Introduction
Osteoporosis is a major health concern throughout
the world. It primarily affects the elderly, particularly
postmenopausal women (1).
Osteoporosis is characterized by low bone mass and deterioration of
bone microarchitecture (2). In
addition, it contributes to an increase in bone fragility,
resulting in disability and mortality of the elderly (3). The worldwide incidence of osteoporotic
hip fracture is ~1.6 million each year and the incidence is
predicted to increase to 6.3 million by 2050(4). With the increasing trend in life
expectancy, the prevalence of osteoporotic hip fractures is
expected to increase. To reduce the onset of severe osteoporosis,
various antiresorptive drugs are currently used, including
alendronate and raloxifene (5).
However, their osteo-protective actions were not conclusive. In a
review, alendronate was reported to be effective in reducing both
vertebral and non-vertebral fractures in postmenopausal women as a
secondary prevention, but it did not exhibit a significant effect
on primary prevention (6). On the
other hand, raloxifene was found to be effective in reducing
vertebral fracture but not non-vertebral fractures (7). Nonetheless, it was recommended to
patients only if bisphosphonates (such as alendronate) were not
suitable for them. Notably, prolonged use of these antiresorptive
drugs may be associated with various adverse effects. For instance,
bisphosphonates may cause odd-fracture and osteonecrosis in jaw
(8), while raloxifene may increase
the risk of deep vein thrombosis (9).
Public attention regarding the use of herbal
medicines is increasing. Numerous studies have explored their
pharmacological functions to prevent or treat osteoporosis. In our
previous studies, various Chinese medicines were found to be
effective in the prevention and treatment of osteoporosis,
including Epimedii Herba, Ligustri Lucidi Fructus and
Psoraleae Fructus (10-12).
Besides herbal medicinal products, green tea may represent another
possible phyto-candidate for maintaining bone health. It is one of
the most extensively studied plants with well-regarded health
benefits and has a long history of consumption with wide safety
margins (13). Common green tea
polyphenols include (-)-epigallocatechin-3-gallate (EGCG),
(-)-epigallocatechin (EGC), (-)-epicatechin-3-gallate (ECG) and
(-)-epicatechin (EC) (14). EGCG
constitutes >50% of the total ingredients in green tea, followed
by EGC and ECG. Epidemiological evidence has demonstrated an
association between tea consumption and prevention of age-related
bone loss (15). Our group reported
that green tea extract (GTE) and common tea polyphenols such as EGC
and EGCG showed positive effects on bone metabolism through a dual
process of promoting osteoblastic activity and inhibiting
osteoclast differentiation using cultured rat osteoblast-like
osteosarcoma UMR-106 and mice monocyte/macrophage-like RAW 264.7
cell lines, respectively (16).
Furthermore, green tea polyphenols also promoted osteogenesis and
inhibited adipocyte formation in human and rat mesenchymal stem
cells (17,18). Shen et al (19,20)
reported that green tea polyphenols mitigated bone loss in
ovariectomized (OVX) and chronic inflammation-induced animal models
via increasing antioxidant capacity, and decreasing oxidative
stress damage and inflammation. Recently, the authors also found
that green tea polyphenols at higher doses suppressed bone turnover
in the trabecular and cortical bone of OVX rats and resulted in
improved cortical bone structural and biomechanical properties,
although it could not prevent the notable cancellous bone loss
induced by OVX (21). Nonetheless,
a previous randomized clinical trial reported that 1-year
supplementation of GTE daily did not modify bone mineral density
(BMD) or adiposity in overweight/obese postmenopausal women with
apparently normal bone mass (22).
The aforementioned reports revealed that GTE supplement itself does
not effectively prevent the development of osteoporosis compared
with antiresorptive drugs, even though positive results had been
demonstrated in animal models.
Both phytochemicals and pharmaceutical agents exert
individual pharmacological properties. Their combination may
complement one another and maximize the ultimate therapeutic
effect, while minimizing the adverse effects of pharmaceutical
agents by reducing their dosages. Previously, a group found that a
Chinese herbal formula (containing Epimedii Herba, Ligustri
Lucidi Fructus and Psoraleae Fructus, named ELP)
synergistically enhanced the therapeutic effect of raloxifene, but
not that of alendronate, in rats with osteoporosis (23). The different responses indicated
that the interaction between each of the herb-drug combinations is
specific and more complex than simply complementing one another.
Considering that GTE and its bioactive polyphenols can promote bone
formation while antiresorptive drugs (alendronate and raloxifene)
can inhibit bone resorption, it was hypothesized that the
combination of green tea and antiresorptive drugs may exert
synergistic effects on inhibiting osteoporosis onset. Given the
increasing popularity of consuming green tea as a health
supplement, information on the efficacy and safety of its
interaction with various pharmaceuticals is essential.
In the present study, it was hypothesized that GTE
could synergistically enhance the efficacy of antiresorptive drugs
at a low dose, and therefore their clinical dosage could be
eventually reduced. The information generated from this project
will provide novel insights for osteoporosis management through a
synergistic intervention between herbal health supplements and
conventional pharmacotherapy. The present study aimed to
investigate the synergistic effects of GTE and antiresorptive drugs
on bone protection in an OVX rat model in relation to BMD and bone
microarchitecture.
Materials and methods
Chemicals
All chemicals were purchased from Sigma-Aldrich
(Merck KGaA) unless otherwise specified. Alendronate sodium and
raloxifene hydrochloride were purchased from Merck KGaA and Eli
Lilly and Company, respectively.
Preparation and characterization of
GTE
Raw green tea leaves materials ‘E Mei Xue Ya’ were
obtained from E Mei Mountain (Sichuan, China). The herbarium
voucher specimen (reference no. GTE-1001) of the tested herb was
deposited in the Institute of Chinese Medicine, The Chinese
University of Hong Kong. For GTE preparation, the tea leaves (100
g) were brewed with 1 l hot distilled water (80˚C) 3 times (15 min
each). The infusion was then cooled to room temperature and
filtered through cellulose filter paper (0.45 µm; EMD Millipore).
The filtrate was concentrated using a vacuum rotary evaporator,
followed by freeze-drying at -50˚C overnight to produce the GTE
powder. The chemical composition of GTE was analyzed using
high-performance liquid chromatography (Fig. S1) and the method is described in
Data S1.
Model establishment and treatment
Three-month-old female Sprague-Dawley rats were used
and housed (n=3/cage) in a room at 22˚C with a 12-h light-dark
cycle. They were maintained on standard rodent chow that contained
0.9% calcium and 0.7% phosphate, and distilled water was available
ad libitum. After 1-week acclimation, the rats were
anaesthetized intraperitoneally using a cocktail of ketamine (70
mg/kg) and xylazine (10 mg/kg) and then OVX bilaterally. Animals in
the sham group underwent the same surgical procedure but without
the ligation of the oviducts or excision of the ovaries. Animal
experimentation ethics approval for this study was obtained from
the Animal Experimental Ethics Committee of The Chinese University
of Hong Kong (approval no. 13/032/MIS-5).
