Introduction
Osteoarthritis (OA) is a major cause of
musculoskeletal disability worldwide, which is associated with
significant loss of work and a high socioeconomic burden (1). The prevalence of symptomatic OA is as
high as 5-7% in the adult population worldwide (2). As one of the typical ‘wear and tear’
diseases, OA is primarily caused by articular cartilage damage or
loss (3,4). Articular cartilage consists of
chondrocytes, which are the sole cell type present in cartilage and
the surrounding extracellular matrix (5). During OA progression, the original
chondrocyte phenotype is altered to accompany a loss of type II
collagen and aggrecan, and an increase in type I and III collagen
(6,7). Matrix metalloproteinases (MMPs) and
interleukin (IL)-1β have been considered as vital components of
cartilage degradation (8). IL-1β
induces cartilage degradation by triggering the expression of MMP9,
MMP13 and other proteases (8-10).
Tumor necrosis factor-α (TNF-α) activates chondrocytes to further
enhance the production of proinflammatory cytokines (11,12).
In addition, due to the low metabolic activity of chondrocytes,
articular cartilage has no intrinsic repair capabilities (3,13).
Therefore, despite various strategies of OA management (14), there are no effective therapeutic
strategies for the disease.
Notch is a single-pass transmembrane cell surface
receptor that serves a vital role in vascular development and
cell-cell communication (15,16).
Notch is as an important regulator of chondrocyte proliferation
during cartilage development (17).
Evidence has shown that the expression levels of Notch family
members are increased in chondrocytes during OA progression
(18). Yu et al (19) indicated that microRNA (miRNA/miR)-9
could facilitate cartilage regeneration of OA in rabbits by
mediating Notch signaling.
Baicalein (BAI) is isolated from Scutellaria
baicalensis Georgi (8,20) and possesses anti-inflammatory
(21), antioxidative (22) and anticarcinogenic properties
(23,24). The anti-OA abilities of BAI have
also been investigated in previous in vitro and in
vivo studies (8,20,25).
One study demonstrated that BAI triggers the expression of
antiapoptotic genes and decreases the expression of proapoptotic
and proinflammatory gene products in rat chondrocytes (20). Another study illustrated that BAI
significantly reduces the expression of MMPs, inhibiting
IL-1β-induced cartilage degradation in vitro and in
vivo (8).
As a family of endogenous small non-coding RNAs,
miRNAs are post-transcriptional modulators that serve a pivotal
role in regulating key cellular processes, including biogenesis and
genomic organization, by preferentially binding to the 3'
untranslated regions of target mRNAs (26-29).
The regulatory functions of miRNAs during the pathogenesis of OA
have been reported (30-32).
Numerous miRNAs, including miR-30a, miR-29, miR-105, miR-146a,
miR-221-3p and miR-145, are involved in regulating the pathogenesis
of OA (32). For example, Guan
et al (33) reported that
miR-164a expression was decreased in OA lesions of human articular
cartilage, and miR-146a-deficient mice developed early onset OA
characterized by cartilage degeneration and osteophytes. Moreover,
Hu et al (34) indicated
that miR-145 attenuates TNF-α-induced cartilage matrix degradation.
Moreover, the overexpression or intra-articular injection of
miR-106a-5p attenuated the progression of OA in vitro and
in vivo, respectively (32).
Although BAI and miR-106a-5p have been separately
reported to reduce articular cartilage injury, the combined effect
of BAI and miR-106a-5p is not completely understood. Therefore, the
present study aimed to investigate the effects of BAI + miR-106a-5p
combination therapy on the progression of OA using an in
vitro chondrocyte injury model.
Materials and methods
Cell culture
The CHON-001 human chondrocyte cell line was
obtained from American Type Culture Collection. Cells were cultured
in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in
a humidified incubator at 37˚C with 5% CO2. To establish
an in vitro OA model, 5x104 CHON-001 cells were
stimulated with recombinant human IL-1β (0, 5, 10 or 20 ng/ml) for
24 h at 37˚C (cat. no. SRP6169; Sigma-Aldrich; Merck KGaA).
Transfection
miR-106a-5p mimics and negative control (NC) were
obtained from Shanghai GenePharma Co., Ltd. CHON-001 cells were
seeded into 6-well plates and cultured overnight to ~70%
confluence. Subsequently, 5x104 CHON-001 cells were
transfected with 10 nM miR-106a-5p mimics or 10 nM NC using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 24 h according to the manufacturer's
protocol.
For combination treatment, 5x104 CHON-001
cells were transfected with miR-106a-5p mimics (2, 4, 6 or 10 nM)
for 24 h at 37˚C, and subsequently treated with 20 µM BAI (cat. no.
465119; Sigma-Aldrich; Merck KGaA) for a further 24 h at 37˚C.
Cells were then stimulated with 20 ng/ml IL-1β for another 24 h at
37˚C to induce the OA phenotype.
Cell viability assay
CHON-001 cells were seeded into 96-well plates
(5x103 cells/well) and incubated at 37˚C overnight.
Following transfection and treatment with BAI and IL-1β, cell
viability was assessed using the Cell Counting Kit-8 (CCK-8) assay
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. Briefly, 10 µl CCK-8 solution was added to
each well and incubated at 37˚C for 2 h. The absorbance of each
well was measured at a wavelength of 450 nm using a microplate
reader (Bio-Rad Laboratories, Inc.).
Flow cytometry
CHON-001 cells were seeded (5x104
cells/well) and cultured to ~80% confluence. Cells were treated
with different concentrations of IL-1β (0, 5, 10 or 20 ng/ml) for
24 h 37˚C. Subsequently, early apoptotic, late apoptotic and
necrotic cells were assessed using the Annexin V-FITC Apoptosis
Staining Detection kit (Abcam) according to the manufacturer's
protocol. Briefly, cells were harvested and centrifuged at 626 x g
for 5 min at 4˚C. After rinsing twice with PBS, cells were
centrifuged at 626 x g for 5 min at 4˚C. Subsequently, cells
(1x105) were collected from each group, resuspended in
binding buffer, and subsequently stained with 5 µl Annexin V-FITC
and 5 µl propidium iodide for 5 min in the dark at room
temperature. Within 1 h, cell apoptosis was assessed via flow
cytometry using a BD FACSAria flow cytometer (BD Biosciences) and
CellQuest Pro software (version 5.1; BD Biosciences).
