Introduction
In neonatology, preterm birth is traditionally
classified by gestational age in weeks and birth weight of the
infant. These two markers are well-established core indicators for
monitoring and evaluating perinatal health under routine health
statistics (1,2). However, both indicators remain
descriptive and non-functional, and do not allow for functional
conclusions regarding the individual biological maturity and
outcome of a preterm infant. Based on the gestational age, preterm
birth is divided into the following three sub-categories: i)
Extremely preterm, <28 weeks; ii) very preterm, 28-32 weeks; and
iii) moderate to late preterm, 32-37 weeks (1). Despite the growing knowledge on the
pathophysiology of this phenomenon, preterm birth still remains a
global challenge, with preterm birth rates increasing in almost all
countries according to reliable data (3), thus representing the leading cause of
neonatal morbidity and mortality (4). Additionally, preterm birth is also
associated with long-term increased risk of adverse health outcome.
Long-term effects of preterm birth include visual, hearing and
neurocognitive impairment as well as an increased risk of chronic
diseases such as insulin resistance (5), respiratory (6) and cardiovascular diseases (7), typical diseases of the elderly
population. The aforementioned reports indicate that preterm birth
may be involved in premature aging, however, further studies
identifying age-associated molecular markers as potential
biomarkers in preterm infants are urgently needed.
Telomeres consist of repeats of the DNA sequence
TTAGGG and are located at the end of each individual chromosome arm
(8). With each somatic cell
division, telomere length undergoes replicative shortening as DNA
polymerase is unable to fully copy telomeric DNA, the so-called
end-replication problem (9).
Therefore, telomeres reflect the replicative history of a cell and
provide a well-established marker for organismal aging and stem
cell turnover (10,11). Furthermore, short telomeres have
been associated with genomic instability and aging. It has been
also reported that diseases characterized by disturbed telomere
maintenance such as dyskeratosis congenita, are known to reflect
the phenotype of premature aging (12).
Epigenetic alterations represent an additional
established hallmark of aging (13). It has been well documented that DNA
methylation patterns are modified with age. Therefore, several
epigenetic aging signatures (EASs) have been applied to accurately
estimate chronological age in children and adults (14-17).
However, only few studies have been conducted on the identification
of EASs designed especially for the neonatal population and these
studies exhibited limited accuracy and precision ability due to the
extremes of the population distribution (18).
The aim of the present study in preterm infants was
to systematically investigate telomere length at birth and apply a
previously established EAS as a potential novel biomarker,
alternative to gestational age and birth weight.
Patients and methods
Samples and clinical
characteristics
Cord blood from 46 neonates, including 35 preterm
(28-37 weeks) and 11 full-term (>37 weeks) ones, was obtained
immediately postpartum by midwives of the University Hospital of
Aachen. All parents signed an informed consent form prior the
collection of the samples and the study was approved by the ethics
committee of the University Hospital of Aachen. Immediately after
birth, the length, weight and head diameter of neonates were
measured during routine follow-up. Detailed characteristics of the
neonates are presented in Table I.
Follow-up weight measurements were available for 9/46 of preterm
neonates.
 | Table IClinical characteristics of the 46
analyzed neonates |
Table I
Clinical characteristics of the 46
analyzed neonates
| Value | 28-37 w1
n=35 | >37 w2
n=11 |
|---|
| Sex | | |
|
Female | 14 | 5 |
|
Male | 21 | 6 |
| Birth weight | | |
|
SGA
(<10th percentile) | 4 | 3 |
|
AGA
(10-90th percentile) | 30 | 8 |
|
LGA
(>90th percentile) | 1 | 0 |
| Birth length
(cm) | | |
|
Average | 45.5 | 49.9 |
|
SD | 3.2 | 3.3 |
| Birth head diameter
(cm) | | |
|
Average | 31.5 | 33.8 |
|
SD | 2.2 | 2.1 |
| Maternal age
(years) | | |
|
<25 | 3 | 0 |
|
25-34 | 18 | 3 |
|
≥35 | 12 | 7 |
|
NA | 2 | 1 |
Flow-fluorescence in situ
hybridization (FISH) analysis
Telomere length was prospectively analyzed in all 46
cord blood samples. The mean telomere length of lymphocytes and
granulocytes was determined using flow-FISH as previously described
(19-26).