Three weeks after the surgical operation,
osteoporosis was developed in this animal model according to our
previous study, in which a significant decrease in total BMD in
lumbar spine, femur and tibia was induced (23). Next, animals received GTE and two
antiresorptive drugs [alendronate (A) and raloxifene (R)] via oral
administration daily using gavage for 4 weeks (Table I). GTE and both drugs were dissolved
in distilled water. The OVX and sham groups were administered the
same volume (2 ml) of distilled water. The treatment period was
designed based on our previous study, which revealed that a
significant difference in BMD between the OVX and the
alendronate/raloxifene treatment groups was identified after 4
weeks of treatment (23). Body
weight and BMD of the animals were measured weekly. At the end of
study, blood samples were obtained from the abdominal inferior vena
cava of the animals after they had been anaesthetized as mentioned
above, and the animals were then euthanized immediately via
cervical dislocation. The confirmation of death was assessed via
direct cardiac palpation to confirm lack of cardiac activity.
Femora were then harvested for microarchitectural analyses.
Uteruses were also harvested and weighed immediately (data not
shown). Completed ovariectomy was confirmed at necropsy by marked
atrophy of the uterine horns and absence of ovarian tissue.
 | Table IGrouping of the animals in the
present study (n=8/group). |
Table I
Grouping of the animals in the
present study (n=8/group).
| Group number | Group name | Treatment
received |
|---|
| 1 | Sham | Sham-operated
group |
| 2 | OVX | OVX only group |
| 3 | GTE (L) | OVX treated with
400 mg/kg GTE |
| 4 | GTE (M) | OVX treated with
800 mg/kg GTE |
| 5 | GTE (H) | OVX treated with
1,600 mg/kg GTE |
| 6 | A (L) | OVX treated with
0.05 mg/kg/day alendronate |
| 7 | A (H) | OVX treated with
0.5 mg/kg/day alendronate |
| 8 | GTE (M) + A
(L) | OVX treated with
GTE (800 mg/kg) + 0.05 mg/kg/day alendronate |
| 9 | GTE (M) + A
(H) | OVX treated with
GTE (800 mg/kg) + 0.5 mg/kg/day alendronate |
| 10 | R (L) | OVX treated with
0.62 mg/kg/day raloxifene |
| 11 | R (H) | OVX treated with
6.2 mg/kg/day raloxifene |
| 12 | GTE (M) + R
(L) | OVX treated with
GTE (800 mg/kg) + 0.62 mg/kg/day raloxifene |
| 13 | GTE (M) + R
(H) | OVX treated with
GTE (800 mg/kg) + 6.2 mg/kg/day raloxifene |
The animals were divided into 13 groups (n=8/group)
as shown in Table I. Group 1 was
sham operated. The other 12 groups of rats were OVX. Three doses of
GTE (group 3, 400 mg/kg; group 4, 800 mg/kg; and group 5, 1,600
mg/kg) were tested, according to a previous study (24), and then the optimal dose was
selected for combination studies. High dose of alendronate [group
7, A (H), 0.5 mg/kg/day] and raloxifene [group 11, R (H), 6.2
mg/kg/day] were equivalent to clinical dose [calculated from the
human equivalent dose table (25)].
Low dose (1/10 of the high dose) of alendronate [group 6, A (L)]
and raloxifene [group 10, R (L)] were also tested to determine the
dose-dependent effect of each drug. For the remaining groups,
combined treatment of GTE with high or low dose of alendronate or
raloxifene was administered to study the interactions between GTE
and various drug combinations.
Monitoring changes in BMD
From the start of the treatment (day 0), changes in
BMD at lumbar vertebra (L5), proximal tibial metaphyses and distal
femoral metaphyses of the rats were monitored weekly for 4 weeks
using peripheral quantitative computed tomography (pQCT; XCT2000;
Stratec Medizintechnik GmbH). Briefly, the animals were
anesthetized as described in the ovariectomy section. They were
then placed and secured on a custom-made translucent plastic
holder. Lumbar spine (L5), right proximal tibia and distal femurs
were scanned under the built-in research mode of pQCT. The scan
speed was 25 mm/sec with a voxel resolution of 0.2 mm. Total BMD
(BMD including both cortical and trabecular areas) was generated
and presented. The coefficient of variation of standard
measurements was <4%.
Bone microarchitecture analysis
The microarchitecture of the left distal femur was
analyzed using micro-CT (Micro CT 40; Scanco Medical AG). Briefly,
the femur was aligned perpendicularly to the scanning axis. The
scanning was conducted at 55 kVp and 144 µA with a resolution of 16
µm per voxel. The trabecular bone in the distal femur was
identified using drawn contour at each two-dimensional section
semi-automatically. Segmentation parameters were fixed as follows:
σ=0.5, support=1.0 and threshold=245. The volume of interest (VOI)
was determined within 50 continuous slices. The microarchitectural
parameters of the VOI were obtained via three-dimensional
reconstructed images using the built-in software of the micro-CT
workstation. Parameters from the direct model [bone volume fraction
(BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th) and
trabecular plate separation (Tb.Sp)] were analyzed.
Assessment of serum bone turnover
markers
Serum was obtained by centrifuging the blood samples
at 1,630 x g for 20 min at 4˚C, and was stored at -80˚C until
analysis. Osteocalcin (OC) is secreted solely by osteoblasts, and
its concentration in serum is often used as a measure of bone
formation. C-terminal telopeptide (CTX) is released into the
bloodstream during bone resorption and hence can serve as a
specific marker for the degradation of mature type I collagen in
bone. In addition, tartrate-resistant acid phosphatase 5b (TRAcP
5b) is a specific marker of osteoclasts, which are known to mediate
bone resorption (26). Hence, TRAcP
5b can also serve as an indicator of the extent of bone
resorption.
To elucidate the mechanism by which bone metabolism
is involved in the potential synergistic effect between GTE and
alendronate or raloxifene, the serum concentrations of OC, TRAcP 5b
and CTX in the groups treated with GTE in combination with drugs
exhibiting a synergistic bone protective effect at various
concentrations were measured using ELISA kits [Rat-MID™ Osteocalcin
EIA (cat. no. AC-12F1), RatTRAP™ (TRAcP 5b) ELISA (cat. no.
SB-TR102) and Serum CrossLaps® (CTX-I) (cat. no.
AC-02F1), respectively, Immunodiagnostic Systems Holdings],
according to the manufacturer's instructions. A standard curve was
generated from each kit, and the concentration of each bone
turnover marker was calculated from the corresponding standard
curve.
