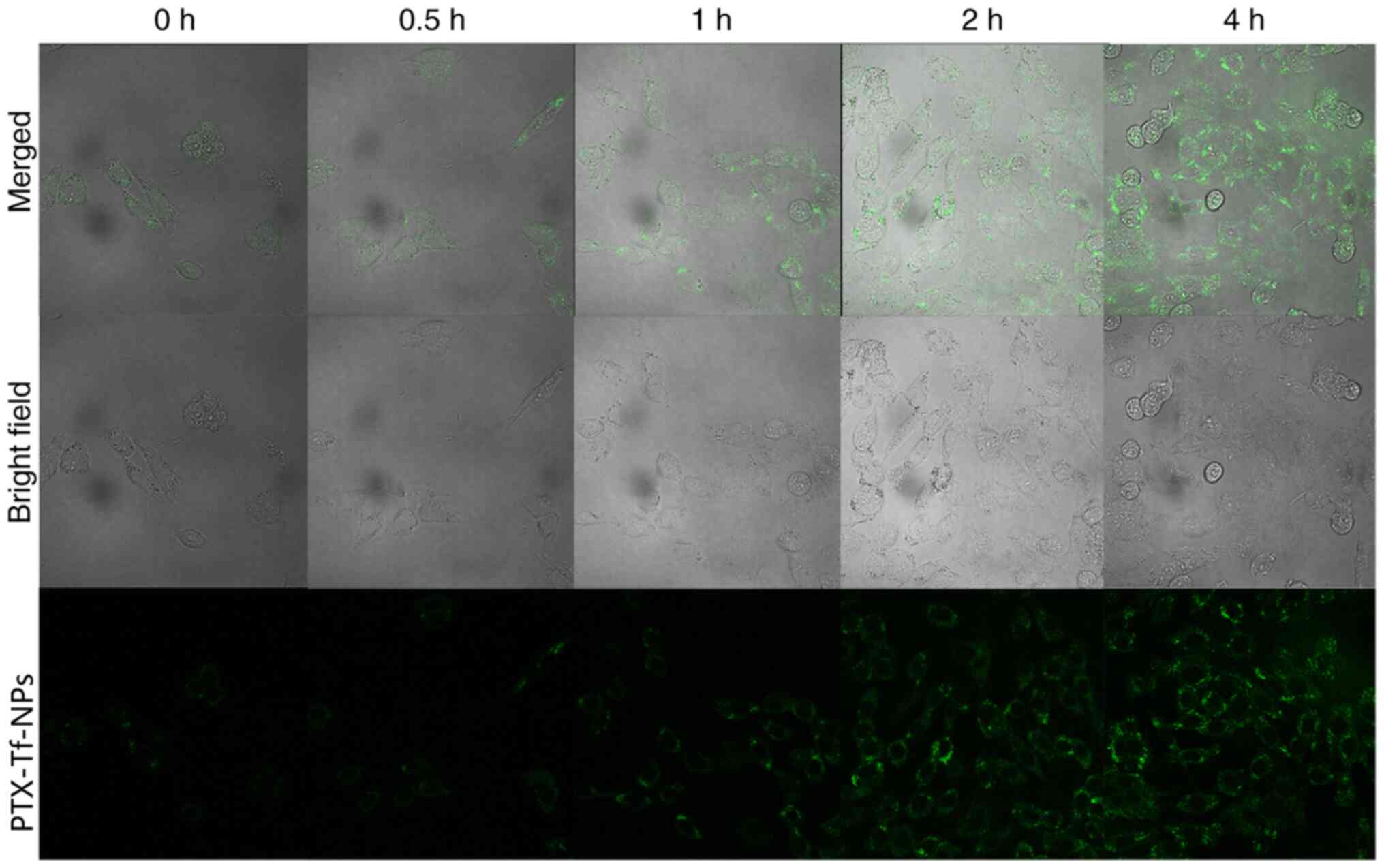
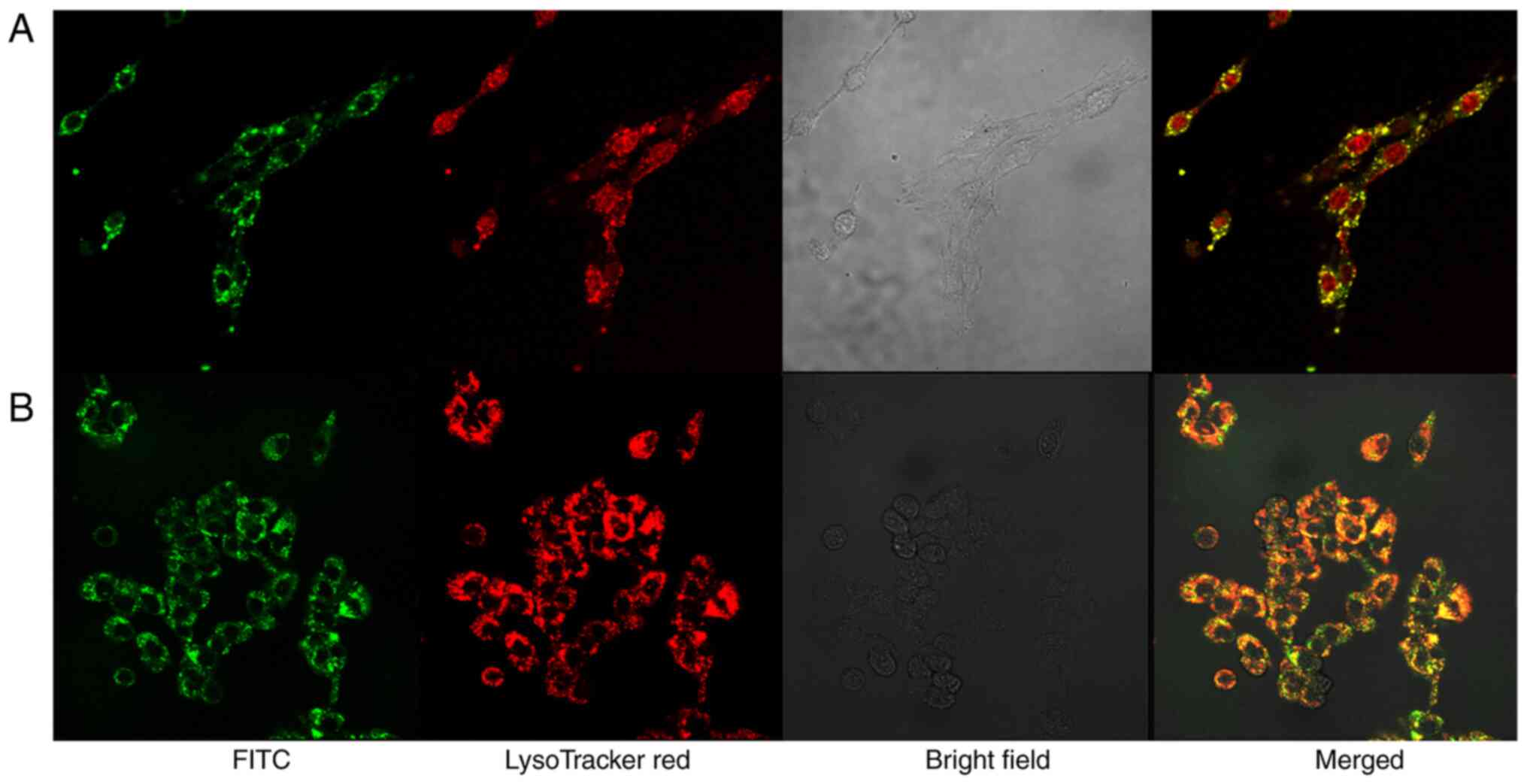
|
1
|
Ostrom QT, Gittleman H, Fulop J, Liu M,
Blanda R, Kromer C, Wolinsky Y, Kruchko C and Barnholtz-Sloan JS:
CBTRUS Statistical Report: Primary Brain and Central Nervous System
Tumors Diagnosed in the United States in 2008-2012. Neuro-oncol. 17
(Suppl 4):iv1–iv62. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang X, Zhang W, Cao WD, Cheng G and
Zhang YQ: Glioblastoma multiforme: Molecular characterization and
current treatment strategy (Review). Exp Ther Med. 3:9–14.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Magrath JW and Kim Y: Salinomycin's
potential to eliminate glioblastoma stem cells and treat
glioblastoma multiforme (Review). Int J Oncol. 51:753–759.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jovčevska I, Kočevar N and Komel R: Glioma
and glioblastoma - how much do we (not) know? Mol Clin Oncol.
1:935–941. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Stupp R, Taillibert S, Kanner AA, Kesari
S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink
KL, et al: Maintenance Therapy With Tumor-Treating Fields Plus
Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized
Clinical Trial. JAMA. 314:2535–2543. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Toms SA and Tapinos N: Recent Advances in
the Treatment of Gliomas - Comprehensive Brain Tumor Center. R I
Med J (2013). 100:43–46. 2017.PubMed/NCBI
|
|
8
|
Chakroun RW, Zhang P, Lin R, Schiapparelli
P, Quinones-Hinojosa A and Cui H: Nanotherapeutic systems for local
treatment of brain tumors. Wiley Interdiscip Rev Nanomed
Nanobiotechnol. 10(e1479)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bryukhovetskiy IS, Dyuizen IV, Shevchenko
VE, Bryukhovetskiy AS, Mischenko PV, Milkina EV and Khotimchenko
YS: Hematopoietic stem cells as a tool for the treatment of
glioblastoma multiforme. Mol Med Rep. 14:4511–4520. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bryukhovetskiy I, Bryukhovetsky A,
Khotimchenko Y, Mischenko P, Tolok E and Khotimchenko R:
Combination of the multipotent mesenchymal stromal cell
transplantation with administration of temozolomide increases
survival of rats with experimental glioblastoma. Mol Med Rep.
