Introduction
Acute lung injury (ALI) is characterized by
pulmonary edema and atelectasis as a result of diffuse
alveolar-capillary membrane damage, which manifests as respiratory
distress and refractory hypoxemia in patients (1). ALI is a common but serious consequence
of a number of pathological conditions, including severe infection,
trauma, shock and harmful gas inhalation (2). Acute respiratory distress syndrome
(ARDS) is a severe form of ALI that can rapidly progress into
multiple organ failure, which has a poor prognosis among patients
(3). Anti-inflammatory treatments,
such as corticosteroids, are currently the primary method for the
clinical treatment of ALI (4).
However, despite exhibiting significant inhibitory effects on the
inflammatory response during ALI, clinical trials have previously
demonstrated that hormonal drugs can cause unforeseen adverse side
effects in clinical use (5). In
addition, they do not reduce the mortality rate of patients with
ALI (5). Other inhaled
anti-asthmatic drugs, including activated protein C, albuterol and
surfactants, have all been withdrawn due to poor clinical efficacy
or side effects (6). In recent
years, a new understanding on the pathogenesis and pathophysiology
of ALI/ARDS has emerged (7,8). Clinical and experimental studies have
demonstrated that pulmonary capillary barrier injury followed by
increased pulmonary edema is the most important pathological
feature of ALI/ARDS, providing a basis for the early stages of this
condition (9,10). However, there remains to be a lack
of effective therapeutic strategies for treating increased
pulmonary microvascular permeability (11).
Diammonium glycyrrhizinate lipid ligand (DGLL) is an
extract of the active ingredient of the root of the Chinese
licorice Glycyrrhiza uralensis. The primary active component
of DGLL is the glycyrrhizic acid diammonium glycyrrhizinate
(Fig. 1A), which is a natural major
bioactive pentacyclic triterpenoid glycoside that possesses
comprehensive pharmacological properties, including anti-hepatitis,
antiviral, anti-inflammatory, anti-allergy, antioxidant and
antitumor characteristics (12-14).
This traditional Chinese medicinal licorice has been widely used as
an active component in preparations, such as Mahuang and Maxing
Shigan decoctions, for treating respiratory infection and acute
lung injury (15,16). Modern clinical observations have
demonstrated that glycyrrhizin alone or in combination with other
agents can effectively ameliorate lung injury, improve alveolar gas
exchange and inhibit pulmonary inflammation (17-19).
In addition, previous studies have shown that licorice exhibits
significant inhibitory effects on inflammatory responses to
non-alcoholic fatty liver in rats, by suppressing inflammatory
mediator expression and inhibiting leukocyte adhesion, infiltration
and peroxide release (20,21).
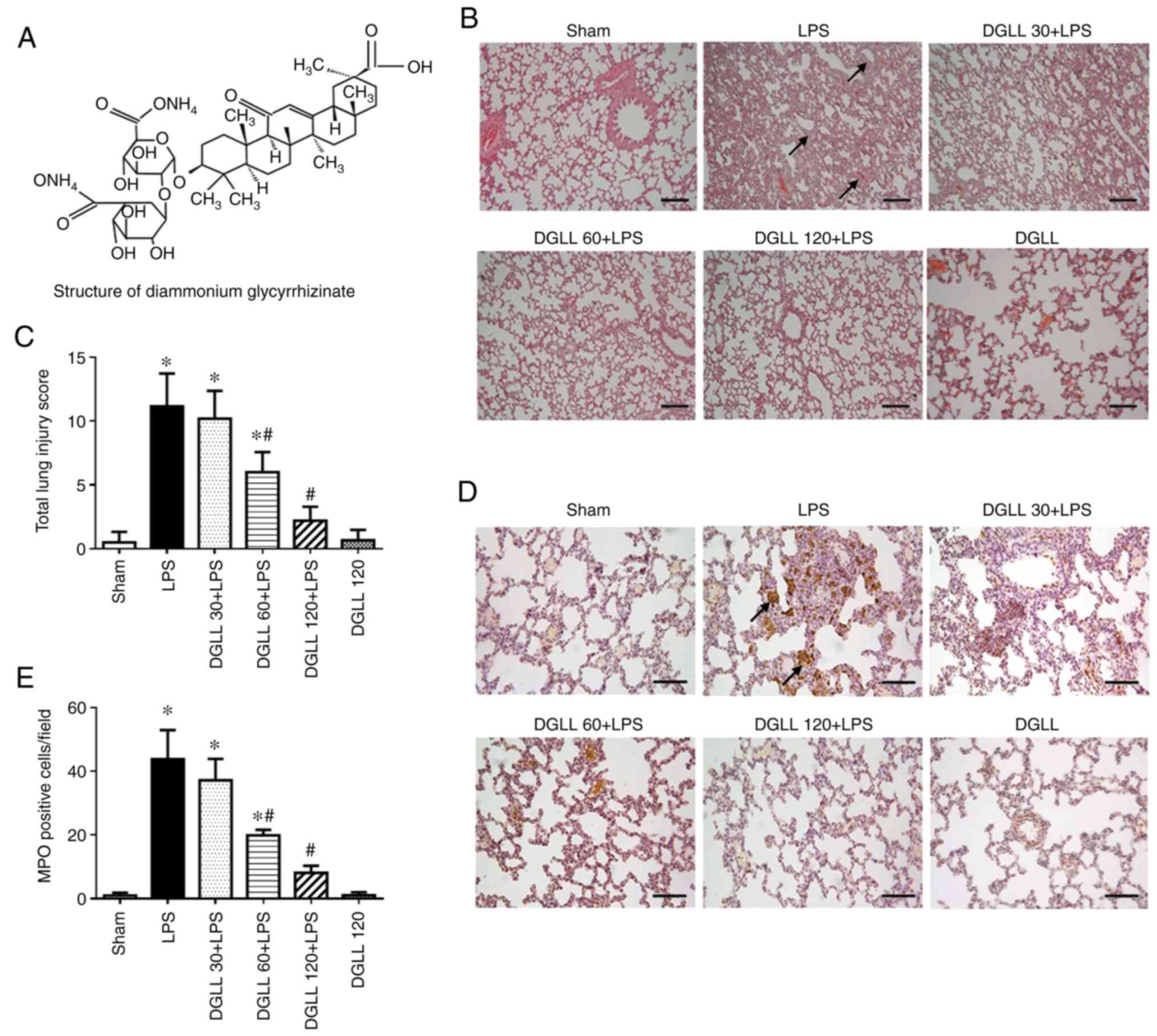 | Figure 1Effect of DGLL on acute lung tissue
injury and MPO expression levels in lung tissue following LPS
stimulation. (A) Structure of diammonium glycyrrhizinate. (B)
Representative H&E staining images of lung tissue in Sham, LPS
and DGLL 30, 60 and 120 + LPS and DGLL-alone groups. Scale bar, 200
µm; arrows represent, interstitial edema and thickening. (C)
Quantified lung injury score. (D) Representative MPO
immunohistochemical staining images of rat lung tissues in Sham,
LPS and DGLL 30, 60 and 120 + LPS and DGLL-alone groups. Scale bar,
200 µm; arrows indicate, MPO-positive cells. (E) Statistical
analysis of MPO-positive cells in lung tissues from each condition.
All data are expressed as the mean ± SEM. *P<0.05 vs.