Statistical analysis
To determine whether BMD changes according to time
and/or different treatments, a mixed two-way ANOVA was conducted,
followed by Bonferroni's correction (n=8/group at each time point).
For other measuring parameters, the differences between treatment
and control groups were evaluated using one-way ANOVA followed by
Bonferroni's correction (n=8/group). The groups with alendronate
and raloxifene were compared separately. All the covariates were
adjusted for statistical analysis, which was performed using the
GraphPad Prism version 6.0 for Windows (GraphPad Software, Inc.).
Data are expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Changes in BMD
BMD has been long regarded as a surrogate measure of
bone strength. The present study revealed that oral GTE treatment
for 4 weeks resulted in a higher BMD on the bone compared with that
of OVX (Fig. 1A). Rats treated with
800 mg/kg GTE exhibited significantly higher total BMD (4.24%) and
trabecular BMD (9.62%) in the distal femur compared with OVX. Those
treated with 1,600 mg/kg GTE significantly increased their total
and trabecular BMD by 10.16 and 23.99%, respectively, in the
proximal tibia compared with the findings in OVX rats. In the
lumbar spine, trabecular BMD in the 800 mg/kg GTE-treated group was
also 7.48% higher than that of OVX, and the difference was
significant. GTE oral administration increased BMD in a
dose-dependent manner in proximal tibia. These results suggested
that GTE reduced the BMD loss of lumbar spine, distal femur and
proximal tibia starting at doses of ≥800 mg/kg.
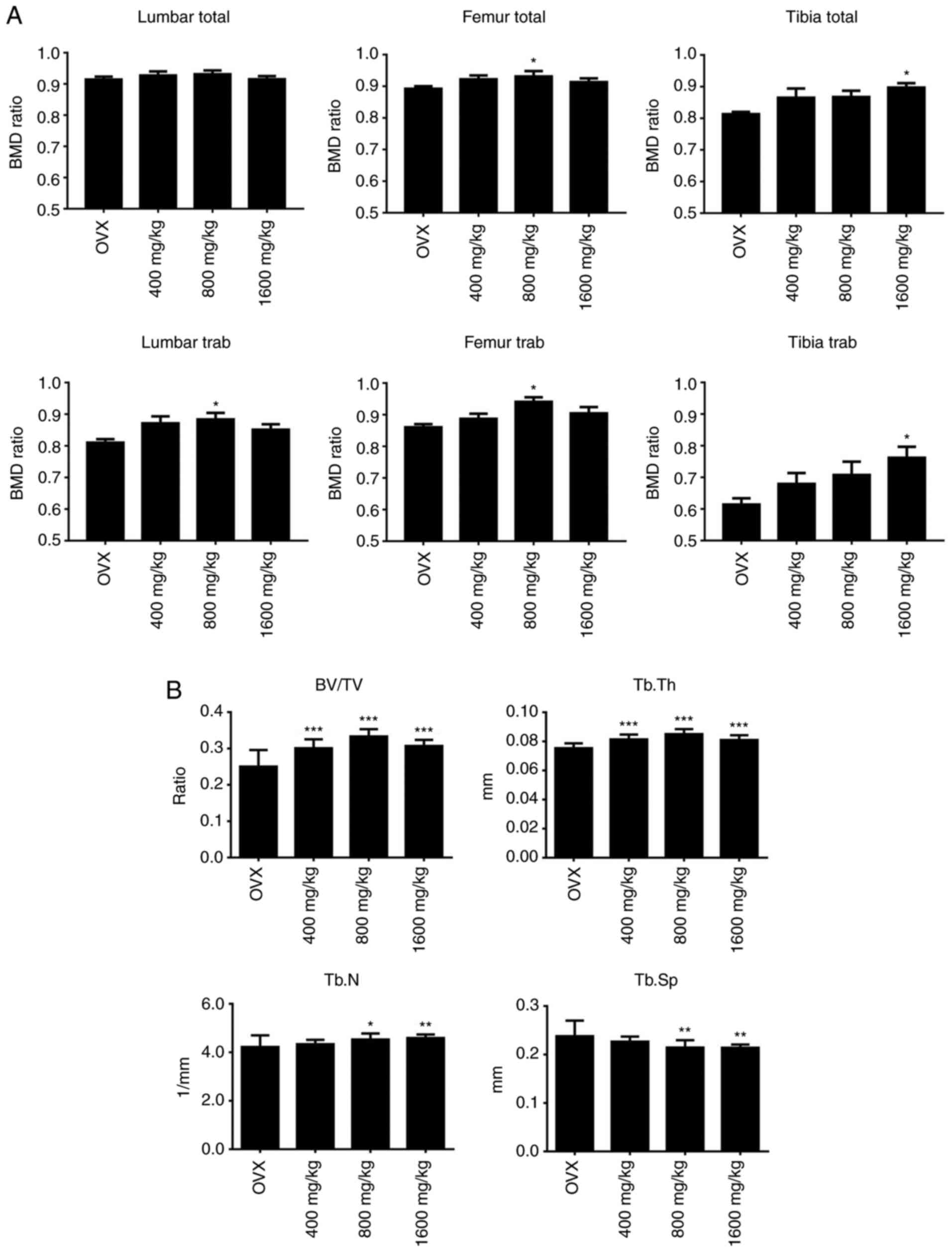 | Figure 1Effect of GTE at different
concentrations on the bone of osteoporotic rat after 4 weeks of
treatment. (A) Mean of the ratio from baseline (Day 0) of total and
Trab BMD in lumbar spine, distal femur and proximal tibia measured
by pQCT; (B) Differences in BV/TV, Tb.N, Tb.Th and Tb.Sp at the
metaphysis of the distal femur measured by micro-CT. The error bar
represents the + SD. *P<0.05; **P<0.01;
***P<0.001 vs. OVX without treatment. GTE, green tea
extract; OVX, ovariectomized; BV/TV, trabecular bone volume; Tb.N,
trabecular number; Tb.Th, trabecular thickness; Tb.Sp, trabecular
separation; Trab, trabecular; BMD, bone mineral density. |
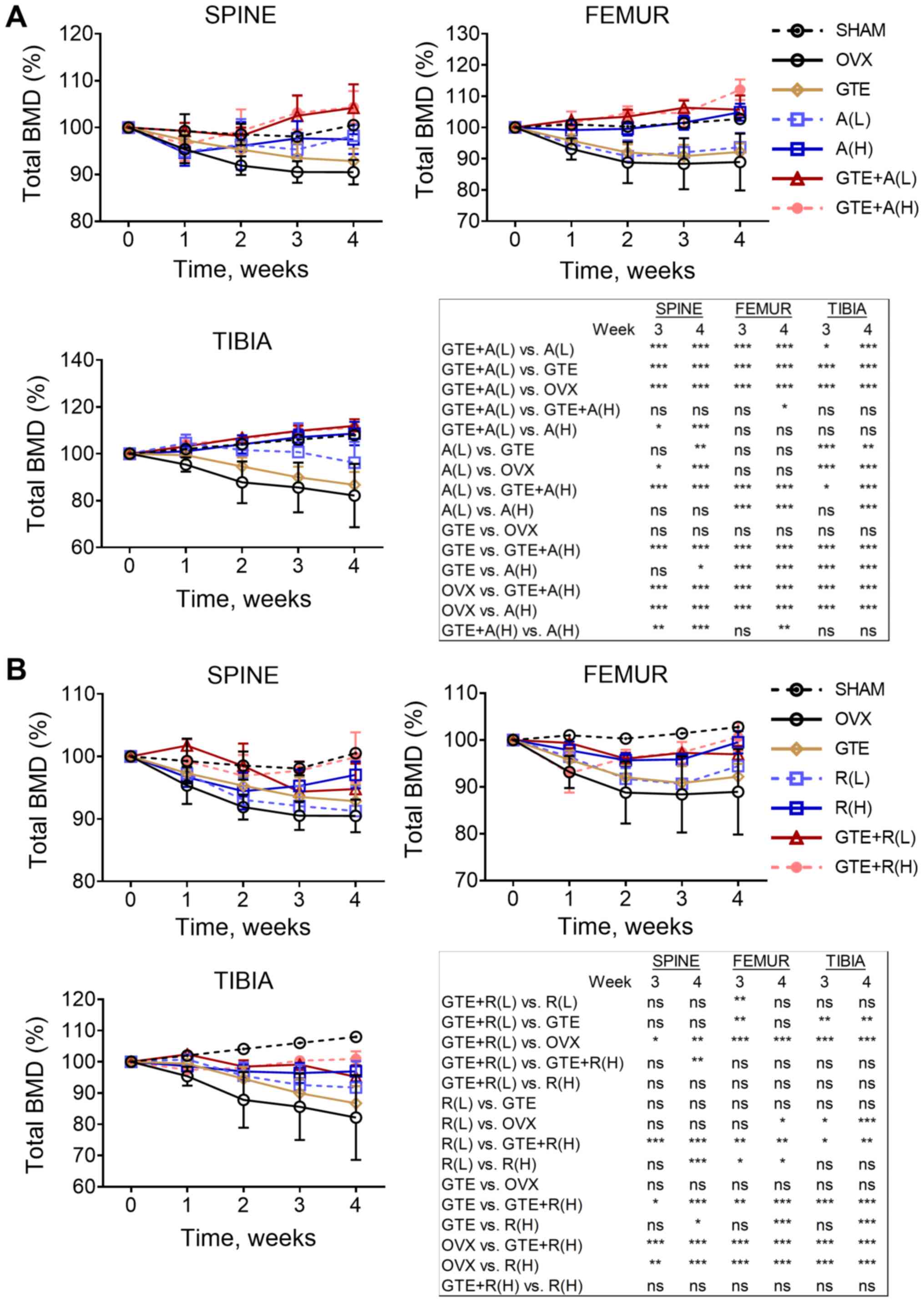
In the sham group, an overall increase in total BMD
was observed in lumbar spine, distal femur and proximal tibia
(Fig. 2). The effect of ovariectomy
on the reduction of total BMD was prominent in these regions of the
OVX group. Total BMD of the OVX group continued to decrease
throughout the 4 weeks of treatment, particularly in proximal tibia
(Fig. 2). Those 11 treatment groups
had a significantly higher BMD at the lumbar spine than the OVX
group from week 2 onwards. Regarding treatment with the
antiresorptive drug alone, alendronate exhibited a dose-dependent
protective effect on BMD in both femur and tibia (Fig. 2A), in contrast to raloxifene
(Fig. 2B). Both A (H) and R (H)
