Introduction
Rheumatic heart disease frequently causes mitral
stenosis and/or aortic regurgitation, which poses a serious threat
to life. Heart valve replacement surgery under cardiopulmonary
bypass (CPB) is a common form of surgical treatment for patients
with rheumatic heart disease. However, the pathophysiological
process of myocardial ischemia-reperfusion injury (MIRI) is
inevitable during general anesthesia for CPB (1). MIRI is a complication of CPB and may
worsen the prognosis of patients undergoing this surgical
treatment. Surgeons should therefore reduce the risk of MIRI during
cardiac surgery with CPB. Optimizing perioperative medications may
represent an important strategy to decrease the risk of MIRI.
Previous studies have reported that ischemia
preconditioning (IPC) is able to mitigate MIRI and improve cardiac
function. The role of IPC in myocardial protection was first
identified by Murry et al (2) using animal models and it was defined
as one or more transient ischemic and reperfusion stimulations
prior to ischemia. IPC is now considered to be the most effective
endogenous protective measure against MIRI (3). However, the clinical application of
IPC is limited due to the fact that there are no strict guidelines
for the timing and duration of ischemia and the invasive nature of
this complex operation. In 1997, Cope et al (4) investigated the effectiveness of drug
pretreatment, which involves simulating endogenous protective
factors in order to induce myocardial protection mimicking IPC.
Therefore, the use of drug pretreatment to reduce MIRI has become a
popular research topic.
Dexmedetomidine (Dex) is a highly selective
α2-adrenoceptor agonist, the effects of which are
characterized by sedation, analgesic and anxiolytic effects, as
well as inhibition of the release of serum catecholamines (5). Dex is widely used in clinical
scenarios and recent studies have indicated that the use of Dex is
advantageous in reducing anesthetic requirements and enhancing
hemodynamic stability following cardiac surgery (6). Dex pre-conditioning and Dex perfusion
during the whole surgery have been examined in a variety of animal
models of ischemia-reperfusion and have been demonstrated to
protect multiple organs and provide a protective effect against
MIRI (7-9).
Furthermore, clinical study have suggested that Dex perfusion
during the whole cardiac surgery under CPB reduces the risk of
postoperative MIRI (10). However,
few studies have investigated whether Dex pre-conditioning can
reduce MIRI. Therefore, it has remained elusive whether Dex
pre-conditioning is able to attenuate MIRI in patients undergoing
rheumatic heart valve replacement surgery under CPB.
In the present study, a prospective trial was
designed to investigate whether Dex pre-conditioning and Dex
perfusion exert myocardial protective effects. The effects of these
methods were investigated by assessing myocardial injury markers,
inflammatory factors, oxidative stress and stress response in order
to determine the best method of Dex administration.
Materials and methods
Study population
The present clinical study was a randomized
double-blinded placebo-controlled trial that was approved by the
Ethics Committee of the Affiliated Hospital of Zunyi Medical
College (Zunyi, China; approval no. 8). All patients included in
the present study or their relatives provided written informed
consent prior to the onset of the study. In total, 105 patients
were treated at the Affiliated Hospital of Zunyi Medical College
(Zunyi, China) between May 2016 and August 2017. All patients were
aged 18-65 years, underwent cardiac valve replacement surgery under
CPB and were classified based on the American Society of
Anesthesiologists (ASA) classification II or III and New York Heart
Association (NYHA) classification II or III (11,12),
and with an estimated operation time of ≥2 or ≤6 h. The exclusion
criteria included patients with myocardial infarction occurring in
the previous month, history of heart surgery, rheumatic activity
prior to surgery, heparin resistance, history of drug allergy,
preoperative severe anemia, termination of anticoagulant therapy
<3 days prior to surgery, abnormal coagulation function, severe
liver and kidney damage, preoperative inflammatory disease,
systemic disease with increased catecholamine secretion, relapse
issues (defibrillation >4 times), difficulty in stopping CPB,
refusal to participate, lack of signed informed consent, withdrawal
during the trial or missing data.
Patient grouping
The eligible patients were numbered sequentially
according to the daily surgical schedule of the Affiliated Hospital
of Zunyi Medical College (Zunyi, China) and were then randomly
divided into the three study groups. Subsequently, a nurse prepared
the appropriate drug and syringe, and they did not know the
corresponding code. The patients and anesthesiologist were blinded
to the contents of the syringe. The sample size was estimated using
a statistical formula for estimating sample size for a complete
randomized design with multiple sample means. The mean and standard
deviation (SD) of each indicator [heart rate (HR), mean artery
pressure (MAP), cardiac troponin I (cTnI), interleukin (IL)-8,
IL-10, malondialdehyde (MDA) and blood glucose] in the three groups
were used in the statistical formula to obtain an estimated
required sample size of ≥27 cases per group. In total, 90 patients
were included in the trial (30 per group). These patients were
blinded to their group identity. In the operating room, patients
were anesthetized by the same anesthesiologist who was blinded to
the patient group.
In the Dex group, patients received Dex
intravenously at 1.0 µg/kg 10 min prior to the induction of
anesthesia and then 0.5 µg/kg/h Dex during the entire operation. In
the Dex pre-conditioning group, patients received Dex intravenously
at 1.0 µg/kg until 10 min prior to the induction of anesthesia and
then 0.9% normal saline (at the same dose as the maintenance dose
of Dex in the Dex group) during the operation. In the control
group, patients were injected with 0.9% normal saline at the same
doses and time-points as Dex in the Dex group (prior to the
induction of anesthesia and during the operation).
Surgery
Patients who met the inclusion criteria were
routinely monitored in terms of electrocardiography (ECG), pulse
oxygen saturation and non-invasive blood pressure after entering
the operating room. In addition, the patients were monitored using
a bispectral index (BIS) monitor to assess the depth of anesthesia.
MAP was monitored by cannulation of the left radial artery under
local anesthesia. A double-lumen central venous catheter was
inserted to monitor the central venous pressure. In addition,
patients were monitored for nasopharyngeal and rectal temperature
and by arterial blood gas analysis, which was also used to
determine the level of blood glucose.
Anesthesia was induced with 0.01 mg/kg penehyclidine
hydrochloride, 0.1 mg/kg midazolam, 1.0 µg/kg sufentanil, 0.15-0.3
mg/kg etomidate and 1 mg/kg rocuronium. Drug use was reduced as
appropriate in patients with severe conditions (such as ASA III and
NYHA III), and tracheal intubation was performed when BIS ranged
between 40 and 50. After successful tracheal intubation,
volume-controlled mechanical ventilation was performed and the
tidal volume was adjusted to 8-10 ml/kg, the
inspiratory-to-respiratory ratio was maintained at 1:1.5-2 and the
respiratory rate was maintained at 12-20 breaths/min to ensure an
end-tidal carbon dioxide partial pressure of 35-45 mmHg.
