Introduction
Epilepsy is a group of brain disorders characterized
by recurrent epileptic seizures, which is related to the abnormal
synchronous discharge of brain neurons (1,2).
Epilepsy has become one of the most common chronic brain diseases,
affecting ~1% of the world population (3). Repeated seizures can cause severe
physiological and mental damage to patients (4). Previous studies have demonstrated that
the pathogenesis of epilepsy might be related to structural or
functional damage in the limbic system (5-7).
Cell death and synaptic strength and plasticity are the primary
causes of epileptic injuries (8-10).
Nerve cell apoptosis is the primary form of epilepsy-induced cell
death, and is accompanied by metabolic abnormalities, gene
expression abnormalities and activation of cell death-related
proteases (11,12).
MicroRNAs (miRNAs/miRs) are endogenous non-coding
RNAs that are typically composed of 18-23 nucleotides (13). miRNAs bind with target mRNA, leading
to the inhibition of protein translation or degradation of the
target mRNA. By regulating target genes, miRNAs are involved in
multiple biological processes, including energy metabolism, cell
proliferation and apoptosis (14).
Previous studies have reported that miRNAs participating in
epilepsy cause brain damage; thus, miRNAs have been widely
investigated in epilepsy therapy (15-17).
For example, miR-132 was reported to affect hippocampal neuron
plasticity and contribute to epilepsy induction (18), and miR-141 was reported to protect
against neural apoptosis in epilepsy via suppressing p53(19). It was also demonstrated that
miR-135a-5p expression in children with temporal lobe epilepsy
(TLE) is significantly increased (20). Moreover, miR-135a-5p was reported to
induce the apoptosis of glioma, ovarian cancer and cardiomyocyte
cells, whereas miR-135a-5p inhibition significantly prevented
apoptosis (21,22). However, whether miR-135a-5p
participates in epilepsy-induced nerve cell apoptosis, as well as
the molecules that are involved in the process, are not completely
understood. Hence, the current study aimed to investigate the
function of miR-135a-5p in epilepsy.
Materials and methods
Cell culture and transfection
The BV2 microglia cell line (American Type Culture
Collection) was cultured in DMEM (Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (HyClone; Cytiva), 100
U/ml penicillin and 100 µg/ml streptomycin (Invitrogen; Thermo
Fisher Scientific, Inc.) at 5% CO2 and 37˚C.
BV2 cells (2x105 cells/well) were seeded
into six-well plates. miRNA-negative control (NC) inhibitor (100
nM; 5'-CUCGAUAGCGCAUGGUCCGAGCUA-3'), miR-135a-5p inhibitor (100 nM;
5'-AUACCGAAAAAUAAGGAUACACU-3'), miR-NC mimic (50 nM;
5'-CUGAACUGCUAGGACGCGUA-3'), miR-135a-5p mimic (50 nM;
5'-AGUGUAUCCUUAUUUUUCGGUAU-3'), si-NC (50 nM; sense,
5'-CGCCAACGCACGCGUAAC-3' and antisense,
5'-AAGCCGCUUCGACGACCGUGC-3'), and siRNA-sirtuin 1 (SIRT1; 50 nM;
sense, 5'-UGGUCUAGAUAAGCUGUUCCC-3' and antisense,
5'-GGGAACAGCUUAUCUAGACCA-3') were purchased from Shanghai
GenePharma Co., Ltd. Transfections into BV2 cells were performed
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After incubation at 37˚C for 48 h, cells were
collected for subsequent experiments. For treatment with KA, BV2
microglia cells were stimulated with 0.6 µm KA (Abcam) for 2 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from BV2 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA was reverse transcribed into cDNA using the
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.)
according to the manufacturer's protocol. Subsequently, qPCR was
performed using ABI Prism 7500 (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and SYBR-Green mix (Roche Diagnostics, Shanghai,
China). The thermocycling conditions were as follows: Initial
denaturation at 95˚C for 5 min, followed by 40 cycles of 95˚C for
30 sec, 55˚C for 30 sec, 72˚C for 45 sec, with a final extension at
72˚C for 10 min. The primer sequences were as follows: U6 forward,
5'-CGCAAGGATGACACGCAAAT-3' and reverse, 5'-GCAGGGTCCGAGGTATTC-3';
miR-135a-5p forward, 5'-AGTGUATCCTTATTTTTCGGTAT-3' and reverse,
5'-ATACCGAAAAATAAGGATACACT-3'; β-actin forward,
5'-ATGTGCGACGAAGACGAGAC-3' and reverse,
5'-CCTTCTGACCCATACCTACCAT-3' and SIRT1 forward,
5'-TTTTAAAGTTTTCAAAAGCCATT-3' and reverse,
5'-AATGGCTTTTGAAAACTTTAAA-3'. Relative expression levels of
miR-135a-5p and SIRT1 were quantified using the 2-∆∆Cq
method (23) and normalized to the
internal reference gene U6 and β-actin, respectively.
Dual luciferase reporter assay
The binding site between miR-135a-5p and SIRT1 was
predicted using TargetScan (version 7.1; www.targetscan.org/vert_71). BV2 cells
(1x105 cells/well) were seeded into 24-well plates and
co-transfected with pGL3-luciferase reporter plasmids (Promega
Corporation) containing either wild-type (WT; 0.5 µg) or mutant
(Mut; 0.5 µg) SIRT1 3'-untranslated region (UTR) and miR-135a-5p
mimic (50 nM) or negative control (NC) mimic (50 nM) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After incubation at 37˚C for 48 h, luciferase
activities were measured using a Dual Luciferase Assay system
(Promega Corporation). Firefly luciferase activities were
normalized to Renilla luciferase activities (Promega
Corporation).
Caspase-3/9 activity
Cultured BV2 cells were washed and harvested with
PBS and lysed using RIPA buffer [Roche Diagnostics (Shanghai) Co.,
Ltd.] with protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA).
Total protein concentration was measured using a BCA protein assay
kit (Beyotime Institute of Biotechnology). Proteins (5 µg) were
used to measure caspase activities using caspase-3 (cat. no. C1116)
and caspase-9 (cat. no. C1158) activity assay kits (Beyotime
Institute of Biotechnology) according to the manufacturer's
protocols.
Western blotting
Cell harvest and lysis were performed as
aforementioned. Protein concentration was determined using a BCA
assay (Beyotime Institute of Biotechnology). Protein samples were
mixed with SDS loading buffer. Proteins (20 µg per lane) were
separated via SDS-PAGE (8%) and electrotransferred onto PVDF
membranes. Following blocking with 5% dry milk in 0.1% TBS-Tween-20
(TBS-T) at room temperature for 2 h, the membranes were incubated
overnight at 4˚C with anti-SIRT1 (cat. no. 9475; 1:1,000; Cell
Signaling Technology, Inc.) and anti-GAPDH (cat. no. 2118; 1:1,000;
Cell Signaling Technology, Inc.) antibodies in TBS-T. Following
washing with 0.1% TBS-T and incubation with goat anti-rabbit IgG
HRP-conjugated secondary antibody (cat. no. 7074; 1:2,000; Cell
Signaling Technology, Inc.) for 1 h at 37˚C, the membranes were
thoroughly washed again. Protein bands were visualized using ECL
Prime Western blot analysis detection reagent (EMD Millipore).
