Introduction
Suboptimal-health is a state between health and
disease (1). Long-term stress,
fatigue, environmental pollution and other factors can cause
suboptimal-health (2). A previous
study suggested that an increasing number of people have a
suboptimal level of health and that the worldwide proportion of the
population with suboptimal-health is as high as 75% (3). Study of an ethnically diverse
community in the US indicated that 2-11% of people experience
severe fatigue with a duration of at least six months (4). As a principal manifestation and
symptom of suboptimal-health, chronic fatigue appears likely to
become one of the predominant factors affecting human health
(5,6). Continuous excessive physiological
activities, such as strenuous exercise, can cause a large number of
free radicals, resulting in oxidative stress injury that can in
turn induce chronic fatigue (7,8). The
occurrence of chronic fatigue is frequently accompanied with
reductions in organ function, especially that in the brain, which
is characterized by decreases in learning and memory capabilities
(4,9,10), and
greatly reduces overall quality of life. Therefore, research and
development into agents and health foods that can protect against
fatigue will likely have a global impact.
Schisandra chinensis is the mature fruit of
Schisandra chinensis in the Magnoliacea family that
was first recorded in the Shennong materia medica >2,000 years
ago, which has been used as a therapeutic agent or nutritional
supplement in the United States, Japan, South Korea and China
(11,12). Schizantherin A (SCA) is one of the
main active monomer components of the Schisandra chinensis
lignans and has been reported to confer anti-inflammatory,
antioxidant and memory improving effects (13). A previous study found that SCA not
only significantly enhanced the exercise endurance of chronic
fatigue mice (14), but also
improved the learning and memory abilities of aging mice, in a
D-galactose induced manner (15).
However, to the best of our knowledge, no previous reports
currently exist regarding the effects of SCA on the learning and
memory abilities on chronic fatigue mouse models. Therefore, the
present study aimed to investigate the effects of SCA on the
learning and memory abilities of chronic fatigue mice, to provide a
theoretical basis for the research and development into exploiting
the use of Schisandra in functional health foods.
Materials and methods
Reagents and materials
A total of 40 male ICR mice (age, 4-6 weeks),
weighing 19±2 g, were provided by the Experimental Animal Research
Center, Jilin University [Production license no. of experimental
animals: SCXK (Ji)-2016-0003]. The research design was approved by
the Animal Ethics Committee of Beihua University (Jilin City,
China) and all experiments were conducted in accordance with the
established guidelines for animal research [The 2010/63/EU
directive (2010) on the Protection of Animals] (16,17).
Mice were maintained at a temperature of 18-22˚C and in a 50-60%
humidity-controlled environment with a light/dark cycle of 12-h and
under specific pathogen-free conditions, with free access to food
and water.
SCA was provided by Sichuan Weikeqi Biotechnology
Co., Ltd. Superoxide dismutase (SOD; cat. no. A001-3-2),
malondialdehyde (MDA; cat. no. A003-1-2), glutathione (GSH; cat.
no. A006-2-1) and catalase (CAT; cat. no. A007-1-1) activity assay
kits were purchased from Nanjing Jiancheng Bioengineering Research
Institute. RIPA lysis buffer (cat. no. WB-0071) was purchased from
Beijing Dingguo Changsheng Biotechnology Co., Ltd. Kelch-like
ECH-associated protein 1 (Keap1; 1:1,000; diluent, TBST containing
1‰ Tween-20; cat. no. A17061), Nuclear factor (erythroid-derived
2)-like 2 (Nrf2; 1:1,000; diluent, TBST containing 1% Tween-20;
cat. no. A0674), heme oxygenase-1 (HO-1, 1:1,000; diluent, TBST
containing 1% Tween-20; cat. no. A1346), Bcl2 (1:1,000; diluent,
TBST containing 1% Tween-20, cat. no. A16776), Bax (1:1,000;
diluent, TBST containing 1‰% Tween 20; cat. no. A15646),
cleaved-caspase-3 (1:1,000; diluent, TBST containing 1% Tween-20;
cat. no. A0214), GAPDH (1:50,000; diluent, TBST containing 1‰
Tween-20; cat. no. AC033), horseradish peroxidase (HRP)-conjugated
goat anti-rabbit IgG H + L (1:5,000; diluent, TBST containing 1%
Tween-20; cat. no. AS014) and HRP goat anti-mouse IgG H + L
(1:5,000; diluent, TBST containing 1% Tween-20; cat. no. AS003)
antibodies were purchased from ABclonal Biotech Co., Ltd. ECL
chromogenic solution was purchased from Vazyme Biotech Co.,
Ltd.
Instruments
The mouse passive avoidance system (cat. no. 080057,
model no. BA-200) and rat Morris water maze video analysis system
(cat. no. 43513, model no. WMT-100, software version no. 1.0.0.1)
were supplied by Chengdu Taimeng Software Co., Ltd. Western blot
gel electrophoresis apparatus and transfer instruments were
purchased from Bio-Rad Laboratories, Inc. The automatic gel imaging
and analysis system was purchased from Beijing Sage Creation
Science Co., Ltd. The Infinite M200 automatic plate reader was
obtained from Tecan Group, Ltd.
