Introduction
Silicosis is an interstitial pulmonary fibrosis
disease that is caused by inhalation of crystalline silica and is
characterized by inflammation and fibrosis of the lung (1,2).
Silicosis is the most common occupational disease in developing
countries, has become a global threat to human health and caused
wide public concern over recent years, with increasing incidence
and prevalence (3). In China, there
are >20,000 new cases of silicosis each year (4). Once silicosis occurs the damage is
irreversible, and the condition may continue to progress even after
the individual is removed from exposure to silica (5).
The crystalline silica, alpha quartz, is the major
cause of silicosis. Inhaled silica particles are deposited in lung
and distal airways and engulfed by alveolar macrophages. In turn,
macrophages are activated, and these provoke the abundant
generation of reactive oxygen species (ROS) and inflammatory
cytokines (6). The oxygen-based
free radicals generated from silica exposure are important
initiators of the fibrotic process, and the persistent inflammation
induced serves a key role in the pathogenesis of silica-induced
diseases (7,8). TGF-β1 is a pivotal mediator of
fibrosis and the TGF-β1 signal is transduced through the activation
of its down-stream effectors, the Smad proteins (9,10). It
has been previously demonstrated that TGF-β1 was able to induce
alveolar epithelial cells to undergo Epithelial to mesenchymal
transition (EMT) in vivo and in vitro via Smad2
activation (11). Together, these
factors stimulate the proliferation of lung fibroblasts, the
production of collagen and subsequently the formation of fibrosis
(2,12-14).
However, previous studies have demonstrated that antioxidants,
including vitamins, carotenoids and tannins provide protection
against oxidative damage, and the negative regulation of
inflammatory cytokines and/or the inflammatory signaling pathway
may attenuate the progression of some pulmonary diseases (15-18).
At present, there is no effective treatment for
silicosis. The conventional treatment used for patients is
symptomatic treatment. If the symptoms are serious, glucocorticoids
are used, and lung transplantation is the last treatment option
available for pulmonary fibrosis (19). Recently, increasing attention has
been paid to the anti-fibrotic effect of pirfenidone (PFD), which
is one of two approved therapies for the treatment of idiopathic
pulmonary fibrosis (IPF) (20). In
2014, PFD was approved for the treatment of the IPF by the U.S.
Food and Drug Administration (6).
However, PFD has not been widely used because of its side effects
and high price (21). It is
necessary to find more effective drugs for the treatment of
silicosis.
According to the pathogenesis of silicosis,
enhancing antioxidative and anti-inflammatory capacity may be a
promising approach to preventing silica-induced lung injury and
fibrosis (22). Currently, natural
products present a promising approach for the treatment of a
variety of acute and chronic inflammatory and fibrotic diseases
(23). Hesperetin (HSP) is a
natural flavonoid that exhibits a number of properties including
anti-inflammatory, antioxidative, anti-bacterial anti-tumoral
effects (24). HSP can target
multiple cell proteins that inhibit tumor growth, including
caspases, Bcl-2 and Bax-induced apoptosis, and is a promoter of
cellular antioxidant defence-related enzyme activity (25). Furthermore, HSP has been
demonstrated to exhibit beneficial effects in the treatment of
hypertension, diabetes and dyslipidemia, and has become a promising
drug candidate for the treatment of a cardiovascular diseases
(26). HSP has also been reported
to be a potential therapy for the treatment of inflammatory liver
diseases via its anti-inflammatory effects (27).
To the best of our knowledge, there have been no
studies performed that have investigated the effect of HSP on
silica-induced lung injury. Therefore, the current study aimed to
assess whether HSP exhibited protective effects against
silica-induced lung injury and fibrosis by alleviating oxidative
stress and inflammation.
Materials and methods
Animals and treatment
A total of 72 healthy male Wistar rats [specific
pathogen-free grade (SPF); 180-200 g; 6-8 weeks old] were provided
by Beijing Victoria Tong Lihua Experimental Animal Technology Co.,
Ltd. The rats were housed in SPF-grade laboratory conditions at
22±2˚C with an average relative humidity of 40-70% and a 12/12 h
light/dark cycle. All rats were kept in separate cages and had free
access to water and standard laboratory food. According to animal
ethics requirements (28),
appropriate measures were taken using pain management protocols to
reduce pain in the animals, and the relief of pain and distress
received careful attention during the experiment.
Rats were randomly divided into 6 groups with 12
rats in each, and each group was divided into 2 time points (7 and
28 days). The groups were as follows: Negative control group,
silica model group, PFD positive control group and 100, 200, 400
mg/kg HSP treatment groups. The µm-sized silicon dioxide was
prepared with normal saline as a 50 mg/ml silica suspension. All
groups excluding for the negative control group were injected with
1 ml 50 mg/kg silicon dioxide suspension into the lung once using a
non-exposed tracheal intubation, and the rats in the negative
control group were injected with the same volume of normal saline
solution. After 24 h, the clinical signs were examined, including
hair, breath and weight, and the rats in the PFD positive control
group and HSP treatment groups were given a daily intragastric
administration of 100 mg/kg PFD (Beijing Kangdini Pharmaceutical
Co., Ltd.) and 100, 200, 400 mg/kg HSP (Chengdu Kanghua
Pharmaceutical Co., Ltd.) for 7 and 28 days. The rats in the
negative control and silica model groups were treated with saline
only. On day 7 and 28 following silica exposure, 6 rats in each
group were euthanized with an overdose of 150 mg/kg sodium
pentobarbital (Merck & Co, Inc.) via intraperitoneal injection,
and death in all rats was via observation of the cessation of
respiration and palpation of the heartbeat. The lungs of each rat
were harvested and used for histological examination and the
determination of oxidative stress and inflammatory factors.
Histopathological examination of lung
tissue
Lungs of rats were fixed in 4% paraformaldehyde
solution at 4˚C for 24 h, embedded in paraffin and sectioned into 5
µm-thick slices. Subsequently, the tissue samples were stained with
hematoxylin and eosin (H&E; hematoxylin staining solution, 3-5
min; eosin staining solution, 5 min; both room temperature) and
Masson's trichrome (Weigert's iron hematoxylin solution, 5-10 min;
ponceau fuchsin acid solution for 5-10 min; 1% phosphomolybdic acid
aqueous solution for 3-5 min; aniline blue for 5 min; immersion in
0.2% acetic acid aqueous solution for 1 min; all at room
temperature). Slides were then examined under a light microscope to
assess the general morphology of the lung tissue. The degree of
alveolitis and pulmonary fibrosis was evaluated according to the
scoring system outlined in Szapiel et al (29). Alveolitis was graded using the
following criteria: None (0), no alveolitis; mild (1+), thickening
of the alveolar septum by a mononuclear cell infiltrate; moderate
(2+), a more widespread alveolitis; severe (3+), a diffuse
alveolitis. The extent of fibrosis was graded using the following
criteria: None (0), no fibrosis; mild (1+), focal regions of
fibrosis, alveolar architecture has some distortion; moderate (2+),
more extensive fibrosis and fibrotic still focal; severe (3+),
widespread fibrosis, confluent lesions with extensive derangement
of parenchymal architecture.
Determination of hydroxyproline (HYP)
content in lung tissue
As an important indicator of collagen level and the
severity of fibrosis, the content of HYP in the lung tissue was
assessed. The tissue was heated at a 95˚C water bath for 30 min and
HYP was measured using hydroxyproline assay kit as per the
manufacturer's protocol (cat. no. A030-2-1; Nanjing Jiancheng
Bioengineering Institute). For the control and standard samples,
water and 5 µg/ml kit standard were used, respectively. Each sample
was repeated three times. A spectrophotometer was used to measure
the absorbance of each sample at 550 nm.
Determination of oxidation indexes and
inflammatory factors
Rat lung tissue was homogenized, centrifuged at 4˚C
at 12,000 x g for 15 min and the supernatant was extracted. The
levels of malondialdehyde (MDA), the activities of superoxide
dismutase (SOD), glutathione peroxidase (GSH-PX), catalase (CAT)
and total antioxidant capacity (T-AOC) in the homogenate were
determined using commercial kits as per the manufacturer's
instructions (Nanjing Jiancheng Bioengineering Institute). The MDA
assay kit (cat. no. A003-1-2), the T-SOD assay kit (cat. no.
A001-1-2), the GSH-PX assay kit (cat. no. A005-1-2), the CAT assay
kit (cat. no. A007-1-1) and the T-AOC assay kit (cat. no. A015-1-2)
were used for the detection of oxidation indexes. The levels of
TGF-β1, IL-1β, IL-4, IL-10, TNF-α and IFN-γ in the lung tissue
homogenate of rats in each group were determined using ELISA as per
the manufacturer's instructions (eBioscience; Thermo Fisher
Scientific, Inc.). The TGF-β1 Rat ELISA kit (cat. no. BMS623-3),
the IL-1β Rat ELISA kit (cat. no. BMS630), the IL-4 Rat ELISA kit
(cat. no. ERA29RB), the IL-10 Rat ELISA kit (cat. no. ERA23RB), the
TNF-α Rat ELISA kit (cat. no. ERA56RB) and the IFN-γ Rat ELISA kit
(cat. no. BMS621) were used for the detection of inflammatory
factors.
Statistical analysis
All data were presented as mean ± SD, and were
analyzed using SPSS 22.0 software (IBM Corp.). Group comparisons
were performed using a one-way ANOVA followed by Tukey test for
normally distributed data and Kruskal-Wallis test and
Dunn-Bonferroni post-hoc test for non-normally distributed data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Rat phenotypes
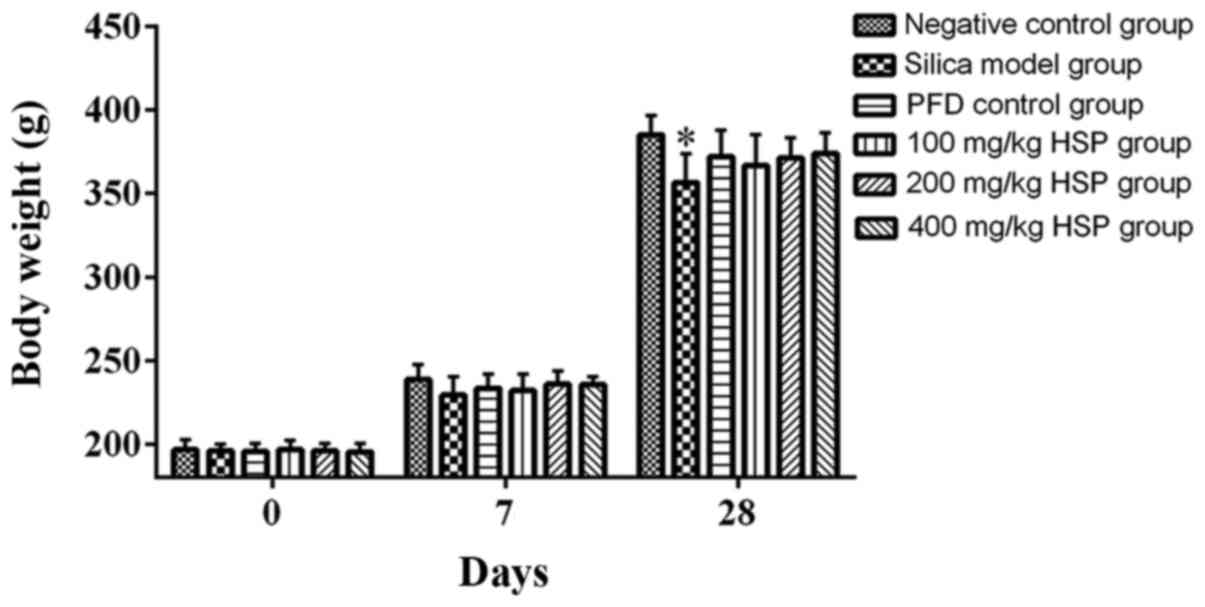
Following exposure to silica, compared with the
negative control group, the weight of the rats in the silica model
group was considerably reduced, but this was statistically
significant only on day 28 (P<0.05). However, the weights of the
rats from the PFD and HSP treatment groups were markedly increased
compared with the silica model group but there was no statistical
difference (P>0.05; Fig. 1).