ELISA
Following transfection and treatment with BAI and
IL-1β, culture supernatants were collected from 6-well plates and
then centrifuged for 10 min at 626 x g at 4˚C. Subsequently,
cellular IL-6 (cat. no. ES4078) and TNF-α (cat. no. ELK1190)
concentrations were determined using ELISA kits (ELK Biotechnology
Co., Ltd.) according to the manufacturer's protocol. IL-6 and TNF-α
concentrations in cell supernatants (100 µl) were assessed for the
following groups: i) Control; ii) 20 ng/ml IL-1β; iii) 20 ng/ml
IL-1β + 20 µM BAI; iv) 20 ng/ml IL-1β + miR-106a-5p mimics; and v)
20 ng/ml IL-1β + miR-106a-5p mimics + 20 µM BAI.
Reverse transcription-quantitative PCR
(RT-qPCR)
To confirm successful transfection of miR-106a-5p
mimics, total RNA was extracted from transfected CHON-001 cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) Total RNA was reverse transcribed into cDNA using
M-MLV Reverse Transcriptase (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. The RT reaction
comprised the following: 1 µg RNA, 10 µl reaction solution, 1 µl RT
primers and 1 µl dNTPs in a final volume of 15 µl. The mixture was
heated at 70˚C for 5 min and then it was quickly placed on ice to
cool. Subsequently, 4 µl RT buffer, 1 µl M-MLV Reverse
Transcriptase and 1 µl RNase inhibitor were added into the mixture
on ice. The RT reactions were subsequently performed as follows:
42˚C for 60 min; 85˚C for 5 min. The following primers were used
for RT: U6, 5'-AACGCTTCACGAATTTGCGT-3'; miR-183-5p,
5'-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAGTGAATT-3'.
Subsequently, qPCR was performed using GoTaq qPCR
Master Mix (Promega Corporation). miRNA expression levels were
normalized to the internal reference gene U6 using the U6 snRNA
normalization RT-PCR quantitation kit (Shanghai GenePharma Co.,
Ltd.) and the ABI Prism7700 detection system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The qPCR amplification reactions
were subsequently performed as follows: 95˚C for 3 min; and 40
cycles at 95˚C for 10 sec, 58˚C for 30 sec and 72˚C for 30 sec. The
following primers were used for qPCR: U6 forward,
5'-GCTTCGGCAGCACATATACTAAAAT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'; and miR-106a-5p forward,
5'-AAAAGTGCTTACAGTGCAGGTAG-3' and reverse,
5'-GAAAAGTGCTTACAGTGCAGGT-3'. miRNA expression levels were
quantified using the 2-ΔΔCq method (35).
Western blotting
Following transfection and treatment with BAI and
IL-1β, total protein was extracted from CHON-001 cells using RIPA
buffer (Beyotime Institute of Biotechnology) for 30 min on ice.
Total protein was quantified using a BCA protein kit (Thermo Fisher
Scientific, Inc.). Proteins (30 µg) were separated via 10% SDS-PAGE
(Pierce; Thermo Fisher Scientific, Inc.), transferred onto PVDF
membranes and blocked with 5% non-fat milk (diluted in 0.05%
TBS-Tween-20) for 1 h at room temperature. Subsequently, the
membranes were incubated at 4˚C overnight with the following
primary antibodies: Rabbit anti-Bax (1:1,000; cat. no. ab32503),
rabbit anti-caspase-3 (1:1,000; cat. no. ab2302), rabbit anti-Bcl-2
(1:1,000; cat. no. ab32124), rabbit anti-collagen I (1:5,000; cat.
no. ab34710), rabbit anti-collagen III (1:1,000; cat. no.
ab184993), rabbit anti-aggrecan (1:1,000; cat. no. ab36861), rabbit
anti-MMP-13 (1:5,000; cat. no. ab39012), rabbit anti-MMP-9
(1:1,000; cat. no. ab38898), rabbit anti-Notch1 (1:1,000; cat. no.
ab52627), rabbit anti-Hes family BHLH transcription factor 1 (Hes1;
1:1,000; cat. no. ab71559) and anti-β-actin (1:1,000; cat. no.
ab8227). The membranes were washed with TBS buffer containing 0.05%
Tween-20 for 30 min and incubated with a horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:5,000;
cat. no. ab205718) for 1 h at room temperature. All primary and
secondary antibodies were purchased from Abcam. Protein bands were
visualized using the Pierce™ ECL Western Blotting
Substrate kit (Thermo Fisher Scientific, Inc.) and the Bio-Rad
ChemiDoc Imaging system (Bio-Rad Laboratories, Inc.). Protein
expression levels were quantified using Image Lab software (version
5.2.1; Bio-Rad Laboratories, Inc.) with β-actin as the loading
control.