Briefly, bovine thymocytes were used as an internal control and
were added to the peripheral blood cells. Samples were prepared for
cell denaturation and mixed with a FITC labeled (CCCTAA)3 peptide
nucleic acid (PNA) probe (Panagene Inc.) for DNA-hybridization
followed by DNA counterstaining with LDS 751 (Sigma-Aldrich; Merck
KGaA). Telomeric fluorescence analysis was carried out on FC-500 or
Navios (both from Becton-Dickinson and Company) using forward
scatter (cell volume) and LDS 751 staining for the identification
of cell subsets (thymocytes, lymphocytes and granulocytes)
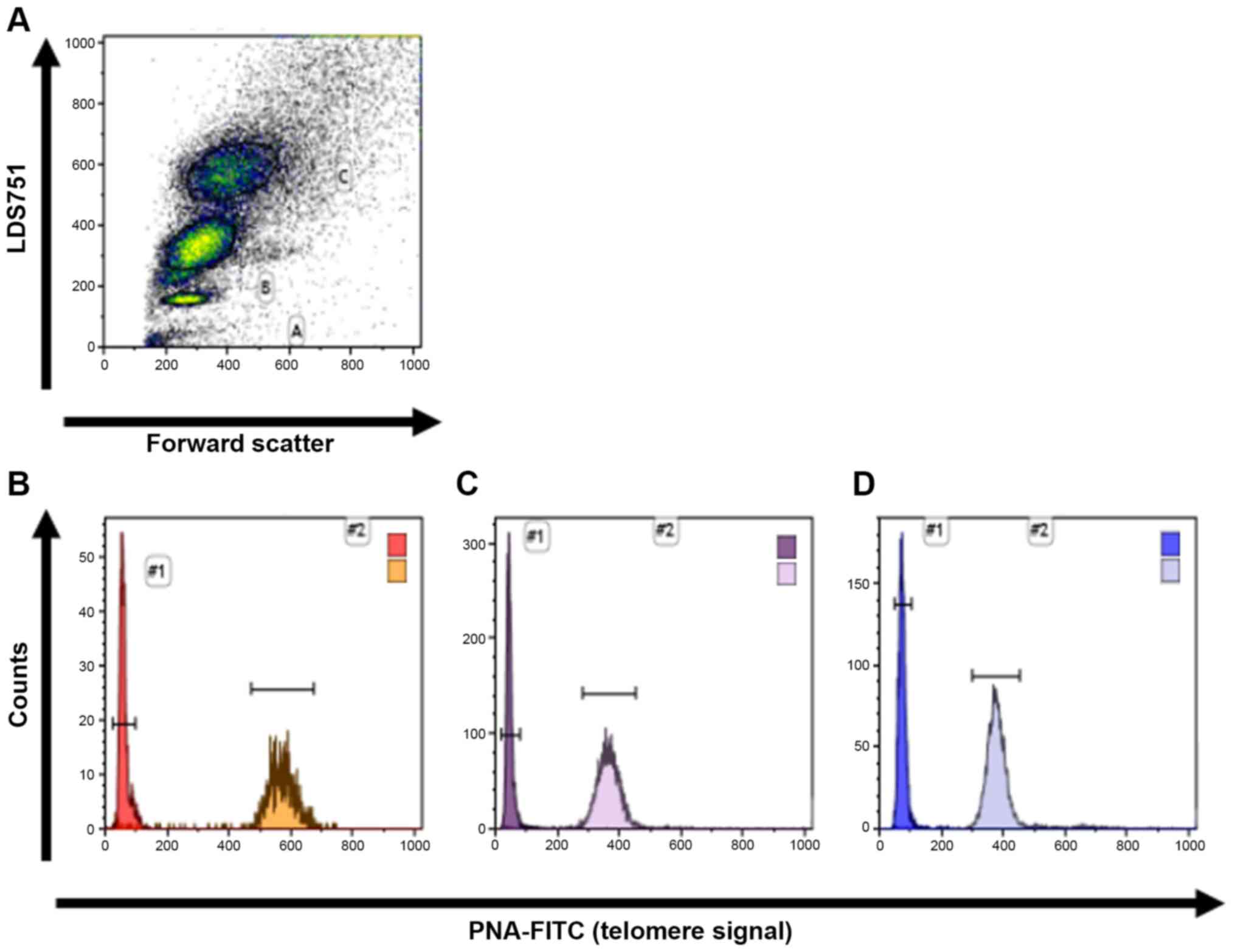
(Fig. 1). The autofluorescence
value of the respective unstained lymphocytes, granulocytes or
thymocytes was subtracted from stained samples and the mean
telomere length was calculated in relation to the internal control
with a known telomere length. All measurements were performed
single-blinded in triplicate.