Histopathological examination
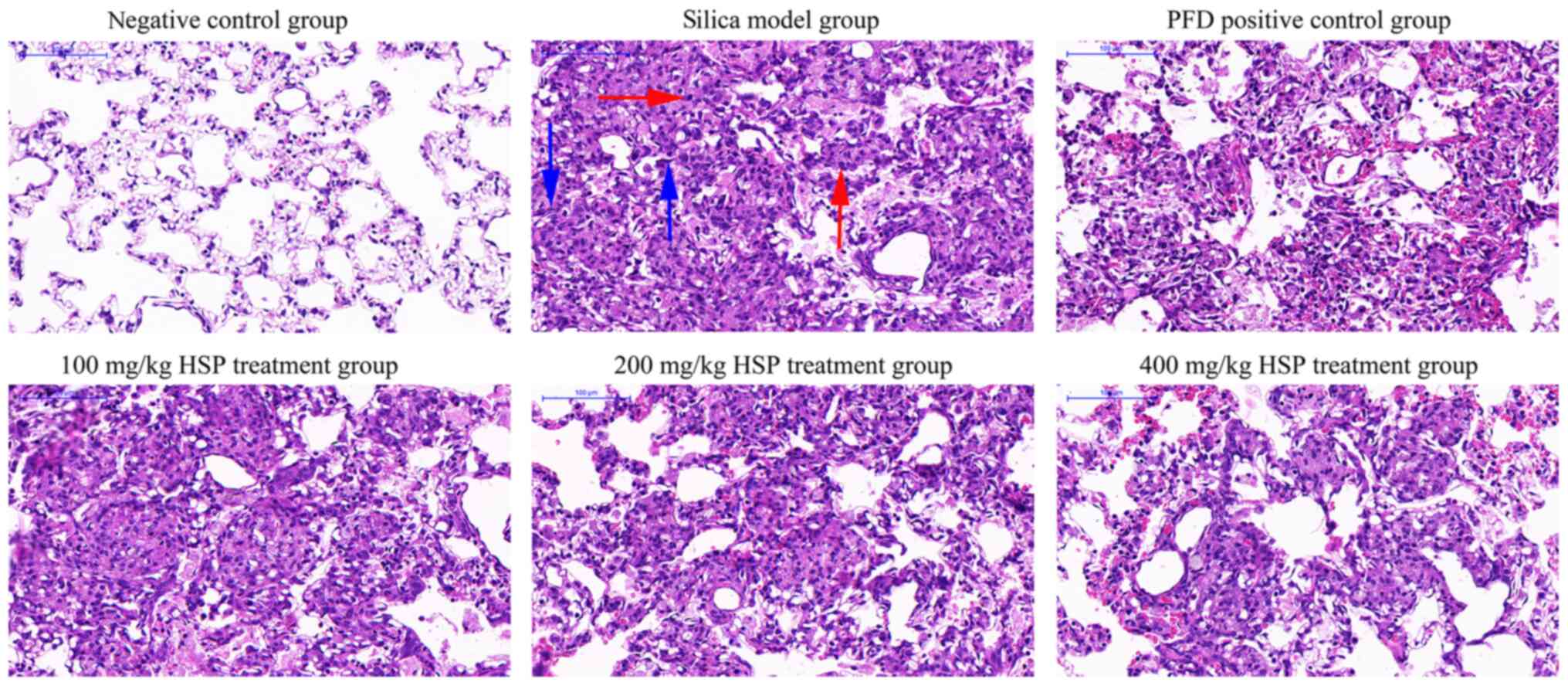
As presented in Fig.
2, after 7 days of silica exposure, histopathological
examination of rat lungs showed that lung tissues from the negative
control group were morphologically normal and the alveolar
structure was intact with a few inflammatory cells that had been
induced by saline. Conversely, tissue structure from rats in the
silica model and the PFD and HSP treatment groups were disrupted.
The walls of the alveoli and the alveolar septum were thickened,
and severe inflammatory cell infiltration was observed. The degree
of inflammatory cell infiltration in the lungs of rats in each
treatment group was reduced and the alveolar structure was more
intact compared with those in the silica model group. As presented
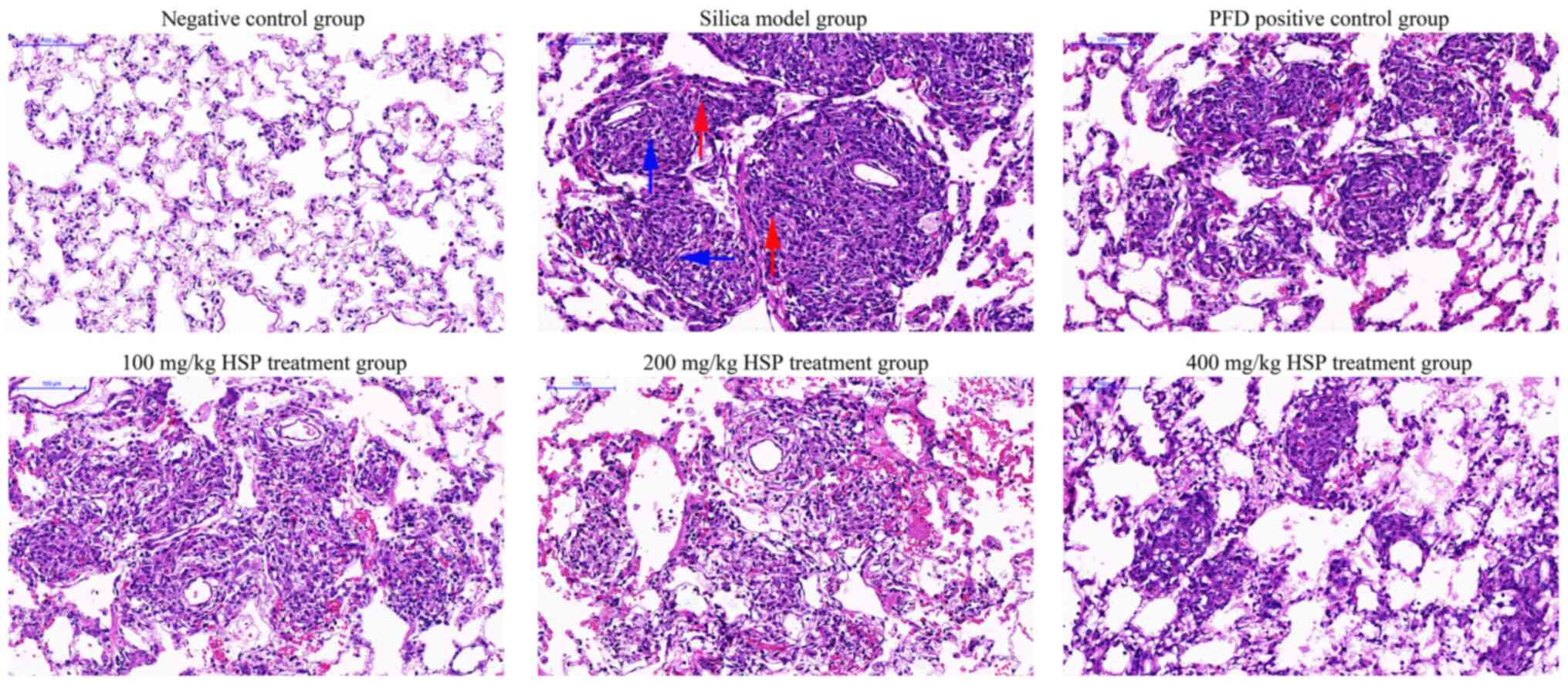
in Fig. 3, after 28 days of silica
exposure, H&E staining demonstrated that rat lung tissues in
the model group exhibited increased alveolar inflammation and
fibroblast development, and the alveolar structure was further
disrupted. The lung tissues from rats in the intervention groups
showed improvement compared with those from the silica model group.
Administration of PFD and HSP for 28 days ameliorated the
inflammatory infiltration and the damaged structure in the lung
tissue compared with the silica model group. Furthermore,
alleviation of lung injury was observed following increasing doses
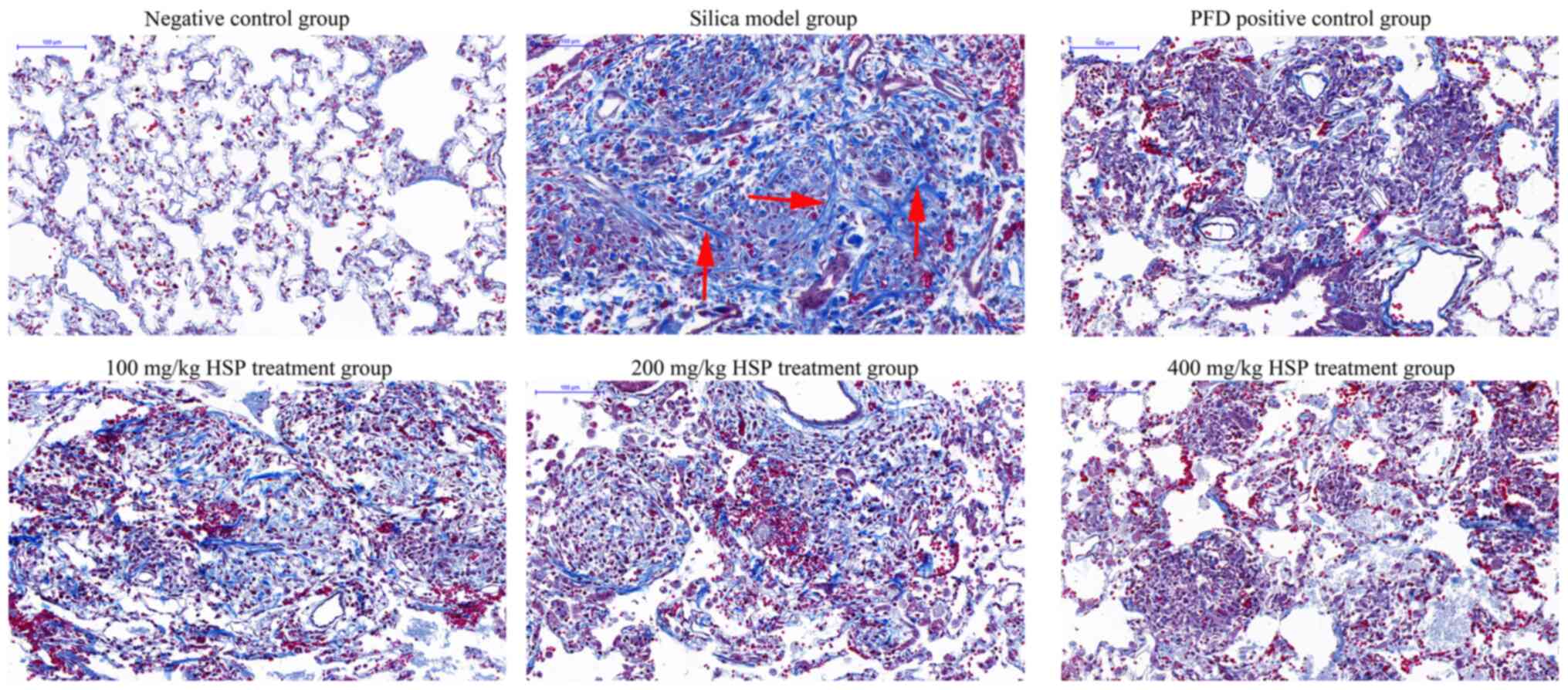
of HSP. As presented in Fig. 4,
Masson's trichrome stain revealed the alveolar walls were thickened
with more collagen deposition revealed in rats from the silica
model group compared with those in the negative control group on
the 28th day. In lung tissues from rats in the PFD and HSP
treatment groups, markedly less collagen deposition was observed
compared with the silica model group. A decrease in the deposition
of collagen in the lungs with increasing dosages of HSP was also
observed.