GAPDH was used as the loading control. Densitometry was analyzed
using ImageJ (version 1.50; National Institutes of Health).
Flow cytometric apoptosis assay
Cell apoptosis was assessed using the Annexin V/Dead
Cell Apoptosis kit (Thermo Fisher Scientific, Inc.). Cultured BV2
cells (1x106) from each group were harvested and washed
with PBS. Following centrifugation at 2,000 x g for 3 min at room
temperature, cells were suspended in 100 µl PBS, mixed with 5 µl
Annexin V-FITC and 5 µl PI and incubated for 10-15 min in the dark
at room temperature. Apoptotic cells at early
(FITC+/PI-) and advanced stages
(FITC+/PI+) were considered as apoptotic
cells. The apoptotic cell percentage were measured by FACSCalibur
flow cytometry (BD Biosciences) and data were analyzed by FlowJo
(version 7.6.3; FlowJo LLC).
MTT assay
BV2 cells (1x104 cells/well) were
cultured at 37˚C in 96-well plates for 0, 24, 48 or 72 h. Cells
were incubated with 20 µl MTT solution at 37˚C for 3 h.
Subsequently, the supernatant was removed, and 150 µl DMSO
(Sigma-Aldrich; Merck KGaA) was added to dissolve the formazan
products. Following gentle agitation for 15 min, the absorbance was
measured using a microplate reader at a wavelength of 570 nm.
Statistical analysis
Each experiment was conducted three times. Data are
analyzed by SPSS (version 19; IBM Corp.). Data are presented as the
mean ± SEM. Comparisons between two groups were analyzed using a
Student's t-test. Comparisons among multiple groups were analyzed
using one-way ANOVA followed by Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
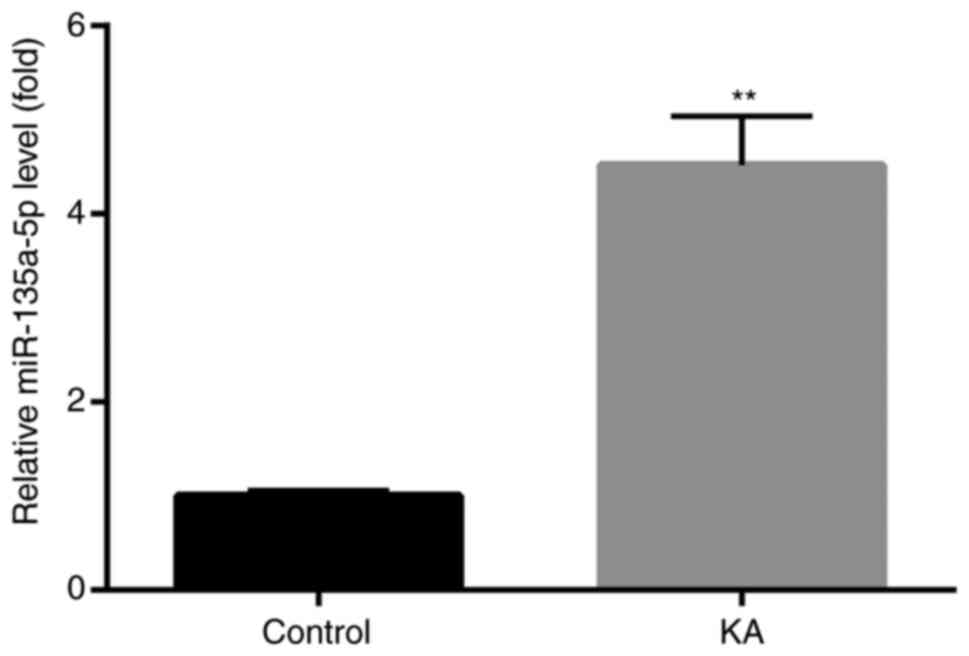
Increased miR-135a-5p expression in
KA-induced in vitro epilepsy models
To assess whether miR-135a-5p was involved in
epilepsy, changes of miR-135a-5p expression in BV2 microglia were
investigated. KA-treated BV2 microglia have been reported to
effectively induce abnormal protein expression, cell apoptosis and
other typical post-epileptic reactions (24,25).
The results demonstrated that miR-135a-5p expression levels were
significantly increased in KA-treated cells compared with controls
(Fig. 1).
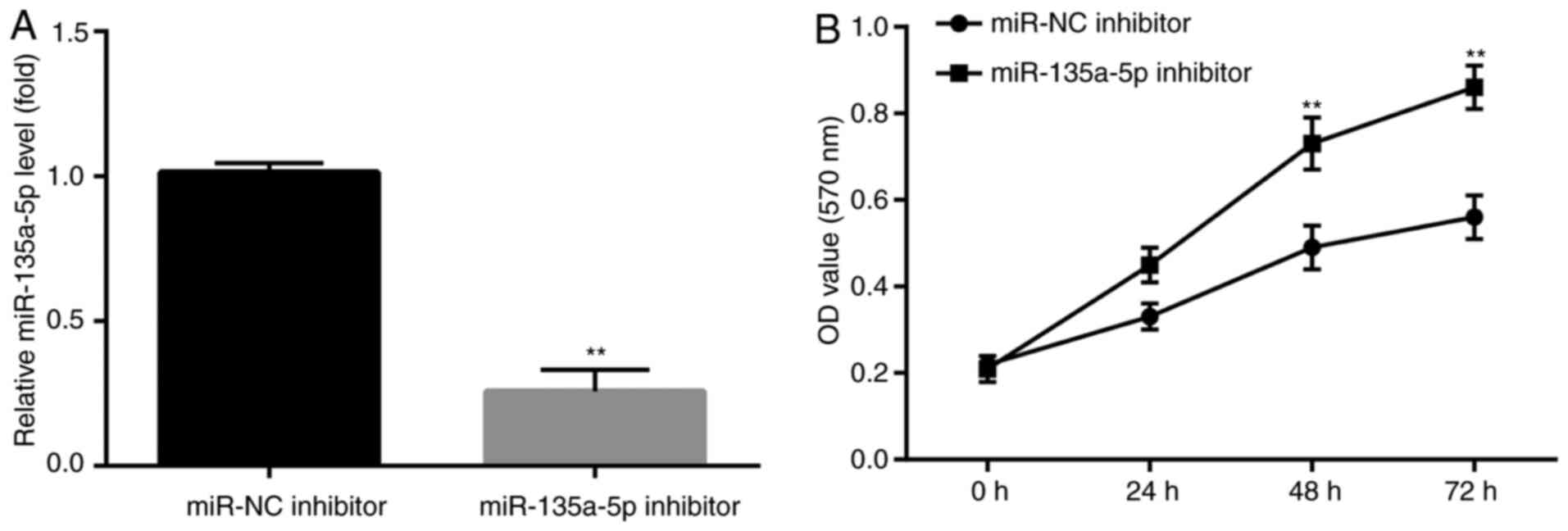
miR-135a-5p inhibitor blocks
KA-induced apoptosis
To further investigate whether miR-135a-5p
participated in KA-induced post-epileptic responses, BV2 cells were
transfected with miR-135a-5p inhibitor prior to KA treatment.