DNA methylation profiles using
bisulfite pyrosequencing
To determine epigenetic age, 39 cord blood samples
were analyzed based on previously published EASs (14) using bisulfite pyrosequencing. This
technique is used to determine the DNA methylation levels at three
CG dinucleotides (CpG sites) located in ASPA, ITGA2B and
PDE4C genes. Genomic DNA was isolated with the DNA Blood and
Tissue kit (Qiagen). A total of 500 ng genomic DNA were used for
further experiments such as DNA bisulfite conversion and
pyrosequencing. Both experiments were performed as described
previously (14). Subsequently, age
prediction was performed using the pyrosequencing results obtained
from an improved multivariate model that was better adjusted for
cord blood samples. This model was applied on recently described
pyrosequencing results form a total of 156 blood samples
particularly derived from adult donors (14). Age was predicted using the following
equation: Predicted age (in
years)=-0.27001α-0.30611β+1.77018γ+38.76516, where α indicated the
methylation frequency of cg02228185 in ASPA gene; β
the methylation frequency of cg25809905 in ITGA2B gene; and
γ the methylation frequency of CpG upstream of cg17861230 in
PDE4C gene.
Statistical analysis
Statistical analyses were performed using the
GraphPad Prism v5.0 software (GraphPad Software Inc.).
Subsequently, a linear regression model was applied to determine
the approximate correlation between telomere length and gestational
age and birth weight. P<0.05 was considered to indicate a
statistically significant difference.
Results
Telomere length
Telomere length was prospectively analyzed in 46
cord blood samples derived from 35 preterm (28-37 weeks) and 11
full-term (>37 weeks) neonates. The results indicated that
increasing birth weight was highly correlated with increasing
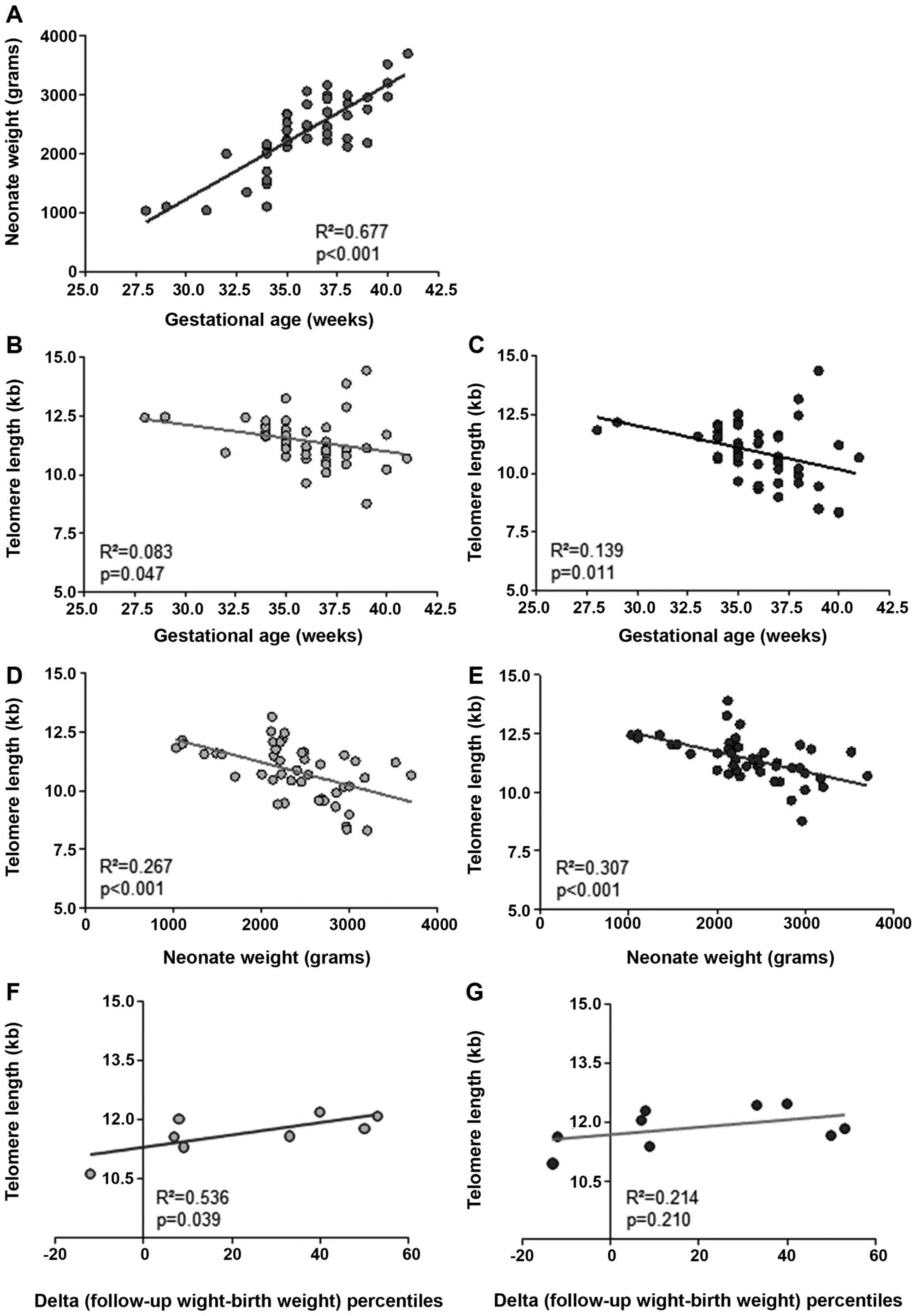
gestational age (Fig. 2A:
R2=0.677; P<0.001). Subsequently, telomere length of
granulocytes and lymphocytes from all neonates was determined by