Lung tissue alveolitis and fibrosis
severity score
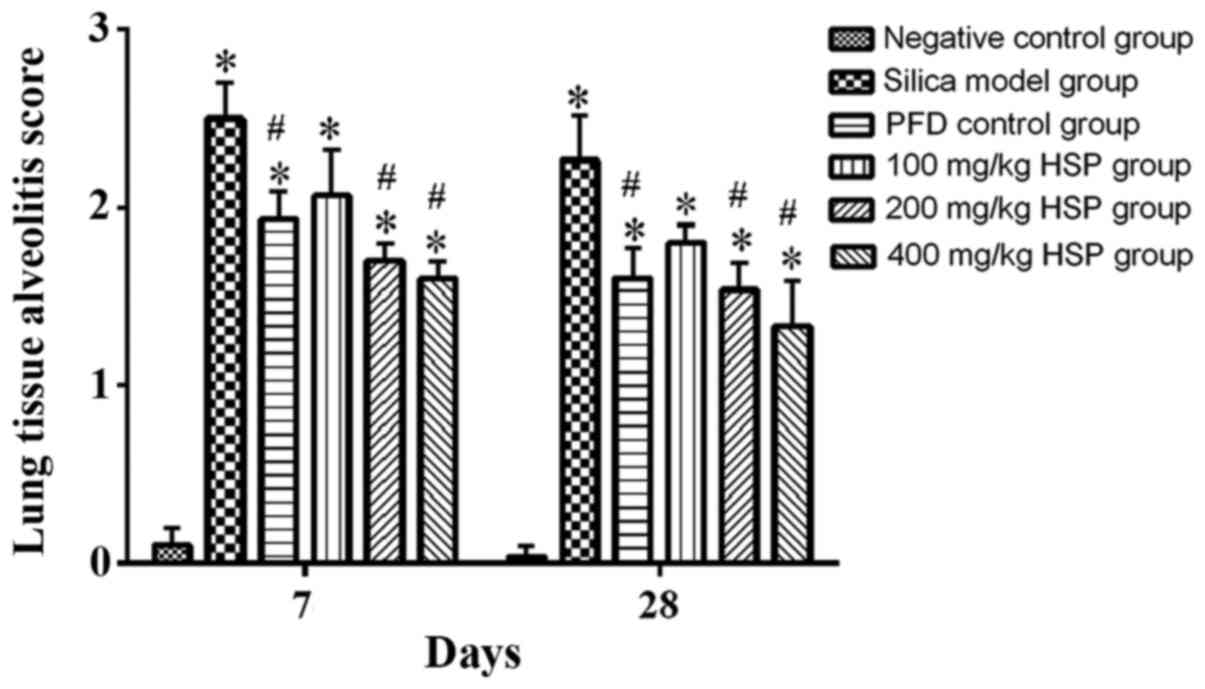
According to the method proposed by Szapiel et
al (29), the degree of
alveolitis and fibrosis in rat lung tissues with silica was
evaluated. The alveolitis score of the silica model group was
significantly higher compared with the negative control group
(P<0.05). The alveolitis scores of the PFD positive control
group, 100, 200 and 400 mg/kg HSP treatment groups were lower
compared with the silica model group on the 7 and 28th day
following treatment, but there was no statistical difference
between the 100 mg/kg HSP treatment group and the silica model
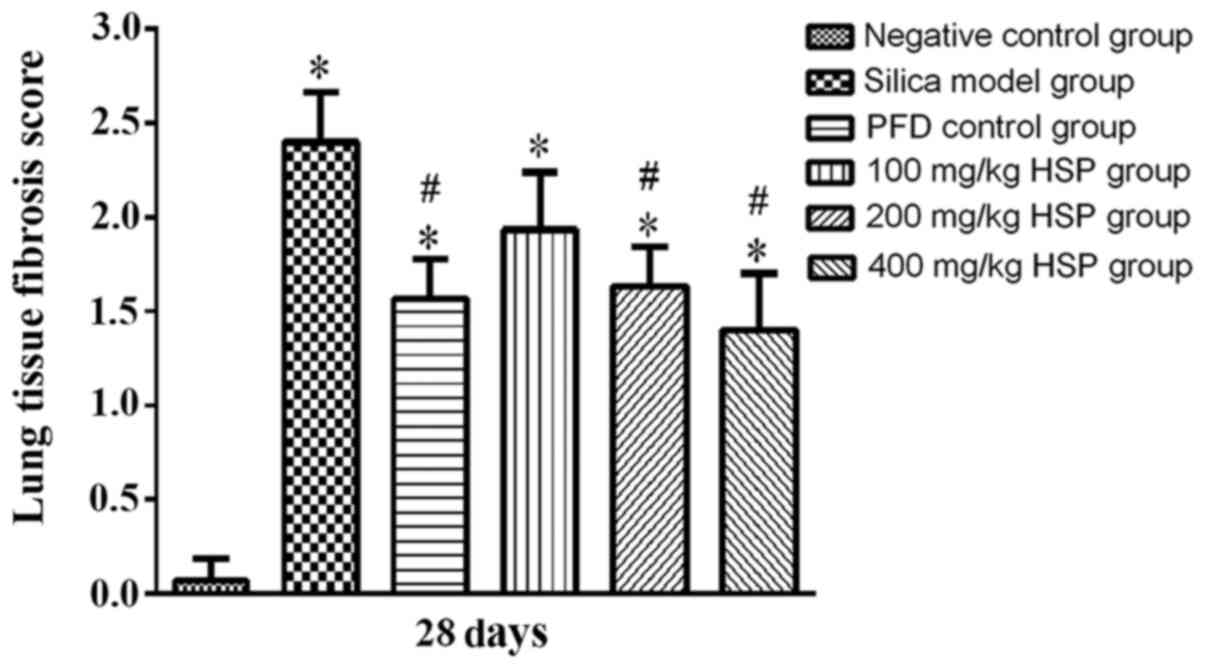
group on the 7 and 28th day (P>0.05; Fig. 5). The pulmonary fibrosis score of
the silica model group on the 28th day was significantly higher
compared with the negative control group (P<0.05). The pulmonary
fibrosis scores of the PFD positive control group and 200 and 400
mg/kg HSP treatment groups on the 28th day were lower compared with
the silica model group (P<0.05). The pulmonary fibrosis score of
the 100 mg/kg HSP treatment group was lower compared with the model
group, but there was no statistical difference (P>0.05; Fig. 6). In addition, numerous infiltrating
inflammatory cells and inflammatory reactions in the lung tissues
exposed to silica for 7 days have been reported, while in the lungs
exposed to silica for 28 days, massive proliferation of collagen
fibers and pulmonary fibrosis has been observed (30). Therefore, the pulmonary fibrosis
score was only assessed on the 28th day in this experiment.
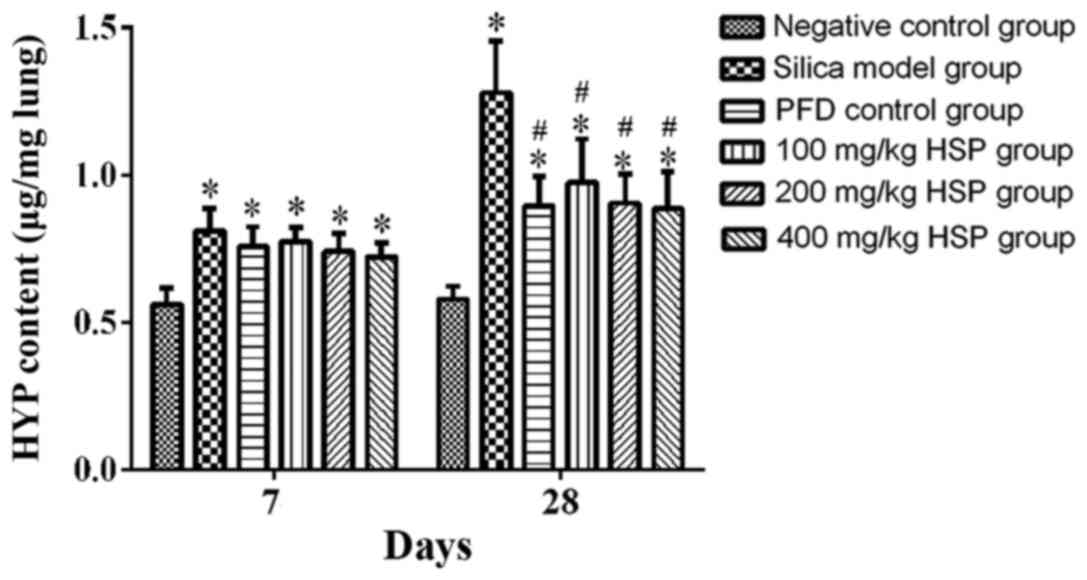
Effect of HSP on the content of HYP in
rat lung tissue
The content of HYP in the lung tissue from rats in
the silica model group was significantly higher compared with the
negative control group (P<0.05) on the 7 and 28th day following
treatment. Following PFD and HSP treatment, the content of HYP in
the lungs of rats was lower compared with the silica model group on
the 28th day (P<0.05; Fig.
7).