12:2828–2834. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Preusser M, de Ribaupierre S, Wöhrer A,
Erridge SC, Hegi M, Weller M and Stupp R: Current concepts and
management of glioblastoma. Ann Neurol. 70:9–21. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bryukhovetskiy I, Ponomarenko A, Lyakhova
I, Zaitsev S, Zayats Y, Korneyko M, Eliseikina M, Mischenko P,
Shevchenko V, Shanker Sharma H, et al: Personalized regulation of
glioblastoma cancer stem cells based on biomedical technologies:
From theory to experiment (Review). Int J Mol Med. 42:691–702.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hundsberger T, Reardon DA and Wen PY:
Angiogenesis inhibitors in tackling recurrent glioblastoma. Expert
Rev Anticancer Ther. 17:507–515. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim SS, Harford JB, Pirollo KF and Chang
EH: Effective treatment of glioblastoma requires crossing the
blood-brain barrier and targeting tumors including cancer stem
cells: The promise of nanomedicine. Biochem Biophys Res Commun.
468:485–489. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Karim R, Palazzo C, Evrard B and Piel G:
Nanocarriers for the treatment of glioblastoma multiforme: Current
state-of-the-art. J Controll Release. 227:23–37. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Glaser T, Han I, Wu L and Zeng X: Targeted
Nanotechnology in Glioblastoma Multiforme. Front Pharmacol.
8(166)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fang K, Liu P, Dong S, Guo Y, Cui X, Zhu
X, Li X, Jiang L, Liu T and Wu Y: Magnetofection based on
superparamagnetic iron oxide nanoparticle-mediated low lncRNA
HOTAIR expression decreases the proliferation and invasion of
glioma stem cells. Int J Oncol. 49:509–518. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee JK, Nam DH and Lee J: Repurposing
antipsychotics as glioblastoma therapeutics: Potentials and
challenges. Oncol Lett. 11:1281–1286. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tivnan A, Zakaria Z, O'Leary C, Kögel D,
Pokorny JL, Sarkaria JN and Prehn JH: Inhibition of multidrug
resistance protein 1 (MRP1) improves chemotherapy drug response in
primary and recurrent glioblastoma multiforme. Front Neurosci.
9(218)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhao C, Liu X, Liu J, Yang Z, Rong X, Li
M, Liang X and Wu Y: Transferrin conjugated poly (γ-glutamic
acid-maleimide-co-L-lactide)-1,2-dipalmitoylsn-glycero-3-phosphoethanolamine
copolymer nanoparticles for targeting drug delivery. Colloids Surf
B Biointerfaces. 123:787–796. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pierzyńska-Mach A, Janowski PA and
Dobrucki JW: Evaluation of acridine orange, LysoTracker Red, and
quinacrine as fluorescent probes for long-term tracking of acidic
vesicles. Cytometry A. 85:729–737. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Choudhury H, Pandey M, Chin PX, Phang YL,
Cheah JY, Ooi SC, Mak KK, Pichika MR, Kesharwani P, Hussain Z, et
al: Transferrin receptors-targeting nanocarriers for efficient
targeted delivery and transcytosis of drugs into the brain tumors:
A review of recent advancements and emerging trends. Drug Deliv
Transl Res. 8:1545–1563. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gkouvatsos K, Papanikolaou G and
Pantopoulos K: Regulation of iron transport and the role of
transferrin. Biochim Biophys Acta. 1820:188–202. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Skjørringe T, Burkhart A, Johnsen KB and
Moos T: Divalent metal transporter 1 (DMT1) in the brain:
Implications for a role in iron transport at the blood-brain
barrier, and neuronal and glial pathology. Front Mol Neurosci.
8(19)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yan F, Wang Y, He S, Ku S, Gu W and Ye L:
Transferrin-conjugated, fluorescein-loaded magnetic nanoparticles
for targeted delivery across the blood-brain barrier. J Mater Sci
Mater Med. 24:2371–2379. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C,
Suzuki T, Kamiie J and Terasaki T: Quantitative targeted absolute
proteomics of human blood-brain barrier transporters and receptors.
