarapurkar DN: Utilization of antihepatocyte clone OCH1E5
(Hep Par 1) in histological evaluation of liver tumors. Indian J
Pathol Microbiol. 49:341–344. 2006.PubMed/NCBI
|
|
3
|
Negrut N, Khan SA, Bungau S, Zaha DC, Aron
CR, Bratu O, Diaconu CC and Ionita-Radu F: Diagnostic challenges in
gastrointestinal infections. Rom J Mil Med. 123:83–90. 2020.
|
|
4
|
Draghici T, Negreanu L, Bratu OG, Stoian
AP, Socea B, Neagu TP, Stanescu AM, Manuc D and Diaconu CC:
Paraneoplastic syndromes in digestive tumors: A review. Rom
Biotechnol Lett. 24:813–819. 2019.
|
|
5
|
Quaglia A: Hepatocellular carcinoma: A
review of diagnostic challenges for the pathologist. J Hepatocell
Carcinoma. 5:99–108. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lai YS, Cheng CC, Lee MT, Chao WT, Lai YC,
Hsu YH and Liu YH: The prognostic value of cytokeratin and sal-like
protein 4 expression in hepatocellular carcinoma and intra-hepatic
cholangiocarcinoma in Taiwan. Int J Med Sci. 15:1746–1756.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ferrell LD: Hepatocellular carcinoma and
its variants. In: Odze and Goldblum Surgical Pathology of the GI
Tract, Liver, Biliary Tract, and Pancreas. 3rd edition. Elsevier
Saunders Publishing House, pp1549-1557, 2015.
|
|
8
|
Torbenson MS, Ng IOL, Park YN, Roncalli M
and Sakamoto M: Hepatocellular carcinoma, chapter 8: Tumours of
tumours of the liver and intrahepatic bile ducts. In: WHO
Classification of Tumours, Digestive System. 5th edition,
pp229-239, 2019.
|
|
9
|
Kutlesic C, Katic V, Veliekovic L and
Katic K: Atypical adenomatous hyperplasia in liver cirrhosis. Arch
Oncol. 12 (Suppl 1):S10–S11. 2004.
|
|
10
|
Shafizadeh N and Kakar S: Diagnosis of
well-differentiated hepatocellular lesions: role of
immunohistochemistry and other ancillary techniques. Adv Anat
Pathol. 18:438–445. 2011.
|
|
11
|
Epîngeac ME, Găman MA, Diaconu CC, Gad M
and Găman AM: The evaluation of oxidative stress in obesity. Rev
Chim (Bucharest). 70:2241–2244. 2019.
|
|
12
|
Dumitru N, Cocolos A, Caragheorgheopol A,
Dumitrache C, Bratu OG, Neagu TP, Diaconu CC and Ghemigian A:
Collagen-the ultrastructural element of the bone matrix. Rev Chim
(Bucharest). 69:1706–1709. 2018.
|
|
13
|
Van Eyken P, Sciot R, Paterson A, Callea
F, Kew MC and Desmet VJ: Cytokeratin expression in hepatocellular
carcinoma: An immunohistochemical study. Hum Pathol. 19:562–568.
1988.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Witjes CD, ten Kate FJ, Verhoef C, de Man
RA and Jzermans JN: Immunohistochemical characteristics of
hepatocellular carcinoma in non-cirrhotic livers. J Clin Pathol.
66:687–691. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fan Z, van de Rijn M, Montgomery K and
Rouse RV: Hep Par 1 antibody stain for the differential diagnosis
of hepatocellular carcinoma: 676 tumors tested using tissue
microarrays and conventional tissue sections. Mod Pathol.
16:137–144. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hanif R and Mansoor S: Hep par-1: A novel
immunohistochemical marker for differentiating hepatocellular
carcinoma from metastatic carcinoma. J Coll Physicians Surg Pak.