Subsequently, the effects of miR-135a-5p inhibitor
pre-administration on KA-induced cells were assessed. miR-135a-5p
inhibitor significantly decreased miR-135a-5p expression compared
with the NC group (Fig. 2A). In
addition, at 48 and 72 h post-KA treatment, the MTT assay results
demonstrated that miR-135a-5p inhibitor significantly increased BV2
cell numbers compared with the NC group (Fig. 2B).
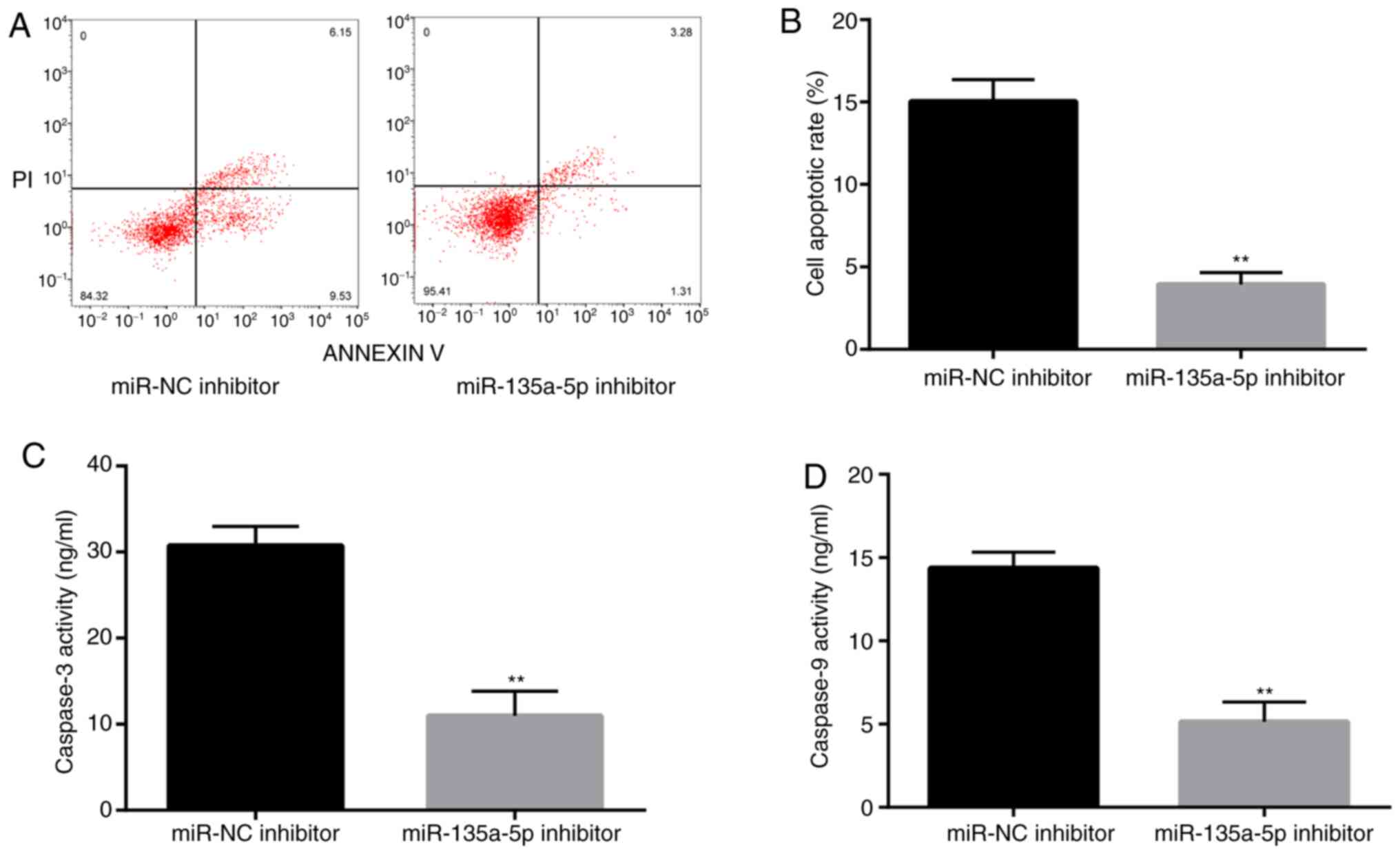
To assess whether miR-135a-5p is involved in
epilepsy-induced apoptosis, flow cytometry was performed to detect
the rate of apoptosis in KA-treated BV2 cells. The rate of
apoptosis in miR-135a-5p inhibitor-transfected cells was
significantly decreased compared with the NC group (Fig. 3A and B). Subsequently, caspase-3 and caspase-9
activities, which are closely related to apoptosis, were measured
(26,27). The results demonstrated that
caspase-3 and caspase-9 activities were significantly suppressed in
the miR-135a-5p inhibitor group compared with the NC group
(Fig. 3C and D). The results indicated that miR-135a-5p
participated in the pathological response of KA-induced epilepsy
models by promoting apoptosis.
miR-135a-5p regulates SIRT1
expression
To detect how miR-135a-5p participates in cell
apoptosis, the potential target genes of miR-135-5p were identified
using TargetScan (www.targetscan.org/vert_71). The results indicated
that the 3'-UTR of SIRT1 mRNA might be targeted by miR-135a-5p
(Fig. 4A). To assess whether
miR-135a-5p regulated SIRT1 expression, a mutant SIRT1 3'-UTR
sequence, which was unable to bind to miR-135a-5p, was designed
(Fig. 4A). Subsequently, BV2 cells
were co-transfected with miR-135a-5p or NC mimic and WT or Mut
SIRT1 3'-UTR-containing luciferase reporter plasmids. In
miR-135a-5p mimic-transfected BV2 cells, a significant increase in
miR-135a-5p expression was detected (Fig. 4B). The dual luciferase assay results
demonstrated that miR-135a-5p mimic suppressed the luciferase
reporter activities of WT SIRT1 3'-UTR-transfected BV2 cells, but
not Mut SIRT1 3'-UTR-transfected BV2 cells, which indicated that
miR-135a-5p targeted the 3'-UTR of SIRT1 (Fig. 4C).
