Coronavirus pneumonia is a respiratory infectious
disease caused by coronavirus infection. Since the severe acute
respiratory syndrome coronavirus (SARS-CoV) spread and caused
infection globally in 2003, coronaviruses have gradually attracted
public attention and have caused several serious epidemics
(1-3).
Coronaviruses are a group of single-stranded
positive-sense RNA viruses, of which 26 species are currently known
(4,5). Based on their differences in antigen
cross-reactivity and genetic composition, they are divided into 4
genera (α, β, γ and δ), of which only genera α and β contain
strains that are pathogenic to humans (6-8).
SARS-CoV-2 (the 2019 novel coronavirus), SARS-CoV and Middle East
respiratory syndrome coronavirus (MERS-CoV) belong to the
β-coronavirus family (9,10). There are seven known coronaviruses
that may cause human diseases, including HCoV-229E, HCoV-OC43,
HCoV-NL63, HCoV-HKU1, SARS-CoV and MERS-CoV (11,12)
and the newly discovered SARS-CoV-2(13). These viruses may cause a variety of
clinically critical conditions, including kidney injury. The aim of
the present systematic review was to summarize the knowledge of
coronavirus infection from the perspective of kidney injury.
SARS was first reported in Asia in early 2003, and
similar diseases were subsequently reported in North America and
Europe (14,15). Of a total of 8,422 patients
diagnosed with SARS, 916 succumbed to the disease, bringing the
case fatality rate to 10.87% (16).
SARS-CoV was found to be the main pathogen of SARS based on the
findings from a macaque infection model (17). SARS-associated coronavirus was the
SARS pathogen identified from the Macaca fascicularis
infection experiment (18). During
the SARS-CoV infection, ~100% of adult and pediatric patients had
fever, approximately half of all patients had cough and/or myalgia,
and a small number of patients experienced upper respiratory
symptoms (19,20). In 10-20% of the patients, blood urea
nitrogen and urine creatinine levels were increased, indicating
that SARS may directly or indirectly cause kidney injury (Table I) (21-26).
Chu et al (21) reported
that kidney involvement in SARS was significantly correlated with
the severity of the disease, and that patients with chronic
diseases were more likely to suffer from kidney injury. Autopsy
reports of some patients with SARS indicated local renal hemorrhage
and varying degrees of acute tubular necrosis (27). In situ hybridization and
electron microscopy indicated the presence of viral sequences and
particles, respectively, in distal renal tubular epithelial cells
(27-29).
The presence of the virus in the distal tubules may explain the
findings of viral RNA and isolation of SARS-CoV from urine samples
(30-32).
The earliest reports of MERS can be traced back to
June 2012, when MERS-CoV was isolated from a patient in Saudi
Arabia who succumbed to severe respiratory disease (33). By December 2019, there were 868
reported deaths among 2,496 MERS cases worldwide, with a case
fatality rate of 34.77% (16).
Researchers indicated that MERS-CoV, the causative agent of the
disease, may originate from bats (11), with dromedary as its intermediate
host (34). The clinical
manifestations in patients with MERS-CoV infection range from
asymptomatic to severe infectious pneumonia, acute respiratory
distress, septic shock and multiple organ failure leading to death
(11). Approximately 40% of
patients exhibited increased urine creatinine levels, suggesting
that MERS-CoV may cause kidney injury in some patients (Table I) (35-37).
In vitro infection experiments with primary human renal
epithelial cells (PromoCell) revealed that MERS-CoV robustly
replicated in culture and produced more infectious virions
(38). Poissy et al
(39) reported that the virus could
be detected in the blood and the urine of their most severely ill
patients with MERS-CoV infection. Patients with MERS-CoV infection
usually manifested with early and rapid-onset acute renal failure,
which adversely affected the disease progression (38-41).
Alsaad et al (42) indicated
that, in patients with MERS-CoV infection, the kidney displayed the
characteristics of renal tubular epithelial cell degeneration and
regeneration/acute kidney injury (AKI). Ng et al (43) found that, in patients with MERS-CoV
infection, the kidney exhibited an increase in global sclerosing
glomeruli, affecting 5-10% of the total glomeruli; thickening
Bowman capsules; severe atherosclerosis and hyaline
arteriolosclerosis; patchy interstitial inflammation; and
intratubular proteinaceous and granular casts.
Coronavirus disease 2019 (COVID-19) is an infectious
disease caused by a novel coronavirus (44,45).
The pathogen of this disease, SARS-CoV-2, shows 75-80% similarity
to the nucleotide sequence of SARS-CoV (45-47).
The bat is presumed to be its animal host and an intermediate host
(48-50).
Although the main target organ of SARS-CoV-2 is the lung, several
studies have demonstrated that SARS-CoV-2 may also induce kidney
injury; 5-30% of patients exhibit increase blood urea nitrogen and
urine creatinine levels and kidney injury, indicating that the
kidney is also targeted by SARS-CoV-2 (Table I) (13,51-56).
During the current COVID-19 pandemic, 4-7% of patients infected
with SARS-CoV-2 developed AKI, and the AKI incidence may be even
higher among patients with severe symptoms admitted to the
intensive care unit (ICU) (51).
Huang et al (54) analyzed
41 patients with SARS-CoV-2 infection and found that >10% had
elevated creatinine levels. Among patients treated in the ICU, 23%
had AKI. Patients with kidney injury (including increased
creatinine and urea nitrogen, proteinuria, hematuria and AKI) were
more likely to die in the hospital in a study of 710 patients with
COVID-19. Cox regression analysis confirmed that kidney injury is
one of the independent risk factors for poor prognosis (57). Su et al analyzed renal
pathologies in 26 autopsies of patients with COVID-19 and found
prominent acute proximal tubular injury, peritubular erythrocyte
aggregation and glomerular fibrin thrombi with ischemic collapse
(58). In another study, 251 of 333
patients (75.4%) presented with renal complications, including
proteinuria, hematuria and AKI. Although renal complications often
resolved within 3 weeks after the onset of symptoms, renal
complications in COVID-19 were associated with higher mortality
(59).
