Introduction
Lysophosphatidic acid (LPA,
1-acyl-2-hydroxy-sn-glycero-3-phosphate) is a simple phospholipid
present in the animal system, having diverse in vitro and
in vivo biological effects in the neuronal and non-neuronal
cells and organs as a lipid-derived growth factor (1). The primary cellular actions of LPA
include intracellular calcium concentration or [Ca2+]i
transient induction as well as Ca2+-mediated cell
proliferation, differentiation, morphological changes, migration,
and survival (1). These effects are
mediated by the activation of G protein-coupled LPA receptors
(LPARs), of which there are six different subtypes (2). Early in vitro and in
vivo studies have shown that LPA1 receptor is involved in the
early brain development and cortical growth through neurogenesis
(3). Recent studies have
demonstrated that LPA-LPA1 receptor axis are also involved in many
adult brain functions, including emotion and cognition (4). Further, LPAR1-6 is also expressed in
the endothelial cells of blood vessel (5,6). LPA
exists in different body fluids, such as plasma, and it takes part
in angiogenesis (7,8). Although LPA plays an important role in
angiogenesis, little is known on the effects of LPA on maintenance
of microvascular integrity including blood-brain barrier (BBB) in
Alzheimer's disease (AD). Although accumulating evidence shows that
AD patients exhibit BBB leakage due to alterations of BBB
permeability (9-11),
the main causes of vascular dysfunctions in AD are due to β-amyloid
accumulation in the brain (12). It
is currently unknown whether LPA affects endothelial cell
junctional proteins that regulate BBB functions.
Panax ginseng C.A. Meyer is one of medicinal
plant having diverse biological effects. Recent reports show that
ginseng contains a novel exogenous LPAR ligand, gintonin (13), since gintonin includes a large
amount of LPAs such as LPA C18:2, LPA C18:1,
and LPA C16:0, as an biologically active ingredients
(14). At the cellular level,
gintonin induces intracellular Ca2+ transients with low
effective concentration 50 values in cells expressing LPAR1, LPAR2,
LPAR3 or LPAR5, but with high effective concentration 50 in cells
expressing LPAR4 and LPAR6, indicating that gintonin exhibits a
differential affinity for LPARs (13). Previously, we have demonstrated that
gintonin regulates various Ca2+-dependent ion channels
and receptors (14). Furthermore,
short-term treatment with gintonin has been found to enhance
synaptic transmission by stimulating glutamate release in
hippocampal slices and increase long-term potentiation (15). An in vivo study on normal
mice demonstrated that long-term administration of gintonin
enhanced hippocampus-dependent memory (16). Previous reports also showed that
long-term treatment of gintonin attenuated β-amyloid plaque
deposition in the cortex and hippocampus and restored
β-amyloid-induced memory dysfunction. Moreover, long-term
administration of gintonin-enriched fraction (GEF) also restored
β-amyloid-induced cholinergic brain dysfunction by increasing
acetylcholine synthesis and stimulating hippocampal neurogenesis in
an APPswe/PSEN-1 double-Tg mouse model of AD (AD Tg mice) (16-18).
Although LPA is involved in brain functions in normal and AD animal
model, it is unknown whether gintonin can protect the brain
microvessels from damages induced by β-amyloid plaque accumulation
in a Tg mouse model of AD.
In the present study, we investigated the effects of
GEF on β-amyloid plaque depositions, disruptions of brain
microvascular permeability, and microvascular endothelial cell
protein expression in the brain of AD Tg mice. We found that
long-term oral administration of GEF attenuated depositions of
β-amyloid plaques, the increased Evans blue permeability, reduced
expression of platelet endothelial cell adhesion molecule
(PECAM-1), and enhanced occludin, claudin-5, and zonula occludens
(ZO)-1 in the cortex and hippocampus of AD Tg mice. We further
discussed the pharmacological roles of GEF on brain microvascular
integrity in AD.
Materials and methods
GEF preparation
Four-year-old Korean ginsengs were purchased from
local ginseng market (Geumsan Ginseng Cooperative, Geumsan,
Republic of Korea) and identified and the specimen (voucher no.
NIBRVP0000730014) was deposited at the herbarium of the National
Institute of Biological Resources (Herbarium code: KB, http://sweetgum.nybg.org/science/ih/herbarium-details/?irn=138656).
GEF was prepared as described previously (19). One kilogram of ginseng was ground
into small pieces (>3 mm) and refluxed eight times with 70%
fermented ethanol for 8 h at 80˚C. The extract (350 g) was
concentrated, dissolved in distilled cold water at a ratio of 1 to
10, and stored at 4˚C for 24-96 h. The supernatant and precipitate
from the water fractionation after ethanol extraction was separated
by centrifugation at 3,000 rpm for 20 min. After centrifugation,
the precipitate was lyophilized.
The main active ingredient
compositions of GEF
The detailed compositions of this fraction including
LPAs as an active ingredient of GEF were identified in previous
report through LC-MS/MS analysis (20). Thus, GEF contains ~7.5% linoleic
(C18:2), 2.8% palmitic (C16:0), and 1.5%
oleic acids (C18:1). GEF contains ~0.2% LPA
C18:2, 0.06% LPA C16:0, and 0.02% LPA
C18:1. GEF contains 0.08% lysophosphatidylcholine, 0.03%
lysophosphatidylethanolamine, and 0.13% lysophosphatidylinositols.
GEF also contains ~1% phosphatidic acid (PA) 16:0-18:2, 0.5% PA
18:2-18:2, and 0.2% PA 16:0-18:1(20).
