Introduction
Endometrial cancers (ECs) are a group of epithelial
malignant tumors that occur in the endometrium, accounting for
20-30% of all malignancies of the female genital tract (1). The average age of onset of EC is 60
years and 75% of EC cases occur in women aged >50 years
(2). At present, EC treatment is
primarily based on surgery supplemented with radiotherapy,
chemotherapy and hormone therapy (3). However, due to the side effects of
radiotherapy and chemotherapy, patients suffer greatly (3). Therefore, the aim of the present study
was to identify novel therapeutic targets to provide potential new
treatment strategies for EC.
Nodal growth differentiation factor (NODAL) is an
important member of the TGF-β family and an important morphogenetic
molecule in embryonic development (4). Previously, it was thought that NODAL
was only expressed in embryonic tissues (5); however, in 2006, Topczewska et
al (6) reported that NODAL is
highly expressed in melanoma and is closely associated with
occurrence and metastasis, serving a protumor effect. Since then,
abnormal expression of NODAL has been identified in breast,
prostate, pancreatic, liver and other types of cancer (7-10).
Additionally, studies have reported that NODAL promotes tumor
development (11,12); however, other studies have indicated
that NODAL inhibits tumor cell proliferation and promotes
apoptosis, as evidenced by dose-dependent studies on prostate and
pancreatic cancer (13,14). To the best of our knowledge, the
role of NODAL in EC has not been previously reported.
Activin receptor-like kinase 7 (ALK7) is one of
seven type I receptors in the TGF-β family (15). A previous study demonstrated that
the TGF-β family members NODAL, Activin A/B and growth
differentiation factor 3 are all ligands of ALK7(16). Compared with NODAL, few studies have
investigated ALK7 and the studies on its function primarily focused
on two aspects. Firstly, ALK7 has been reported to inhibit cell
proliferation and promote cell apoptosis following activation
(17). Secondly, ALK7 is involved
in sugar and lipid metabolism (18). Furthermore, a recent study has
demonstrated that ALK7, as a tumor suppressor gene, is a barrier to
tumor occurrence and metastasis (19). However, to the best of our
knowledge, the expression, effect and underlying mechanisms of ALK7
in EC are not completely understood.
NODAL is the ligand of ALK7 and promotes breast
cancer cell apoptosis via ALK7(20). Bioinformatics analysis (ualcan.path.uab.edu) indicated that the expression
levels of NODAL and ALK7 in EC cell lines were decreased. However,
the expression of NODAL and ALK7 in EC and their effects on tumor
cell proliferation, invasion and migration are not completely
understood. Therefore, the present study investigated the effects
of NODAL and ALK7 on EC cell proliferation, invasion, migration and
apoptosis, as well as the underlying mechanisms, to identify novel
therapeutic targets for EC.
Materials and methods
Cell culture and treatment
EC cell lines (Ishikawa, KLE, RL95-2 and AN3 CA) and
a novel immortalized human endometrial stromal cell line (THESCs;
referred to as ESC in the present study; RRID: CVCL_C464; NCBI
Taxonomy: 9606) were purchased from The Cell Bank of Type Culture
Collection of the Chinese Academy of Sciences. Cells were cultured
in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) at 37˚C with 5%
CO2. Solid SB431542 (cat. no. HY-10431) was obtained
from MedChemExpress and was dissolved in 1 ml DMEM (Gibco; Thermo
Fisher Scientific, Inc.) at a final concentration of 10 mmol/l. The
induction time of SB431542 is 24 h at 37˚C with 5%
CO2.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using RNAzol RT
(Sigma-Aldrich; Merck KGaA), according to the manufacturer's
protocol. RNA concentration and quantification were assessed using
a NanoDrop spectrophotometer (Thermo Fisher Scientific, Inc.).
Following DNase I digestion, total RNA was reverse transcribed into
cDNA using a QuantiTect Reverse Transcription kit (Qiagen GmbH),
according to the manufacturer's protocol. Subsequently, qPCR was
performed using a QuantiTect SYBR Green PCR kit (Qiagen GmbH),
according to the manufacturer's protocol. The following
thermocycling conditions were used for qPCR: 95˚C for 10 min;
followed by 40 cycles of 95˚C for 10 sec and 60˚C for 60 sec. The
following primers (GenScript) were used for qPCR: ALK7 forward,
5'-ATGACCCCAGCGCGCGGCTCCGCACT-3' and reverse,
5'-CTTCCTGTATGTGCACTGGCGGTCCT-3'; NODAL forward,
5'-ACCGAGTCCCTTCCACTTGT-3' and reverse, 5'-CAGAGGCACCCACATTCTTC-3';
and GAPDH forward, 5'-AGCCACATCGCTCAGACAC-3' and reverse,
5'-GCCCAATACGACCAAATCC-3'. mRNA expression levels were quantified
using the 2-ΔΔCq method (21) and normalized to the internal
reference gene GAPDH.
Bioinformatics website
The expression levels of NODAL and ALK7 in EC were
predicted using a bioinformatics website (ualcan.path.uab.edu; release date, 03/13/2019).
Western blotting
Cells were washed twice with cold PBS, lysed with
RIPA lysis buffer (Beyotime Institute of Biotechnology) and
incubated for 30 min on ice. Cell lysates were centrifuged at 300 x
g at 4˚C for 20 min and the protein supernatant was transferred
into Eppendorf tubes. Total protein was quantified using a BCA
protein assay kit (Bio-Rad Laboratories, Inc.). Proteins (40 µg)
were separated via 10% SDS-PAGE and transferred to PVDF membranes
(GE Healthcare), which were blocked with 10% skimmed milk for 1 h
at room temperature. Subsequently, the membranes were incubated
overnight at 4˚C with the following primary antibodies (all
purchased from Abcam): Anti-NODAL (1:1,000; cat. no. ab55676),
anti-ALK7 (1:1,000; cat. no. ab111121), anti-matrix
metallopeptidase (MMP)2 (1:1,000; cat. no. ab215986), anti-MMP7
(1:1,000; cat. no. ab205525), anti-MMP9 (1:1,000; cat. no.
ab219372), anti-caspase-3 (1:1,000; cat. no. ab13847),
anti-caspase-9 (1:1,000; cat. no. ab65608), anti-cleaved-caspase-3
(1:1,000; cat. no. 9953S), anti-cleaved-caspase-9 (1:1,000; cat.
no. ab2324), anti-Bcl2 (1:1,000; cat. no. ab32124), anti-Bax
(1:1,000; cat. no. ab32503) and anti-GAPDH (1:1,000; cat. no.
ab181602). Following primary incubation, the membranes were
incubated with a goat anti-rabbit horseradish peroxidase-conjugated
IgG secondary antibodies (1:5,000; cat. no. AA24142; Abcam) at room
temperature for 1 h. Protein bands were visualized using enhanced
chemiluminescence reagent (GE Healthcare). Protein expression
levels were semi-quantified using ImageJ software (version 1.46;
National Institutes of Health) with GAPDH as the loading
control.