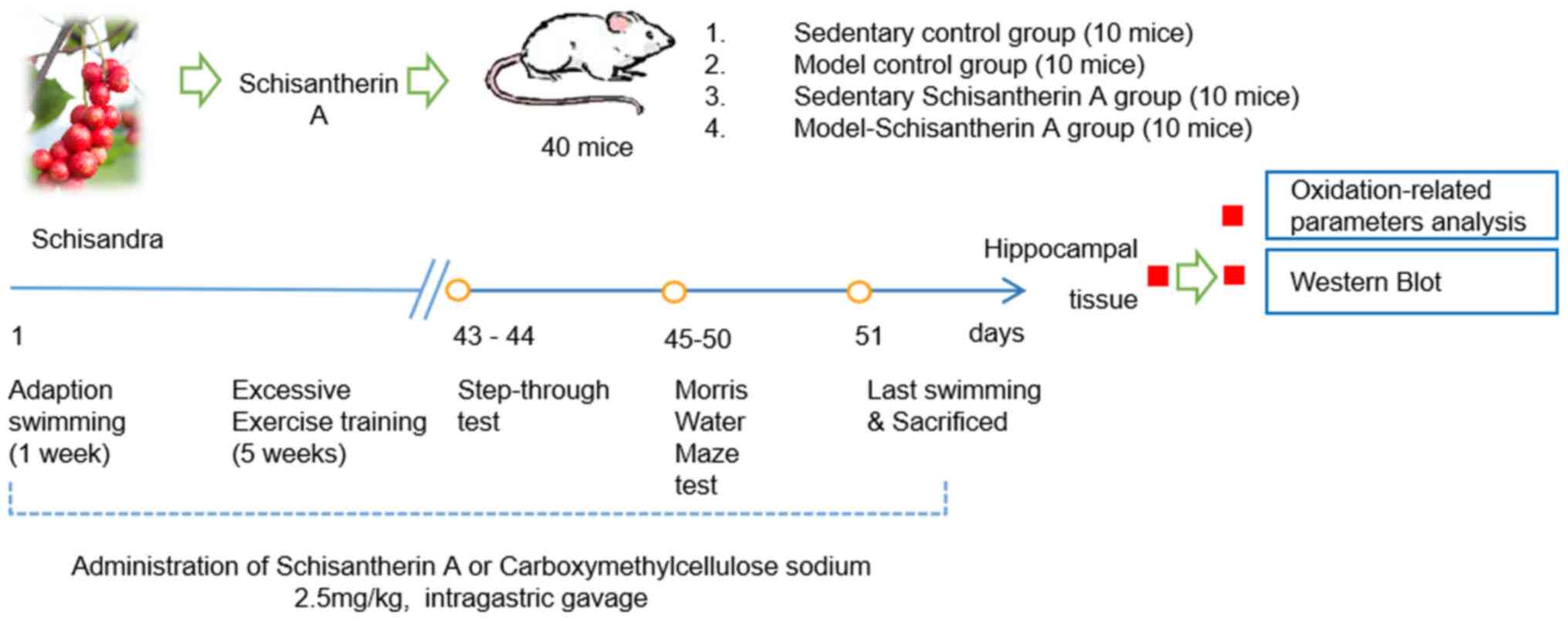
Animal grouping and
administration
The mice were allowed to acclimatize to the
laboratory environment for 7 days and then randomly divided into
the following four groups (n=10 mice per group): i) Control group,
(CON), which were treated with distilled water by gavage and
subjected to sedentary experiments; ii) chronic fatigue model group
(MOD), which were treated with distilled water by gavage and
underwent exhaustive exercise training; iii) SCA control group [SCA
(C)], which received 2.5 mg/kg SCA by gavage and was subjected to
sedentary experiment; and iv) SCA model group [SCA (M)], which were
treated with 2.5 mg/kg SCA by gavage followed by exhaustive
exercise training. Mice in the SCA (C) and SCA (M) groups were
given 2.5 mg/kg SCA once a day by gavage continuously for 6 weeks,
whilst those in CON and MOD groups were given an equal volume of
distilled water using the same approach. Subsequently, behavior of
mice was observed and the related biochemical indicators were
investigated. The specific experimental processes are shown in
Fig. 1.
Loaded swimming training
The chronic fatigue mouse model was established
using a loaded swimming training procedure as previously described
(14,18,19).
The mice were first placed in a plastic container, with a water
depth of 20 cm at 15±2˚C. In the first week, mice in each group
were trained for adaptation. On weeks 2-6, the mice were then
burdened with a 10% lead block attached to the tail. The training
on the first day lasted 30 min, 45 min on the second day and then
60 min on each of the following days. The mice were trained for 5
days per week. Subsequently, daily swimming training sessions each
lasting 60 min were performed from weeks 2-6, in which the body
weight of mice was measured weekly and the loaded weight was
adjusted to 10% of their body weight.
Learning and memory test Step-through
test
On day 43 of the experiment, step-through training
was performed. After a further 24 h, the step-through test began.
Darkness avoidance latency and the number of errors were observed
and recorded over a 5 min period to investigate the effect of SCA
on the learning and memory ability of chronic fatigue mice.
Morris water maze test
Morris water maze test was performed between days 45
and 50. The mice were placed in the MWT-100 Morris water maze video
tracking test system. The training time was set as 120 sec maximum.
If the mice were unable to reach the platform within 120 sec, this
was recorded as 120 sec. The mice were trained once every 24 h
successively for 5 days. On day 6, the platform for testing spatial
search function was removed, following which the number of times
the mice crossed the point where the platform would have been and
the effective area that the mice passed through were recorded.
Detection of SOD, CAT, GSH and MDA
levels in hippocampal tissue
On day 51 of the experiment, mice in all groups were
euthanized with a lethal dose of pentobarbital (210 mg/kg), and
their hippocampi were taken to prepare hippocampal homogenate. The
hippocampus was homogenized in a glass homogenizer with x10 saline
on ice, centrifuged at 160 x g, for 10 min, and then the
supernatant was collected. Hippocampal tissue homogenate was then
diluted with x10 saline for the determination of the activities of
SOD and CAT, as well as the concentration of GSH and MDA, in the
hippocampus homogenate were then determined according to the
protocols of the kits.
Detection of Keap1, Nrf2, HO-1, Bcl2,
Bax and cleaved-caspase-3 protein expression levels in the
hippocampus using western blotting
Protein lysis buffer (RIPA Lysis Buffer) was added
to the hippocampus homogenates. Subsequently, the protein
concentration was detected using bicinchoninic acid protein assay
and 10% SDS-PAGE gel electrophoresis was performed on the samples
(60 µg of protein per lane). Proteins were then transferred onto
PVDF membranes and blocked with blocking buffer (TBST buffer
containing 5% skim milk powder) for 1 h at room temperature before
the primary antibodies (1:1,000) were added and incubated overnight
at 4˚C. Secondary antibodies (1:5,000) were then added onto the
membranes after washing with TBST and incubated at room temperature
for 1 h. ECL chromogenic solution was used to develop the bands
after the membranes were washed. ImageJ (version 1.51j8; National
Institutes of Health) was used to perform the western blotting
densitometric analysis.
Statistical analysis
SPSS 20.0 software (IBM Corp.) was used to analyze
the data. Mice behavioral data, SOD and CAT activities, in addition
to GSH and MDA expression levels in the hippocampus tissue of mice
and protein expression levels, were all expressed as the mean ± SD.