Subsequently, 1-2 µg/kg/h sufentanil, 4-6 mg/kg/h propofol and
intermittent intravenous injections of rocuronium and midazolam,
along with compound inhalation of isoflurane (if necessary), were
used to maintain the appropriate depth of anesthesia. The target
range of BIS was 40-50 and the fluctuation of blood pressure was
maintained at <20%.
Furthermore, the induction and maintenance of
anesthesia in the three groups were using the same protocols in
order to limit variability among patients. All operative procedures
were performed by the same surgical team, using a CPB machine
(Jostra; Sorin Group Co., Ltd.), an extracorporeal membrane
oxygenator (CAPIOX RXO5, Terumo Co., Ltd.), a blood ultrafilter
(SORIN DHF0.2; Sorin Group Co., Ltd.), the domestic Ningbo Filar
corresponding type of arterial microthrombus filter (Ningbo Filar
Medical Products Co., Ltd.) and the corresponding type of
non-heparin coated circulation pipeline (Ningbo Filar Medical
Products Co., Ltd.). Surgery was performed under standard
hypothermic CPB (28-30˚C), with cannulation of the superior vena
cava, inferior vena cava and ascending aorta. During CPB, the
following parameters were maintained: Activated clotting time of
>480 sec, hematocrit of 25-28%, MAP of 50-80 mmHg, hemoglobin of
>80 g/l, pH of 7.35-7.45 and partial pressure of CO2
of 35-45 mmHg. Adrenaline, dopamine and nitroglycerin were
intravenously pumped to maintain hemodynamic stability after
stopping the CPB.
Measurement of hemodynamic and blood
parameters
HR and MAP were assessed in the three groups of
patients based on ECG and invasive artery blood pressure at eight
time-points: i) T1, pre-medication; ii) T2, 10 min post-medication;
iii) T3, immediately post-intubation; iv) T4, upon skin incision;
v) T5, upon sawing the sternum; vi) T6, immediately post-CPB; vii)
T7, immediately post-operation; and viii) T8, 24 h post-operation.
Blood samples (5 ml) were obtained from the radial artery at four
time-points (T1, T6, T7 and T8), centrifuged and then stored in a
refrigerator at 4˚C. After centrifugation and storage at -80˚C, the
supernatant was collected to determine the serum levels of cTnI,
IL-8, IL-10 and MDA. The concentrations of serum cTnI, IL-8 and
IL-10 were assayed by ELISA using the cTnI, IL-8 and IL-10
detection kits (Beijing Biosic Biomedical Technology Co., Ltd.)
according to the manufacturer's protocols. The serum level of MDA
was measured using the thiobarbituric acid assay with the MDA
determination kit (Beijing Solarbio Technology Co., Ltd.),
according to the manufacturer's protocol. The concentration of
blood glucose was detected by arterial blood gas analysis at four
time-points: T1, T5, T6 and T7 (Fig.
1). All measurements were performed in duplicate.
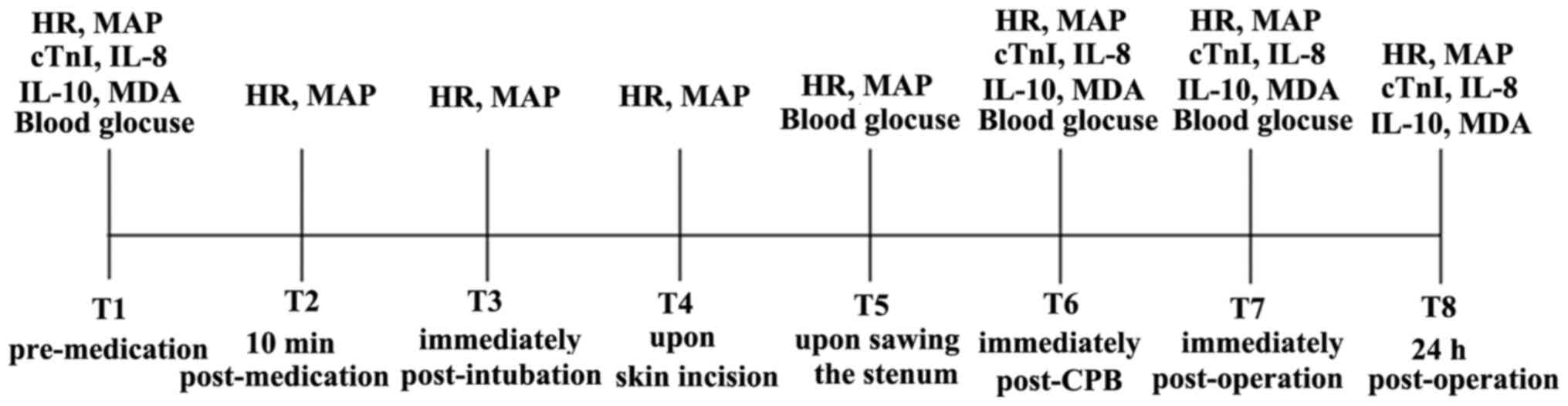 | Figure 1Timeline sketch indicating time-points
and indicators detected. The time-points were as follows: i) T1,
pre-medication; ii) T2, 10 min post-medication; iii) T3,
immediately post-intubation; iv) T4, upon skin incision; v) T5,
upon sawing the sternum; vi) T6, immediately post-CPB; vii) T7,
immediately post-operation; and viii) T8, 24 h post-operation. HR,
heart rate; MAP, mean artery pressure; cTnI, cardiac troponin I;
IL, interleukin; MDA, malondialdehyde; CPB, cardiopulmonary
bypass. |
Statistical analysis
Data analysis and statistical analyses were
performed using SPSS software version 18.0 (SPSS, Inc.). Data with
a normal distribution are presented as the mean ± SD. Mixed ANOVA
was used to assess the main effect of treatment, main effect of
time point and the interaction between these two main effects.
Afterwards, Bonferroni's correction is used for post hoc
inspection. The significance of differences among the three groups
was determined by one-way analysis of variance (ANOVA), when
obtaining a significant result, Bonferroni's correction is used for
post hoc inspection between the two groups. All tests were
two-tailed. Differences in categorical variables were assessed
using the χ2 or Fisher's exact test. All tests were
two-tailed. P<0.05 was considered to indicate statistical
significance.