Cell transfection
Cells (1x105 cells/well) were seeded into
6-well plates and cultured for 24 h at 37˚C with 5% CO2.
Subsequently, cells were transfected with NODAL overexpression
vector (Ov-NODAL), empty vector (NC; Ov-NC), two different
ALK7-targeting short hairpin (sh)RNA (shRNA-ALK7-1 and
shRNA-ALK7-2) or shRNA-NC at a concentration of 20 nM. All plasmids
were obtained from Shanghai GenePharma Co., Ltd. and transfected
using Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. Cells
in the blank control group (Control) were untreated. At 48 h
post-transfection, transfection efficiency was assessed via
RT-qPCR.
Cell Counting Kit-8 (CCK-8) assay
Cells (1x103 cells/well) were seeded into
96-well plates and incubated at 37˚C with 5% CO2. Cell
proliferation was determined using CCK-8 reagent (Dojindo Molecular
Technologies, Inc.), according to the manufacturer's protocol.
Following transfection for 24, 48 or 72 h, 10 µl CCK-8 solution was
added to each well for 4 h at 37˚C. Absorbance was measured at a
wavelength of 450 nm using a microplate reader. SB431542 was added
and induced for 24 h, followed by cell transfection. CCK-8
experiment was performed again 72 h later.
Wound healing assay
Cells were seeded (1x105 cells/well) into
12-well plates. At 80% confluence, the medium was replaced with
serum-free DMEM and cells were incubated at 37˚C overnight.
Subsequently, a 200-µl pipette tip was used to scratch the cell
monolayer. Following washing with PBS to remove free-floating cells
and debris, the plates were maintained at 37˚C with 5%
CO2. Following incubation for 48 h, the wounds were
observed using a BX51 inverted microscope (Olympus Corporation;
magnification, x100). Cell migration was quantified as follows: (0
h scratch width-scratch width following culturing)/0 h scratch
width.
Cell invasion assay
To assess cell invasion, 24-well Transwell plates
(Corning, Inc.) with 8-µm pore inserts were coated with Matrigel
(BD Biosciences) at 37˚C for 30 min. Cells (5x104
cells/ml) in 200 µl serum-free medi plated into the upper chamber
and 600 µl DMEM supplemented with 10% FBS was added to the lower
chamber. Following incubation for 24 h at 37˚C with 5%
CO2, non-invading cells were removed using a
cotton-tipped swab. Invading cells were fixed with 4% formaldehyde
for 15 min at 25˚C and stained with 0.1% crystal violet solution