Statistical analysis
Experiments were repeated ≥3 times. Data are
presented as the mean ± standard deviation. Statistical analyses
were conducted using GraphPad Prism software (version 7; GraphPad
Software, Inc.). Comparisons between two groups were evaluated
using unpaired Student's t-test. Comparisons among multiple groups
were analyzed by one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Establishment of an in vitro OA
model
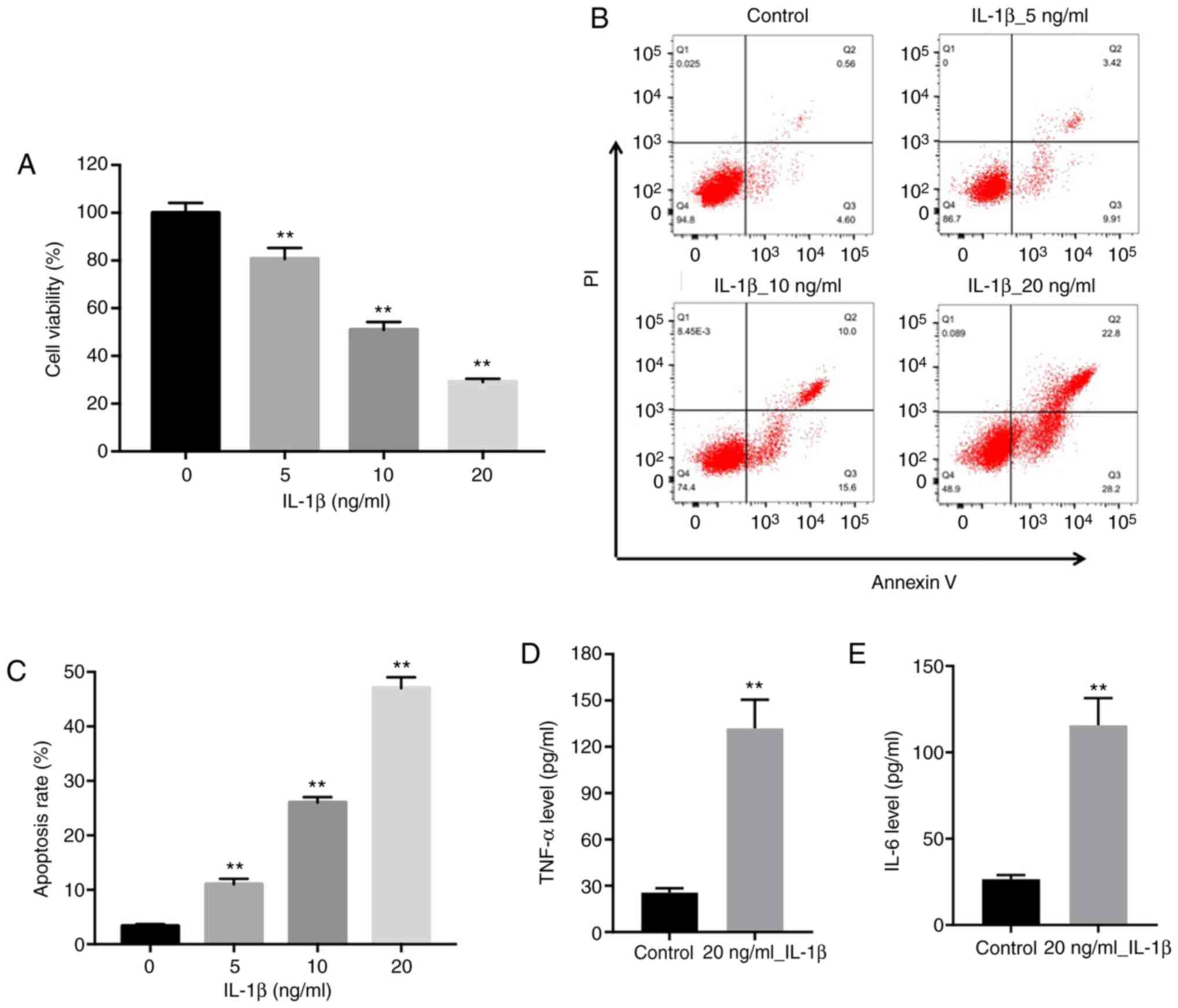
To construct an in vitro OA model, CHON-001
cells were treated with different concentrations of IL-1β (0, 5, 10
and 20 ng/ml) for 24 h. The CCK-8 assay was conducted to assess the
effects of IL-1β on chondrocyte viability. IL-1β inhibited CHON-001
cell viability in a dose-dependent manner compared with the control
group (0 ng/ml IL-1β), and 20 ng/ml IL-1β inhibited cell viability
by ~70%; therefore, 20 ng/ml IL-1β was selected to establish the
in vitro OA model in CHON-001 cells (Fig. 1A). The flow cytometry results
indicated that 5, 10 and 20 ng/ml IL-1β significantly induced
CHON-001 cell apoptosis compared with the control group (0 ng/ml
IL-1β), with the 20 ng/ml IL-1β group displaying the highest level
of apoptosis among the groups (Fig.
1B). As indicators of a functional cellular OA model, the
expression levels of inflammatory cytokines, such as TNF-α and
IL-6, were significantly increased by 20 ng/ml IL-1β compared with
the control group (Fig. 1D and
E). Collectively, the results
indicated the successful establishment of an in vitro OA
cell model.
