Introduction
Myopia is one of the most commonly reported ocular
disorders worldwide, and there has recently been a significant
increase in the myopic population in China (1). The costs of examinations and surgical
corrections of myopia are significant, and this disorder has been
associated with other pathological eye conditions. High myopia is
associated with reduced retinal perfusion. In fundus photography,
retinal vessel density and blood flow are markedly reduced in
highly myopic eyes (2). Using a
dynamic vessel analyzer, it was previously demonstrated that there
was a narrowing of retinal large vessels in high myopia (3). Since the retinal microvasculature
directly supplies O2 and nutrients to the retinal
tissues, this vasculature is more susceptible to myopia-related
alterations (4). Understanding
these changes to the retinal large vessels and microvasculature
will ultimately aid in the early diagnosis and monitoring of
retinopathy in patients with high myopia. However, previous studies
are limited due to the imaging modalities used. For instance, only
large vessels could be imaged, and invasive methods were required,
such as fluorescein angiography (5). These imaging modalities may prevent
researchers from investigating alterations to the retinal
microvasculature as early indicators of the onset of retinopathy.
However, with the recent advances in optical coherence tomography
angiography (OCTA), the vasculature can be measured quantitatively
in a noninvasive manner in terms of morphological information,
which can provide depth-resolved visualization of the retinal
microvasculature (6).
Morphological changes to the optic disc in myopic
eyes are induced by posterior scleral remodeling during axial
elongation, including β-zone parapapillary atrophy (β-PPA), optic
disc tilt and optic disc rotation (7,8). Since
the optic disc becomes smaller as myopia progresses (9), it was hypothesized that myopic
deformation of the optic disc may be associated with macular and
disc perfusion. Therefore, the purpose of the current study was to
determine the correlation between optic disc deformation and
retinal vasculature in non-pathological high myopia using OCTA
imaging.
Materials and methods
Participants
The current study was approved by the Beijing
Friendship Hospital Affiliated to Capital Medical University
(Beijing, China), and was conducted in accordance with the ethical
standards stated in the Declaration of Helsinki and the Health
Insurance Portability and Accountability Act. Written informed
consent was obtained from all the examined patients and volunteers
participating in the study prior to OCTA imaging. Patients from the
Beijing Friendship Hospital (Beijing, China) were recruited from
April 2018 to September 2018. A total of 77 patients were included
in the study. The mean age was 35.24±8.45 years (age range, 20-55).
Subjects comprised 37 males and 40 females.
Each patient underwent a complete ocular examination
that included best-corrected visual acuity testing, intraocular
pressure (IOP) evaluations using an automatic tonometer, slit-lamp
examinations, funduscopy and axial length (AL) measurements using
optical biometry (IOLMaster®; Carl Zeiss AG).
A total of 130 eyes with non-pathological high
myopia were included in the current cross-sectional study. Patients
with high myopia and a refraction of <-6 diopters or ALs
>26.5 mm were included in the current study. Furthermore,
patients with a history of prior vitreous or retinal surgery, an
IOP of >21 mmHg or evidence of retinal disease (other than
myopic degeneration) that affected the retinal or choroidal
vasculature as evidenced by history or examination were excluded.
Additionally, eyes that exhibited diffuse retinal pigment
epitheliopathy (RPE) atrophy due to high myopia or any structural
changes, including myopic choroidal neovascularization, were
excluded from the analyses.