Sham; #P<0.05 vs. LPS. DGLL, diammonium
glycyrrhizinate lipid ligand; MPO, myeloperoxidase; LPS,
lipopolysaccharide. |
However, the potential effects of DGLL on pulmonary
edema, is role in ALI and underlying mechanism remain to be fully
elucidated. Therefore, the present study investigated the effects
and underlying mechanisms of DGLL on ALI and pulmonary edema
induced by lipopolysaccharide (LPS) in rat models.
Materials and methods
Animals and drugs
Male Sprague-Dawley (SD) rats (113 in total)
weighing 180-200 g (age, 6±1 weeks) were provided by Anhui Medical
University Experimental Animal Center (Hefei, China). The animals
were given tap water ad libitum and housed at 24±1˚C and relative
humidity of 50±1% with a light/dark cycle of 12 h. Animals were fed
with standard grain forage and kept at room temperature (18-22˚C)
for 1 week with free access to food and water before the
experiments and animal health and behavior were monitored once a
day. All animals were handled according to the guidelines of the
Anhui Medical University Animal Research Committee (22). The protocols were approved by the
Committee on the Ethics of Animal Experiments of the Anhui Medical
University (approval nos. IACUC20180724-18 and IACUC20200710-11;
Hefei, China).
DGLL enteric-coated capsules (cat. no. 20180126AD),
also known as Tianqing Ganping, were provided by Jiangsu Zhengda
Datianqing Pharmacy Co., Ltd.
Experimental protocol
The present study was divided into two parts. In the
first set of experiments, SD rats were randomly divided into the
following six groups: i) Sham group (n=18); ii) DGLL group (n=3),
which received 120 mg/kg alone; iii) LPS (Escherichia coli
serotype O55:B5, Sigma-Aldrich; Merck KGaA) group (n=18), which
received 10 mg/kg LPS for 6 h; iv) DGLL 30 + LPS group (n=18),
which received DGLL 30 mg/kg + 10 mg/kg LPS; v) DGLL 60 + LPS group
(n=18), which received DGLL 60 mg/kg + 10 mg/kg LPS; and vi) DGLL
120 + LPS group (n=18), which received DGLL 120 mg/kg + 10 mg/kg
LPS (Table I).
 | Table IAllocation of animals into the
different experimental groups and parameters measured at 6 h after
LPS injection. |
Table I
Allocation of animals into the
different experimental groups and parameters measured at 6 h after
LPS injection.
| Experimental
group | Wet-to-dry
ratio | ELISA | Western
blotting | H&E | IHC and IF | Evans blue
extravasation | BALF analysis | Total |
|---|
| Sham | 6 | 6a | 4a | 3a | 3a | 6 | 6 | 18 |
| LPS | 6 | 6a | 4a | 3a | 3a | 6 | 6 | 18 |
| DGLL 30 + LPS | 6 | 6a | 4a | 3a | 3a | 6 | 6 | 18 |
| DGLL 60 + LPS | 6 | 6a | 4a | 3a | 3a | 6 | 6 | 18 |
| DGLL 120 + LPS | 6 | 6a | 4a | 3a | 3a | 6 | 6 | 18 |
| DGLL | | | | 3 | 3b | | | 3 |
| Total | 30 | | | 3 | | 30 | 30 | 93 |
In the Sham group, physiological saline (5 ml/kg)
was given by oral gavage 1 h after intraperitoneal saline injection
(5 ml/kg). In the DGLL group, DGLL (120 mg/kg) dissolved in
physiological saline was given by oral gavage 1 h before
intraperitoneal saline injection (5 ml/kg). In LPS group,
physiological saline (5 ml/kg) was given by oral gavage 1 h before
intraperitoneal LPS (10 mg/kg) injection. In the three DGLL
pre-treatment groups, DGLL (30, 60 and 120 mg/kg) dissolved in
physiological saline was administered by oral gavage 1 h before
intraperitoneal LPS (10 mg/kg) injection. In total, 6 h after LPS
injection, rats were anesthetized with 2% pentobarbital (60 mg/kg)
by intraperitoneal injection, where the successful induction of
anesthesia was defined as immobility and the absence of motor
response of rats to a noxious stimulus (such as a pinch). The rats
were euthanized by exsanguination followed by cardiac arrest under
anesthesia at the indicated endpoint of experiment.
In the second set of experiments, rats were randomly
divided into the following five groups: i) Sham group; ii) LPS
group, which received 10 mg/kg LPS alone for 1 h; iii) DGLL 30 +
LPS group, which received 30 mg/kg DGLL and + 10 mg/kg LPS for 1 h;
iv) DGLL 60 + LPS group, which received 60 mg/kg DGLL and 10 mg/kg
LPS for 1 h; and v) DGLL 120 + LPS group, which received 120 mg/kg
DGLL and 10 mg/kg LPS for 1 h (Table
II). The animal treatment protocol was the same as that for
that described for the LPS 6 h groups aforementioned. A total of 1
h after LPS injection, rats were sacrificed for parameter
detection.
 | Table IINumber of animals allocated into each
of the different experimental groups for analysis 1 h after LPS
injection. |
Table II
Number of animals allocated into each
of the different experimental groups for analysis 1 h after LPS
injection.
| Experimental
group | Western blotting
analysis for phosphorylated-VE cadherin | Total |
|---|
| Sham | 4 | 4 |
| LPS | 4 | 4 |
| DGLL 30 + LPS | 4 | 4 |
| DGLL 60 + LPS | 4 | 4 |
| DGLL 120 + LPS | 4 | 4 |
| Total | 20 | 20 |
Cell culture
RAW 264.7 murine macrophages were obtained from
ScienCell Research Laboratories, Inc. The cells were cultured at a
density of 1x105 cells/cm2 in DMEM
supplemented with 10% of FBS (both from ScienCell Research
Laboratories, Inc.), streptomycin (100 µg/ml) and penicillin (100
U/ml). The cells were cultured to confluence under a humidified
atmosphere of 5% CO2 and 95% air at 37˚C and incubated
with LPS (100 ng/ml) for 6 h at 37˚C. In the DGLL pre-treatment
groups, DGLL was added 1 h before LPS stimulation to a
concentration of 50, 100 and 200 µg/ml.
Hematoxylin and eosin (H&E)
staining
At 6 h after LPS stimulation, rats were anesthetized
and euthanatized before the middle right lobe of the lung was cut
from each rat for fixation in 4% paraformaldehyde for 48 h at 4˚C
and processed for paraffin sectioning (5 µm). H&E staining was
performed to evaluate lung tissue injury. In brief, after
deparaffinization by xylene and ethanol gradient, sections were
stained by sequential hematoxylin for 5 min and eosin for 15 sec,
both at room temperature. Using a light microscope (Olympus, Tokyo,
Japan), the morphological changes of lung tissue were observed
under a x10 objective lens. Five visual fields were selected
randomly from each section, and lung injury score was determined
based on the following histological features: i) Focal alveolar
membrane thickening; ii) capillary congestion; iii) intra-alveolar
hemorrhage; iv) interstitial edema; and v) intra-alveolar leukocyte
infiltration. Each feature was scored from 0 to 3 based on its
presence: i) Score 0, 0% of the section areas; ii) mild, score 1,
0-25% of the section areas; iii) moderate, score 2, 25-50% of the
section areas; and iv) severe, score 3 >50% of the section
areas. The total lung injury score was the sum of the score of each
feature (15 represents the maximum and the most severe) (23).