Previous studies have reported that patients
infected with SARS-CoV, MERS-CoV or SARS-CoV-2 may present with
AKI, but the incidence across studies was not consistent. AKI was
reported to develop in 5-15% cases of SARS and MERS-CoV infections,
whereas early reports suggested a lower incidence of AKI among
patients with COVID-19 infection (13,51).
Chen et al (60) found that
the mortality rate of AKI was highest in SARS (86.6%), followed by
COVID-19 (76.5%) and MERS (68.5%). Autopsy results in patients with
SARS-CoV infection revealed that the kidney exhibited local
hemorrhage and different degrees of acute tubular necrosis instead
of glomerular lesions (21,27). Unlike SARS, a MERS autopsy report
revealed that the kidney had the characteristic of epithelial cell
degeneration and regeneration, but the size and shape of the
glomeruli were normal, with only minor ischemic changes (42). Su et al (58) analyzed renal pathologies in 26
autopsies of patients with COVID-19 and found prominent acute
proximal tubular injury, peritubular erythrocyte aggregation and
glomerular fibrin thrombi with ischemic collapse. Ding et al
(29) reported that SARS-CoV was
detected in distal convoluted renal tubules. In situ
hybridization and electron microscopy also indicated the presence
of viral sequences and particles, respectively, in distal renal
tubular epithelial cells (27-29).
MERS-CoV particles were localized in renal proximal tubular
epithelial cells (42). SARS-CoV-2
particles were identified by electron microscopy in the cytoplasm
of renal proximal tubular epithelial cells and podocytes, but less
so in the distal tubules (58).
Interestingly, all three coronaviruses were isolated from urine
samples (30-32,61,62).
Kidney injury in coronavirus infection is mainly due
to the ability of coronavirus proteins to bind to specific cell
surface receptors (63-65).
To date, two major functional receptors for coronavirus have been
identified:
Angiotensin-converting enzyme 2 (ACE2) is mainly
expressed in the lung, kidney, heart and other tissues; in the
kidney, this protein is prominently expressed in the proximal
tubule and at a lower level in the glomeruli (66,67).
Dipeptidyl peptidase 4 (DPP4; also referred to as CD26) is also
highly expressed in the kidney, small intestine and lung (68-70).
DPP4 is also one of the renal tubular brush border membrane
proteins and is present in glomerular podocytes and capillaries
(71). The expression levels of
ACE2 and DPP4 in normal tissues were examined by searching two
public databases, A Database of Hepatocellular Carcinoma Expression
Atlas (http://lifeome.net/database/hccdb/home.html) (72) and the Human Protein Atlas (HPA;
https://www.proteinatlas.org/) (73,74).
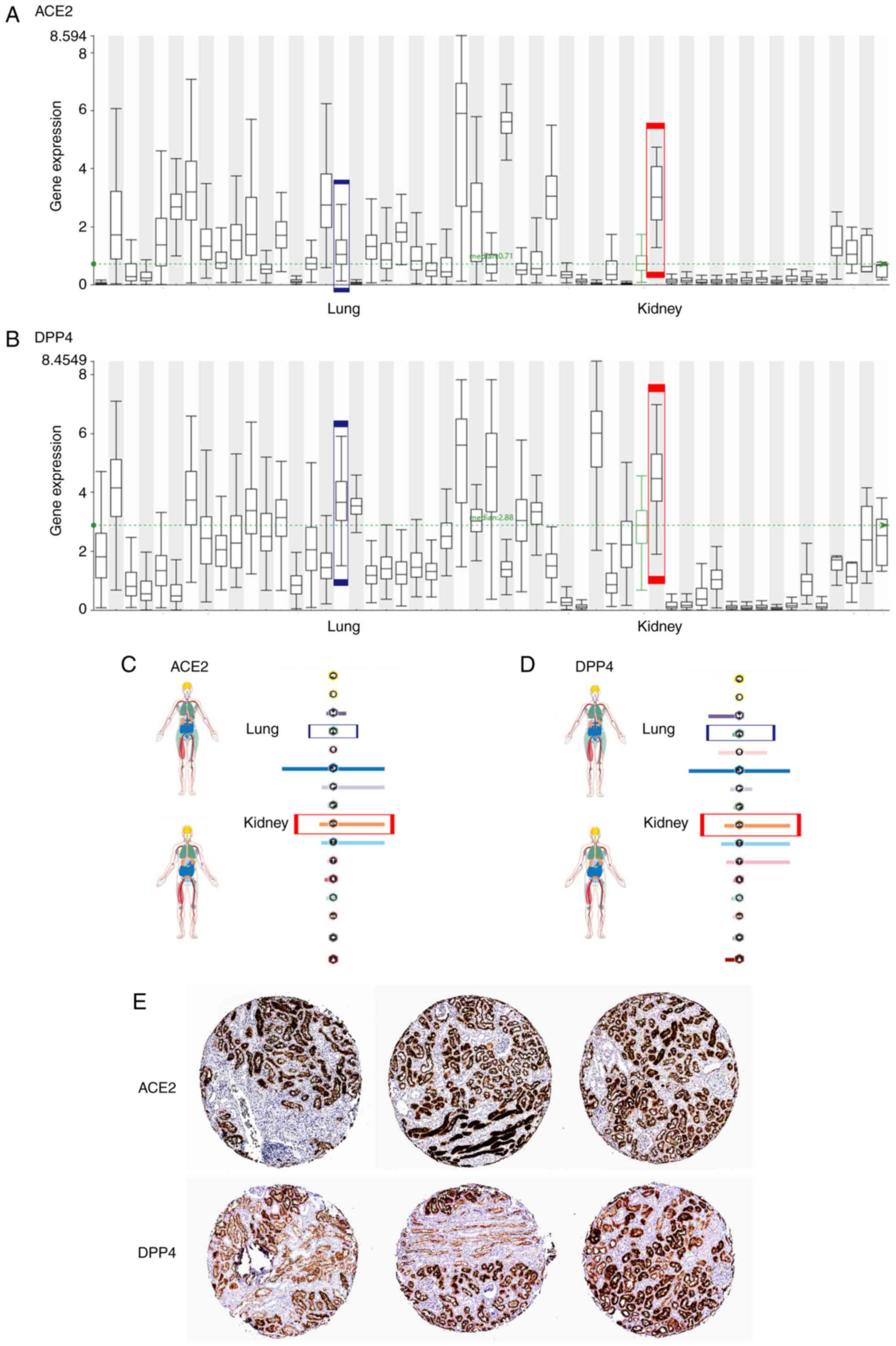
At the RNA level, the expression level of ACE2 in kidney tissues
was higher compared with that in lung tissues (Fig. 1A and C), which is consistent with previous
reports by Xu et al (75)
and Hoffmann et al (76).