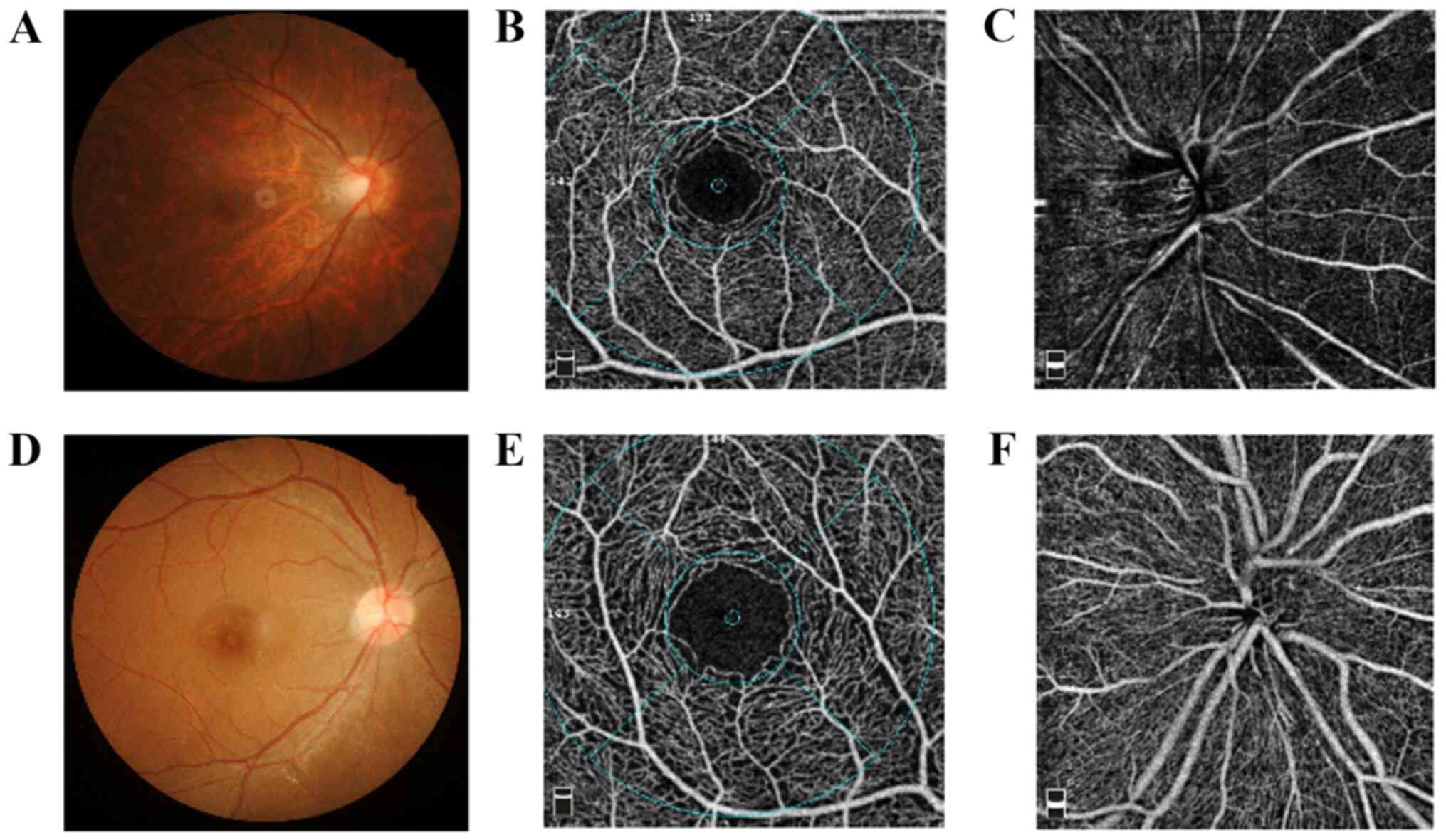
Image acquisition and analysis
OCTA imaging was performed using an RTVue XR Avanti
system with AngioVue version 2016.2.0.35 (Optovue, Inc.) at a
scanning speed of 70,000 A-scans/sec. All imaging was performed by
a single operator (JS). The scan protocol examined a
3.0x3.0-mm2 area focused on the macula and a
4.5x4.5-mm2 area focused on the optic disc. Vessel flow
density (VFD) and fractal dimension of the retina, as well as the
foveal avascular zone (FAZ), were analyzed and quantified using en
face projection images. Enhanced-depth imaging of the fovea was
also acquired by OCTA (Fig. 1). VFD
was measured at the SRP and DRP in the macular region. Each layer
was divided into four sectors: i) Nasal; ii) temporal; iii)
superior and iv) inferior. The perfusion of the optic disc was
divided into three layers: i) Optic disc head (ONH); ii) radial
peripapillary capillary (RPC) and iii) choroid. Horizontal and
vertical priorities in ~2.9 sec for each of the two raster scans
were obtained. The superficial retinal plexus (SRP) was segmented
from the outer boundary of the inner limiting membrane (ILM) to the
outer boundary of the inner plexiform layer (IPL), which extends
from 3 µm below the ILM to 15 µm below the IPL. The deep retinal
plexus (DRP) was segmented from the outer boundary of the IPL to
the outer boundary of the outer plexiform layer, which extends from
15 to 70 µm below the IPL.