flow-FISH (27,28). Telomere length was inversely
correlated with gestational age in both cell subpopulations
(Fig. 2B and C: Granulocytes, R2=0.083;
P=0.047; n=46; and lymphocytes, R2=0.139; P=0.011;
n=45). In addition, telomere length was significantly correlated
with birth weight (Fig. 2D and
E: Granulocytes,
R2=0.267; P<0.001; n=46; and lymphocytes,
R2=0.307; P<0.001; n=45).
To further compare telomere shortening with known
values from children and adult cohorts, the estimated telomere
attrition per week and per 500 g weight gain was calculated using a
linear regression model. Telomere shortening per week was estimated
to 0.126 and 0.186 kb for peripheral blood granulocytes and
lymphocytes, respectively. Additionally, telomere shortening per
500 g of weight gain was measured to 0.424 and 0.497 kb in the
neonates' granulocytes and lymphocytes, respectively (Table II).
 | Table IICalculated telomere shortening in the
lymphocyte and granulocyte subpopulation |
Table II
Calculated telomere shortening in the
lymphocyte and granulocyte subpopulation
| | Telomere shortening
per week (Kb) | Telomere shortening
per 500 g (Kb) |
|---|
| Granulocytes | 0.1257±0.0617 | 0.42395±0.0970 |
| Lymphocytes | 0.1862±0.0699 | 0.49680±0.1246 |
In the present study, data on weight gain from 9
preterm neonates born within the 32-37th week of gestational age
were also available. Therefore, the postnatal weight development
percentile, indicating the difference between follow-up weight
percentile and initial birth weight percentile, was correlated with
telomere length at the time of birth. The results revealed a
positive correlation between telomere length at birth and the
difference in weight development percentiles (Fig. 2F and G). More specifically, a significant
correlation was observed for peripheral blood granulocytes
(Fig. 1F: R2=0.536;
P=0.039; n=8), but not for peripheral blood lymphocytes, where no
significant trend was obtained (Fig.
2G: R2=0.214; P=0.210; n=9).