The DPP4 expression in the kidney was also higher compared with
that in the lung (Fig. 1B and
D). ACE2 was abundantly expressed
in the kidney (77), mainly in the
brush border of the proximal tubule (65,78),
which was consistent with immunohistochemistry results in the HPA
(Fig. 1E). Pala et al
(79) found that DPP4 was
abundantly expressed in human glomerular endothelial cells, which
was also consistent with the immunohistochemistry results in the
HPA (Fig. 1E).
SARS-CoV-2 and SARS-CoV exhibited high homology (up
to 79%) on bioinformatics analysis (50). The affinity of SARS-CoV-2 was
markedly higher compared with that of SARS-CoV when the S protein
bound to the human ACE2(85).
SARS-CoV-2 can use ACE2 to enter the recipient cells and activate
the S protein by the serine protease TMPRSS2 on the host cell
surface (76). Two studies that
recently published online investigated the mechanism of how
SARS-CoV-2 identifies and binds to human ACE2 and the composite
crystal structure, which enhanced our understanding of the
ACE2-mediated SARS-CoV-2 recognition and cell infection processes
(86,87). Pan et al (88) concluded that the cytopathic effects
of SARS-CoV-2 on podocytes and proximal straight tubule cells may
cause AKI in patients with COVID-19, particularly those with
evidence of SARS-CoV-2 infection in blood samples. Electron
microscopic examination revealed that coronavirus particles were
present in podocytes and renal tubular epithelial cells. In
addition, immunostaining with SARS-CoV nucleoprotein antibody was
positive in the tubules (58).
SARS-CoV-2 nucleocapsid protein was detected in the renal tubular
structure, and nucleocapsid protein-positive inclusion bodies were
also observed in the renal cell cytoplasm (58). Researchers reported the presence of
particles on the renal tubular epithelium, which were
morphologically identical to SARS-CoV-2, and with viral arrays and
other features of virus assembly, which constituted evidence of
direct infection of the kidney by SARS-CoV-2. This finding
confirmed that direct renal infection occurs in the setting of AKI
in COVID-19(89). Patients infected
with SARS-CoV and SARS-CoV-2 developed kidney injury that may be
caused by a direct attack on kidney cells through ACE2. It remains
unclear how the virus causes AKI after infecting the kidney
cells.
In addition, immune activation caused by viral
infection may release a large amount of inflammatory mediators
(such as IL-1, IL-6 and TNF), resulting in kidney injury (92,93).
During the SARS outbreak, some critically ill patients experienced
an inflammatory storm characterized by elevated IL-1β, IL-6, IL-12,
IFN-γ, IP10 and MCP-1 levels (94).
The ‘cytokine storm’ caused by MERS coronavirus is primarily
associated with IFN-γ, TNF-α, IL-15 and IL-17(95). SARS and MERS both induce a ‘cytokine
storm’ in critically ill patients (96-98).
COVID-19 patients may be affected by both the cytopathic effects
directly induced by the virus as well as the systemic inflammatory
responses caused by the cytokine storm, which may result in
pathological changes in renal podocytes and proximal tubular cells
and lead to AKI (88). Researchers
analyzed the clinical characteristics of COVID-19 patients and
found that, in patients with pneumonia, particularly in severe
cases, there was a significant decrease in the lymphocyte count,
and that a number of inflammatory factors (such as IL-6 and TNF)
were increased significantly and that these may have caused kidney
and other organ failure (51,54,99).
Researchers also indicated that the virus may enter the blood
circulation after lung infection, accumulate in the kidneys and
cause kidney damage (100).
Patients with viral infections suffered from anorexia, diarrhea and
excessive perspiration, which may lead to hypovolemia and renal
hypoperfusion, eventually causing kidney injury (101). Notably, certain antibiotics and
antiviral drugs are also likely to cause drug-related kidney injury
(102,103).
In addition to antiviral therapy and respiratory
support, blood purification is also an important modality for
treating coronavirus infections. According to the Kidney Diseases
Improving Global Outcomes AKI guidelines, when continuous renal
replacement therapy (CRRT) is used to treat COVID-19 patients, the
therapeutic dose is 20-25 ml/kg/h post-dilution and 25-30 ml/kg/h
predilution (104). Clinical
studies demonstrated that the AKI incidence in COVID-19 patients
was 3-7%, and the proportion of patients on CRRT was 1.5-9.0%; the
AKI incidence in severe and critically ill patients admitted to the
ICU was significantly increased, ranging from 8.3 to 23.0%, and
CRRT was required for 5.6-23.0% of the patients, whereas CRRT was
required for 66.7-100% of patients with AKI (13,51,54).
It was previously demonstrated that 6.7-11.1% of patients with SARS
developed AKI and 1.8% received CRRT (21). The incidence of AKI in MERS was 26.7
and 13.5-20% patients with AKI received CRRT (41,96).
Up to 50% of MERS patients received CRRT (105). In addition, extracorporeal
membrane oxygenation combined with CRRT was reported to effectively
improve the patient's volume load and prognosis (106,107). However, it is worth noting that
patients receiving maintenance hemodialysis are susceptible to
COVID-19 and that hemodialysis centers are high-risk settings for
COVID-19(108).
CRRT eliminates the overexpressed inflammatory
factors and anti-inflammatory transmitters in the blood circulation
non-selectively, reducing the peak concentrations of these factors
and downregulating the body's inflammatory responses (109). Plasma replacement, adsorption,
perfusion and other special blood purification treatment
technologies are mainly used in the early and middle stages of
cytokine storms in severe and critically ill patients with
COVID-19, mainly to block disease progression by reducing IL-6
levels (110,111). In addition to using antibodies
against inflammatory factors, such as tocilizumab, to combat the
cytokine storm (112,113), blood purification treatment may
also effectively suppress the cytokine storm and reduce the
mortality rate of patients with severe COVID-19 (110,114,115). However, according to a recent
research, tocilizumab was not effective in preventing intubation or
death in moderately ill hospitalized patients with
COVID-19(116). A benefit of
dexamethasone was demonstrated in hospitalized patients with
COVID-19 who were treated with either invasive mechanical
ventilation or oxygen alone (117).