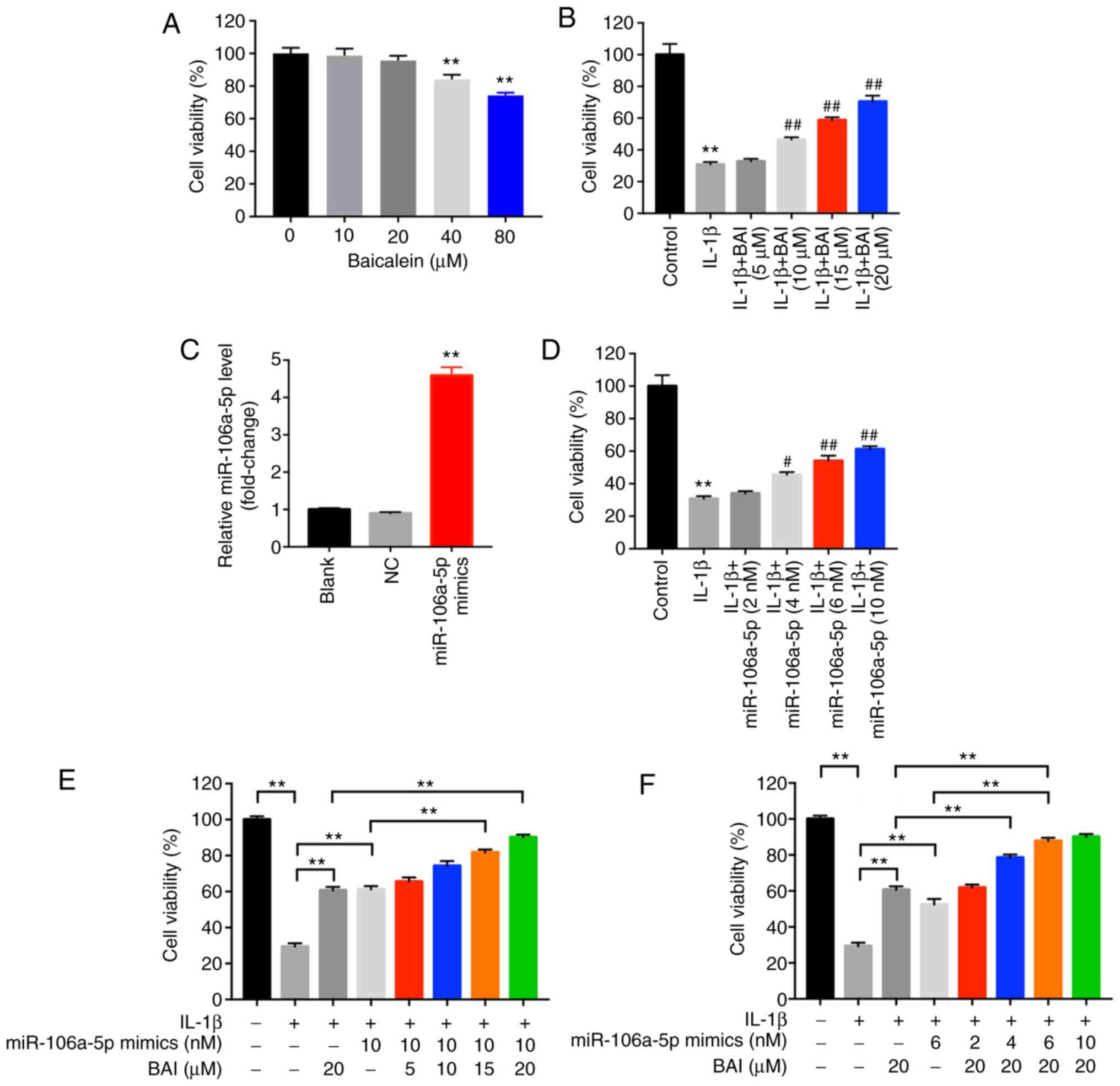
BAI and miR-106a-5p combination
treatment significantly alleviates IL-1β-induced inhibition of cell
viability
CHON-001 cells were cultured with various
concentrations of BAI (0, 10, 20, 40 and 80 µM). The CCK-8 assay
results indicated that 40 µM BAI significantly reduced cell
viability compared with the control group (0 µg/ml BAI), so 20 µM
BAI was used for subsequent experiments (Fig. 1A). The effect of BAI on the OA model
was assessed by measuring cell viability. BAI dose-dependently
reversed IL-1β-induced reductions in cell viability. In addition,
CHON-001 cells were transfected with miR-106a-5p mimics and the
RT-qPCR results indicated transfection efficiency (Fig. 2C). miR-106a-5p mimics also
dose-dependently reversed IL-1β-induced reductions in cell
viability (Fig. 2D).
Subsequently, the anti-OA effects of BAI and
miR-106a-5p mimics were assessed (Fig.
2E and F). The results
indicated that 4 nM miR-106a-5p mimics + 20 µM BAI and 6 nM
miR-106a-5p mimics + 20 µM BAI displayed an enhanced protective
effect in IL-1β-induced CHON-001 cells compared with 20 µM BAI
treatment alone. Therefore, the results suggested that combination
therapy may synergistically alleviate IL-1β-induced CHON-001
cytotoxicity; however, some combination strategies may only exhibit
additive therapeutic effect in OA.
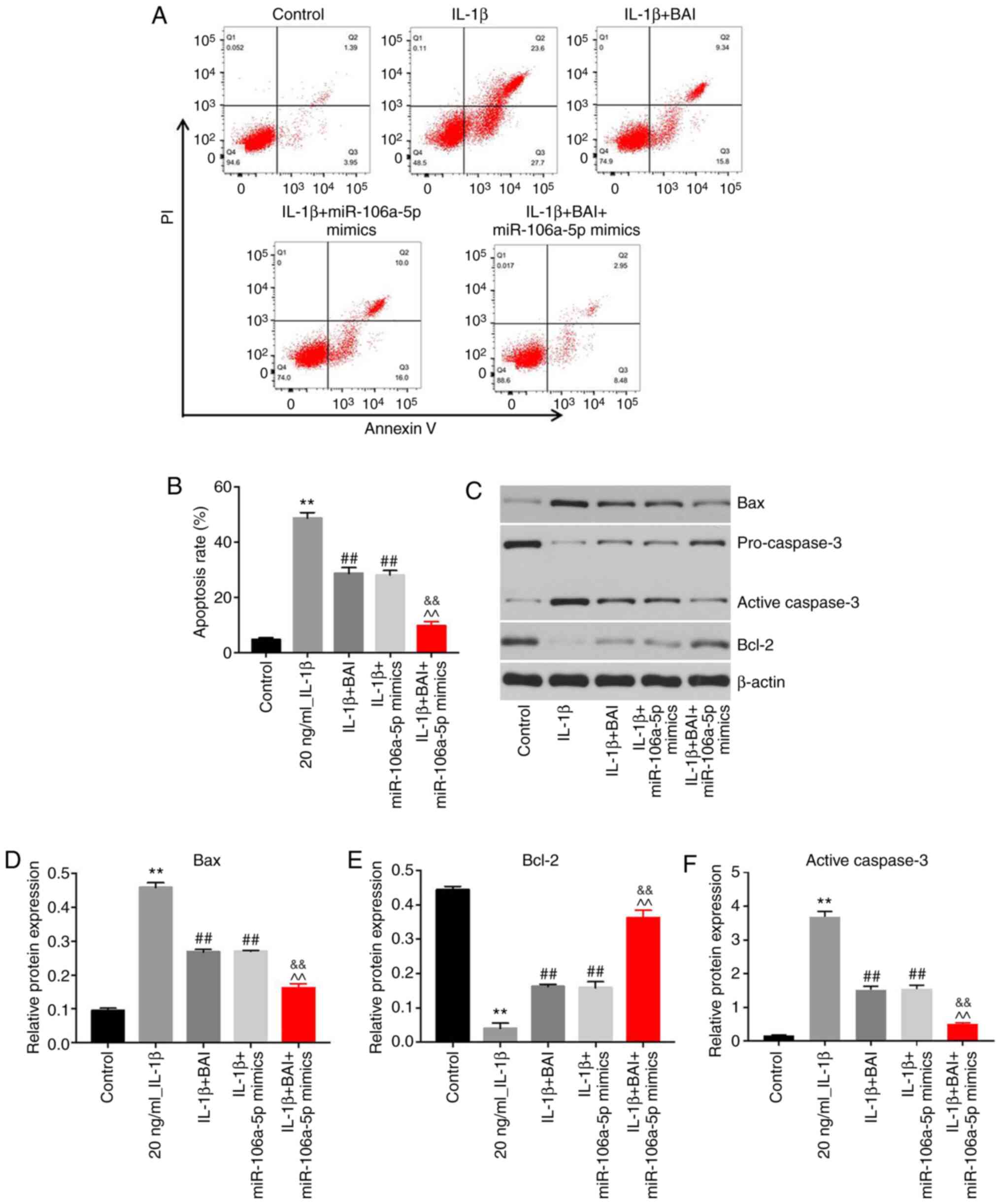
BAI and miR-106a-5p combination
treatment significantly alleviates IL-1β-induced apoptosis
An in vitro OA model was established and