J Neurochem. 117:333–345. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wiley DT, Webster P, Gale A and Davis ME:
Transcytosis and brain uptake of transferrin-containing
nanoparticles by tuning avidity to transferrin receptor. Proc Natl
Acad Sci USA. 110:8662–8667. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Clark AJ and Davis ME: Increased brain
uptake of targeted nanoparticles by adding an acid-cleavable
linkage between transferrin and the nanoparticle core. Proc Natl
Acad Sci USA. 112:12486–12491. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sonali Singh RP, Singh N, Sharma G,
Vijayakumar MR, Koch B, Singh S, Singh U, Dash D, Pandey BL, et al:
Transferrin liposomes of docetaxel for brain-targeted cancer
applications: Formulation and brain theranostics. Drug Deliv.
23:1261–1271. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Recht L, Torres CO, Smith TW, Raso V and
Griffin TW: Transferrin receptor in normal and neoplastic brain
tissue: Implications for brain-tumor immunotherapy. J Neurosurg.
72:941–945. 1990.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Voth B, Nagasawa DT, Pelargos PE, Chung
LK, Ung N, Gopen Q, Tenn S, Kamei DT and Yang I: Transferrin
receptors and glioblastoma multiforme: Current findings and
potential for treatment. J Clin Neurosci. 22:1071–1076.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Abe T, Hasegawa S, Taniguchi K, Yokomizo
A, Kuwano T, Ono M, Mori T, Hori S, Kohno K and Kuwano M: Possible
involvement of multidrug-resistance-associated protein (MRP) gene
expression in spontaneous drug resistance to vincristine, etoposide
and adriamycin in human glioma cells. Int J Cancer. 58:860–864.
1994.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Garrido W, Muñoz M, San Martín R and
Quezada C: FK506 confers chemosensitivity to anticancer drugs in
glioblastoma multiforme cells by decreasing the expression of the
multiple resistance-associated protein-1. Biochem Biophys Res
Commun. 411:62–68. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Peigñan L, Garrido W, Segura R, Melo R,
Rojas D, Cárcamo JG, San Martín R and Quezada C: Combined use of
anticancer drugs and an inhibitor of multiple drug
resistance-associated protein-1 increases sensitivity and decreases
survival of glioblastoma multiforme cells in vitro. Neurochem Res.
36:1397–1406. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pang Z, Gao H, Yu Y, Guo L, Chen J, Pan S,
Ren J, Wen Z and Jiang X: Enhanced intracellular delivery and
chemotherapy for glioma rats by transferrin-conjugated
biodegradable polymersomes loaded with doxorubicin. Bioconjug Chem.
22:1171–1180. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu G, Mao J, Jiang Z, Sun T, Hu Y, Jiang
Z, Zhang C, Dong J, Huang Q and Lan Q: Transferrin-modified
Doxorubicin-loaded biodegradable nanoparticles exhibit enhanced
efficacy in treating brain glioma-bearing rats. Cancer Biother
Radiopharm. 28:691–696. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Porru M, Zappavigna S, Salzano G, Luce A,
Stoppacciaro A, Balestrieri ML, Artuso S, Lusa S, De Rosa G,
Leonetti C, et al: Medical treatment of orthotopic glioblastoma
with transferrin-conjugated nanoparticles encapsulating zoledronic
acid. Oncotarget. 5:10446–10459. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li Y, He H, Jia X, Lu WL, Lou J and Wei Y:
A dual-targeting nanocarrier based on poly(amidoamine) dendrimers
conjugated with transferrin and tamoxifen for treating brain
gliomas. Biomaterials. 33:3899–3908. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Khalil IR, Burns AT, Radecka I, Kowalczuk
M, Khalaf T, Adamus G, Johnston B and Khechara MP:
Bacterial-Derived Polymer Poly-y-Glutamic Acid (y-PGA)-Based
Micro/Nanoparticles as a Delivery System for Antimicrobials and
Other Biomedical Applications. Int J Mol Sci.
18(313)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pavot V, Berthet M, Rességuier J, Legaz S,
Handké N, Gilbert SC, Paul S and Verrier B: Poly(lactic acid) and
poly(lactic-co-glycolic acid) particles as versatile carrier
platforms for vaccine delivery. Nanomedicine (Lond). 9:2703–2718.
2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Navarro G, Essex S, Sawant RR, Biswas S,
Nagesha D, Sridhar S, de ILarduya CT and Torchilin VP:
Phospholipid-modified polyethylenimine-based nanopreparations for
siRNA-mediated gene silencing: Implications for transfection and
the role of lipid components. Nanomedicine (Lond). 10:411–419.
2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik
AM, Agarwal RG and Lam KS: The effect of surface charge on in vivo
biodistribution of PEG-oligocholic acid based micellar
nanoparticles. Biomaterials. 32:3435–3446. 2011.PubMed/NCBI View Article : Google Scholar
|