EAS
To further extent the biomarker analysis, a cord
blood-optimized EAS was applied in 39 cord blood samples. As
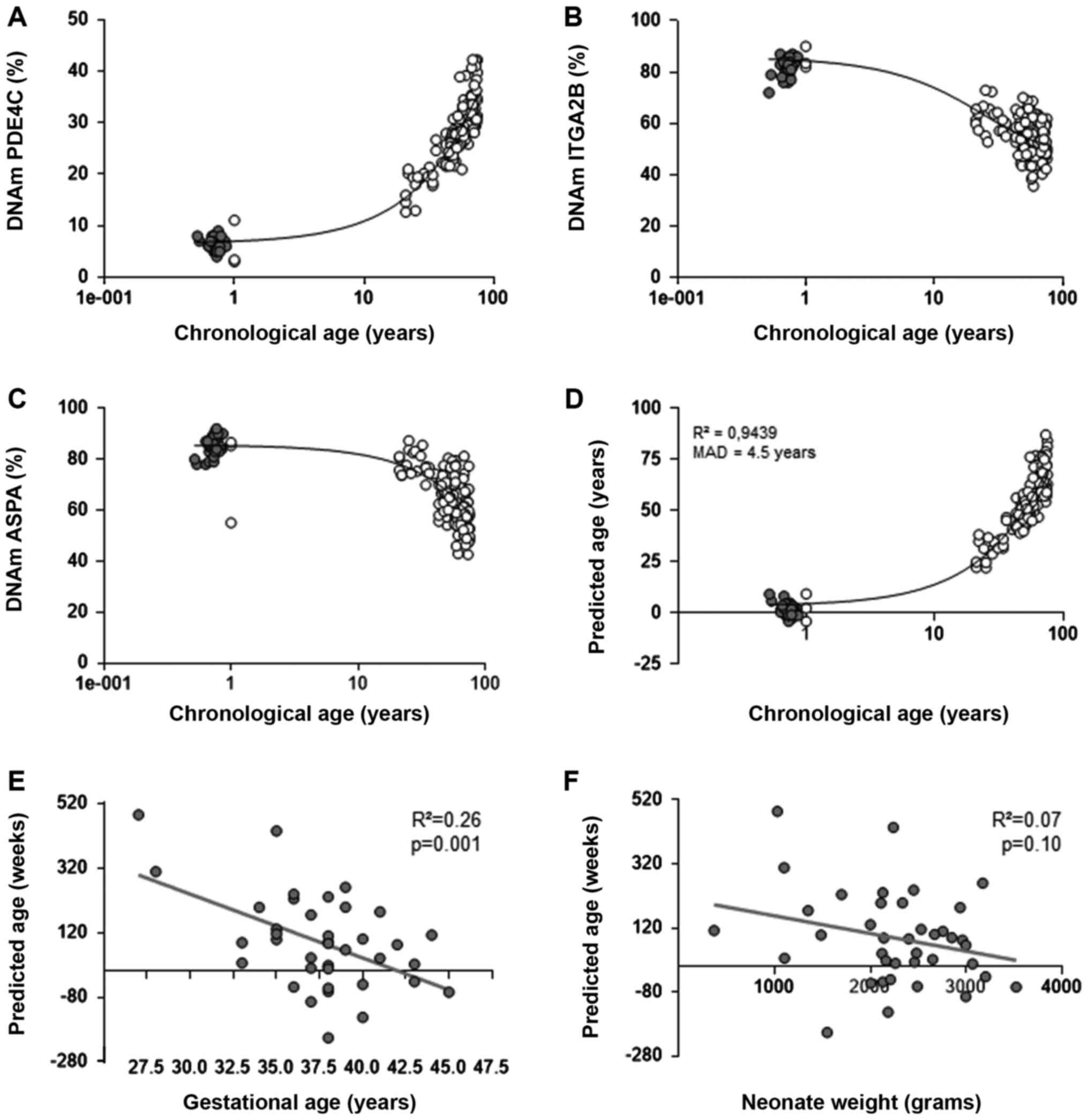
expected, DNA methylation status of PDE4C, ITGA2B and
ASPA genes at the three CpGs showed age-associated DNA
methylation changes, which were consistent with previous
measurements in cord blood (Fig.
3A-D). Our previously published multivariate model for
age predictions was not customized for cord blood samples,
therefore, the epigenetic age was systematically overestimated. To
avoid bias, the multivariate model was adjusted and 39 cord blood
samples were analyzed. This adjusted model provided a mean average
deviation (MAD) between predicted and chronological age of 4.5
years for all samples and 2.2 years for cord blood samples.