for 30 min at room temperature. Invading cells in five randomly
selected fields of view were observed using an inverted microscope
(Olympus Corporation; magnification, x100).
TUNEL assay
Cells were collected and washed three times with
PBS. Following fixing with 4% paraformaldehyde at room temperature
for 20 min, the cells were washed twice with PBS. Then, 0.2% Triton
X-100 was added to the cells at room temperature for 5 min.
Subsequently, 50 µl TUNEL assay solution (Boehringer Mannheim) was
added to the cells and incubated at 37˚C in the dark for 60 min.
The detection solution was discarded and cells were washed three
times with PBS. Subsequently, three fields of view were selected at
random, each with about 300-500 cells and then 300-500 cells were
sealed with anti-fluorescence quenched sealing solution for
observation under a fluorescence microscope (Zeiss GmbH). The
available excitation wavelength range was 450-500 nm and the
emission wavelength range was 515-565 nm (green fluorescence).
Statistical analysis
Data are expressed as the mean ± standard deviation
from ≥3 independent experiments. Statistical analyses were
performed using SPSS statistical software (version 22.0; IBM
Corp.). Comparisons among multiple groups were analyzed using
one-way ANOVA followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
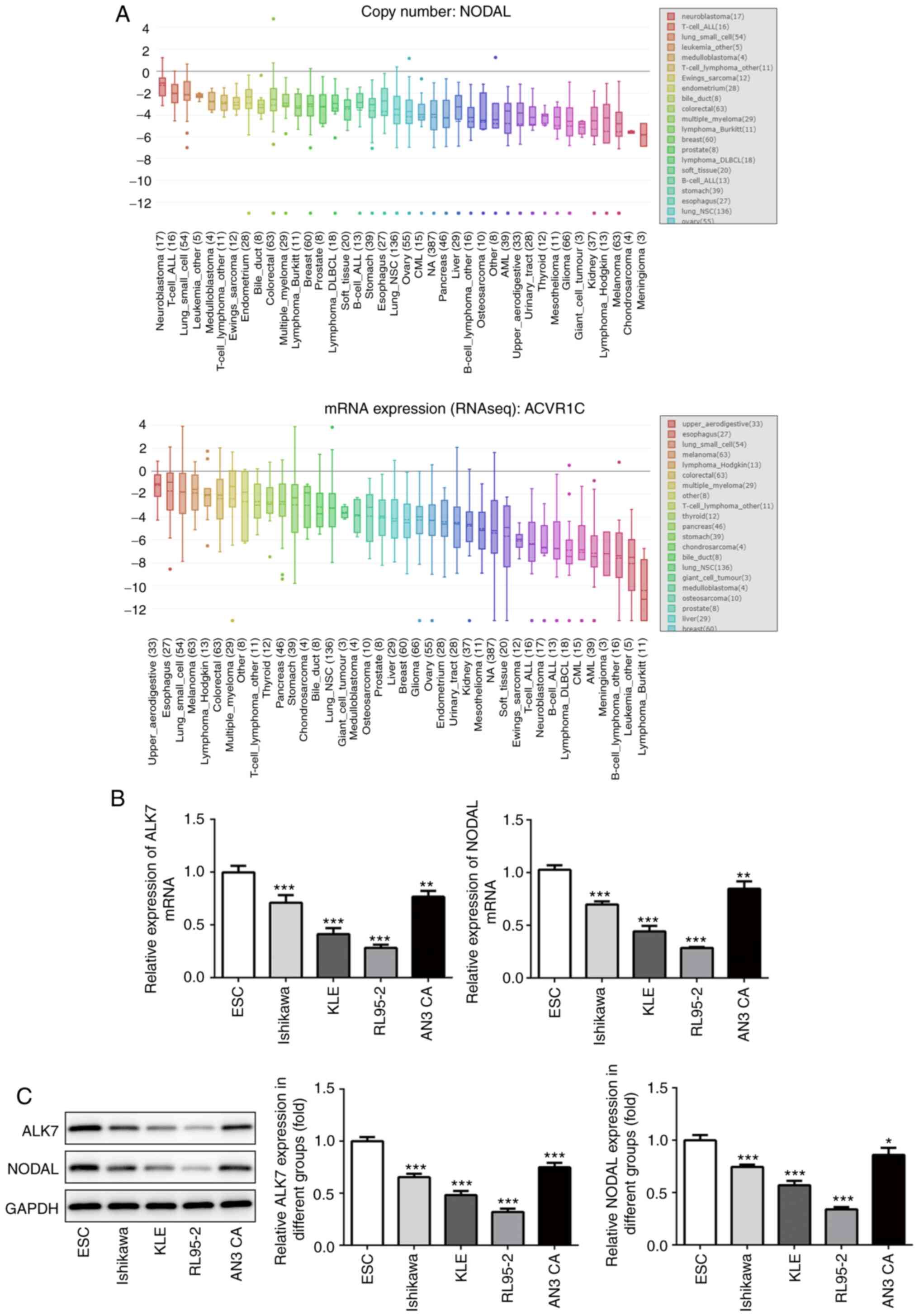
NODAL and ALK7 expression levels are
lower in EC cell lines
The expression levels of NODAL and ALK7 in EC were
predicted using a bioinformatics website (ualcan.path.uab.edu). Compared with normal tissues,
NODAL and ALK7 expression levels were significantly decreased in EC
(Fig. 1A). Subsequently, the
expression levels of NODAL and ALK7 in EC cell lines were detected
via RT-qPCR (Fig. 1B) and western
blotting (Fig. 1C). Compared with
normal endometrial cells, the expression levels of NODAL and ALK7
in EC cell lines were significantly decreased. RL95-2 cells
displayed the lowest expression levels of NODAL and ALK7 among the
EC cell lines. Therefore, RL95-2 cells were selected for subsequent
experiments.
NODAL overexpression inhibits EC cell
proliferation, invasion and migration
To evaluate the specific role of NODAL in EC cells,
NODAL overexpression was performed and confirmed via RT-qPCR
(Fig. 2A) and western blotting