The data between the two groups were compared using one-way ANOVA
followed by Dunnett's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of SCA on the learning and
memory abilities of mice
Step-through tests and water maze tests are classic
methods that can be used to assess the learning and memory ability
of animals (20). The results of
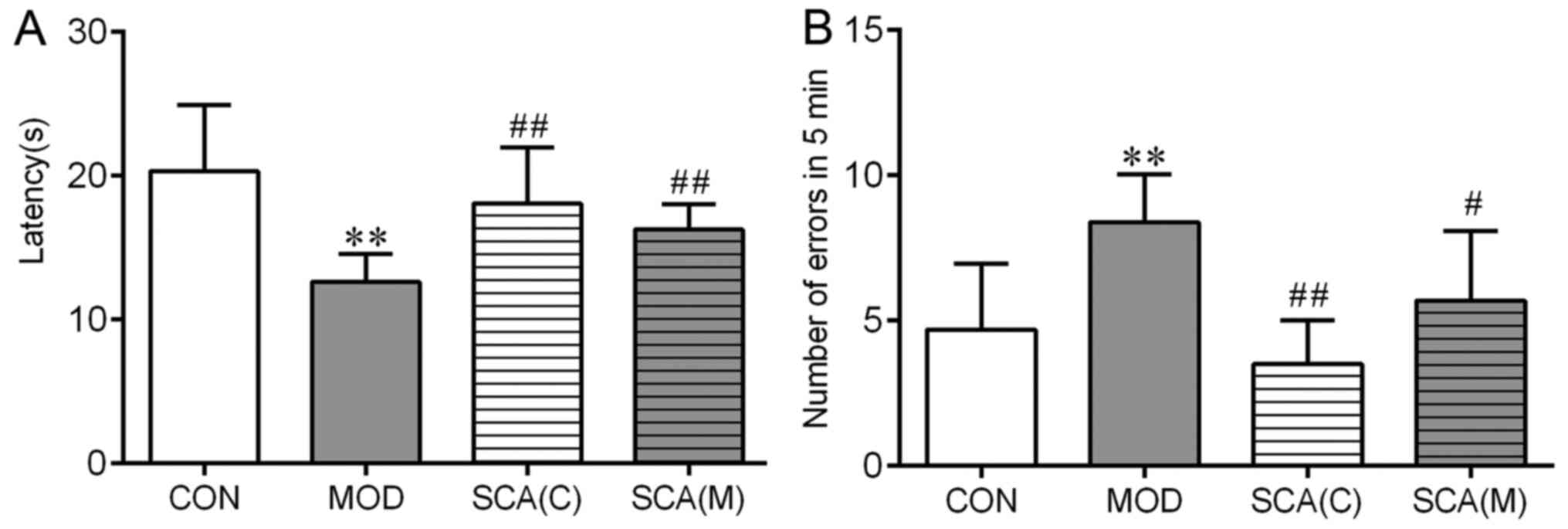
the step-through test (Fig. 2)
showed that compared with that in the CON group, in the MOD group,
the darkness avoidance latency of mice was significantly shortened
(P<0.01), whereas the number of errors was significantly
increased (P<0.01). No significant difference was observed
between the number of errors committed and the latency time in the
SCA (C) group or the SCA (M) group compared with that in the CON
group. Compared with that in the MOD group, the latency time was
found to be significantly prolonged (P<0.01 and P<0.01) and
the number of errors was significantly decreased (P<0.01 and
P<0.05) in the SCA (C) group and SCA (M) groups respectively. No
significant difference was observed between the SCA (M) group and
the SCA (C) group in the number of errors committed or the latency
time.
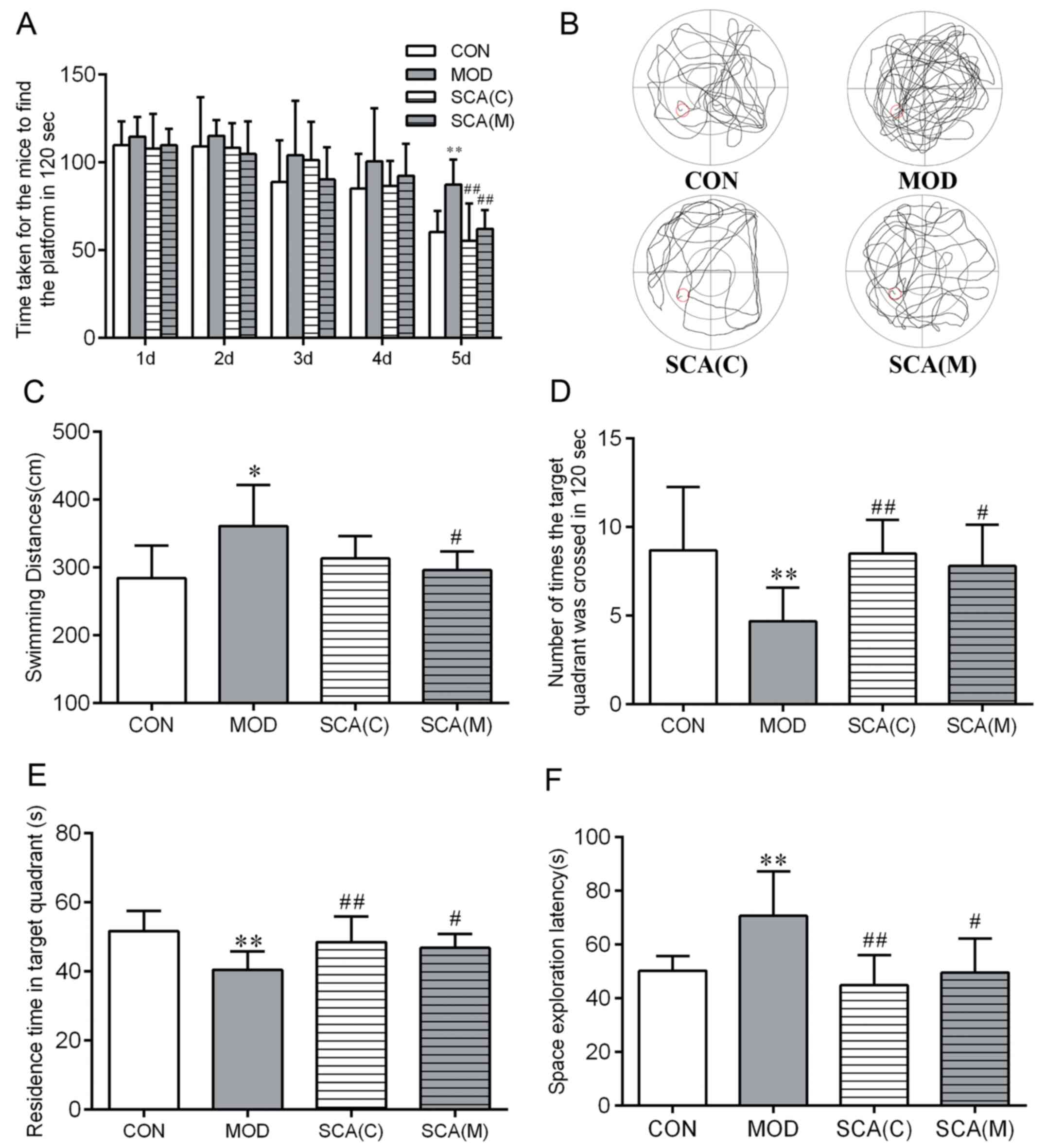
Morris water maze test results (Fig. 3) showed that in the orientation
navigation test, compared with that in the CON group, the time that
mice took to find the platform on day 5 (P<0.01) and the total
swimming distance (P<0.05) were significantly prolonged in the
MOD group. Compared with the CON group, there were no significant
differences between the time that the mice took to find the
platform on day 5 or the latency time in either the SCA (C) group
or the SCA (M) group. However, compared with those in the MOD
group, the time that mice took to find the platform on the 5th day
(P<0.01) was significantly shorter in the SCA (C) group and both
the time that mice took to find the platform on the 5th day
(P<0.01) and the total swimming distance (P<0.05) were
significantly shorter in the SCA (M). There was no significant
difference between the SCA (C) group and the SCA (M) group in the
time that the mice took to find the platform on the 5th day or the
total swimming distance. In the space exploration test, compared
with that in the CON group, the number of mice crossing the
platform was significantly reduced (P<0.01), whilst the
residence time in the target quadrant was significantly shortened
(P<0.01) and the latency of space exploration was significantly
prolonged (P<0.01) in the MOD group. In comparison with the CON
group, the number of mice crossing the platform, the residence time
in the target quadrant and the latency of space exploration of both
the SCA (C) group and the SCA (M) were not significantly different.