Results
General information of the patients
and surgical conditions
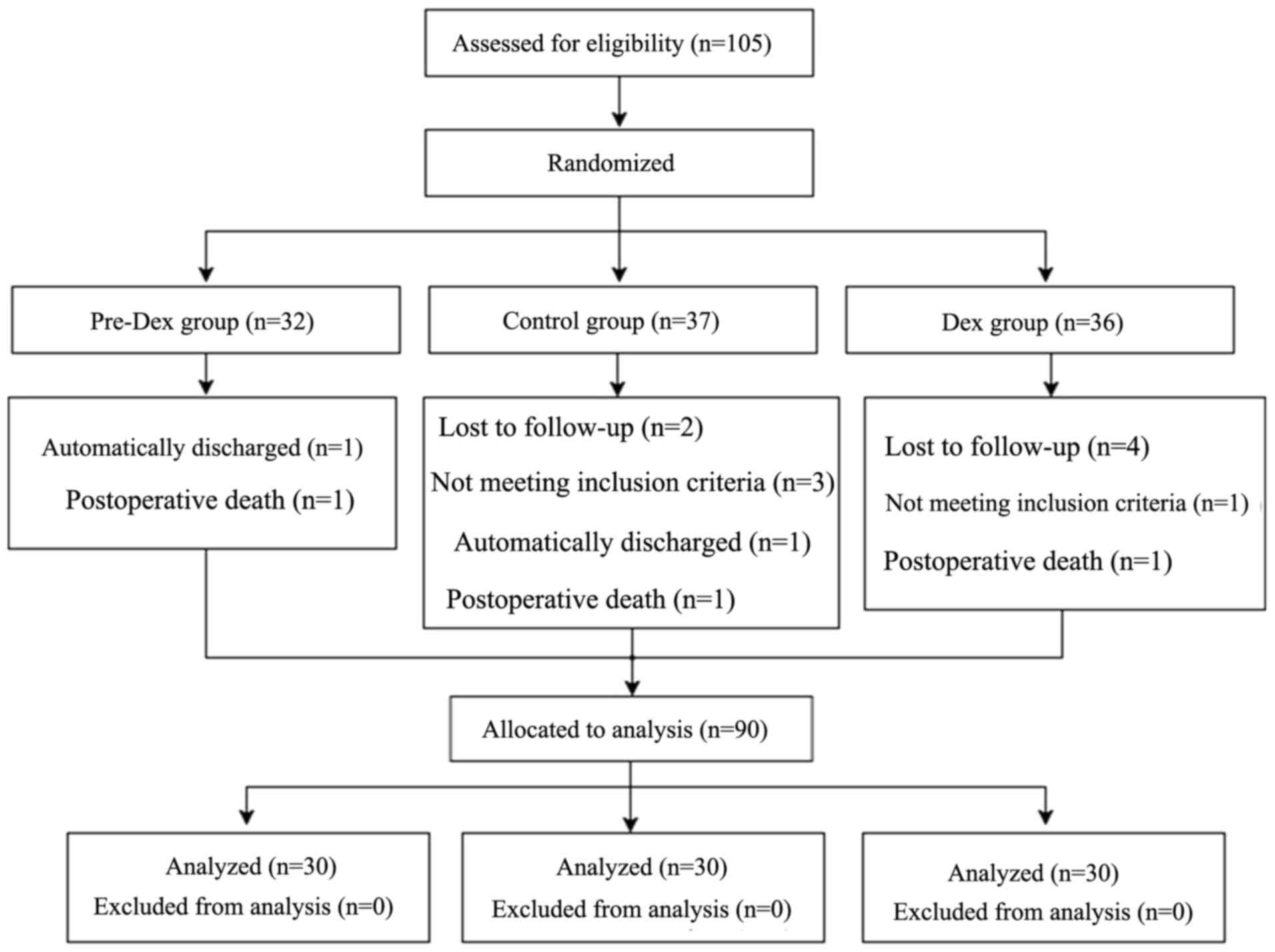
A total of 105 patients were randomly allocated to
the Control group (n=37), Pre-Dex group (n=32) and Dex group
(n=36). After randomization, four patients (three in the Control
group and one in the Dex group) did not meet the inclusion
criteria, since three patients in the Control group had an
operation time of >6 h and one patient in the Dex group had an
operation time of <2 h. Therefore, these three patients were
excluded. In addition, six patients were not followed up due to
their families refused to participate after the operation and were
therefore excluded due to incomplete data. Furthermore, three
patients could not be followed up due to death of heart failure
after operation, so they were excluded from this study. Mortality
of each group [pre-Dex group, 1/32 (3.1%), Control group, 1/37
(2.7%) and Dex group 1/36 (2.8%)]. Of note, the cause of death was
mostly due to heart failure and were not related to Dex treatment.
In addition, two patients were discharged from hospital due to
serious illness and poor prognosis and were therefore excluded.
Finally, 90 cases were included in the present analysis and 30
patients were allocated in each of the three groups. All patients
were surgically treated and none of the patients withdrew from the
study (Fig. 2). As presented in
Table I, the mean age of the
patients in the Pre-Dex, Dex and Control groups was 50.01±9.97,
47.24±7.93 and 51.39±6.78 years, respectively. Male patients
accounted for 87.5% of the Dex group and 76.5% of both the Pre-Dex
and Control groups (P>0.05). The baseline demographic
characteristics were similar among the three groups (P>0.05).
There were no significant differences in ascending aortic
cross-clamping duration, CPB duration, incidence of ventricular
fibrillation after reperfusion, cardiac self-rejuvenation rate or
operation duration across the three groups (P>0.05; Table I).
 | Table IBaseline demographic characteristics,
aortic cross-clamping duration, CPB duration, VF after reperfusion,
self-rejuvenation rate and operation duration in the different
groups. |
Table I
Baseline demographic characteristics,
aortic cross-clamping duration, CPB duration, VF after reperfusion,
self-rejuvenation rate and operation duration in the different
groups.