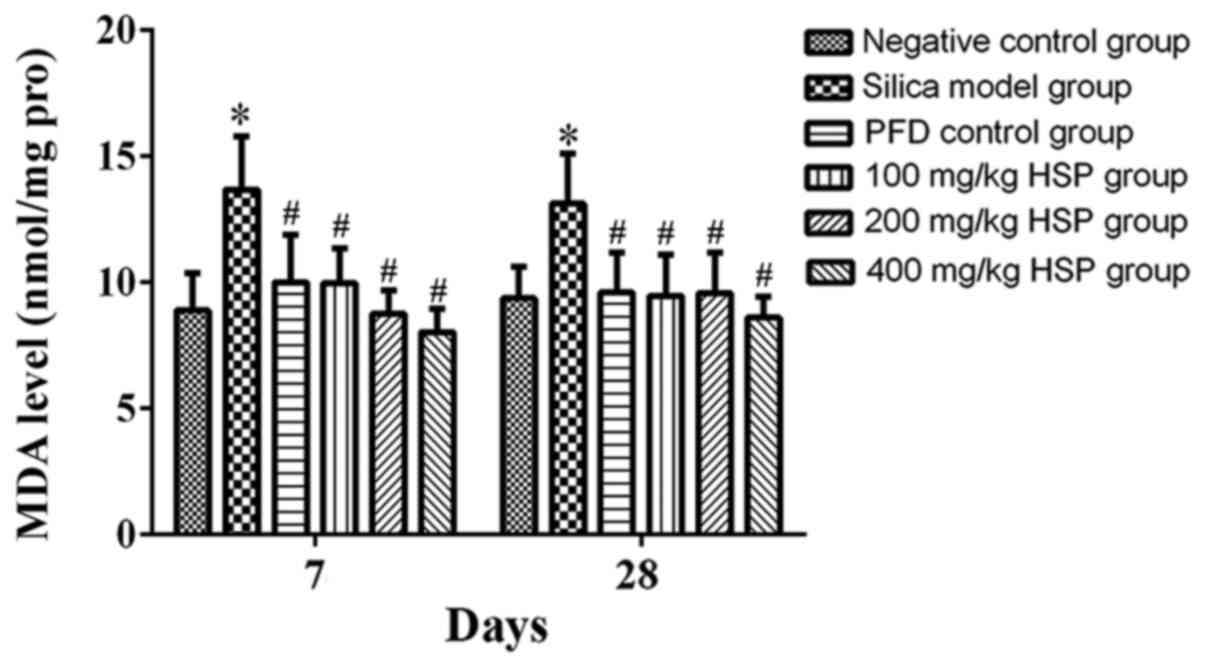
Analysis of MDA level, SOD, CAT,
GSH-Px and T-AOC activity in lung tissue Effect of HSP on the level
of MDA in lung tissue of silica exposed rats
On the 7 and 28th days after exposure, the level of
MDA in the silica model group was significantly higher compared
with the negative control group (P<0.05). The level of MDA in
the PFD and all HSP treatment groups was significantly decreased
(P<0.05) compared with the silica model group (Fig. 8).
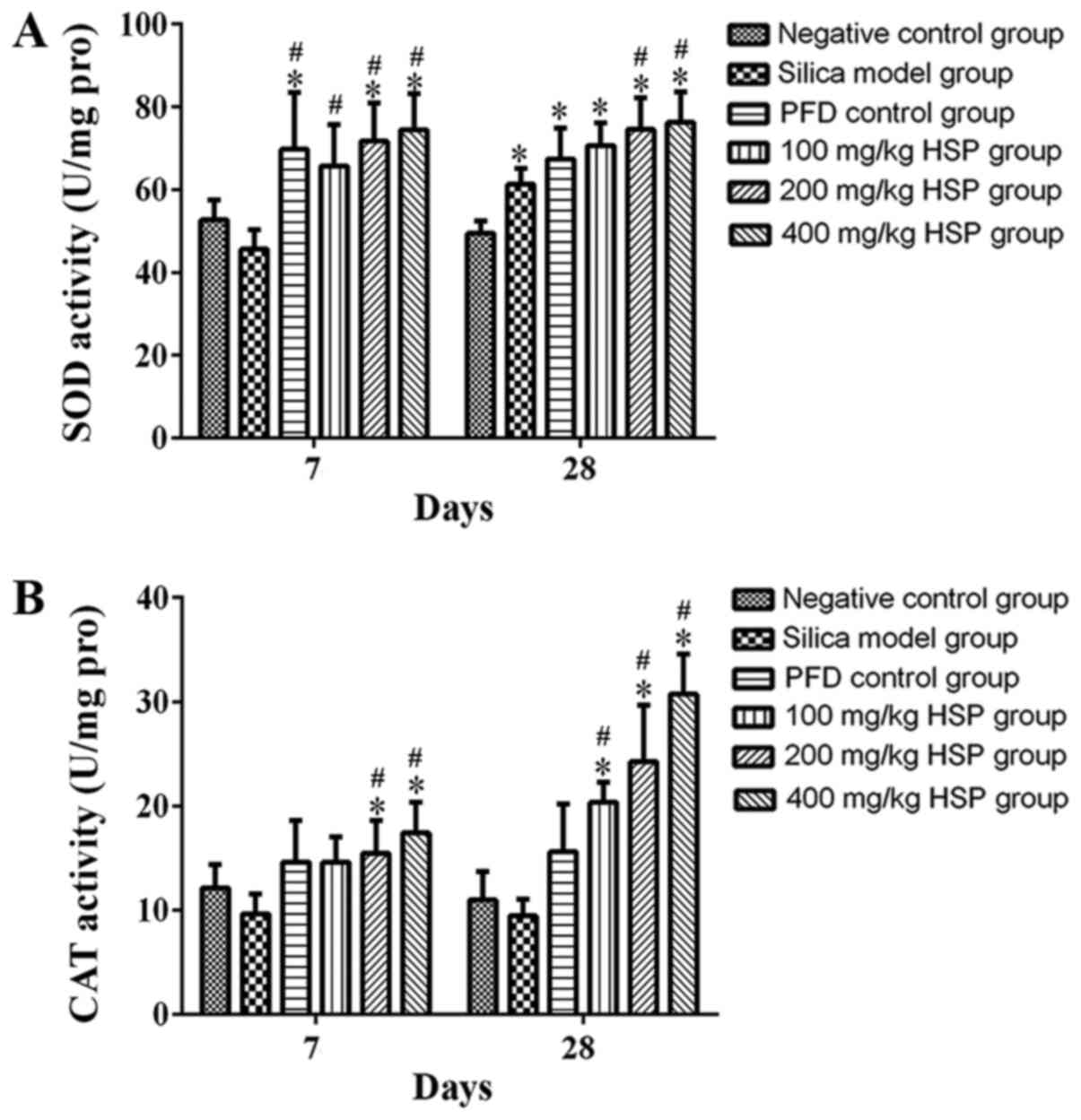
Effect of HSP on the activity of SOD
and CAT in lung tissue of silica exposed rats
Compared with the negative control group, the SOD
activity in the silica model group was markedly lower on the 7th
day, but there was no statistical difference (P>0.05). However,
the SOD activity in silica model group was significantly higher
compared with the negative control group on the 28th day
(P<0.05; Fig. 9A). Compared with
the silica model group, no statistically significant difference in
SOD activity was observed in the PFD and 100 mg/kg HSP groups on
the 28th day, but in the PFD and all the three dosages of HSP
treatment group on the 7th day and 200 and 400 mg/kg HSP groups on
the 28th day, the activity of SOD was significantly increased
(P<0.05). There was no statistical difference in the CAT
activity between the silica model group and the negative control
group on the 7 and 28th days (P>0.05). Following treatment with
200 and 400 mg/kg HSP on the 7th day and with all the three dosages
of HSP on the 28th day, the activity of CAT significantly increased
(P<0.05). On the contrary, in the PFD and 100 mg/kg HSP groups,
the activity of CAT was markedly higher than the silica model group
on the 7th day, but there was no statistical difference (P>0.05;
Fig. 9A and B).
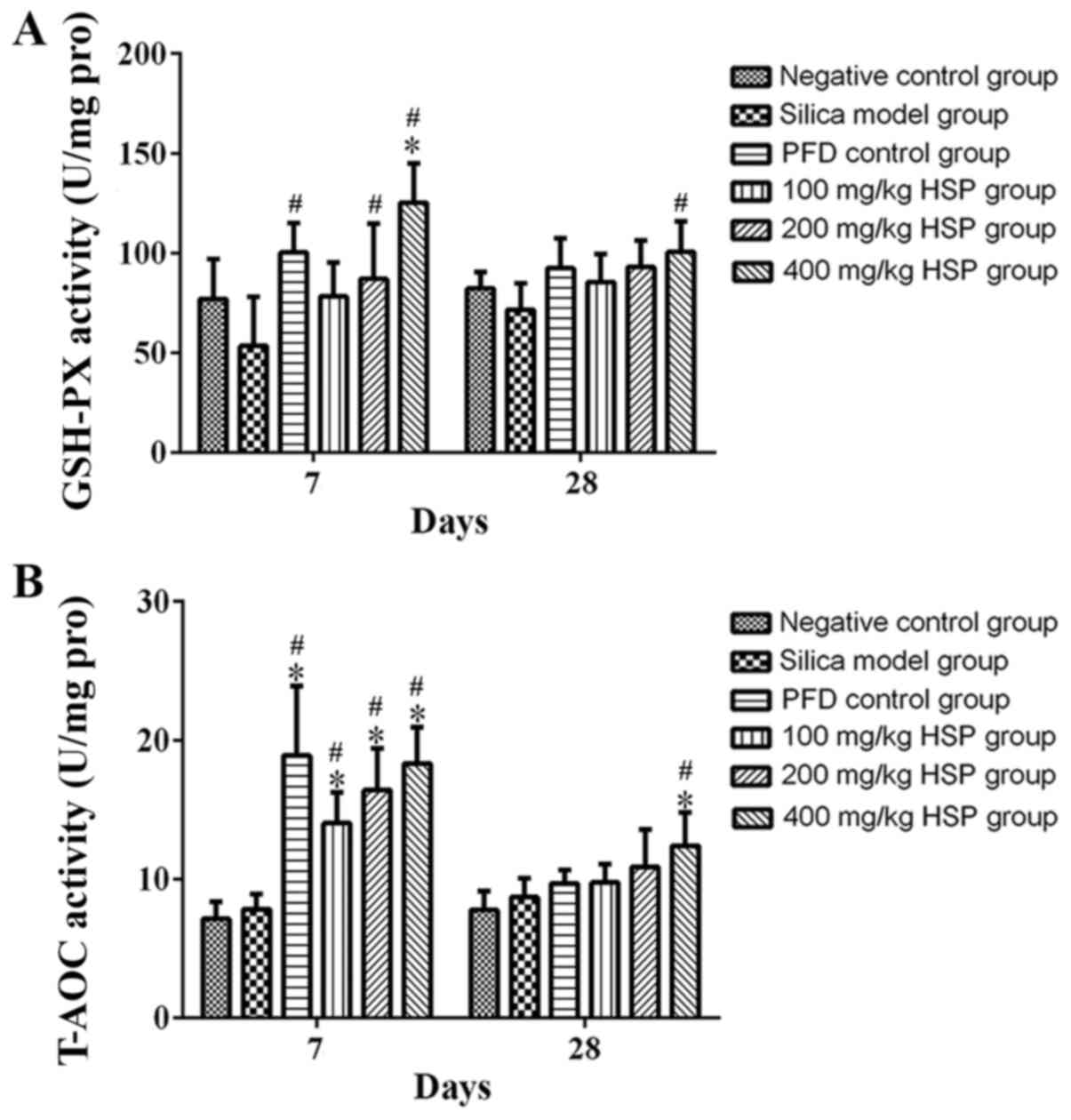
Effect of HSP on the activity of
GSH-Px and T-AOC in lung tissue of silica exposed rats
On the 7 and 28th days after exposure, there was no
statistical difference in the activity of GSH-Px and T-AOC between
silica model group and the negative control group (P>0.05).
After HSP treatment, it was revealed that GSH-Px activity in each
group increased, and a significant difference was observed between
the PFD positive control group and the 200 and 400 mg/kg HSP groups
compared with silica model group on the 7th day (P<0.05), but
there was no statistical difference between the silica model group
and the 100 mg/kg HSP group (P>0.05). It was clear that as the
dose of HSP increased, the activity of GSH-Px also increased. An
increasing trend of T-AOC levels in the 400 mg/kg HSP group was
observed compared with the negative control group and silica model
group on the 28th day (P<0.05), while it was also increased in
the PFD positive control group and 100 and 200 mg/kg HSP groups on
the 28th day, but there was no statistical difference compared with
the silica model group (P>0.05). In addition, compared with the
model group, the T-AOC activity of the PFD positive control group
and all the three dosages of HSP treatment group on the 7th day was
significantly increased (P<0.05; Fig. 10A and B).