treated with BAI, miR-106a-5p mimics or a combination. The effects
of the combination treatment on apoptosis were assessed via flow
cytometry. Monotherapy with BAI or miR-106a-5p mimics significantly
alleviated IL-1β-induced CHON-001 cell apoptosis (Fig. 3A and B). Moreover, combination treatment with
BAI and miR-106a-5p mimics further reversed IL-1β-induced CHON-001
cell apoptosis compared with either treatment alone. Furthermore,
the expression levels of apoptosis-related proteins (Bax and active
caspase-3) were significantly downregulated in the combination
treatment group compared with the BAI and miR-106a-5p mimics
monotherapy groups (Fig. 3C,
D and F). By contrast, the expression level of
the anti-apoptotic protein Bcl-2 was significantly upregulated in
the combination treatment group compared with the BAI and
miR-106a-5p mimics monotherapy groups (Fig. 3E).
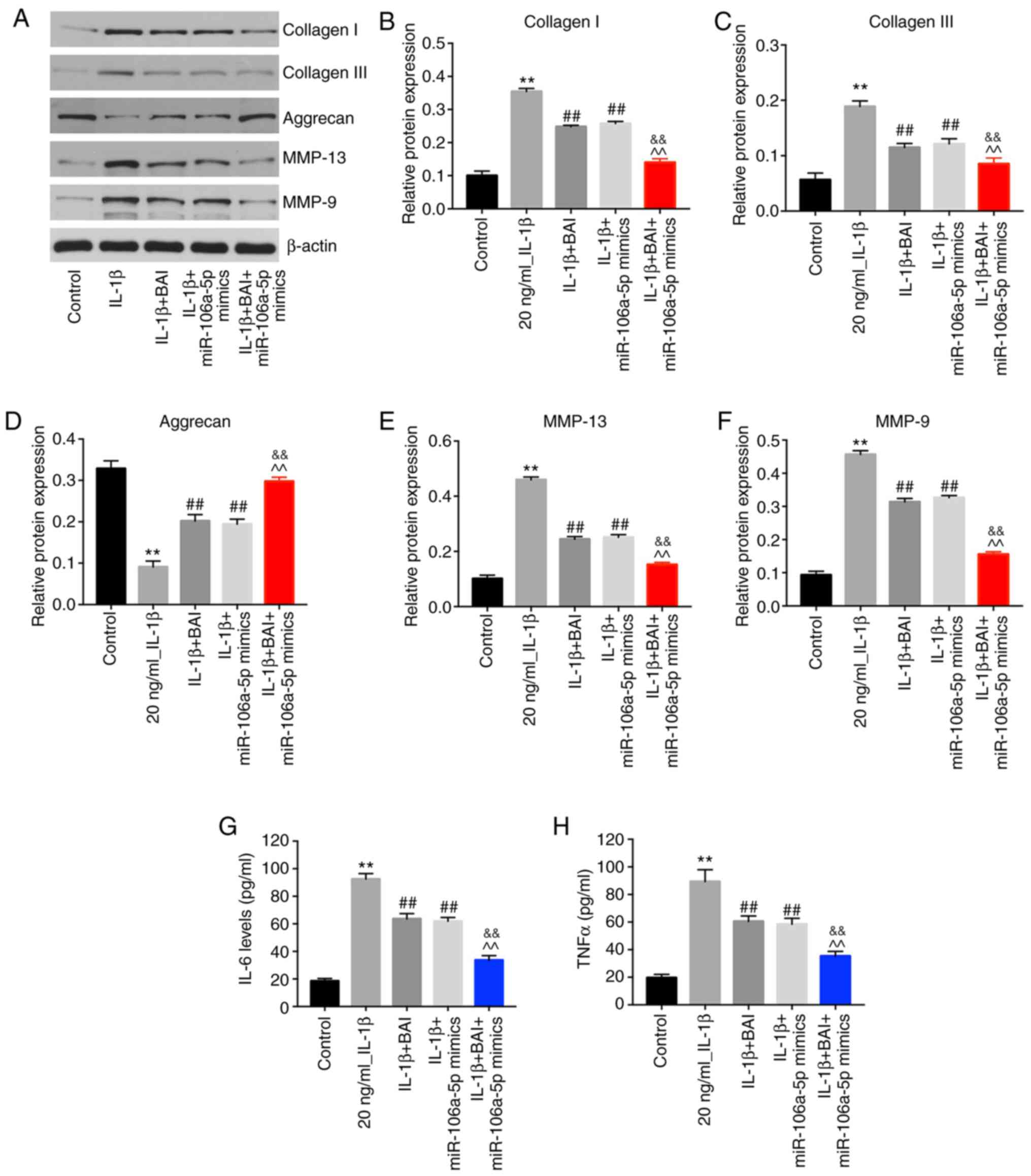
BAI and miR-106a-5p combination
treatment significantly alleviates the OA phenotype and attenuates
the inflammatory response
To determine the phenotype of the OA model, the
expression levels of collagen I and III, aggrecan, MMP-13 and MMP-9
were detected by western blotting. BAI or miR-106a-5p mimics
monotherapy reversed IL-1β-induced aggrecan degradation, but
combination therapy was more effective compared with either
monotherapy (Fig. 4A and D). Moreover, IL-1β-induced upregulation of
collagen I and III was alleviated to a greater extent in the
combination treatment group compared with the BAI and miR-106a-5p
mimics monotherapy groups (Fig.