Myeloperoxidase (MPO)
immunohistochemical staining
MPO expression levels in the lung tissues were
determined by immunohistochemistry. Briefly, sections were
deparaffinized using xylene and ethanol gradient followed by
heat-mediated antigen retrieval in 0.01 M citrate buffer (pH 6.0)
and hydrogen peroxide (0.3%) blocking at room temperature for 30
min. Then, sections were blocked with goat serum (cat. no.
ZLI-9056; OriGene Technologies, Inc.) for 30 min at room
temperature and incubated overnight with the rabbit MPO polyclonal
primary antibody (1:200; Abcam; cat. no. ab9535) at 4˚C. The
sections were then incubated with biotinylated anti-rabbit
IgG-horseradish peroxidase (HRP) (cat. no. SP-9001, OriGene
Technologies, Inc.) at room temperature for 30 min and visualized
using a DAB substrate kit (OriGene Technologies, Inc.). The
location and expression levels of MPO-positive cells in lung tissue
samples were observed under a light microscope under a x20
objective lens. A total of five visual fields were selected from
each section for analysis of the numbers of positive cells.
Occludin immunofluorescence
staining
Tissue sections were deparaffinized by xylene and
ethanol gradient followed by heat-mediated antigen retrieval in
0.01 M citrate buffer (pH 6.0), washed with PBS and permeabilized
with 0.3% Triton X-100 for 30 min at 37˚C. Following blocking with
3% goat serum (cat. no. ZLI-9056; OriGene Technologies, Inc.) at
room temperature for 30 min, sections were incubated with primary
antibodies against Occludin (1:50, Invitrogen; Thermo Fisher
Scientific, Inc.; cat. no. 33-1500) and von Willebrand factor
(1:50; Abcam; cat. no. ab6994) diluted in PBS overnight at 4˚C.
After being rinsed with PBS, sections were incubated with Dylight™
488-labeled goat-anti rabbit secondary antibodies (1:100; KPL,
Inc.; cat. no. 5230-0385) and Dylight™ 549-labeled goat-anti mouse
secondary antibodies (1:100; KPL, Inc.; cat. no. 072-04-18-03) for
2 h at 37˚C. All sections were counterstained with Hoechst 33342
(1:50; Dojindo Molecular Technologies, Inc.) for 20 min at room
temperature to stain the nuclei. Images were acquired using a laser
scanning confocal microscope under a x63 objective lens (Leica
Microsystems GmbH).
ELISA
At 6 h after LPS stimulation, rats were anesthetized
and the right middle lobe of the lung from each rat was collected.
The one-step RIPA (Applygene Technologies, Inc.) method was used to
extract total protein from lung tissues. The expression levels of
tumor necrosis factor-α (TNF-α; cat. no. RTA00) and interleukin
(IL)-1β (cat. no. RLB00) in the lung tissues and cell culture
supernatants of RAW 264.7 cells were measured using an ELISA kit
(R&D Systems, Inc.) according to the manufacturer's
protocols.
Determination of the lung dry-wet
weight ratio
At 6 h after LPS stimulation, rats were anesthetized
and ~100 mg lung tissue from each rat was sampled from the right
upper lobe. The lung tissue was then weighed before the tissue was
dried in a vacuum oven at 80˚C for 48 h and the lung tissue was
weighed again. The ratio of the lung weight before and after oven
drying was then calculated.
Pulmonary microvascular permeability
test
At 6 h after the intraperitoneal injection of LPS,
rats were anesthetized with 2% pentobarbital (60 mg/kg) by
peritoneal injection, before being injected with 2% Evans blue (EB)
solution (30 mg/kg) via the jugular vein. After 30 min, rats were
sacrificed by perfusion with saline via the right ventricle until
cardiac arrest to rinse intravascular EB in the lung tissue and
part of the lower left lobe lung tissue of each rat was collected,
weighed and placed in a clean centrifuge tube. A total of ~100 mg
wet weight of the lower left lobe lung tissue was added to 1 ml
100% formamide, placed in a water bath at 37˚C for 24 h and
centrifuged at 1,000 x g at room temperature for 30 min before the
supernatant was extracted. Absorbance values of 200 µl supernatant
were then measured using a spectrophotometer at the wavelength of
620 nm. The standard curve method was used to calculate the EB
content of each sample. Pulmonary microvascular permeability was
expressed as the ratio of EB content to the wet lung weight.
Bronchoalveolar lavage fluid (BALF)
collection and analysis
At 6 h after intraperitoneal injection of LPS, rats
were anesthetized before a plastic cannula was inserted into the
trachea to collect BALF by sterile physiological saline aspiration,
which was performed three times. The rats were then euthanized by
exsanguination until cardiac arrest. The BALF sample was
centrifuged at 1,300 x g at 4˚C for 10 min and the supernatant was
extracted. Bicinchoninic acid protein assay (BCA) method was used
to detect total protein concentration in the BALF samples, whilst
the cell pellet was resuspended in PBS. The cells were then stained
with Wright-Giemsa stain for 1 min at room temperature and the
total cell counts were assessed using a cell count chamber and
observed under a light microscope under a x4 objective lens.
Western blot analysis
At 6 h after LPS stimulation, rats were anesthetized
and euthanatized, and the right lower lobe of each rat was sampled.
The one-step RIPA (Applygene Technologies, Inc.) method was used to
extract total protein from lung tissues and RAW 264.7 macrophages
before protein concentration was measured using the BCA method.
Following separation by 8% SDS-PAGE electrophoresis (100 mg protein
for each group), the protein samples were transferred onto PVDF
membranes for blocking by 5% non-fat milk at room temperature for 1
h. The following primary antibodies and concentrations were used:
intercellular adhesion molecule (ICAM)-1 (1:1,000; Abcam; cat. no.
ab206398), vascular endothelial (VE)-cadherin (1:500; Invitrogen;
Thermo Fisher Scientific, Inc.; cat. no. 36-1900), zonula occludens
(ZO)-1 (1:1,000; Invitrogen; Thermo Fisher Scientific, Inc.; cat.
no. 40-2200), Occludin (1:500; Invitrogen; Thermo Fisher
Scientific, Inc.; cat. no. 33-1500), junctional adhesion molecule
(JAM)-1 (1:1,000; Santa Cruz Biotechnology, Inc.; cat. no.
sc-53623) and GAPDH (1:5,000; Cell Signaling Technology, Inc.; cat.
no. 2118). All primary antibodies were incubated overnight at 4˚C.
In a separate experiment, rats were anesthetized 1 h after LPS
stimulation before the right lower lobe was collected and the
phosphorylation (p-) of VE-Cadherin (1:1,000; Abcam; cat. no.
ab119785) and total VE-cadherin (1:500; Invitrogen; Thermo Fisher
Scientific, Inc.; cat. no. 36-1900) were detected. Following
incubation with HRP-conjugated anti-rabbit IgG secondary antibody
(1:5,000; Cell Signaling Technology, Inc.; cat. no. 7074) or
HRP-conjugated anti-mouse IgG secondary antibody (1:5,000; Cell
Signaling Technology, Inc.; cat. no. 7076) for 1 h at room
temperature, the bands were visualized using the SuperEnhanced
chemiluminescence detection reagents (Applygene Technologies,
Inc.). GAPDH was used as the internal reference. The average
optical density of western blot bands was measured using Quantity
One image analyzer software (v4.6.6; Bio-Rad Laboratories, Inc.)
where the ratio of the average optical density of the target band
to that of GAPDH was calculated. The results are expressed as the
ratio with respect to those of the Sham group.