Animals, drug treatments, and ethical
approval
The breeding pairs of double-Tg male mice expressing
the mutant swe-AβPP (AβPPswe) and mutant presenilin-1
(PSEN-1) genes (deletion of exon 9) [AβPPswe/PSEN-1
double-Tg mice, B6C3-Tg (AβPPswe/PSEN1dE9) 85Dbo/J; The Jackson
Laboratory] were housed and bred in an approved animal facility at
the Kangwon National University, Republic of Korea. Four animals
were housed in each cage under constant temperature (23±1˚C) and
humidity (55±5%). The animals were provided access to food and
water ad libitum and were maintained under a 12-h light/dark
cycle (lights on 07:00-19:00). The mice were divided into wild-type
(WT, n=8), AD Tg (n=8), and AD Tg + GEF (50 or 100 mg/kg, p.o.,
n=8) groups. The six-month-old AD Tg mice were treated with saline
or gintonin (50 or 100 mg/kg, orally) 3 times a week for 3 months
(17). All analyses were performed
when the mice were 9 months old. The number of mice which died
during 9 months old was 0, 1, 2 and 1 in wild-type, AD Tg treated
with saline, AD Tg + GEF (50 mg/kg), and AD Tg + GEF (100 mg/kg)
group, respectively. All experimental procedures were conducted in
a blinded manner in accordance with the Guide for the Care and Use
of Laboratory Animals of the National Institutes of Health. The
protocol was approved by the Institutional Animal Care and Use
Committees of Kangwon (Permit no. 13-156) and Konkuk (Permit no.
14-956) Universities. Sodium pentobarbital (50 mg/kg, i.p.) was
used for mouse anesthesia and mice were sacrificed by cervical
dislocation when they did not respond to paw stimuli.
Brain and section preparation
The brains and sections were prepared as previously
described with slight modifications (19). Mice from WT (n=4), AD Tg (n=4), and
AD Tg + 50 or 100 mg/kg GEF (n=4, each) groups were sacrificed
using diethyl ether and were perfused intracardially with 0.9%
saline and fixative with 4% paraformaldehyde in 0.1 M phosphate
buffer (pH 7.4). The brains were fixed with the same fixative
overnight at 4˚C. The brains were serially cryoprotected in 10, 20
and 30% sucrose in phosphate-buffered saline for 48 h at 4˚C and
coronally sliced into 30-µm-thick sections with a freezing
microtome (model: CM3050S, Leica Biosystems) for
immunohistochemical and immunofluorescent staining.
Quantification of BBB
permeability
The level of BBB disruption was detected by the
quantitative measurement of Evans blue content as previously
described (21). Briefly, 2%
sterilized Evans blue dye (Sigma-Aldrich; Merck KGaA) solution was
intravenously injected at a dose of 4.0 ml/kg per mouse in WT
(n=4), AD Tg (n=4), and AD Tg + 50 or 100 mg/kg GEF (n=4, each)
groups. Thirty minutes after the injection, the mice were perfused
with saline to remove Evans blue dye from the vascular system. The
brains were immediately removed, sliced into 2 mm thickness using
brain matrix (Cell Point Scientific), and fixed with 4%
paraformaldehyde solution. Cryosections of 30 µm thickness were
prepared and immunofluorescent staining of PECAM-1 was performed as
previously described (21).
Staining with Evans blue and immunofluorescent staining of PECAM-1
were used to detect albumin extravasation (BBB permeability) and
BBB disruption, respectively.
Quantitative real-time PCR
analysis
For real-time PCR analysis, the hippocampus from the
right hemisphere of each mouse groups were used after harvest and
the total RNA was extracted using TRIsure reagent according to the
manufacturer's instructions (Bioline). Real-time PCR analysis was
performed as previously described (21). Briefly, the hippocampus were reacted
with the following primer sets: ICAM-1, 5'-TGC GTT TTG GAG CTA GCG
GAC CA-3' and 5'-CGA GGA CCA TAC AGC ACG TGC AG-3'; VCAM-1, 5'-CCT
CAC TTG CAG CAC TAC GGG CT-3' and 5'-TTT TCC AAT ATC CTC AAT GAC
GGG-3'; claudin-3, 5'-CTG GGA GGG CCT GTG GAT GAA CT-3' and 5'-TCG
CGG CGC AGA ATA GAG GAT-3'; and glyceraldehyde 3-phosphate
dehydrogenase, 5'-AGG TCA TCC CAG AGC TGA ACG-3' and 5'-CAC CCT GTT
GCT GTA GCC GTA T-3'. All real-time PCR experiments were performed
at least three times, and the expression of each gene was
normalized to that of glyceraldehyde 3-phosphate dehydrogenase.
Immunohistochemical and
immunofluorescence evaluations
Immunohistochemical staining was performed as
previously described (21).
Briefly, the free-floating sections (n=4 per brain) of each mouse
from WT (n=4), AD Tg (n=4), and AD Tg + 50 or 100 mg/kg GEF (n=4,
each) groups were reacted with rabbit anti-β-amyloid antibody
(1:200; Invitrogen; Thermo Fisher Scientific, Inc.) and
biotinylated secondary antibody (1:200; Vector Laboratories), and
were visualized using an avidin-biotinylated horseradish peroxidase
complex Elite® kit (Vector Laboratories) and
3,3'-diaminobenzidine. The sections were analyzed using a DP70
image analysis system (Olympus Co.). β-amyloid plaque deposition in
the parietal cortex and hippocampus were quantified using ImageJ
(http://rsb.info.nih.gov/ij/). The plaque
deposition was also quantified as previously described (17). The images of β-amyloid-stained
sections were compared to 6-grade plaque photographic reference
panels (17) and assigned a
numerical grade ranging from 0 to 6. Immunofluorescence staining
was performed as previously described (21). Briefly, the floating brain sections
from all mice (n=3 per brain) were incubated with rabbit
anti-PECAM-1 (1:500; Santa Cruz Biotechnology, Inc.), mouse
anti-occludin, claudin-5, and ZO-1 (1:500; Invitrogen; Thermo
Fisher Scientific, Inc.) antibodies and secondary antibody (1:200;
Vector Laboratories), and examined with a confocal imaging system
(LSM 5 PASCAL; Carl Zeiss). The captured color images were
converted to 8-bit gray-scale images and the specific signals were
detected from a nonspecific background by a threshold value that
was set between 25 and 30. The area densities of immunofluorescent
positive area were calculated as the proportion of pixels having
higher fluorescent intensities than threshold value.
Statistical analyses
All statistical analyses were performed using SPSS
package version 21.0 (SPSS Inc.) for Windows. Multiple comparisons
were performed using one-way ANOVA with Tukey's post hoc test for
the data in Figs. 2, 3 and S1
and non-parametric Kruskal Wallis test with post hoc test for the
data in Fig. 1. All data are
presented as the mean ± SEM, and the statistical difference was
detected at a 5% level unless otherwise indicated.