The number of platform crossings was found to be significantly
increased (P<0.01, P<0.05) and the residence time in the
target quadrant was significantly increased in the SCA (C) group
and the SCA (M) group (P<0.01, P<0.05) compared with those in
the MOD group. In addition, compared with that in MOD group, the
latency of space exploration was also significantly shortened
(P<0.01, P<0.05) in the SCA (C) group and the SCA (M) group.
No significant differences were observed between the SCA (M) group
and the SCA (C) group in these results.
Effects of SCA on the activity of SOD
and CAT, in addition to the content of GSH and MDA in the
hippocampus tissue of mice
SOD, CAT and GSH are important antioxidants in the
body whereas MDA is the product of lipid peroxidation (21). The activity and content of these
biomarkers reflects the degree of oxidative damage (22). At the end of the behavioral
experiments, the hippocampal tissue of mice in each treatment group
was taken for the detection of SOD, CAT, GSH and MDA. The results
showed that compared with those in the CON group, the activities of
SOD (P<0.05) and CAT (P<0.01), as well as the concentration
of GSH (P<0.01), were significantly decreased in the MOD group.
However, MDA levels were significantly increased (P<0.01) in the
MOD group compared with those in the CON group (Fig. 4). The activities of SOD and CAT, as
well as the GSH and MDA levels in the SCA (C) group and the SCA (M)
group were not significantly different from those in the CON group.
SOD (P<0.01, P<0.05) and CAT (P<0.01, P<0.05)
activities and GSH concentration (P<0.01, P<0.05) were
significantly increased, whilst the MDA concentration was
significantly decreased (P<0.01, P<0.05) in the SCA (C) group
and the SCA (M) group respectively. No significant difference was
observed between the SCA (M) group and the SCA (C) group in the
SOD, CAT, GSH or MDA levels.
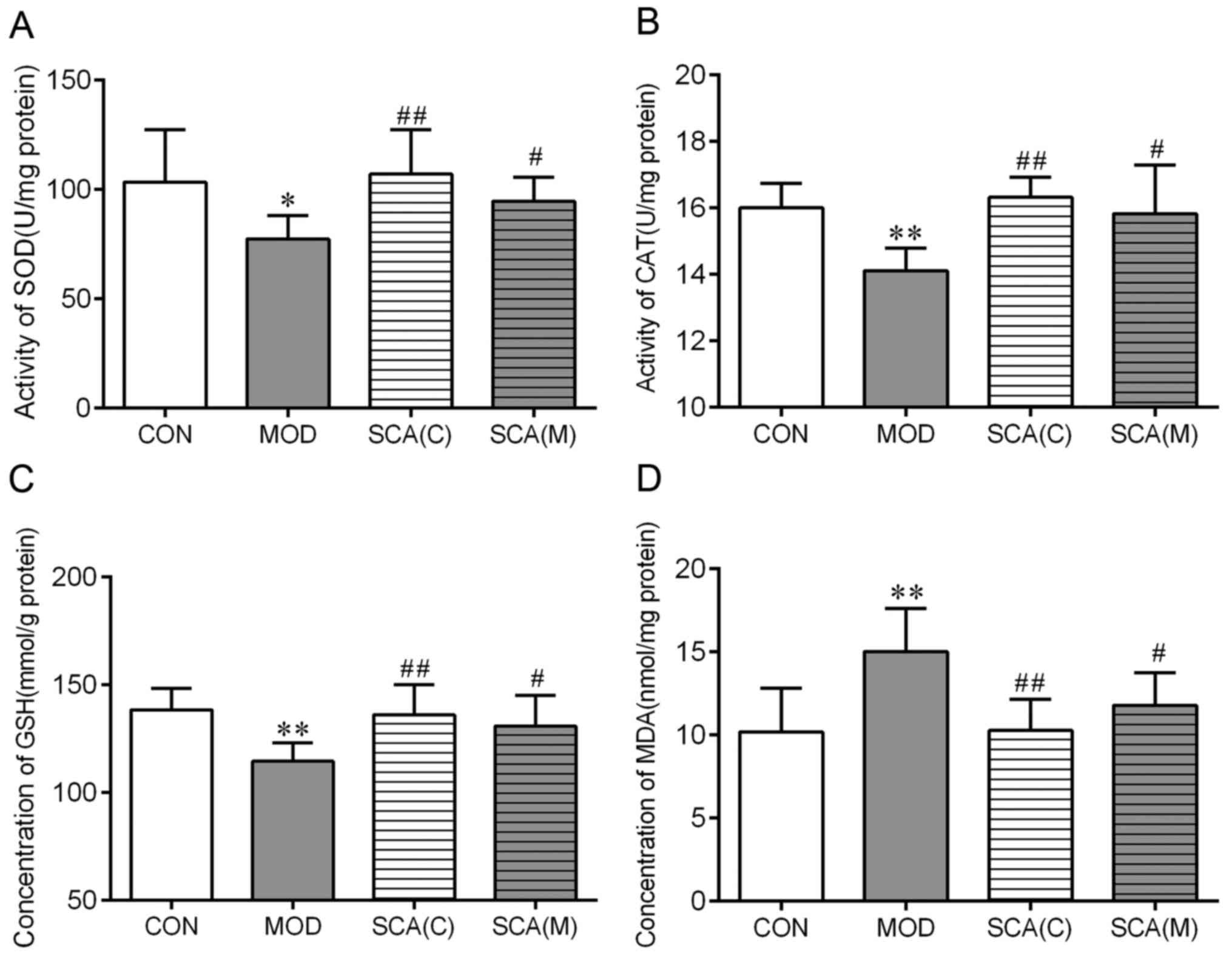 | Figure 4Effects of SCA on the parameters of
antioxidants in mouse hippocampal tissues. Activities of (A) SOD
and (B) CAT, as well as the concentration (C) of GSH and (D) MDA
were measured in the hippocampus tissue of chronic fatigue mice.