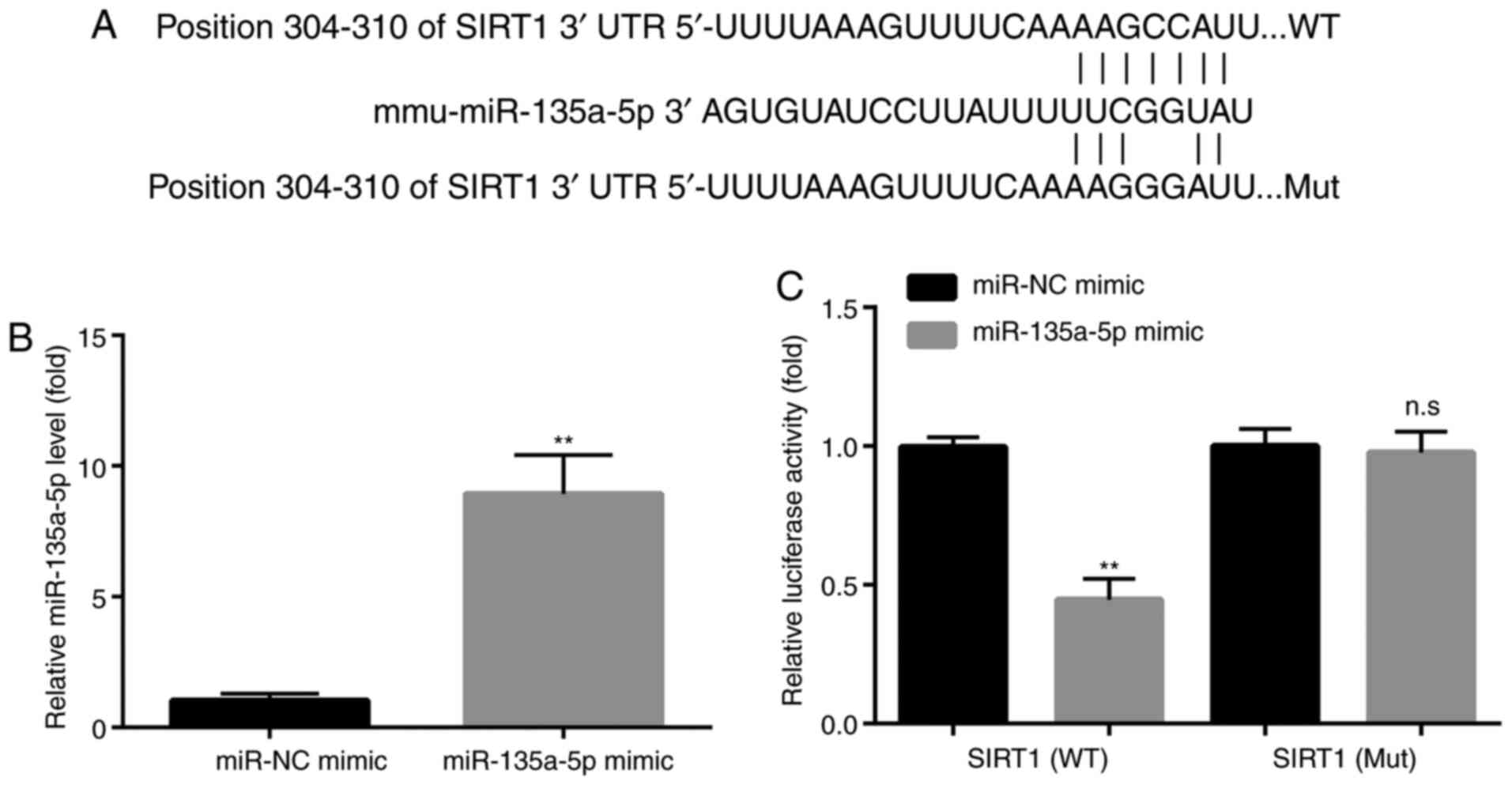 | Figure 4miR-135a-5p targets SIRT1. (A) SIRT1
is predicted to be a target of miR-135a-5p via TargetScan analysis.
(B) Reverse transcription-quantitative PCR showed that compared
with the miR-NC mimic group, miR-135a-5p mimic significantly
increased the levels of miR-135a-5p in BV2 microglia cells,
indicating the successful transfection of miR-135a-5p mimic in BV2
microglia cells. (C) Dual luciferase assay showed that miR-135a-5p
mimic decreased the luciferase activity in BV2 microglia cells
transfected with WT SIRT1 3'-UTR but not mutant SIRT1 3'-UTR,
indicating that miR-135a-5p targeted the 3'-UTR of SIRT1.
**P<0.01 vs. miR-NC mimic. n.s, no significant
difference; SIRT1, sirtuin 1; miR, microRNA; NC, negative control;
UTR, untranslated region; WT, wild-type; Mut, mutant. |
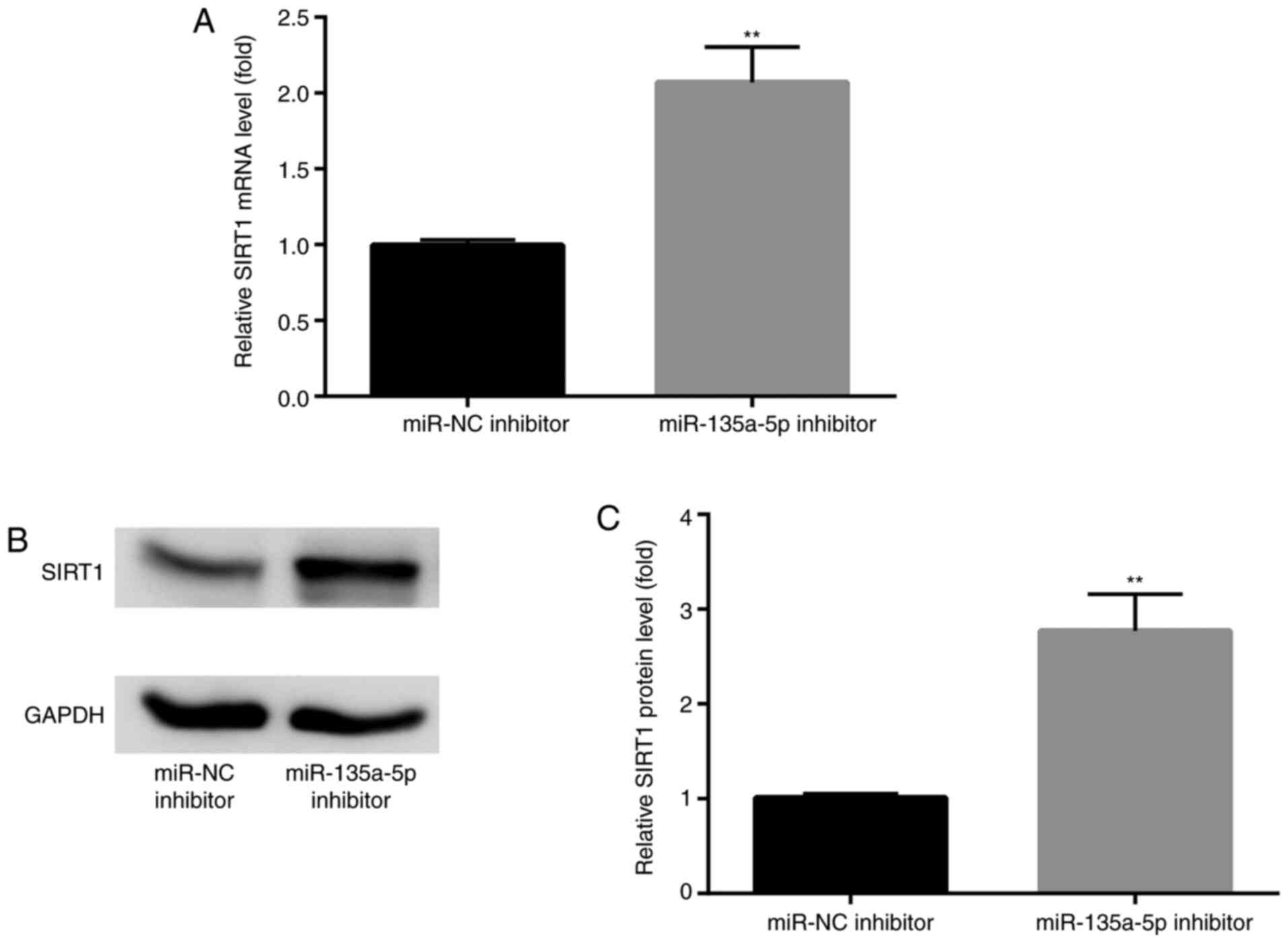
To further detect the effects of miR-135a-5p on
SIRT1 expression, BV2 cells were transfected with miR-135a-5p
inhibitor. The results indicated that SIRT1 mRNA and protein
expression levels were significantly enhanced compared with NC
groups (Fig. 5A-C).