| Variables | Control group | Dex group | Pre-Dex group | P-value |
|---|
| Age (years) | 51.39±6.78 | 47.24±7.93 | 50.01±9.97 | 0.151 |
| Sex (M/F) | 13(43)/17(57) | 14(47)/16(53) | 13(43)/17(57) | 1.000 |
| Bodyweight
(kg) | 55.51±8.43 | 53.79±8.92 | 57.81±10.06 | 0.239 |
| NYHA (II/III) | 11(37)/19(63) | 14(47)/16(53) | 12(40)/18(60) | 1.000 |
| LVEF (%) | 57.87±7.14 | 58.60±5.54 | 61.33±6.58 | 0.097 |
| Aortic cross-clamp
duration (min) | 71.58±17.25 | 68.52±20.65 | 71.77±19.07 | 0.760 |
| CPB duration
(min) | 106.90±20.58 | 99.70±20.70 | 105.80±19.27 | 0.335 |
| VF after
reperfusion | 5(17) | 5(17) | 6(20) | 1.000 |
| Self-rejuvenation
rate | 25(83) | 25(83) | 24(80) | 1.000 |
| Operation duration
(min) | 249.60±42.39 | 254.07±50.78 | 254.87±44.00 | 0.892 |
Dex perfusion and Dex pre-conditioning
improve heart function after CPB
The HR was significantly reduced in the Pre-Dex and
Dex groups at T2, T3, T4 and T5 compared with that of the Control
group (P<0.00625), demonstrating the positive effects of DEX on
myocardial oxygen consumption. However, the HR was not
statistically significant among the three groups after CPB (T6, T7
and T8), suggesting that Dex had no influence on cardiac
rejuvenation and HR after CPB (Fig.
3A). The HR was increased in the Pre-Dex and Dex groups after
CPB (T6, T7 and T8) compared with that prior to CPB (T2-T5;
P<0.00625). Furthermore, the HR was increased in control group
after CPB (T6, T7) compared with that prior to CPB (T2;
P<0.00625).
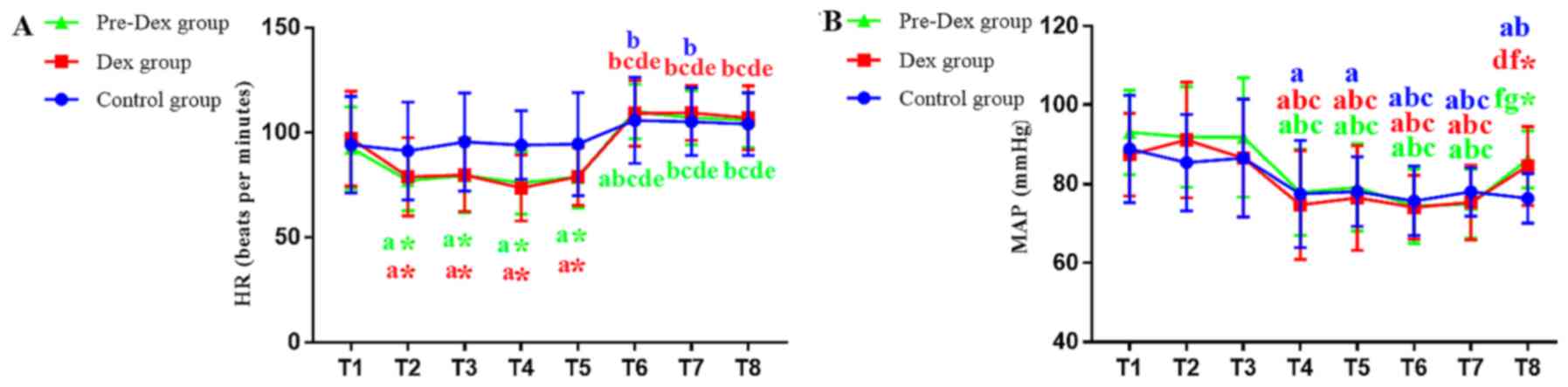 | Figure 3(A and B) Changes in HR and MAP in the
three groups are shown respectively. HR and MAP in the three groups
were recorded at eight time-points: i) T1, pre-medication; ii) T2,
10 min post-medication; iii) T3, immediately post-intubation; iv)
T4, upon skin incision; v) T5, upon sawing the sternum; vi) T6,
immediately post-cardiopulmonary bypass; vii) T7, immediately
post-operation; and viii) T8, 24 h post-operation. Values are
expressed as the mean ± standard deviation. aP<0.05
vs. T1, bP<0.05 vs. T2, cP<0.05 vs. T3,
dP<0.05 vs. T4, eP<0.05 vs. T5,
fP<0.05 vs. T6, gP<0.05 vs. T7.
*P<0.05 vs. Control group at the same time point.
Bonferroni's correction. HR, heart rate; MAP, mean artery pressure;
Pre-Dex group, Dex pre-conditioning group; Dex,
dexmedetomidine. |
The MAP was reduced in three groups at T4, T5, T6
and T7 compared with T1 (P<0.00625), and there was no difference
among the three groups. The MAP was reduced in the Control group at
T8 compared with T1 (P<0.00625), while there were no differences
in the Pre-Dex and Dex groups (P>0.05). Compared with the
Control group, the MAP in Pre-Dex and Dex groups was increased at
T8 (P<0.00625), suggesting that DEX treatment improved the MAP,
thus enhancing cardiac perfusion after surgery, and had no
influence on MAP during surgery (Fig.
3B).
Dex perfusion and Dex pre-conditioning
decrease the levels of myocardial injury-associated markers
In comparison with T1, the cTnI levels were
significantly increased at T6, T7 and T8 in three groups,
indicating that CPB induced cardiac damage (P<0.0125). In
comparison with the Control group, cTnI levels were decreased at
T6, T7 and T8 in the Pre-Dex and Dex groups (P<0.0125). Compared
with the Pre-Dex group, the cTnI levels were decreased in the Dex
group at T7 (P<0.0125). It was indicated that Pre-Dex and Dex
treatments are able to prevent myocardial injury. In addition, the
present results suggested that Dex treatment was more effective
than Pre-Dex treatment (Fig.
4A).