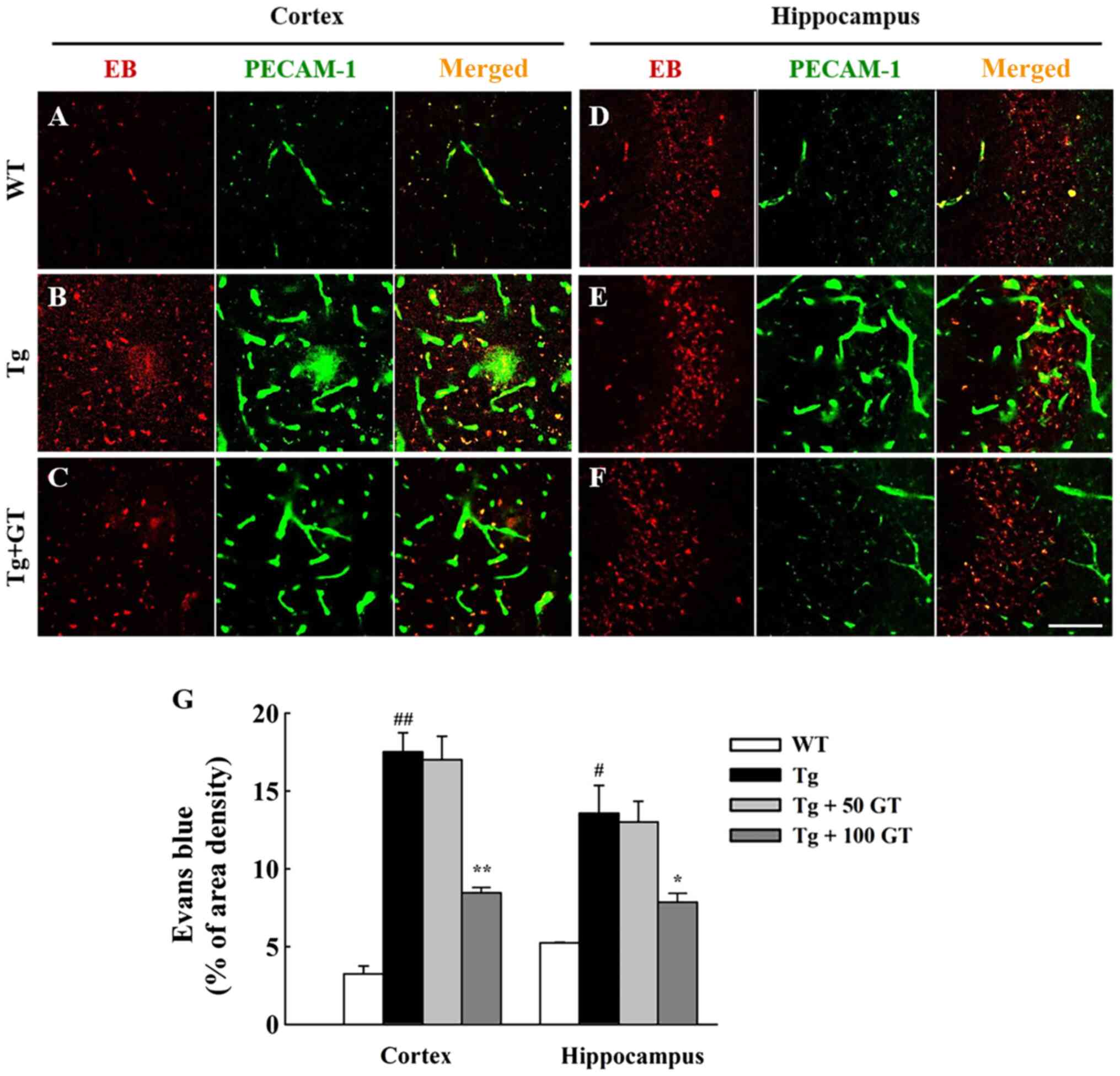 | Figure 2GEF reduced BBB permeability in the
brain of AD mice. Three months after GEF treatment (mice were 9
months old), sterilized Evans blue solution was intravenously
injected to mice of WT (n=4), AD Tg (n=4), and AD Tg + GEF (n=4)
groups. A period of 30 min after injection, brain sections (n=3 per
brain) were prepared, immunofluorescence staining was performed
using an antibody against PECAM-1. The representative pictures of
Evans blue (left panel of A-C and D-F) and PECAM-1 (middle panel of
A-C and D-F) were captured and quantified with (G) two different
dosages of GEF. Scale bar, 50 µm. #P<0.05 and
##P<0.01 vs. WT mice; *P<0.05 and
**P<0.01 vs. AD Tg mice alone. GEF, gintonin-enriched
fraction; BBB, brain-blood barrier; AD, Alzheimer's disease; WT,
wild-type; ZO-1, zonula occludens-1. |
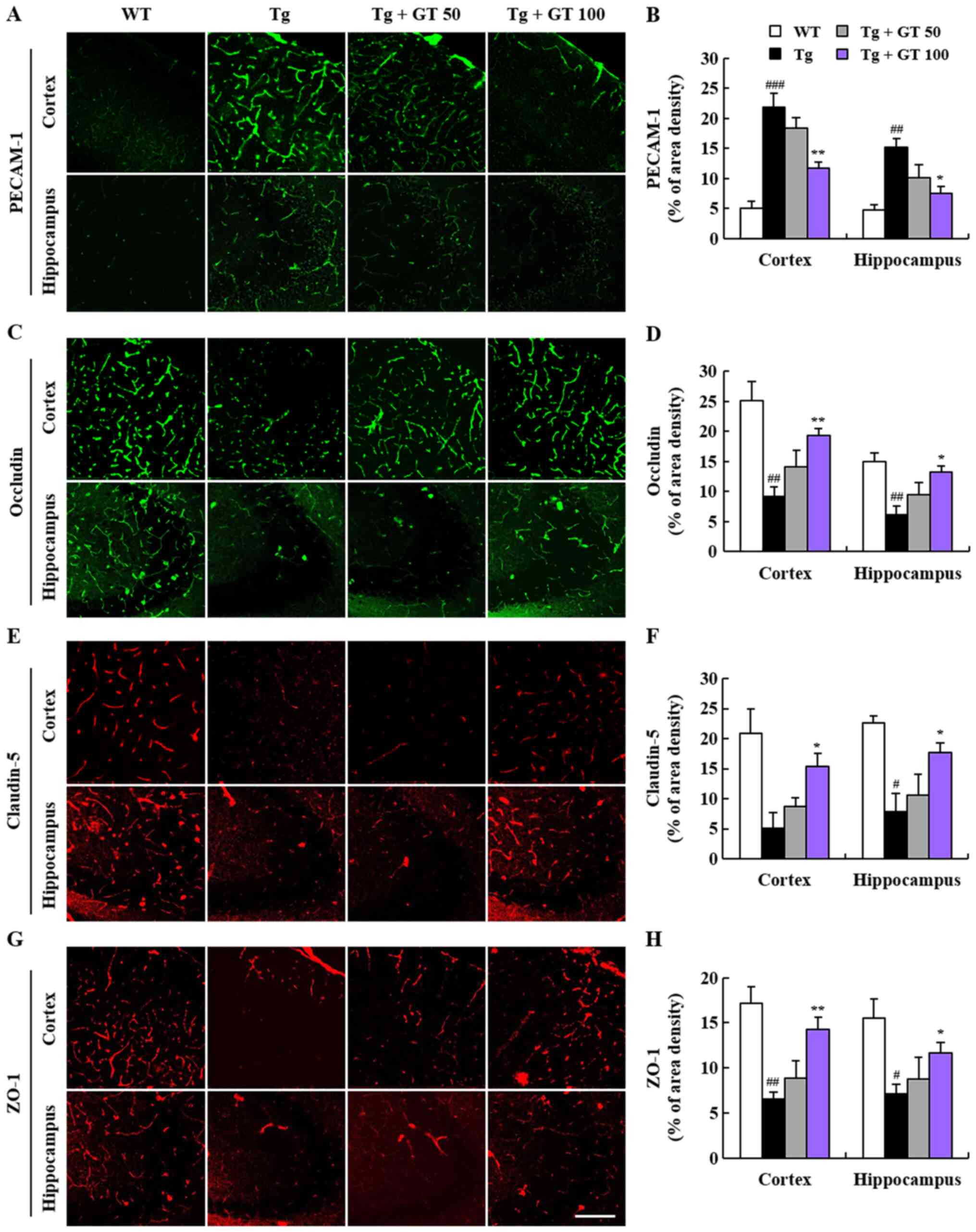 | Figure 3GEF maintained the BBB integrity in