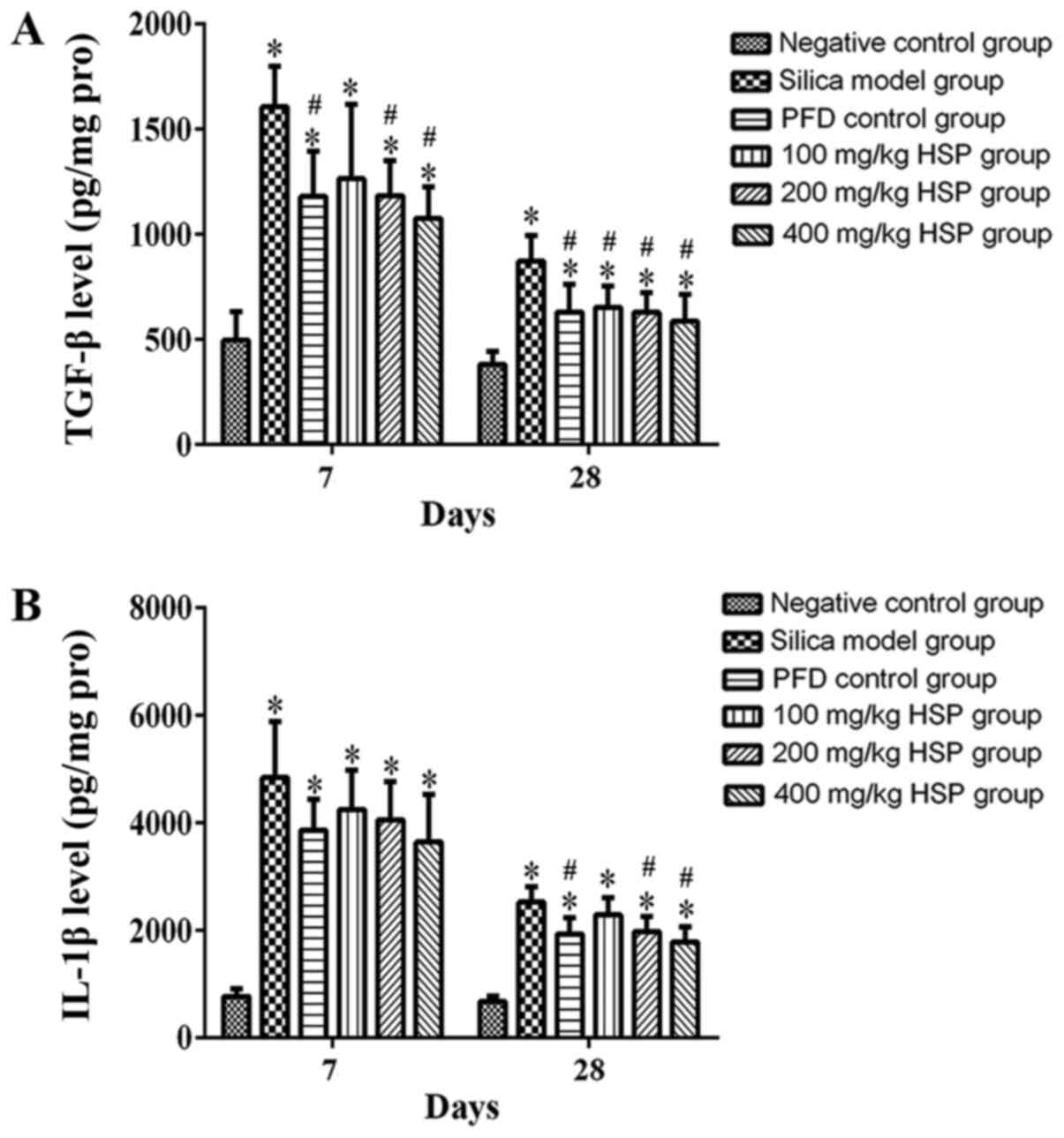
Analysis of TGF-β1, IL-1β, IL-4,
IL-10, TNF-α and IFN-γ levels in lung tissue Effect of HSP on the
levels of TGF-β1 and IL-1β in lung tissue of silica exposed
rats
On the 7 and 28th days after silica exposure,
significantly higher levels of TGF-β1 and IL-1β were observed in
the silica model group compared with the negative control group
(Fig. 11A and B). Compared with the silica model group,
except for the 100 mg/kg HSP group on the 7th day, after treatment
with PFD and HSP, the level of TGF-β1 was significantly lower
compared with the silica model group (P<0.05). The level of
IL-1β decreased on the 7th day after treatment with PFD and HSP,
but there was no statistical difference compared with the silica
model group (P>0.05). On the 28th day, except for the 100 mg/kg
HSP group, no significant difference was detected, and the levels
of IL-1β in the PFD, 200 and 400 mg/kg groups were significantly
reduced (P<0.05; Fig. 11A and
B).
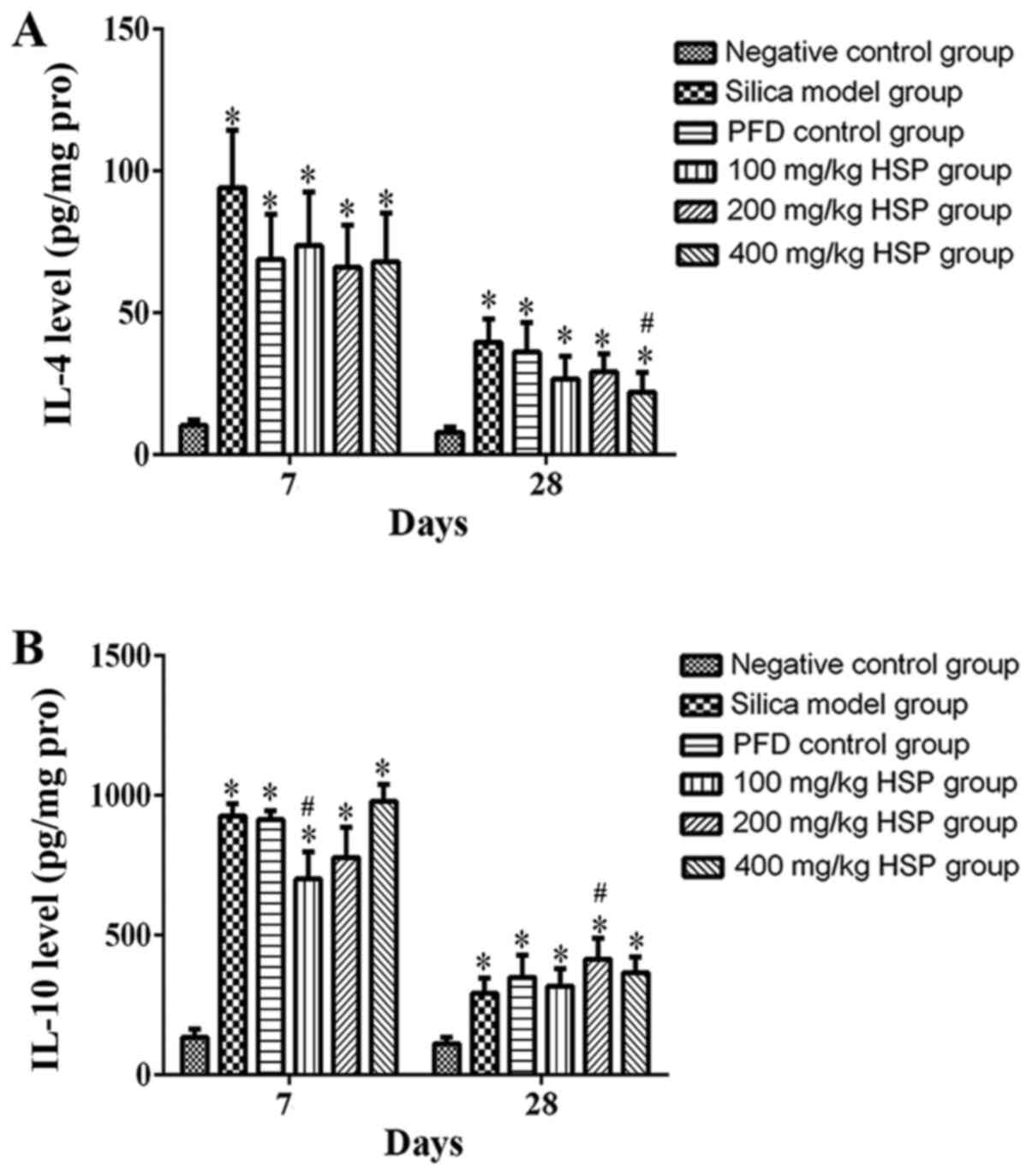
Effect of HSP on the levels of IL-4
and IL-10 in lung tissue of silica exposed rats
On the 7 and 28th days after silica exposure, the
levels of IL-4 and IL-10 in the lung tissue of rats in the silica
model group were significantly higher compared with the negative
control group (P<0.05). On the 7 and 28th days after HSP
treatment, the level of IL-4 decreased significantly, but there was
only a statistical difference between the 400 mg/kg HSP group and
the silica model group on the 28th day (P<0.05). Compared with
silica model group, the levels of IL-10 in the 100 mg/kg HSP
treatment group decreased on the 7th day (P<0.05), but there
were no significant differences in the PFD, 200 and 400 mg/kg HSP
groups on the 7th day and in the PFD, 100 and 400 mg/kg HSP groups
on the 28th day. (P>0.05; Fig.
12A and B).