24:186–189. 2014.PubMed/NCBI
|
|
17
|
Kakar S, Gown AM, Goodman ZD and Ferrell
LD: Best practices in diagnostic immunohistochemistry:
Hepatocellular carcinoma versus metastatic neoplasms. Arch Pathol
Lab Med. 131:1648–1654. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang XY, Degos F, Dubois S, Tessiore S,
Allegretta M, Guttmann RD, Jothy S, Belghiti J, Bedossa P and
Paradis V: Glypican-3 expression in hepatocellular tumors:
Diagnostic value for preneoplastic lesions and hepatocellular
carcinomas. Hum Pathol. 37:1435–1441. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
DU JL, Wei LX and Wang YL: Expression and
clinicopathologic significance of GPC3 and other antibodies in
well-differentiated hepatocellular carcinoma. Zhonghua Bing Li Xue
Za Zhi. 40:11–16. 2011.PubMed/NCBI(In Chinese).
|
|
20
|
Coston WM, Loera S, Lau SK, Ishizawa S,
Jiang Z, Wu CL, Yen Y, Weiss LM and Chu PG: Distinction of
hepatocellular carcinoma from benign hepatic mimickers using
Glypican-3 and CD34 immunohistochemistry. Am J Surg Pathol.
32:433–444. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Suceveanu AI, Stoian AP, Mazilu L, Voinea
F, Hainarosie R, Diaconu CC, Pituru S, Nitipir C, Badiu DC, Ceausu
I and Suceveanu AP: Interferon-free therapy is not a trigger for
hepatocellular carcinoma in patients with chronic infection with
hepatitis C virus. Farmacia. 66:904–908. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gheorghe G, Pantea Stoian A, Gaman MA,
Socea B, Neagu TP, Stanescu AMA, Bratu OG, Mischianu DLD, Suceveanu
AI and Diaconu CC: The benefits and risks of antioxidant treatment
in liver diseases. Rev Chim (Bucharest). 70:651–655. 2019.
|
|
23
|
Segatelli V, de Oliveira EC, Boin IF,
Ataide EC and Escanhoela CA: Evaluation and comparison of
microvessel density using the markers CD34 and CD105 in
regenerative nodules, dysplastic nodules and hepatocellular
carcinoma. Hepatol Int. 8:260–265. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lau SK, Prakash S, Geller SA and Alsabeh
R: Comparative immunohistochemical profile of hepatocellular
carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum
Pathol. 33:1175–1181. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Du JL, Wang YL, Shi HY, Guo AT and Wei LX:
Expression of glypican-3, hepatocyte antigen, alpha-fetoprotein,
CD34 and CD10 in hepatocellular carcinoma: A clinicopathologic
analysis of 375 cases. Zhonghua Bing Li Xue Za Zhi. 41:309–13.
2012.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
26
|
Cui DJ, Wu Y and Wen DH: CD34, PCNA and
CK19 expressions in AFP-hepatocellular carcinoma. Eur Rev Med
Pharmacol Sci. 22:5200–5205. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Surcel M, Huică RI, Munteanu AN, Isvoranu
G, Pîrvu IR, Ciotaru D, Constantin C, Bratu O, Căruntu C, Neagu M
and Ursaciuc C: Phenotypic changes of lymphocyte populations in
psoriasiform dermatitis animal model. Exp Ther Med. 17:1030–1038.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Plotogea O, Ilie M, Sandru V, Chiotoroiu
A, Bratu O and Diaconu C: Cardiovascular and metabolic consequences
of liver transplantation: A review. Medicina (Kaunas).
55(E489)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nasir A, Lehrke HD, Mounajjed T, Said S,
Zhang L, Yasir S, Shah SS, Chandan VS, Smyrk TC, Moreira RK, et al:
Albumin in situ hybridization can be positive in adenocarcinomas
and other tumors from diverse sites. Am J Clin Pathol. 152:190–199.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kakar S, Muir T, Murphy LM, Lloyd RV and
Burgart LJ: Immunoreactivity of Hep Par 1 in hepatic and
extrahepatic tumours and its correlation with albumin in situ
hybridization in hepatocellular carcinoma. Am J Clin Pathol.
119:361–366. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shahid M, Mubeen A, Tse J, Kakar S,
Bateman AC, Borger D, Rivera MN, Ting DT and Deshpande V: Branched
chain in situ hybridization for albumin as a marker of
hepatocellular differentiation: Evaluation of manual and automated
in situ hybridization platforms. Am J Surg Pathol. 39:25–34.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fang YW: Expression and significance of
hTERT, c-myc and Ki-67 in hepatocellular carcinoma. Xi Bao Yu Fen
Zi Mian Yi Xue Za Zhi. 25:338–340. 2009.PubMed/NCBI(In Chinese).
|
|
33
|
Zhou X, Zhu H and Lu J: PTEN and hTERT
gene expression and the correlation with human hepatocellular
carcinoma. Pathol Res Pract. 211:316–319. 2015.PubMed/NCBI View Article : Google Scholar
|