the brain of AD mice. A period of 3 months after GEF treatment
(mice were 9 months old), brain sections (n=3 per brain) including
the parietal cortex and hippocampus (CA3) from WT (n=4), AD Tg
(n=4), and AD Tg + 50 or 100 mg/kg GEF (n=4, each) groups were
analyzed by the immunofluorescence staining using an antibodies
against (A) PECAM-1, (C) occludin, (E) claudin-5 and (G) ZO-1 and
quantified (B, D, F and H). Scale bar, 100 µm.
#P<0.05 and ##P<0.01 vs. WT mice;
###P<0.001 vs. WT mice; *P<0.05 and
**P<0.01 vs. AD Tg mice alone. GEF, gintonin-enriched
fraction; BBB, brain-blood barrier; AD, Alzheimer's disease; WT,
wild-type; ZO-1, zonula occludens-1. |
Results
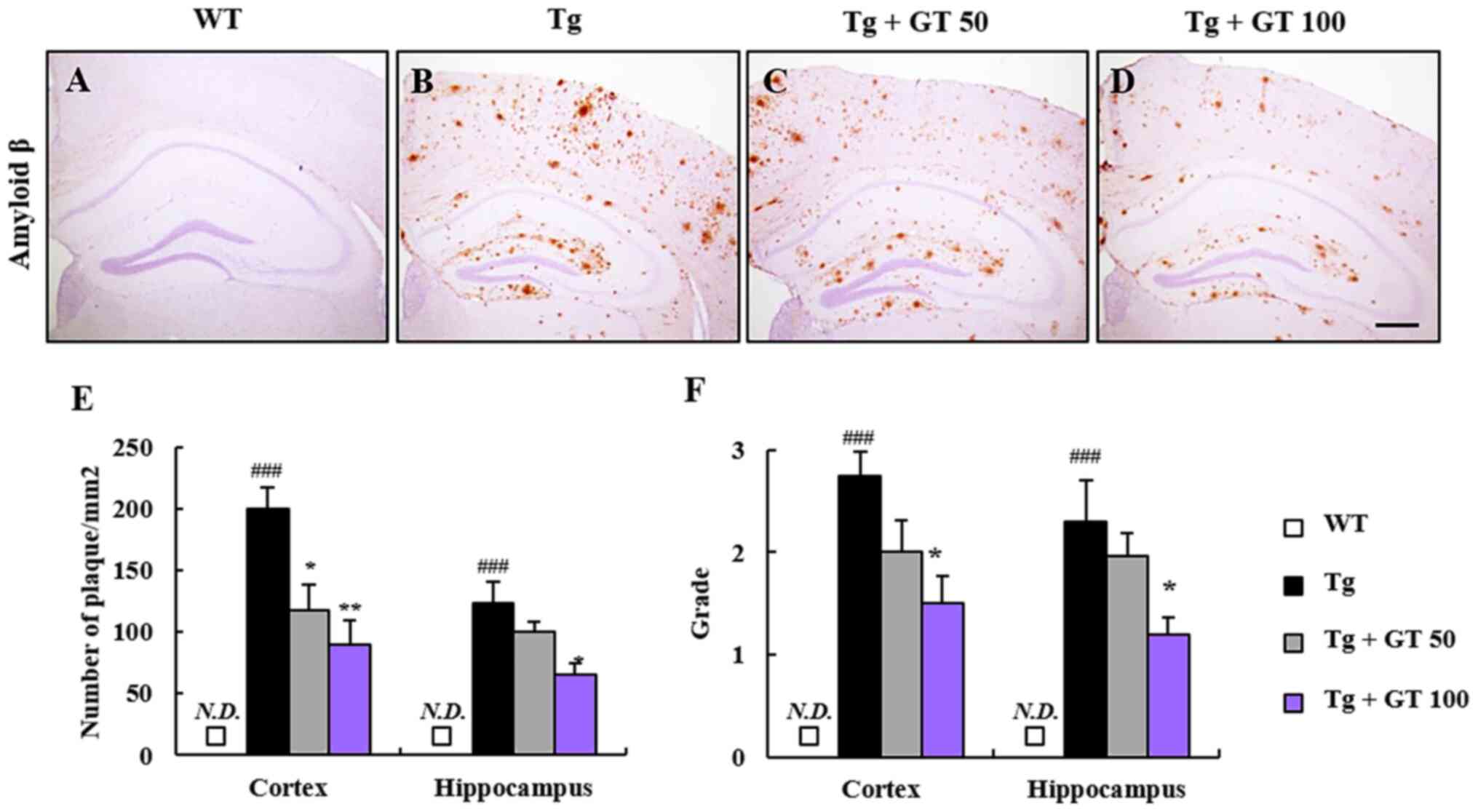
Effects of GEF on β-amyloid plaque
deposition in the brain of AD Tg mice
The six-month-old AD Tg mice were first treated with
saline or GEF (50 or 100 mg/kg, p.o.) 3 times a week for 3 months.