Statistical analysis
All data are expressed as the mean ± SEM, n=3 for
RAW264.7 cell experiments; n=3-6 for corresponding animal
experiments as indicate in Tables I
and II. One-way ANOVA and Tukey's
post hoc test or Kruskal-Wallis test followed by Dunn's test were
performed using SPSS 15.0 mathematical statistics software (SPSS,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
DGLL treatment inhibits LPS-induced
ALI and pulmonary inflammation in rats
H&E staining results showed that in the Sham and
DGLL-alone groups, the rat lung structure exhibited completely
clear alveolar spaces, where there was no edema or inflammatory
cell infiltration in the alveolar septa. By contrast, in the LPS
group, notable edema and thickening (Fig. 1B, arrow) occurred in the pulmonary
interstitial tissue, which are accompanied by alveolar atrophy.
DGLL pre-treatment prevented LPS-induced ALI and insult to the
alveolar structure, which were significant at 120 mg/kg (Fig. 1B and C).
Subsequent immunohistochemistry examination showed
that compared with that in the Sham and DGLL-alone groups,
MPO-positive cell staining was significantly increased in rat
pulmonary tissues following LPS stimulation. MPO-positive staining
in the DGLL pretreatment groups was weaker compared with that in
the LPS group, where the number of MPO-positive cells appeared to
have decreased in a dose-dependent manner (Fig. 1D and E). This suggests that DGLL prevented
LPS-induced leukocyte infiltration and damage to the rat pulmonary
tissue.
DGLL inhibits LPS-induced expression
of cytokines and adhesion molecules in rat lung tissues and
macrophages
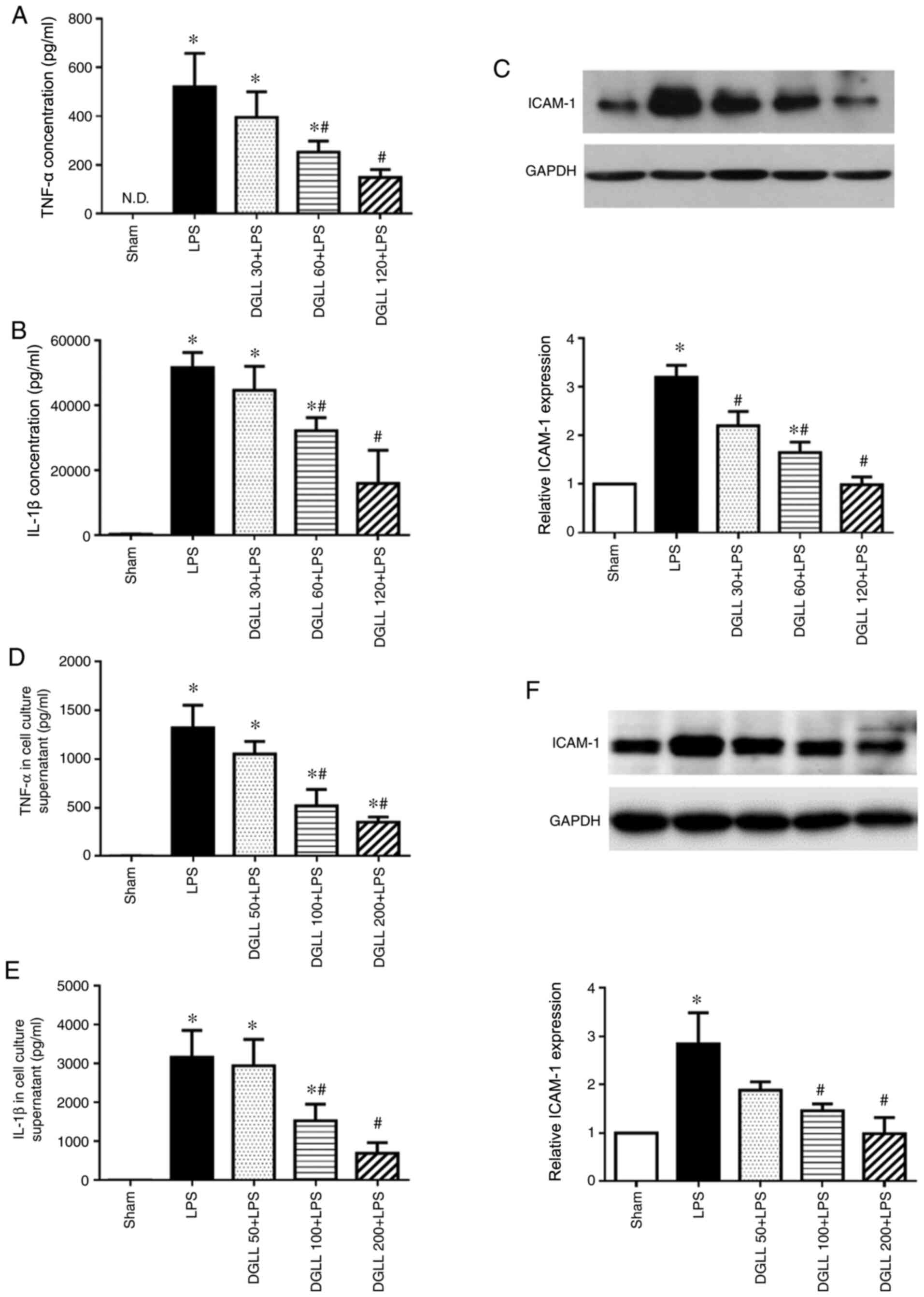
ELISA data showed that LPS significantly increased
inflammatory cytokine TNF-α and IL-1β levels in the lung tissue
compared with those in the Sham group (Fig. 2A and B). Pretreatment with 60 and 120 mg/kg DGLL
significantly prevented this increase in LPS-induced inflammatory
factor expression in the lung tissue, whilst pretreatment with 30
mg/kg DGLL exhibited no notable effects (Fig. 2A and B). Similarly, western blot analysis
demonstrated that endothelial adhesion molecule ICAM-1 expression
levels in the LPS group were significantly increased compared with
those in the Sham group. However, pretreatment with 60 and 120
mg/kg DGLL significantly prevented the LPS-induced upregulation of
ICAM-1 levels in the rat lung tissues (Fig. 2C). To validate the anti-inflammatory
effects of DGLL, the levels of TNF-α and IL-1β in the cell culture
supernatants of RAW 264.7 murine macrophages were next detected. In
parallel with the in vivo data, DGLL pretreatment
significantly abrogated LPS-induced inflammatory cytokine release,
in addition to ICAM-1 expression, by RAW 264.7 cells, at 100 and
200 µg/ml (Fig. 2D-F).