4A-C). Similarly, combination treatment reversed IL-1β-mediated
upregulation of MMP-9 and MMP-13 to a greater extent compared with
the BAI and miR-106a-5p mimics monotherapy groups (Fig. 4A, E
and F). In addition, the
combination treatment group displayed lower levels of TNF-α and
IL-6 compared with the BAI and miR-106a-mimics monotherapy groups
(Fig. 4G and H). Collectively, the results indicated
that the inflammatory response of the OA model was reversed to a
greater degree by combination treatment compared with BAI or
miR-106a-5p mimics monotherapy.
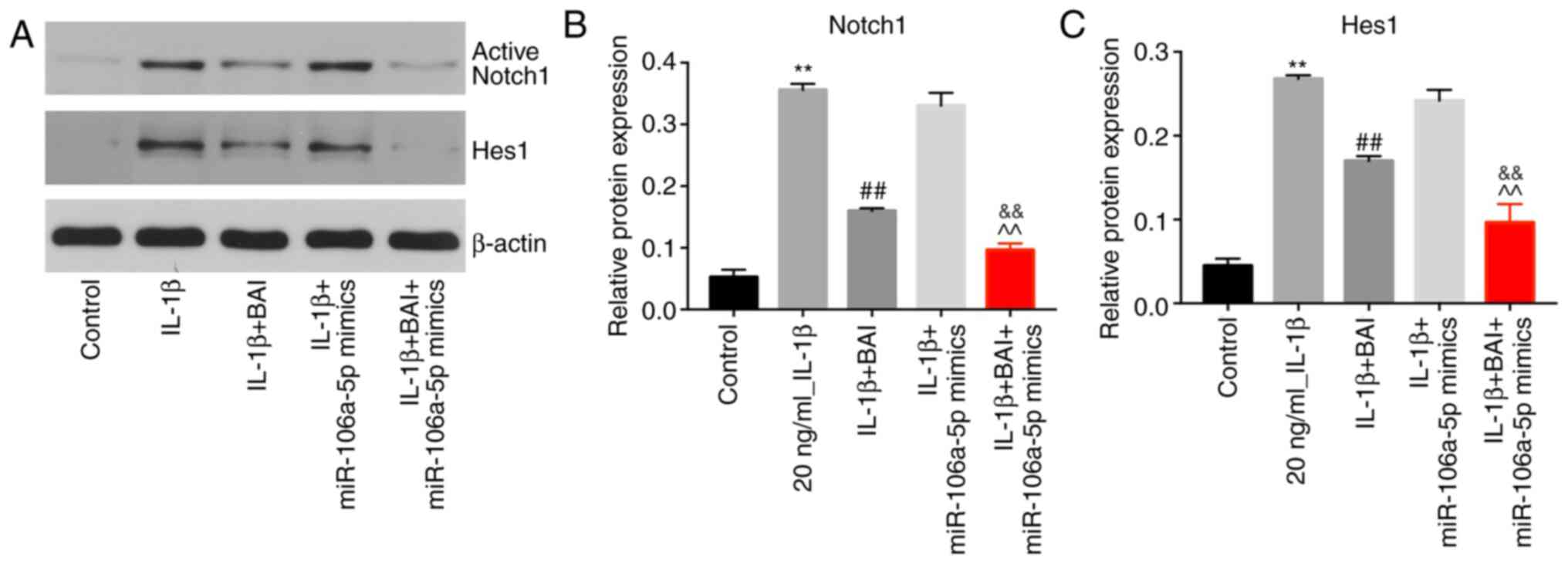
BAI and miR-106a-5p combination
treatment reverses IL-1β-induced upregulation of active Notch1 and
Hes1
IL-1β significantly upregulated active Notch1
expression in CHON-001 cells compared with the control group, but
BAI treatment significantly reversed IL-1β-mediated upregulation in
the in vitro OA model. By contrast, miR-106a-5p mimics did
not inhibit IL-1β-induced upregulation of active Notch1. BAI and
miR-106a-5p combination treatment alleviated IL-1β-mediated
upregulation of active Notch1 to a greater degree compared with BAI
treatment alone (Fig. 5A and
B). The protein expression level of
Hes1 was also upregulated in the IL-1β group compared with the
control group (Fig. 5A and C), which was reversed following treatment
with BAI, but not miR-106a-5p mimics. However, the combination of
BAI and miR-106a-5p mimics had a greater inhibitory effect on
IL-1β-mediated Hes1 upregulation compared with BAI alone (Fig. 5A and C).