In summary, blood purification is a key therapeutic
strategy against COVID-19, particularly in critically ill patients
with or without renal failure, and it may improve the prognosis and
outcome of these patients.
Kidney injury is an important clinical issue in
coronavirus infection. The two currently identified receptors for
coronavirus infection, ACE2 and DPP4, may be the key mediators
triggering direct kidney injury by the coronavirus, while it
remains unclear how the coronavirus causes kidney injury after
entering renal cells. ACE2 and DPP4 are potential therapeutic
targets, and target drugs are developed based on their structure to
block virus invasion before injury occurs. Therefore, it is
necessary to carry out further basic and clinical research to guide
clinical practice. Blood purification is an important treatment
measure in coronavirus infection with or without kidney injury.
Early and timely blood purification therapy may reduce or prevent
disease progression in patients with coronavirus infection.
The current COVID-19 epidemic is still not under
control globally. Although the vaccine is currently used on
patients, it is still necessary to focus on infection prevention in
patients with kidney disease, study the pathogenic mechanism of
COVID-19 in depth, and optimize the treatment strategies for
severely ill patients with AKI in order to improve their
prognosis.
Not applicable.
Funding: The present study was supported by the National Natural
Sciences Foundation of China (grant nos. 81870498 and 81900633) and
the National Natural Sciences Foundation of Hunan Province (grant
no. 2017JJ2342).
Not applicable.
ZF, HZ and WZ conceived and designed the study. YC,
YW and JW contributed to drafted the manuscript and revised it
critically for important intellectual content. ZF prepared the
manuscript. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Kapoor M, Pringle K, Kumar A, Dearth S,
Liu L, Lovchik J, Perez O, Pontones P, Richards S, Yeadon-Fagbohun
J, et al: Clinical and laboratory findings of the first imported
case of Middle East respiratory syndrome coronavirus to the United
States. Clin Infect Dis. 59:1511–1518. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhong NS, Zheng BJ, Li YM, Poon Xie ZH,
Chan KH, Li PH, Tan SY, Chang Q, Xie JP, et al: Epidemiology and
cause of severe acute respiratory syndrome (SARS) in Guangdong,
People's Republic of China, in February, 2003. Lancet.
362:1353–1358. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Plipat T, Buathong R, Wacharapluesadee S,
Siriarayapon P, Pittayawonganon C, Sangsajja C, Kaewpom T,
Petcharat S, Ponpinit T, Jumpasri J, et al: Imported case of Middle
East respiratory syndrome coronavirus (MERS-CoV) infection from
Oman to Thailand, June 2015. Euro Surveill.
22(30598)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vijayanand P, Wilkins E and Woodhead M:
Severe acute respiratory syndrome (SARS): A review. Clin Med.
4:152–160. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Muthumani K, Falzarano D, Reuschel EL,
Tingey C, Flingai S, Villarreal DO, Wise M, Patel A, Izmirly A,
Aljuaid A, et al: A synthetic consensus anti-spike protein DNA
vaccine induces protective immunity against Middle East respiratory
syndrome coronavirus in nonhuman primates. Sci Transl Med.
7(301ra132)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Paules CI, Marston HD and Fauci AS:
Coronavirus infections-More Than Just the common cold. JAMA.
323:707–708. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fehr AR and Perlman S: Coronaviruses: An
overview of their replication and pathogenesis. Methods Mol Biol.
1282:1–23. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang SF, Tuo JL, Huang XB, Zhu X, Zhang
DM, Zhou K, Yuan L, Luo HJ, Zheng BJ, Yuen KY, et al: Epidemiology
characteristics of human coronaviruses in patients with respiratory
infection symptoms and phylogenetic analysis of HCoV-OC43 during
2010-2015 in Guangzhou. PLoS One. 13(e0191789)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Berry M, Gamieldien J and Fielding BC:
Identification of new respiratory viruses in the new millennium.
Viruses. 7:996–1019. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Chan PK and Chan MC: Tracing the
SARS-coronavirus. J Thorac Dis. 5 (Suppl 2):S118–S121.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zumla A, Hui DS and Perlman S: Middle East
respiratory syndrome. Lancet. 386:995–1007. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Skariyachan S, Challapilli SB, Packirisamy
S, Kumargowda ST and Sridhar VS: Recent aspects on the pathogenesis
mechanism, animal models and novel therapeutic interventions for
middle east respiratory syndrome coronavirus infections. Front
Microbiol. 10(569)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen N, Zhou M, Dong X, Qu J, Gong F, Han
Y, Qiu Y, Wang J, Liu Y, Wei Y, et al: Epidemiological and clinical
characteristics of 99 cases of 2019 novel coronavirus pneumonia in
Wuhan, China: A descriptive study. Lancet. 395:507–513.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Heymann DL, Mackenzie JS and Peiris M:
SARS legacy: Outbreak reporting is expected and respected. Lancet.
381:779–781. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Anderson LJ and Tong S: Update on SARS
research and other possibly zoonotic coronaviruses. Int J
Antimicrob Agents. 36 (Suppl 1):S21–S25. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Meo SA, Alhowikan AM, Al-Khlaiwi T, Meo
IM, Halepoto DM, Iqbal M, Usmani AM, Hajjar W and Ahmed N: Novel
coronavirus 2019-nCoV: Prevalence, biological and clinical
characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med
Pharmacol Sci. 24:2012–2019. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kuiken T, Fouchier RA, Schutten M,
Rimmelzwaan GF, van Amerongen G, van Riel D, Laman JD, de Jong T,
van Doornum G, Lim W, et al: Newly discovered coronavirus as the
primary cause of severe acute respiratory syndrome. Lancet.