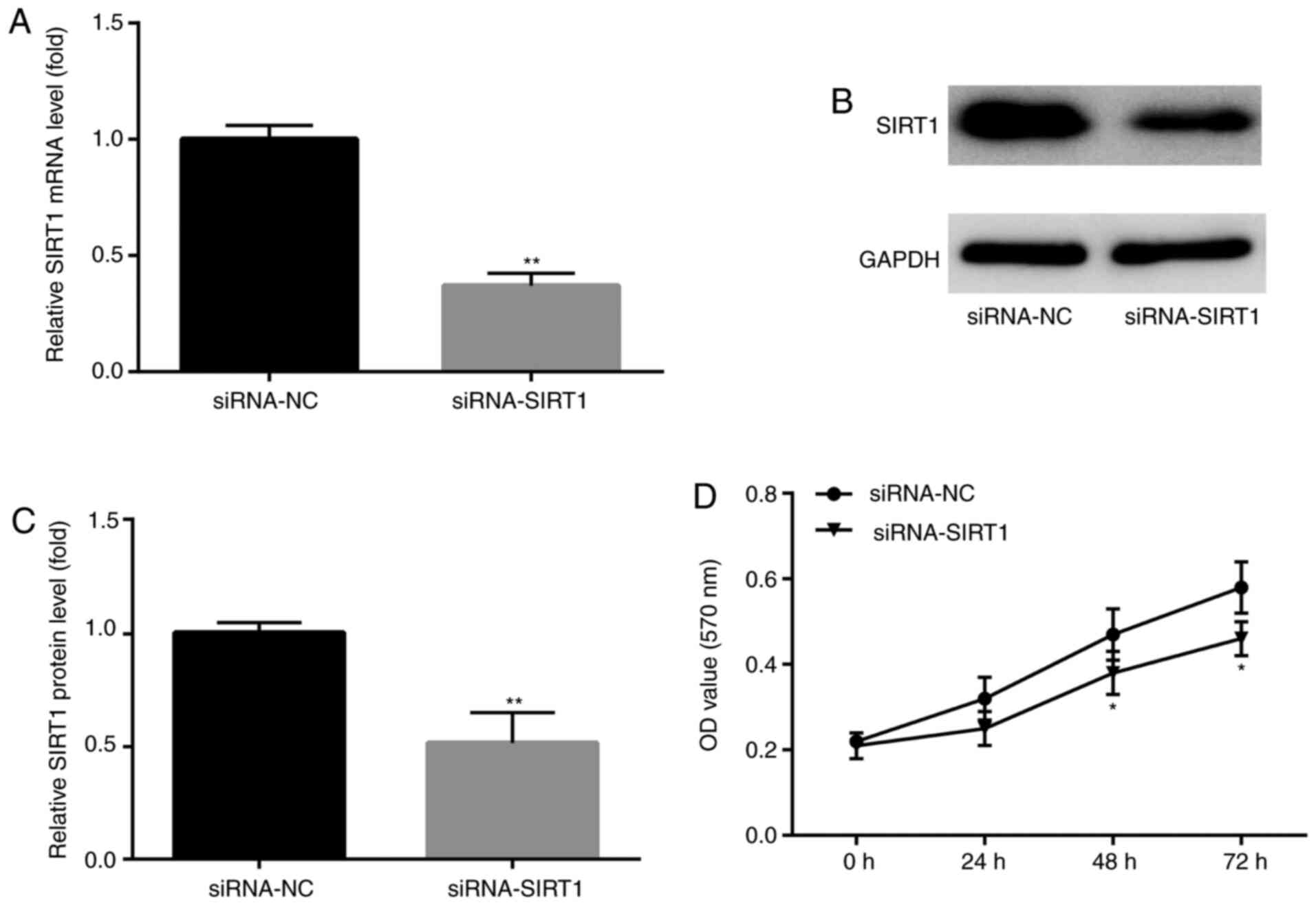
siRNA-SIRT1 enhances KA-induced
apoptosis
SIRT1 participates in a variety of important
biological processes, including cell apoptosis and survival
(28,29). To assess whether SIRT1 participated
in KA-induced post-epileptic responses, siRNA-SIRT1 was designed
and transfected into BV2 cells prior to KA treatment to investigate
the effects of siRNA-SIRT1 pre-administration on KA-induced
epilepsy. SIRT1 mRNA and protein expression levels were
significantly decreased in SIRT1 siRNA-transfected BV2 cells
compared with NC groups (Fig.
6A-C). In addition, at 48 and 72 h after KA treatment, the MTT
assay results demonstrated that siRNA-SIRT1 significantly decreased
BV2 cell numbers compared with the siRNA-NC group (Fig. 6D).