DGLL prevents increases in LPS-induced
pulmonary microvascular permeability and pulmonary edema
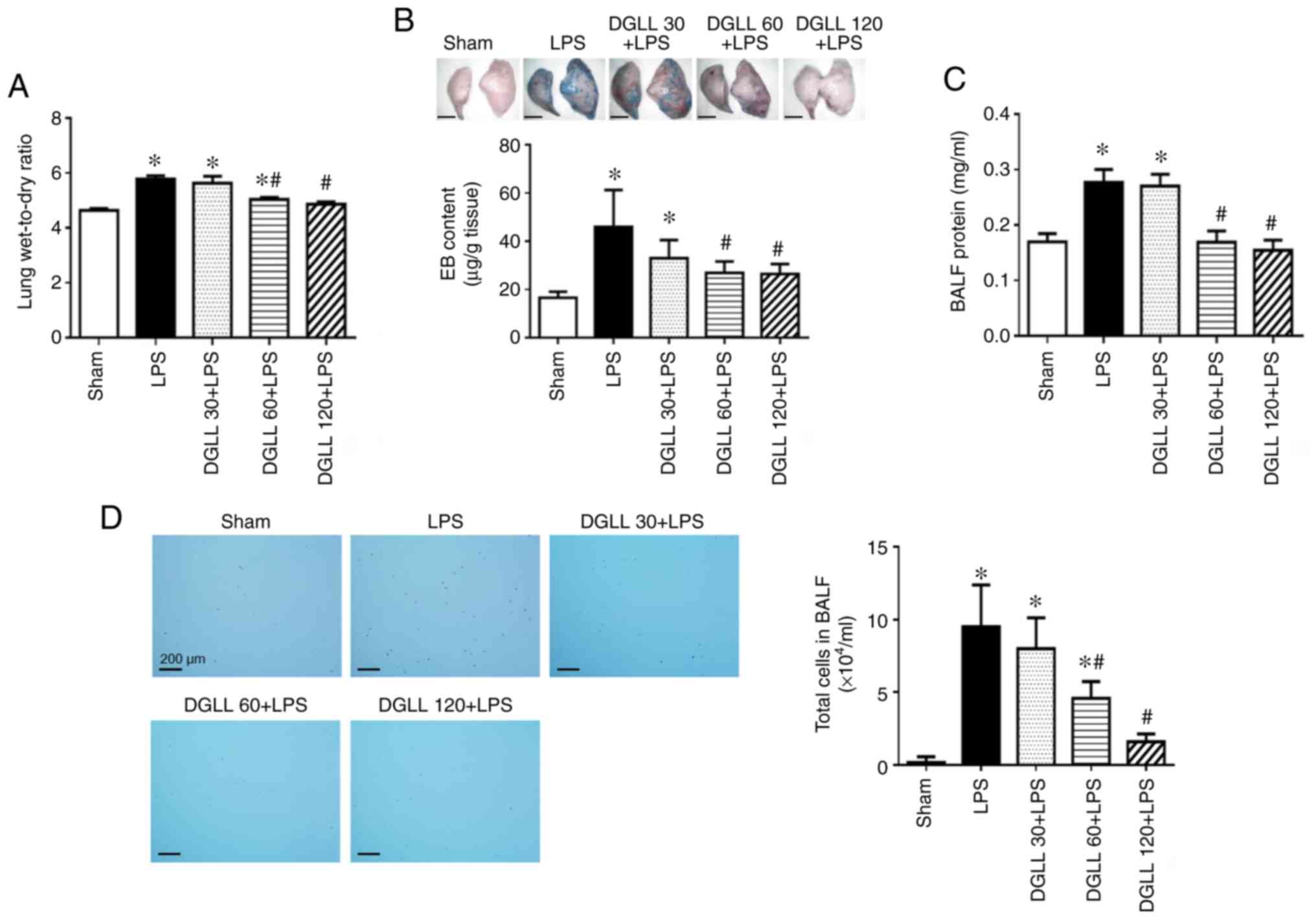
Compared with that in the Sham group, lung tissue
edema was significantly increased following LPS induction for 6 h,
which was demonstrated by the significant elevation in the
wet-to-dry weight ratio of rat lung tissues (Fig. 3A). Extravasation of EB in the lung
tissue and the protein content in BALF in the LPS group were also
found to be significantly higher compared with those in the Sham
group (Fig. 3B and C), suggesting that LPS impaired the
integrity of the pulmonary vascular and alveolar epithelial
barrier. In addition, a significant increase in the total cell
number in BALF was observed following LPS stimulation compared with
that in the Sham group (Fig. 3D).
All of the aforementioned changes, namely the lung wet/dry weight
ratio, EB extravasation, BALF protein concentration and total cell
number in BALF, were all significantly lower in the DGLL 60 and 120
mg/kg groups compared with those in the LPS group (Fig. 3D). These data indicate that
pretreatment with DGLL effectively inhibited LPS-induced pulmonary
edema and increases in pulmonary microvascular permeability
(Fig. 3).
DGLL prevents LPS-induced VE-cadherin
phosphorylation and reduction in VE-cadherin expression in rat lung
tissues
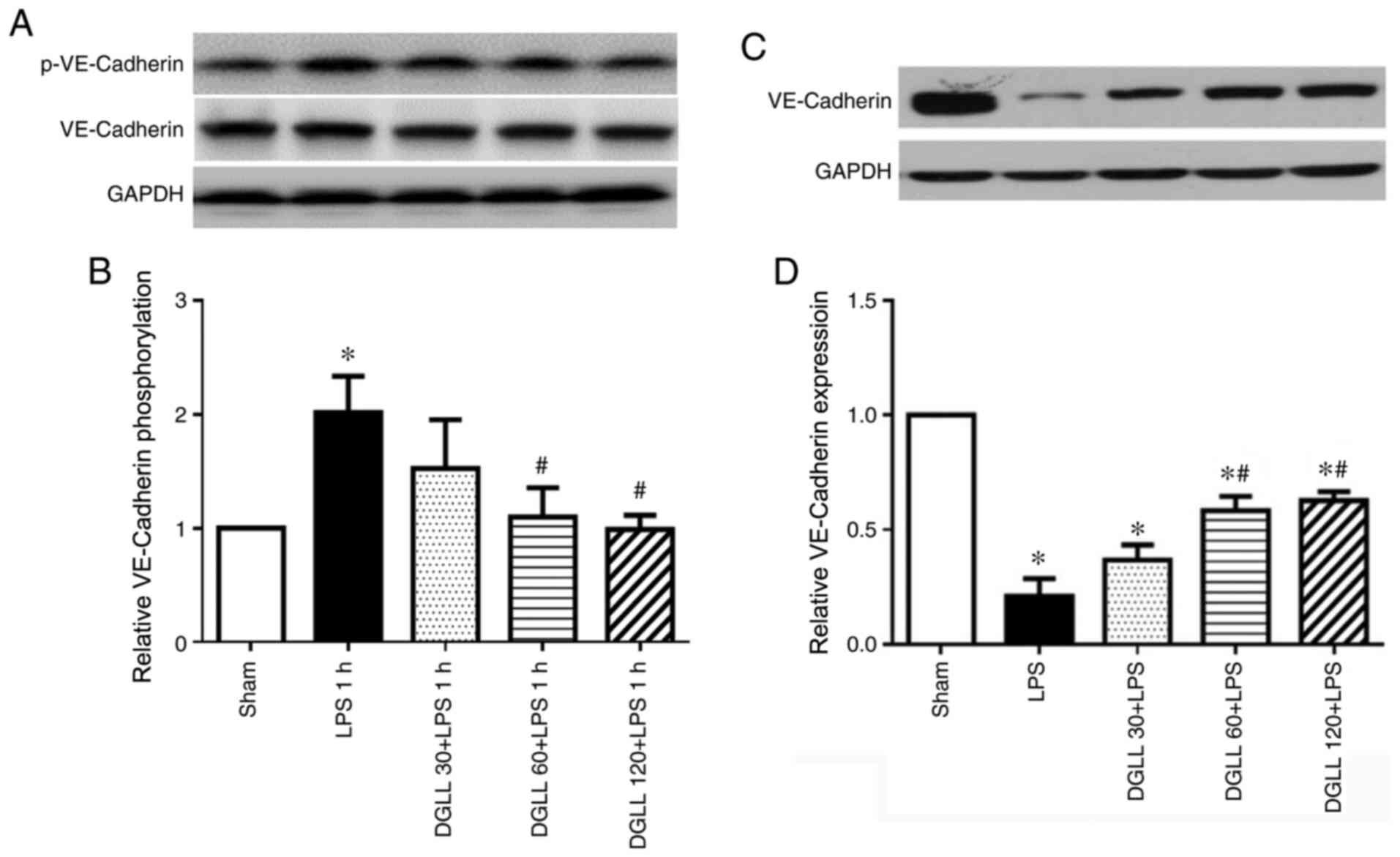
At 6 h after LPS induction, western blot analysis
showed that the expression levels of the adhesion junction protein
VE-Cadherin were significantly reduced in the LPS group, which were
significantly prevented by pretreatment with 60 and 120 mg/kg DGLL
(Fig. 4C and D). LPS mediates disruption of VE-Cadherin
through a series of transcellular events, starting with the
tyrosine phosphorylation of VE-Cadherin (24). Therefore, p-VE-cadherin was next
measured in lung tissues 1 h after LPS stimulation. The results
showed that levels of VE-cadherin phosphorylation were
significantly higher in the LPS group compared with those in the
Sham group, which were significantly abrogated by 60 and 120 mg/kg
DGLL pretreatment (Fig. 4A and
B). This suggests that DGLL
prevented the reductions in VE-cadherin expression by inhibiting
LPS-induced VE-cadherin phosphorylation.