362:263–270. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fouchier RA, Kuiken T, Schutten M, van
Amerongen G, van Doornum GJ, van den Hoogen BG, Peiris M, Lim W,
Stöhr K and Osterhaus AD: Aetiology: Koch's postulates fulfilled
for SARS virus. Nature. 423(240)2003.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Peiris JS, Yuen KY, Osterhaus AD and Stöhr
K: The severe acute respiratory syndrome. N Engl J Med.
349:2431–2441. 2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Woodhead M, Ewig S and Torres A: Severe
acute respiratory syndrome (SARS). Eur Respir J. 21:739–740.
2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chu KH, Tsang WK, Tang CS, Lam MF, Lai FM,
To KF, Fung KS, Tang HL, Yan WW, Chan HW, et al: Acute renal
impairment in coronavirus-associated severe acute respiratory
syndrome. Kidney Int. 67:698–705. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lu HY, Xu XY, Lei Y, Wu YF, Chen BW, Xiao
F, Xie GQ and Han DM: Clinical features of probable severe acute
respiratory syndrome in Beijing. World J Gastroenterol.
11:2971–2974. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee N, Hui D, Wu A, Chan P, Cameron P,
Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, et al: A major
outbreak of severe acute respiratory syndrome in Hong Kong. N Engl
J Med. 348:1986–1994. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hsu LY, Lee CC, Green JA, Ang B, Paton NI,
Lee L, Villacian JS, Lim PL, Earnest A and Leo YS: Severe acute
respiratory syndrome (SARS) in Singapore: Clinical features of
index patient and initial contacts. Emerg Infect Dis. 9:713–717.
2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jang TN, Yeh DY, Shen SH, Huang CH, Jiang
JS and Kao SJ: Severe acute respiratory syndrome in Taiwan:
Analysis of epidemiological characteristics in 29 cases. J Infect.
48:23–31. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cheng VC, Hung IF, Tang BS, Chu CM, Wong
MM, Chan KH, Wu AK, Tse DM, Chan KS, Zheng BJ, et al: Viral
replication in the nasopharynx is associated with diarrhea in
patients with severe acute respiratory syndrome. Clin Infect Dis.
38:467–475. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Gu J, Gong E, Zhang B, Zheng J, Gao Z,
Zhong Y, Zou W, Zhan J, Wang S, Xie Z, et al: Multiple organ
infection and the pathogenesis of SARS. J Exp Med. 202:415–424.
2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lang ZW, Zhang LJ, Zhang SJ, Meng X, Li
JQ, Song CZ, Sun L, Zhou YS and Dwyer DE: A clinicopathological
study of three cases of severe acute respiratory syndrome (SARS).
Pathology. 35:526–531. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ding Y, He L, Zhang Q, Huang Z, Che X, Hou
J, Wang H, Shen H, Qiu L, Li Z, et al: Organ distribution of severe
acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV)
in SARS patients: Implications for pathogenesis and virus
transmission pathways. J Pathol. 203:622–630. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Peiris JS, Chu CM, Cheng VC, Chan KS, Hung
IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, et al: Clinical
progression and viral load in a community outbreak of
coronavirus-associated SARS pneumonia: A prospective study. Lancet.
361:1767–1772. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chan KH, Poon LL, Cheng VC, Guan Y, Hung
IF, Kong J, Yam LY, Seto WH, Yuen KY and Peiris JS: Detection of
SARS coronavirus in patients with suspected SARS. Emerg Infect Dis.
10:294–299. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cheng PK, Wong DA, Tong LK, Ip SM, Lo AC,
Lau CS, Yeung EY and Lim WW: Viral shedding patterns of coronavirus
in patients with probable severe acute respiratory syndrome.
Lancet. 363:1699–1700. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zaki AM, van Boheemen S, Bestebroer TM,
Osterhaus AD and Fouchier RA: Isolation of a novel coronavirus from
a man with pneumonia in Saudi Arabia. N Engl J Med. 367:1814–1820.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cho H, Excler JL, Kim JH and Yoon IK:
Development of Middle East respiratory syndrome coronavirus
vaccines-advances and challenges. Hum Vaccin Immunother.
14:304–313. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sun B, He H, Wang Z, Qu J, Li X, Ban C,
Wan J, Cao B, Tong Z and Wang C: Emergent severe acute respiratory
distress syndrome caused by adenovirus type 55 in immunocompetent
adults in 2013: A prospective observational study. Crit Care.
18(456)2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Al Ghamdi M, Alghamdi KM, Ghandoora Y,
Alzahrani A, Salah F, Alsulami A, Bawayan MF, Vaidya D, Perl TM and
Sood G: Treatment outcomes for patients with Middle Eastern
respiratory syndrome coronavirus (MERS CoV) infection at a
coronavirus referral center in the Kingdom of Saudi Arabia. BMC
Infect Dis. 16(174)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sherbini N, Iskandrani A, Kharaba A,
Khalid G, Abduljawad M and Al-Jahdali H: Middle East respiratory
syndrome coronavirus in Al-Madinah City, Saudi Arabia: Demographic,
clinical and survival data. J Epidemiol Glob Health. 7:29–36.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Eckerle I, Muller MA, Kallies S, Gotthardt
DN and Drosten C: In-vitro renal epithelial cell infection reveals
a viral kidney tropism as a potential mechanism for acute renal
failure during Middle East Respiratory Syndrome (MERS) Coronavirus
infection. Virol J. 10(359)2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Poissy J, Goffard A, Parmentier-Decrucq E,
Favory R, Kauv M, Kipnis E, Mathieu D, van der Werf S and Guery B:
MERS-CoV Biology Group. Kinetics and pattern of viral excretion in
biological specimens of two MERS-CoV cases. J Clin Virol.
61:275–278. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nassar MS, Bakhrebah MA, Meo SA, Alsuabeyl
MS and Zaher WA: Middle East respiratory syndrome coronavirus
(MERS-CoV) infection: Epidemiology, pathogenesis and clinical
characteristics. Eur Rev Med Pharmacol Sci. 22:4956–4961.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cha RH, Joh JS, Jeong I, Lee JY, Shin HS,
Kim G and Kim Y: Critical Care Team of National Medical Center.