Discussion
The present study indicated that combination
treatment of BAI and miR-106a-5p mimics attenuated the OA phenotype
by reversing IL-1β-induced upregulation of active Notch 1 and Hes
1. Consistent with the results of the present study, previous
studies have reported that BAI exhibited an antitumor effect via
downregulation of the Notch 1/Hes 1 signaling pathway. Lian et
al (36) indicated that BAI
could inhibit cervical cancer cell proliferation via inhibition of
the Notch 1/Hes signaling pathway. Similarly, Su et al
(37) reported that the antitumor
effect of BAI was also mediated via downregulation of Notch1 and
Hes 1 expression in non-small cell lung carcinoma A549 and H1299
cells. The aforementioned studies and the present study identified
a common mechanism underlying BAI-mediated therapeutic effects in
multiple diseases. However, Ji et al (32) suggested that different signaling
pathways and targets are involved in miR-106a-5p-associated OA
pathogenesis. According to the aforementioned study, miR-106a-5p
downregulation was accompanied by decreased paired box protein
PAX-5 expression and increased GLI-similar 3 expression in patients
with OA. The discrepancy between studies may be due to the
complexity of the signaling pathways involved in OA
pathogenesis.
Interestingly, Pu et al (38) demonstrated that BAI inhibited
acinar-to-ductal metaplasia of AR42J pancreatic acinal cells via
inhibiting NF-κB activation. In the present study, it was revealed
that BAI treatment exhibited an effect on Notch signaling in
IL-1β-treated CHON-001 cells. The Notch 1/Hes 1 signaling pathway
might be a common mechanism underlying several protective effects
of BAI against a number of diseases. Different signaling pathways
might be involved when BAI is used for the treatment of other
diseases. Therefore, the results of the present study provide an
insight into investigating the different mechanisms underlying BAI
in different diseases. Since the signaling pathways and targets
involved in OA pathogenesis are numerous and complex (39,40),
further in vitro and in vivo studies are required to
determine the mechanisms underlying the effects of combined
treatment with BAI and miR-106a-5p mimics.
In conclusion, the present study suggested that the
combined treatment of BAI and miR-106a-5p mimics might achieve an
improved anti-inflammatory and antiapoptotic effect in
IL-1β-stimulated CHON-001cells.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Jiangsu Province
‘333’ project (grant no. BRA2018249).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QX made major contributions to the conception,
design and manuscript drafting of the present study. JW and TW were
responsible for data acquisition, data analysis and data
interpretation. HZ made substantial contributions to conception and
design of the study and revised the manuscript. All authors agreed
to be accountable for all aspects of the work. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rustenburg CME, Emanuel KS, Peeters M,
Lems WF, Vergroesen PA and Smit TH: Osteoarthritis and
intervertebral disc degeneration: Quite different, quite similar.
JOR Spine. 1(e1033)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gezginaslan O, Ozturk EA, Cengiz M,
Mirzaoglu T and Cakci FA: Effects of isokinetic muscle
strengthening on balance, proprioception, and physical function in
bilateral knee osteoarthritis patients with moderate fall risk.
Turk J Phys Med Rehabil. 64:353–361. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Berenbaum F: Osteoarthritis as an
inflammatory disease (osteoarthritis is not osteoarthrosis!).
Osteoarthritis Cartilage. 21:16–21. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Blagojevic M, Jinks C, Jeffery A and
Jordan KP: Risk factors for onset of osteoarthritis of the knee in
older adults: A systematic review and meta-analysis. Osteoarthritis
Cartilage. 18:24–33. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fang Y, Wang P, Xia L, Bai S, Shen Y, Li
Q, Wang Y, Zhu J, Du J and Shen B: Aberrantly hydroxymethylated
differentially expressed genes and the associated protein pathways
in osteoarthritis. PeerJ. 7(e6425)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Duval E, Leclercq S, Elissalde JM, Demoor
M, Galera P and Boumediene K: Hypoxia-inducible factor 1alpha
inhibits the fibroblast-like markers type I and type III collagen
during hypoxia-induced chondrocyte redifferentiation: Hypoxia not
only induces type II collagen and aggrecan, but it also inhibits
type I and type III collagen In the hypoxia-inducible factor
1alpha-dependent redifferentiation of chondrocytes. Arthritis
Rheum. 60:3038–3048. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Egloff C, Hart DA, Hewitt C, Vavken P,
Valderrabano V and Herzog W: Joint instability leads to long-term
alterations to knee synovium and osteoarthritis in a rabbit model.
Osteoarthritis Cartilage. 24:1054–1060. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen WP, Xiong Y, Hu PF, Bao JP and Wu LD:
Baicalein inhibits MMPs expression via a MAPK-dependent mechanism
in chondrocytes. Cell Physiol Biochem. 36:325–333. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ruan G, Xu J, Wang K, Wu J, Zhu Q, Ren J,
Bian F, Chang B, Bai X, Han W and Ding C: Associations between knee
structural measures, circulating inflammatory factors and MMP13 in
patients with knee osteoarthritis. Osteoarthritis Cartilage.
26:1063–1069. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fei J, Liang B, Jiang C, Ni H and Wang L:
Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and
attenuates osteoarthritis progression in a rat model. Biomed
Pharmacother. 109:1586–1592. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Marchand F, Perretti M and McMahon SB:
Role of the immune system in chronic pain. Nat Rev Neurosci.
6:521–532. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Stassen M, Hultner L and Schmitt E:
Classical and alternative pathways of mast cell activation. Crit
Rev Immunol. 22:115–140. 2002.PubMed/NCBI
|
|
13
|
Shen J, Abu-Amer Y, O'Keefe RJ and
McAlinden A: Inflammation and epigenetic regulation in
osteoarthritis. Connect Tissue Res. 58:49–63. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim L and Kim JY: Chondroprotective effect
of curcumin and lecithin complex in human chondrocytes stimulated
by IL-1β via an anti-inflammatory mechanism. Food Sci Biotechnol.