(Fig. 2B). Compared with the Ov-NC
group, NODAL expression levels were significantly increased in the
Ov-NODAL group. Following NODAL overexpression, CCK-8, wound
healing and Transwell assays were performed to assess cell
proliferation, migration and invasion, respectively. Compared with
the Ov-NC group, cell proliferation (Fig. 2C), migration (Fig. 2D and E) and invasion (Fig. 2F and G) were significantly decreased in the
Ov-NODAL group. Furthermore, the expression levels of invasion and
migration-related proteins (MMP2, MMP7 and MMP9) were detected. The
results indicated that MMP2, MMP7 and MMP9 expression levels were
significantly decreased in the Ov-NODAL group compared with the
Ov-NC group (Fig. 2H). The results
indicated that NODAL inhibited EC cell proliferation, invasion and
migration.
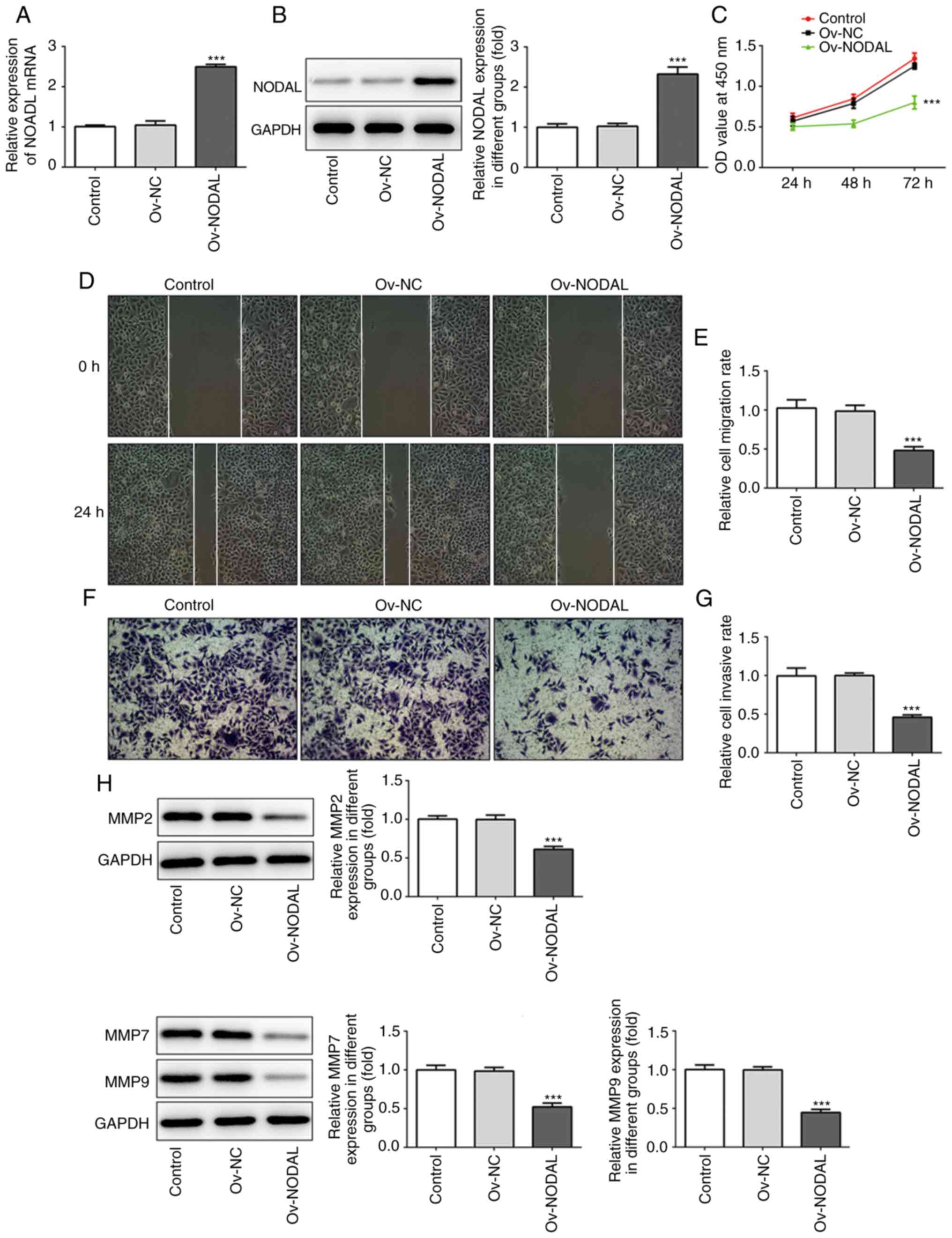 | Figure 2NODAL overexpression inhibits EC cell
proliferation, invasion and migration. NODAL (A) mRNA and (B)
protein expression levels following NODAL overexpression in EC
cells. (C) Cell proliferation was assessed by performing a Cell
Counting Kit-8 assay. (D) Representative images of the wound
healing assay (magnification, x100). (E) Quantification of cell
migration. (F) Representative images of the Transwell assay
(magnification, x100). (G) Quantification of cell invasion. (H)
MMP2, MMP7 and MMP9 protein expression levels were assessed via
western blotting. ***P<0.001 vs. Ov-NC. NODAL, nodal
growth differentiation factor; EC, endometrial cancer; MMP, matrix
metallopeptidase; Ov, overexpression; NC, negative control; OD,
optical density. |
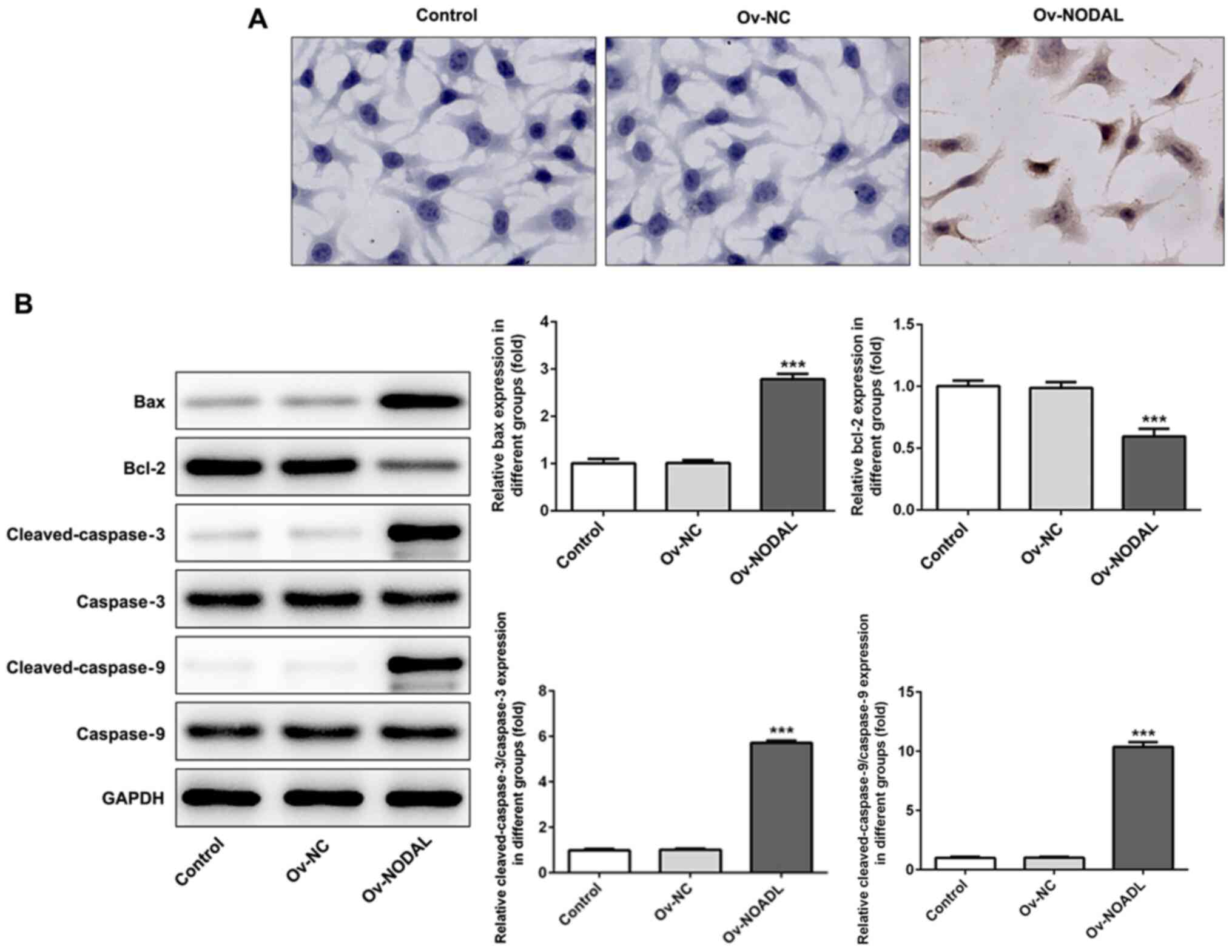
NODAL overexpression promotes EC cell
apoptosis
Following NODAL overexpression, apoptosis was
detected by performing a TUNEL assay. Compared with the Ov-NC
group, cell apoptosis was markedly increased in the Ov-NODAL group
(Fig. 3A). Subsequently, the
expression levels of apoptosis-related proteins were detected via
western blotting. Compared with the Ov-NC group, the expression
levels of the antiapoptotic protein Bcl-2 were significantly
decreased, whereas the expression levels of the proapoptotic
proteins Bax, cleaved-caspase-3 and cleaved-caspase-9 were
significantly increased in the Ov-NODAL group. The results
indicated that NODAL promoted EC cell apoptosis (Fig. 3B).