Next, to evaluate whether long-term oral administration of GEF
reduced brain β-amyloid depositions, we first examined the plaque
burdens in the parietal cortex and hippocampus of AD Tg mice by
immunohistochemical analyses. We observed that the number of
β-amyloid plaques and grade of β-amyloid plaque depositions were
significantly higher in AD Tg mice than that in wild-type mice
(Fig. 1A, B, E and
F), whereas the depositions were
significantly inhibited by long-term oral administration of GEF
with dose-dependent manner (Fig.
1C, D, E and F).
These results indicated that GEF has inhibitory effects against
β-amyloid plaque depositions in AD Tg mice, which is well
consistent with previous reports (17,18).
Effects of GEF on Evans blue
permeability in AD Tg mice
Since the amyloid plaque accumulations in AD brain
are associated with brain blood vessel disruptions including BBB
(12), we further examined whether
brain microvascular permeability was increased in AD Tg mice and
whether long-term oral administration of GEF can attenuate the
increased brain microvascular leakage. We used Evans blue as a
marker of BBB integrity (22). In
the present study, we found that compared to cortex and hippocampus
of WT mice (Fig. 2A, D and G),
the level of Evans blue staining was significantly higher around
the PECAM-1 positive endothelial cells in the cortex and
hippocampus of AD Tg mice (Fig. 2B,
E and G), whereas long-term oral administration
of GEF significantly inhibited the increased magnitude of Evans
blue staining and amount of the dye in cortex and hippocampus with
dose-dependent manner (Fig. 2C,
F and G). The GEF effect corroborated with the
alteration in PECAM-1 (Figs. 2 and
3), indicating that long-term
administration of GEF might assist in the maintenance of BBB
integrity in the AD animal model.
Effects of GEF on maintenance of BBB
integrity in AD Tg mice
Since long-term administration of GEF reduced the
leakage of brain microvessels in AD as observed in the Evans blue
staining (Fig. 2), we further
evaluated the effects of GEF on the disruptions of BBB components
in cortex and hippocampus that form the BBB. We first compared
PECAM-1 (CD31) protein expression in AD Tg mice with that in WT
mice as an indicator of BBB disruption (23,24).
We found that compared to WT mice, the immunofluorescence level of
PECAM-1 was higher in the cortex and hippocampus of AD Tg mice
(Fig. 3A and B), although this elevated expression was
significantly inhibited by long-term oral treatment with GEF with
dose-dependent manner (Fig. 3A and
B). Since BBB disruption is also
related to the alterations in the levels of intercellular tight
junction molecules (25,26), we also investigated whether GEF had
further protective effects on the protein expression of junctional
molecules using immunofluorescence staining. The expression levels
of occludin, claudin-5, and ZO-1, the main junctional proteins, was
downregulated in the cortex and hippocampus of AD Tg mice as
compared to WT mice (Fig. 3C-H).
However, long-term oral GEF treatment significantly prevented this
downregulation (Fig. 3C-H). We
additionally investigated whether GEF had further protective
effects on the gene expression of the representative molecules
using quantitative real-time PCR. The mRNA expression of claudin-3,
a junctional protein, was downregulated in the hippocampus of AD Tg
mice as compared to WT mice, whereas long-term GEF treatment
prevented this downregulation (Fig.
S1A). The mRNA expression of endothelial ICAM-1 and VCAM-1, two
intercellular adherens junction proteins, were upregulated in the
hippocampus of AD Tg mice as compared to WT mice, whereas GEF
treatment attenuated the upregulation of ICAM-1 and VCAM-1
expression (Fig. S1B and C). These results suggest that GEF might
exert protective effects on BBB disruption induced by β-amyloid
plaque depositions in AD Tg mice through regulation of the
differential gene or protein expression of adherens and junctional
molecules.
Discussion
The endothelium is located inside most of the blood
vessel, including the brain microvessels which consists of a
monolayer of endothelial cells. Inter-endothelial connections, such
as adherens junctions and tight junctions contain a variety of
junctional proteins (27). These
inter-connecting proteins in the endothelium contribute to the
integrity of the BBB. Considering that in vitro deposition
of β-amyloid leads to BBB disintegration and BBB dysfunctions in AD
resulting in an alteration in BBB permeability (9,28), we
investigated whether long-term treatment via oral GEF could alter
β-amyloid plaque deposition, leakage of brain microvessels, and
expression of intercellular tight junction proteins in AD Tg
mice.
In the present study, we showed that accumulations
of amyloid plaques in the brain are closely associated with
disruption of brain microvascular integrity. First, we found that
GEF administration attenuated β-amyloid plaque accumulations in
cortex and hippocampus, of which is comparable with donepezil used
as a positive drug in previous study (17). Next, we showed that GEF-mediated
attenuation of β-amyloid plaque accumulations helped in restoring
the brain microvascular integrity as observed by Evans blue test
and immunohistochemical studies with endothelial junctional
proteins such as PECAM-1, occludin, claudin-5 and ZO-1 (Figs.