28:547–553. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hosaka Y, Saito T, Sugita S, Hikata T,
Kobayashi H, Fukai A, Taniguchi Y, Hirata M, Akiyama H, Chung UI
and Kawaguchi H: Notch signaling in chondrocytes modulates
endochondral ossification and osteoarthritis development. Proc Natl
Acad Sci USA. 110:1875–1880. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gao W, Sweeney C, Walsh C, Rooney P,
McCormick J, Veale DJ and Fearon U: Notch signalling pathways
mediate synovial angiogenesis in response to vascular endothelial
growth factor and angiopoietin 2. Ann Rheum Dis. 72:1080–1088.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mirando AJ, Liu Z, Moore T, Lang A, Kohn
A, Osinski AM, O'Keefe RJ, Mooney RA, Zuscik MJ and Hilton MJ:
RBP-Jκ-dependent Notch signaling is required for murine articular
cartilage and joint maintenance. Arthritis Rheum. 65:2623–2633.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang W, Zeng L, Wang ZM, Zhang S, Rong XF
and Li RH: Ginsenoside Rb1 inhibits matrix metalloproteinase 13
through down-regulating Notch signaling pathway in osteoarthritis.
Exp Biol Med (Maywood). 240:1614–1621. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yu HT, Gu CZ and Chen JQ: MiR-9
facilitates cartilage regeneration of osteoarthritis in rabbits
through regulating Notch signaling pathway. Eur Rev Med Pharmacol
Sci. 23:5051–5058. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li Y, Wang J, Song X, Bai H, Ma T, Zhang
Z, Li X, Jiang R, Wang G, Fan X, et al: Effects of baicalein on
IL-1β-induced inflammation and apoptosis in rat articular
chondrocytes. Oncotarget. 8:90781–90795. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chi YS, Lim H, Park H and Kim HP: Effects
of wogonin, a plant flavone from Scutellaria radix, on skin
inflammation: In vivo regulation of inflammation-associated gene
expression. Biochem Pharmacol. 66:1271–1278. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gao Z, Huang K, Yang X and Xu H: Free
radical scavenging and antioxidant activities of flavonoids
extracted from the radix of Scutellaria baicalensis Georgi.
Biochim Biophys Acta. 1472:643–650. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Huang Y, Tsang SY, Yao X and Chen ZY:
Biological properties of baicalein in cardiovascular system. Curr
Drug Targets Cardiovasc Haematol Disord. 5:177–184. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang CZ, Mehendale SR and Yuan CS:
Commonly used antioxidant botanicals: Active constituents and their
potential role in cardiovascular illness. Am J Chin Med.
35:543–558. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang X, Zhu Y, Chen X, Zhang Y, Zhang Y,
Jia Y, Wang H, Liu Y and Xiao L: Baicalein ameliorates
inflammatory-related apoptotic and catabolic phenotypes in human
chondrocytes. Int Immunopharmacol. 21:301–308. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hamam R, Hamam D, Alsaleh KA, Kassem M,
Zaher W, Alfayez M, Aldahmash A and Alajez NM: Circulating
microRNAs in breast cancer: Novel diagnostic and prognostic
biomarkers. Cell Death Dis. 8(e3045)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Skommer J, Rana I, Marques FZ, Zhu W, Du Z
and Charchar FJ: Small molecules, big effects: The role of
microRNAs in regulation of cardiomyocyte death. Cell Death Dis.
5(e1325)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
McDermott R, Gabikian P, Sarvaiya P,
Ulasov I and Lesniak MS: MicroRNAs in brain metastases: Big things
come in small packages. J Mol Med (Berl). 91:5–13. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bak RO, Hollensen AK and Mikkelsen JG:
Managing microRNAs with vector-encoded decoy-type inhibitors. Mol
Ther. 21:1478–1485. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xu JF, Zhang SJ, Zhao C, Qiu BS, Gu HF,
Hong JF, Cao L, Chen Y, Xia B, Bi Q and Wang YP: Altered microRNA
expression profile in synovial fluid from patients with knee
osteoarthritis with treatment of hyaluronic acid. Mol Diagn Ther.
19:299–308. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Le LT, Swingler TE, Crowe N, Vincent TL,
Barter MJ, Donell ST, Delany AM, Dalmay T, Young DA and Clark IM:
The microRNA-29 family in cartilage homeostasis and osteoarthritis.
J Mol Med (Berl). 94:583–596. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ji Q, Qi D, Xu X, Xu Y, Goodman SB, Kang
L, Song Q, Fan Z, Maloney WJ and Wang Y: Cryptotanshinone protects
cartilage against developing osteoarthritis through the
miR-106a-5p/GLIS3 axis. Mol Ther Nucleic Acids. 11:170–179.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Guan YJ, Li J, Yang X, Du S, Ding J, Gao
Y, Zhang Y, Yang K and Chen Q: Evidence that miR-146a attenuates
aging- and trauma-induced osteoarthritis by inhibiting Notch1,
IL-6, and IL-1 mediated catabolism. Aging Cell.
17(e12752)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hu G, Zhao X, Wang C, Geng Y, Zhao J, Xu
J, Zuo B, Zhao C, Wang C and Zhang X: MicroRNA-145 attenuates
TNF-α-driven cartilage matrix degradation in osteoarthritis via
direct suppression of MKK4. Cell Death Dis. 8(e3140)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lian H, Hui Y, Xiaoping T, Wei T, Jiyi X
and Xiaolan Y: Baicalein suppresses the proliferation of human
cervical cancer cells via Notch 1/Hes signaling pathway. J Cancer
Res Ther. 15:1216–1220. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Su G, Chen H and Sun X: Baicalein
suppresses non small cell lung cancer cell proliferation, invasion
and Notch signaling pathway. Cancer Biomark. 22:13–18.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pu WL, Luo YY, Bai RY, Guo AW, Zhou K,
Zhang YS, Miao L, Rüegg C, Hottiger MO, Gao XM and Sun LK:
Baicalein inhibits acinar-to-ductal metaplasia of pancreatic acinal
cell AR42J via improving the inflammatory microenvironment. J Cell
Physiol. 233:5747–5755. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Saito T and Tanaka S: Molecular mechanisms
underlying osteoarthritis development: Notch and NF-κB. Arthritis
Res Ther. 19(94)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhou Y, Wang T, Hamilton JL and Chen D:
Wnt/β-catenin signaling in osteoarthritis and in other forms of
arthritis. Curr Rheumatol Rep. 19(53)2017.PubMed/NCBI View Article : Google Scholar
|