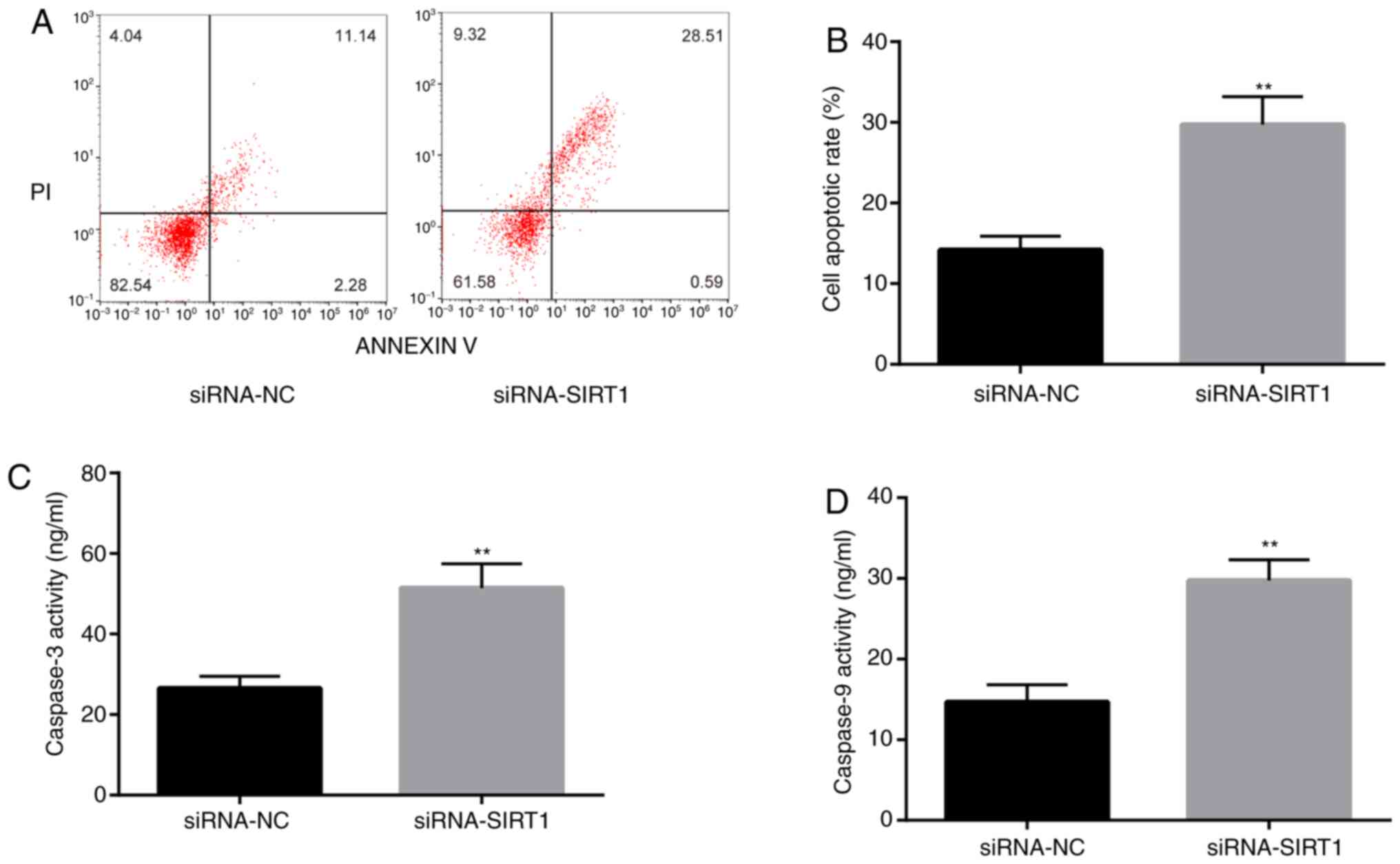
To confirm that SIRT1 was involved in
epilepsy-induced apoptosis, flow cytometry was performed to detect
the rate of apoptosis in KA-treated BV2 cells. The rate of
apoptosis of siRNA-SIRT1-transfected cells was significantly higher
compared with the siRNA-NC group (Fig.
7A and B). Moreover, caspase-3
and caspase-9 activities were increased in the siRNA-SIRT1 group
compared with the siRNA-NC group (Fig.
7C and D). The results
suggested that SIRT1 might be involved in the pathological response
of KA-induced epilepsy model by inhibiting apoptosis.
SIRT1 participates in the regulation
of miR-135a-5p on epilepsy
To study the relationship between SIRT1 and the
biological phenotype induced by miR-135a-5p inhibitor, KA-treated
BV2 cells were co-transfected with miR-135a-5p inhibitor and SIRT1
siRNA. Subsequently, flow cytometry was performed (Fig. 8A and B). miR-135a-5p inhibitor-induced
reductions in the rate of apoptosis were attenuated by SIRT1
knockdown. In addition, miR-135a-5p inhibitor-mediated reductions
in caspase-3 and caspase-9 activities were also reversed by
co-transfection with SIRT1 siRNA (Fig.
8C and D). As presented in
Fig. 8E, miR-135a-5p
inhibitor-induced increases in cell number were inhibited by
co-transfection with siRNA-SIRT1. The aforementioned results
suggested that the effects of the miR-135a-5p inhibitor on the
KA-induced epilepsy model were impaired by SIRT1 knockdown.
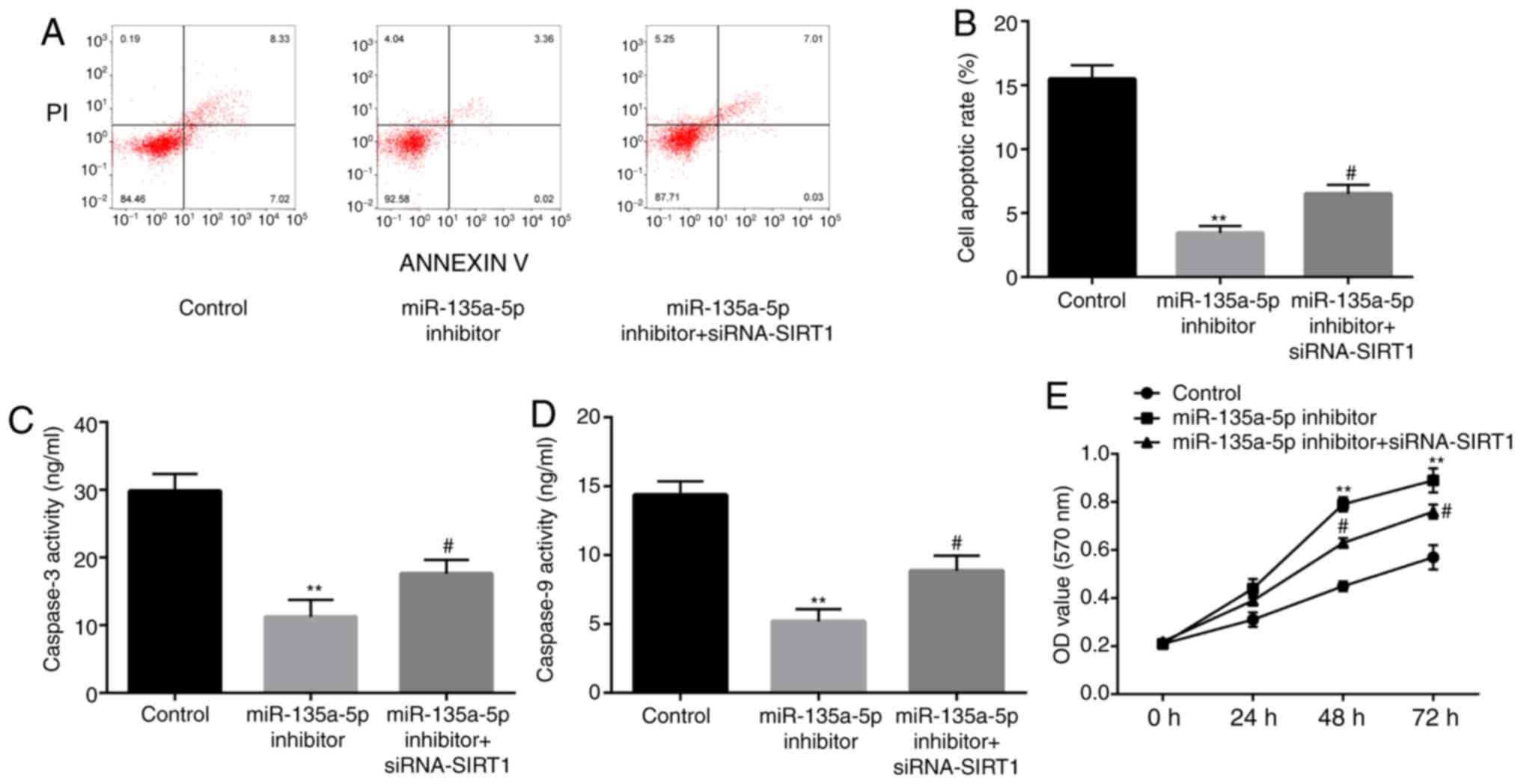 | Figure 8SIRT1 inhibitor reverses the effects
of miR-135-5p inhibitor on the reduction of nerve cell apoptosis.