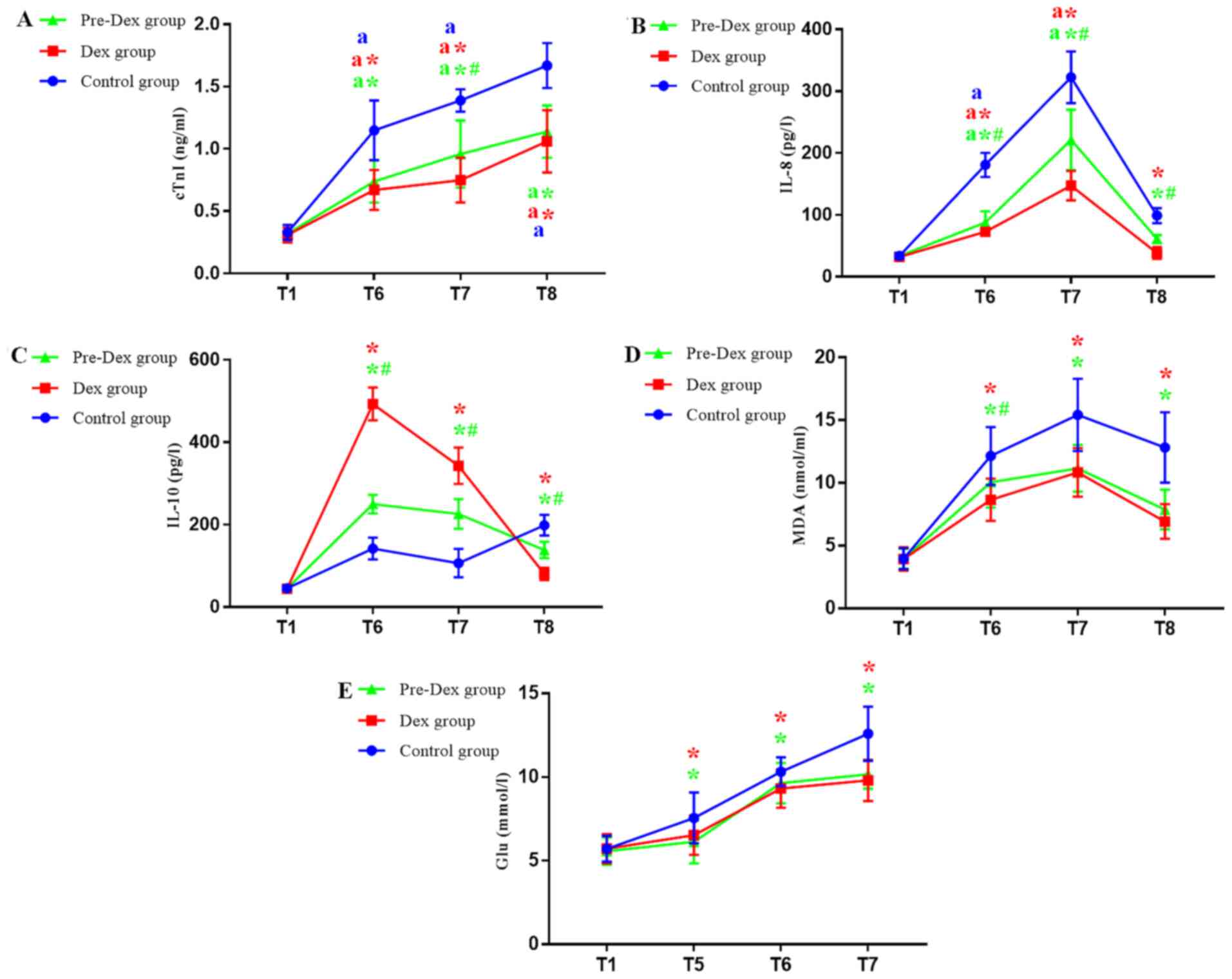 | Figure 4(A-E) Changes in the levels of cTnI,
IL-8, IL-10, MDA and blood glucose in the three study groups are
shown respectively. Levels of cTnI, IL-8, IL-10 and MDA in the
three groups were measured at eight time-points: i) T1,
pre-medication i.e. the time point before dexmedetomidine was used;
ii) T2, 10 min post-medication; iii) T3, immediately
post-intubation; iv) T4, upon skin incision; v) T5, upon sawing the
sternum; vi) T6, immediately post-cardiopulmonary bypass; and vii)
T7, immediately post-operation. Each reaction was performed three
times for each patient at each time point. Values are expressed as
the mean ± standard deviation. #P<0.05 vs. Dex group
at the same time-point; aP<0.05 T5-T8 vs. T1.
*P<0.05 vs. the control group at the same time-point.
Bonferroni's correction. cTnI, cardiac troponin I; IL, interleukin;
MDA, malondialdehyde; Glu, blood glucose; Pre-Dex group, Dex
pre-conditioning group; Dex, dexmedetomidine. |
Dex perfusion and Dex pre-conditioning
regulates the levels of inflammatory factors
IL-8 gradually increased during the surgical
procedure, reached a maximum at T7 and decreased at T8. During the
whole procedure, in the three groups, IL-8 levels increased
significantly at T6 and T7 time points compared to T1. In the
control group, the level of IL-8 was significantly higher compared
with that in the Pre-Dex and Dex groups at the same time point. and
the IL-8 levels in the Dex group were decreased compared with those
in the Pre-Dex group (P<0.0125). IL-8 returned to baseline
levels at 24 h post-operation, suggesting that CPB rheumatic heart
valve replacement surgery stimulated the inflammatory response.
However, Pre-Dex and Dex treatment downregulated the inflammatory
response and Dex treatment significantly decreased the expression
levels of the inflammatory marker IL-8.
IL-10 was increased at T6 in the three groups. A
significant increase was identified in groups subjected to Dex
treatment compared with the Control group (P<0.0125), suggesting
that, after CPB, Pre-Dex and Dex treatment increased the release of
anti-inflammatory factors and Dex treatment was more effective than
Pre-Dex treatment. Compared with T6, the IL-10 levels were
decreased in the three groups at the subsequent time-points, but
the Pre-Dex and Dex groups presented with lower levels of IL-10
compared with those in the Control group (P<0.0125). At T8, the
levels of IL-10 reached a maximum in the Control group and its
levels were decreased in the Pre-Dex and Dex groups (P<0.0125;
Fig. 4B and C).
Dex perfusion and Dex pre-conditioning
suppress oxidative stress and the stress response
MDA, an indicator of oxidative stress, in the three
groups, it increased from T1 to T7 and reached a maximum at the T7
time point before slightly decreasing at T8. In comparison with the
Control group, the groups subjected to Dex treatment exhibited
reduced MDA levels at T6, T7 and T8. Treatment by Dex perfusion
during the whole surgery led to a more significant reduction
compared with that in the Pre-Dex group at T6 (P<0.0125;
Fig. 4D).
The glucose levels, an indicator of the stress
response, increased during the whole procedure and reached a
maximum at 24 h after surgery in all three groups. Compared with
those in the Control group, the glucose levels in Pre-Dex and Dex
groups were decreased (P<0.0125). The glucose levels were
similar between the Pre-Dex and Dex groups (Fig. 4E).
Discussion
MIRI is a common pathophysiological process that
occurs after cardiac surgery under CPB and involves multiple
mechanisms, including cardiomyocyte apoptosis, inflammatory
reactions, oxidative stress and stress responses (13-15).