Since magnification is different in myopic eyes, the
imaging sampling density used in myopic eyes must be lower than
that used in normal eyes. Therefore, the magnification of the
obtained images was corrected for highly myopic eyes using
Bennett's formula (10). Two
independent examiners (JS and JW) reviewed each image. Poor-quality
images were excluded based on the following criteria: i) Evidence
of poor fixation, including a double vessel pattern and motion
artifacts; ii) presence of motion artifacts that could not be
corrected by motion correction technology; iii) media opacity, as
exhibited by shadowing or obscuration of the vessel signal in the
field of view or a signal strength index of <40; and iv)
segmentation error in the defining vascular layers.
OCTA data were analyzed using Optovue software
(version no. 2016.2.0.35; Optovue, Inc.). The foveal avascular zone
(FAZ) area for each superficial plexus image was determined and
measured using the built-in non-flow automatic measurement tool of
the AngioVue review software version 2016.2.0.35 (Optovue,
Inc.).
High-resolution digital color fundus photographs
were obtained using a digital retina camera (Kowa Nonmyd WX; Kowa
Company, Ltd.). Image processing of the β-PPA area, optic disc tilt
ratio, and horizontal and vertical optic disc diameter measurements
were performed using the public domain ImageJ software (version no.
1.50i; National Institutes of Health). Two examiners (JS and JW)
measured each image three times to assess the reproducibility of
the technique.
Statistical analysis
Statistical analysis was performed using a
commercially available statistical software program (SPSS for
Microsoft; version 24.0; IBM Corp.). Firstly, the mean and standard
deviation of the main outcome parameters were calculated. Following
this, a regression analysis using the angiographic parameters as
the dependent variables was performed. The parameters that were
significantly associated with the angiographic parameters following
the univariate analysis were used as independent variables. For all
analyses, P<0.05 was considered to indicate a statistically
significant difference.
Results
Demographics
The demographic and ocular characteristics of the
participants are presented in Table
I. A total of 130 eyes from 77 participants with
nonpathological high myopia were analysed in this study. The mean
age was 35.24±8.45 years, and the mean spherical equivalent (SE)
refractive error was -10.03±3.57 D. The demographics of these
participants are shown in Table
I.
 | Table IDemographic and ocular characteristics
of the participants. |
Table I
Demographic and ocular characteristics
of the participants.
| Characteristic | Mean ± SD (n=77) | Median (n=77) | Range (n=77) |
|---|
| Age (years) | 35.24±8.45 | 34.50 | 20.00-55.00 |
| Sex | | | |
| Male | 37 | | |
| Female | 40 | | |
| SE (D) | 10.03±3.57 | 8.94 | 6.15-20.13 |
| AL (mm) | 27.43±1.68 | 27.28 | 26.10-34.46 |
| SP (mmHg) | 119.95±11.90 | 122.00 | 91.00-149.00 |
| DP (mmHg) | 74.67±8.75 | 75.00 | 56.00-103.00 |
| HR (bpm) | 76.11±8.33 | 76.00 | 59.00-93.00 |
| IOP (mmHg) | 15.62±3.25 | 15.15 | 9.90-20.80 |
| Optic tilt ratio | 1.29±0.18 | 1.26 | 0.91-1.76 |
| Horizontal optic disc
diameter (mm) | 1.33±0.17 | 1.33 | 1.01-2.04 |
| Vertical optic disc
diameter (mm) | 1.05±0.19 | 1.05 | 0.67-1.57 |
| β-PPA
(mm2) | 1.09±0.62 | 0.88 | 0.19-2.85 |
| RNFL (µm) | 95.69±9.49 | 95.00 | 72.00-117.00 |
| C/D | 0.28±0.18 | 0.28 | 0.03-0.65 |
| Disc area
(mm2) | 2.01±0.59 | 1.93 | 0.94-4.38 |
Association between optic disc
deformation and macular perfusion parameters
Vessel flow density was measured at the SRP and DRP
in the macular region. Each layer was divided into four sectors: i)
Nasal; ii) temporal; iii) superior; and iv) inferior. The results
from the regression analysis revealed that each sector of the
macular superficial layer was negatively correlated with β-PPA
(mean, R=-2.805; P=0.006; Table
II), while the nasal sector was negatively correlated with age
(R=-2.116; P=0.038). Furthermore, FAZ was not correlated with age,
optic tilt ratio, horizontal optic disc diameter and β-PPA in the
current study.