Inhibiting ALK7 reverses the
inhibitory effect of NODAL overexpression on EC cell proliferation,
invasion and migration
NODAL overexpression significantly increased ALK7
expression levels compared with Ov-NC (Fig. 4A), indicating that ALK7 was
activated. An ALK7-targeting shRNA was constructed and transfected
into EC cells and western blotting was performed to detect ALK7
expression levels (Fig. 4A).
Compared with the shRNA-NC group, the expression of ALK7 was
significantly decreased in the shRNA-ALK7-1 and shRNA-ALK7-2
groups. Moreover, cells were co-transfected with shRNA-ALK7 and
Ov-NODAL. RT-qPCR and western blotting were performed to measure
ALK7 expression levels (Fig. 4B and
C). Compared with the Ov-NODAL-NC
group, the expression of ALK7 was significantly lower in the
Ov-NODAL-shRNA-ALK7-1 and Ov-NODAL-shRNA-ALK7-2 groups.
shRNA-ALK7-1 plasmid was selected for subsequent experiments since
the inhibition of ALK7 with shRNA-ALK7-1 was markedly increased
compared with shRNA-ALK7-2. Subsequently, an ALK7 inhibitor
(SB431542) was used and the cells were divided into the following
six groups: i) Control; ii) Ov-NC; iii) Ov-NODAL; iv) Ov-NODAL-NC;
v) Ov-NODAL + shRNA-ALK7; and vi) Ov-NODAL + SB431542. Following
this, cell proliferation (Fig. 4D),
migration (Fig. 4E and F) and invasion (Fig. 4G and H) were assessed. Compared with the
Ov-NODAL-NC group, the Ov-NODAL + shRNA-ALK7 displayed
significantly increased cell proliferation, migration and invasion,
and significantly upregulated expression levels of MMP2, MMP7 and
MMP9 (Fig. 4I). Furthermore,
compared with the Ov-NODAL group, the Ov-NODAL + SB431542 groups
displayed significantly increased cell proliferation, migration and
invasion, and significantly upregulated expression levels of MMP2,
MMP7 and MMP9. The results suggested that NODAL overexpression
activated ALK7 and inhibited EC cell proliferation, migration and
invasion. Moreover, ALK7 inhibition reversed NODAL
overexpression-mediated inhibition of EC cell proliferation,
migration and invasion, indicating that NODAL inhibited EC cell
proliferation, invasion and migration by activating ALK7.
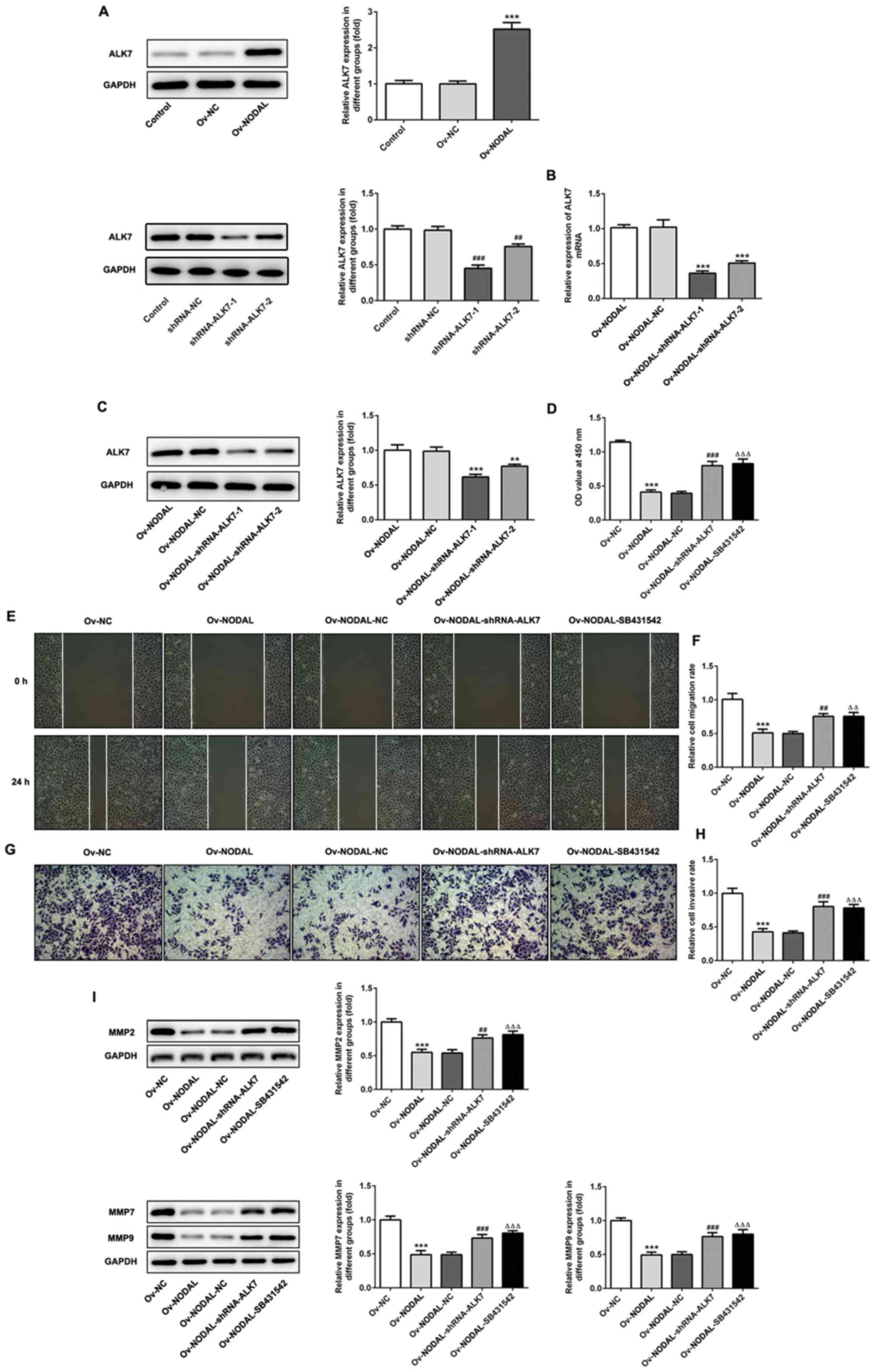 | Figure 4ALK7 inhibition reverses NODAL
overexpression-mediated effects on endometrial cancer cell
proliferation. (A and B) ALK7 protein expression levels in
transfected cells were measured via western blotting.