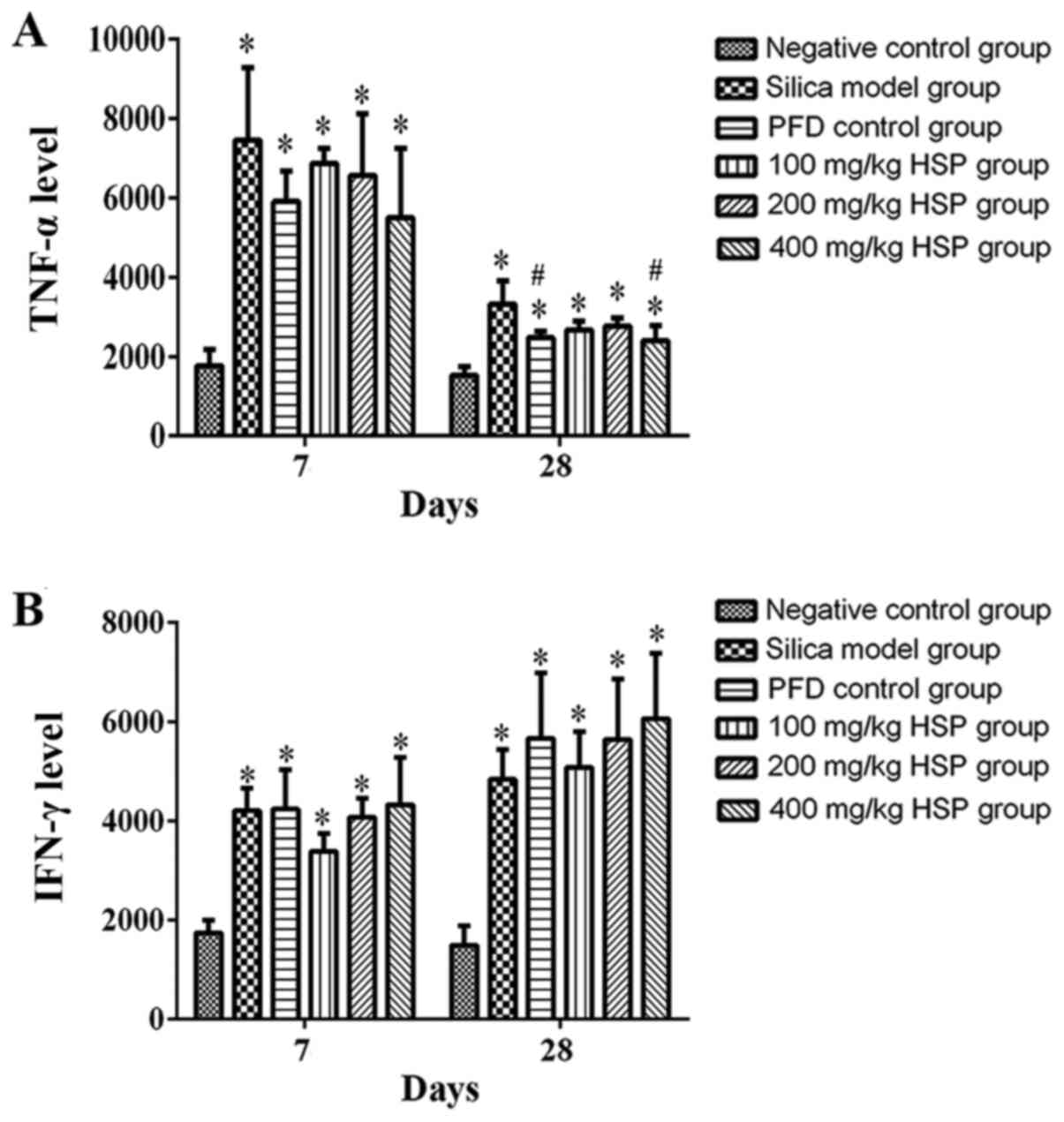
Effect of HSP on the levels of TNF-α
and IFN-γ in lung tissue of silica exposed rats
On the 7 and 28th days following silica exposure,
the levels of TNF-α and IFN-γ in lung tissue of rats in the silica
model group was significantly higher compared with the negative
control group (P<0.05). Following treatment with PFD and all HSP
levels, the level of TNF-α was markedly lower compared with the
silica model group. This was only significant in the high (400
mg/kg) dose group of HSP and the PFD group as compared with the
silica model group (P<0.05) on the 28th day. Except for the 100
mg/kg HSP group on the 7th day, the IFN-γ level of each treatment
group was markedly higher compared with the silica model group, but
there were no statistical differences observed (P>0.05). The
general increases between the silica model and the treatment groups
were markedly higher on the 28th day following silica exposure
compared with the 7th day (Fig.
13A and B).
Discussion
The pathological characteristics of silica-induced
pulmonary fibrosis at the primary stage include alveolitis,
pulmonary edema and infiltration of inflammatory cells (6,31).
Developments of this disease include increased fibroblast
proliferation and excessive collagen deposition leading to
pulmonary fibrosis (32,33). In the present study, two different
time points in the animal model were used: Days 7 and 28
post-silica exposure. The 7th day following silica exposure
represents the early inflammatory stage, and the 28th day
represents the later fibrotic stage (34,35).
The aim of the current study was to demonstrate the pathological
process of silica exposure. Oxidative stress and inflammatory
response are considered to be two important mechanisms of
silica-induced lung injury (36).
Oxidative stress can induce a inflammatory response by activating
specific transcription factors, including NF-κB and Activator
protein 1 (37,38), and the inflammatory response in
return exacerbates oxidative stress, contributing to excessive ROS
generation in a number of different cell types following stimuli
(9). An inflammatory response can
accelerate the progression of fibrosis through mast cells, which
promote fibrosis by recruiting inflammatory cells to sites of
damage (39). In the process of
silica exposure, the cytokines interact and depend on each other to
form a complex cytokine network that participates in the process of
lung tissue injury (40). HYP is an
amino acid that forms a major component of the protein collagen and
is commonly used as a marker to measure the levels of collagen
present (41). The results of the
current study indicated that the content of HYP in rat lung tissues
increased significantly following silica exposure. This was further
increased in the fibrotic stage as compared to that during early
inflammatory stage, which supports the observation that silica
exposure leads to pulmonary fibrosis in rats. HSP treatment was
shown to effectively reduce the content of HYP possibly by
inhibiting the release of inflammatory factors to prevent the
proliferation of fibroblasts. This is consistent with the
histopathological findings of the current study.
As previously reported, when respirable crystalline
silica particles are inhaled, they are able to reach the alveoli,
inducing oxidative stress via the formation of ROS and nitrogen
species (42,43). MDA, which is a secondary product of
lipid peroxidation, is a useful biomarker to evaluate oxidative
stress (31). The current study
demonstrated that the level of MDA in lung tissue significantly
increased following 7 and 28 days of exposure to silica. SOD, CAT
and GSH-Px are important antioxidant enzymes in cells and their
activities can affect cellular capacity to scavenge free radicals
and regulative oxidative stress (44). A previous study revealed that with a
significant increase in lipid peroxidation in the venous blood
samples, the activities of SOD and CAT were decreased in silicosis
workers (45). In the present
study, the activity of SOD was indicated to be markedly lower in
lung tissue from rats in silica model group compared with the
negative control group on the 7th day. However, activity of SOD
increased in the silica model group compared with the negative
control group on the 28th day. However, after treatment with
different dosages of HSP, and especially with 400 mg/kg HSP, the
activity of SOD increased compared with the model group. The
present study also revealed that early invention using HSP was more
effective on SOD activity during the early stage of lung injury
compared with at the later stage of fibrosis. The results also
demonstrated that the activity of CAT increased significantly in a
dose-dependent manner following HSP treatment. This suggests that
HSP inhibits oxidative damage by increasing the activity of
antioxidant enzymes in the early stages of lung injury.
GSH is a major cellular antioxidant defense and
serves an important role in scavenging free radicals and other ROS
(46). Enhancing GSH and associated
enzymes has previously been used as a therapeutic strategy for a
number of different diseases including Alzheimer's disease, cancer,
liver diseases, cardiovascular diseases, arthritis and diabetes
(47-51).
The current study indicated that silica-induced oxidative stress
may result in the depletion of GSH, and the lack of GSH renders
cells to be more susceptible to the effects of oxidants (52,53).
It was also revealed that silica decreased the activity of GSH-Px,
which in turn aggravated lung toxicity. In the early stage of lung
injury, it was clear that GSH-Px activity increased after treatment
with 200 and 400 mg/kg doses of HSP. T-AOC is an index used to
reflect the body's antioxidant capacity (54). The results of the present study
indicated that the activity level of T-AOC in the HSP treatment
group was significantly higher compared with the negative control
group and silica model group on the 7th day of the model. This
suggested that HSP was able to maintain the antioxidant capacity of
the body at a high level. HSP has previously been considered to
exhibit potent antioxidant activities. There has been previous
evidence that HSP increases cellular antioxidant defense capacity
via ERK/Nrf2 signaling and enhances the activity levels of
antioxidant enzymes, such as CAT, SOD and GST in oxidative stress
related-hepatocyte injury and liver dysfunction (55). HSP can also inhibit STZ-induced
oxidative stress by increasing the activities of antioxidant
enzymes (SOD, GSH-Px, GPX, GRX and CAT) and reducing the level of
MDA in the hippocampus, providing evidence that it is of potential
therapeutic value for Alzheimer's disease (56). The current study also demonstrated
that HSP exhibits therapeutic potential for silica exposure via its
strong antioxidant capacity.
In addition to oxidative stress, inflammation has
been reported to be a contributing mechanism of lung injury
(57). Under oxidative stress,
immune cells are induced to produce and secrete inflammatory
factors to stimulate inflammation, leading to pneumonia and
fibrosis (58). At present, a
number of studies have confirmed that a variety of cytokines are
associated with the pathogenesis of silica exposure and these
include TGF-β1, IL-1β and TNF-α (59,60).
TGF-β1 serves an important role in the formation and development of
pulmonary fibrosis, and acts as a stimulus signal in the repair of
cell injury and the formation of fibrosis (61,62).
TGF-β1 can induce and promote fibroblasts to transform into
myofibroblasts, promote the precipitation of extracellular matrix,
such as collagen, and inhibit its degradation (63). TGF-β1 can also induce EMT of lung
epithelial cells via the TGF-β/Smad2 signaling pathway (64). The results of the present study
demonstrated that in the early injury stage of silica exposure, the
level of TGF-β1 in the silica model group was significantly higher
compared with the negative control group. The inhibitory effect of
HSP in each treatment group was more significant with increasing
dosages, suggesting that HSP can inhibit the synthesis and
secretion of fibrogenic factor TGF-β1 in a dose-dependent manner.
IL-1β and TNF-α are produced by macrophages as proinflammatory
factors that can mediate the release of other cytokines and
inflammatory mediators, thereby promoting the proliferation and
differentiation of fibroblasts (65). In the current study, the levels of
IL-1β and TNF-α increased following silica exposure, and the levels
of these inflammatory factors decreased in the 400 mg/kg HSP group
on the 28th day. Previous studies have also revealed that HSP can
reduce the levels of TNF-α and IL-6, and protect TNBS-induced
colitis model via its antioxidation, anti-inflammatory and
anti-apoptotic effects (66). It is
considered that HSP exhibits a protective effect on many diseases
by exerting anti-inflammatory effects (67). Recent evidence has suggested the use
of HSP derivatives to evaluate anti-inflammatory effects (68). IL-4 is a proinflammatory factor,
which can antagonize IFN-γ to promote the proliferation of
fibroblasts, the production of extracellular matrix and increase
the synthesis of collagen fibers (69-72).
IFN-γ is an effective anti-fibrotic factor due to its
anti-proliferation and immunosuppressive effects in the regulation
of inflammation (73,74). IL-10, which is another
anti-inflammatory cytokine, has been revealed to serve a role in
blocking macrophage metabolism, promoting mitochondrial autophagy
and inhibiting the synthesis of cytokines (75). IL-10 also exhibits an anti-fibrotic
activity by reducing the production of type I collagen that is
stimulated by TGF-β (76,77). The results of the current study
demonstrated that the level of IL-4 in the model group during the
early injury stage was significantly higher compared with the
negative control, PFD and all HSP treatment groups. Additionally,
the levels of IL-10 and IFN-γ in lung tissue of model group were
markedly increased in the early injury stage, suggesting that they
increased in a compensatory manner and inhibited the levels of
inflammatory factors in lung tissue of early silica exposed rats
following injury. After treatment with 400 mg/kg HSP on the 28th
day, the level of IL-4 decreased compared with the silica model
group. During the late fibrotic stage (28th day), IFN-γ levels in
rat lung tissue increased compared with the model group, suggesting
that PFD and HSP may serve an anti-fibrotic role by increasing
IFN-γ levels. Combined with the results of lung histopathology, it
can be suggested that inflammatory factors serve a key role in the
early stages of lung injury, and HSP may alleviate silica-induced
lung injury in rats by reducing the level of inflammatory factors
and increasing anti-inflammatory factors.