 | Table IICorrelation between age, optic disc
deformation, superficial retinal plexus perfusion parameters and
FAZ. |
Table II
Correlation between age, optic disc
deformation, superficial retinal plexus perfusion parameters and
FAZ.
| | Mean | Temporal | Superior | Nasal | Inferior | FAZ |
|---|
| Parameter | R | P-value | R | P-value | R | P-value | R | P-value | R | P-value | R | P-value |
|---|
| Age (years) | -1.271 | 0.207 | -1.064 | 0.291 | -0.702 | 0.485 | -2.116 | 0.038 | -1.672 | 0.099 | 1.020 | 0.311 |
| Optic tilt ratio | -0.365 | 0.723 | -0.377 | 0.707 | -0.431 | 0.668 | 0.336 | 0.738 | -0.750 | 0.456 | -0.592 | 0.556 |
| Horizontal optic disc
diameter (mm) | 0.134 | 0.894 | 0.162 | 0.871 | 0.559 | 0.578 | 0.724 | 0.472 | 0.245 | 0.807 | 0.879 | 0.382 |
| AL (mm) | -0.307 | 0.001 | -0.346 | <0.001 | -0.248 | 0.006 | -0.331 | <0.001 | -0.315 | <0.001 | -0.252 | 0.006 |
| β-PPA | -2.805 | 0.006 | -2.337 | 0.022 | -2.130 | 0.036 | -2.865 | 0.005 | -2.958 | 0.004 | -0.002 | 0.998 |
Table III presents
the correlations between optic disc deformation and DRP parameters.
There was a negative correlation between β-PPA and the mean
(R=-2.801; P=0.006), nasal (R=-2.743; P=0.008) and temporal sectors
(R=-2.740; P=0.008). Additionally, there was a negative correlation
between optic disc tilt and DRP perfusion parameters in the mean
(R=-2.291; P=0.025) and inferior (R=-3.667; P<0.001) regions.
Furthermore, the horizontal optic disc diameter was negatively
correlated with superior retinal vessel density (R=-1.995;
P=0.050). Table III presents a
negative association between age and the mean (R=-2.429; P=0.017),
nasal (R=-2.341; P=0.022) and inferior (R=-3.883; P<0.001)
regions.
 | Table IIICorrelation between age, optic disc
deformation and deep retinal plexus perfusion parameters. |
Table III
Correlation between age, optic disc
deformation and deep retinal plexus perfusion parameters.
| | Mean | Temporal | Superior | Nasal | Inferior |
|---|
| Parameter | R | P-value | R | P-value | R | P-value | R | P-value | R | P-value |
|---|
| Age | -2.429 | 0.017 | -1.630 | 0.107 | -0.695 | 0.489 | -2.341 | 0.022 | -3.883 | <0.001 |
| Optic tilt
ratio | -2.291 | 0.025 | -1.512 | 0.135 | -0.229 | 0.819 | -1.910 | 0.060 | -3.667 | <0.001 |
| Horizontal optic
disc diameter (mm) | -1.065 | 0.290 | -1.344 | 0.183 | -1.995 | 0.050 | -0.765 | 0.447 | 1.298 | 0.198 |
| AL (mm) | -0.400 | <0.001 | -0.275 | 0.002 | -0.404 | <0.001 | -0.307 | 0.001 | -0.400 | <0.001 |
| β-PPA | -2.801 | 0.006 | -2.740 | 0.008 | -0.264 | 0.792 | -2.743 | 0.008 | -1.489 | 0.141 |
Association between optic disc
deformation and perfusion parameters
The results of the regression analysis between optic
disc deformation and optic disc perfusion parameters are presented
in Table IV. The perfusion of the
optic disc was divided into three layers: i) Optic disc head (ONH);
ii) radial peripapillary capillary (RPC); and iii) choroid. The RPC
was negatively correlated with β-PPA (R=-3.936; P<0.001), while
the subfoveal choroidal thickness was negatively correlated with
age (R=-4.234; P<0.001), β-PPA (R=-2.161; P=0.034) and
horizontal optic disc diameter (R=-2.281; P=0.025).