significantly increased total BMD in all bone regions compared with
the findings in the OVX group. The protective effect of alendronate
was higher than that of raloxifene. Compared with the baseline
value, total BMD in femur and tibia of rats treated with A (H) was
significantly higher at weeks 3 and 4 [104.88% (P<0.001) and
108.59% (P=0.004) for femur and tibia, respectively], but these
significant differences were not found in rats treated with R (H)
[99.52 and 96.94% for femur and tibia, respectively, at week 4
(P>0.05 for both)].
In the combination studies, the data demonstrated
that GTE worked synergistically with alendronate in increasing BMD.
Compared with the findings in the OVX group, co-treatment with GTE
and A (L) significantly increased total BMD in all bone regions at
weeks 3 and 4. However, A (L) alone did not exhibit a significant
effect in reducing total BMD in distal femur. GTE was also found to
enhance the effect of A (H) on total BMD compared with that of A
(H), and significant differences were found in spine (weeks 3 and
4) and femur (week 4) (Fig. 2A).
Overall, co-treatment of GTE and A (L) was found to be the most
effective combination to reduce total BMD loss among the groups.
Notably, the combination of GTE and raloxifene did not result in
any synergistic effect on the reduction of total BMD loss,
regardless of the concentrations of raloxifene (Fig. 2B).
Differences in bone
microarchitecture
To further evaluate the impact of different GTE-drug
combinations on the quality of trabecular bone, bone
microarchitectural properties of the distal femur were analyzed.
All the GTE treatment groups showed improvements in the
microarchitectural properties of the trabecular bone over the OVX
group. Treatment with 400 mg/kg GTE significantly increased the
BV/TV and Tb.Th compared with the OVX group. Treatment of 800 and
1,600 mg/kg GTE significantly increased all the BV/TV, Tb.N and
Tb.Th, whereas it decreased the Tb.Sp, compared with the OVX group
(Fig. 1B).
Both alendronate and raloxifene treatment resulted
in an improvement in the bone microarchitectural properties at the
distal femur (Fig. 3). Similar to
the total BMD analysis, alendronate treatment resulted in
increasing trends in BV/TV, Tb.N and Tb.Th, and a decreasing trend
in Tb.Sp, as the dosage of alendronate increased [A(H) compared
with A(L)] (Fig. 3A), while
raloxifene treatment did not (Fig.
3B). Notably, the combination of GTE and A (L) or R (L)
significantly increased Tb.N compared with the findings in the OVX
group. This osteo-protective effect could not be observed either in
GTE, A (L) or R (L) alone. Rats treated with low-dose alendronate
and GTE simultaneously further increased their Tb.N compared with
that of rats treated with low-dose alendronate alone. The effect of
low-dose alendronate plus GTE on bone microarchitecture was
comparable to that of treatment with high-dose alendronate alone,
but no statistically significant difference was observed.