1-3). In the Evans blue test, we observed that long-term
administration of GEF restored BBB permeability near to wild-type
level with 100 mg/kg GEF (Fig. 2).
Regarding the endothelial adherens proteins, long-term GEF
treatment reduced the expression of PECAM-1, ICAM-1, and VCAM-1, of
which expressions are increased by β-amyloids (11, 28). In
addition, β-amyloid induced inflammation in the brain led to
activation of microglia, increased ICAM-1 expression, and
disruption of BBB integrity (28).
GEF, however, attenuated the increased gene expression of
claudin-3, ICAM-1, and VCAM-1 near to the wild-type level in the
hippocampus of AD Tg mice (Fig.
S1). In addition, β-amyloid accumulations affected PECAM-1,
occludin, claudin-5, and ZO-1 expression level, resulting in
disruption of BBB integrity (28).
GEF, however, also attenuated the increased expression of PECAM-1
near to the wild-type level and also restored the decreased
occludin, claudin-5 and ZO-1 protein expression level in the cortex
and hippocampus of AD Tg mice near to the wild-type (Fig. 3), demonstrating that GEF-mediated
attenuation of amyloid plaque accumulations was closely related to
brain microvascular integrity.
Occludin, claudin-5, and ZO-1, members of junctional
protein, participates in the formation of tight junctions among the
brain microvascular endothelial cells. Although the selective loss
of occludin, claudin-5, and ZO-1 from BBB tight junctions is
demonstrated under different pathological conditions, such as
experimental autoimmune encephalomyelitis and human glioblastoma
multiforme (29,30), alteration in claudin-5 expression by
in vivo β-amyloid plaque accumulation is not currently
unknown. In the present study, occludin, claudin-5, and ZO-1,
expression was reduced in the cortex and hippocampus of AD Tg mice,
whereas oral administration of GEF increased the expression of
occludin, claudin-5, and ZO-1. These findings supported that
claudin-5, occludin, and ZO-1 might also be involved in the
pathology of AD by affecting tight junction integrity and GEF
treatment could restore the claudin-5, occludin and ZO-1 level
(Fig. 3).
It is noteworthy to consider the molecular
mechanisms which are involved in the beneficial effects of GEF on
the integrity of brain microvascular endothelium in AD Tg mice.
First, LPA plays a key role in angiogenesis via LPARs (8). In a previous study, we have shown that
gintonin stimulated proliferation, migration, and tube formation in
human umbilical vein endothelial cells via LPA1/3 receptor
activations (31). GEF also
increased the release of vascular endothelial growth factors (VEGF)
from human umbilical vein endothelial cells and astrocytes through
LPAR membrane signaling (31,32).
VEGF is involved in maintaining the integrity of BBB and its
ability to significantly prevent β-amyloid-induced endothelial cell
apoptosis (33,34). Thus, the GEF-mediated release of
VEGF from the brain microvascular endothelial cells and astrocytes
might contribute to the restoration of AD-induced changes in the
blood vessels and junctional proteins of the brain. Second,
β-amyloid, which induces neuroinflammation and oxidative stress in
the brain microvessels can disrupt the integrity of the brain
microvascular endothelium by modifying the inter-junctional protein
expression and microvascular permeability (35,36).
GEF-mediated anti-neuroinflammatory and anti-oxidative effects via
LPA receptors might protect the brain microvascular endothelium
from disintegrations following β-amyloid plaque deposition. In
fact, we showed that both in vitro treatment of RAW 264.7
cells with gintonin and in vivo administration of gintonin
to AD Tg and Parkinson's disease and Huntington's disease model
mice were found to inhibit microglial activation and formation of
reactive oxygen species (17,37-41).
Thus, gintonin-mediated VEGF release in brain microvessels and
astrocytes and gintonin-mediated anti-neuroinflammatory and
anti-oxidant effects might contribute to the brain microvascular
integrity in AD Tg mice.
In addition, it is worthwhile to note the
differential regulations of gintonin on the brain microvascular
functions. In a previous report, we have shown that acute
intravenous administration of gintonin opened BBB transiently and
increased BBB permeability through LPA1/3 receptor signaling
pathways (42). We further showed
that gintonin itself could enter the brain and bind to the neuronal
cells (42). Thus, previous and
present studies demonstrate that gintonin could exert differential
regulatory functions on brain BBB that might be dependent on the
route and duration of its administration. This hypothesis is
supported by the fact that after acute oral administration of the
same dose of gintonin that was used for the long-term treatment in
the AD mouse model, we could not observe any effect of gintonin on
the brain permeability through transient BBB opening (data not
shown). Thus, although it is likely that acute intravenous but not
oral administration of gintonin opens BBB transiently, further
studies are required to elucidate the molecular mechanisms of the
differential effects of gintonin on brain BBB regulation.
Taken together, the present study demonstrated that
β-amyloid plaque depositions might induce changes in the junctional
protein expressions eventually disrupting the integrity of BBB,
which is linked to the increased BBB permeability. Long-term oral
administration of GEF to AD Tg mice, however, exerted protective
effects against brain microvascular disruptions as well as
β-amyloid plaque depositions (Figs.
1-3). The present study is well-consistent with previous report
that long-term oral administration of GEF to Parkinson's disease
animal model mice also protect from BBB disruptions induced by
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (40). Thus, the present study might further
expand the previous molecular knowledges on the gintonin-mediated
anti-AD effects.
In summary, the present study showed that long-term
oral treatment of GEF attenuated the disruptions of BBB
permeability and endothelial inter-junctional proteins observed in
the brain microvessels of AD Tg mice. Taken together, the present
study demonstrated a possibility that gintonin-mediated anti-AD
effects might be achieved through mitigations of the microvascular
damages and attenuations of amyloid plaque accumulation in the
brain.
Supplementary Material
GEF maintained the BBB integrity in
the brain of AD mice. (A) Claudin-3, (B) ICAM-1 and (C) VCAM-1 mRNA
expression in the hippocampus of WT (n=4), AD Tg (n=4), and AD Tg +
50 or 100 mg/kg GEF (n=4) groups of mice were estimated using RT
PCR. #P<0.05 vs. WT mice; *P<0.05 vs.