***P<0.001 vs. Ov-NC; ##P<0.01,
###P<0.001 vs. shRNA-NC. (C) ALK7 expression levels
in co-transfected cells were measured via reverse
transcription-quantitative PCR. **P<0.01 and
***P<0.001 vs. Ov-NODAL-NC. (D) Cell proliferation
was assessed by performing Cell Counting Kit-8 assays. (E)
Representative images of the wound healing assay (magnification,
x100). (F) Quantification of cell migration. (G) Representative
images of the Transwell assay (magnification, x100). (H)
Quantification of cell invasion. (I) MMP2, MMP7 and MMP9 protein
expression levels were measured via western blotting.
***P<0.001 vs. Ov-NC; ##P<0.01 and
###P<0.001 vs. Ov-NODAL-NC; ∆∆P<0.01
and ∆∆∆P<0.001 vs. Ov-NODAL. ALK7, activin A receptor
type 1C; NODAL, nodal growth differentiation factor; Ov,
overexpression; NC, negative control; MMP, matrix metallopeptidase;
shRNA, short hairpin RNA; OD, optical density. |
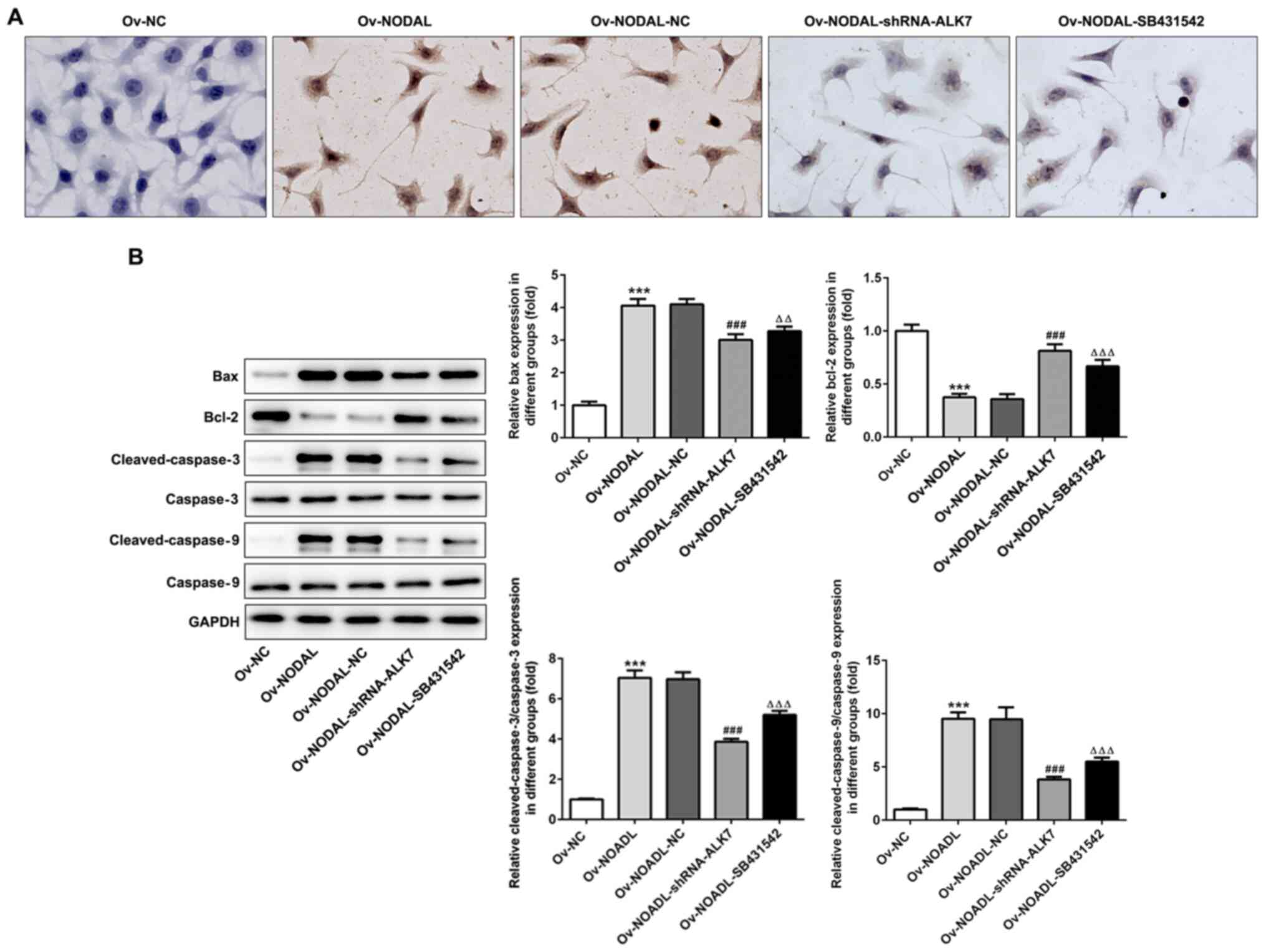
ALK7 inhibition reverses the effect of
NODAL overexpression on EC cell apoptosis
The effect of ALK7 inhibition on cell apoptosis was
assessed. Compared with the Ov-NC group, the rates of apoptosis in
the Ov-NODAL + shRNA-ALK7 and Ov-NODAL + SB431542 groups were
notably increased (Fig. 5A).
Additionally, the expression levels of the apoptosis-related
proteins Bax, cleaved-caspase-3 and cleaved-caspase-9 were
significantly decreased in the Ov-NODAL-shRNA-ALK7 group compared
with the Ov-NODAL-NC group (Fig.
5B). The results suggested that NODAL promoted EC cell
apoptosis by activating ALK7.