 | Table IVCorrelation between optic disc
deformation, perfusion parameters and subfoveal choroidal
thickness. |
Table IV
Correlation between optic disc
deformation, perfusion parameters and subfoveal choroidal
thickness.
| | RPC | Subfoveal choroidal
thickness (µm) |
|---|
| Parameter | R | P-value | R | P-value |
|---|
| Age (years) | -1.656 | 0.102 | -4.234 | <0.001 |
| Optic tilt
ratio | -1.870 | 0.065 | -1.797 | 0.076 |
| Horizontal optic
disc diameter (mm) | 0.139 | 0.890 | -2.281 | 0.025 |
| AL (mm) | -0.053 | 0.175 | -0.431 | <0.001 |
| β-PPA | -3.936 | <0.001 | -2.161 | 0.034 |
Discussion
The current study demonstrated that there were
significant correlations between optic disc deformation and
vascular parameters, including the vessel density of SRP, DRP and
RPC. Compared to a previous report (11), the present study recruited
participants whose SE are -10.03±3.57 D and performed regression
analysis separately in each direction (superior, inferior, nasal
and temporal).
The results demonstrated that the optic disc tilt
ratio was correlated with the mean and inferior vessel density of
the DRP. Furthermore, since disc tilt and torsion were
significantly more frequent in the inferior direction, it is
possible that changes in optic disc morphology may be associated
with changes in inferior scleral thinning (12). Since it is difficult to precisely
measure the true horizontal diameter of the optic disc, previous
study has evaluated the amount of tilt by calculating the ratio
between the minimum and maximum diameters of the nerve, a value
termed the index of tilt (13).
In the current study, the superior vessel density of
the DRP became lower as the horizontal disc diameter increased.
However, Dai et al (14)
reported that β-PPA and γ-PPA were associated with vertical disc
diameter, and that the associations between β-PPA or γ-PPA and
horizontal disc diameter were unclear and not significant. By
contrast, Guo et al (15)
demonstrated that the horizontal and vertical disc diameters were
positively associated with the enlargement of γ-PPA.
The results of the present study revealed that
vessel density in the RPC was negatively correlated with β-PPA.
However, Fan et al (5)
demonstrated that there were no differences in vascular density in
the optic disc region among the three groups (control, moderate and
high myopia), and vascular density in the optic disc region was not
associated with AL, spherical equivalent or RNFL thickness.
Furthermore, the current study demonstrated that FAZ
was not correlated with optic disc deformations. Wang et al
(6) did not identify any
differences in the area and diameter of the FAZ in healthy Chinese
volunteers. This finding may indicate that the FAZ is not a
suitable outcome to study changes in the microvessel network
density of myopic eyes. Notably, the FAZ area did not significantly
change in response to hyperoxia. However, most of the O2
supplied to the retina from the FAZ area is derived from the
choroidal vessel, rather than from the retinal circulation, which
may explain the lack of changes in the FAZ area in response to
hyperoxia (16).
Garg et al (17) reported that choroidal thinning was
associated with β-PPA. The current study demonstrated that
subfoveal choroidal thickness was thinner in eyes with higher β-PPA
than that in eyes with lower β-PPA. Furthermore, Wang et al
(6) revealed that the density of
the macular vascular networks in superior and deep layers and the
choriocapillaris decreased with age.