Mean ± SD, n=10. *P<0.05, **P<0.01 vs.
CON; #P<0.05 and ##P<0.01 vs. MOD. CAT,
catalase; GSH, glutathione; MDA, malondialdehyde; SCA,
Schizantherin A; SOD, superoxide dismutase; CON, control; MOD,
model; SCA (C), Schizantherin A (control); SCA (M), Schizantherin A
(model). |
Effects of SCA on the protein
expression levels of Keap1, Nrf2 and HO-1 in the hippocampus
tissue
Western blotting was used to detect the protein
expression levels of Keap1, Nrf2 and HO-1 in the hippocampus tissue
of the mice used in the present study. The results showed that
compared with those in the CON group, the protein expression levels
of Keap1 were significantly higher (P<0.05), whilst Nrf2
(P<0.05) and HO-1 (P<0.01) protein expression levels were
significantly lower, in the hippocampal tissue of mice in the MOD
group (Fig. 5). Additionally, the
expression levels of Keap1, HO-1 and Nrf2 were not significantly
different in the hippocampal tissue of mice in the SCA (C) group
and SCA (M) group compared with those in the CON group. The
expression levels of Keap1 were significantly lower (P<0.05),
whilst those of Nrf2 (P<0.01) were significantly higher in the
SCA (C) group compared with those in the MOD group. The expression
levels of Keap1 were significantly lower (P<0.05), whilst those
of Nrf2 (P<0.05) and HO-1 (P<0.05) were significantly higher,
in the hippocampus tissue of mice in the SCA (M) group compared
with those in the MOD group. There was no significant difference
between the SCA (M) group and the SCA (C) group in the Keap1, Nrf2
and HO-1 levels.
 | Figure 5Effects of SCA on the parameters of
redox signaling in mouse hippocampal tissues. (A) Representative
western blotting images showing Keap1, Nrf2 and HO-1 expression in
the hippocampal tissue of chronic fatigue mice. Protein expression
levels of (B) Keap1, (C) Nrf2 and (D) HO-1 in the hippocampal
tissue of chronic fatigue mice were then quantified. Mean ± SD,
n=3. *P<0.05, **P<0.01 vs. CON;
#P<0.05, ##P<0.01 vs. MOD. HO-1, heme
oxygenase 1; Keap1, kelch like ECH associated protein 1; Nrf2,
Nuclear factor (erythroid-derived 2)-like 2; SCA, Schizantherin A;
CON, control; MOD, model; SCA (C), Schizantherin A (control); SCA
(M), Schizantherin A (model). |
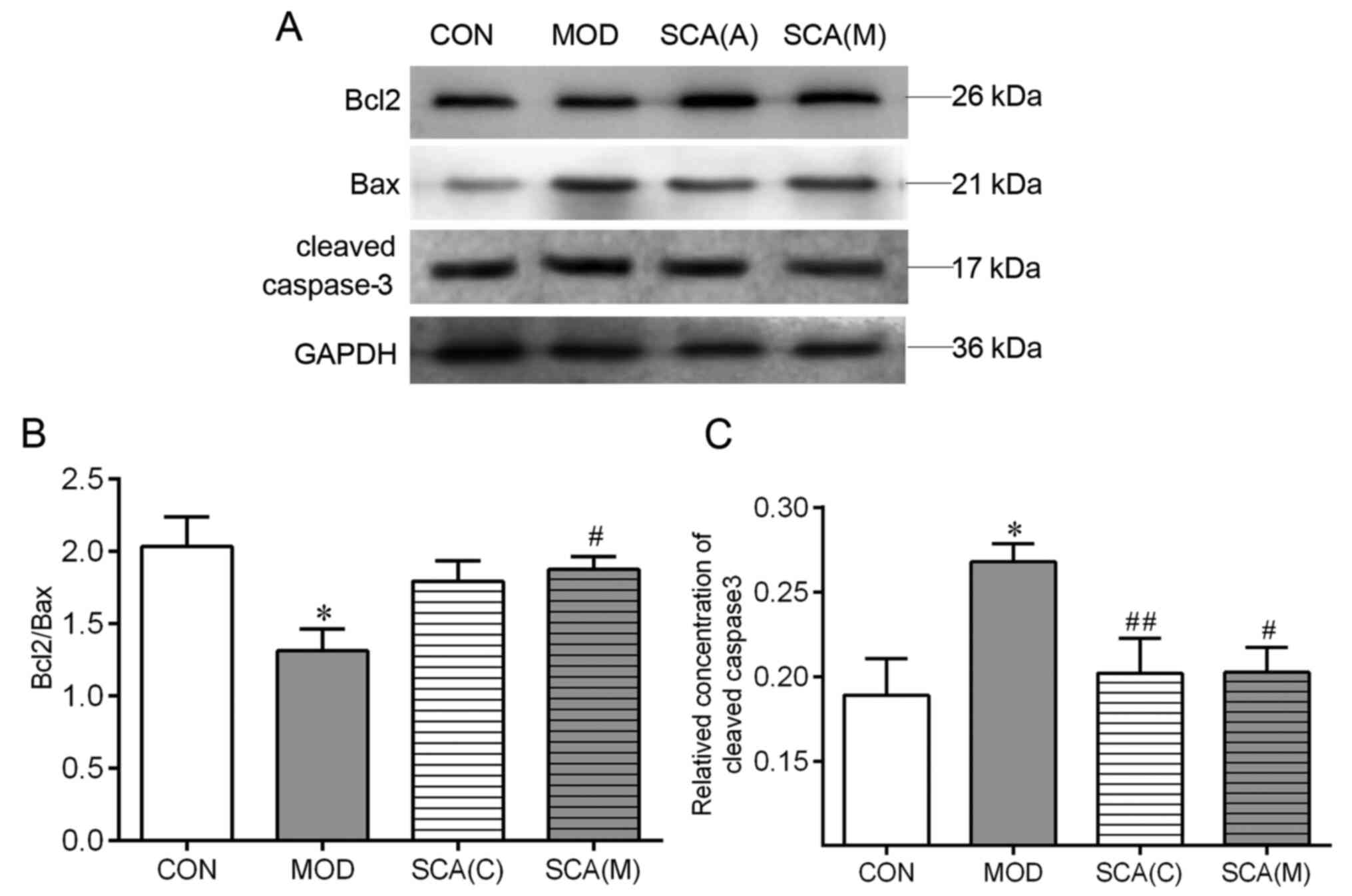
Effects of SCA on the expression
levels of Bcl2, Bax and cleaved caspase-3 in the hippocampus
tissue
Western blotting was used to detect the expression
levels of Bcl2, Bax and cleaved caspase-3 in the hippocampus tissue
of the chronic fatigue mice. As shown in Fig. 6, compared with that in the CON
group, the ratio of Bcl2/Bax was significantly decreased
(P<0.05) whereas the protein expression levels of cleaved
caspase-3 were significantly increased (P<0.05) in the
hippocampus tissue of mice in the MOD group. The ratio of Bcl2/Bax
and the protein expression levels of cleaved caspase-3 in the SCA
(C) group and the SCA (M) group were not significantly different
from the CON group. Compared with the MOD group, the expression
level of cleaved caspase-3 was significantly decreased (P<0.05)
in the hippocampal tissue of mice in the SCA (C) group. The ratio
of Bcl2/Bax was significantly increased (P<0.05) and the
expression levels of cleaved caspase-3 were significantly decreased
(P<0.05) in the hippocampal tissue of mice in the SCA (M) group
compared with those in the MOD group. There was no significant
difference between the SCA (M) group and the SCA (C) group in the
ratio of Bcl2/Bax or the protein expression levels of cleaved
caspase-3.