DGLL inhibits LPS-induced
downregulation of tight junction proteins in rat lung tissues
The role of DGLL in the expression of the tight
junction protein occludin was assessed by confocal microscopy at 6
h after LPS administration (Fig.
5A). Compared with those in the Sham group, reduced occluding
expression levels and discontinuous localization of occludin were
observed in the rat lung tissues from the LPS group (Fig. 5A-b). These aforementioned effects
were markedly prevented by pretreatment with DGLL, particularly at
the 60 and 120 mg/kg (Fig. 5A-d and
A-e). Similarly, western blotting
results also demonstrated that the expression levels of tight
junction proteins ZO-1, occludin and JAM-1 in the rat lung tissue
were significantly decreased following LPS induction (Fig. 5B-E). In the DGLL 60 and 120 mg/kg
dose groups, lung tissue tight junction expression levels were
significantly higher compared with those in the LPS group (Fig. 5B-E). This demonstrated that DGLL
prevented the reductions in the expression of pulmonary tight
junction proteins caused by LPS.
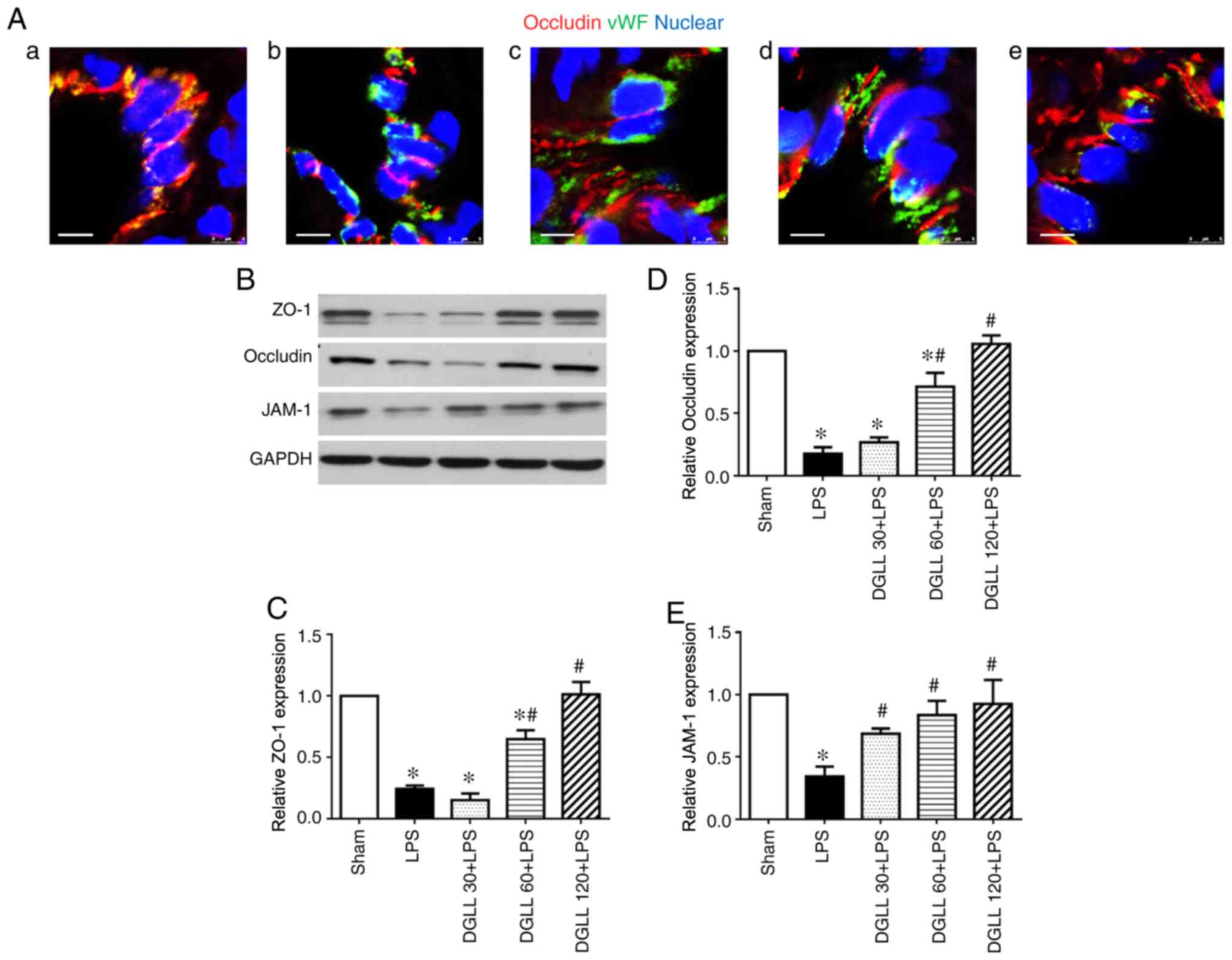 | Figure 5Effect of DGLL on the regulation of
tight junction protein expression in rat lung tissue after LPS
treatment. (A) Representative immunofluorescence confocal images
for Occludin expression in lung tissues in (a) Sham, (b) LPS, (c)
DGLL 30 + LPS, (d) DGLL 60 + LPS and (e) DGLL 120 + LPS groups.
Red, Occludin; green, vWF; blue, Hoeschst 33342 (nuclear). Scale
bar, 5 µm. (B) Representative western blot image and quantitative
analysis of (C) ZO-1, (D) occludin and (E) JAM-1 in rat lung
tissue. All data are expressed as the mean ± SEM.
*P<0.05 vs. Sham; #P<0.05 vs. LPS.
DGLL, diammonium glycyrrhizinate lipid ligand; LPS,
lipopolysaccharide; JAM-1, junction adhesion molecule-1; ZO-1,
zonula occludens-1; vWF, von Willebrand factor. |
Discussion
The present study demonstrated that pretreatment
with DGLL prevented LPS-induced ALI in rats, reduced MPO expression
levels, reduced cytokine production and downregulated adhesion
molecule expression in LPS-inflamed lung tissues. DGLL also
attenuated LPS-induced pulmonary edema and pulmonary microvascular
permeability in addition to preventing the reductions in the
expression of the adhesion junction protein VE-Cadherin and tight
junction proteins ZO-1, occludin and JAM-1 in rat lung tissues.