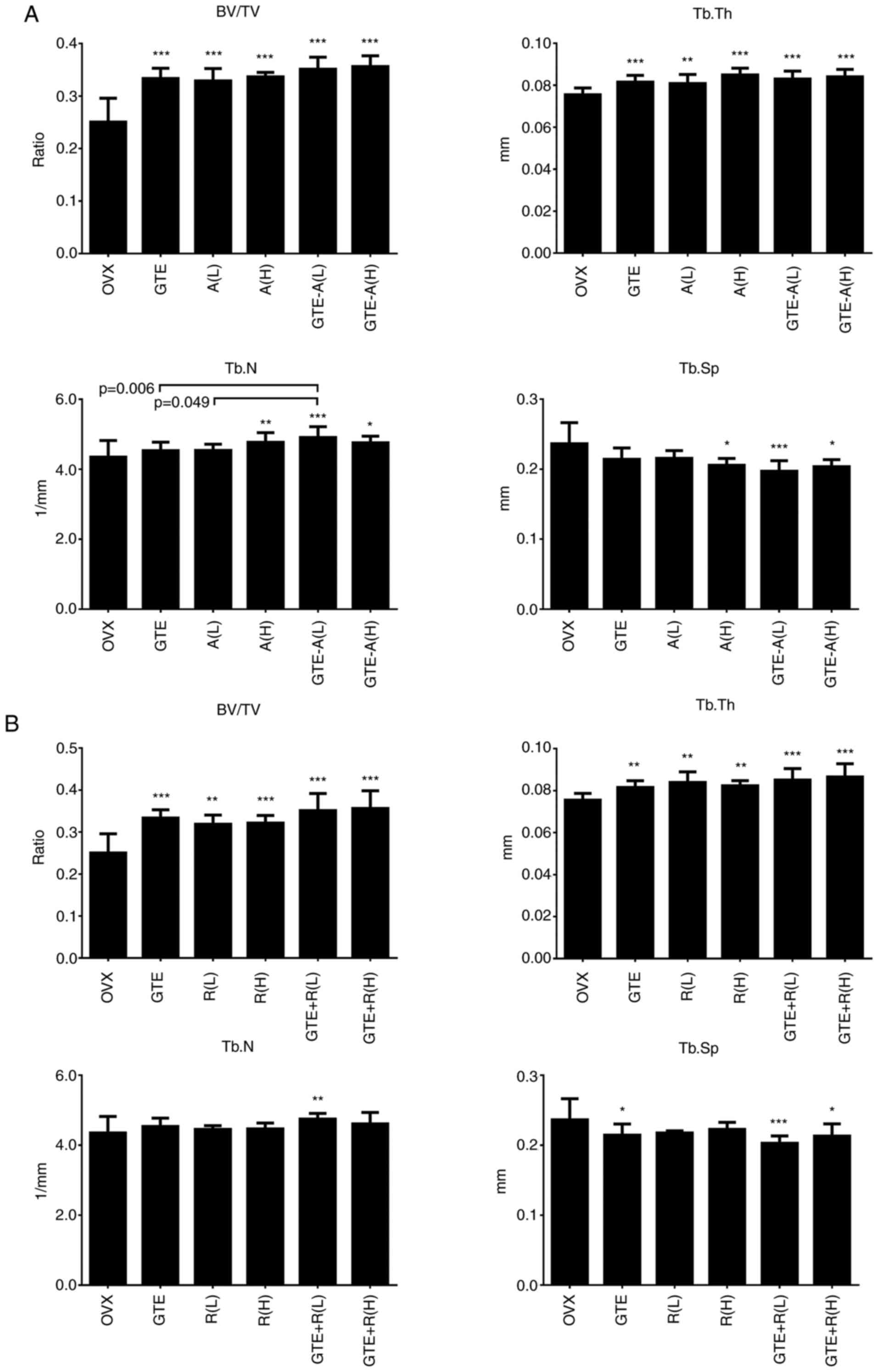 | Figure 3Difference in microarchitectural
properties at the distal femur of rats co-treated with GTE and
antiresorptive drugs. Mean of trabecular bone volume (BV/TV),
trabecular number (Tb.N), trabecular thickness (Tb.Th), and
trabecular separation (Tb.Sp) after 4 weeks of co-treatment with
(A) A and (B) R. The error bar represents the + SD.
*P<0.05; **P<0.01;
***P<0.001 vs. OVX without treatment. GTE, green tea
extract; OVX, ovariectomized; BV/TV, trabecular bone volume; Tb.N,
trabecular number; Tb.Th, trabecular thickness; Tb.Sp, trabecular
separation; L, low dose; H, high dose; A, alendronate; R,
raloxifene. |
Differences in serum biochemical
markers
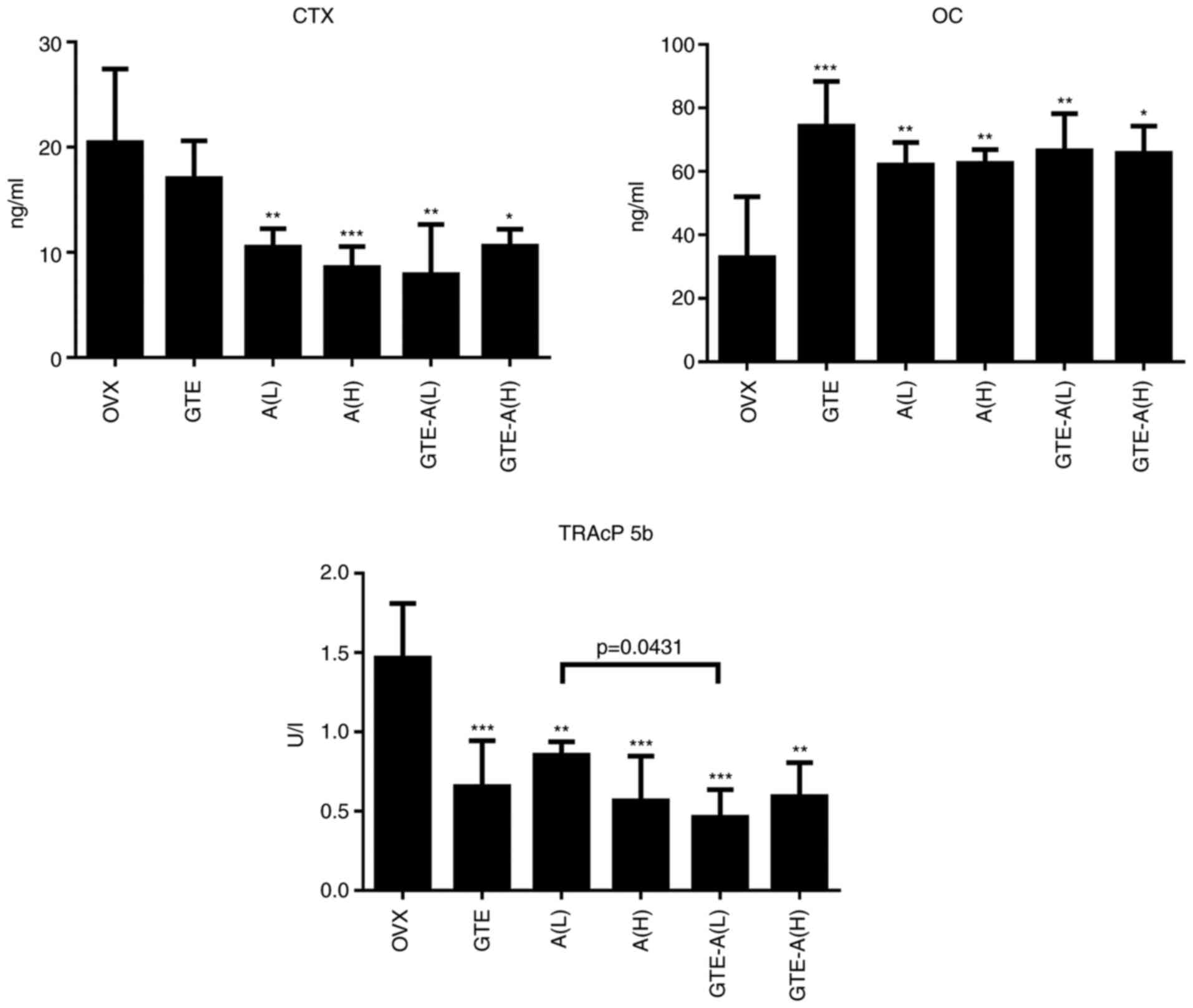
A prominent synergistic protective effect on bones
was observed following treatment with GTE and alendronate.