Discussion
Lignans is the main active component within
Schisandra chinensis (23).
Lignans has been reported to confer multiple properties, including
protection against liver injury, reduction in lipid levels,
anti-inflammation, anti-oxidation and improvements in learning and
memory (13). Among different types
of lignans, SCA is one of the main active components that can be
found in Schisandra chinensis (14). A previous study found that SCA can
significantly enhance exercise endurance in chronic fatigue mice,
enhance glycogen content in the liver and muscles, reduce the
content of urea nitrogen and reduce MDA content in the blood,
resulting in a significant anti-fatigue effect (14). In the present study, a mouse
classical chronic fatigue model was established using a previously
established procedure of 6-week loaded swimming training (14,18,19).
The effects of SCA on learning and memory abilities of chronic
fatigue mice were observed and the underlying mechanism of action
was then explored from two perspectives of anti-oxidation and
anti-apoptosis.
Step-through test and water maze test are classic
methods that can be used to evaluate the learning and memory
ability of animals (21). In the
present study, the learning and memory abilities of mice were
evaluated using the two aforementioned methods. The results showed
that the learning and memory ability of mice in the MOD group was
significantly lower compared with that in the CON group in both the
step-through and in the Morris water maze tests, consistent with
previous reports on the effects of chronic fatigue on learning and
memory disorders (24,25). Results from the present study also
showed that the learning and memory ability of chronic fatigue mice
was significantly improved after the administration of SCA,
suggesting that SCA can improve learning and memory abilities. A
previous study showed that learning and memory disorders of mice
induced by D-galactose were improved after the administration of
SCA (15). These findings, along
with results of the present study, suggest that SCA can be used as
a supplement for the prevention and treatment of chronic fatigue-
and D-galactose-induced learning and memory disorders.
Vigorous exercise for an extended period will
enhance the metabolism and oxygen consumption of the body and
increase the production of excessive free radicals in various
tissues (26). This can exceed the
clearance ability of the body's own antioxidant defense system,
causing oxidative stress damage. Oxidative stress in brain tissues
will lead to impairments in learning and memory (27). Antioxidants serve an important role
in the prevention of oxidative stress-induced memory defects
(28,29). SOD, CAT and GSH are important
anti-oxidants for scavenging free radicals in the body (21). MDA is the product of lipid
peroxidation, the content of which can reflect the degree of lipid
peroxidation and in turn the degree of cell damage (20). Since the hippocampal region of the
brain is closely associated with learning and memory (30), hippocampal tissue of chronic fatigue
mice was selected for the detection of the aforementioned
indicators in the present study. The results showed that SCA could
increase the activities of SOD and CAT, as well as the GSH content
but reduce the levels of MDA, suggesting increased antioxidant
capacity. It has been previously found that SCA can significantly
improve antioxidant capacity in both livers in fatigue mice and
brain of aging mice induced by D-galactose (14,15).
These results suggest that SCA improves the learning and memory
abilities of chronic fatigue mice by enhancing antioxidant capacity
to protect the brain from oxidative damage.
The Nrf2/ARE signaling pathway is an important
regulatory pathway involved in the antioxidant response (31). Nrf2 is a key regulator of cell
oxidation (32). it can effectively
resist oxidative stress injuries when activated (32,33).
By contrast, Keap1 is a negative regulator of Nrf2, and its
ubiquitination and phosphorylation can activate Nrf2 (34,35).
HO-1 is a strong antioxidant in the body that is regulated by
Nrf2(36). When Nrf2 is activated,
HO-1 expression has been indicated to be significantly increased
(37). In the present study, the
activity of these three components of the Nrf2/ARE signaling
pathway in the hippocampus tissue of mice was investigated. The
results showed that SCA could downregulate the protein expression
levels of Keap1 whilst upregulating the protein expression levels
of Nrf2 and HO-1 in the hippocampus of chronic fatigue mice. This
suggest that SCA may alleviate learning and memory disorders caused
by chronic fatigue through the Nrf2/ARE signaling pathway. However,
the exact binding site and the precise mode of action on this
pathway induced by SCA remain unknown, which is a limitation of the
present study.
The accumulation of a large number of free radicals
can lead to apoptosis through lipid peroxidation, protein
denaturation and DNA damage (38).
A direct consequence of neuronal apoptosis in the brain is
impairments in learning and memory (39). Therefore, protection against this
process is important for the protection of brain functions
(39). Bcl2 and Bax are important
apoptotic regulators, with Bcl2 being anti-apoptotic and Bax being
pro-apoptotic, rendering the ratio of Bcl2/Bax useful for
determining the extent of apoptosis (40). Previous studies have shown that Nrf2
can regulate the expression levels of Bcl2 and the process of
apoptosis by binding to the anti-oxidant response element of the
Bcl2 gene (41,42). Administration of Nrf2 inhibitors has
also been found to reduce the expression levels of Bcl2 and the
Bcl2/Bax ratio (42), suggesting
that changes in Nrf2 expression levels may directly affect
apoptosis. Consistent with these previous observations, the results
of the present study showed that SCA could upregulate the protein
expression levels of Nrf2 and Bcl2 whilst downregulating the
protein expression levels of Bax and increasing the Bcl2/Bax ratio
in the hippocampus of chronic fatigue mice, suggesting that SCA
exerts an anti-apoptotic effect. Cleaved caspase-3 is another key
factor for inducing apoptosis, with levels of cleaved caspase-3
directly associated with the degree of apoptosis (43,44).