Recent studies have shown that damage to the
pulmonary microvascular barrier and the resulting pulmonary edema
is the primary pathological feature of early-stage ALI, serving as
a key target for treating ALI/ARDS (25,26).
Epidemiological studies have previously revealed that LPS, which is
a major component of the cell wall in gram-negative bacteria, is
the most common cause of lung injury and has been widely used to
establish animal models of ALI (27,28).
Previous studies have shown that LPS activates NF-κB by binding to
leukocyte toll-like receptor-4 receptors, inducing the expression
of a number of inflammatory factors, including TNF-α, IL-1β and
IL-6, leading to the destruction of paracellular junctions
(29,30). Consequently, by increasing the
expression levels of adhesion molecules on the endothelium, LPS
facilitates leukocyte adhesion to the microvascular endothelium
(31). Adherent leukocytes
indirectly damage the microvessels by releasing proteases and
peroxides, which increases pulmonary microvascular permeability and
lung edema, eventually resulting in decreased lung compliance and
functional impairment (32).
Previous pharmacological studies have documented that diammonium
glycyrrhizinate decreases lung injury by decreasing inflammation in
the respiratory tract, inhibiting protein and mRNA expression of
inflammatory factors in the lung tissue and by suppressing the
expression and activation of NF-κB (33,34).
The present study verified further that DGLL inhibited LPS-induced
inflammatory cell infiltration into the lung tissue and decreased
the upregulation of MPO expression, which is a leukocyte
infiltration marker (35).
Additionally, DGLL also reduced the levels of inflammatory
cytokines TNF-α and IL-1β and the expression of adhesion molecule
ICAM-1 in lung tissues. In vitro data demonstrated that DGLL
also inhibited LPS-induced cytokine release from RAW264.7
macrophages dose-dependently. These results suggest that DGLL
inhibited the increase in pulmonary microvascular permeability by
inhibiting the excessive activation of leukocytes in LPS-challenged
lung tissues.
In addition to indirectly damaging blood vessels via
leukocyte hyperactivation, LPS directly damages microvascular
barrier function, which results in increased microvascular
permeability and pulmonary edema (36,37).
Results of the present study demonstrated that the wet-to-dry
weight ratio, BALF protein content and extravasation of EB in lung
tissues in the DGLL pretreatment groups were significantly lower
compared with those in the LPS group. These observations suggest
that DGLL inhibited LPS-induced pulmonary microvascular
hyperpermeability and pulmonary tissue edema. The microvascular
barrier is primarily regulated by tight and adhesion junctions
between microvascular endothelial cells (38). Adherens junctions are primarily
formed by VE-cadherin protein via the formation of homodimers,
which are linked to catenin proteins in the cytoplasm by
cytoskeletal proteins, such as F-actin (39). Tight junction proteins, including
claudin, occludin and JAM, serve key roles in stabilizing the tight
interactions between cells by linking with the ZO family of
proteins in the cytoplasm (38).
The present study demonstrated that DGLL significantly prevented
the LPS-induced phosphorylation of VE-cadherin in the rat lung
tissues and LPS-induced reductions in VE-cadherin expression. A
possible limitation of this study was that only the full-length of
VE-cadherin was measured, where the analysis of VE-degradation
fragment expression was missing. In addition, DGLL stabilized lung
microvascular tight junction proteins occludin (Fig. 5A), as well as increasing the
expression levels of JAM-1 and ZO-1 protein. DGLL regulated
microvascular barriers in the lung tissue by increasing the
expression levels of tight junction and adhesion proteins, thereby
preventing LPS-induced pulmonary microvascular hyperpermeability
and pulmonary tissue edema. However, the regulatory mechanism of
DGLL on tight junction and adhesion proteins in microvascular
endothelial cells, as well as the therapeutical effects of DGLL
after ALI induction require further investigation.
In conclusion, DGLL exhibited significant protective
effects against LPS-induced ALI in rats, with the mechanism of
action of which found to be associated with the inhibition of
inflammatory cell infiltration and reduced expression of
intercellular junction proteins. These results provided a novel
theoretical basis for the clinical treatment of ALI using DGLL.
Acknowledgements
Not applicable.
Funding
The present study was supported financially by the
Key Projects of Natural Science Research of Anhui Province (grant
no. KJ2016A374).
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MML performed and funded the research and analyzed
the data. JZ and DJ contributed to animal experiments. JY and YPH
performed the immunochemistry analysis. QW designed the research,
interpreted the data, wrote the manuscript and finally approved the
submission of this manuscript. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
All animals were handled according to the guidelines
of the Anhui Medical University Animal Research Committee. The
protocols were approved by the Committee on the Ethics of Animal
Experiments of the Anhui Medical University (approval nos.
IACUC20180724-18 and IACUC20200710-11; Hefei, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Piantadosi CA and Schwartz DA: The acute
respiratory distress syndrome. Ann Intern Med. 141:460–470.
2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rezoagli E, Fumagalli R and Bellani G:
Definition and epidemiology of acute respiratory distress syndrome.
Ann Transl Med. 5(282)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bellani G, Laffey JG, Pham T, Fan E,
Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley
DF, et al: LUNG SAFE Investigators; ESICM Trials Group:
Epidemiology, patterns of care, and mortality for patients with
acute respiratory distress syndrome in intensive care units in 50
countries. JAMA. 315:788–800. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Meduri GU, Golden E, Freire AX, Taylor E,
Zaman M, Carson SJ, Gibson M and Umberger R: Methylprednisolone
infusion in early severe ARDS: Results of a randomized controlled
trial. Chest. 131:954–963. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Meduri GU, Headley AS, Golden E, Carson
SJ, Umberger RA, Kelso T and Tolley EA: Effect of prolonged
methylprednisolone therapy in unresolving acute respiratory
distress syndrome: A randomized controlled trial. JAMA.
280:159–165. 1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Matthay MA, Ware LB and Zimmerman GA: The
acute respiratory distress syndrome. J Clin Invest. 122:2731–2740.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Matthay MA, Zemans RL, Zimmerman GA, Arabi
YM, Beitler JR, Mercat A, Herridge M, Randolph AG and Calfee CS:
Acute respiratory distress syndrome. Nat Rev Dis Primers.
5(18)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fan E, Brodie D and Slutsky AS: Acute
respiratory distress syndrome: Advances in diagnosis and treatment.
JAMA. 319:698–710. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Matthay MA and Zimmerman GA: Acute lung
injury and the acute respiratory distress syndrome: Four decades of
inquiry into pathogenesis and rational management. Am J Respir Cell
Mol Biol. 33:319–327. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cardinal-Fernández P, Bajwa EK,
Dominguez-Calvo A, Menéndez JM, Papazian L and Thompson BT: The
presence of diffuse alveolar damage on open lung biopsy is
associated with mortality in patients with acute respiratory
distress syndrome: A systematic review and meta-analysis. Chest.