Renal Complications and their prognosis in Korean patients with
Middle East respiratory syndrome-coronavirus from the central
MERS-CoV designated hospital. J Korean Med Sci. 30:1807–1814.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Alsaad KO, Hajeer AH, Al Balwi M, Al
Moaiqel M, Al Oudah N, Al Ajlan A, AlJohani S, Alsolamy S, Gmati
GE, Balkhy H, et al: 2. Histopathology. 72:516–524. 2018.
|
|
43
|
Ng DL, Al Hosani F, Keating MK, Gerber SI,
Jones TL, Metcalfe MG, Tong S, Tao Y, Alami NN, Haynes LM, et al:
Clinicopathologic, immunohistochemical, and Ultrastructural
findings of a fatal case of Middle East respiratory syndrome
coronavirus infection in the United Arab Emirates, April 2014. Am J
Pathol. 186:652–658. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Munster VJ, Koopmans M, van Doremalen N,
van Riel D and de Wit E: A novel coronavirus emerging in china-key
questions for impact assessment. N Engl J Med. 382:692–694.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhu N, Zhang D, Wang W, Li X, Yang B, Song
J, Zhao X, Huang B, Shi W, Lu R, et al: A Novel coronavirus from
patients with pneumonia in China, 2019. N Engl J Med. 382:727–733.
2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Perlman S: Another decade, another
coronavirus. N Engl J Med. 382:760–762. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hui DS, I Azhar E, Madani TA, Ntoumi F,
Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, et al:
The continuing 2019-nCoV epidemic threat of novel coronaviruses to
global health-The latest 2019 novel coronavirus outbreak in Wuhan,
China. Int J Infect Dis. 91:264–266. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H,
Wang W, Song H, Huang B, Zhu N, et al: Genomic characterisation and
epidemiology of 2019 novel coronavirus: Implications for virus
origins and receptor binding. Lancet. 395:565–574. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan
S and Yuen KY: Genomic characterization of the 2019 novel
human-pathogenic coronavirus isolated from a patient with atypical
pneumonia after visiting Wuhan. Emerg Microbes Infect. 9:221–236.
2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhou P, Yang XL, Wang XG, Hu B, Zhang L,
Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al: A pneumonia outbreak
associated with a new coronavirus of probable bat origin. Nature.
579:270–273. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J,
Wang B, Xiang H, Cheng Z, Xiong Y, et al: Clinical characteristics
of 138 hospitalized patients with 2019 novel coronavirus-infected
pneumonia in Wuhan, China. JAMA. 323:1061–1069. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He
JX, Liu L, Shan H, Lei CL, Hui DSC, et al: Clinical characteristics
of coronavirus disease 2019 in China. N Engl J Med. 382:1708–1720.
2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H,
Wu Y, Zhang L, Yu Z, Fang M, et al: Clinical course and outcomes of
critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China:
A single-centered, retrospective, observational study. Lancet
Respir Med. 8:475–481. 2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma
CL, Li SB, Wang HY, Zhang S, Gao HN, et al: Clinical findings in a
group of patients infected with the 2019 novel coronavirus
(SARS-Cov-2) outside of Wuhan, China: Retrospective case series.
BMJ. 368(m606)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z,
Su Y, Ma Z, Zhang Y, Li Z, et al: COVID-19 in a designated
infectious diseases hospital outside Hubei Province, China.
Allergy. 75:1742–1752. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Cheng Y, Luo R, Wang K, Zhang M, Wang Z,
Dong L, Li J, Yao Y, Ge S and Xu G: Kidney disease is associated
with in-hospital death of patients with COVID-19. Kidney Int.
97:829–838. 2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Su H, Yang M, Wan C, Yi LX, Tang F, Zhu
HY, Yi F, Yang HC, Fogo AB, Nie X and Zhang C: Renal
histopathological analysis of 26 postmortem findings of patients
with COVID-19 in China. Kidney Int. 98:219–227. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu
C, Ma Z, Huang Y, Liu W, Yao Y, et al: Renal involvement and Early
prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol.
31:1157–1165. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Chen YT, Shao SC, Lai EC, Hung MJ and Chen
YC: Mortality rate of acute kidney injury in SARS, MERS, and
COVID-19 infection: A systematic review and meta-analysis. Crit
Care. 24(439)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Guery B, Poissy J, el Mansouf L, Séjourné
C, Ettahar N, Lemaire X, Vuotto F, Goffard A, Behillil S, Enouf V,
et al: Clinical features and viral diagnosis of two cases of
infection with Middle East Respiratory Syndrome coronavirus: A
report of nosocomial transmission. Lancet. 381:2265–2272.
2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Chan VW, Chiu PK, Yee CH, Yuan Y, Ng CF
and Teoh JY: A systematic review on COVID-19: Urological
manifestations, viral RNA detection and special considerations in
urological conditions. World J Urol: May 27, 2020 (Epub ahead of
print). doi: 10.1007/s00345-020-03246-4.
|
|
63
|
Müller MA, Raj VS, Muth D, Meyer B,
Kallies S, Smits SL, Wollny R, Bestebroer TM, Specht S, Suliman T,
et al: Human coronavirus EMC does not require the SARS-coronavirus
receptor and maintains broad replicative capability in mammalian
cell lines. mBio. 3:e00515–12. 2012.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Monteil V, Kwon H, Prado P, Hagelkrüys A,
Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C,
Prosper F, et al: Inhibition of SARS-CoV-2 infections in engineered
human tissues using clinical-grade soluble human ACE2. Cell.
181:905–913.e7. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Hamming I, Timens W, Bulthuis ML, Lely AT,
Navis G and van Goor H: Tissue distribution of ACE2 protein, the
functional receptor for SARS coronavirus. A first step in
understanding SARS pathogenesis. J Pathol. 203:631–637.
2004.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Rakušan D, Bürgelová M, Vaněčková I,
Vaňourková Z, Husková Z, Skaroupková P, Mrázová I, Opočenský M,
Kramer HJ, Netuka I, et al: Knockout of angiotensin 1-7 receptor
Mas worsens the course of two-kidney, one-clip Goldblatt
hypertension: Roles of nitric oxide deficiency and enhanced
vascular responsiveness to angiotensin II. Kidney Blood Press Res.