β-PPA is associated with myopic eyeball axial
elongation and temporal pulling of the optic nerve. The adjacent
retinal tissue extends externally, and this mechanical stretching
results in morphological changes in vessel and tissue thickness
(18). During β-PPA, the shape of
vessels becomes straighter and thinner, which may affect the vessel
flow in the macular region (19).
Furthermore, changes in vessel thickness may damage endothelial
cells and subsequently reduce the concentration of VEGF (20,21).
Chui et al (22) revealed
that retinal stretching may not mirror scleral growth, and that
there is a difference between the photoreceptor margin and RPE
margin in certain eyes, indicating that slippage may occur during
eye growth within the retina. This may result in retinoschisis and
subsequently reduce perfusion in the macula.
There is interplay between genetic factors and
environmental stressors in the development of myopia. Myopia is
typically exhibited with apparent familial aggregation; however,
genetic factors alone cannot explain the rapid increase in the
prevalence of myopia over the past one or two generations (23). Bredrup et al (24) reported that the clinical
characteristics of a family with a distinctly excavated optic disc
anomaly exhibited optic nerve dysplasia, high-grade myopia and
increased ALs. Additionally, genetic analysis revealed a
co-segregation of this optic disc anomaly with a mutation in the
MYC-binding protein 2 gene. Overall, the underlying mechanisms of
myopia remain to be elucidated.
The current study had certain limitations. Firstly,
the present study was limited by its cross-sectional design.
Therefore, additional studies that include frequent follow-ups of
these patients are warranted. Secondly, participants did not
present with pathological myopia. Thus, further studies are
needed.
In conclusion, a correlation between optic disc
deformation and retinal vasculature in non-pathological highly
myopic eyes was observed using OCTA. This association may explain
the reduced peripapillary and macular vessel density in high
myopia. According to the results of the present study, disc
deformation (particularly optic disc tilt and β-PPA) may occur
earlier than changes in the macular region in myopia retinopathy.
Therefore, changes in the optic disc may be early signs of retinal
changes in myopic eyes.
Acknowledgements
Not applicable.
Funding
Funding: The current study was supported by the Research
Foundation of Beijing Friendship Hospital Affiliated to Capital
Medical University, Beijing, China (grant no. yyqdkt2019-29).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW contributed to the acquisition and analysis of
data. JW designed the current study and revised the manuscript. JS
designed the study, analyzed data and drafted the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of the Beijing Friendship Hospital (Beijing, China).
Written informed consent was obtained from all the examined
patients and volunteering participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jonas JB, Xu L, Wei WB, Wang XY, Jiang WJ,
Bi HS and Jonas SP: Myopia in China: A population-based
cross-sectional, histological, and experimental study. Lancet. 388
(Suppl 1)(S20)2016.
|
|
2
|
Shimada N, Ohno-Matsui K, Harino S,
Yoshida T, Yasuzumi K, Kojima A, Kobayashi K, Futagami S, Tokoro T
and Mochizuki M: Reduction of retinal blood flow in high myopia.
Graefes Arch Clin Exp Ophthalmol. 242:284–288. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
La Spina C, Corvi F, Bandello F and
Querques G: Static characteristics and dynamic functionality of
retinal vessels in longer eyes with or without pathologic myopia.
Graefes Arch Clin Exp Ophthalmol. 254:827–834. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li M, Yang Y, Jiang H, Giovanni G, Luiz R,
Zheng F, Ke B, Qu DY and Wang JH: Retinal microvascular network and
microcirculation assessments in high myopia. Am J Ophthalmol.
174:56–67. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fan H, Chen HY, Ma HJ, Chang Z, Yin HQ, Ng
DS, Cheung CY, Hu S, Xiang X, Tang SB and Li SN: Reduced macular
vascular density in myopic eyes. Chin Med J (Engl). 130:445–451.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang Q, Chan S, Yang JY, You B, Wang YX,
Jonas JB and Wei WB: Vascular density in retina and
choriocapillaris as measured by optical coherence tomography
angiography. Am J Ophthalmol. 168:95–109. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Samarawickrama C, Mitchell P, Tong L,
Gazzard G, Lim L, Wong TY and Saw SM: Myopia-related optic disc and
retinal changes in adolescent children from Singapore.