(A and B) In a KA-induced in vitro model of epilepsy in
microglia cells, at 24 h after incubation, flow cytometry assay
showed that compared with the control group, miR-135a-5p inhibitor
significantly decreased the cell apoptosis rate of BV2 microglia
cells, which is attenuated by siRNA-SIRT1. BCA protein assay showed
that that compared with the control group, miR-135a-5p inhibitor
significantly decreased the activity of (C) caspase-3 and (D)
caspase-9 in BV2 microglia cells, which is attenuated by
siRNA-SIRT1. (E) After incubation of BV2 microglia cells for 24, 48
and 72 h, MTT assay showed that compared with the control group,
miR-135a-5p inhibitor significantly increased the cell
proliferation, which is attenuated by siRNA-SIRT1.
**P<0.01 vs. control; #P<0.05 vs.
miR-135a-5p inhibitor. SIRT1, sirtuin 1; siRNA, small interfering
RNA; NC, negative control; miR, microRNA; OD, optical density. |
Discussion
miRNAs are widely involved in neural damage
following seizures (30), but the
molecular mechanisms are not completely understood. In the present
study, miR-135a-5p expression was significantly upregulated in a
KA-induced in vitro epilepsy model, and miR-135a-5p
inhibitor effectively increased BV2 cell proliferation and
inhibited cell apoptosis. Moreover, the results indicated that
SIRT1 expression was regulated by miR-135a-5p, and SIRT1 knockdown
impaired the effects of the miR-135a-5p inhibitor on KA-treated BV2
cells.
Numerous miRNAs have been reported to be abnormally
expressed in patients with epilepsy and epilepsy models, and
certain miRNAs have been confirmed to be involved in neural damage
following seizures (21,22,31).
In the present study, miR-135a-5p expression levels in a KA-induced
epilepsy model were markedly increased. Similarly, Wu et al
(20) reported an abnormal increase
in miR-135a-5p expression levels in children with TLE, which was
also observed in a rat model. Alsharafi and Xiao (32) also confirmed that miR-135a-5p was
upregulated in adult patients with TLE and animal models. The
aforementioned results indicated that increased miR-135a-5p
expression was a co-occurrence and characteristic phenotype in
different epilepsy models, which suggested that miR-135a-5p might
serve as a potential biomarker of epilepsy. In addition, previous
studies demonstrated that miR-135a-5p inhibition effectively
alleviated epilepsy-induced nerve injury (20,33).
In the present study, the number of BV2 cells was significantly
increased and the rate of apoptosis was notably decreased by
miR-135a-5p inhibitor transfection, which suggested that
miR-135a-5p might serve as a potential therapeutic target for
epilepsy. However, the mRNA targets of miR-135a-5p require further
investigation.
Using TargetScan, SIRT1 was predicted as a potential
mRNA target for miR-135a-5p. Additionally, the dual luciferase
reporter assay results further suggested that SIRT1 was targeted by
miR-135a-5p. SIRT1 expression levels were increased by miR-135a-5p
inhibitor. Collectively, the results indicated that SIRT1 was a
target of miR-135a-5p in a KA-induced in vitro model of
epilepsy. Subsequently, the role of SIRT1 and the interaction
between SIRT1 and miR-135a-5p in epilepsy were investigated.
SIRT1 is an important type III histone deacetylase
that regulates gene expression by catalyzing histone deacetylation
and is necessary for SIRT1 to exert its deacetylase activity in the
presence of NAD+ (34,35).
SIRT1 is involved in numerous important physiological processes,
including chromosome remodeling, transcriptional inhibition, energy
metabolism, cell survival and apoptosis (28,29).
SIRT1 has also been reported to be involved in epilepsy,
Huntington's disease and other mental diseases (36). Moreover, SIRT1 protein expression
levels and activities are altered at different time points
following seizure (37-40).
A study demonstrated that SIRT1 protein expression levels and
activity levels are increased in patients and rats at 1 h
post-seizure (37). However,
another study indicated that SIRT1 protein expression was decreased
at a longer time after seizure (38), which was consistent with the results
of the present study. Collectively, the aforementioned results
suggested that SIRT1 might serve different functions at different
time points after a seizure and might also participate in
epilepsy-induced cell apoptosis.
In addition, siRNA-SIRT1 effectively inhibited BV2
microglia proliferation and promoted microglia apoptosis. Moreover,
SIRT1 inhibition attenuated, but did not completely block the
effects mediated by miR-135a-5p inhibitor. There are a number of
potential explanations for the aforementioned findings: i) There
was a high level of SIRT1 protein expression following siRNA
transfection, and the residual SIRT1 protein might be sufficient to
regulate cell survival; ii) miR-135a-5p might serve roles via other
target proteins, for example, it has been reported that potassium
voltage-gated channel subfamily Q member 3, caspase activity and
apoptosis inhibitor 1 (CAAP1) and other proteins are regulated by
miR-135a-5p, and participate in cell proliferation and apoptosis
(20,33); and iii) as aforementioned, SIRT1
protein expression levels and activity levels were dynamically
altered post-seizure, thus inhibiting SIRT1 protein expression
might trigger other cellular mechanisms, resulting in a
compensatory effect.
In conclusion, the results of the present study
suggested that miR-135a-5p participated in epilepsy-induced cell
apoptosis via a SIRT1-related signaling pathway in a KA-induced BV2
microglia epilepsy model. Therefore, the present study suggested a
potential therapeutic target and biomarker for post-epileptic
intervention and provided an important experimental basis for the
treatment of post-epileptic neural damage.
However, the present study had two key limitations:
i) miR-135a-5p and SIRT1 overexpression experiments were not
conducted and ii) experiments were not performed in multiple cell
lines or in vivo.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
YW, ZhiY, KZ, YW and YZ performed the experiments
and the data analyses. ZhuY designed the experiments and prepared
the manuscript. YW and ZhuY confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de Kinderen RJ, Lambrechts DA, Wijnen BF,
Postulart D, Aldenkamp AP, Majoie MH and Evers SM: An economic
evaluation of the ketogenic diet versus care as usual in children
and adolescents with intractable epilepsy: An interim analysis.
Epilepsia. 57:41–50. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Proposal for revised classification of
epilepsies and epileptic syndromes. Commission on classification
and terminology of the international league against epilepsy.
Epilepsia. 30:389–399. 1989.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fiest KM, Sauro KM, Wiebe S, Patten SB,
Kwon CS, Dykeman J, Pringsheim T, Lorenzetti DL and Jetté N:
Prevalence and incidence of epilepsy: A systematic review and
meta-analysis of international studies. Neurology. 88:296–303.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fazel S, Wolf A, Långström N, Newton CR
and Lichtenstein P: Premature mortality in epilepsy and the role of
psychiatric comorbidity: A total population study. Lancet.