The present results showed that SCA could reduce the expression
levels of cleaved caspase-3 in the hippocampus of chronic fatigue
mice, further supporting the notion that SCA may enhance learning
and memory abilities by inhibiting the apoptosis of hippocampal
neurons.
In conclusion, the present study suggests that SCA
treatment may improve the learning and memory abilities of chronic
fatigue mice. This may be associated with its observed modulation
of the Nrf2/ARE signaling pathway and antioxidant role, in addition
to the inhibition of apoptosis of hippocampal neurons in chronic
fatigue mice. The present study may provide an experimental basis
for the application of Schisandra or SCA as drugs and health
foods to alleviate fatigue and to improve learning and memory.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Jilin Provincial
Department of science and Technology (grant nos. 20170309006YY,
20200201521JC, 20200404053YY and 20200404022YY), Jilin Science and
technology innovation development plan project (grant no.
20190601177), Jilin provincial health and Family Planning
Commission (grant no. 2018J089), Jilin Provincial Development and
Reform Commission (grant no. 2020C033-2) and Jilin Administration
of traditional Chinese Medicine (grant no. 2020121).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SJ and HL conceived and designed the study. HJL, XZ,
JLL, LY and JL performed the animal experiments. HL, CW, JS and JC
performed the data analysis. The final version of the manuscript
was read and approved by all authors.
Ethics approval and consent to
participate
The animal experiments were approved by the
Institutional Animal Care and Use Committee of Beihua University
(Jilin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feng S, Liu W, Zuo S, Xie T, Deng H, Zhang
Q and Zhong B: Impaired function of the intestinal barrier in a
novel sub-health rat model. Mol Med Rep. 13:3459–3465.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bi JL, Chen J, Sun XM, Nie XL, Liu YY, Luo
R and Zhao XS: The development and evaluation of a sub-health
self-rating scale for university students in China. BMC Public
Health. 19(330)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xu X, Zeng Q, Ding H, Feng L and Deng L:
Correlation between women's sub-health and reproductive diseases
with pregnancies and labors. J Tradit Chin Med. 34:465–469.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lenaert B, Boddez Y, Vlaeyen JWS and
Caroline MH: Learning to feel tired: A learning trajectory towards
chronic fatigue. Behav Res Ther. 100:54–66. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhao R, Hao W, Ma B and Chen Z:
Improvement effect of Lycium barbarum polysaccharide on
sub-health mice. Iran J Basic Med Sci. 18:1245–1252.
2015.PubMed/NCBI
|
|
6
|
Lamou B, Taiwe GS, Hamadou A, Abene
Houlray J, Atour MM and Tan PV: Antioxidant and antifatigue
properties of the aqueous extract of Moringa oleifera in
rats subjected to forced swimming endurance test. Oxid Med Cell
Longev. 2016(3517824)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Duan FF, Guo Y, Li JW and Yuan K:
Antifatigue effect of luteolin-6-C-neohesperidoside on oxidative
stress injury induced by forced swimming of rats through modulation
of Nrf2/ARE signaling pathways. Oxid Med Cell Longev.
2017(3159358)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hsiao CY, Hsu YJ, Tung YT, Lee MC, Huang
CC and Hsieh CC: Effects of Antrodia camphorata and Panax
ginseng supplementation on anti-fatigue properties in mice. J
Vet Med Sci. 80:284–291. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu XL, Zhu CC, Qiao DC, Hou LJ and Kang
DF: Effect of exercise-induced fatigue on rat learning and memory
ability and expressions of CaN in the brain. Energy Procedia.
1:248–251. 2011.
|
|
10
|
van't Leven M, Zielhuis GA, van der Meer
JW, Verbeek AL and Bleijenberg G: Fatigue and chronic fatigue
syndrome-like complaints in the general population. Eur J Public
Health. 20:251–257. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qi Y, Cheng X, Jing H, Yan T, Xiao F, Wu
B, Bi K and Jia Y: Effect of Alpinia oxyphylla-Schisandra
chinensis herb pair on inflammation and apoptosis in Alzheimer'
s disease mice model. J Ethnopharmacol. 237:28–38. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gao Y, Wu S, Cong R, Xiao J and Ma F:
Characterization of lignans in Schisandra chinensis oil with
a single analysis process by UPLC-Q/TOF-MS. Chem Phys Lipids.
218:158–167. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang X, Yu J, Li W, Wang C, Li H, Ju W,
Chen J and Sun J: Characteristics and antioxidant activity of
lignans in Schisandra chinensis and Schisandra
sphenanthera from different locations. Chem Biodivers.
15(e1800030)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang XY, Lin HJ, Li X, Jing S, Sun W,
Jiang WH, Wang CM, Sun JH, Li H and Chen JG: Schisantherin A
improves fatigue of mice by regulating the antioxidant pathway of
liver Nrf2/ARE. Food Sci. 41:1–10. 2020.
|
|
15
|
Liu C, Sun W, Li N, Gao J, Yu C, Wang C,
Sun J, Jing S, Chen J and Li H: Schisantherin A improves learning
and memory of mice with D-galactose-induced learning and memory
impairment through its antioxidation and regulation of
p19/p53/p21/Cyclin D1/CDK4/RB gene expressions. J Med Food.