33:476–488. 2010.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Li W, Moore MJ, Vasilieva N, Sui J, Wong
SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough
TC, et al: Angiotensin-converting enzyme 2 is a functional receptor
for the SARS coronavirus. Nature. 426:450–454. 2003.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Raj VS, Mou H, Smits SL, Dekkers DH,
Müller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, et
al: Dipeptidyl peptidase 4 is a functional receptor for the
emerging human coronavirus-EMC. Nature. 495:251–254.
2013.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Li F and Du L: MERS coronavirus: An
emerging zoonotic virus. Viruses. 11(663)2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Abdel-Moneim AS: Middle East respiratory
syndrome coronavirus (MERS-CoV): Evidence and speculations. Arch
Virol. 159:1575–1584. 2014.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Kenny AJ, Booth AG, George SG, Ingram J,
Kershaw D, Wood EJ and Young AR: Dipeptidyl peptidase IV, a kidney
brush-border serine peptidase. Biochem J. 157:169–182.
1976.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Lian Q, Wang S, Zhang G, Wang D, Luo G,
Tang J, Chen L and Gu J: HCCDB: A database of hepatocellular
carcinoma expression atlas. Genomics Proteomics Bioinformatics.
16:269–275. 2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Ponten F, Jirstrom K and Uhlen M: The
human protein atlas-a tool for pathology. J Pathol. 216:387–393.
2008.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Uhlen M, Fagerberg L, Hallstrom BM,
Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C,
Sjöstedt E, Asplund A, et al: Proteomics. Tissue-based map of the
human proteome. Science. 347(1260419)2015.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng
X, Li T and Chen Q: High expression of ACE2 receptor of 2019-nCoV
on the epithelial cells of oral mucosa. Int J Oral Sci.
12(8)2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Hoffmann M, Kleine-Weber H, Schroeder S,
Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH,
Nitsche A, et al: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2
and is blocked by a clinically proven protease inhibitor. Cell.
181:271–280.e8. 2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Harmer D, Gilbert M, Borman R and Clark
KL: Quantitative mRNA expression profiling of ACE 2, a novel
homologue of angiotensin converting enzyme. FEBS Lett. 532:107–110.
2002.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Lely AT, Hamming I, van Goor H and Navis
GJ: Renal ACE2 expression in human kidney disease. J Pathol.
204:587–593. 2004.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Pala L, Mannucci E, Pezzatini A, Ciani S,
Sardi J, Raimondi L, Ognibene A, Cappadona A, Vannelli BG and
Rotella CM: Dipeptidyl peptidase-IV expression and activity in
human glomerular endothelial cells. Biochem Biophys Res Commun.
310:28–31. 2003.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan
B, Huan Y, Yang P, Zhang Y, Deng W, et al: A crucial role of
angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced
lung injury. Nat Med. 11:875–879. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
81
|
Ge XY, Li JL, Yang XL, Chmura AA, Zhu G,
Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, et al: Isolation and
characterization of a bat SARS-like coronavirus that uses the ACE2
receptor. Nature. 503:535–538. 2013.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Strawn WB, Richmond RS, Ann Tallant E,
Gallagher PE and Ferrario CM: Renin-angiotensin system expression
in rat bone marrow haematopoietic and stromal cells. Br J Haematol.
126:120–126. 2004.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Batlle D, Wysocki J and Satchell K:
Soluble angiotensin-converting enzyme 2: A potential approach for
coronavirus infection therapy? Clin Sci. 134:543–545.
2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Yang XH, Deng W, Tong Z, Liu YX, Zhang LF,
Zhu H, Gao H, Huang L, Liu YL, Ma CM, et al: Mice transgenic for
human angiotensin-converting enzyme 2 provide a model for SARS
coronavirus infection. Comp Med. 57:450–459. 2007.PubMed/NCBI
|
|
85
|
Wrapp D, Wang N, Corbett KS, Goldsmith JA,
Hsieh CL, Abiona O, Graham BS and McLellan JS: Cryo-EM structure of
the 2019-nCoV spike in the prefusion conformation. Science.
367:1260–1263. 2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara
H, Geng Q, Auerbach A and Li F: Structural basis of receptor
recognition by SARS-CoV-2. Nature. 581:221–224. 2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S,
Zhang Q, Shi X, Wang Q, Zhang L and Wang X: Structure of the
SARS-CoV-2 spike receptor-binding domain bound to the ACE2
receptor. Nature. 581:215–220. 2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Pan XW, Xu D, Zhang H, Zhou W, Wang LH and
Cui XG: Identification of a potential mechanism of acute kidney
injury during the COVID-19 outbreak: A study based on single-cell
transcriptome analysis. Intensive Care Med. 46:1114–1116.
2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Farkash EA, Wilson AM and Jentzen JM:
Ultrastructural evidence for direct renal infection with
SARS-CoV-2. J Am Soc Nephrol. 31:1683–1687. 2020.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Iwata-Yoshikawa N, Okamura T, Shimizu Y,
Kotani O, Sato H, Sekimukai H, Fukushi S, Suzuki T, Sato Y, Takeda
M, et al: Acute respiratory infection in human Dipeptidyl Peptidase
4-transgenic mice infected with Middle East respiratory syndrome
coronavirus. J Virol. 93:e01818–18. 2019.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Lu G, Hu Y, Wang Q, Qi J, Gao F, Li Y,
Zhang Y, Zhang W, Yuan Y, Bao J, et al: Molecular basis of binding
between novel human coronavirus MERS-CoV and its receptor CD26.
Nature. 500:227–231. 2013.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Deeks SG, Tracy R and Douek DC: Systemic
effects of inflammation on health during chronic HIV infection.
Immunity. 39:633–645. 2013.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Wang W, Li G, De Wu Luo Z, Pan P, Tian M,
Wang Y, Xiao F, Li A, Wu K, et al: Zika virus infection induces
host inflammatory responses by facilitating NLRP3 inflammasome
assembly and interleukin-1β secretion. Nat Commun.
9(106)2018.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Wong CK, Lam CW, Wu AK, Ip WK, Lee NL,
Chan IH, Lit LC, Hui DS, Chan MH, Chung SS and Sung JJ: Plasma
inflammatory cytokines and chemokines in severe acute respiratory
syndrome. Clin Exp Immunol. 136:95–103. 2004.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Mahallawi WH, Khabour OF, Zhang Q,
Makhdoum HM and Suliman BA: MERS-CoV infection in humans is
associated with a pro-inflammatory Th1 and Th17 cytokine profile.