However, the application of this model requires further validation
in the future using independent datasets (Fig. 3D). Furthermore, the estimated
epigenetic age in the cord blood of preterm and full-term neonates
was inversely correlated with gestational age (Fig. 3E: R2=0.26; P=0.001;
n=39). These findings were consistent with our previous study,
where the aforementioned multivariate model was performed (14). By contrast, no statistically
significant correlation between predicted age and birth weight was
observed (Fig. 3F:
R2=0.07; P=0.10; n=39).
Discussion
Preterm birth has an impact on the molecular markers
of aging. The results of the present study were consistent with
previously published data by Friedrich et al, demonstrating
a significant correlation between birth weight and telomere length
in extremely preterm infants. In the present study an accelerated
rate of telomere shortening was also observed, with 0.126 and 0.186
kb per week in granulocytes and lymphocytes, respectively.
Consistent with our results, a previous study demonstrated an
estimated weekly telomere shortening rate of 0.041 kb in leucocytes
from overall preterm (<37 week) infants and 0.238 kb in extreme
to very preterm (27-32 week) born infants (29). In addition, other studies showed
increased telomere shortening range during the first years of
development, which was also consistent with the results of the
current study (22,30).
This study also suggested that telomere length was
strongly correlated with birth weight, but not with gestational
age. Okuda et al reported a significant correlation between
telomere length in different fetal tissues and cord blood (31). Therefore, it was hypothesized that
telomere length in cord blood could be considered as a more robust
surrogate marker for organismal growth/maturation and weight gain
compared with gestational age. The estimated telomere length
shortening was approximately 0.5 kb/500 g or 1 bp/1 g of weight
gain. Therefore, the longitudinally followed neonates with longer
telomeres as opposed to those with shorter telomeres, reached
normal weight percentiles during the first year of development. The
close correlation of telomere length and weight could explain the
increasing variability of telomere length with increased week of
gestation. However, other factors such as food consumption or
parental body mass index may influence weight gain. Therefore, it
was suggested that organismal growth and weight gain could be
considered as additional factors contributing to the variability of
telomere length.
Regarding DNA methylation in neonates, there is
limited information on epigenetic changes during gestation. The
present study also revealed a significant correlation with
gestational age, but not with birth weight using the optimized EAS.
A previous study by Javed et al did not report any
association between gestational age, birth weight and methylation
profile at birth based on 353 CpG sites and Horvath predictor for
age estimation using cord blood (32). However, Knight et al
established a predictor model for gestational age based on 148 CpG
sites similar to the present data (18). In general, wider aging signatures
may be more precise, however, pyrosequencing of few CpGs is more
cost effective and provides higher site-specific precision of the
DNA methylation levels. More interestingly, the finding that
epigenetic and gestational age were inversely correlated was
somewhat unexpected, therefore, further validation in independent
and larger cohorts is urgently needed. It has been suggested that
age-related DNA methylation changes in peripheral blood occur more
rapidly during childhood and are imperfectly accounted for
statistical corrections that are linear in age (33). Therefore, it is conceivable that
preterm birth is associated with aberrant epigenetic age and
vice versa.
In summary, this study highlighted the predictive
value of aging biomarkers at birth. While telomere length is
correlated with the organismal growth, DNA methylation changes are
correlated with maturity based on gestational age. As preterm birth
still remains a great challenge for pediatricians, reliable
biomarkers with high prognostic value are needed for an efficient
decision-making in a clinical setting. Furthermore, the findings of
the present study supported the additive use of telomere length as
a possible biomarker for therapy strategy in preterm neonates, as
previously proposed (34). However,
the number of cases was too low to suggest any strong clinical
recommendations. Therefore, further research is needed to establish
telomere length as a valuable prognostic biomarker for clinicians
in predicting organismal growth and development of preterm born
infants. The present study reinforced not only the importance of
cellular aging during fetal development, but also the critical role
of telomere length in predicting newborns' health outcome (growth
and development).