The pipeline for extracorporeal circulation is responsible for the
process of cooling and rewarming, which causes cytokine release and
promotes the occurrence and development of inflammatory and stress
responses (16). Furthermore, when
the blocked ascending aorta is re-opened, recanalization may
promote additional production of inflammatory chemokines due to
oxidative stress. The release of cytokines eventually causes damage
to tissues and organs (17,18). For patients with rheumatic heart
disease, cardiac reserve function is reduced and hemodynamic
fluctuations tend to occur during the perioperative period
(19). Therefore, reducing the
stress response and inflammation, and stabilizing the hemodynamics,
may reduce the detrimental effects induced by MIRI.
It has been previously reported that Dex is able to
inhibit the sympathetic activity of the nervous system and relieve
oxidative stress reactions, inhibiting inflammation and stress
response (20-22).
This effect reduces the severe hemodynamic fluctuations during
surgery and hemodynamic stability has a positive effect on the
postoperative outcomes (23,24).
Dex has an HR-slowing effect prior to CPB and at the concentration
used in the present study, the HR in patients in the Pre-Dex and
Dex groups was lower than that in the Control group at T2, T3, T4
and T5, but only had a negligible impact on MAP. After the
ascending aorta opens (recovers blood flow to the heart), it has no
effect on the heart's rebound rate and the HR. Therefore, reducing
myocardial oxygen consumption prior to CPB may be beneficial for
MIRI. Kehlet (25) pointed out that
the application of Dex during the perioperative period may have
important implications for Enhanced Recovery After Surgery (ERAS).
Of note, using minimally invasive techniques and fast-track cardiac
anesthesia for congenital heart disease surgery was reported to
promote early extubation and accelerate patient recovery, which
promoted ERAS (26,27).
Previous studies have indicated that Dex is able to
reduce the levels of cTnI after myocardial ischemia-reperfusion,
inhibit the release of the pro-inflammatory cytokine IL-8, promote
the production of the anti-inflammatory cytokine IL-10 and exert
antioxidant effects by reducing the level of MDA, thereby exerting
cardioprotective effects (5,22,28,29).
The most widely used clinical markers of myocardial injury are cTnI
and creatine kinase-MB. Transesophageal echocardiography cardiac
function monitoring and pro-brain natriuretic peptide may also be
used as indicators of myocardial function in response surgery
(30). However, cTnI is a highly
sensitive and specific biomarker in the diagnosis of myocardial
injury in the clinic (31). In
addition, Dex inhibits the release of catecholamines to reduce HR
(32), which prolongs coronary
perfusion and simultaneously reduces myocardial oxygen consumption,
thus improving the maintenance of the myocardial oxygen
supply-demand balance (33,34). Dex is also able to increase the
ischemic to non-ischemic myocardial blood flow ratio during
myocardial ischemia in the perioperative period (35,36).
The effect of Dex may be associated with the activation of
α2-adrenoceptors and its bidirectional regulation of the
coronary artery diameter (37),
thus preventing further aggravation of MIRI. The results of the
present trial indicated that the levels of cTnI in the Pre-Dex and
Dex groups were lower than those in the Control group at T6, T7 and
T8, which means the application of DEX may reduce myocardial injury
after CPB in patients with heart valve replacement surgery. This
effect may be due to alterations in the timing of the release of
myocardial injury markers into the blood, the peak time and the
elimination half-life.
A systemic inflammatory response is able to
aggravate myocardial damage (38).
Previous studies have indicated that infiltration of inflammatory
cells into the myocardium during MIRI leads to overproduction of
reactive oxygen species, which are key in MIRI (39). IL-8 is a pro-inflammatory cytokine;
mainly pro-inflammatory cells release a series of active products,
leading to the occurrence of inflammatory reactions. IL-10 is
mainly an anti-inflammatory cytokine whose major role is to inhibit
inflammatory responses. The present study indicated that the levels
of IL-8 in the Pre-Dex and Dex groups were lower than those in the
Control group at T6, T7 and T8, while the levels of IL-10 in the
Pre-Dex and Dex groups were higher than those in the Control group
at T6 and T7. Collectively, Dex administration alleviated MIRI by
inhibiting the inflammatory response. In the Pre-Dex and Dex
groups, IL-10 was lower compared with that in the control group at
T8. This may be due to the application of DEX promoting the release
of anti-inflammatory factor IL-10, inhibiting the pro-inflammatory
reactions and reducing MIRI, thereby maintaining the inflammation
at low levels. At T8, the level of IL-10 in the Dex group was
suddenly significantly lower than that of the Pre-Dex group. This
may be because the half-life of IL-10 in the body was only a few
hours. The Dex group promoted IL-10 secretion during surgery. At T8
time, most were likely metabolized. In the Pre-Dex group,
preconditioning induces myocardial the protection mechanism. At the
T8 time point, new IL-10 is produced. Therefore, at the T8 time
point, the IL-10 level of the Dex group is suddenly significantly
lower than that of the Pre-Dex group. The control group
demonstrated a strong inflammatory response, inducing
anti-inflammatory defense mechanisms and maintaining high levels of
IL-10. However, compared with those in the Dex group, the levels of
IL-10 in the Pre-Dex group decreased at T6 and T7. The present
results revealed that Dex perfusion is able to minimize the
inflammatory response, which may protect cardiomyocytes from MIRI
damage by inhibiting inflammatory factors.
MDA is an important product of lipid peroxidation in
cell membranes and is associated with the level of oxygen free
radicals. Due to defects in antioxidant defense mechanisms, an
increased level of oxygen free radicals may promote the release of
chemokines and cytokines via an inflammatory response, thus
resulting in myocardial cell injury (40). The results of the present study
suggested that the levels of MDA in the Pre-Dex and Dex groups were
lower than those in the Control group at T6, T7 and T8. The present
results suggested that the two Dex administration methods are able
to attenuate the oxidative stress reaction and may serve a role in
myocardial protection. However, the present results suggested that
Dex administration during the whole surgery is more effective than
Dex pre-conditioning. This effect may be caused by Dex-mediated
improvements in antioxidant defense mechanisms, thereby reducing
the production of MDA.
Previous studies have shown that reduced insulin
sensitivity leads to elevated blood glucose in patients undergoing
intracardiac surgery under CPB (41). The results of the present study
suggested that the concentration of blood glucose in the Pre-Dex
and Dex groups was lower than that in the Control group at T5, T6
and T7. This may be due to the fact that Dex application during the
perioperative period may increase insulin sensitivity, thereby
reducing the blood glucose levels.