Therefore, measurement of serum biochemical markers in the groups
treated with GTE and alendronate (alone or in combination) at
various concentrations was conducted.
After 4 weeks of treatment, the serum CTX
concentration was reduced effectively by alendronate at both low
(0.05 mg/kg/day) and high (0.5 mg/kg/day) concentrations (Fig. 4). GTE alone significantly increased
the serum OC level, which was similar to the effect of alendronate
at both low and high concentrations. Nevertheless, no synergistic
effect was observed when GTE was co-administered with alendronate.
GTE nor alendronate (at both concentrations) alone could
significantly reduce the serum TRAcP 5b level compared with the
findings in the OVX group. Notably, the combination of GTE and
alendronate at low concentration synergistically decreased the
TRAcP 5b level significantly when compared with the effect of
alendronate at low level alone. Collectively, these findings
indicated an important role of GTE in reinforcing the effects of
alendronate on enhancing bone formation and inhibiting bone
resorption.
Discussion
In the present study, a synergistic effect between
GTE and alendronate on reduction of osteoporotic bone loss caused
by ovariectomy was identified. Particularly, GTE was demonstrated
to enhance the effect of a low dose of alendronate on inhibiting
bone resorption, as indicated by a decrease in TRAcP 5b level. The
combination of GTE and alendronate at low doses improved the bone
microarchitectural properties (BV/TV, Tb.Th and Tb.N of the
trabecular bone in distal femur) as well as BMD, and significant
differences were found between GTE + A (L) and either A (L) or GTE
alone.
Regarding the antiresorptive drug raloxifene, it was
observed that the addition of GTE to raloxifene at all
concentrations prevented BMD loss at lumbar spine, distal femur and
proximal tibia, and improved the bone microarchitectural properties
in the femur compared with the findings in the OVX group. Compared
with the effects of treatment with raloxifene alone, however, the
presence of GTE did not result in significant differences in any of
the parameters evaluated. Notably, our group previously observed
that the extract of a Chinese herbal formula (ELP) worked
synergistically with raloxifene in increasing the BMD of osteopenic
bone in an OVX rat model (23).
This synergistic effect was further substantiated by bone
microarchitecture analysis. The discrepancy between the results of
the two studies may be due to the different compositions of the two
herbal extracts. The main composition of green tea is tea
polyphenols, which is absent in ELP. In the present study, the
composition of the tea polyphenols in the GTE was similar to that
of the green tea polyphenols in previous studies conducted by Shen
et al (20,21). In all of these studies, the most
abundant catechin was EGCG, followed by ECG and EGC. A small
quantity of catechin was also identified. On the other hand, some
estrogenic compounds in ELP may work synergistically with
raloxifene to result in osteo-protection. Raloxifene is an oral
selective estrogen receptor modulator that has estrogenic actions
on inhibiting bone resorption (5,27).
When ELP is combined with raloxifene, the stimulation of
osteoblasts by ELP may have an additive effect in the
anti-osteoclastic action of raloxifene. Besides, the difference in
treatment period and osteoporotic conditions between the two
studies may also attribute to the discrepancy in the results. The
treatment period of the ELP study was 8 weeks, compared with the
4-week GTE treatment period in the current study.
A study by Wu et al (24) reported that the highest dose of GTE
selected was 370 mg/kg. It was also the effective dose to improve
femoral BMD of OVX rats. However, in the current study, the lowest
dose selected was 400 mg/kg and effective dose was 800 mg/kg.
Considering that BMD was the primary outcome of our study and with
reference to the study by Wu et al, it was hypothesized that
370 mg/kg as the minimum effective dose. To make the calculation
simple, our team started the minimum dose from 400 mg/kg, and then
doubled the concentration in order to see if there will be a
dose-dependent effect of the GTE on improving BMD. The difference
in the effective dose between the two studies might be due to the
differences in: i) Species of green tea-Wu et al studied
‘Yunnan Daye’ (Camellia sinensis [Linn.] var. assamica
[Masters] Kitamura) from the Yunnan province of China while our
team studied ‘E Mei Xue Ya’ from the Sichuan province of China.
They may have different chemical composition (different
concentrations of alkaloids and catechins); ii) Extraction method:
Wu et al extracted 100 g of green tea twice using 1,200 ml
of water each time (1.5 h) under reflux (100˚C) while our team
brewed 100 g of green tea with 1,000 ml hot distilled water (80˚C)
3 times. This difference may alter the concentrations of chemical
composition of GTE; iii) species of animal model; and iv) treatment
protocol-Wu et al started the GTE treatment after 2 weeks of
the OVX and the treatment this lasted for 13 weeks. However, the
present study started the GTE treatment after 3 weeks of the OVX
and the treatment period was only 4 weeks. This meant that
osteoporosis had reached a more severe condition than Wu et
al while the treatment period was shorted than the duration
selected. A significant difference in femoral BMD may have been
observed at 400 mg/kg of GTE in the current study, if the treatment
period was extended to 13 weeks.
Raloxifene is not considered the first-line
preventive measure against osteoporosis, compared with
bisphosphonates (28). The current
data supported this clinical observation. As a preventive measure,
alendronate is more effective than raloxifene, with or without GTE.