Cytokine. 104:8–13. 2018.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Al-Jasser FS, Nouh RM and Youssef RM:
Epidemiology and predictors of survival of MERS-CoV infections in
Riyadh region, 2014-2015. J Infect Public Health. 12:171–177.
2019.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Reichsoellner M, Raggam RB, Wagner J,
Krause R and Hoenigl M: Clinical evaluation of multiple
inflammation biomarkers for diagnosis and prognosis for patients
with systemic inflammatory response syndrome. J Clin Microbiol.
52:4063–4066. 2014.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Hui DSC and Zumla A: Severe acute
respiratory syndrome: Historical, epidemiologic, and clinical
features. Infect Dis Clin North Am. 33:869–889. 2019.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Tisoncik JR, Korth MJ, Simmons CP, Farrar
J, Martin TR and Katze MG: Into the eye of the cytokine storm.
Microbiol Mol Biol Rev. 76:16–32. 2012.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Fani F, Regolisti G, Delsante M,
Cantaluppi V, Castellano G, Gesualdo L, Villa G and Fiaccadori E:
Recent advances in the pathogenetic mechanisms of sepsis-associated
acute kidney injury. J Nephrol. 31:351–359. 2018.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Martinez-Garcia JJ, Leon-Sicairos NM,
Canizalez-Roman A and García-Arellano BA: Fluid balance and acute
kidney injury in septic shock. Bol Med Hosp Infant Mex. 74:282–288.
2017.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
102
|
Jia X, Liu B, Bao L, Lv Q, Li F, Li H, An
Y, Zhang X, Cao B and Wang C: Delayed oseltamivir plus sirolimus
treatment attenuates H1N1 virus-induced severe lung injury
correlated with repressed NLRP3 inflammasome activation and
inflammatory cell infiltration. PLoS Pathog.
14(e1007428)2018.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Lorz C, Justo P, Sanz A, Subirá D, Egido J
and Ortiz A: Paracetamol-induced renal tubular injury: A role for
ER stress. J Am Soc Nephrol. 15:380–389. 2004.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Khwaja A: KDIGO clinical practice
guidelines for acute kidney injury. Nephron. Clin Pract.
120:c179–c184. 2012.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Al-Dorzi HM, Aldawood AS, Khan R, Baharoon
S, Alchin JD, Matroud AA, Al Johany SM, Balkhy HH and Arabi YM: The
critical care response to a hospital outbreak of Middle East
respiratory syndrome coronavirus (MERS-CoV) infection: An
observational study. Ann Intensive Care. 6(101)2016.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Li Y, Cao C, Huang L, Xiong H, Mao H, Yin
Q and Luo X: ‘Awake’ extracorporeal membrane oxygenation combined
with continuous renal replacement therapy for the treatment of
severe chemical gas inhalation lung injury. J Burn Care Res.
41:908–912. 2020.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Ostermann M, Connor M Jr and Kashani K:
Continuous renal replacement therapy during extracorporeal membrane
oxygenation: Why, when and how? Curr Opin Crit Care. 24:493–503.
2018.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Xiong F, Tang H, Liu L, Tu C, Tian JB, Lei
CT, Liu J, Dong JW, Chen WL, Wang XH, et al: Clinical
characteristics of and medical interventions for COVID-19 in
hemodialysis patients in Wuhan, China. J Am Soc Nephrol.
31:1387–1397. 2020.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Ronco C, Tetta C, Mariano F, Wratten ML,
Bonello M, Bordoni V, Cardona X, Inguaggiato P, Pilotto L, d'Intini
V and Bellomo R: Interpreting the mechanisms of continuous renal
replacement therapy in sepsis: The peak concentration hypothesis.
Artif Organs. 27:792–801. 2003.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Ma J, Xia P, Zhou Y, Liu Z, Zhou X, Wang
J, Li T, Yan X, Chen L, Zhang S, et al: Potential effect of blood
purification therapy in reducing cytokine storm as a late
complication of critically ill COVID-19. Clin Immunol.
214(108408)2020.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Tang B, Li S, Xiong Y, Tian M, Yu J, Xu L,
Zhang L, Li Z, Ma J, Wen F, et al: COVID-19 pneumonia in a
hemodialysis patient. Kidney Med. 2:354–358. 2020.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Xu X, Han M, Li T, Sun W, Wang D, Fu B,
Zhou Y, Zheng X, Yang Y, Li X, et al: Effective treatment of severe
COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA.
117:10970–10975. 2020.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Fu B, Xu X and Wei H: Why tocilizumab
could be an effective treatment for severe COVID-19? J Transl Med.
18(164)2020.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Yang XH, Sun RH, Zhao MY, Chen EZ, Liu J,
Wang HL, Yang RL and Chen DC: Expert recommendations on blood
purification treatment protocol for patients with severe COVID-19:
Recommendation and consensus. Chronic Dis Transl Med. 6:106–114.
2020.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Zhang Y, Yu L, Tang L, Zhu M, Jin Y, Wang
Z and Li L: A promising anti-cytokine-storm targeted therapy for
COVID-19: The artificial-liver blood-purification system.
Engineering (Beijing): Mar 20, 2020 (Epub ahead of print). doi:
10.1016/j.eng.2020.03.006.
|
|
116
|
Stone JH, Frigault MJ, Serling-Boyd NJ,
Fernandes AD, Harvey L, Foulkes AS, Horick NK, Healy BC, Shah R,
Bensaci AM, et al: Efficacy of tocilizumab in patients hospitalized
with Covid-19. N Engl J Med. 383:2333–2344. 2020.PubMed/NCBI View Article : Google Scholar
|
|
117
|
RECOVERY Collaborative Group. Horby P, Lim
WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N,
Brightling C, Ustianowski A, et al: Dexamethasone in hospitalized
patients with Covid-19-preliminary report. N Engl J Med: Jul 17,
2020 (Epub ahead of print). doi: 10.1056/NEJMoa2021436.
|