AD Tg mice alone. GEF, gintonin-enriched fraction; BBB, brain-blood
barrier; AD, Alzheimer's disease; WT, wild-type; RT, reverse
transcription.
Acknowledgements
Not applicable.
Funding
This research was supported by the Basic Science
Research Program and Brain Research Program through the National
Research Foundation of Korea (NRF) funded by the Ministry of
Science and ICT (grant nos. NRF-2012R1A1A2008505,
NRF-2016M3C7A1905074, NRF-2016M3C7A1913845, NRF-2017R1A2A2A05069493
and NRF-2020R1F1A1058460). The current study was supported by
Konkuk University Researcher Fund in 2019.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MJ, SHC, IHC, and SYN conceived and designed the
study and drafted the manuscript. MJ, SHC, JHC, and JO performed
the experiments. MJ, SHC, JHC, RML, NEL, YJC, JO and SYN performed
the investigation. RML, NEL and YJC analyzed data and prepared
figures. HR and HCK provided the resources. HR, HCK and JO
supervised the research. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
All experimental procedures were conducted in a
blinded manner in accordance with the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The
protocol was approved by the Institutional Animal Care and Use
Committees of Kangwon (no. 13-156) and Konkuk (no. 14-956)
Universities.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yung YC, Stoddard NC, Mirendil H and Chun
J: Lysophosphatidic Acid signaling in the nervous system. Neuron.
85:669–682. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Choi JW, Herr DR, Noguchi K, Yung YC, Lee
CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, et al: LPA
receptors: Subtypes and biological actions. Annu Rev Pharmacol
Toxicol. 50:157–186. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hecht JH, Weiner JA, Post SR and Chun J:
Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid
receptor expressed in neurogenic regions of the developing cerebral
cortex. J Cell Biol. 135:1071–1083. 1996.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ladrón de Guevara-Miranda D,
Moreno-Fernández RD, Gil-Rodríguez S, Rosell-Valle C,
Estivill-Torrús G, Serrano A, Pavón FJ, Rodríguez de Fonseca F,
Santín LJ and Castilla-Ortega E: Lysophosphatidic acid-induced
increase in adult hippocampal neurogenesis facilitates the
forgetting of cocaine-contextual memory. Addict Biol. 24:458–470.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lin C-I, Chen C-N, Lin P-W, Chang K-J,
Hsieh F-J and Lee H: Lysophosphatidic acid regulates
inflammation-related genes in human endothelial cells through LPA1
and LPA3. Biochem Biophys Res Commun. 363:1001–1008.
2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ren Y, Guo L, Tang X, Apparsundaram S,
Kitson C, Deguzman J, Fuentes ME, Coyle L, Majmudar R, Allard J, et
al: Comparing the differential effects of LPA on the barrier
function of human pulmonary endothelial cells. Microvasc Res.
85:59–67. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Baker DL, Desiderio DM, Miller DD, Tolley
B and Tigyi GJ: Direct quantitative analysis of lysophosphatidic
acid molecular species by stable isotope dilution electrospray
ionization liquid chromatography-mass spectrometry. Anal Biochem.
292:287–295. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rivera-Lopez CM, Tucker AL and Lynch KR:
Lysophosphatidic acid (LPA) and angiogenesis. Angiogenesis.
11:301–310. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rosenberg GA: Blood-brain barrier
permeability in aging and Alzheimer's disease. J Prev Alzheimers
Dis. 1:138–139. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
van de Haar HJ, Burgmans S, Jansen JF, van
Osch MJ, van Buchem MA, Muller M, Hofman PA, Verhey FR and Backes
WH: Blood-brain barrier leakage in patients with early Alzheimer
disease. Radiology. 281:527–535. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zenaro E, Piacentino G and Constantin G:
The blood-brain barrier in Alzheimer's disease. Neurobiol Dis.
107:41–56. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chakraborty A, de Wit NM, van der Flier WM
and de Vries HE: The blood brain barrier in Alzheimer's disease.
Vascul Pharmacol. 89:12–18. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hwang SH, Shin TJ, Choi SH, Cho HJ, Lee
BH, Pyo MK, Lee JH, Kang J, Kim HJ, Park CW, et al: Gintonin, newly
identified compounds from ginseng, is novel lysophosphatidic
acids-protein complexes and activates G protein-coupled
lysophosphatidic acid receptors with high affinity. Mol Cells.
33:151–162. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Choi SH, Jung SW, Lee BH, Kim HJ, Hwang
SH, Kim HK and Nah SY: Ginseng pharmacology: A new paradigm based
on gintonin-lysophosphatidic acid receptor interactions. Front
Pharmacol. 6(245)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Park H, Kim S, Rhee J, Kim HJ, Han JS, Nah
SY and Chung C: Synaptic enhancement induced by gintonin via
lysophosphatidic acid receptor activation in central synapses. J
Neurophysiol. 113:1493–1500. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim S, Kim MS, Park K, Kim HJ, Jung SW,
Nah SY, Han JS and Chung C: Hippocampus-dependent cognitive
enhancement induced by systemic gintonin administration. J Ginseng
Res. 40:55–61. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hwang SH, Shin EJ, Shin TJ, Lee BH, Choi
SH, Kang J, Kim HJ, Kwon SH, Jang CG, Lee JH, et al: Gintonin, a
ginseng-derived lysophosphatidic acid receptor ligand, attenuates
Alzheimer's disease-related neuropathies: Involvement of
non-amyloidogenic processing. J Alzheimers Dis. 31:207–223.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kim HJ, Shin EJ, Lee BH, Choi SH, Jung SW,
Cho IH, Hwang SH, Kim JY, Han JS, Chung C, et al: Oral
Administration of gintonin attenuates cholinergic impairments by
scopolamine, amyloid-β protein, and mouse model of Alzheimer's
disease. Mol Cells. 38:796–805. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Choi JH, Jang M, Nah SY, Oh S and Cho IH:
Multitarget effects of Korean Red Ginseng in animal model of
Parkinson's disease: Antiapoptosis, antioxidant, antiinflammation,
and maintenance of blood-brain barrier integrity. J Ginseng Res.