21:678–688. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cheluvappa R, Scowen P and Eri R: Ethics
of animal research in human disease remediation, its institutional
teaching; and alternatives to animal experimentation. Pharmacol Res
Perspect. 5(e00332)2017.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Smith AJ, Clutton RE, Lilley E, Hansen KEA
and Brattelid T: PREPARE: Guidelines for planning animal research
and testing. Lab Anim. 52:135–141. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yeh TS, Chuang HL, Huang WC, Chen YM,
Huang CC and Hsu MC: Astragalus membranaceus improves
exercise performance and ameliorates exercise-induced fatigue in
trained mice. Molecules. 19:2793–2807. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Falavigna G, Alves de Araújo J, Rogero MM,
Pires IS, Pedrosa RG, Martins E Jr, Alves de Castro I and Tirapegui
J: Effects of diets supplemented with branched-chain amino acids on
the performance and fatigue mechanisms of rats submitted to
prolonged physical exercise. Nutrients. 4:1767–1780.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu C, Wen Y, Huang H, Lin W, Huang M, Lin
R and Ma Y: Over-expression of 5-HT6 receptor and activated
Jab-1/p-c-Jun play important roles in pilocarpine-induced seizures
and learning-memory impairment. J Mol Neurosci. 67:388–399.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhou J, Liu X, Chen T, Cheng G and Cai S:
Preventive effect of ethanol extract from Chinese sumac fruits
against tetrachloromethane-induced liver fibrosis in mice. Food
Funct. 11:7061–7072. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Huang CC, Hsu MC, Huang WC, Yang HR and
Hou CC: Triterpenoid-rich extract from Antrodia camphorata
improves physical fatigue and exercise performance in mice. Evid
Based Complement Alternat Med. 2012(364741)2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sowndhararajan K, Deepa P, Kim M, Park SJ
and Kim S: An overview of neuroprotective and cognitive enhancement
properties of lignans from. Schisandra chinensis. Biomed
Pharmacother. 97:958–968. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rasouli O, Gotaas ME, Stensdotter AK,
Skovlund E, Landrø NI, Dåstøl P and Fors EA: Neuropsychological
dysfunction in chronic fatigue syndrome and the relation between
objective and subjective findings. Neuropsychology. 33:658–669.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fan XL, Li F and Song YH: Effect of sihui
mixture on the learning and memory ability, mRNA expressions of
hippocampus NMDA subunit NR2A and NR2B, and EphB2 receptor in
fatigue rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 31:512–516.
2011.PubMed/NCBI(In Chinese).
|
|
26
|
Ramos D, Martins EG, Viana-Gomes D,
Casimiro-Lopes G and Salerno VP: Biomarkers of oxidative stress and
tissue damage released by muscle and liver after a single bout of
swimming exercise. Appl Physiol Nutr Metab. 38:507–511.
2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Baghcheghi Y, Salmani H, Beheshti F,
Shafei MN, Sadeghnia HR and Soukhtanloo M: Effects of PPAR-γ
agonist, pioglitazone on brain tissues oxidative damage and
learning and memory impairment in juvenile hypothyroid rats. Int J
Neurosci. 129:1024–1038. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Halder S, Kar R, Galav V, Mehta AK,
Bhattacharya SK, Mediratta PK and Banerjee BD: Cadmium exposure
during lactation causes learning and memory-impairment in F1
generation mice: Amelioration by quercetin. Drug Chem Toxicol.
39:272–278. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Haddadi M, Jahromi SR, Sagar BK, Patil RK,
Shivanandappa T and Ramesh SR: Brain aging memory impairment and
oxidative stress: A study in Drosophila melanogaster. Behav
Brain Res. 259:60–69. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Schurman LD, Carper MC, Moncayo LV,
Ogasawara D, Richardson K, Yu L, Liu X, Poklis JL, Liu QS, Cravatt
BF and Lichtman AH: Diacylglycerol lipase-alpha regulates
hippocampal-dependent learning and memory processes in mice. J
Neurosci. 39:5949–5965. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tu WJ, Wang H, Li S, Liu Q and Sha H: The
anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE
signaling pathway in chronic diseases. Aging Dis. 10:637–651.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen K, You J, Tang Y, Zhou Y, Liu P, Zou
D, Zhou Q, Zhang T, Zhu J and Mi M: Supplementation of superfine
powder prepared from Chaenomeles speciosa fruit increases
endurance capacity in rats via antioxidant and Nrf2/ARE signaling
pathway. Evid Based Complement Alternat Med.
2014(976438)2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Taguchi K, Maher JM, Suzuki T, Kawatani Y,
Motohashi H and Yamamoto M: Genetic analysis of cytoprotective
functions supported by graded expression of Keap1. Mol Cell Biol.
30:3016–3026. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kopacz A, Kloska D, Forman HJ, Jozkowicz A
and Grochot-Przeczek A: Beyond repression of Nrf2: An update on
Keap1. Free Radic Biol Med. 157:63–74. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Baird L and Yamamoto M: The molecular
mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol.
40:e00099–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhong L, Zhao L, Yang F, Yang W, Sun Y and
Hu Q: Evaluation of anti-fatigue property of the extruded product
of cereal grains mixed with cordyceps militaris on mice. J Int Soc
Sports Nutr. 14(15)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Surhio MM, Wang YF, Fang S, Li J and Ye M:
Anti-fatigue activity of a lachnum polysaccharide and its
carboxymethylated derivative in mice. Bioorg Med Chem Lett.
27:4777–4780. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang K, Chen Z, Huang J, Huang L, Luo N,
Liang X, Liang M and Xie W: Naringenin prevents ischaemic stroke
damage via anti-apoptotic and anti-oxidant effects. Clin Exp
Pharmacol Physiol. 44:862–871. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jiang XM, Hu JH, Wang LL, Ma C, Wang X and
Liu XL: Ulinastatin alleviates neurological deficiencies evoked by
transient cerebral ischemia via improving autophagy, Nrf-2-ARE and
apoptosis signals in hippocampus. Physiol Res. 67:637–646.
2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yoo ES, Choo GS, Kim SH, Woo JS, Kim HJ,
Park YS, Kim BS, Kim SK, Park BK, Cho SD, et al: Antitumor and
apoptosis-inducing effects of piperine on human melanoma cells.
Anticancer Res. 39:1883–1892. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Niture SK and Jaiswal AK: Nrf2
up-regulates anti-apoptotic protein Bcl-2 and prevents cellular
apoptosis. J Biol Chem. 287:9873–9886. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhang J, Su L, Ye Q, Zhang S, Kung H,
Jiang F, Jiang G, Miao J and Zhao B: Discovery of a novel Nrf2
inhibitor that induces apoptosis of human acute myeloid leukemia
cells. Oncotarget. 8:7625–7636. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ye J, Jiang R, Cui M, Zhu B, Sun L, Wang
Y, Zohaib A, Dong Q, Ruan X, Song Y, et al: Etanercept reduces
neuroinflammation and lethality in mouse model of Japanese
encephalitis. J Infect Dis. 210:875–889. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gu Y, Yang Y, Cao X, Zhao Y, Gao X, Sun C,
Zhang F, Yuan Y, Xu Y, Zhang J, et al: Plin3 protects against
alcoholic liver injury by facilitating lipid export from the
endoplasmic reticulum. J Cell Biochem. 120:16075–16087.
2019.PubMed/NCBI View Article : Google Scholar
|