Discussion
The role of NODAL in cancer has received increasing
attention (22). NODAL is
abnormally expressed in melanoma, participating in occurrence and
metastasis (6). Later, it was
reported that NODAL is abnormally expressed in glioma (23), pancreatic cancer (11), breast cancer (24) and other tumor cells, where it
participates in the occurrence and development of tumors. Quail
et al (25) demonstrated
that the expression of NODAL is positively correlated with high
vascular densities in breast lesions. Additionally, an in
vitro study identified that NODAL promoted the migration of
endothelial cells and vascular formation in breast cancer (25). The expression of NODAL in liver
cancer tissues is increased and NODAL promotes migration, invasion
and vascular formation in liver cancer cells (26). However, the expression and specific
roles of NODAL in EC are not completely understood. Therefore, the
present study predicted the expression of NODAL in EC using
bioinformatics software, which indicated that NODAL was expressed
at low levels in EC. Additionally, the in vitro cell
experiments indicated that the expression of NODAL in EC cell lines
was significantly decreased compared with normal endometrial cells.
The present study demonstrated that NODAL overexpression inhibited
EC cell proliferation, migration and invasion compared with Ov-NC,
demonstrating that NODAL may inhibit tumorigenesis and development
in EC.
NODAL inhibits cell proliferation and induces
apoptosis in human trophoblast cells, which can be blocked by a
lack of the kinase ALK7. In other words, NODAL inhibits cell
proliferation and induces apoptosis by activating ALK7(27). Li et al (28) reported that in bladder cancer
tissues and cell lines, NODAL knockdown blocked the expression of
ALK7. Additionally, an ALK7 inhibitor reversed the effect of NODAL
overexpression on bladder cancer cell proliferation, invasion and
migration. Furthermore, a previous study indicated that the
overexpression of NODAL and its receptor ALK7 induced ovarian
follicle cell apoptosis, indicating that the expression of NODAL
and ALK7 is crucial in gynecological diseases (29). However, to the best of our
knowledge, the expression of NODAL and ALK7 in EC and whether NODAL
affects EC cell proliferation, invasion, migration and apoptosis
has not been previously reported. The results of the present study
indicated that NODAL overexpression activated ALK7, inhibited cell
proliferation, invasion and migration, and promoted apoptosis in EC
cells compared with Ov-NC. Additionally, ALK7 inhibition reversed
NODAL overexpression-mediated inhibition of EC cell proliferation,
invasion and migration, and promotion of EC cell apoptosis.
Therefore, it was hypothesized that anticancer drugs targeting
NODAL or ALK7 may inhibit tumor cell proliferation, invasion and
migration, and induce apoptosis by activating NODAL and ALK7. The
results of the present study were consistent with the results of a
study conducted by Xu et al (21), which investigated NODAL and ALK7 in
ovarian cancer. The aforementioned study indicated that NODAL
inhibited cell proliferation and promoted cell apoptosis in ovarian
cancer by activating ALK7. This finding provided a potential
explanation for the occurrence of endometriosis near the ovaries of
women with ovarian cancer, indicating an inextricable association
between the two (30).
In the present study, ALK7 knockdown or inhibition
did not completely reverse NODAL overexpression-mediated effects on
EC cells. It was hypothesized that a potential reason was that
NODAL acted on EC cells not only via the ALK7 signaling pathway,
but also via other signaling pathways. For instance, NODAL promoted
renal cell carcinoma cell proliferation by activating the Smad and
ERK1/2 signaling pathways (31).
Additionally, NODAL promotes non-small cell lung cancer cell
malignancy via activation of the NF-κB/IL-6 signaling pathway
(32). Therefore, whether NODAL
acts via other signaling pathways in EC requires further
investigation.
The present study only investigated the effect of
the NODAL/ALK7 signaling pathway on EC cells. Therefore, other
mechanisms underlying NODAL in EC cells should be explored in
future studies. Furthermore, the present study only conducted in
vitro experiments, but in vivo experiments. Therefore,
future studies should investigate the effect of NODAL on EC with
animal models.
In conclusion, the present study indicated that
NODAL inhibited EC cell proliferation, invasion and migration, and
promoted EC cell apoptosis, potentially via activating ALK7.
Therefore, the present study identified potential novel therapeutic
targets for EC.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Guangdong
Medical Science and Technology Research Foundation (grant no.
A2018059).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ, XL and HZ conceptualized and designed the
current study. XH, BW, YL and HW acquired, analyzed and interpreted
data. XH, HZ, YL and HW drafted the manuscript and revised it
critically for important intellectual content. All authors agreed
to be held accountable for the current study in ensuring questions
related to the integrity of any part of the work are appropriately
investigated and resolved. All authors read approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhong Y, Wang Y, Dang H and Wu X: LncRNA
AFAP1-AS1 contributes to the progression of endometrial carcinoma
by regulating miR-545-3p/VEGFA pathway. Mol Cell Probes.
53(101606)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Moore K and Brewer MA: Endometrial cancer:
Is this a new disease? Am Soc Clin Oncol Educ Book. 37:435–442.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lee YC, Lheureux S and Oza AM: Treatment
strategies for endometrial cancer: Current practice and
perspective. Curr Opin Obstet Gynecol. 29:47–58. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zinski J, Tajer B and Mullins MC: TGF-beta
family signaling in early vertebrate development. Cold Spring Harb
Perspect Biol. 10(a033274)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Martyn I, Kanno TY, Ruzo A, Siggia ED and
Brivanlou AH: Self-organization of a human organizer by combined
Wnt and Nodal signalling. Nature. 558:132–135. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Topczewska JM, Postovit LM, Margaryan NV,
Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J and Hendrix
MJ: Embryonic and tumorigenic pathways converge via Nodal
signaling: Role in melanoma aggressiveness. Nat Med. 12:925–932.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Gong W, Sun B, Zhao X, Zhang D, Sun J, Liu
T, Gu Q, Dong X, Liu F, Wang Y, et al: Nodal signaling promotes
vasculogenic mimicry formation in breast cancer via the Smad2/3
pathway. Oncotarget. 7:70152–70167. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Qi YF, Wu L, Li ZQ, Wu ML, Wang HF, Chan
KY, Lu LL, Cai SH, Wang HS and Du J: Nodal signaling modulates the
expression of Oct-4 via nuclear translocation of β-catenin in lung
and prostate cancer cells. Arch Biochem Biophys. 608:34–41.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kong B, Wang W, Esposito I, Friess H,
Michalski CW and Kleeff J: Increased expression of Nodal correlates
with reduced patient survival in pancreatic cancer. Pancreatology.