A previous report has been carried out on the
treatment of silica-induced lung injury; however the treatment
methods used are still not effective (78). PFD is an anti-fibrotic drug that is
used clinically to treat mild to moderate IPF in a number of
different countries (79).
Recently, Zou et al (80)
revealed that PFD could inhibit the expression of Wnt/β-catenin
signaling proteins and decrease the risk of lung cancer in patients
with pulmonary fibrosis. A previous study has indicated that
although PFD can reduce pulmonary fibrosis in rats with silica
exposure, it cannot significantly increase their survival rate
(81). However, PFD's mechanism of
action remains largely unknown, and it is contraindicated in
patients with severe hepatic injury and severe chronic renal
failure (21). A previous clinical
study has indicated that treatment of patients with this drug may
bring about side effects associated with the gastrointestinal tract
(nausea, emesis, abdominal discomfort, dyspepsia and diarrhea) and
the skin (photosensitivity and rash) (21). Building on those previous studies,
and in order to facilitate comparison, the current study
established a PFD group as a positive control group to investigate
the function of HSP. In recent years certain native compounds have
been used to examine protective effect to silica-induced lung
injury. For examples, previous studies have reported that HSP is a
native compound derived from citrus fruit, which is reported to
possess a number of different properties, including antitumor,
antioxidant, anti-inflammatory and lipid lowering effects (82-84).
Kumar et al (44) indicated
that the therapeutic effects of HSP rectified retinal
neuroinflammation, oxidative stress and oedema caused by chronic
uncontrolled hyperglycaemic. Furthermore, HSP has been reported to
be a potential anti-inflammatory and neuroprotective agent. A
similar study has indicated that treatment with HSP can inhibit the
NF-κB and ERK signaling pathways through its antioxidant and
anti-inflammatory activities, attenuating neuropathic pain that is
induced by partial sciatic nerve ligation in rats (85). The current study demonstrated that
HSP exhibits protective effects on lung injury in silica exposed
rats via antioxidative and anti-inflammatory effects.
In conclusion, the results of the current study
revealed that HSP can effectively inhibit the secretion of
oxidative and inflammatory factors while increasing antioxidant and
anti-inflammatory capacity to protect lung injury. According to the
results of the present study, with increasing doses of HSP, the
degree of lung injury gradually improved, and the medium (200
mg/kg) and high (400 mg/kg) doses of HSP were more effective in
preventing lung injury. The current study provides a reliable
pharmacological basis for the development and application of HSP in
the future and as a potential therapeutic agent for the treatment
of silicosis.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Key Research
and Development Plan of Shandong Province (grant no.
2016GSF201047), Shandong Traditional Chinese Medicine Science and
Technology Development Plan Project (grant no. 2015-328), National
Science Foundation of Shandong Province (grant no. ZR2019MH102),
Science and Technology Development Plan of Jinan City (grant no.
201907061), and the Innovation Project of Shandong Academy of
Medical Sciences.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GY and HS designed the study and confirmed the
authenticity of the raw data. LS and SL performed the experiments
and wrote the manuscript. JF, JZ and YC analyzed the data. AJY and
MFL revised the manuscript critically for important intellectual
content and were also involved in the conception of the study. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of Shandong Academy of Occupational Health and
Occupational Medicine (Jinan, China; approval no. 2018DL023).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bissonnette E and Rola-Pleszczynski M:
Pulmonary inflammation and fibrosis in a murine model of asbestosis
and silicosis. Possible role of tumor necrosis factor.
Inflammation. 13:329–339. 1989.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fubini B and Hubbard A: Reactive oxygen
species (ROS) and reactive nitrogen species (RNS) generation by
silica in inflammation and fibrosis. Free Radic Biol Med.
34:1507–1516. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lopes-Pacheco M, Bandeira E and Morales
MM: Cell-based therapy for silicosis. Stem Cells Int.
2016(5091838)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen JJ, Chen L, Liu W and Wang SX:
Effects of Gymnadenia conopse alcohol extract on early protein
profiles in lung tissue of rats exposed to silica. Zhonghua Lao
Dong Wei Sheng Zhi Ye Bing Za Zhi. 30:432–435. 2012.PubMed/NCBI(In Chinese).
|
|
5
|
Fernandez Alvarez R, Martinez Gonzalez C,
Quero Martinez A, Blanco Perez JJ, Carazo Fernandez L and Prieto
Fernandez A: Guidelines for the diagnosis and monitoring of
silicosis. Arch Bronconeumol. 51:86–93. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rimal B, Greenberg AK and Rom WN: Basic
pathogenetic mechanisms in silicosis: Current understanding. Curr
Opin Pulm Med. 11:169–173. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vallyathan V, Shi XL, Dalal NS, Irr W and
Castranova V: Generation of free radicals from freshly fractured
silica dust. Potential role in acute silica-induced lung injury. Am
Rev Respir Dis. 138:1213–1219. 1988.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ghio AJ, Kennedy TP, Whorton AR, Crumbliss
AL, Hatch GE and Hoidal JR: Role of surface complexed iron in
oxidant generation and lung inflammation induced by silicates. Am J
Physiol. 263:L511–L518. 1992.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Elmarakby AA and Sullivan JC: Relationship
between oxidative stress and inflammatory cytokines in diabetic
nephropathy. Cardiovasc Ther. 30:49–59. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu MW, Liu R, Wu HY, Li YY, Su MX, Dong
MN, Zhang W and Qian CY: Radix puerariae extracts ameliorate
paraquat-induced pulmonary fibrosis by attenuating follistatin-like
1 and nuclear factor erythroid 2p45-related factor-2 signalling
pathways through downregulation of miRNA-21 expression. BMC
Complement Altern Med. 16(11)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G,
Vaziri ND and Zhao YY: New insights into TGF-β/Smad signaling in
tissue fibrosis. Chem Biol Interact. 292:76–83. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Borensztajn K, Crestani B and Kolb M:
Idiopathic pulmonary fibrosis: From epithelial injury to
biomarkers-insights from the bench side. Respiration. 86:441–452.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kusaka T, Nakayama M, Nakamura K, Ishimiya
M, Furusawa E and Ogasawara K: Effect of silica particle size on
macrophage inflammatory responses. PLoS One.
9(e92634)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wintergerst ES, Maggini S and Hornig DH:
Contribution of selected vitamins and trace elements to immune
function. Ann Nutr Metab. 51:301–323. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ribeiro D, Freitas M, Silva AMS, Carvalho
F and Fernandes E: Antioxidant and pro-oxidant activities of
carotenoids and their oxidation products. Food Chem Toxicol.
120:681–699. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Koleckar V, Kubikova K, Rehakova Z, Kuca
K, Jun D, Jahodar L and Opletal L: Condensed and hydrolysable
tannins as antioxidants influencing the health. Mini Rev Med Chem.
8:436–447. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vitenberga Z and Pilmane M: Inflammatory,
anti-inflammatory and regulatory cytokines in relatively healthy
lung tissue as an essential part of the local immune system. Biomed
Pap Med Fac Univ Palacky Olomouc Czech Repub. 161:164–173.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Joubert KD, Awori Hayanga J, Strollo DC,
Lendermon EA, Yousem SA, Luketich JD, Ensor CR and Shigemura N:
Outcomes after lung transplantation for patients with occupational
lung diseases. Clin Transplant. 33(e13460)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Amirshahrokhi K and Bohlooli S: Effect of
methylsulfonylmethane on paraquat-induced acute lung and liver
injury in mice. Inflammation. 36:1111–1121. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sathiyamoorthy G, Sehgal S and Ashton RW:
Pirfenidone and nintedanib for treatment of idiopathic pulmonary
fibrosis. South Med J. 110:393–398. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Meyer KC and Decker CA: Role of
pirfenidone in the management of pulmonary fibrosis. Ther Clin Risk
Manag. 13:427–437. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
King TE Jr, Bradford WZ, Castro-Bernardini
S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM,
Kardatzke D, Lancaster L, et al: A phase 3 trial of pirfenidone in
patients with idiopathic pulmonary fibrosis. N Engl J Med.
370:2083–2092. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bahri S, Mies F, Ben Ali R, Mlika M,
Jameleddine S, Entee KM and Shlyonsky V: Rosmarinic acid
potentiates carnosic acid induced apoptosis in lung fibroblasts.
PLoS One. 12(e0184368)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Iranshahi M, Rezaee R, Parhiz H,
Roohbakhsh A and Soltani F: Protective effects of flavonoids
against microbes and toxins: The cases of hesperidin and
hesperetin. Life Sci. 137:125–132. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ma H, Feng X and Ding S: Hesperetin
attenuates ventilator-induced acute lung injury through inhibition
of NF-kB-mediated inflammation. Eur J Pharmacol. 769:333–341.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Roohbakhsh A, Parhiz H, Soltani F, Rezaee
R and Iranshahi M: Molecular mechanisms behind the biological
effects of hesperidin and hesperetin for the prevention of cancer
and cardiovascular diseases. Life Sci. 124:64–74. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bai X, Yang P, Zhou Q, Cai B, Buist-Homan
M, Cheng H, Jiang J, Shen D, Li L, Luo X, et al: The protective
effect of the natural compound hesperetin against fulminant
hepatitis in vivo and in vitro. Br J Pharmacol. 174:41–56.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Guide for the Care and Use of Laboratory
Animals. 8th edition. National Academies Press, Washington, DC,
2011.
|
|
29
|
Szapiel SV, Elson NA, Fulmer JD,
Hunninghake GW and Crystal RG: Bleomycin-induced interstitial
pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis.
120:893–899. 1979.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang YT, Zhang Y, Yu GC, Chen YJ, Bo CX,
Jia Q and Shao H: Lung fibrosis and changes in autophagy-related
proteins in rats exposed to silica dust. Zhonghua Lao Dong Wei
Sheng Zhi Ye Bing Za Zhi. 36:890–895. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
31
|
Nardi J, Nascimento S, Goethel G, Gauer B,
Sauer E, Fão N, Cestonaro L, Peruzzi C, Souza J and Garcia SC:
Inflammatory and oxidative stress parameters as potential early
biomarkers for silicosis. Clin Chim Acta. 484:305–313.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Castranova V and Vallyathan V: Silicosis
and coal workers' pneumoconiosis. Environ Health Perspect. 108
(Suppl 4):S675–S684. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lapp NL and Castranova V: How silicosis
and coal workers' pneumoconiosis develop-a cellular assessment.
Occup Med. 8:35–56. 1993.PubMed/NCBI
|
|
34
|
Liu N, Cao F, Li Q, Zhang Y, Zhang Z and
Guan W: Study of quercetin on pulmonary fibrosis by silica
particles. Wei Sheng Yan Jiu. 43:814–818. 2014.PubMed/NCBI(In Chinese).
|
|
35
|
Wang JY, Yu GC, Jia Q, Li C, Shao LL, Sai
LL and Shao H: Preliminary analysis of differential expression of
miRNA-423-5p and miRNA-26a-5p in lung tissue of early silicotic
rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 37:7–12.