To the best of our knowledge, the present study was the first to
demonstrate that GTE and alendronate have a synergistic effect on
preventing the reduction in total BMD, suggesting the adjuvant use
of this combination in the prevention of osteoporosis. Although the
present findings demonstrated that the synergistic effect of GTE
and raloxifene on osteo-protection was lower than that of GTE and
alendronate, it was confirmed that the combination treatment (GTE +
R) has beneficial effects compared with R treatment alone.
It is well documented that green tea exhibits
antiobese (29), hypolipidemic and
hypoglycaemic properties, hence ameliorating cardiovascular
diseases (CVD) (30). However, the
negative effects of caffeine in green tea on human behaviors and
sleep deprivation (31) are
concerned by the majority of people. The present study demonstrated
that green tea is beneficial for maintaining bone health in a rat
model. Despite this, a high dose of green tea should still be
avoided for individuals with multiple chronic conditions. The
primary goal of this study was to examine potential drug
interactions with GTE. It was found that GTE could synergistically
enhance the osteo-protective effects of alendronate and reduce the
dose of alendronate required to achieve its biological effects.
Therefore, GTE may be employed in a novel combination with
bisphosphonate agents for the management of osteoporotic
progression in osteopenic individuals. The current findings justify
clinical studies using GTE and standard antiresorptive agents
together in an attempt to counteract osteoporosis.
It is generally considered that green tea is safe,
based on its nature and long dietary history. However, previous
studies indicated that green tea may reduce the anti-coagulant
effect of warfarin, and the absorption of folic acid and statin
(32,33). The present study indicated that the
adverse events caused by green tea to the interaction with
antiresorptive drugs were minimal, based on observation no loss of
body weight or abnormal behavior (Fig.
S2). From the measurement of the body weight within the 4 weeks
of the treatment period, the results indicated that various doses
of GTE (Fig. S2A) and the
combinations of the medium dose of GTE with alendronate or
raloxifene (Fig. S2B) did not
cause significant change in body weight when compared with OVX
without treatment. Further in vivo studies are still
required to evaluate the effect of GTE on the pharmacokinetics of
alendronate and raloxifene, and on the activities of drug
metabolizing enzymes (such as CYP3A) and uptake of efflux
transporters (such as P-glycoprotein).
Due to the limited sample size, histomorphometrical
analysis or molecular assessment to support the results of the
changes in bone remodeling markers and to elucidate the molecular
pathway of bone turnover could not be conducted.
In conclusion, to the best of our knowledge, this
was the first comprehensive study to demonstrate the synergistic
effect of GTE and anti-osteoporosis drugs on preserving BMD,
improving bone microarchitecture and ultimately preventing
osteoporosis in a rat model. The findings of the present study may
justify the initiation of clinical trials on supplementing green
tea in addition to standard antiresorptive agents to promote bone
health. With the new synergistic intervention between food therapy
and pharmacotherapy, these trials are expected to have a long-term
impact on the elderly by alleviating the increasing trend in the
incidence of osteoporosis.
Supplementary Material
Green tea extract preparation and
characterization. High-performance liquid chromatography (HPLC)
analysis was performed using Agilent 1100 series HPLC System,
equipped with G1329A ALS Auto-sampler and G1315A Diode Array
Detector (Agilent Technologies, Inc.). Sample solution was injected
onto a Supelco Discovery RP Amide C16 guard column (15 cm x 4.6 mm,
5 μm; Sigma-Aldrich; Merck KGaA). A gradient elution was
carried out using the following solvent systems: Mobile phase A,
0.05 -phosphoric acid; mobile phase B, acetonitrile. The elution
was performed with a gradient procedure: 0-1 min, 2% B; 2-30 min,
from 2% B to 50% B. The sample injection volume was 10 μl.
Elution was performed at a solvent flow rate of 0.8 ml/min. A
standard mixture which contains caffeine (CAF), epigallocatechin
(EGC), catechin (C), epigallocatechin gallate (EGCG) and
epicatechin gallate (ECG) in methanol was prepared and analyzed.
Purine alkaloid and catechin compounds were identified by comparing
the retention time and spectral data with those of authentic
standards. All analyses were repeated three times. The HPLC
chemical profile of GTE was shown in Fig. S1. The main alkaloid in GTE was
caffeine (CAF; 3.12±0.05%) while the major catechin was EGCG
(9.52±0.05%). Particularly, the relative compositions of catechins
in green tea increased in the order: C<EGC<ECG<EGCG. The
yield of GTE was 32.05% (g/10 g).
Alkaloids and catechins contents in
GTE. Detection was performed using Agilent 1100 series HPLC System
with a Supelco Discovery RP Amide C16 guard column at UV 210 nm.
High Performance Liquid Chromatography of GTE is depicted. CAF,
caffeine; EGC, epigallocatechin; C, catechin; EGCC,
epigallocatechin gallate; ECG, epicatechin gallate.
Change of rat mean body weight from
week 0 (baseline) to week 4. Rat body weight with different
treatment was illustrated: (A) GTE only in L, M or H doses; and (B)
with anti-resorptives A or R. The error bar represents the SEM for
each treatment group (n=8 per group). GTE, green tea extract; OVX,
ovariectomized; A, alendronate; R, raloxifene; L, low dose; M,
medium dose; H, high dose.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Health and
Medical Research Fund of Food and Health Bureau, Hong Kong Special
Administration Region (grant no. 11120301).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WSS verified the methodology, established the animal
model, analyzed and interpreted the data and was a major
contributor in writing, revision and editing of the manuscript. CHK
designed the experiments, analyzed the data and contributed in the
writing of the original manuscript. HTS performed the assessment of
the serum bone turnover markers, analyzed the data and contributed
in the writing of the original manuscript. KKL sourced the green
tea, prepared and characterized the green tea extract, performed
the animal experiment and BMD measurement. WTS performed
measurement and analyzed the bone microarchitecture. PCL formed the
concept and idea of the study, acquired funding source and
supervised the overall study. JFZ formed the concept and idea of
the study and help in acquisition of funding source. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Animal Experimentation Ethics Approval was obtained
from the Animal Experimental Ethics Committee of The Chinese
University of Hong Kong (ref no. 13/032/MIS-5) for the present
study. All the animal experiments complied with the ARRIVE
guidelines and were carried out in accordance with the U.K. Animals
(Scientific Procedures) Act, 1986 and associated guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Riggs BL and Melton LJ III: The worldwide
problem of osteoporosis: Insights afforded by epidemiology. Bone.