42:379–388. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cho HJ, Choi SH, Kim HJ, Lee BH, Rhim H,
Kim HC, Hwang SH and Nah SY: Bioactive lipids in gintonin-enriched
fraction from ginseng. J Ginseng Res. 43:209–217. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee MJ, Jang M, Choi J, Chang BS, Kim DY,
Kim SH, Kwak YS, Oh S, Lee JH, Chang BJ, et al: Korean red ginseng
and ginsenoside-Rb1/-Rg1 alleviate experimental autoimmune
encephalomyelitis by suppressing Th1 and Th17 cells and
upregulating regulatory T cells. Mol Neurobiol. 53:1977–2002.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Saunders NR, Dziegielewska KM, Møllgård K
and Habgood MD: Markers for blood-brain barrier integrity: How
appropriate is Evans blue in the twenty-first century and what are
the alternatives? Front Neurosci. 9(385)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Flynn KM, Michaud M, Canosa S and Madri
JA: CD44 regulates vascular endothelial barrier integrity via a
PECAM-1 dependent mechanism. Angiogenesis. 16:689–705.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kalinowska A and Losy J: PECAM-1, a key
player in neuroinflammation. Eur J Neurol. 13:1284–1290.
2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Al-Obaidi MM and Desa MN: Mechanisms of
blood brain barrier disruption by different types of bacteria, and
bacterial-host interactions facilitate the bacterial pathogen
invading the brain. Cell Mol Neurobiol. 38:1349–1368.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Almutairi MM, Gong C, Xu YG, Chang Y and
Shi H: Factors controlling permeability of the blood-brain barrier.
Cell Mol Life Sci. 73:57–77. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wallez Y and Huber P: Endothelial adherens
and tight junctions in vascular homeostasis, inflammation and
angiogenesis. Biochim Biophys Acta. 1778:794–809. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Marco S and Skaper SD: Amyloid
beta-peptide1-42 alters tight junction protein distribution and
expression in brain microvessel endothelial cells. Neurosci Lett.
401:219–224. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu Z, Liu J, Wang S, Liu S and Zhao Y:
Neuronal uptake of serum albumin is associated with neuron damage
during the development of epilepsy. Exp Ther Med. 12:695–701.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wolburg H, Wolburg-Buchholz K, Kraus J,
Rascher-Eggstein G, Liebner S, Hamm S, Duffner F, Grote EH, Risau W
and Engelhardt B: Localization of claudin-3 in tight junctions of
the blood-brain barrier is selectively lost during experimental
autoimmune encephalomyelitis and human glioblastoma multiforme.
Acta Neuropathol. 105:586–592. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hwang SH, Lee BH, Choi SH, Kim HJ, Won KJ,
Lee HM, Rhim H, Kim H-C and Nah SY: Effects of gintonin on the
proliferation, migration, and tube formation of human
umbilical-vein endothelial cells: Involvement of
lysophosphatidic-acid receptors and
vascular-endothelial-growth-factor signaling. J Ginseng Res.
40:325–333. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Choi SH, Kim HJ, Cho HJ, Park SD, Lee NE,
Hwang SH, Rhim H, Kim HC, Cho IH and Nah SY: Gintonin-mediated
release of astrocytic vascular endothelial growth factor protects
cortical astrocytes from hypoxia-induced cell damages. J Ginseng
Res. 43:305–311. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Religa P, Cao R, Religa D, Xue Y,
Bogdanovic N, Westaway D, Marti HH, Winblad B and Cao Y: VEGF
significantly restores impaired memory behavior in Alzheimer's mice
by improvement of vascular survival. Sci Rep.
3(2053)2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shen F, Walker EJ, Jiang L, Degos V, Li J,
Sun B, Heriyanto F, Young WL and Su H: Coexpression of
angiopoietin-1 with VEGF increases the structural integrity of the
blood-brain barrier and reduces atrophy volume. J Cereb Blood Flow
Metab. 31:2343–2351. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Balducci C and Forloni G: Novel targets in
Alzheimer's disease: A special focus on microglia. Pharmacol Res.
130:402–413. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cai Z, Zhao B and Ratka A: Oxidative
stress and β-amyloid protein in Alzheimer's disease. Neuromolecular
Med. 13:223–250. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Saba E, Jeon BR, Jeong DH, Lee K, Goo YK,
Kwak D, Kim S, Roh SS, Kim SD, Nah SY, et al: A novel Korean red
ginseng compound gintonin inhibited inflammation by MAPK and NF-κB
pathways and recovered the levels of mir-34a and mir-93 in RAW
264.7 cells. Evid Based Complement Alternat Med.
2015(624132)2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jo MG, Ikram M, Jo MH, Yoo L, Chung KC,
Nah SY, Hwang H, Rhim H and Kim MO: Gintonin mitigates MPTP-induced
loss of nigrostriatal dopaminergic neurons and accumulation of
α-synuclein via the Nrf2/HO-1 pathway. Mol Neurobiol. 56:39–55.
2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kim HJ, Kim DJ, Shin EJ, Lee BH, Choi SH,
Hwang SH, Rhim H, Cho IH, Kim HC and Nah SY: Effects of
gintonin-enriched fraction on hippocampal cell proliferation in
wild-type mice and an APPswe/PSEN-1 double Tg mouse model of
Alzheimer's disease. Neurochem Int. 101:56–65. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Choi JH, Jang M, Oh S, Nah SY and Cho IH:
Multi-target protective effects of gintonin in
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-mediated model of
Parkinson's disease via lysophosphatidic acid receptors. Front
Pharmacol. 9(515)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jang M, Choi JH, Chang Y, Lee SJ and Nah
SY and IH: Gintonin, a ginseng-derived ingredient, as a novel
therapeutic strategy for Huntington's disease: Activation of the
Nrf2 pathway through lysophosphatidic acid receptors. Brain Behav
Immun. 80:146–162. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kim DG, Jang M, Choi SH, Kim HJ, Jhun H,
Kim HC, Rhim H, Cho IH and Nah SY: Gintonin, a ginseng-derived
exogenous lysophosphatidic acid receptor ligand, enhances
blood-brain barrier permeability and brain delivery. Int J Biol
Macromol. 114:1325–1337. 2018.PubMed/NCBI View Article : Google Scholar
|