382:1646–1654. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Engel J Jr, Thompson PM, Stern JM, Staba
RJ, Bragin A and Mody I: Connectomics and epilepsy. Curr Opin
Neurol. 26:186–194. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Goldberg EM and Coulter DA: Mechanisms of
epileptogenesis: A convergence on neural circuit dysfunction. Nat
Rev Neurosci. 14:337–349. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Lillis KP, Wang Z, Mail M, Zhao GQ,
Berdichevsky Y, Bacskai B and Staley KJ: Evolution of network
synchronization during early epileptogenesis parallels synaptic
circuit alterations. J Neurosci. 35:9920–9934. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Leite JP, Neder L, Arisi GM, Carlotti CG
Jr, Assirati JA and Moreira JE: Plasticity, synaptic strength, and
epilepsy: What can we learn from ultrastructural data? Epilepsia.
46 (Suppl 5):S134–S141. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Toth Z, Yan XX, Haftoglou S, Ribak CE and
Baram TZ: Seizure-induced neuronal injury: Vulnerability to febrile
seizures in an immature rat model. J Neurosci. 18:4285–4294.
1998.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dingledine R, Varvel NH and Dudek FE: When
and how do seizures kill neurons, and is cell death relevant to
epileptogenesis? Adv Exp Med Biol. 813:109–122. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Henshall DC and Simon RP: Epilepsy and
apoptosis pathways. J Cereb Blood Flow Metab. 25:1557–1572.
2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chelyshev IuA, Cherepnev GV and Saĭtkulov
KI: Apoptosis in the nervous system. Ontogenez. 32:118–129.
2001.PubMed/NCBI(In Russian).
|
|
13
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Winden KD, Karsten SL, Bragin A, Kudo LC,
Gehman L, Ruidera J, Geschwind DH and Engel J Jr: A systems level,
functional genomics analysis of chronic epilepsy. PLoS One.
6(e20763)2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Henshall DC: MicroRNA and epilepsy:
Profiling, functions and potential clinical applications. Curr Opin
Neurol. 27:199–205. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Reschke CR and Henshall DC: microRNA and
epilepsy. Adv Exp Med Biol. 888:41–70. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guo J, Wang H, Wang Q, Chen Y and Chen S:
Expression of p-CREB and activity-dependent miR-132 in temporal
lobe epilepsy. Int J Clin Exp Med. 7:1297–306. 2014.PubMed/NCBI
|
|
19
|
Liu D, Li S, Gong L, Yang Y, Han Y, Xie M
and Zhang C: Suppression of microRNA-141 suppressed p53 to protect
against neural apoptosis in epilepsy by SIRT1 expression. J Cell
Biochem. 120:9409–9420. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu X, Wang Y, Sun Z, Ren S, Yang W, Deng
Y, Tian C, Yu Y and Gao B: Molecular expression and functional
analysis of genes in children with temporal lobe epilepsy. J Integr
Neurosci. 18:71–77. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wu S, Lin Y, Xu D, Chen J, Shu M, Zhou Y,
Zhu W, Su X, Zhou Y, Qiu P and Yan G: MiR-135a functions as a
selective killer of malignant glioma. Oncogene. 31:3866–3874.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang T, Shao Y, Chu TY, Huang HS, Liou
YL, Li Q and Zhou H: MiR-135a and MRP1 play pivotal roles in the
selective lethality of phenethyl isothiocyanate to malignant glioma
cells. Am J Cancer Res. 6:957–972. 2016.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Reddy DS and Kuruba R: Experimental models
of status epilepticus and neuronal injury for evaluation of
therapeutic interventions. Int J Mol Sci. 14:18284–18318.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ben-Ari Y: Limbic seizure and brain damage
produced by kainic acid: Mechanisms and relevance to human temporal
lobe epilepsy. Neuroscience. 14:375–403. 1985.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hakem R, Hakem A, Duncan GS, Henderson JT,
Woo M, Soengas MS, Elia A, de la Pompa JL, Kagi D, Khoo W, et al:
Differential requirement for caspase 9 in apoptotic pathways in
vivo. Cell. 94:339–352. 1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shi Y: Mechanisms of caspase activation
and inhibition during apoptosis. Mol Cell. 9:459–470.
2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yamakuchi M, Ferlito M and Lowenstein CJ:
miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci
USA. 105:13421–13426. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hall AM, Brennan GP, Nguyen TM,
Singh-Taylor A, Mun HS, Sargious MJ and Baram TZ: The role of Sirt1
in Epileptogenesis. eNeuro. 4(ENEURO.0301-16.2017)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cava C, Manna I, Gambardella A, Bertoli G
and Castiglioni I: Potential role of miRNAs as theranostic
biomarkers of epilepsy. Mol Ther Nucleic Acids. 13:275–290.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu DZ, Tian Y, Ander BP, Xu H, Stamova
BS, Zhan X, Turner RJ, Jickling G and Sharp FR: Brain and blood
microRNA expression profiling of ischemic stroke, intracerebral
hemorrhage, and kainate seizures. J Cereb Blood Flow Metab.
30:92–101. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Alsharafi W and Xiao B: Dynamic expression
of MicroRNAs (183, 135a, 125b, 128, 30c and 27a) in the Rat
Pilocarpine model and temporal lobe epilepsy patients. CNS Neurol
Disord Drug Targets. 14:1096–1102. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li BG, Wu WJ, Zheng HC, Yang HF, Zuo YX
and Cui XP: Long noncoding RNA GAS5 silencing inhibits the
expression of KCNQ3 by sponging miR-135a-5p to prevent the
progression of epilepsy. Kaohsiung J Med Sci. 35:527–534.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gräff J, Kahn M, Samiei A, Gao J, Ota KT,
Rei D and Tsai LH: A dietary regimen of caloric restriction or
pharmacological activation of SIRT1 to delay the onset of
neurodegeneration. J Neurosci. 33:8951–8960. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cantó C and Auwerx J: NAD+ as a signaling
molecule modulating metabolism. Cold Spring Harb Symp Quant Biol.
76:291–298. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Maiese K: The mechanistic target of
rapamycin (mTOR) and the silent mating-type information regulation
2 homolog 1 (SIRT1): Oversight for neurodegenerative disorders.
Biochem Soc Trans. 46:351–360. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang SJ, Zhao XH, Chen W, Bo N, Wang XJ,
Chi ZF and Wu W: Sirtuin 1 activation enhances the
PGC-1α/mitochondrial antioxidant system pathway in status
epilepticus. Mol Med Rep. 11:521–526. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang D, Li Z, Zhang Y, Wang G, Wei M, Hu
Y, Ma S, Jiang Y, Che N, Wang X, et al: Targeting of
microRNA-199a-5p protects against pilocarpine-induced status
epilepticus and seizure damage via SIRT1-p53 cascade. Epilepsia.
57:706–716. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kim D, Nguyen MD, Dobbin MM, Fischer A,
Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, et
al: SIRT1 deacetylase protects against neurodegeneration in models
for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J.
26:3169–3179. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jiang M, Wang J, Fu J, Du L, Jeong H, West
T, Xiang L, Peng Q, Hou Z, Cai H, et al: Neuroprotective role of
Sirt1 in mammalian models of Huntington's disease through
activation of multiple Sirt1 targets. Nat Med. 18:153–158.
2011.PubMed/NCBI View Article : Google Scholar
|