17 (17 Suppl):505S–511S. 1995.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lane NE: Epidemiology, etiology, and
diagnosis of osteoporosis. Am J Obstet Gynecol. 194 (2
Suppl):S3–S11. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Varacallo MA and Fox EJ: Osteoporosis and
its complications. Med Clin North Am. 98:817–831. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
World Health Organization (WHO): WHO
Scientific Group on the Assessment of Osteoporosis at Primary
Health Care Level. Summary Meeting Report, Brussels, Belgium, 5-7
May 2004. WHO Press, Geneva, 2007.
|
|
5
|
Kling JM, Clarke BL and Sandhu NP:
Osteoporosis prevention, screening, and treatment: A review. J
Womens Health (Larchmt). 23:563–572. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wells GA, Cranney A, Peterson J, Boucher
M, Shea B, Robinson V, Coyle D and Tugwell P: Alendronate for the
primary and secondary prevention of osteoporotic fractures in
postmenopausal women. Cochrane Database Syst Rev.
23(CD001155)2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nelson HD, Haney EM, Dana T, Bougatsos C
and Chou R: Screening for osteoporosis: An update for the U.S.
Preventive services task force. Ann Intern Med. 153:99–111.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pendrys DG and Silverman SL: Osteonecrosis
of the jaws and bisphosphonates. Curr Osteoporos Rep. 6:31–38.
2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Andreopoulou P and Bockman RS: Management
of postmenopausal osteoporosis. Annu Rev Med. 66:329–342.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ko CH, Siu WS, Lau CP, Lau CBS, Fung KP
and Leung PC: Osteoprotective effects of fructus ligustri lucidi
aqueous extract in aged ovariectomized rats. Chin Med.
5(39)2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li G, Zhang XA, Zhang JF, Chan CY, Yew
DTW, He ML, Lin MCM, Leung PC and Kung HF: Ethanol extract of
fructus ligustri lucidi promotes osteogenesis of mesenchymal stem
cells. Phyther Res. 24:571–576. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Zhang JF, Li G, Chan CY, Meng CL, Lin MCM,
Chen YC, He ML, Leung PC and Kung HF: Flavonoids of herba epimedii
regulate osteogenesis of human mesenchymal stem cells through BMP
and Wnt/β-catenin signaling pathway. Mol Cell Endocrinol.
314:70–74. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hu J, Webster D, Cao J and Shao A: The
safety of green tea and green tea extract consumption in
adults-results of a systematic review. Regul Toxicol Pharmacol.
95:412–433. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Khan N and Mukhtar H: Tea and health:
Studies in humans. Curr Pharm Des. 19:6141–6147. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hegarty VM, May HM and Khaw KT: Tea
drinking and bone mineral density in older women. Am J Clin Nutr.
71:1003–1007. 2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ko CH, Lau KM, Choy WY and Leung PC:
Effects of tea catechins, epigallocatechin, gallocatechin, and
gallocatechin gallate, on bone metabolism. J Agric Food Chem.
57:7293–7297. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kashiwa K, Kotobuki N, Tadokoro M,
Matsumura K, Hyon SH, Yoshiya S and Ohgushi H: Effects of
epigallocatechin gallate on osteogenic capability of human
mesenchymal stem cells after suspension in phosphate-buffered
saline. Tissue Eng Part A. 16:91–100. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ko CH, Siu WS, Wong HL, Shum WT, Fung KP,
Lau CBS and Leung PC: Pro-bone and antifat effects of green tea and
its polyphenol, epigallocatechin, in rat mesenchymal stem cells in
vitro. J Agric Food Chem. 59:9870–9876. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shen CL, Wang P, Guerrieri J, Yeh JK and
Wang JS: Protective effect of green tea polyphenols on bone loss in
middle-aged female rats. Osteoporos Int. 19:979–990.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shen CL, Yeh JK, Samathanam C, Cao JJ,
Stoecker BJ, Dagda RY, Chyu MC and Wang JS: Protective actions of
green tea polyphenols and alfacalcidol on bone microstructure in
female rats with chronic inflammation. J Nutr Biochem. 22:673–680.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shen CL, Smith BJ, Li J, Cao JJ, Song X,
Newhardt MF, Corry KA, Tomison MD, Tang L, Wang JS and Chyu MC:
Effect of long-term green tea polyphenol supplementation on bone
architecture, turnover, and mechanical properties in middle-aged
ovariectomized rats. Calcif Tissue Int. 104:285–300.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dostal AM, Arikawa A, Espejo L and Kurzer
MS: Long-term supplementation of green tea extract does not modify
adiposity or bone mineral density in a randomized trial of
overweight and obese postmenopausal women. J Nutr. 146:256–264.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ko CH, Siu WS, Wong HL, Gao S, Shum WT,
Lau CP, Cheng SW, Tam JCW, Hung LK, Fung KP, et al: In vivo study
on the pharmacological interactions between a chinese herbal
formula ELP and antiresorptive drugs to counteract osteoporosis.
Evid Based Complement Altern Med. 2012(203732)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wu X, Xie CQ, Zhu QQ, Wang MY, Sun B,
Huang YP, Shen C, An MF, Zhao YL, Wang XJ and Sheng J: Green tea
(Camellia sinensis) aqueous extract alleviates
postmenopausal osteoporosis in ovariectomized rats and prevents
RANKL-induced osteoclastogenesis in vitro. Food Nutr Res.
8(62)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nair AB and Jacob S: A simple practice
guide for dose conversion between animals and human. J Basic Clin
Pharm. 7:27–31. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Halleen JM, Tiitinen SL, Ylipahkala H,
Fagerlund KM and Väänänen HK: Tartrate-resistant acid phosphatase
5b (TRACP 5b) as a marker of bone resorption. Clin Lab. 52:499–509.
2006.PubMed/NCBI
|
|
27
|
Rey JR, Cervino EV, Rentero ML, Crespo EC,
Alvaro AO and Casillas M: Raloxifene: Mechanism of action, effects
on bone tissue, and applicability in clinical traumatology
practice. Open Orthop J. 3:14–21. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pavone V, Testa G, Giardina SMC, Vescio A,
Restivo DA and Sessa G: Pharmacological therapy of osteoporosis: A
systematic current review of literature. Front Pharmacol.
8(830)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dulloo AG, Seydoux J, Girardier L, Chantre
P and Vandermander J: Green tea and thermogenesis: Interactions
between catechin-polyphenols, caffeine and sympathetic activity.
Int J Obes Relat Metab Disord. 24:252–258. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chacko SM, Thambi PT, Kuttan R and
Nishigaki I: Beneficial effects of green tea: A literature review.
Chin Med. 5(13)2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Smith A: Effects of caffeine on human
behavior. Food Chem Toxicol. 40:1243–1255. 2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cheng TO: Green tea may inhibit warfarin.
Int J Cardiol. 115(236)2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Izzo AA: Interactions between herbs and
conventional drugs: Overview of the clinical data. Med Princ Pract.
21:404–428. 2012.PubMed/NCBI View Article : Google Scholar
|