Acknowledgements
The authors would like to thank Lucia Vankann and
Melanie Coeuru from the Department of Hematology, Oncology,
Hemostaseology and Stem Cell Transplantation) for technical
assistance with flow-FISH.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author contributions
NTS wrote the manuscript, performed experiments,
collected and analyzed data; MSVF performed experiments and
interpreted the data; WW and ME performed experiments and
interpreted the data; SD collected and analyzed the data; THB
interpreted the data and provided financial support; TO and FB
designed the experiments and provided financial support; all
authors reviewed the manuscript.
Ethics approval and consent to
participate
All parents signed an informed consent form prior
the collection of the samples and the study was approved by the
ethics committee of the University Hospital of Aachen (EK
041/15).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization (WHO). WHO
Recommendations on Interventions to Improve Preterm Birth Outcomes.
World Health Organization, Geneva, 2015.
|
|
2
|
Santos JV, Correia C, Cabral F, Bernardes
J, Costa-Pereira A and Freitas A: Should European perinatal
indicators be revisited? Eur J Obstet Gynecol Reprod Biol.
170:85–89. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Blencowe H, Cousens S, Oestergaard MZ,
Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L
and Lawn JE: National, regional, and worldwide estimates of preterm
birth rates in the year 2010 with time trends since 1990 for
selected countries: A systematic analysis and implications. Lancet.
379:2162–2172. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kinney MV, Lawn JE, Howson CP and Belizan
J: 15 Million preterm births annually: What has changed this year?
Reprod Health. 9(28)2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Salis ER, Reith DM, Wheeler BJ, Broadbent
RS and Medlicott NJ: Hyperglycaemic preterm neonates exhibit
insulin resistance and low insulin production. BMJ Paediatr Open.
1(e000160)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kwinta P and Pietrzyk JJ: Preterm birth
and respiratory disease in later life. Expert Rev Respir Med.
4:593–604. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Demerath EW, Cameron N, Gillman MW, Towne
B and Siervogel RM: Telomeres and telomerase in the fetal origins
of cardiovascular disease: A review. Hum Biol. 76:127–146.
2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Greider CW and Blackburn EH: A telomeric
sequence in the RNA of Tetrahymena telomerase required for telomere
repeat synthesis. Nature. 337:331–337. 1989.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
de Lange T: How telomeres solve the
end-protection problem. Science. 326:948–952. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Blasco MA: Telomeres and human disease.
Ageing, cancer and beyond. Nat Rev Genet. 6:611–622.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Brümmendorf TH and Balabanov S: Telomere
length dynamics in normal hematopoiesis and in disease states
characterized by increased stem cell turnover. Leukemia.
20:1706–1716. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kirwan M and Dokal I: Dyskeratosis
congenita, stem cells and telomeres. Biochim Biophys Acta.
1792:371–319. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
López-Otín C, Blasco MA, Partridge L,
Serrano M and Kroemer G: The hallmarks of aging. Cell.
153:1194–1217. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Weidner CI, Lin Q, Koch CM, Eisele L,
Beier F, Ziegler P, Bauerschlag DO, Jöckel KH, Erbel R, Mühleisen
TW, et al: Aging of blood can be tracked by DNA methylation changes
at just three CpG sites. Genome Biol. 15(R24)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Horvath S: DNA methylation age of human
tissues and cell types. Genome Biol. 14(R115)2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hannum G, Guinney J, Zhao L, Zhang L,
Hughes G, Sadda S, Klotzle B, Bibikova M, Fan JB, Gao Y, et al:
Genome-wide methylation profiles reveal quantitative views of human
aging rates. Mol Cell. 49:359–367. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bocklandt S, Lin W, Sehl ME, Sánchez FJ,
Sinsheimer JS, Horvath S and Vilain E: Epigenetic predictor of age.
PLoS One. 6(e14821)2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Knight AK, Craig JM, Theda C,
Bækvad-Hansen M, Bybjerg-Grauholm J, Hansen CS, Hollegaard MV,
Hougaard DM, Mortensen PB, Weinsheimer SM, et al: An epigenetic
clock for gestational age at birth based on blood methylation data.