In summary, the two Dex administration methods in
CPB rheumatic heart valve replacement surgery were demonstrated to
reduce myocardial injury, inhibit the release of pro-inflammatory
factors, promote the release of anti-inflammatory factors, enhance
the activity of antioxidant enzymes and reduce oxidative stress and
stress responses. Dex perfusion during the whole surgery was more
effective compared with Dex pre-conditioning. The study of
perioperative myocardial protection is an important aspect of the
application of ERAS in cardiac surgery. Therefore, the present
study may be of great significance to the development of ERAS in
cardiac surgery.
Acknowledgements
The authors would like to thank the Guizhou
Anesthesia and Organ Protection Laboratory for providing the test
platform. Furthermore, they thank Professor Liu Daxing, director at
the Department of Cardiovascular Surgery, Affiliated Hospital of
Zunyi Medical University (Zunyi, China) for his support.
Specifically, Professor Liu is the chief surgeon of all patients in
the present study.
Funding
The authors gratefully acknowledge the financial support by the
Chinese Society of Cardiothoracic Anesthesiology Pain Branch (grant
no. CSCVA-PM-2017001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. This trial was registered at http://www.ChiCTR.org.cn under the clinical trial
number ChiCTR-INR-17011955 (date of registration, July 12,
2017).
Authors' contributions
WC participated in the design and implementation of
the trial. YW implemented anesthesia, including the preparation of
various drugs for induction and maintenance of anesthesia, parallel
arterial puncture and central venous catheterization and tracheal
intubation after induction of anesthesia. ZP, DL and XC made
substantial contributions to the acquisition of data and analysis
and interpretation of the data. WC, YW, ZP and HW authenticate the
raw data in this study. HW made substantial contributions to
conception and design, provided guidance for the study and revised
it critically for important intellectual content and gave final
approval of the version to be published. All of the authors read
the final manuscript and agreed to its submission.
Ethics approval and consent to
participate
All of the research that was performed on the
subjects followed national regulations in accordance with the
relevant set of ethical principles. The Ethics Committee of the
Affiliated Hospital of Zunyi Medical College (Zunyi, China;
approval no. 8) approved the present study. All subjects provided
written informed consent to participate in this clinical study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li M, Xu S, Geng Y, Sun L, Wang R, Yan Y,
Wang H, Li Y, Yi Q, Zhang Y, et al: The protective effects of
L-carnitine on myocardial ischaemia-reperfusion injury in patients
with rheumatic valvular heart disease undergoing CPB surgery are
associated with the suppression of NF-KB pathway and the activation
of Nrf2 pathway. Clin Exp Pharmacol Physiol. 46:1001–1012.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: A delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Karwi QG, Bice JS and Baxter GF: Pre- and
postconditioning the heart with hydrogen sulfide (H2S) against
ischemia/reperfusion injury in vivo: A systematic review and
meta-analysis. Basic Res Cardiol. 113(6)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cope DK, Impastato WK, Cohen MV and Downey
JM: Volatile anesthetics protect the ischemic rabbit myocardium
from infarction. Anesthesiology. 86:699–709. 1997.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang K, Wu M, Xu J, Wu C, Zhang B, Wang G
and Ma D: Effects of dexmedetomidine on perioperative stress,
inflammation, and immune function: Systematic review and
meta-analysis. Br J Anaesth. 123:777–794. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sheikh TA, Dar BA, Akhter N and Ahmad N: A
Comparative study evaluating effects of intravenous sedation by
dexmedetomidine and propofol on patient hemodynamics and
postoperative outcomes in cardiac surgery. Anesth Essays Res.
12:555–560. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Riquelme JA, Westermeier F, Hall AR,
Vicencio JM, Pedrozo Z, Ibacache M, Fuenzalida B, Sobrevia L,
Davidson SM, Yellon DM, et al: Dexmedetomidine protects the heart
against ischemia-reperfusion injury by an endothelial eNOS/NO
dependent mechanism. Pharmacol Res. 103:318–327. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang JJ, Peng K, Zhang J, Meng XW and Ji
FH: Dexmedetomidine preconditioning may attenuate myocardial
ischemia/reperfusion injury by down-regulating the
HMGB1-TLR4-MyD88-NF-KB signaling pathway. PLoS One.
12(e0172006)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ren J, Li C, Liu Y, Liu H and Dong Z:
Protective effect of dexmedetomidine against myocardial
ischemia-reperfusion injury in rabbits. Acta Cir Bras. 33:22–30.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ammar AS, Mahmoud KM, Kasemy ZA and Helwa
MA: Cardiac and renal protective effects of dexmedetomidine in
cardiac surgeries: A randomized controlled trial. Saudi J Anaesth.
10:395–401. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dripps RD: New classification of physical
status. Anesthesiology. 24(111)1963.PubMed/NCBI
|
|
12
|
Caraballo C, Desai NR, Mulder H, Alhanti
B, Wilson FP, Fiuzat M, Felker GM, Piña IL, O'Connor CM, Lindenfeld
J, et al: Clinical implications of the New York heart Association
Classification. J Am Heart Assoc. 8(e014240)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xiong W, Qu Y, Chen H and Qian J: Insight
into long noncoding RNA-miRNA-mRNA axes in myocardial
ischemia-reperfusion injury: The implications for mechanism and
therapy. Epigenomics. 11:1733–1748. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shen Y, Liu X, Shi J and Wu X: Involvement
of Nrf2 in myocardial ischemia and reperfusion injury. Int J Biol
Macromol. 125:496–502. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ma C, Xu Z and Lv H: Low n-6/n-3 PUFA
ratio improves inflammation and myocardial ischemic reperfusion
injury. Biochem Cell Biol. 97:621–629. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Al-Fares A, Pettenuzzo T and Del Sorbo L:
Extracorporeal life support and systemic inflammation. Intensive
Care Med Exp. 7 (Suppl 1)(S46)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Natanov R, Gueler F, Falk CS, Kühn C, Maus
U, Boyle EC, Siemeni T, Knoefel AK, Cebotari S, Haverich A and
Madrahimov N: Blood cytokine expression correlates with early
multi-organ damage in a mouse model of moderate hypothermia with
circulatory arrest using cardiopulmonary bypass. PLoS One.
13(e0205437)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
O'Neill S, Ross JA, Wigmore SJ and
Harrison EM: The role of heat shock protein 90 in modulating
ischemia-reperfusion injury in the kidney. Expert Opin Investig
Drugs. 21:1535–1548. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mebazaa A, Pitsis AA, Rudiger A, Toller W,
Longrois D, Ricksten SE, Bobek I, De Hert S, Wieselthaler G,
Schirmer U, et al: Clinical review: Practical recommendations on
the management of perioperative heart failure in cardiac surgery.