Ophthalmology. 118:2050–2057. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ohno-Matsui K: Proposed classification of
posterior staphylomas based on analyses of eye shape by
three-dimensional magnetic resonance imaging and wide-field fundus
imaging. Ophthalmology. 121:1798–1809. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang X, Kong X, Jiang C, Li M, Yu J and
Sun X: Is the peripapillary retinal perfusion related to myopia in
healthy eyes? A prospective comparative study. BMJ Open.
6(e010791)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sampson DM, Gong P, An D, Menghini M,
Hansen A, Mackey DA, Sampson DD and Chen FK: Axial length variation
impacts on superficial retinal vessel density and foveal avascular
zone area measurements using optical coherence tomography
angiography. Invest Ophthalmol Vis Sci. 58:3065–3072.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
He J, Chen Q, Yin Y, Zhou H, Fan Y, Zhu
JF, Zou HD and Xu X: Association between retinal microvasculature
and optic disc alterations in high myopia. Eye (Lond).
33:1494–1503. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ohno-Matsui K, Lai TY, Lai CC and Cheung
CM: Updates of pathologic myopia. Prog Retin Eye Res. 52:156–187.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tay E, Seah SK, Chan SP, Lim AT, Chew SJ,
Foster PJ and Aung T: Optic disk ovality as an index of tilt and
its relationship to myopia and perimetry. Am J Ophthalmol.
139:247–252. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dai Y, Jonas JB, Huang H, Wang M and Sun
X: Microstructure of parapapillary atrophy: Beta zone and gamma
zone. Invest Ophthalmol Vis Sci. 54:2013–2018. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Guo Y, Liu LJ, Tang P, Feng Y, Lv YY, Wu
M, Xu L and Jonas JB: Parapapillary gamma zone and progression of
myopia in school children: The Beijing children eye study. Invest
Ophthalmol Vis Sci. 59:1609–1616. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xu H, Deng G, Jiang C, Kong X, Yu J and
Sun X: Microcirculatory responses to hyperoxia in macular and
peripapillary regions. Invest Ophthalmol Vis Sci. 57:4464–4468.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Garg A, Blumberg DM, Al-Aswad LA, Oll M,
Yzer S, Forbes M, Allikmets RL and Bearelly S: Associations between
β-peripapillary atrophy and reticular pseudodrusen in early
age-related macular degeneration. Invest Ophthalmol Vis Sci.
58:2810–2815. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kwon JW, Choi JA, Kim JS and La TY:
Ganglion cell-inner plexiform layer, peripapillary retinal nerve
fiber layer, and macular thickness in eyes with myopic β-zone
parapapillary atrophy. J Ophthalmol. 2016(3746791)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lee KM, Choung HK, Kim M, Oh S and Kim SH:
Positional change of optic nerve head vasculature during axial
elongation as evidence of lamina cribrosa shifting: Boramae myopia
cohort study report 2. Ophthalmology. 125:1224–1233.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang M, Hwang TS, Campbell JP, Bailey ST,
Wilson DJ, Huang D and Jia Y: Projection-resolved optical coherence
tomographic angiography. Biomed Opt Express. 7:816–828.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Landa G and Rosen RB: New patterns of
retinal collateral circulation are exposed by a retinal functional
imager (RFI). Br J Ophthalmol. 94:54–58. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chui TY, Zhong Z and Burns SA: The
relationship between peripapillary crescent and axial length:
Implications for differential eye growth. Vision Res. 51:2132–2138.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cai XB, Shen SR, Chen DF, Zhang Q and Jin
ZB: An overview of myopia genetics. Exp Eye Res.
188(107778)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bredrup C, Johansson S, Bindoff LA,
Sztromwasser P, Kråkenes J, Mellgren AE, Brurås KR, Lind O, Boman
H, Knappskog PM and Rødahl E: High myopia-excavated optic disc
anomaly associated with a frameshift mutation in the MYC-binding
protein 2 gene (MYCBP2). Am J Ophthalmol. 159:973–979.e2.
2015.PubMed/NCBI View Article : Google Scholar
|