Genome Biol. 17(206)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Beier F, Balabanov S, Buckley T, Dietz K,
Hartmann U, Rojewski M, Kanz L, Schrezenmeier H and Brümmendorf TH:
Accelerated telomere shortening in glycosylphosphatidylinositol
(GPI)-negative compared with GPI-positive granulocytes from
patients with paroxysmal nocturnal hemoglobinuria (PNH) detected by
proaerolysin flow-FISH. Blood. 106:531–533. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Beier F, Masouleh BK, Buesche G, Ventura
Ferreira MS, Schneider RK, Ziegler P, Wilop S, Vankann L,
Gattermann N, Platzbecker U, et al: Telomere dynamics in patients
with del (5q) MDS before and under treatment with lenalidomide.
Leuk Res, Sep 21, 2015 (Online ahead of print).
|
|
21
|
Beier F, Foronda M, Martinez P and Blasco
MA: Conditional TRF1 knockout in the hematopoietic compartment
leads to bone marrow failure and recapitulates clinical features of
dyskeratosis congenita. Blood. 120:2990–3000. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Werner B, Beier F, Hummel S, Balabanov S,
Lassay L, Orlikowsky T, Dingli D, Brümmendorf TH and Traulsen A:
Reconstructing the in vivo dynamics of hematopoietic stem cells
from telomere length distributions. Elife. 4(e08687)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bartolović K, Balabanov S, Berner B,
Bühring HJ, Komor M, Becker S, Hoelzer D, Kanz L, Hofmann WK and
Brümmendorf TH: Clonal heterogeneity in growth kinetics of
CD34+CD38-human cord blood cells in vitro is correlated with gene
expression pattern and telomere length. Stem Cells. 23:946–957.
2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Brummendorf TH, Ersoz I, Hartmann U,
Balabanov S, Wolke H, Paschka P, Lahaye T, Berner B, Bartolovic K,
Kreil S, et al: Normalization of previously shortened telomere
length under treatment with imatinib argues against a preexisting
telomere length deficit in normal hematopoietic stem cells from
patients with chronic myeloid leukemia. Ann N Y Acad Sci.
996:26–38. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Brümmendorf TH, Holyoake TL, Rufer N,
Barnett MJ, Schulzer M, Eaves CJ, Eaves AC and Lansdorp PM:
Prognostic implications of differences in telomere length between
normal and malignant cells from patients with chronic myeloid
leukemia measured by flow cytometry. Blood. 95:1883–1890.
2000.PubMed/NCBI
|
|
26
|
Brümmendorf TH, Maciejewski JP, Mak J,
Young NS and Lansdorp PM: Telomere length in leukocyte
subpopulations of patients with aplastic anemia. Blood. 97:895–900.
2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rufer N, Brümmendorf TH, Kolvraa S,
Bischoff C, Christensen K, Wadsworth L, Schulzer M and Lansdorp PM:
Telomere fluorescence measurements in granulocytes and T lymphocyte
subsets point to a high turnover of hematopoietic stem cells and
memory T cells in early childhood. J Exp Med. 190:157–167.
1999.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Baerlocher GM, Vulto I, de Jong G and
Lansdorp PM: Flow cytometry and FISH to measure the average length
of telomeres (flow FISH). Nat Protoc. 1:2365–2376. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Friedrich U, Schwab M, Griese EU, Fritz P
and Klotz U: Telomeres in neonates: New insights in fetal
hematopoiesis. Pediatr Res. 49:252–256. 2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Aubert G, Baerlocher GM, Vulto I, Poon SS
and Lansdorp PM: Collapse of telomere homeostasis in hematopoietic
cells caused by heterozygous mutations in telomerase genes. PLoS
Genet. 8(e1002696)2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Okuda K, Bardeguez A, Gardner JP,
Rodriguez P, Ganesh V, Kimura M, Skurnick J, Awad G and Aviv A:
Telomere length in the newborn. Pediatr Res. 52:377–381.
2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Javed R, Chen W, Lin F and Liang H:
Infant's DNA methylation age at birth and epigenetic aging
accelerators. Biomed Res Int. 2016(4515928)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Alisch RS, Barwick BG, Chopra P, Myrick
LK, Satten GA, Conneely KN and Warren ST: Age-associated DNA
methylation in pediatric populations. Genome Res. 22:623–632.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Turner KJ, Vasu V, Greenall J and Griffin
DK: Telomere length analysis and preterm infant health: The
importance of assay design in the search for novel biomarkers.
Biomark Med. 8:485–498. 2014.PubMed/NCBI View Article : Google Scholar
|