Crit Care. 14(201)2010.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Zhang J, Jiang H, Liu DH and Wang GN:
Effects of dexmedetomidine on myocardial ischemia-reperfusion
injury through PI3K-Akt-mTOR signaling pathway. Eur Rev Med
Pharmacol Sci. 23:6736–6743. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yu J, Yang W, Wang W, Wang Z, Pu Y, Chen
H, Wang F and Qian J: Involvement of miR-665 in protection effect
of dexmedetomidine against oxidative stress injury in myocardial
cells via CB2 and CK1. Biomed Pharmacother.
115(108894)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xu Z, Wang D, Zhou Z, Chen Q, Zhang D,
Chen S, Jiang H, Jia C and Liu X: Dexmedetomidine attenuates renal
and myocardial ischemia/reperfusion injury in a dose-dependent
manner by inhibiting inflammatory response. Ann Clin Lab Sci.
49:31–35. 2019.PubMed/NCBI
|
|
23
|
Vijayan NK, Talwar V and Dayal M:
Comparative evaluation of the effects of pregabalin,
dexmedetomidine, and their combination on the hemodynamic response
and anesthetic requirements in patients undergoing laparoscopic
cholecystectomy: A randomized double-blind prospective study.
Anesth Essays Res. 13:515–521. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ye W, Hu Y, Wu Y, Zhu Z, Jin X and Hu Z:
Retrobulbar dexmedetomidine in pediatric vitreoretinal surgery
eliminates the need for intraoperative fentanyl and postoperative
analgesia: A randomized controlled study. Indian J Ophthalmol.
67:922–927. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kehlet H: Multimodal approach to control
postoperative pathophysiology and rehabilitation. Br J Anaesth.
78:606–617. 1997.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hanada S, Kurosawa A, Randall B, Van Der
Horst T and Ueda K: Impact of high spinal anesthesia technique on
fast-track strategy in cardiac surgery: Retrospective study. Reg
Anesth Pain Med: Nov 25, 2019 doi: 10.1136/rapm-2018-100213 (Epub
ahead of print).
|
|
27
|
Zientara A, Mariotti S, Matter-Ensner S,
Seifert B, Graves K, Dzemali O and Genoni M: Fast-track management
in off-pump coronary artery bypass grafting: Dexmedetomidine
provides rapid extubation and effective pain modulation. Thorac
Cardiovasc Surg. 67:450–457. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kim SH and Choi YS: Effects of
dexmedetomidine on malondialdehyde and proinflammatory cytokines
after tourniquet-induced ischemia-reperfusion injury in total knee
arthroplasty. Minerva Anestesiol. 86:223–224. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang J, Xia F, Zhao H, Peng K, Liu H,
Meng X, Chen C and Ji F: Dexmedetomidine-induced cardioprotection
is mediated by inhibition of high mobility group box-1 and the
cholinergic anti-inflammatory pathway in myocardial
ischemia-reperfusion injury. PLoS One. 14(e0218726)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Papp A, Uusaro A, Parviainen I,
Hartikainen J and Ruokonen E: Myocardial function and haemodynamics
in extensive burn trauma: Evaluation by clinical signs, invasive
monitoring, echocardiography and cytokine concentrations. A
prospective clinical study. Acta Anaesthesiol Scand. 47:1257–1263.
2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Vaz HA, Guimaraes RB and Dutra O:
Challenges in high-sensitive troponin assay interpretation for
intensive therapy. Rev Bras Ter Intensiva. 31:93–105.
2019.PubMed/NCBI View Article : Google Scholar : (In Portuguese,
En).
|
|
32
|
Yu Z, Zhang P, Wang H, Zhang L, Wei W,
Fang W and Mu X: Effects of dexmedetomidine versus remifentanil on
mothers and neonates during cesarean section under general
anesthesia. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
164:417–424. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kundra TS, Thimmarayappa A, Dhananjaya M
and Manjunatha N: Dexmedetomidine for prevention of skeletal muscle
ischaemia-reperfusion injury in patients with chronic limb
ischaemia undergoing aortobifemoral bypass surgery: A prospective
double-blind randomized controlled study. Ann Card Anaesth.
21:22–25. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kundra TS, Nagaraja PS, Singh NG,
Dhananjaya M, Sathish N and Manjunatha N: Effect of dexmedetomidine
on diseased coronary vessel diameter and myocardial protection in
percutaneous coronary interventional patients. Ann Card Anaesth.
19:394–398. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Peng K, Qiu Y, Li J, Zhang ZC and Ji FH:
Dexmedetomidine attenuates hypoxia/reoxygenation injury in primary
neonatal rat cardiomyocytes. Exp Ther Med. 14:689–695.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hashemian M, Ahmadinejad M, Mohajerani SA
and Mirkheshti A: Impact of dexmedetomidine on hemodynamic changes
during and after coronary artery bypass grafting. Ann Card Anaesth.
20:152–157. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Duncan D, Sankar A, Beattie WS and
Wijeysundera DN: Alpha-2 adrenergic agonists for the prevention of
cardiac complications among adults undergoing surgery. Cochrane
Database Syst Rev. 3(CD004126)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yeang C, Hasanally D, Que X, Hung MY,
Stamenkovic A, Chan D, Chaudhary R, Margulets V, Edel AL, Hoshijima
M, et al: Reduction of myocardial ischaemia-reperfusion injury by
inactivating oxidized phospholipids. Cardiovasc Res. 115:179–189.
2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kuznetsov AV, Javadov S, Margreiter R,
Grimm M, Hagenbuchner J and Ausserlechner MJ: The role of
mitochondria in the mechanisms of cardiac ischemia-reperfusion
injury. Antioxidants (Basel). 8(454)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xu X, Rui S, Chen C, Zhang G, Li Z, Wang
J, Luo Y, Zhu H and Ma X: Protective effects of astragalus
polysaccharide nanoparticles on septic cardiac dysfunction through
inhibition of TLR4/NF-KB signaling pathway. Int J Biol Macromol.
153:977–985. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhang DS, Liang GY, Liu DX, Yu J and Wang
F: Role of phosphorylated AMP-activated protein kinase (AMPK) in
myocardial insulin resistance after myocardial ischemia-reperfusion
during cardiopulmonary bypass surgery in dogs. Med Sci Monit.
25:4149–4158. 2019.PubMed/NCBI View Article : Google Scholar
|