2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
36
|
Zhao Y, Xu G, Li H, Chang M, Guan Y, Li Y,
Wu W and Yao S: Overexpression of endogenous lipoic acid synthase
attenuates pulmonary fibrosis induced by crystalline silica in
mice. Toxicol Lett. 323:57–66. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hubbard AK, Thibodeau M and Giardina C:
Cellular and molecular mechanisms regulating silica-induced
adhesion molecule expression in mice. J Environ Pathol Toxicol
Oncol. 20 (Suppl 1):S45–S51. 2001.PubMed/NCBI
|
|
38
|
Hubbard AK, Timblin CR, Shukla A, Rincon M
and Mossman BT: Activation of NF-kappaB-dependent gene expression
by silica in lungs of luciferase reporter mice. Am J Physiol Lung
Cell Mol Physiol. 282:L968–L975. 2002.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Karlmark KR, Weiskirchen R, Zimmermann HW,
Gassler N, Ginhoux F, Weber C, Merad M, Luedde T, Trautwein C and
Tacke F: Hepatic recruitment of the inflammatory Gr1+ monocyte
subset upon liver injury promotes hepatic fibrosis. Hepatology.
50:261–274. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Barrett EG, Johnston C, Oberdorster G and
Finkelstein JN: Antioxidant treatment attenuates cytokine and
chemokine levels in murine macrophages following silica exposure.
Toxicol Appl Pharmacol. 158:211–220. 1999.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kehrer JP, Lee YC and Solem SM: Comparison
of in vitro and in vivo rates of collagen synthesis in normal and
damaged lung tissue. Exp Lung Res. 10:187–201. 1986.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cheresh P, Kim SJ, Tulasiram S and Kamp
DW: Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta.
1832:1028–1040. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Vallyathan V, Mega JF, Shi X and Dalal NS:
Enhanced generation of free radicals from phagocytes induced by
mineral dusts. Am J Respir Cell Mol Biol. 6:404–413.
1992.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kumar B, Gupta SK, Srinivasan BP, Nag TC,
Srivastava S, Saxena R and Jha KA: Hesperetin rescues retinal
oxidative stress, neuroinflammation and apoptosis in diabetic rats.
Microvasc Res. 87:65–74. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Anlar HG, Bacanli M, İritaş S, Bal C, Kurt
T, Tutkun E, Yilmaz OH and Basaran N: Effects of occupational
silica exposure on oxidative stress and immune system parameters in
ceramic workers in Turkey. J Toxicol Environ Health A. 80:688–696.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Pocernich CB and Butterfield DA: Elevation
of glutathione as a therapeutic strategy in Alzheimer disease.
Biochim Biophys Acta. 1822:625–630. 2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Peter C, Braidy N, Zarka M, Welch J and
Bridge W: Therapeutic approaches to modulating glutathione levels
as a pharmacological strategy in Alzheimer's disease. Curr
Alzheimer Res. 12:298–313. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Townsend DM, Tew KD and Tapiero H: The
importance of glutathione in human disease. Biomed Pharmacother.
57:145–155. 2003.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sacco R, Eggenhoffner R and Giacomelli L:
Glutathione in the treatment of liver diseases: Insights from
clinical practice. Minerva Gastroenterol Dietol. 62:316–324.
2016.PubMed/NCBI
|
|
50
|
Nuttall SL, Martin U, Sinclair AJ and
Kendall MJ: Glutathione: In sickness and in health. Lancet.
351:645–646. 1998.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Julius M, Lang CA, Gleiberman L, Harburg
E, DiFranceisco W and Schork A: Glutathione and morbidity in a
community-based sample of elderly. J Clin Epidemiol. 47:1021–1026.
1994.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Karkale S, Khurana A, Saifi MA, Godugu C
and Talla V: Oropharyngeal administration of silica in Swiss mice:
A robust and reproducible model of occupational pulmonary fibrosis.
Pulm Pharmacol Ther. 51:32–40. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kliment CR and Oury TD: Oxidative stress,
extracellular matrix targets, and idiopathic pulmonary fibrosis.
Free Radic Biol Med. 49:707–717. 2010.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wu FJ, Xue Y, Liu XF, Xue CH, Wang JF, Du
L, Takahashi K and Wang YM: The protective effect of
eicosapentaenoic acid-enriched phospholipids from sea cucumber
Cucumaria frondosa on oxidative stress in PC12 cells and SAMP8
mice. Neurochem Int. 64:9–17. 2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Chen MC, Ye YY, Ji G and Liu JW:
Hesperidin upregulates heme oxygenase-1 to attenuate hydrogen
peroxide-induced cell damage in hepatic L02 cells. J Agric Food
Chem. 58:3330–3335. 2010.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kheradmand E, Hajizadeh Moghaddam A and
Zare M: Neuroprotective effect of hesperetin and nano-hesperetin on
recognition memory impairment and the elevated oxygen stress in rat
model of Alzheimer's disease. Biomed Pharmacother. 97:1096–1101.
2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Rahman I and MacNee W: Oxidative stress
and regulation of glutathione in lung inflammation. Eur Respir J.
16:534–554. 2000.PubMed/NCBI View Article : Google Scholar
|
|
58
|
van der Vliet A, Janssen-Heininger YMW and
Anathy V: Oxidative stress in chronic lung disease: From
mitochondrial dysfunction to dysregulated redox signaling. Mol
Aspects Med. 63:59–69. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Dong X, Li X, Li M, Chen M, Fan Q and Wei
W: Antiinflammation and antioxidant effects of thalidomide on
pulmonary fibrosis in mice and human lung fibroblasts.
Inflammation. 40:1836–1846. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Kawasaki H: A mechanistic review of
silica-induced inhalation toxicity. Inhal Toxicol. 27:363–377.
2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Bonner JC: Regulation of PDGF and its
receptors in fibrotic diseases. Cytokine Growth Factor Rev.
15:255–273. 2004.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Phan SH: Genesis of the myofibroblast in
lung injury and fibrosis. Proc Am Thorac Soc. 9:148–152.
2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Saito A and Nagase T: Hippo and TGF-β
interplay in the lung field. Am J Physiol Lung Cell Mol Physiol.
309:L756–L767. 2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Zhu L, Fu X, Chen X, Han X and Dong P: M2
macrophages induce EMT through the TGF-β/Smad2 signaling pathway.
Cell Biol Int. 41:960–968. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Peteranderl C, Sznajder JI, Herold S and
Lecuona E: Inflammatory responses regulating alveolar ion transport
during pulmonary infections. Front Immunol. 8(446)2017.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Polat FR, Karaboga I, Polat MS, Erboga Z,
Yilmaz A and Guzel S: Effect of hesperetin on inflammatory and
oxidative status in trinitrobenzene sulfonic acid-induced
experimental colitis model. Cell Mol Biol (Noisy-le-grand).
64:58–65. 2018.PubMed/NCBI
|
|
67
|
Parhiz H, Roohbakhsh A, Soltani F, Rezaee
R and Iranshahi M: Antioxidant and anti-inflammatory properties of
the citrus flavonoids hesperidin and hesperetin: An updated review
of their molecular mechanisms and experimental models. Phytother
Res. 29:323–331. 2015.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Huang AL, Zhang YL, Ding HW, Li B, Huang
C, Meng XM and Li J: Design, synthesis and investigation of
potential anti-inflammatory activity of O-alkyl and O-benzyl
hesperetin derivatives. Int Immunopharmacol. 61:82–91.
2018.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Hashimoto S, Gon Y, Takeshita I, Maruoka S
and Horie T: IL-4 and IL-13 induce myofibroblastic phenotype of
human lung fibroblasts through c-Jun NH2-terminal kinase-dependent
pathway. J Allergy Clin Immunol. 107:1001–1008. 2001.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Postlethwaite AE, Holness MA, Katai H and
Raghow R: Human fibroblasts synthesize elevated levels of
extracellular matrix proteins in response to interleukin 4. J Clin
Invest. 90:1479–1485. 1992.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Doucet C, Brouty-Boye D, Pottin-Clemenceau
C, Jasmin C, Canonica GW and Azzarone B: IL-4 and IL-13
specifically increase adhesion molecule and inflammatory cytokine
expression in human lung fibroblasts. Int Immunol. 10:1421–1433.
1998.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Doucet C, Brouty-Boye D, Pottin-Clemenceau
C, Canonica GW, Jasmin C and Azzarone B: Interleukin (IL) 4 and
IL-13 act on human lung fibroblasts. Implication in asthma. J Clin
Invest. 101:2129–2139. 1998.PubMed/NCBI View
Article : Google Scholar
|
|
73
|
Antoniou KM, Ferdoutsis E and Bouros D:
Interferons and their application in the diseases of the lung.
Chest. 123:209–216. 2003.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Tzortzaki EG, Antoniou KM, Zervou MI,
Lambiri I, Koutsopoulos A, Tzanakis N, Plataki M, Maltezakis G,
Bouros D and Siafakas NM: Effects of antifibrotic agents on
TGF-beta1, CTGF and IFN-gamma expression in patients with
idiopathic pulmonary fibrosis. Respir Med. 101:1821–1829.
2007.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Ip WKE, Hoshi N, Shouval DS, Snapper S and
Medzhitov R: Anti-inflammatory effect of IL-10 mediated by
metabolic reprogramming of macrophages. Science. 356:513–519.
2017.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Cyktor JC, Carruthers B, Kominsky RA,
Beamer GL, Stromberg P and Turner J: IL-10 inhibits mature fibrotic
granuloma formation during Mycobacterium tuberculosis infection. J
Immunol. 190:2778–2790. 2013.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Palomares O, Martin-Fontecha M, Lauener R,
Traidl-Hoffmann C, Cavkaytar O, Akdis M and Akdis CA: Regulatory T
cells and immune regulation of allergic diseases: Roles of IL-10
and TGF-β. Genes Immun. 15:511–520. 2014.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Mlika M, Adigun R and Bhutta BS:
Silicosis. In: StatPearls. StatPearls Publishing, Treasure Island,
FL, 2020.
|
|
79
|
Xiao H, Zhang GF, Liao XP, Li XJ, Zhang J,
Lin H, Chen Z and Zhang X: Anti-fibrotic effects of pirfenidone by
interference with the hedgehog signalling pathway in patients with
systemic sclerosis-associated interstitial lung disease. Int J
Rheum Dis. 21:477–486. 2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Zou WJ, Huang Z, Jiang TP, Shen YP, Zhao
AS, Zhou S and Zhang S: Pirfenidone inhibits proliferation and
promotes apoptosis of hepatocellular carcinoma cells by inhibiting
the Wnt/β-catenin signaling pathway. Med Sci Monit. 23:6107–6113.
2017.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Seifirad S, Keshavarz A, Taslimi S, Aran
S, Abbasi H and Ghaffari A: Effect of pirfenidone on pulmonary
fibrosis due to paraquat poisoning in rats. Clin Toxicol (Phila).
50:754–758. 2012.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Chahar MK, Sharma N, Dobhal MP and Joshi
YC: Flavonoids: A versatile source of anticancer drugs. Pharmacogn
Rev. 5:1–12. 2011.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Wang J, Zhu H, Yang Z and Liu Z:
Antioxidative effects of hesperetin against lead acetate-induced
oxidative stress in rats. Indian J Pharmacol. 45:395–398.
2013.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Zhang J, Song J, Wu D, Wang J and Dong W:
Hesperetin induces the apoptosis of hepatocellular carcinoma cells
via mitochondrial pathway mediated by the increased intracellular
reactive oxygen species, ATP and calcium. Med Oncol.
32(101)2015.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Aswar M, Kute P, Mahajan S, Mahajan U,
Nerurkar G and Aswar U: Protective effect of hesperetin in rat
model of partial sciatic nerve ligation induced painful neuropathic
pain: An evidence of anti-inflammatory and anti-oxidative activity.
Pharmacol Biochem Behav. 124:101–107. 2014.PubMed/NCBI View Article : Google Scholar
|