149:1155–1164. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Johnson ER and Matthay MA: Acute lung
injury: Epidemiology, pathogenesis, and treatment. J Aerosol Med
Pulm Drug Deliv. 23:243–252. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang R, Cheng K, Xu S, Li S, Zhou Y, Zhou
S, Kong R, Li L, Li J, Feng J, et al: Metformin and diammonium
glycyrrhizinate enteric-coated capsule versus metformin alone
versus diammonium glycyrrhizinate enteric-coated capsule alone in
patients with nonalcoholic fatty liver disease and type 2 diabetes
mellitus. Gastroenterol Res Pract. 2017(8491742)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pang H, Huang T, Song J, Li D, Zhao Y and
Ma X: Inhibiting HMGB1 with glycyrrhizic acid protects brain injury
after DAI via its anti-inflammatory effect. Mediators Inflamm.
2016(4569521)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cai Y, Xu Y, Chan HF, Fang X, He C and
Chen M: Glycyrrhetinic acid mediated drug delivery carriers for
hepatocellular carcinoma therapy. Mol Pharm. 13:699–709.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
He Y, Lou X, Jin Z, Yu L, Deng L and Wan
H: Mahuang decoction mitigates airway inflammation and regulates
IL-21/STAT3 signaling pathway in rat asthma model. J
Ethnopharmacol. 224:373–380. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhong Y, Zhou J, Liang N, Liu B, Lu R, He
Y, Liang C, Wu J, Zhou Y, Hu M, et al: Effect of maxing Shigan Tang
on H1N1 influenza a virus-associated acute lung injury in mice.
Intervirology. 59:267–274. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yao L and Sun T: Glycyrrhizin
administration ameliorates Streptococcus aureus-induced
acute lung injury. Int Immunopharmacol. 70:504–511. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen J, Zhang W, Zhang L, Zhang J, Chen X,
Yang M, Chen T and Hong J: Glycyrrhetinic acid alleviates
radiation-induced lung injury in mice. J Radiat Res (Tokyo).
58:41–47. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang D, Liu B, Cao B, Wei F, Yu X, Li GF,
Chen H, Wei LQ and Wang PL: Synergistic protection of Schizandrin B
and Glycyrrhizic acid against bleomycin-induced pulmonary fibrosis
by inhibiting TGF-β1/Smad2 pathways and overexpression of NOX4. Int
Immunopharmacol. 48:67–75. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jung JC, Lee YH, Kim SH, Kim KJ, Kim KM,
Oh S and Jung YS: Hepatoprotective effect of licorice, the root of
Glycyrrhiza uralensis Fischer, in alcohol-induced fatty
liver disease. BMC Complement Altern Med. 16(19)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tsuruoka N, Abe K, Wake K, Takata M, Hatta
A, Sato T and Inoue H: Hepatic protection by glycyrrhizin and
inhibition of iNOS expression in concanavalin A-induced liver
injury in mice. Inflamm Res. 58:593–599. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kan C, Ding N, Yang J, Tan Z, McGuire TL,
Lu H, Zhang K, Berger DM, Kessler JA and Kan L: BMP-dependent,
injury-induced stem cell niche as a mechanism of heterotopic
ossification. Stem Cell Res Ther. 10(14)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Matute-Bello G, Downey G, Moore BB,
Groshong SD, Matthay MA, Slutsky AS and Kuebler WM: Acute Lung
Injury in Animals Study Group. An official American Thoracic
Society workshop report: Features and measurements of experimental
acute lung injury in animals. Am J Respir Cell Mol Biol.
44:725–738. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chan YH, Harith HH, Israf DA and Tham CL:
Differential regulation of LPS-mediated VE-cadherin disruption in
human endothelial cells and the underlying signaling pathways: A
mini review. Front Cell Dev Biol. 7(280)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dong W, He B, Qian H, Liu Q, Wang D, Li J,
Wei Z, Wang Z, Xu Z, Wu G, et al: RAB26-dependent autophagy
protects adherens junctional integrity in acute lung injury.
Autophagy. 14:1677–1692. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Audard J, Godet T, Blondonnet R, Joffredo
JB, Paquette B, Belville C, Lavergne M, Gross C, Pasteur J, Bouvier
D, et al: Inhibition of the receptor for advanced glycation
end-products in acute respiratory distress syndrome: A randomised
laboratory trial in piglets. Sci Rep. 9(9227)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen H, Bai C and Wang X: The value of the
lipopolysaccharide-induced acute lung injury model in respiratory
medicine. Expert Rev Respir Med. 4:773–783. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cheng KT, Xiong S, Ye Z, Hong Z, Di A,
Tsang KM, Gao X, An S, Mittal M, Vogel SM, et al:
Caspase-11-mediated endothelial pyroptosis underlies
endotoxemia-induced lung injury. J Clin Invest. 127:4124–4135.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Tong W, Chen X, Song X, Chen Y, Jia R, Zou
Y, Li L, Yin L, He C, Liang X, et al: Resveratrol inhibits
LPS-induced inflammation through suppressing the signaling cascades
of TLR4-NF-κB/MAPKs/IRF3. Exp Ther Med. 19:1824–1834.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee YM, Hybertson BM, Cho HG, Terada LS,
Cho O, Repine AJ and Repine JE: Platelet-activating factor
contributes to acute lung leak in rats given interleukin-1
intratracheally. Am J Physiol Lung Cell Mol Physiol. 279:L75–L80.
2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zemans RL, Colgan SP and Downey GP:
Transepithelial migration of neutrophils: Mechanisms and
implications for acute lung injury. Am J Respir Cell Mol Biol.
40:519–535. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Abraham E: Neutrophils and acute lung
injury. Crit Care Med. 31 (Suppl 4):S195–S199. 2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Feng C, Wang H, Yao C, Zhang J and Tian Z:
Diammonium glycyrrhizinate, a component of traditional Chinese
medicine Gan-Cao, prevents murine T-cell-mediated fulminant
hepatitis in IL-10- and IL-6-dependent manners. Int
Immunopharmacol. 7:1292–1298. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jin J, Xiong T, Hou X, Sun X, Liao J,
Huang Z, Huang M and Zhao Z: Role of Nrf2 activation and NF-κB
inhibition in valproic acid induced hepatotoxicity and in
diammonium glycyrrhizinate induced protection in mice. Food Chem
Toxicol. 73:95–104. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ding D, Xu S, Zhang H, Zhao W, Zhang X,
Jiang Y, Wang P, Dai Z and Zhang J: 3-Methyladenine and
dexmedetomidine reverse lipopolysaccharide-induced acute lung
injury through the inhibition of inflammation and autophagy. Exp
Ther Med. 15:3516–3522. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dreymueller D, Martin C, Kogel T,
Pruessmeyer J, Hess FM, Horiuchi K, Uhlig S and Ludwig A: Lung
endothelial ADAM17 regulates the acute inflammatory response to
lipopolysaccharide. EMBO Mol Med. 4:412–423. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Veszelka S, Pásztói M, Farkas AE, Krizbai
I, Ngo TK, Niwa M, Abrahám CS and Deli MA: Pentosan polysulfate
protects brain endothelial cells against bacterial
lipopolysaccharide-induced damages. Neurochem Int. 50:219–228.
2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mehta D and Malik AB: Signaling mechanisms
regulating endothelial permeability. Physiol Rev. 86:279–367.
2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Rimm DL, Koslov ER, Kebriaei P, Cianci CD
and Morrow JS: Alpha 1(E)-catenin is an actin-binding and -bundling
protein mediating the attachment of F-actin to the membrane
adhesion complex. Proc Natl Acad Sci USA. 92:8813–8817.
1995.PubMed/NCBI View Article : Google Scholar
|