15:156–161. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sun C, Sun L, Jiang K, Gao DM, Kang XN,
Wang C, Zhang S, Huang S, Qin X, Li Y and Liu YK: NANOG promotes
liver cancer cell invasion by inducing epithelial-mesenchymal
transition through NODAL/SMAD3 signaling pathway. Int J Biochem
Cell Biol. 45:1099–1108. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Perkhofer L, Walter K, Costa IG, Carrasco
MC, Eiseler T, Hafner S, Genze F, Zenke M, Bergmann W, Illing A, et
al: Tbx3 fosters pancreatic cancer growth by increased angiogenesis
and activin/nodal-dependent induction of stemness. Stem Cell Res.
17:367–378. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bodenstine TM, Chandler GS, Reed DW,
Margaryan NV, Gilgur A, Atkinson J, Ahmed N, Hyser M, Seftor EA,
Strizzi L and Hendrix MJ: Nodal expression in triple-negative
breast cancer: Cellular effects of its inhibition following
doxorubicin treatment. Cell Cycle. 15:1295–1302. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lawrence MG, Margaryan NV, Loessner D,
Collins A, Kerr KM, Turner M, Seftor EA, Stephens CR, Lai J, APC
BioResource, et al: Reactivation of embryonic nodal signaling is
associated with tumor progression and promotes the growth of
prostate cancer cells. Prostate. 71:1198–1209. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wakefield LM and Hill CS: Beyond TGFβ:
Roles of other TGFβ superfamily members in cancer. Nat Rev Cancer.
13:328–341. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Inman GJ, Nicolás FJ, Callahan JF, Harling
JD, Gaster LM, Reith AD, Laping NJ and Hill CS: SB-431542 is a
potent and specific inhibitor of transforming growth factor-beta
superfamily type I activin receptor-like kinase (ALK) receptors
ALK4, ALK5, and ALK7. Mol Pharmacol. 62:65–74. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Roberts HJ, Hu S, Qiu Q, Leung PC,
Caniggia I, Gruslin A, Tsang B and Peng C: Identification of novel
isoforms of activin receptor-like kinase 7 (ALK7) generated by
alternative splicing and expression of ALK7 and its ligand, Nodal,
in human placenta. Biol Reprod. 68:1719–1726. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hu T, Su F, Jiang W and Dart DA:
Overexpression of activin receptor-like kinase 7 in breast cancer
cells is associated with decreased cell growth and adhesion.
Anticancer Res. 37:3441–3451. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu C, Yang Z, Li D, Liu Z, Miao X, Yang
L, Zou Q and Yuan Y: Overexpression of B2M and loss of ALK7
expression are associated with invasion, metastasis, and
poor-prognosis of the pancreatic ductal adenocarcinoma. Cancer
Biomark. 15:735–743. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Michael IP, Saghafinia S, Tichet M,
Zangger N, Marinoni I, Perren A and Hanahan D: ALK7 signaling
manifests a homeostatic tissue barrier that is abrogated during
tumorigenesis and metastasis. Dev Cell. 49:409–424.e6.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhong Y, Xu G, Ye G, Lee D, Modica-Amore J
and Peng C: Nodal and activin receptor-like kinase 7 induce
apoptosis in human breast cancer cell lines: Role of caspase 3. Int
J Physiol Pathophysiol Pharmacol. 1:83–96. 2009.PubMed/NCBI
|
|
21
|
Xu G, Zhong Y, Munir S, Yang BB, Tsang BK
and Peng C: Nodal induces apoptosis and inhibits proliferation in
human epithelial ovarian cancer cells via activin receptor-like
kinase 7. J Clin Endocrinol Metab. 89:5523–5534. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Strizzi L, Hardy KM, Kirschmann DA,
Ahrlund-Richter L and Hendrix MJ: Nodal expression and detection in
cancer: Experience and challenges. Cancer Res. 72:1915–1920.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Venuto S, Castellana S, Monti M, Appolloni
I, Fusilli C, Fusco C, Pucci P, Malatesta P, Mazza T, Merla G and
Micale L: TRIM8-driven transcriptomic profile of neural stem cells
identified glioma-related nodal genes and pathways. Biochim Biophys
Acta Gen Subj. 1863:491–501. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kantor O, Wong S, Weiss A, Metzger O,
Mittendorf EA and King TA: Prognostic significance of residual
nodal disease after neoadjuvant endocrine therapy for hormone
receptor-positive breast cancer. NPJ Breast Cancer.
6(35)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Quail DF, Walsh LA, Zhang G, Findlay SD,
Moreno J, Fung L, Ablack A, Lewis JD, Done SJ, Hess DA and Postovit
LM: Embryonic protein nodal promotes breast cancer vascularization.
Cancer Res. 72:3851–3863. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen J, Liu WB, Jia WD, Xu GL, Ma JL, Ren
Y, Chen H, Sun SN, Huang M and Li JS: Embryonic morphogen nodal is
associated with progression and poor prognosis of hepatocellular
carcinoma. PLoS One. 9(e85840)2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Munir S, Xu G, Wu Y, Yang B, Lala PK and
Peng C: Nodal and ALK7 inhibit proliferation and induce apoptosis
in human trophoblast cells. J Biol Chem. 279:31277–31286.
2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li Y, Zhong W, Zhu M, Hu S and Su X: Nodal
regulates bladder cancer cell migration and invasion via the
ALK/Smad signaling pathway. Onco Targets Ther. 11:6589–6597.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang H, Jiang JY, Zhu C, Peng C and Tsang
BK: Role and regulation of nodal/activin receptor-like kinase 7
signaling pathway in the control of ovarian follicular atresia. Mol
Endocrinol. 20:2469–2482. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ruderman R and Pavone ME: Ovarian cancer
in endometriosis: An update on the clinical and molecular aspects.
Minerva Ginecol. 69:286–294. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang Z, Jiang T, Li Q, Wang J, Yang D, Li
X, Wang Q and Song X: Nodal activates smad and extracellular
signal-regulated kinases 1/2 pathways promoting renal cell
carcinoma proliferation. Mol Med Rep. 12:587–594. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xu X, Zhou X, Gao C, Cao L, Zhang Y, Hu X
and Cui Y: Nodal promotes the malignancy of non-small cell lung
cancer (NSCLC) cells via activation of NF-κB/IL-6 signals. Biol
Chem. 400:777–785. 2019.PubMed/NCBI View Article : Google Scholar
|